Fungal Fermented Palm Kernel Expeller as Feed for Black Soldier Fly Larvae in Producing Protein and Biodiesel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Rhizopus oligosporus Spore Suspension
2.2. Rearing of Black Soldier Fly Larvae
2.3. Growth Performances of Black Soldier Fly Larvae
2.4. Biochemical Analyses
2.4.1. Lipid
2.4.2. Protein
2.4.3. Fatty Acid Methyl Ester
3. Results and Discussion
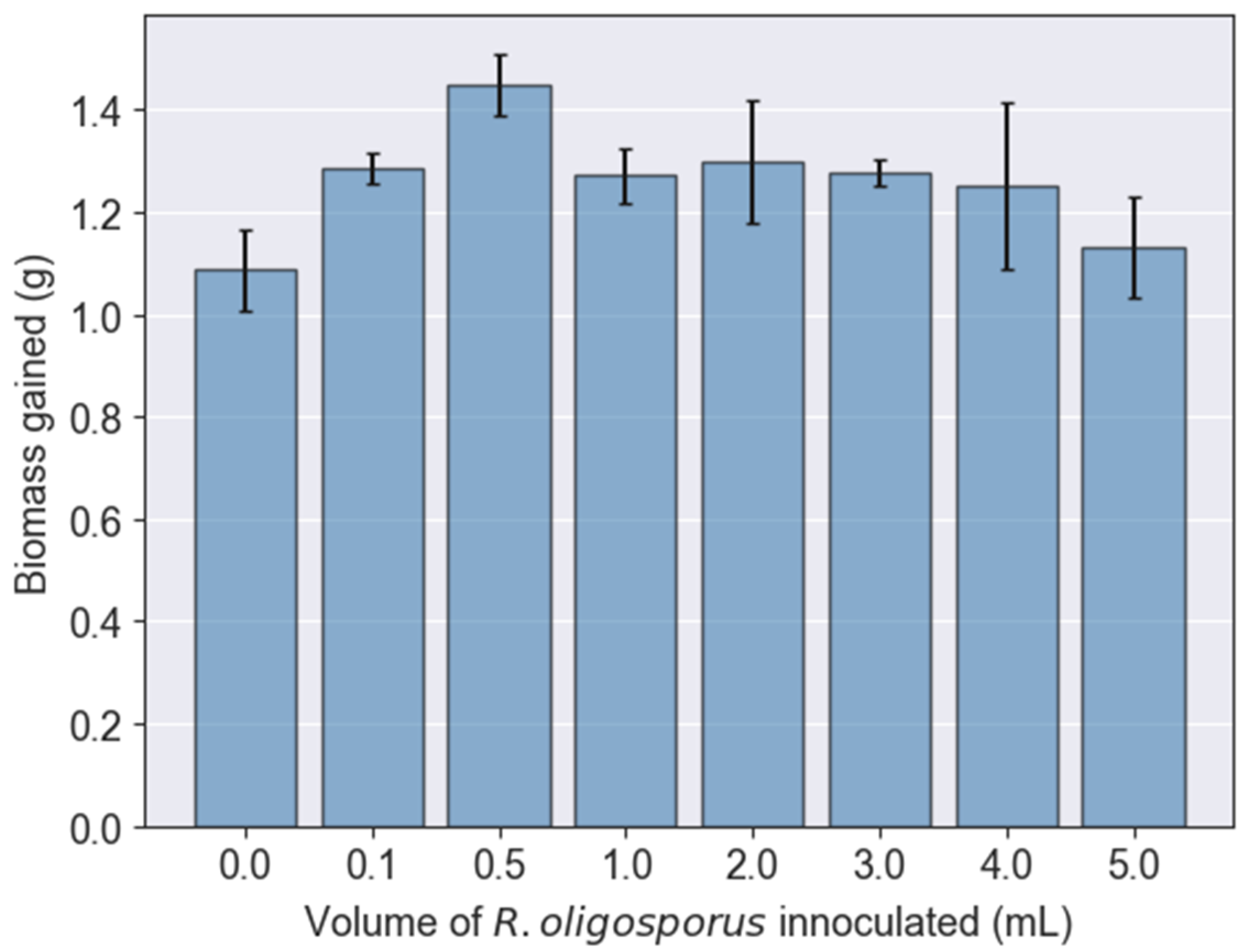
3.1. Growth of BSFL Fed with Various Fermented PKE
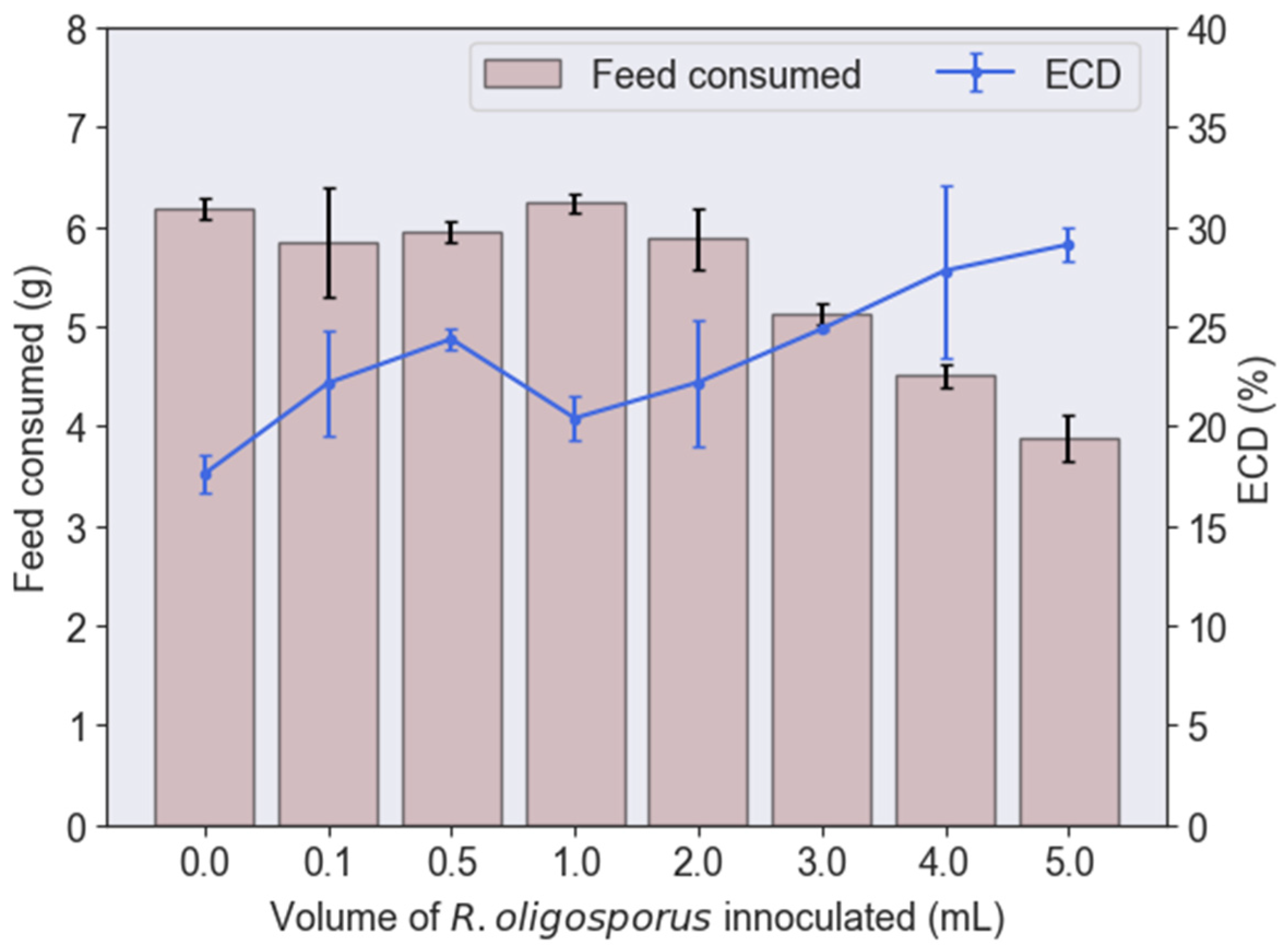
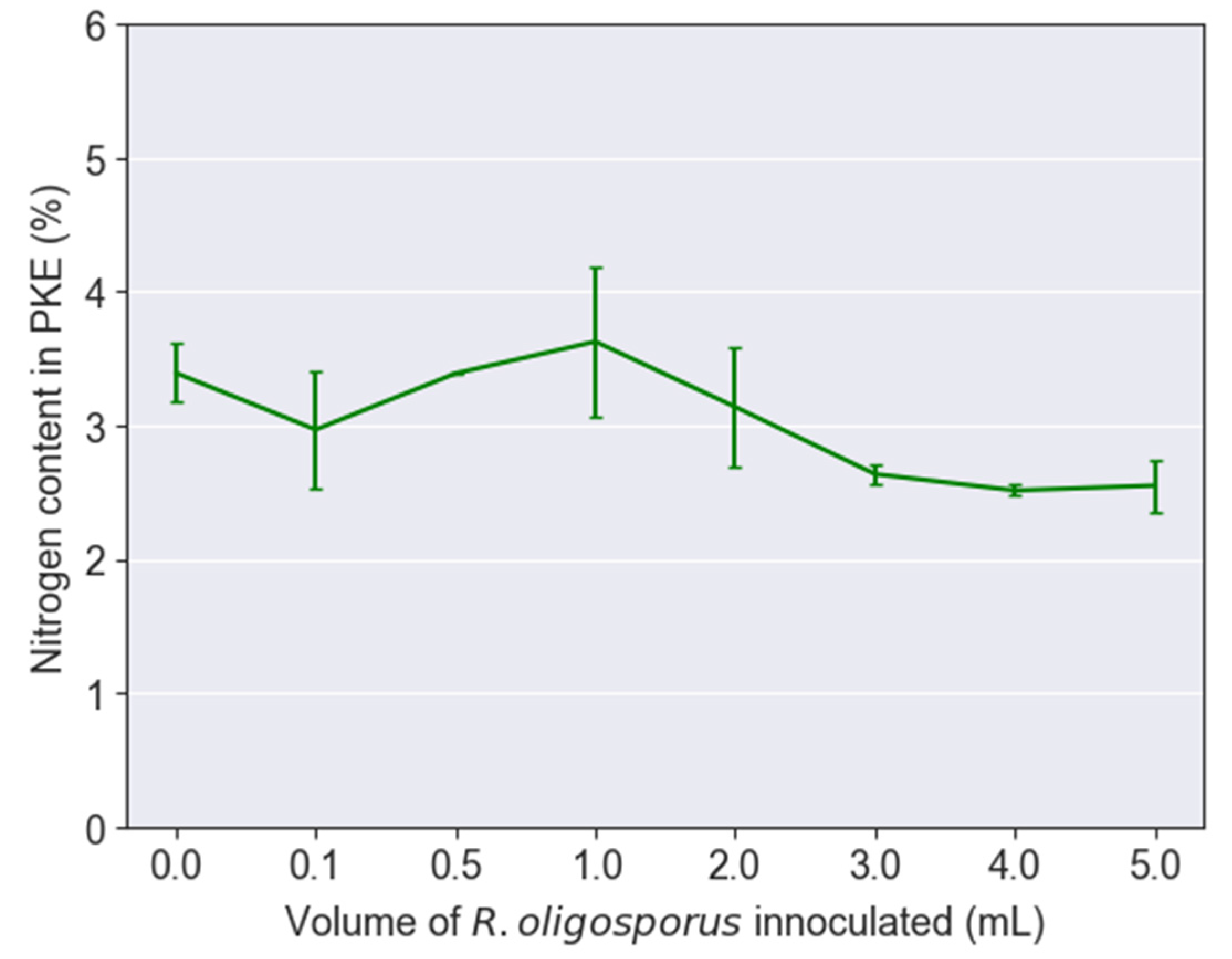
3.2. Protein and Lipid to Biodiesel from BSFL Fed with Various Fermented PKE
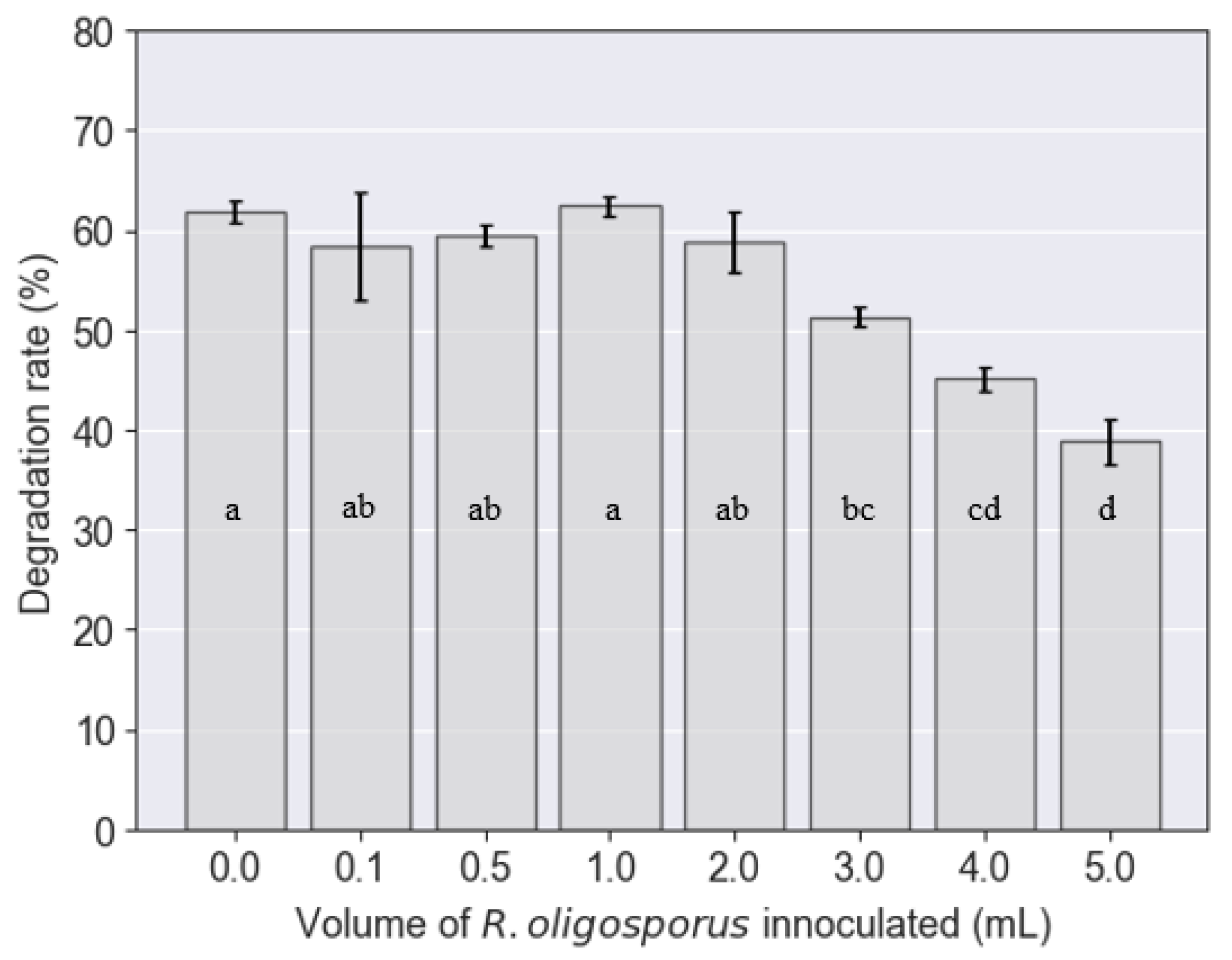
3.3. Degradation Rate of PKE by BSFL
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raksasat, R.; Kiatkittipong, K.; Kiatkittipong, W.; Wong, C.Y.; Lam, M.K.; Ho, Y.C.; Oh, W.D.; Suryawan, I.W.K.; Lim, J.W. Blended sewage sludge–palm kernel expeller to enhance the palatability of black soldier fly larvae for biodiesel production. Processes 2021, 9, 297. [Google Scholar] [CrossRef]
- Lalander, C.; Diener, S.; Zurbrügg, C.; Vinnerås, B. Effects of feedstock on larval development and process efficiency in waste treatment with black soldier fly (Hermetia illucens). J. Clean. Prod. 2019, 208, 211–219. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, W.; Lu, X.; Zhu, F.; Liu, W.; Wang, X.; Lei, C. Bioconversion performance and life table of black soldier fly (Hermetia illucens) on fermented maize straw. J. Clean. Prod. 2019, 230, 974–980. [Google Scholar] [CrossRef]
- Wong, C.Y.; Lim, J.W.; Chong, F.K.; Lam, M.K.; Uemura, Y.; Tan, W.N.; Bashir, M.J.K.; Lam, S.M.; Sin, J.C.; Lam, S.S. Valorization of exo-microbial fermented coconut endosperm waste by black soldier fly larvae for simultaneous biodiesel and protein productions. Environ. Res. 2020, 185, 109458. [Google Scholar] [CrossRef]
- Norgren, R.; Jonsson, A.; Björkqvist, O. Original article: Fermented pulp and paper bio-sludge as feed for black soldier fly larvae. Biomass Convers. Biorefinery 2021, 1–8. [Google Scholar] [CrossRef]
- Howdeshell, T.; Tanaka, T. Recovery of glucose from dried distiller’s grain with solubles, using combinations of solid-state fermentation and insect culture. Can. J. Microbiol. 2018, 64, 706–715. [Google Scholar] [CrossRef]
- Pamintuan, K.R.S.; Agustin, H.A.T.; Deocareza, E.D. Growth and Nutritional Performance of Black Soldier Fly (Hermetia illucens) Larvae Reared in Fermented Rice Straw and Duck Manure. In IOP Conference Series: Earth and Environmental Science, Proceedings of the 2020 6th International Conference on Environment and Renewable Energy, Hanoi, Vietnam, 24–26 February 2020; Institute of Physics Publishing: Bristol, UK, 2020; Volume 505. [Google Scholar]
- Chen, H. Modern Solid State Fermentation: Theory and Practice; Springer: Dordrecht, The Netherlands, 2013; ISBN 9789400760431. [Google Scholar]
- Caplice, E.; Fitzgerald, G.F. Food fermentations: Role of microorganisms in food production and preservation. Int. J. Food Microbiol. 1999, 50, 131–149. [Google Scholar] [CrossRef]
- McFeeters, R.F. Effects of Fermentation on the Nutritional Properties of Food. In Nutritional Evaluation of Food Processing; Springer: Dordrecht, The Netherlands, 1988; pp. 423–446. [Google Scholar]
- Singhania, R.R.; Patel, A.K.; Soccol, C.R.; Pandey, A. Recent advances in solid-state fermentation. Biochem. Eng. J. 2009, 44, 13–18. [Google Scholar] [CrossRef]
- Chan, L.H.M. Handbook of indigenous fermented foods. Food Res. Int. 1997, 30, 464. [Google Scholar] [CrossRef]
- Nowak, J.; Szebiotko, K. Some biochemical changes during soybean and pea tempeh fermentation. Food Microbiol. 1992, 9, 37–43. [Google Scholar] [CrossRef]
- Handoyo, T.; Morita, N. Structural and functional properties of fermented soybean (Tempeh) by using rhizopus oligosporus. Int. J. Food Prop. 2006, 9, 347–355. [Google Scholar] [CrossRef] [Green Version]
- Gerhard, M.; Rad, R.; Prinz, C.; Naumann, M. Pathogenesis of Helicobacter pylori infection. Helicobacter 2002, 7, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Abu-Salem, F.M.; Abou-Arab, E.A. Physico-chemical properties of tempeh produced from chickpea seeds. J. Am. Sci. 2011, 7, 107–118. [Google Scholar]
- Angulo-Bejarano, P.I.; Verdugo-Montoya, N.M.; Cuevas-Rodríguez, E.O.; Milán-Carrillo, J.; Mora-Escobedo, R.; Lopez-Valenzuela, J.A.; Garzón-Tiznado, J.A.; Reyes-Moreno, C. Tempeh flour from chickpea (Cicer arietinum L.) nutritional and physicochemical properties. Food Chem. 2008, 106, 106–112. [Google Scholar] [CrossRef]
- Wong, H.K.; Wan Zahari, M. Utilisation of oil palm by-products as ruminant feed in malaysia. J. Oil Palm Res. 2011, 23, 1029–1035. [Google Scholar]
- Wan Zahari, M.; Alimon, A. Use of Palm Kernel Cake and Oil Palm By-Products in Compound Feed Use of Palm Kernel Cake and Oil Palm By-Products in Compound Feed. Palm Oil Dev. 2003, 40, 5–9. [Google Scholar]
- Navidshad, B.; Liang, J.B.; Faseleh Jahromi, M.; Akhlaghi, A.; Abdullah, N. A comparison between a yeast cell wall extract (Bio-mos®) and palm kernel expeller as mannan-oligosac-charides sources on the performance and ileal microbial population of broiler chickens. Ital. J. Anim. Sci. 2015, 14, 10–15. [Google Scholar] [CrossRef]
- Syakirah, W.; Abdullah, W.; Osman, M.; Zainal, M.; Ab, A. The Potential and Status of Renewable Energy Development in Malaysia. Energies 2019, 12, 2437. [Google Scholar]
- Nelofer, R. Nutritional Enhancement of Barley in Solid State Fermentation by Rhizopus oligosporus ML-10. Nutr. Food Sci. Int. J. 2018, 6, 555700. [Google Scholar] [CrossRef]
- Wong, C.Y.; Kiatkittipong, K.; Kiatkittipong, W.; Lim, J.W.; Lam, M.K.; Wu, T.Y.; Show, P.L.; Daud, H.; Goh, P.S.; Sakuragi, M.; et al. Rhizopus oligosporus-assisted valorization of coconut endosperm waste by black soldier fly larvae for simultaneous protein and lipid to biodiesel production. Processes 2021, 9, 299. [Google Scholar] [CrossRef]
- Wong, C.Y.; Rosli, S.S.; Uemura, Y.; Ho, Y.C.; Leejeerajumnean, A.; Kiatkittipong, W.; Cheng, C.K.; Lam, M.K.; Lim, J.W. Potential protein and biodiesel sources from black soldier fly larvae: Insights of larval harvesting instar and fermented feeding medium. Energies 2019, 12, 1570. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, S.S. Protein Nitrogen Determination. In Food Analysis Laboratory Manual; Springer: Boston, MA, USA, 2010; pp. 39–45. [Google Scholar]
- Lim, J.W.; Mohd-Noor, S.N.; Wong, C.Y.; Lam, M.K.; Goh, P.S.; Beniers, J.J.A.; Da Oh, W.; Jumbri, K.; Ghani, N.A. Palatability of black soldier fly larvae in valorizing mixed waste coconut endosperm and soybean curd residue into larval lipid and protein sources. J. Environ. Manage. 2019, 231, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Lateef, A.; Oloke, J.K.; Gueguim Kana, E.B.; Oyeniyi, S.O.; Onifade, O.R.; Oyeleye, A.O.; Oladosu, O.C.; Oyelami, A.O. Improving the quality of agro-wastes by solid-state fermentation: Enhanced antioxidant activities and nutritional qualities. World J. Microbiol. Biotechnol. 2008, 24, 2369–2374. [Google Scholar] [CrossRef]
- Alshelmani, M.I.; Loh, T.C.; Foo, H.L.; Sazili, A.Q.; Lau, W.H. Effect of solid state fermentation on nutrient content and ileal amino acids digestibility of palm kernel cake in broiler chickens. Indian J. Anim. Sci. 2017, 87, 1135–1140. [Google Scholar]
- Sprangers, T.; Ottoboni, M.; Klootwijk, C.; Ovyn, A.; Deboosere, S.; De Meulenaer, B.; Michiels, J.; Eeckhout, M.; De Clercq, P.; De Smet, S. Nutritional composition of black soldier fly (Hermetia illucens) prepupae reared on different organic waste substrates. J. Sci. Food Agric. 2017, 2594–2600. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.Y.; Aris, M.N.M.; Daud, H.; Lam, M.K.; Yong, C.S.; Hasan, H.A.; Chong, S.; Show, P.L.; Hajoeningtijas, O.D.; Ho, Y.C.; et al. In-situ yeast fermentation to enhance bioconversion of coconut endosperm waste into larval biomass of hermetia illucens: Statistical augmentation of larval lipid content. Sustainability 2020, 12, 1558. [Google Scholar] [CrossRef] [Green Version]
- Manivannan, A.; Bhardwaj, R.; Padmanabhan, S.; Suneja, P.; Hebbar, K.B.; Kanade, S.R. Biochemical and nutritional characterization of coconut (Cocos nucifera L.) haustorium. Food Chem. 2018, 238, 153–159. [Google Scholar] [CrossRef]
- Dias, F.; Burke, J.L.; Pacheco, D.; Holmes, C.W. The effect of palm kernel expeller as a supplement for grazing dairy cows at the end of lactation. Proc. New Zeal. Soc. Anim. Prod. 2008, 68, 111–112. [Google Scholar]
- Hoekman, S.K.; Broch, A.; Robbins, C.; Ceniceros, E.; Natarajan, M. Review of biodiesel composition, properties, and specifications. Renew. Sustain. Energy Rev. 2012, 16, 143–169. [Google Scholar] [CrossRef]
- ur Rehman, K.; Rehman, A.; Cai, M.; Zheng, L.; Xiao, X.; Somroo, A.A.; Wang, H.; Li, W.; Yu, Z.; Zhang, J. Conversion of mixtures of dairy manure and soybean curd residue by black soldier fly larvae (Hermetia illucens L.). J. Clean. Prod. 2017, 154, 366–373. [Google Scholar] [CrossRef]
- Wang, H.; Rehman, K.U.; Liu, X.; Yang, Q.; Zheng, L.; Li, W.; Cai, M.; Li, Q.; Zhang, J.; Yu, Z. Insect biorefinery: A green approach for conversion of crop residues into biodiesel and protein. Biotechnol. Biofuels 2017, 10, 304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, C.H.; Ryu, J.; Lee, J.; Ko, K.; Lee, J.Y.; Park, K.Y.; Chung, H. Use of black soldier fly larvae for food waste treatment and energy production in asian countries: A review. Processes 2021, 9, 161. [Google Scholar] [CrossRef]


| FAME | BSFL (This Work at 0.5 mL) (%) | Soybean (%) [33] | Rapeseed (%) [33] | Oil Palm (%) [33] |
|---|---|---|---|---|
| C10:0 | 1.2 | 0.0 | 0.6 | 0.5 |
| C12:0 | 47.5 | 0.1 | 0.1 | 0.3 |
| C14:0 | 14.4 | 0.1 | 0.0 | 1.1 |
| C16:0 | 14.6 | 11.6 | 4.2 | 42.5 |
| C16:1 | 3.1 | 0.2 | 0.1 | 0.2 |
| C18:0 | 2.1 | 3.9 | 1.6 | 4.2 |
| C18:1 | 11.1 | 23.7 | 59.5 | 41.3 |
| C18:2 | 4.0 | 53.8 | 21.5 | 9.5 |
| C22:1 | 2.0 | 0.1 | 0.5 | 0.0 |
| SFA | 79.8 | 16.5 | 7.4 | 49.0 |
| MUFA | 16.2 | 24.7 | 62.8 | 41.6 |
| PUFA (<4 π bonds) | 4.0 | 59.7 | 30.0 | 9.8 |
| Total | 100.0 | 100.9 | 100.2 | 100.4 |
| Substrate | Degradation Rate (%) | Reference |
|---|---|---|
| Palm kernel expeller | 38.8–62.4 | This work |
| Coconut endosperm waste | 52.0–75.0 | [23,26] |
| Soybean curd residue | 64.0–72.4 | [26,34] |
| Cow manure | 25.8 | [34] |
| Corn stover | 39.9 | [35] |
| Fermented maize straw | 48.4 | [3] |
| Wheat bran | 55.0 | [3] |
| Fruits and vegetable waste | 46.7–49.5 | [2,36] |
| Food waste | 50.3–55.3 | [2,36] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liew, C.S.; Wong, C.Y.; Abdelfattah, E.A.; Raksasat, R.; Rawindran, H.; Lim, J.W.; Kiatkittipong, W.; Kiatkittipong, K.; Mohamad, M.; Yek, P.N.Y.; et al. Fungal Fermented Palm Kernel Expeller as Feed for Black Soldier Fly Larvae in Producing Protein and Biodiesel. J. Fungi 2022, 8, 332. https://doi.org/10.3390/jof8040332
Liew CS, Wong CY, Abdelfattah EA, Raksasat R, Rawindran H, Lim JW, Kiatkittipong W, Kiatkittipong K, Mohamad M, Yek PNY, et al. Fungal Fermented Palm Kernel Expeller as Feed for Black Soldier Fly Larvae in Producing Protein and Biodiesel. Journal of Fungi. 2022; 8(4):332. https://doi.org/10.3390/jof8040332
Chicago/Turabian StyleLiew, Chin Seng, Chung Yiin Wong, Eman A. Abdelfattah, Ratchaprapa Raksasat, Hemamalini Rawindran, Jun Wei Lim, Worapon Kiatkittipong, Kunlanan Kiatkittipong, Mardawani Mohamad, Peter Nai Yuh Yek, and et al. 2022. "Fungal Fermented Palm Kernel Expeller as Feed for Black Soldier Fly Larvae in Producing Protein and Biodiesel" Journal of Fungi 8, no. 4: 332. https://doi.org/10.3390/jof8040332
APA StyleLiew, C. S., Wong, C. Y., Abdelfattah, E. A., Raksasat, R., Rawindran, H., Lim, J. W., Kiatkittipong, W., Kiatkittipong, K., Mohamad, M., Yek, P. N. Y., Setiabudi, H. D., Cheng, C. K., & Lam, S. S. (2022). Fungal Fermented Palm Kernel Expeller as Feed for Black Soldier Fly Larvae in Producing Protein and Biodiesel. Journal of Fungi, 8(4), 332. https://doi.org/10.3390/jof8040332










