Impact of the Arbuscular Mycorrhizal Fungus Funneliformis mosseae on the Physiological and Defence Responses of Canna indica to Copper Oxide Nanoparticles Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Plant, Soil, and Fungal Materials
2.2. Growth Conditions and Experimental Design
2.3. Leaf Chlorophyll Content and Photosynthetic Parameters
2.4. Morphological Parameters
2.5. AM Colonization Rate
2.6. Cu and P Content Analysis
2.7. Antioxidant Enzyme, Lipid Peroxidation (MDA), and Reactive Oxygen Species (ROS)
2.8. Collection and Measurement of Organic Acids Released from Roots
2.9. Gene Expression Analysis
2.10. Statistical Analysis
3. Results
3.1. Plant Growth, AM Colonization, and Cu Content of Seedlings
3.2. Leaf Chlorophyll Contents and Photosynthetic Parameters
3.3. Antioxidant Enzyme Activities, Lipid Peroxidation (MDA), and Reactive Oxygen Species (ROS)
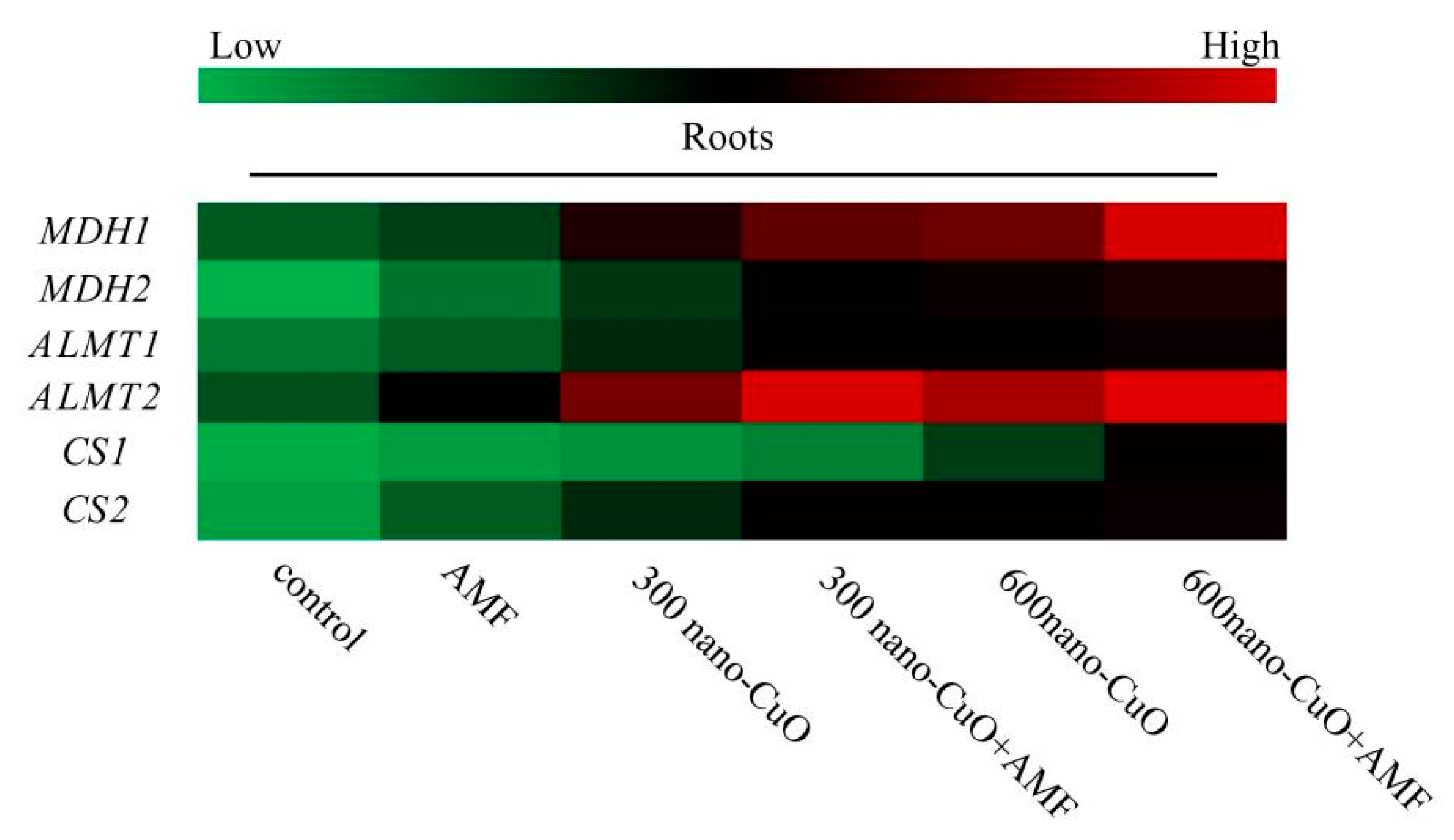
3.4. Impacts on the Organic Acid Content in Roots Arising from Stress
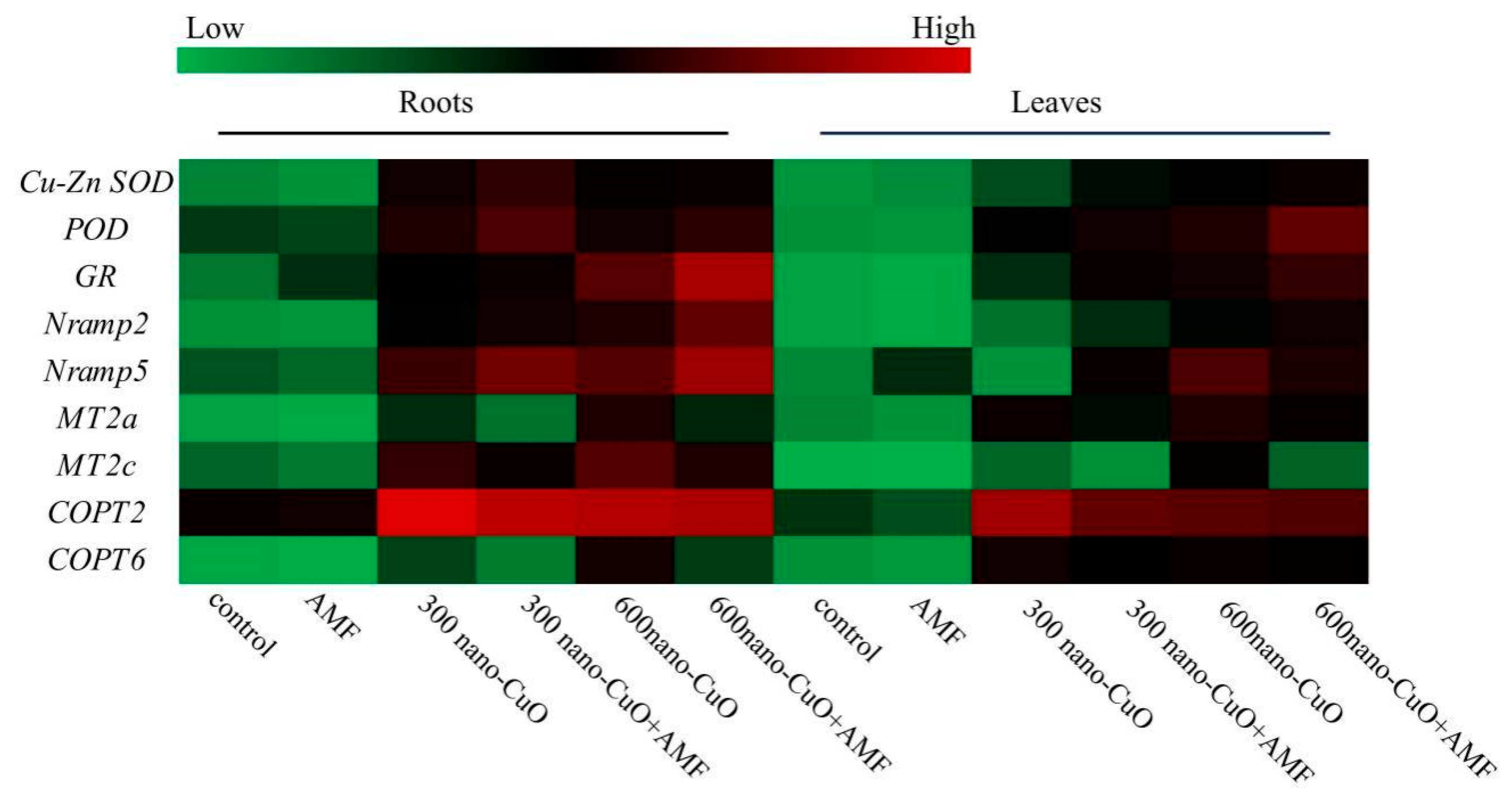
3.5. Impacts on Gene Expression Arising from AMF in Response to Nano-CuO Stresses
4. Discussion
4.1. AMF Significantly Facilitated C. indica Seedling Establishment in Response to Nano-CuO Stress
4.2. AMF Led to a High-Efficiency ROS Scavenging Mechanism for the Protection of the C. indica Seedlings Exposed to Nano-CuO Stress from Oxidative Damage
4.3. AMF Inhibited Cu Uptake in C. indica Seedlings to Alleviate Nano-CuO Stress
4.4. AMF Facilitated Organic Acid Generation and Secretion to Chelate Nano-CuO
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Naz, S.; Gul, A.; Zia, M. Toxicity of copper oxide nanoparticles: A review study. IET Nanobiotechnol. 2020, 14, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Wang, X.X.; Hayat, T.; Wang, X.K. Ecotoxicological effects and mechanism of CuO nanoparticles to individual organisms. Environ. Pollut. 2017, 221, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Assadian, E.; Zarei, M.H.; Gilani, A.G.; Farshin, M.; Degampanah, H.; Pourahmad, J. Toxicity of copper oxide (CuO) nanoparticles on human blood lymphocytes. Biol. Trace Elem. Res. 2018, 184, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Rawat, S.; Pullagurala, V.L.R.; Hernandez-Molina, M.; Sun, Y.P.; Niu, G.H.; Hernandez-Viezcas, J.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Impacts of copper oxide nanoparticles on bell pepper (Capsicum annum L.) plants: A full life cycle study. Environ. Sci.-Nano 2018, 5, 83–95. [Google Scholar] [CrossRef]
- Pu, S.; Yan, C.; Huang, H.; Liu, S.; Deng, D. Toxicity of nano-CuO particles to maize and microbial community largely depends on its bioavailable fractions. Environ. Pollut. 2019, 255, 113248. [Google Scholar] [CrossRef]
- Zuverza-Mena, N.; Medina-Velo, I.A.; Barrios, A.C.; Tan, W.J.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Copper nanoparticles/compounds impact agronomic and physiological parameters in cilantro (Coriandrum sativum). Environ. Sci.-Proc. Imp. 2015, 17, 1783–1793. [Google Scholar] [CrossRef]
- Stampoulis, D.; Sinha, S.K.; White, J.C. Assay-dependent phytotoxicity of nanoparticles to plants. Environ. Sci. Technol. 2009, 43, 9473–9479. [Google Scholar] [CrossRef]
- Nair, P.M.G.; Chung, I.M. Study on the correlation between copper oxide nanoparticles induced growth suppression and enhanced lignification in Indian mustard (Brassica juncea L.). Ecotox. Environ. Safe 2015, 113, 302–313. [Google Scholar] [CrossRef]
- Deng, F.; Wang, S.L.; Xin, H. Toxicity of CuO nanoparticles to structure and metabolic activity of Allium cepa root tips. Bull. Environ. Contam. Toxicol. 2016, 97, 702–708. [Google Scholar] [CrossRef]
- Shi, J.Y.; Peng, C.; Yang, Y.Q.; Yang, J.J.; Zhang, H.; Yuan, X.F.; Chen, Y.X.; Hu, T.D. Phytotoxicity and accumulation of copper oxide nanoparticles to the Cu-tolerant plant Elsholtzia splendens. Nanotoxicology 2014, 8, 179–188. [Google Scholar] [CrossRef]
- Keller, A.A.; Mcferran, S.; Lazareva, A.; Suh, S. Global life cycle releases of engineered nanomaterials. J. Nanoparticle Res. 2013, 15, 1692. [Google Scholar] [CrossRef]
- Gujre, N.; Soni, A.; Rangan, L.; Tsang, D.C.W.; Mitra, S. Sustainable improvement of soil health utilizing biochar and arbuscular mycorrhizal fungi: A review. Environ. Pollut. 2021, 268, 115549. [Google Scholar] [CrossRef] [PubMed]
- Ouziad, F.; Hildebrandt, U.; Schmelzer, E.; Bothe, H. Differential gene expressions in arbuscular mycorrhizal-colonized tomato grown under heavy metal stress. J. Plant Physiol. 2005, 162, 634–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irving, T.B.; Alptekin, B.; Kleven, B.; Ané, J.M. A critical review of 25 years of glomalin research: A better mechanical understanding and robust quantification techniques are required. New Phytol. 2021, 232, 1572–1581. [Google Scholar] [CrossRef]
- Chen, X.W.; Wu, L.; Luo, N.; Mo, C.H.; Wong, M.H.; Li, H. Arbuscular mycorrhizal fungi and the associated bacterial community influence the uptake of cadmium in rice. Geoderma 2019, 337, 749–757. [Google Scholar] [CrossRef]
- Siani, N.G.; Fallah, S.; Pokhrel, L.R.; Rostamnejadi, A. Natural amelioration of Zinc oxide nanoparticle toxicity in fenugreek (Trigonella foenum-gracum) by arbuscular mycorrhizal (Glomus intraradices) secretion of glomalin. Plant Physiol. Biochem. 2017, 112, 227. [Google Scholar] [CrossRef]
- Wang, F.Y.; Liu, X.Q.; Shi, Z.Y.; Tong, R.J.; Adams, C.A. Arbuscular mycorrhizae alleviate negative effects of zinc oxide nanoparticle and zinc accumulation in maize plants—A soil microcosm experiment. Chemosphere 2016, 147, 88–97. [Google Scholar] [CrossRef]
- Dong, J.; Wang, L.; Ma, F.; Qi, S.S.; Zhang, H.X.; Zhao, T. Effects of arbuscular mycorrhizal fungi inoculation on the phytoremediation of herbicide by Canna indica L. var flava Roxb. in water. J. Harbin Inst. Technol. 2017, 49, 37–43. [Google Scholar]
- El Faiz, A.; Duponnois, R.; Winterton, P.; Ouhammou, A.; Meddich, A.; Boularbah, A.; Hafidi, M. Effect of different amendments on growing of Canna indica L. inoculated with AMF on mining substrate. Int. J. Phytoremediat. 2015, 17, 503–513. [Google Scholar] [CrossRef]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. BBA-Bioenerg. 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Kormanik, P.P.; Bryan, W.C.; Schultz, R.C. Procedures and equipment for staining large numbers of plant root samples for endomycorrhizal assay. Can. J. Microbiol. 1980, 26, 536–538. [Google Scholar] [CrossRef] [PubMed]
- Giovannetti, M.; Mosse, B. An evaluation of techniques for measuring vesicular arbuscular arbuscular mycorrhizal infection in roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- Thomas, R.L.; Sheard, R.W.; Moyer, J.R. Comparison of conventional and automated procedures for nitrogen phosphorus and potassium analysis of plant material using a single digestion. Agron. J. 1967, 59, 240–243. [Google Scholar] [CrossRef]
- Li, Y.; Sun, H.; Li, H.; Yang, L.; Ye, B.; Wang, W. Dynamic changes of rhizosphere properties and antioxidant enzyme responses of wheat plants (Triticum aestivum L.) grown in mercury-contaminated soils. Chemosphere 2013, 93, 972–977. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, G.R.; Burchett, M. Photosynthetic pigments and peroxidase activity as indicators of heavy metal stress in the Grey Mangrove, Avicennia marina (Forsk.) Vierh. Mar. Pollut. Bull. 2001, 42, 233–240. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Jiang, M.Y.; Zhang, J.H. Water stress-induced abscisic acid accumulation triggers the increased generation of reactive oxygen species and upregulates the activities of antioxidant enzymes in maize leaves. J. Exp. Bot. 2002, 53, 2401–2410. [Google Scholar] [CrossRef] [Green Version]
- Naeem, M.S.; Rasheed, M.; Liu, D.; Jin, Z.L.; Ming, D.F.; Yoneyama, K.; Takeuchi, Y.; Zhou, W.J. 5-Aminolevulinic acid ameliorates salinity-induced metabolic, water-related and biochemical changes in Brassica napus L. Acta Physiol. Plant. 2011, 33, 517–528. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Ding, H.; Wen, D.; Fu, Z.; Qian, H. The secretion of organic acids is also regulated by factors other than aluminum. Environ. Monit. Assess 2013, 186, 1123–1131. [Google Scholar] [CrossRef]
- Yang, J.L.; You, J.F.; Li, Y.Y.; Wu, P.; Zheng, S.J. Magnesium enhances aluminum-induced citrate secretion in rice bean roots (Vigna umbellata) by restoring plasma membrane H+-ATPase activity. Plant Cell Physiol. 2007, 48, 66–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.S.; Zhang, D.Y.; Li, H.Y.; Gao, B.; Yang, H.L.; Zhang, Y.M.; Wood, A.J. Characterization of reference genes for RT-qPCR in the desert moss Syntrichia caninervis in response to abiotic stress and desiccation/rehydration. Front. Plant Sci. 2015, 6, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Oyetibo, G.O.; Miyauchi, K.; Huang, Y.; Chien, M.F.; Ilori, M.O.; Amund, O.O.; Endo, G. Biotechnological remedies for the estuarine environment polluted with heavy metals and persistent organic pollutants. Int. Biodeterior. Biodegrad. 2017, 119, 614–625. [Google Scholar] [CrossRef]
- Dong, J.; Wang, L.; Ma, F.; Yang, J.X.; Qi, S.S.; Zhao, T. The effect of Funnelliformis mosseae inoculation on the phytoremediation of atrazine by the aquatic plant Canna indica L. var. flava Roxb. RSC Adv. 2016, 6, 22538–22549. [Google Scholar] [CrossRef]
- Fu, D.; Rui, Y.; Zevenbergen, C.; Singh, R.P. Nitrogen Absorption Efficiency and Mechanism in Arbuscular Mycorrhizal Fungi—Canna indica Symbiosis. Chemosphere 2021, 282, 130708. [Google Scholar] [CrossRef]
- Cornejo, P.; Pérez-Tienda, J.; Meier, S.; Valderas, A.; Ferrol, N. Copper compartmentalization in spores as a survival strategy of arbuscular mycorrhizal fungi in Cu-polluted environments. Soil Biol. Biochem. 2013, 57, 925–928. [Google Scholar] [CrossRef]
- Gatahi, D.M.; Wanyika, H.N.; Mumokavoo, A.; Kihurani, A.W.; Miindaateka, E. Enhancement of bacterial wilt resistance and rhizosphere health in tomato using bionanocomposites. Int. J. Hortic. Sci. Technol. 2016, 3, 129–144. [Google Scholar]
- Xu, Z.; Wu, Y.; Xiao, Z.; Ban, Y.; Belvett, N. Positive effects of Funneliformis mosseae inoculation on reed seedlings under water and TiO2 nanoparticles stresses. World J Microb. Biot. 2019, 35, 81. [Google Scholar] [CrossRef]
- Xiao, J.X.; An, J.; Chen, Y.Y.; Hu, C.Y. Improved growth and Cu tolerance of Cu excess-stressed white clover after inoculation with arbuscular mycorrhizal fungi. J. Plant Nutr. 2016, 39, 227–234. [Google Scholar] [CrossRef]
- Cao, J.L.; Feng, Y.Z.; Lin, X.G. Influence of arbuscular mycorrhizal fungi and iron oxide magnetic nanoparticles on maize growth and Fe-uptake. J. Ecol. Rural Environ. 2017, 33, 555–563. [Google Scholar]
- Zhang, S.J.; Wang, L.; Ma, F.; Zhang, X.; Fu, D.F. Arbuscular mycorrhiza improved phosphorus efficiency in paddy fields. Ecol. Eng. 2016, 95, 64–72. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Q.; Yerger, E.H.; Chen, X.; Shi, Q.; Wan, F. AM fungi facilitate the competitive growth of two invasive plant species, Ambrosia artemisiifolia and Bidens pilosa. Mycorrhiza 2018, 28, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Wang, J.; Wan, L.; Dai, Z.; da Silva Matos, D.M.; Du, D.; Egan, S.; Bonser, S.P.; Thomas, T.; Moles, A.T. Arbuscular mycorrhizal fungi contribute to phosphorous uptake and allocation strategies of Solidago canadensis in a phosphorous-deficient environment. Front. Plant Sci. 2022, 13, 831654. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Sarwat, M.; Sharma, S. Reactive oxygen species, antioxidants and signaling in plants. J. Plant Biol. 2008, 51, 167–173. [Google Scholar] [CrossRef]
- Moll, J.; Gogos, A.; Bucheli, T.D.; Widmer, F.; Van, D. Effect of nanoparticles on red clover and its symbiotic microorganisms. J. Nanobiotechnol. 2016, 14, 36. [Google Scholar] [CrossRef] [Green Version]
- Noctor, G.; Mhamdi, A.; Foyer, C.H. The roles of reactive oxygen metabolism in drought: Not so cut and dried. Plant Physiol. 2014, 164, 1636–1648. [Google Scholar] [CrossRef] [Green Version]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef]
- Mo, Y.; Wang, Y.; Yang, R.; Zheng, J.; Liu, C.; Li, H.; Ma, J.; Zhang, Y.; Wei, C.; Xian, Z. Regulation of plant growth, photosynthesis, antioxidation and osmosis by an arbuscular mycorrhizal fungus in watermelon seedlings under well-watered and drought conditions. Front. Plant Sci. 2016, 7, 644. [Google Scholar] [CrossRef] [Green Version]
- Fan, Q.J.; Liu, J. Colonization with arbuscular mycorrhizal fungus affects growth, drought tolerance and expression of stress-responsive genes in Poncirus trifoliata. Acta Physiol. Plant 2011, 33, 1533–1542. [Google Scholar] [CrossRef]
- Patel, A.; Tiwari, S.; Prasad, S.M. Arsenate and arsenite-induced inhibition and recovery in two diazotrophic cyanobacteria Nostoc muscorum and Anabaena sp.: Study on time-dependent toxicity regulation. Environ. Sci. Pollut. Res. 2021, 28, 51088–51104. [Google Scholar] [CrossRef] [PubMed]
- Danouche, M.; El Ghachtouli, N.; El Baouchi, A.; El Arroussi, H. Heavy metals phycoremediation using tolerant green microalgae: Enzymatic and non-enzymatic antioxidant systems for the management of oxidative stress. J. Environ. Chem. Eng. 2020, 8, 104460. [Google Scholar] [CrossRef]
- Andrade, S.; Gratão, P.L.; Azevedo, R.A.; Silveira, A.; Schiavinato, M.A.; Mazzafera, P. Biochemical and physiological changes in jack bean under mycorrhizal symbiosis growing in soil with increasing Cu concentrations. Environ. Exp. Bot. 2010, 68, 198–207. [Google Scholar] [CrossRef]
- Ferrol, N.; González-Guerrero, M.; Valderas, A.; Benabdellah, K.; Azcón-Aguilar, C. Survival strategies of arbuscular mycorrhizal fungi in Cu-polluted environments. Phytochem. Rev. 2009, 8, 551–559. [Google Scholar] [CrossRef]
- Purin, S.; Rillig, M.C. The arbuscular mycorrhizal fungal protein glomalin: Limitations, progress, and a new hypothesis for its function. Pedobiologia 2008, 51, 123–130. [Google Scholar] [CrossRef]
- Balestrini, R.; Lanfranco, L. Fungal and plant gene expression in arbuscular mycorrhizal symbiosis. Mycorrhiza 2006, 16, 509–524. [Google Scholar] [CrossRef]
- Sasaki, A.; Yamaji, N.; Ma, J.F. Overexpression of OsHMA3 enhances Cd tolerance and expression of Zn transporter genes in rice. J. Exp. Bot. 2014, 65, 6013–6021. [Google Scholar] [CrossRef] [Green Version]
- Navarro, E.; Baun, A.; Behra, R.; Hartmann, N.B.; Filser, J.; Miao, A.J.; Quigg, A.; Santschi, P.H.; Sigg, L. Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology 2008, 17, 372–386. [Google Scholar] [CrossRef] [Green Version]
- Noori, A.; White, J.C.; Newman, L.A. Mycorrhizal fungi influence on silver uptake and membrane protein gene expression following silver nanoparticle exposure. J. Nanopart. Res. 2017, 19, 66. [Google Scholar] [CrossRef]
- Hassinen, V.H.; Tervahauta, A.I.; Schat, H.; Kärenlampi, S.O. Plant metallothioneins-metal chelators with ROS scavenging activity? Plant Biol. 2011, 13, 225–232. [Google Scholar] [CrossRef]
- Ma, J.F. Role of organic acids in detoxification of aluminum in higher plants. Plant Cell Physiol. 2000, 41, 383–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kollmeier, M.; Dietrich, P.; Bauer, C.S.; Hedrich, H.R. Aluminum activates a citrate-permeable anion channel in the aluminum-sensitive zone of the maize root apex. A comparison between an aluminum-sensitive and an aluminumresistant cultivar. Plant Physiol. 2001, 126, 397–410. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.L.; Zhang, L.; Ying, L.Y.; You, J.F.; Ping, W.U.; Zheng, S.J. Citrate transporters play a critical role in aluminium-stimulated citrate efflux in rice bean (Vigna umbellata) roots. Ann. Bot. 2006, 97, 579–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.F.; Ma, J.F.; Matsumoto, H. Pattern of aluminum-induced secretion of organic acids differs between rye and wheat. Plant Physiol. 2000, 123, 1537–1544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, J.S.; Ma, F.J.; Matsumoto, H. High aluminum resistance in buckwheat. I. Al-induced specific secretion of oxalic acid from root tips. Plant Physiol. 1998, 117, 745–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.Y.; Lou, H.Q.; Chen, W.W.; Piñeros, M.A.; Xu, J.M.; Fan, W.; Kochian, L.V.; Zheng, S.J.; Yang, J.L. Two citrate transporters coordinately regulate citrate secretion from rice bean root tip under aluminum stress. Plant Cell Environ. 2018, 41, 809–822. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.P.; Magalhaes, J.V.; Shaff, J.; Kochian, L.V. Aluminum-activated citrate and malate transporters from the MATE and ALMT families function independently to confer Arabidopsis aluminum tolerance. Plant J. 2009, 57, 389–399. [Google Scholar] [CrossRef] [Green Version]
- Liang, C.; Pineros, M.A.; Tian, J.; Yao, Z.; Sun, L.; Liu, J.; Shaff, J.; Coluccio, A.; Kochian, L.V.; Liao, H. Low pH, aluminum, and phosphorus coordinately regulate malate exudation through GmALMT1 to improve soybean adaptation to acid soils. Plant Physiol. 2013, 161, 1347–1361. [Google Scholar] [CrossRef] [Green Version]
- Sharma, T.; Dreyer, I.; Kochian, L.; Piñeros, M.A. The ALMT family of organic acid transporters in plants and their involvement in detoxification and nutrient security. Front. Plant Sci. 2016, 7, 1488. [Google Scholar] [CrossRef] [Green Version]
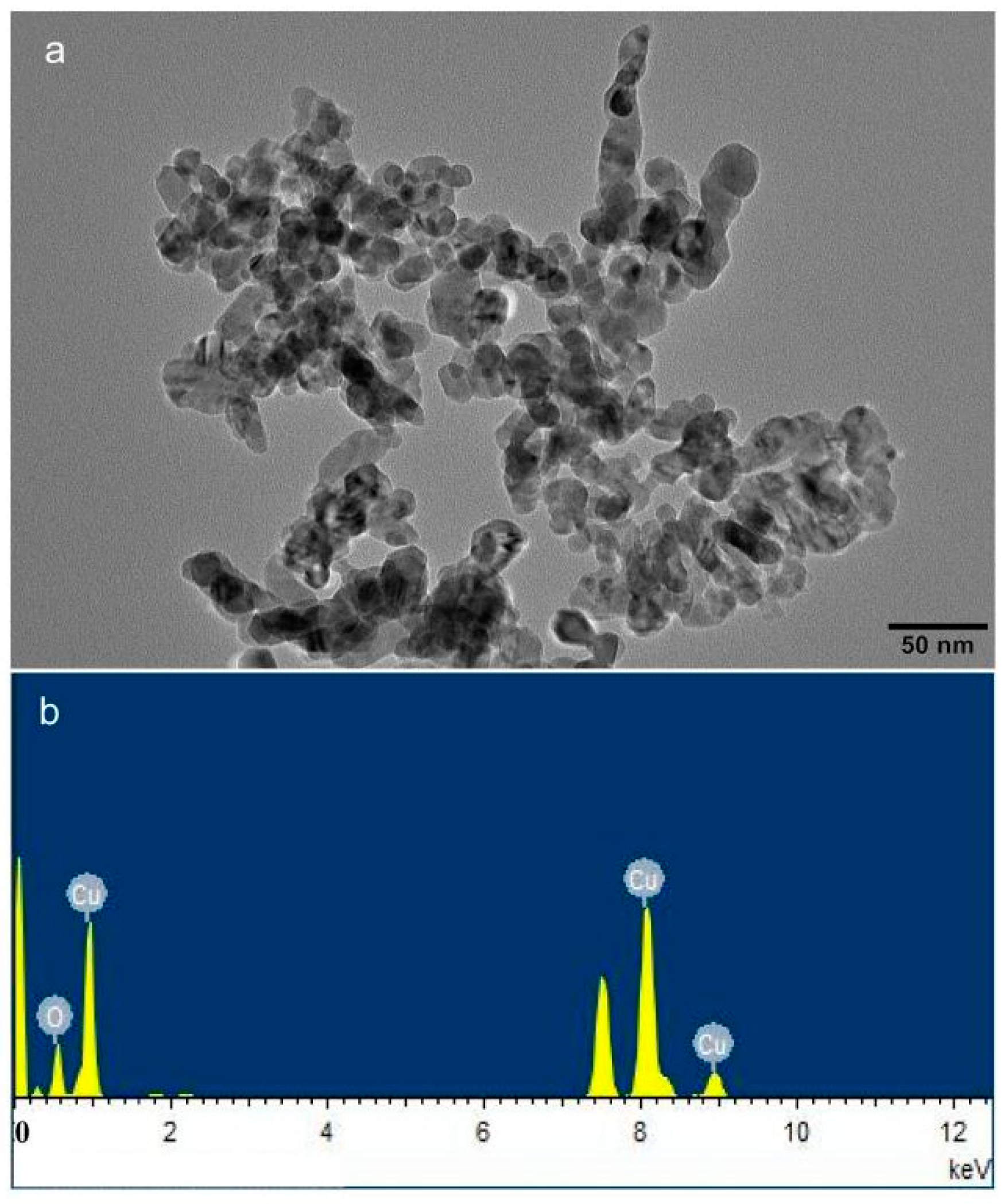

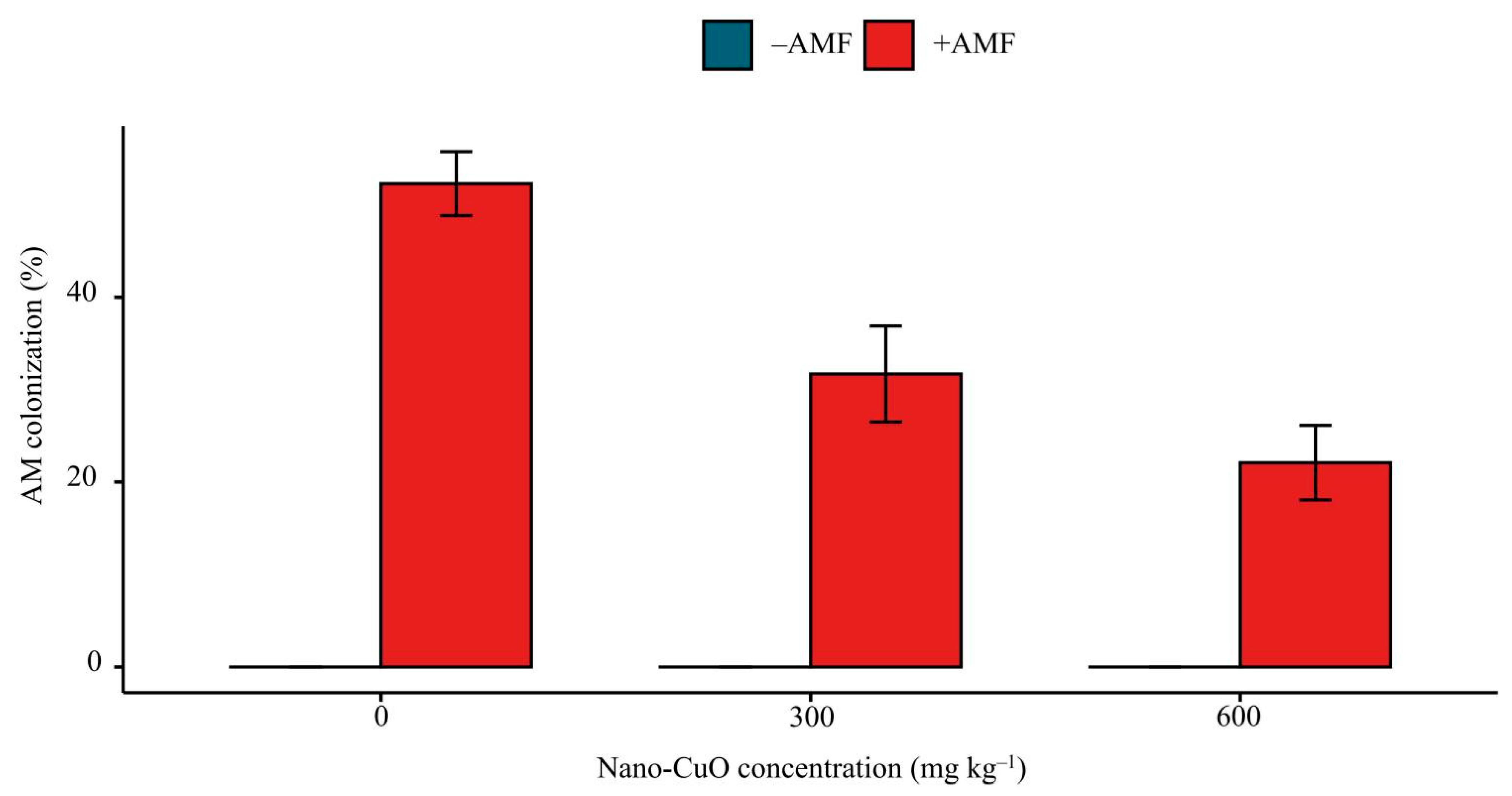

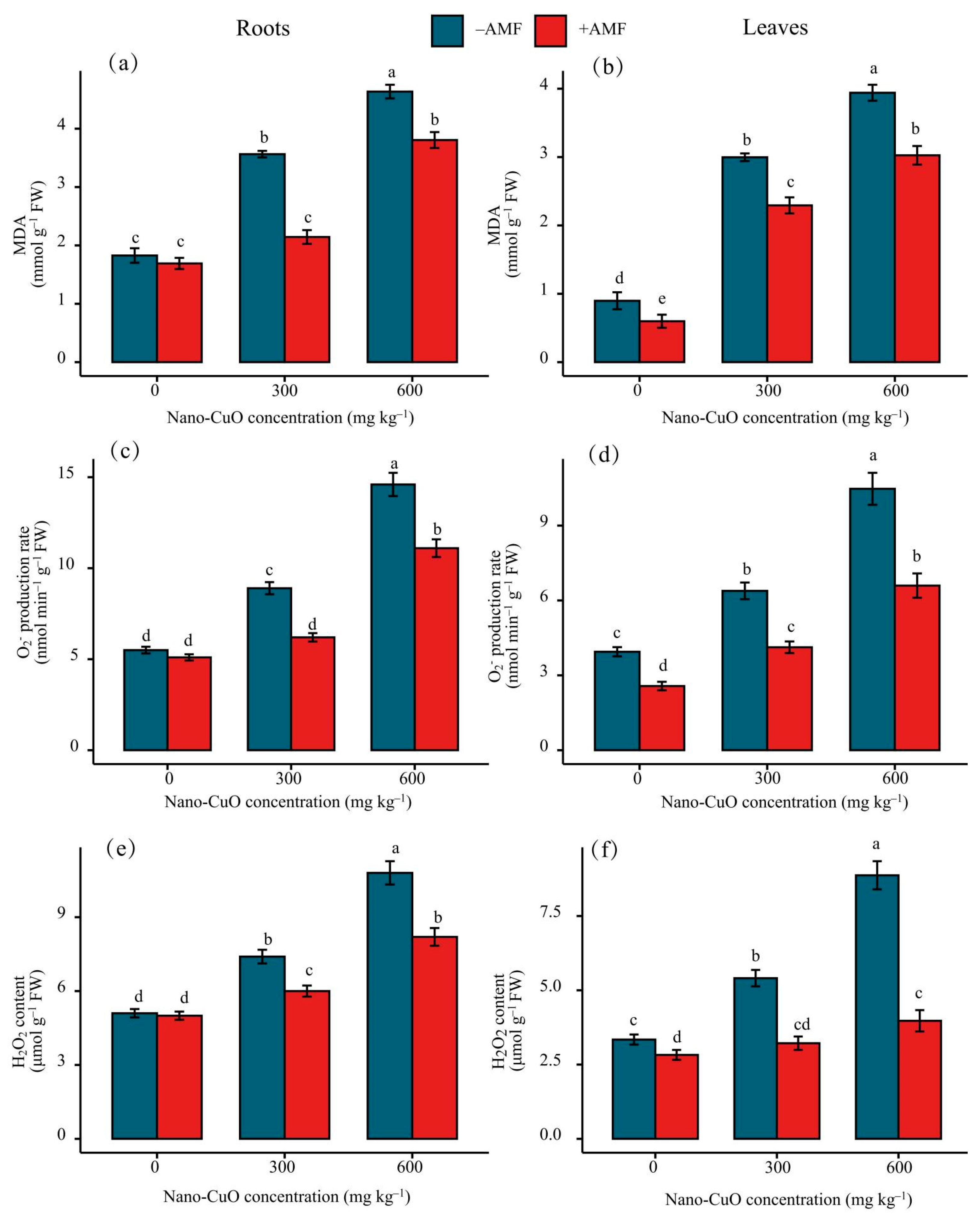
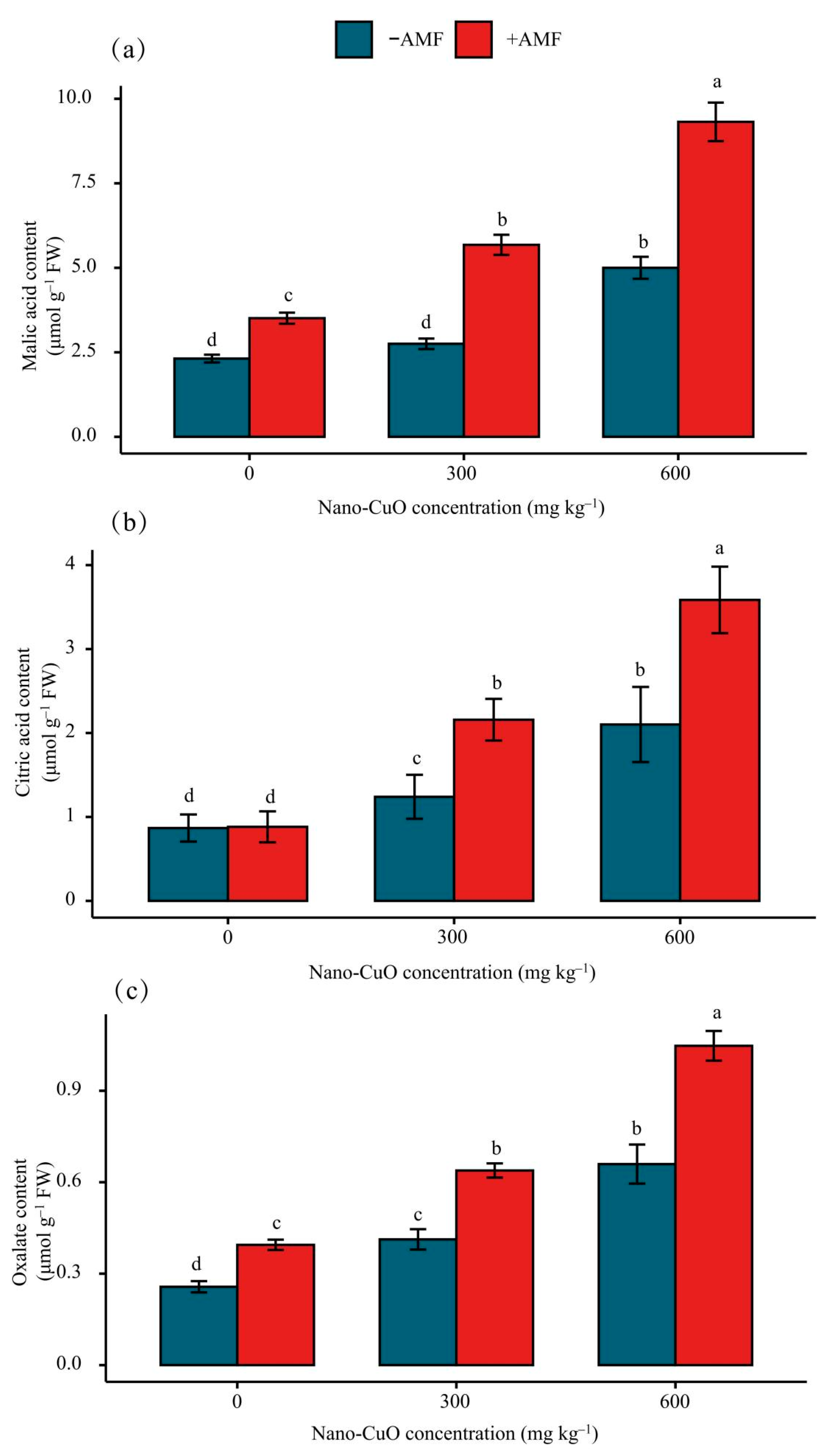
| Nano-CuO | Treatment | Shoot dry | Root Dry | Shoot Height | Root Length | Cu Content in Shoots | Cu Content in Roots | P Content in Shoots | P Content in Roots |
|---|---|---|---|---|---|---|---|---|---|
| Levels mg kg−1 | Mass (g) | mass (g) | (cm) | (cm) | (mg kg−1, DW) | (mg kg−1, DW) | (mg g−1, DW) | (mg g−1, DW) | |
| 0 | −AMF | 2.63 ± 0.19b | 0.65 ± 0.09b | 36.9 ± 1.8a | 22.3 ± 1.3a | 8.45 ± 0.21c | 33.52 ± 0.3e | 2.41 ± 0.08bc | 2.06 ± 0.04a |
| +AMF | 3.13 ± 0.21a | 0.79 ± 0.06a | 38.6 ± 1.9a | 24.5 ± 1.5a | 8.19 ± 0.11c | 33.92 ± 0.056e | 2.79 ± 0.05a | 2.11 ± 0.04a | |
| 300 | −AMF | 2.06 ± 0.13d | 0.46 ± 0.07c | 27.5 ± 1.4c | 15.1 ± 0.9c | 68.37 ± 3.11b | 218.73 ± 0.34c | 2.32 ± 0.07c | 1.94 ± 0.07ab |
| +AMF | 2.35 ± 0.08c | 0.60 ± 0.06b | 31.2 ± 1.6b | 16.8 ± 1.0b | 57.16 ± 4.62b | 174.33 ± 1.72d | 2.55 ± 0.08b | 2.01 ± 0.06a | |
| 600 | −AMF | 1.59 ± 0.05e | 0.31 ± 0.04d | 22.6 ± 1.1d | 10.9 ± 0.6d | 88.05 ± 2.71a | 278.3 ± 3.31a | 2.02 ± 0.04d | 1.74 ± 0.05c |
| +AMF | 1.71 ± 0.04e | 0.39 ± 0.02d | 24.5 ± 1.2cd | 12.3 ± 0.7d | 80.61 ± 2.52a | 245.71 ± 5.01b | 2.11 ± 0.05d | 1.80 ± 0.07bc |
| Nano-CuO Content | Treatment | Pn | GS | Ci | Tr | Chlorophyll a | Chlorophyll b | Total Chlorophyll |
|---|---|---|---|---|---|---|---|---|
| mg kg−1 | μmol m−2 s−1 | μmol m−2 s−1 | mmol m−2 s−1 | mmol m−2 s−1 | mg g−1 | mg g−1 | mg g−1 | |
| 0 | −AMF | 7.9 ± 1.6a | 0.7 ± 0.07a | 333.3 ± 8.9c | 4.0 ± 0.2a | 4.7 ± 0.21a | 2.6 ± 0.17a | 7.3 ± 0.32a |
| +AMF | 8.5 ± 0.76a | 0.8 ± 0.03a | 332.7 ± 3.9c | 3.9 ± 0.07a | 4.8 ± 0.17a | 2.8 ± 0.07a | 7.6 ± 0.16a | |
| 300 | −AMF | 4.5 ± 0.65c | 0.5 ± 0.03b | 348.09 ± 6.7b | 3.1 ± 0.2b | 3.7 ± 0.16b | 1.9 ± 0.06b | 5.6 ± 0.14c |
| +AMF | 7.3 ± 0.4ab | 0.8 ± 0.06a | 326.4 ± 6.9c | 4.2 ± 0.2a | 4.4 ± 0.27a | 2.4 ± 0.1a | 6.8 ± 0.32ab | |
| 600 | −AMF | 3.5 ± 0.51d | 0.3 ± 0.004c | 365.2 ± 5.4a | 1.9 ± 0.1c | 2.9 ± 0.14c | 1.5 ± 0.1c | 4.4 ± 0.24d |
| +AMF | 4.8 ± 0.13c | 0.5 ± 0.05b | 342.8 ± 3.9b | 2.8 ± 0.2b | 3.1 ± 0.22c | 1.7 ± 0.1bc | 4.8 ± 0.28d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, J.; Yan, Q.; Yang, G.; Wang, Y. Impact of the Arbuscular Mycorrhizal Fungus Funneliformis mosseae on the Physiological and Defence Responses of Canna indica to Copper Oxide Nanoparticles Stress. J. Fungi 2022, 8, 513. https://doi.org/10.3390/jof8050513
Luo J, Yan Q, Yang G, Wang Y. Impact of the Arbuscular Mycorrhizal Fungus Funneliformis mosseae on the Physiological and Defence Responses of Canna indica to Copper Oxide Nanoparticles Stress. Journal of Fungi. 2022; 8(5):513. https://doi.org/10.3390/jof8050513
Chicago/Turabian StyleLuo, Jie, Qiuxia Yan, Guo Yang, and Youbao Wang. 2022. "Impact of the Arbuscular Mycorrhizal Fungus Funneliformis mosseae on the Physiological and Defence Responses of Canna indica to Copper Oxide Nanoparticles Stress" Journal of Fungi 8, no. 5: 513. https://doi.org/10.3390/jof8050513
APA StyleLuo, J., Yan, Q., Yang, G., & Wang, Y. (2022). Impact of the Arbuscular Mycorrhizal Fungus Funneliformis mosseae on the Physiological and Defence Responses of Canna indica to Copper Oxide Nanoparticles Stress. Journal of Fungi, 8(5), 513. https://doi.org/10.3390/jof8050513





