Polysaccharides from Volvariella volvacea Mushroom: Extraction, Biological Activities and Cosmetic Efficacy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Chemical Composition Analysis
2.4. Polysaccharide Extraction
2.4.1. Polysaccharide Extraction Using Hot Water Shaking (HS)
2.4.2. Polysaccharide Extraction Using Ultrasonic Assistance (UA)
2.4.3. Polysaccharide Extraction Using Microwave Assistance (MA)
2.5. Determination of Polysaccharide Contents
2.6. Determination of Beta-Glucan Content
2.7. Determination of Antioxidant Capacity
2.7.1. ABTS Radical Scavenging Capacity Assay
2.7.2. Ferric-Reducing Antioxidant Power (FRAP) Assay
2.7.3. Lipid Peroxidation Inhibition Activity Assay
2.8. Determination of Tyrosinase Inhibitory Activity
2.9. Determination of Elastase Inhibitory Activity
2.10. UV Absorption Scanning
2.11. Cytotoxicity Test
2.11.1. Cell Culture
2.11.2. MTT Cytotoxicity Test
2.12. Development of Gel Cream Containing VVP
2.12.1. Stability Test for Gel Cream Containing VVP under Various Conditions
2.12.2. Stability Evaluation
2.13. Determination of Sun Protection Factor
2.14. Irritation Test Measurement
2.15. In Vivo Efficacy Test on Gel Cream Containing VVP
2.16. Statistical Analysis
3. Results
3.1. Chemical Composition
3.2. Extraction Yield
3.3. Solubility of VVP
3.4. Polysaccharide Content
3.5. Beta-Glucan Content
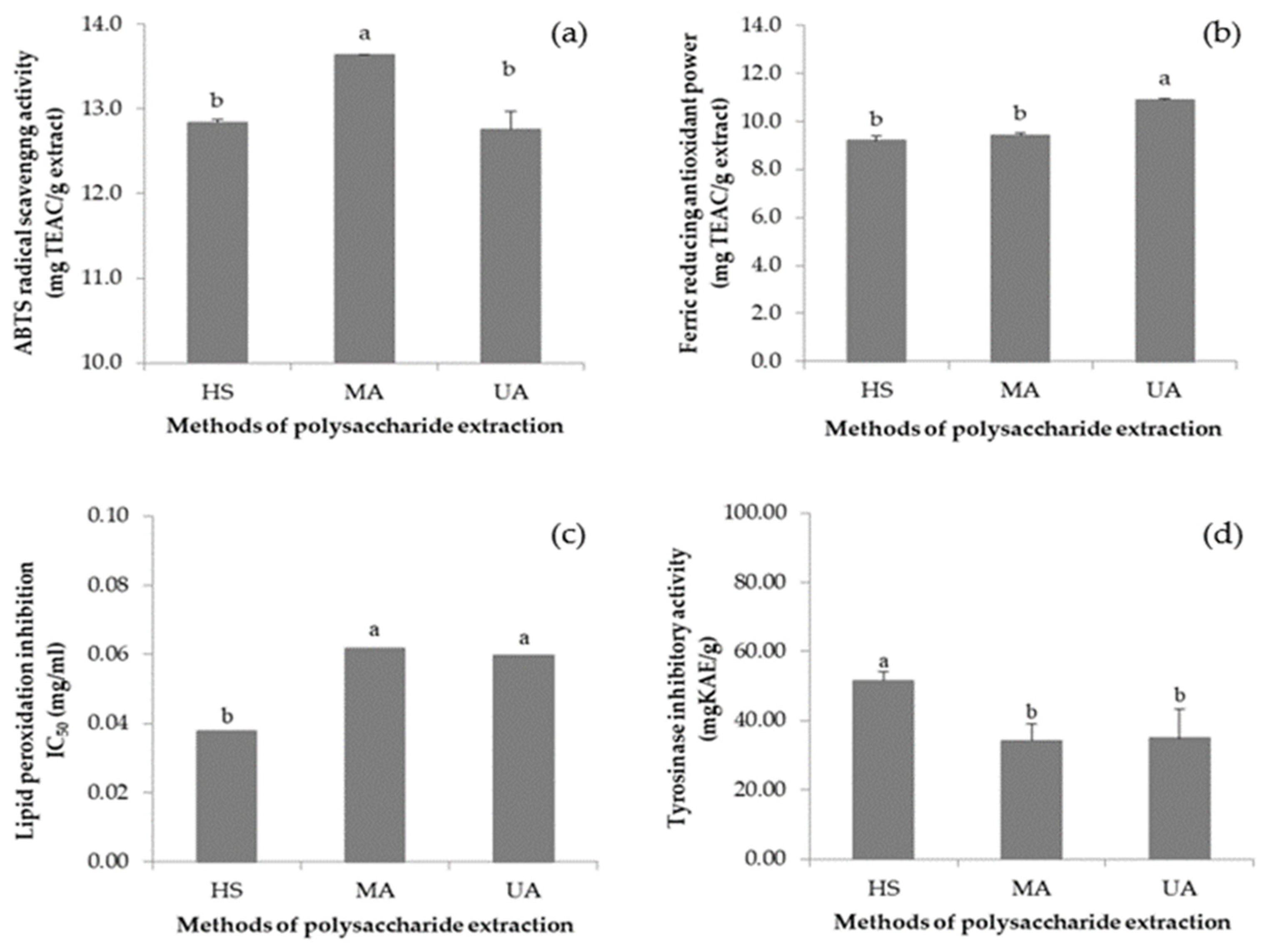
3.6. VVP Antioxidant Capacity
3.7. VVP Tyrosinase Inhibitory Activity
3.8. VVP Elastase Inhibitory Activity
3.9. VVP UV Absorption Scanning
3.10. VVP Cell Toxicity
3.11. Development of Facial Gel Cream Containing VVP
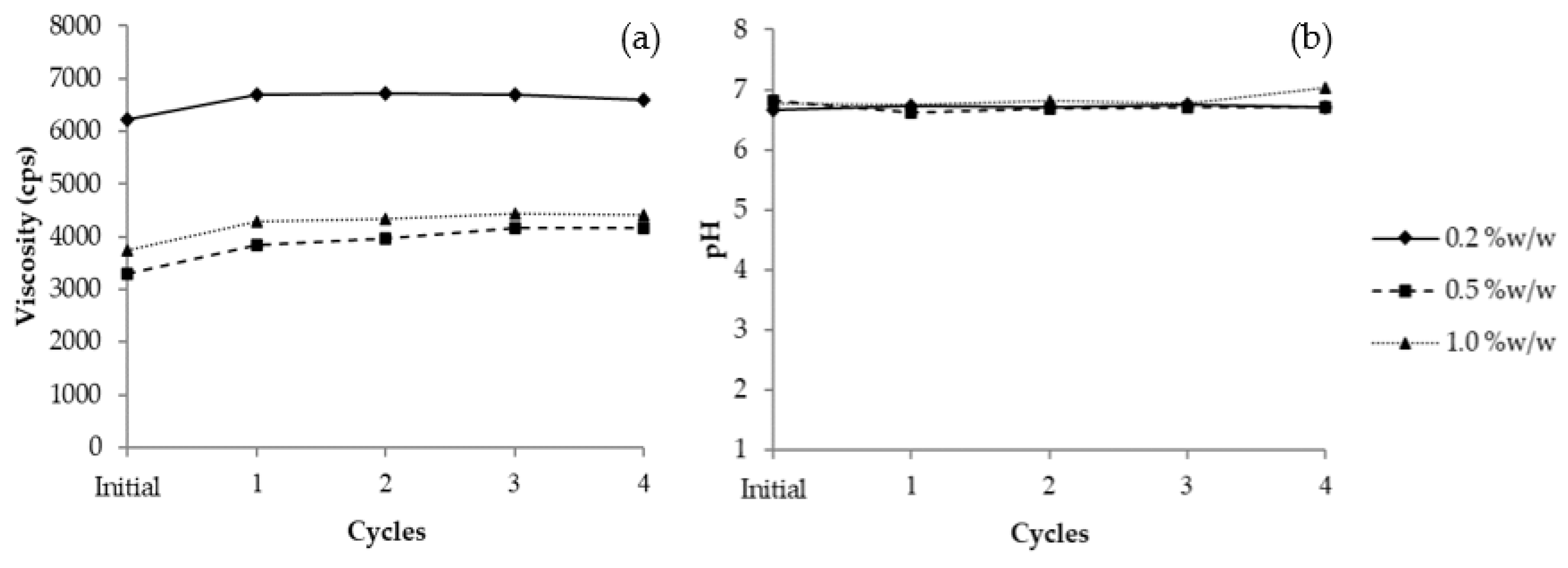
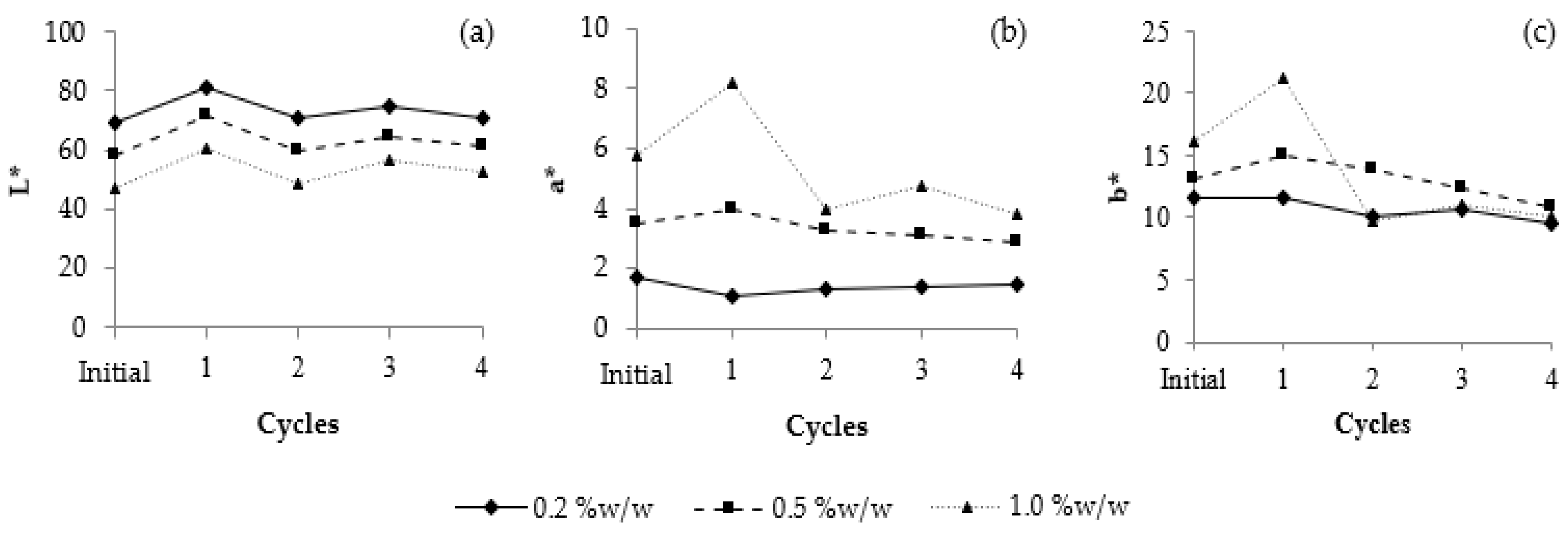
3.11.1. Heating–Cooling Stability
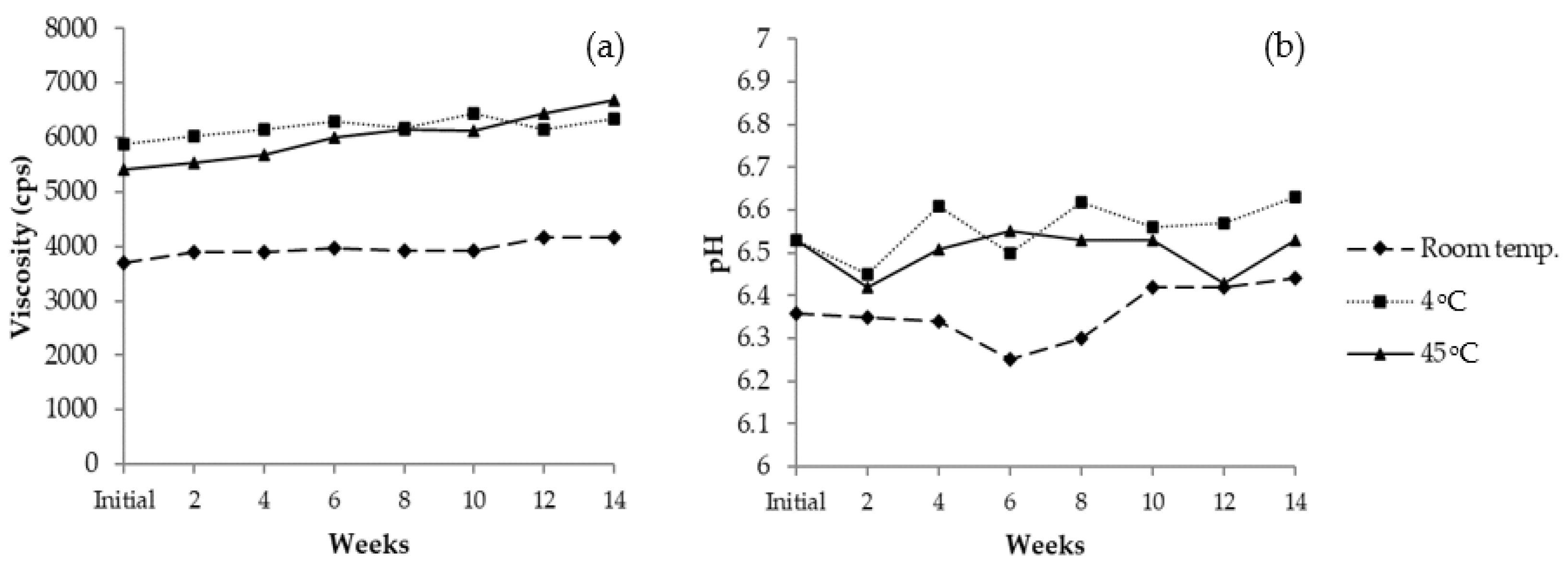
3.11.2. Long-Term Stability
3.12. Irritation Test
3.13. Sun Protection Factor (SPF)
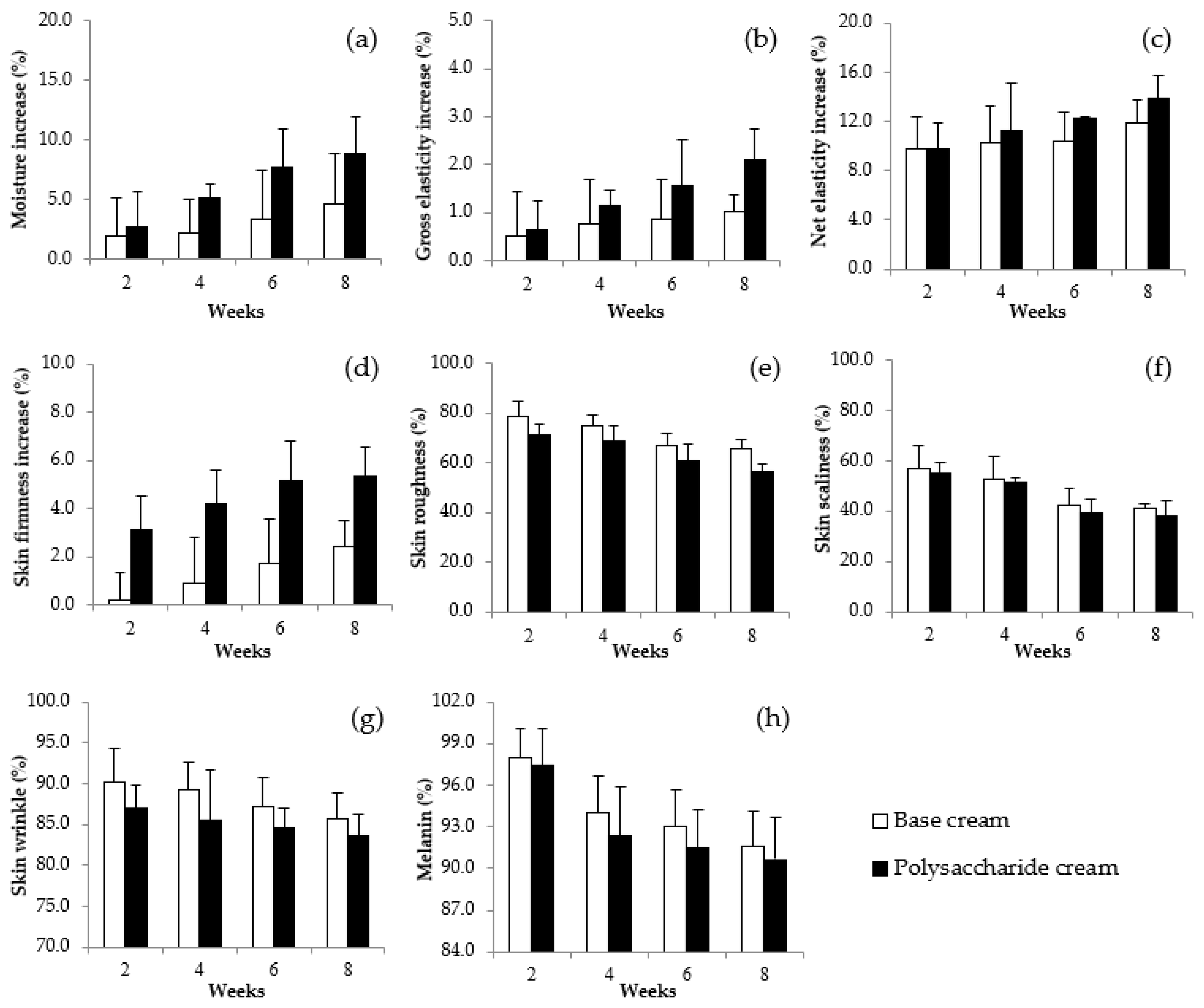
3.14. Anti-Wrinkle, Moisturization and Whitening Efficiencies of Gel Cream Containing VVP
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Beluhan, S.; Ranogajec, A. Chemical composition and non-volatile components of Croatian wild edible mushrooms. Food Chem. 2011, 124, 1076–1082. [Google Scholar] [CrossRef]
- Ragucci, S.; Landi, N.; Russo, R.; Valletta, M.; Pedone, P.V.; Chambery, A.; Di Maro, A. Ageritin from Pioppino Mushroom: The Prototype of Ribotoxin-Like Proteins, a Novel Family of Specific Ribonucleases in Edible Mushrooms. Toxins 2021, 13, 263. [Google Scholar] [CrossRef] [PubMed]
- Landi, N.; Clemente, A.; Pedone, P.V.; Ragucci, S.; Di Maro, A. An Updated Review of Bioactive Peptides from Mushrooms in a Well-Defined Molecular Weight Range. Toxins 2022, 14, 84. [Google Scholar] [CrossRef]
- Yada, D.; Negi, S.P. Bioactive components of mushrooms: Processing effects and health benefits. Food Res. Int. 2021, 148, 110599. [Google Scholar] [CrossRef] [PubMed]
- Tominac, V.P.; Krpan, V.Z.; Grba, S.; Srecec, S.; Krbavcic, I.P.; Vidovic, L. Biological effects of yeast β-glucans. Agric. Conspec. Sci. 2010, 75, 149–158. [Google Scholar]
- Browder, W.; Williams, D.; Pretus, H.; Oilvero, G.; Enrichens, F.; Mao, P.; Franchello, A. Beneficial effect of enhanced macrophage function in the trauma patient. Ann. Surg. 1990, 211, 605–612. [Google Scholar]
- Portera, C.A.; Love, E.J.; Memore, L.; Zhang, L.; Muller, A.; Browder, W.; Williams, D.L. Effect of macrophage stimulation on collagen biosynthesis in the healing wound. Am. J. Surg. 1997, 63, 125–131. [Google Scholar]
- Tsai, S.Y.; Hwang, B.F.; Wang, Y.H.; Lin, C.P. Moisture desorption and thermal properties of polysaccharide from pulsed light irradiated Flammulina velutipes. J. Therm. Anal. Calorim. 2017, 127, 469–481. [Google Scholar] [CrossRef]
- Liu, H.; He, L. Comparison of the moisture retention capacity of Tremella polysaccharides and hyaluronic acid. J. Anhui Agric. Sci. 2012, 40, 13093–13094. [Google Scholar]
- Kanlayavattanakul, M.; Lourith, N. Biopolysaccharides for Skin Hydrating Cosmetics. In Polysaccharides; Ramawat, K., Mérillon, J.M., Eds.; Springer: Cham, Switzerland, 2015; pp. 1867–1892. [Google Scholar]
- Liu, Z.; Zhang, K.; Lin, J.F.; Guo, L.H. Breeding cold tolerance strain by chemical mutagenesis in Volvariella volvacea. Sci. Hortic. 2011, 130, 18–24. [Google Scholar] [CrossRef]
- Yao, Q.Z.; Yu, M.M.; Ooi, L.S.; Ng, T.B.; Chang, S.T.; Sun, S.S.; Ooi, V.E. Isolation and Characterization of a Type 1 Ribosome-Inactivating Protein from Fruiting Bodies of the Edible Mushroom (Volvariella volvacea). J. Agric. Food Chem. 1998, 46, 788–792. [Google Scholar] [CrossRef] [PubMed]
- Kishida, E.; Sone, Y.; Misaki, A. Purification of an antitumor-active, branched (1-3)-beta-D-glucan from Volvariella volvacea, and elucidation of its fine structure. Carbohydr. Res. 1989, 193, 227–239. [Google Scholar] [CrossRef]
- Ruksiriwanich, W.; Sirithunyalug, J.; Boonpisuttinant, K.; Jantrawut, P. Potent in vitro collagen biosynthesis stimulating and antioxidant activities of edible mushroom Volvariella volvacea aqueous extract. Int. J. Pharm. Pharm. Sci. 2014, 6, 406–412. [Google Scholar]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of AOAC International, 16th ed.; AOAC International: Washington, DC, USA, 1995. [Google Scholar]
- Landi, N.; Pacifico, S.; Ragucci, S.; MA Di Giuseppe, A.; Iannuzzi, F.; Zarrelli, A.; Piccolella, S.; Di Maro, A. Pioppino mushroom in southern Italy: An undervalued source of nutrients and bioactive compounds. J. Sci. Food Agric. 2017, 97, 5388–5397. [Google Scholar] [CrossRef]
- Yap, A.T.; Ng, M.L.M. An Improved Method for the Isolation of Lentinan from the Edible and Medicinal Shiitake Mushroom, Lentinus edodes (Berk.) Sing. (Agaricomycetideae). Int. J. Med. Mushroom 2001, 3, 6–19. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.Y.; Zhang, Z.; Wang, X. Purified Auricularia auricular-judae polysaccharide (AAP I-a) prevents oxidative stress in an ageing mouse model. Carbohydr. Polym. 2011, 84, 638–648. [Google Scholar] [CrossRef]
- Zeng, W.C.; Zhang, Z.; Gao, H.; Jia, L.R.; Chen, W.Y. Characterization of antioxidant polysaccharides from Auricularia auricular using microwave-assisted extraction. Carbohydr. Polym. 2012, 89, 694–700. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Bak, W.C.; Park, J.H.; Park, Y.A.; Ka, K.H. Determination of glucan contents in the fruiting bodies and mycelia of Lentinula edodes cultivars. Mycobiology 2014, 42, 301–304. [Google Scholar] [CrossRef] [Green Version]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevalolos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP and ORAC assays for estimating antioxidant activity from guava fruit extract. J. Food Comp. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar] [PubMed]
- Zhang, J.; Kirkham, M.B. Antioxidant responses to drought in sunflower and sorghum seedlings. New Phytol. 1996, 132, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Onar, H.C.; Yusufoglu, A.; Turker, G.; Yanardag, R. Elastase, tyrosinase and lipoxygenase inhibition and antioxidant activity of an aqueous extract from Epilobium angustifolium L. leaves. J. Med. Plants Res. 2012, 6, 716–726. [Google Scholar]
- Lee, K.K.; Cho, J.J.; Park, E.J.; Choi, J.D. Anti-elastase and anti-hyaluronidase of phenolic substance from Areca catechu as a new anti-ageing agent. Int. J. Cosmet. Sci. 2001, 23, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Bandasak, C.; Rawdkuen, S.; Pintathong, P.; Chaiwut, P. Bioactivities of Carica papaya latex extract. Thai J. Agric. Sci. 2011, 44, 106–112. [Google Scholar]
- Chen, A.M.; Ning, W.; Yu, R.H.; Li, B.C.; Li, F. The research of polysaccharide extraction method from purslane. Amino Acids Biot. Resour. 2006, 28, 10–11. [Google Scholar]
- Zhang, Z.F.; Lv, G.Y.; He, W.Q.; Shi, L.G.; Pan, H.G.; Fan, L.F. Effects of extraction methods on the antioxidant activities of polysaccharides obtained from Flammulina velutipes. Carbohydr. Polym. 2013, 98, 1524–1531. [Google Scholar] [CrossRef]
- Smolskaite, L.; Venskutonis, P.R.; Talou, T. Comprehensive evaluation of antioxidant and antimicrobial properties of different mushroom species. LWT-Food Sci. Technol. 2015, 60, 462–471. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y. Extraction of purslane polysaccharides by microwave assisted process optimization. J. Guizhou Agric. Sci. 2012, 40, 148–150. [Google Scholar]
- Zhao, Y.T.; Tang, Y.Q.; Liu, K.Z.; Zhang, Y. A Study about Microwave-Assisted Extraction of Polysaccharides Form Medicinal Mushrooms Fomitopsis ulmaria (Sor.: For.) Bond. et Sing. Adv. Mater. Res. 2015, 1073–1076, 1837–1840. [Google Scholar] [CrossRef]
- Gu, C.; Pan, S. The Comparison and Analysis of Three Extraction Methods for Polysaccharides in Purslane. J. Food Nutr. Res. 2014, 2, 401–405. [Google Scholar] [CrossRef]
- Qu, C.; Yu, S.; Luo, L.; Zhao, Y.; Huang, Y. Optimization of ultrasonic extraction of polysaccharides from Ziziphus jujuba Mill. by response surface methodology. Chem. Cent. J. 2013, 7, 160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, Y.; Zeng, H.; Xu, Z.; Zheng, B.; Lin, Y.; Gan, C.; Lo, Y.M. Ultrasonic-assisted extraction and antioxidant activity of polysaccharides recovered from white button mushroom (Agaricus bisporus). Carbohydr. Polym. 2012, 88, 522–529. [Google Scholar] [CrossRef]
- Kadajji, V.G.; Betageri, G.V. Water soluble polymers for pharmaceutical application. Polymers 2011, 3, 1972–2009. [Google Scholar] [CrossRef] [Green Version]
- Rop, O.; Mlcek, J.; Jurikova, T. Beta-glucans in higher fungi and their health effects. Nutr. Rev. 2009, 67, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Kozarski, M.; Klaus, A.; Niksic, M.; Vrvic, M.M.; Todorovic, N.; Jakovljevic, D.; Griensven, L.J.L.D. Antioxidative activities and chemical characterization of polysaccharide extracts from the widely used mushrooms Ganoderma applanatum, Ganoderma lucidum, Lentinus edodes and Trametes versicolor. J. Food Compos. Anal. 2012, 26, 144–153. [Google Scholar] [CrossRef]
- Du, B.; Bian, Z.; Xu, B. Skin health promotion effects of natural beta-glucan derived from cereals and microorganisms: A review. Phytother. Res. 2014, 28, 159–166. [Google Scholar] [CrossRef]
- Wu, Y.; Choi, M.-H.; Li, J.; Yang, H.; Shin, H.-J. Mushroom Cosmetics: The Present and Future. Cosmetics 2016, 3, 22. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, X.; Yan, L.; Zhang, Q.; Zhu, J.; Huang, N.; Wang, Z. Chemical compositions and antioxidant activities of polysaccharides from the sporophores and cultured products of Armillaria mellea. Molecules 2015, 20, 5680–5697. [Google Scholar] [CrossRef] [Green Version]
- Verma, N.; Behera, B.C.; Sonone, A.; Makhija, U. Lipid peroxidation and tyrosinase inhibition by lichen symbionts grown in vitro. Afr. J. Biochem. Res. 2008, 2, 225–231. [Google Scholar]
- Alam, N.; Yoon, K.N.; Lee, K.R.; Shin, P.G.; Cheong, J.C.; Yoo, Y.B.; Shim, M.J.; Lee, M.W.; Lee, U.Y.; Lee, T.S. Antioxidant Activities and Tyrosinase Inhibitory Effects of Different Extracts from Pleurotus ostreatus Fruiting Bodies. Mycobiology 2010, 38, 295–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Lai, P.; Shen, H.; Zhen, H.; Fang, R. Effect of Extraction Methods on Polysaccharide of Clitocybe maxima Stipe. Adv. J. Food Sci. Technol. 2013, 5, 370–373. [Google Scholar] [CrossRef]
- Nguyen, T.K.; Shin, D.B.; Lee, K.R.; Shin, P.G.; Cheong, J.C.; Yoo, Y.B.; Lee, M.W.; Jin, G.-H.; Kim, H.Y.; Im, K.H.; et al. Antioxidant and anti-inflammatory activities of fruiting bodies of Dyctiophora indusiata. J. Mushroom 2013, 11, 269–277. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.Y.; Go, K.C.; Song, Y.S.; Jeong, Y.S.; Kim, E.J.; Kim, B.J. Extract of the mycelium of T. matsutake inhibits elastase activity and TPA-induced MMP-1 expression in human fibroblasts. Int. J. Mol. Med. 2014, 34, 1613–1621. [Google Scholar] [CrossRef] [PubMed]
- Saewan, N.; Jimtaisong, A. Photoprotection of natural flavonoids. J. Appl. Pharm. Sci. 2013, 3, 129–141. [Google Scholar]
- Pelizzo, M.; Zattra, E.; Nicolosi, P.; Peserico, A.; Garoli, D.; Alaibac, M. In vitro evaluation of sunscreens: An update for the clinicians. ISRN Dermatol. 2012, 2012, 352135. [Google Scholar] [CrossRef] [Green Version]
- Monsalve-Bustamante, Y.; Rincón-Valencia, S.; Mejía-Giraldo, J.; Moreno-Tirado, D.; Puertas-Mejía, M. Screening of the UV absorption capacity, proximal and chemical characterization of extracts, and polysaccharide fractions of the Gracilariopsis tenuifrons cultivated in Colombia. J. Appl. Pharm. Sci. 2019, 9, 103–109. [Google Scholar]
- Lin, J.-W.; Chiang, H.-M.; Lin, Y.-C.; Wen, K.-C. Natural products with skin—Whitening effects. J. Food Drug Anal. 2008, 16, 8. [Google Scholar] [CrossRef]




| Part | Ingredient | Formula | |||
|---|---|---|---|---|---|
| Base | 0.2% | 0.5% | 1.0% | ||
| A | Deionized water | 85.25 | 85.05 | 84.75 | 84.25 |
| Sodium Acrylates/Beheneth-25 Methacrylate Crosspolymer (and) Hydrogenated Polydecene (and) Lauryl Glucoside | 2.00 | 2.00 | 2.00 | 2.00 | |
| Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Taurate Copolymer (and) squalane (and) polysorbate 60 | 1.00 | 1.00 | 1.00 | 1.00 | |
| B | Deionized water | 10.00 | 10.00 | 10.00 | 10.00 |
| Citric acid | 0.05 | 0.05 | 0.05 | 0.05 | |
| C | Isopropyl myristate | 1.50 | 1.50 | 1.50 | 1.50 |
| D | V. Volvacea polysaccharide (VVP) | - | 0.20 | 0.50 | 1.00 |
| E | Phenoxyethanol | 0.20 | 0.20 | 0.20 | 0.20 |
| Composition | Percentage (% Dried Weight Basis) |
|---|---|
| Crude fat | 2.49 ± 0.18 |
| Protein | 19.40 ± 0.29 |
| Carbohydrate | 43.16 ± 0.38 |
| Fiber | 15.10 ± 0.16 |
| Ash | 11.71 ± 0.28 |
| Moisture | 8.15 ± 0.28 |
| Analyses | Hot Water Shaking (HS) | Microwave Assisted (MA) | Ultrasonic Assisted (UA) |
|---|---|---|---|
| Yield (%) | 15.58 ± 0.96 a | 11.05 ± 1.47 b | 9.06 ± 0.34 b |
| Total polysaccharide (g GE/g) | 0.46 ± 0.02 a | 0.58 ± 0.06 a | 0.59 ± 0.13 a |
| Beta-glucans (%w/w) | 18.80 ± 0.81 a | 9.75 ± 0.53 b | 14.29 ± 0.73 c |
| Parameters | Sample | |
|---|---|---|
| Gel Cream Base | Gel Cream with 0.2% VVP | |
| SPF mean | 0.99 | 1.02 |
| Critical wavelength | 54.8 | 212.9 |
| UVA/UVB ratio | 0.015 | −0.463 |
| Boots Star Rating | Not detected | Not detected |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sangthong, S.; Pintathong, P.; Pongsua, P.; Jirarat, A.; Chaiwut, P. Polysaccharides from Volvariella volvacea Mushroom: Extraction, Biological Activities and Cosmetic Efficacy. J. Fungi 2022, 8, 572. https://doi.org/10.3390/jof8060572
Sangthong S, Pintathong P, Pongsua P, Jirarat A, Chaiwut P. Polysaccharides from Volvariella volvacea Mushroom: Extraction, Biological Activities and Cosmetic Efficacy. Journal of Fungi. 2022; 8(6):572. https://doi.org/10.3390/jof8060572
Chicago/Turabian StyleSangthong, Sarita, Punyawatt Pintathong, Patcharee Pongsua, Areeya Jirarat, and Phanuphong Chaiwut. 2022. "Polysaccharides from Volvariella volvacea Mushroom: Extraction, Biological Activities and Cosmetic Efficacy" Journal of Fungi 8, no. 6: 572. https://doi.org/10.3390/jof8060572
APA StyleSangthong, S., Pintathong, P., Pongsua, P., Jirarat, A., & Chaiwut, P. (2022). Polysaccharides from Volvariella volvacea Mushroom: Extraction, Biological Activities and Cosmetic Efficacy. Journal of Fungi, 8(6), 572. https://doi.org/10.3390/jof8060572







