Minimal versus Intensive: How the Pruning Intensity Affects Occurrence of Grapevine Leaf Stripe Disease, Wood Integrity, and the Mycobiome in Grapevine Trunks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Sites and Plant Material
2.2. Monitoring
2.3. Grapevine Trunk Properties
2.4. Grapevine Wood Integrity
2.5. Local Climate
2.6. Wood-Sample Preparation and DNA Extraction
2.7. Next-Generation Sequencing (NGS)
2.8. Mycobiome—Raw Data Processing
2.9. Mycobiome—Statistical Analysis
3. Results
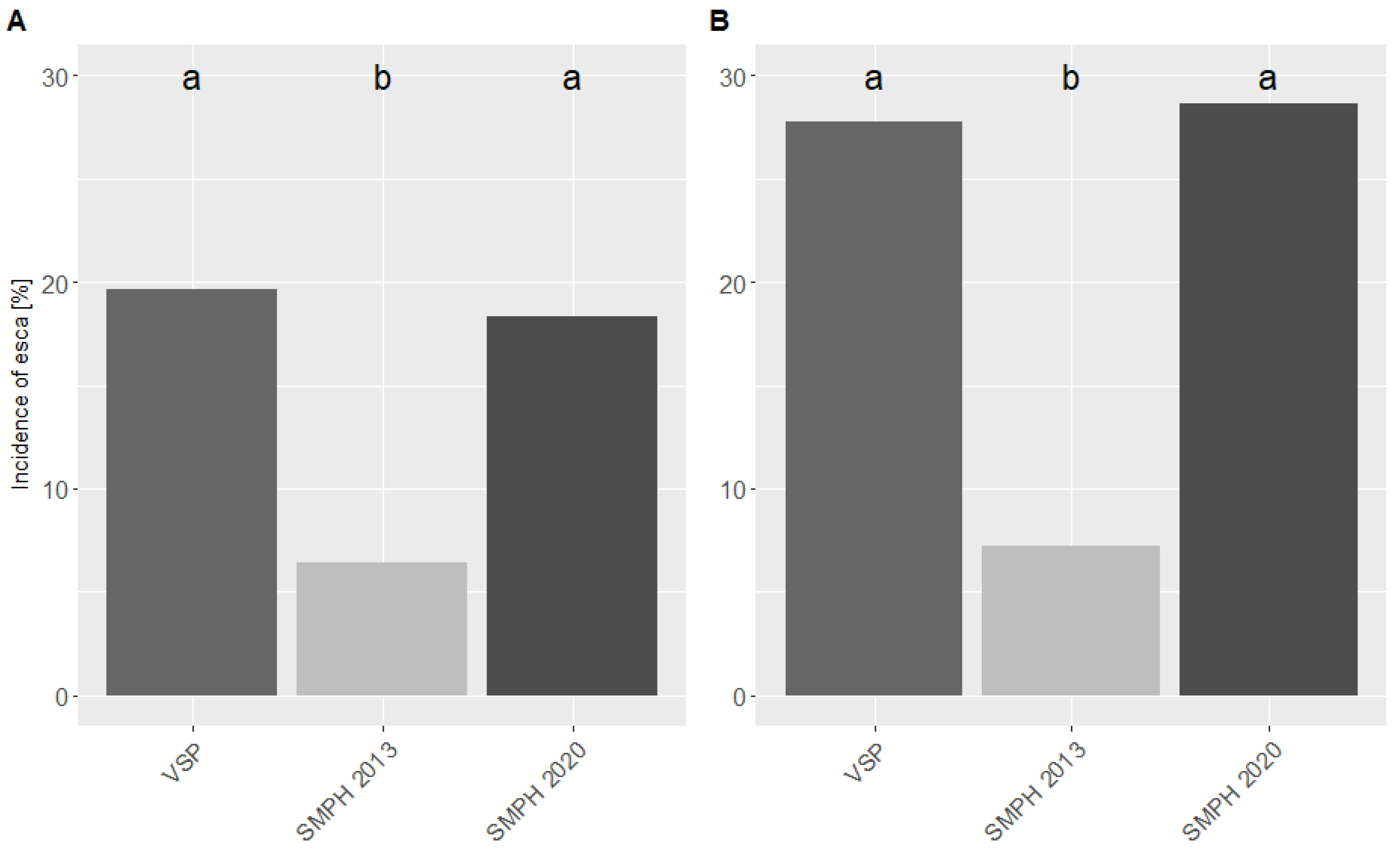
3.1. Esca Incidence
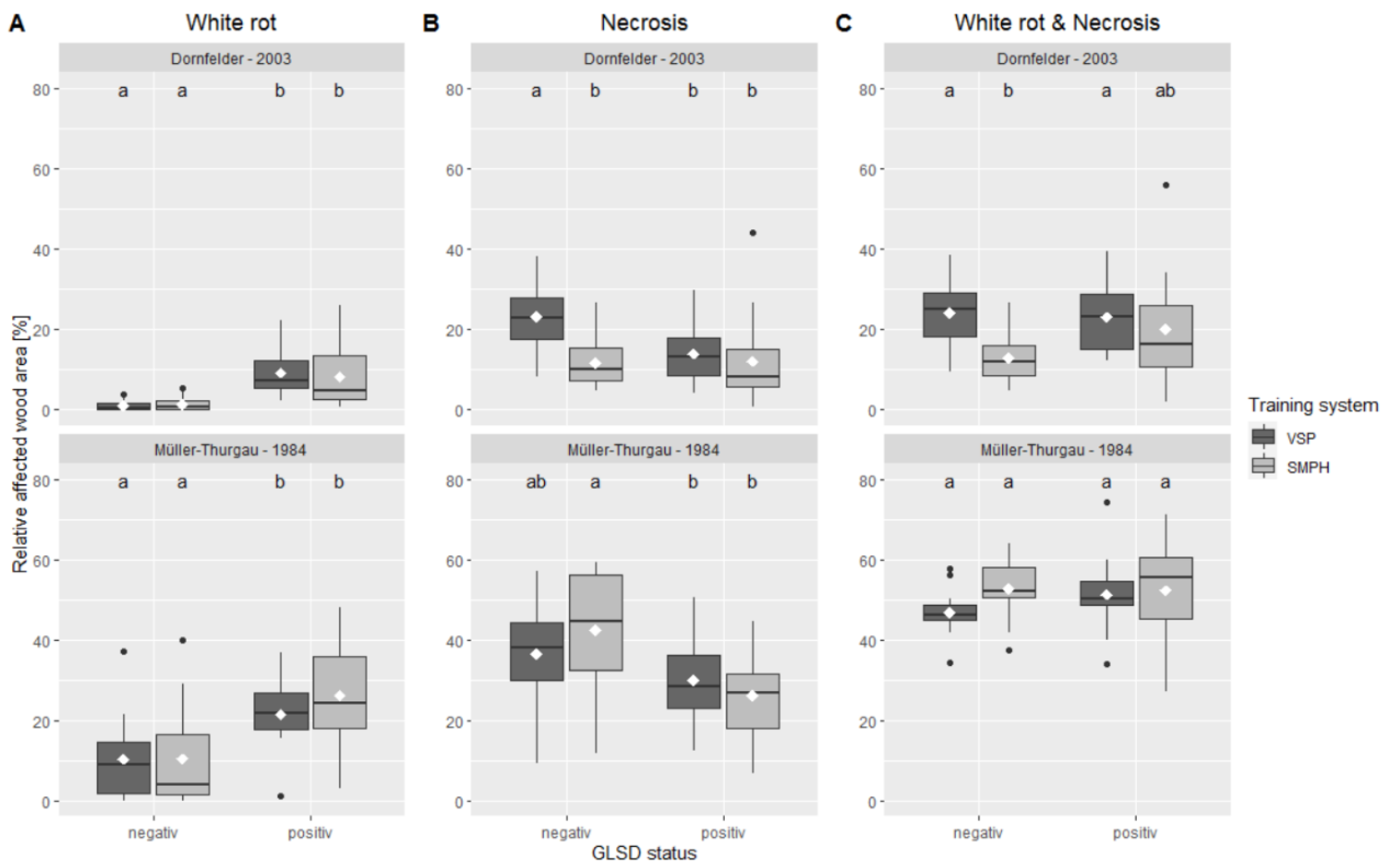
3.2. Grapevine Trunk Integrity
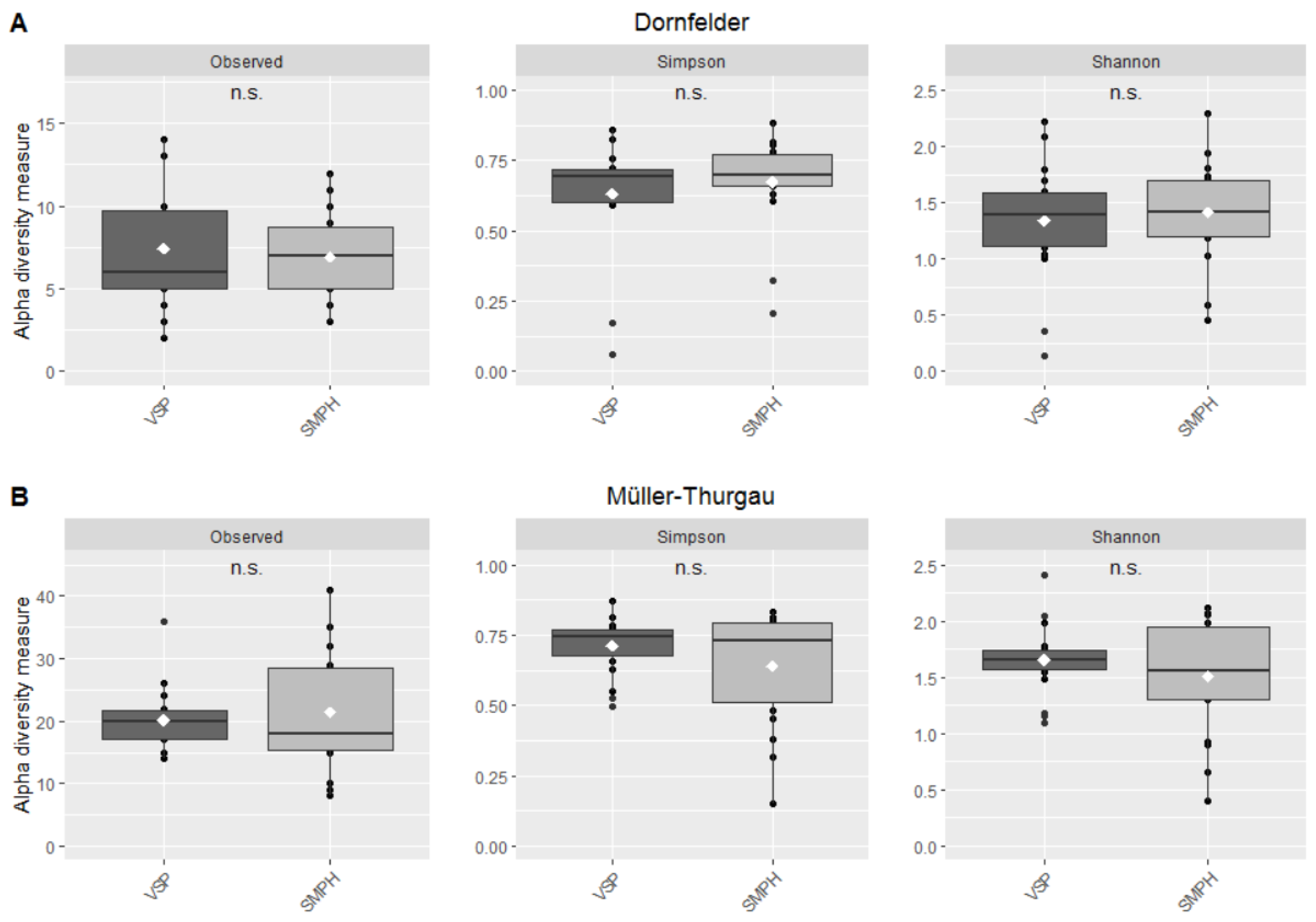
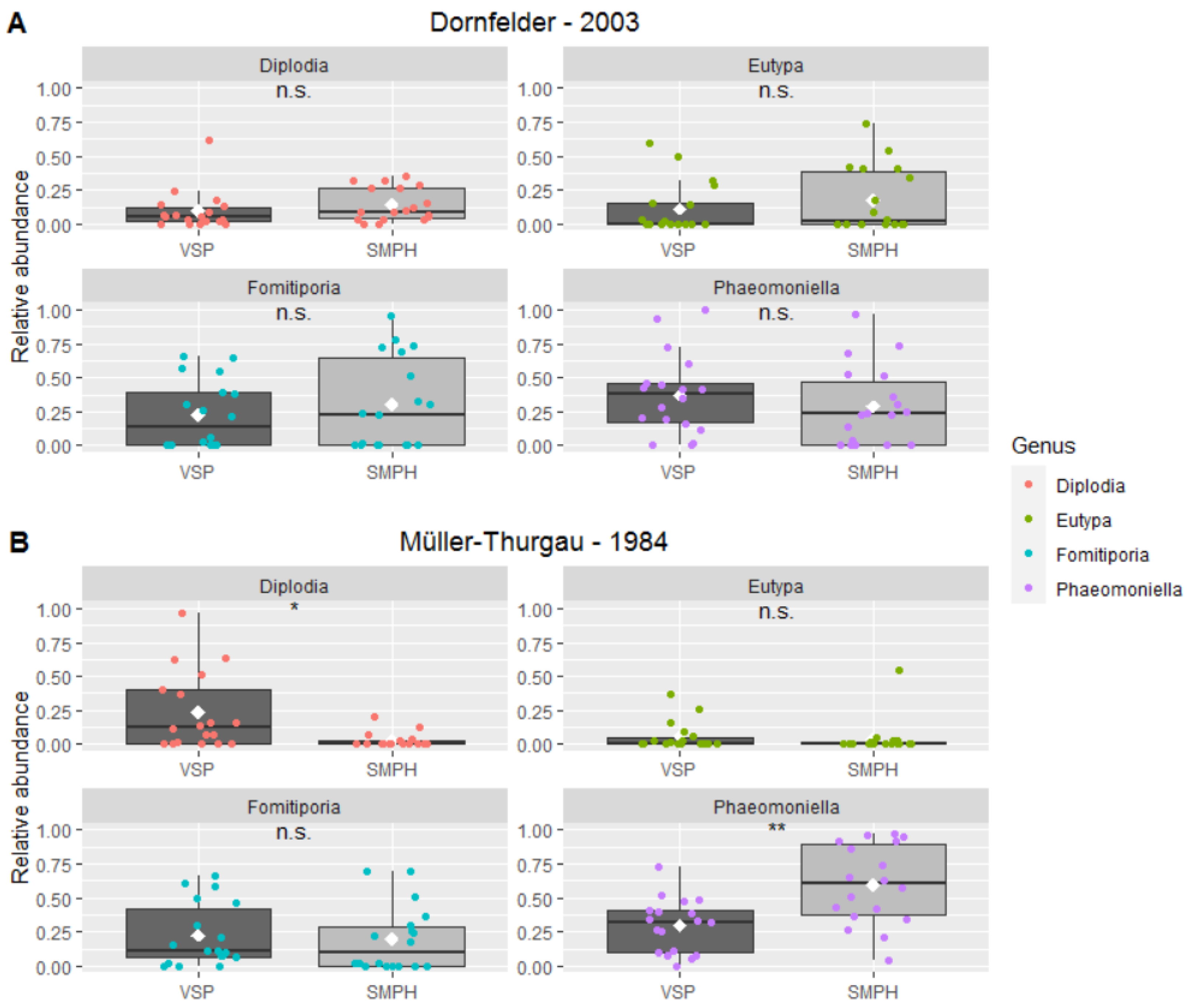
3.3. Mycobiome in the Trunk
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Gramaje, D.; Úrbez-Torres, J.R.; Sosnowski, M.R. Managing grapevine trunk diseases with respect to etiology and epidemiology: Current strategies and future prospects. Plant Dis. 2018, 102, 12–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mondello, V.; Songy, A.; Battiston, E.; Pinto, C.; Coppin, C.; Trotel-Aziz, P.; Clément, C.; Mugnai, L.; Fontaine, F. Grapevine trunk diseases: A review of fifteen years of trials for their control with chemicals and biocontrol agents. Plant Dis. 2018, 102, 1189–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovács, C.; Sándor, E. The increasing importance of grapevine trunk diseases. Int. J. Hortic. Sci. 2016, 22, 21–30. [Google Scholar] [CrossRef] [Green Version]
- Pouzoulet, J.; Scudiero, E.; Schiavon, M.; Rolshausen, P.E. Xylem vessel diameter affects the compartmentalization of the vascular pathogen Phaeomoniella chlamydospora in grapevine. Front. Plant Sci. 2017, 8, 1442. [Google Scholar] [CrossRef] [Green Version]
- Bertsch, C.; Ramírez-Suero, M.; Magnin-Robert, M.; Larignon, P.; Chong, J.; Abou-Mansour, E.; Spagnolo, A.; Clément, C.; Fontaine, F. Grapevine trunk diseases: Complex and still poorly understood. Plant Pathol. 2013, 62, 243–265. [Google Scholar] [CrossRef] [Green Version]
- Pouzoulet, J.; Scudiero, E.; Schiavon, M.; Santiago, L.S.; Rolshausen, P.E. Modeling of xylem vessel occlusion in grapevine. Tree Physiol. 2019, 39, 1438–1445. [Google Scholar] [CrossRef]
- Rolshausen, P.E.; Mahoney, N.E.; Molyneux, R.J.; Gubler, W.D. A reassessment of the species concept in Eutypa lata, the causal agent of eutypa dieback of grapevine. Phytopathology 2006, 96, 369–377. [Google Scholar] [CrossRef] [Green Version]
- Moyo, P.; Mostert, L.; Spies, C.F.J.; Damm, U.; Halleen, F. Diversity of Diatrypaceae species associated with dieback of grapevines in South Africa, with the description of Eutypa cremea sp. nov. Plant Dis. 2018, 102, 220–230. [Google Scholar] [CrossRef]
- Trouillas, F.P.; Urbez-Torres, J.R.; Gubler, W.D. Diversity of diatrypaceous fungi associated with grapevine canker diseases in California. Mycologia 2010, 102, 319–336. [Google Scholar] [CrossRef]
- Carter, M.V. The Status of Eutypa Lata as a Pathogen; CAB International: Wallingford, UK, 1991; ISBN 9780851986951. [Google Scholar]
- Úrbez-Torres, J.R.; Peduto, F.; Striegler, R.K.; Urrea-Romero, K.E.; Rupe, J.C.; Cartwright, R.D.; Gubler, W.D. Characterization of fungal pathogens associated with grapevine trunk diseases in Arkansas and Missouri. Fungal Divers. 2012, 52, 169–189. [Google Scholar] [CrossRef]
- Martos, S.; Andolfi, A.; Luque, J.; Mugnai, L.; Surico, G.; Evidente, A. Production of phytotoxic metabolites by five species of Botryosphaeriaceae causing decline on grapevines, with special interest in the species Neofusicoccum luteum and N. parvum. Eur. J. Plant Pathol. 2008, 121, 451–461. [Google Scholar] [CrossRef]
- Úrbez-Torres, J.R. The status of Botryosphaeriaceae species infecting grapevines. Phytopathol. Mediterr. 2011, 50, S5–S45. [Google Scholar]
- Surico, G. Towards a redefinition of the diseases within the esca complex of grapevine. Phytopathol. Mediterr. 2009, 48, 5–10. [Google Scholar]
- Surico, G.; Mugnai, L.; Marchi, G. The esca disease complex. In Integrated Management of Diseases Caused by Fungi, Phytoplasma and Bacteria; Ciancio, A., Mukerji, K.G., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 119–136. ISBN 978-1-4020-8570-3. [Google Scholar]
- Graniti, A.; Surico, G.; Mugnai, L. Esca of grapevine: A disease complex or a complex of diseases. Phytopathol. Mediterr. 2000, 39, 16–20. [Google Scholar]
- White, C.-L.; Halleen, F.; Mostert, L. Symptoms and fungi associated with esca in South African vineyards. Phytopathol. Mediterr. 2011, 50, S236–S246. [Google Scholar] [CrossRef]
- Kuntzmann, P.; Villaume, S.; Larignon, P.; Bertsch, C. Esca, BDA and Eutypiosis: Foliar symptoms, trunk lesions and fungi observed in diseased vinestocks in two vineyards in Alsace. Vitis 2010, 49, 71–76. [Google Scholar] [CrossRef]
- Mugnai, L.; Graniti, A.; Surico, G. Esca (black measles) and brown wood-streaking: Two old and elusive diseases of grapevines. Plant Dis. 1999, 83, 404–418. [Google Scholar] [CrossRef] [Green Version]
- Crous, P.W.; Gams, W. Phaeomoniella chlamydospora gen. et comb. nov., A causal organism of Petri grapevine decline and esca. Phytopathol. Mediterr. 2000, 39, 112–118. [Google Scholar]
- Fischer, M.; Kassemeyer, H.H. Fungi associated with esca disease of grapevine in Germany. Phytopathol. Mediterr. 2003, 42, 109–116. [Google Scholar] [CrossRef]
- Úrbez-Torres, J.R.; Haag, P.; Bowen, P.; O’Gorman, D.T. Grapevine trunk diseases in British Columbia: Incidence and characterization of the fungal pathogens associated with esca and Petri diseases of grapevine. Plant Dis. 2014, 98, 469–482. [Google Scholar] [CrossRef] [Green Version]
- Brown, A.A.; Lawrence, D.P.; Baumgartner, K. Role of basidiomycete fungi in the grapevine trunk disease esca. Plant Pathol. 2020, 69, 205–220. [Google Scholar] [CrossRef]
- Fischer, M.; García, V.G. An annotated checklist of European basidiomycetes related to white rot of grapevine (Vitis vinifera). Phytopathol. Mediterr. 2015, 54, 281–298. [Google Scholar]
- Fischer, M. A new wood-decaying basidiomycete species associated with esca of grapevine: Fomitiporia mediterranea (Hymenochaetales). Mycol. Prog. 2002, 1, 315–324. [Google Scholar] [CrossRef]
- Cloete, M.; Fischer, M.; Mostert, L.; Halleen, F. A novel Fomitiporia species associated with esca on grapevine in South Africa. Mycol. Prog. 2014, 13, 303–311. [Google Scholar] [CrossRef]
- Del Frari, G.; Oliveira, H.; Ferreira, R.B. White rot fungi (Hymenochaetales) and esca of grapevine: Insights from recent microbiome studies. J. Fungi 2021, 7, 770. [Google Scholar] [CrossRef]
- Pintos, C.; Redondo, D.C.; Aguín, O.; Mansilla, P. Fungi associated with grapevine trunk diseases in nursery-produced Vitis vinifera plants. Phytopathol. Mediterr. 2018, 57, 407–424. [Google Scholar] [CrossRef]
- Camele, I.; Mang, S.M. First report of Seimatosporium vitis associated with grapevine trunk diseases on Vitis vinifera in Italy. Plant Dis. 2019, 103, 771. [Google Scholar] [CrossRef]
- Abed-Ashtiani, F.; Narmani, A.; Arzanlou, M. Analysis of Kalmusia variispora associated with grapevine decline in Iran. Eur. J. Plant Pathol. 2019, 154, 787–799. [Google Scholar] [CrossRef]
- Fischer, M. Biodiversity and geographic distribution of Basidiomycetes causing esca-associates white rot in grapevine: A worldwide perspective. Phytopathol. Mediterr. 2006, 45, 30–42. [Google Scholar] [CrossRef]
- Cloete, M.; Fischer, M.; Mostert, L.; Halleen, F. Hymenochaetales associated with esca-related wood rots on grapevine with a special emphasis on the status of esca in South African vineyards. Phytopathol. Mediterr. 2015, 54, 299–312. [Google Scholar] [CrossRef]
- Maharachchikumbura, S.S.N.; Larignon, P.; Hyde, K.D.; Al-Sadi, A.; Liu, Z.-Y. Characterization of Neopestalotiopsis, Pestalotiopsis and Truncatella species associated with grapevine trunk diseases in France. Phytopathol. Mediterr. 2016, 55, 380–390. [Google Scholar] [CrossRef]
- Fischer, M.; Peighami-Ashnaei, S. Grapevine, esca complex, and environment: The disease triangle. Phytopathol. Mediterr. 2019, 58, 17–37. [Google Scholar] [CrossRef]
- Hrycan, J.; Hart, M.; Bowen, P.; Forge, T.; Úrbez-Torres, J.R. Grapevine trunk disease fungi: Their roles as latent pathogens and stress factors that favour disease develop-ment and symptom expression. Phytopathol. Mediterr. 2020, 59, 395–424. [Google Scholar]
- Kovács, C.; Balling, P.; Bihari, Z.; Nagy, A.; Sándor, E. Incidence of grapevine trunk diseases is influenced by soil, topology and vineyard age, but not by Diplodia seriata infection rate in the Tokaj Wine Region, Hungary. Phytoparasitica 2017, 45, 21–32. [Google Scholar] [CrossRef] [Green Version]
- Surico, G.; Mugnai, L.; Marchi, G. Older and more recent observations on esca: A critical overview. Phytopathol. Mediterr. 2006, 45, S68–S86. [Google Scholar] [CrossRef]
- Fontaine, F.; Gramaje, D.; Armengol, J.; Smart, R.; Nagy, Z.A.; Borgo, M.; Rego, C.; Corio-Costet, M.-F. Grapevine Trunk Diseases. A Review; OIV Publications: Paris, France, 2016; p. 24. [Google Scholar]
- Bruez, E.; Lecomte, P.; Grosman, J.; Doublet, B.; Bertsch, C.; Fontaine, F.; Ugaglia, A.; Teissedre, P.-L.; Da Costa, J.-P.; Guerin-Dubrana, L.; et al. Overview of grapevine trunk diseases in France in the 2000s. Phytopathol. Mediterr. 2013, 52, 262–275. [Google Scholar]
- Murolo, S.; Romanazzi, G. Effects of grapevine cultivar, rootstock and clone on esca disease. Australas. Plant Pathol. 2014, 43, 215–221. [Google Scholar] [CrossRef]
- Marchi, G. Susceptibility to esca of various grapevine (“Vitis vinifera”) cultivars grafted on different rootstock in a vineyard in the province of Siena (Italy). Phytopathol. Mediterr. 2001, 40, 27–36. [Google Scholar]
- Quaglia, M.; Covarelli, L.; Zazzerini, A. Epidemiological survey on esca disease in Umbria, Central Italy. Phytopathol. Mediterr. 2009, 48, 84–91. [Google Scholar]
- Pouzoulet, J.; Pivovaroff, A.L.; Santiago, L.S.; Rolshausen, P.E. Can vessel dimension explain tolerance toward fungal vascular wilt diseases in woody plants? Lessons from Dutch elm disease and esca disease in grapevine. Front. Plant Sci. 2014, 5, 253. [Google Scholar] [CrossRef]
- Ramsing, C.K.; Gramaje, D.; Mocholí, S.; Agustí, J.; de Santa María, F.C.S.; Armengol, J.; Berbegal, M. Relationship between the xylem anatomy of grapevine rootstocks and their susceptibility to Phaeoacremonium minimum and Phaeomoniella chlamydospora. Front. Plant Sci. 2021, 12, 726461. [Google Scholar] [CrossRef] [PubMed]
- Foglia, R.; Landi, L.; Romanazzi, G. Analyses of xylem vessel size on grapevine cultivars and relationship with incidence of esca disease, a threat to grape quality. Appl. Sci. 2022, 12, 1177. [Google Scholar] [CrossRef]
- Alsina, M.M.; de Herralde, F.; Aranda, X.; Savé, R.; Biel, C.C. Water relations and vulnerability to embolism are not related: Experiments with eight grapevine cultivars. Vitis 2007, 46, 1–6. [Google Scholar] [CrossRef]
- Marchi, G.; Peduto, F.; Mugnai, L.; di Marco, S.; Calzarano, F.; Surico, G. Some observations on the relationship of manifest and hidden esca to rainfall. Phytopathol. Mediterr. 2006, 45, S117–S126. [Google Scholar]
- Calzarano, F.; Osti, F.; Baránek, M.; di Marco, S. Rainfall and temperature influence expression of foliar symptoms of grapevine leaf stripe disease (esca complex) in vineyards. Phytopathol. Mediterr. 2018, 57, 488–505. [Google Scholar] [CrossRef]
- van Niekerk, J.M.; Bester, W.; Halleen, F.; Crous, P.W.; Fourie, P.H. The distribution and symptomatology of grapevine trunk disease pathogens are influenced by climate. Phytopathol. Mediterr. 2011, 50, S98–S111. [Google Scholar]
- Surico, G.; Marchi, G.; Braccini, P.; Mugnai, L. Epidemiology of esca in some vineyards in Tuscany (Italy). Phytopathol. Mediterr. 2000, 39, 190–205. [Google Scholar]
- Kraus, C.; Voegele, R.T.; Fischer, M. The esca complex in German vineyards: Does the training system influence occurrence of GLSD symptoms? Eur. J. Plant Pathol. 2019, 155, 265–279. [Google Scholar] [CrossRef]
- Mondello, V.; Larignon, P.; Armengol, J.; Kortekamp, A.; Vaczy, K.; Prezman, F.; Serrano, E.; Rego, C.; Mugnai, L.; Fontaine, F. Management of grapevine trunk diseases: Knowledge transfer, current strategies and innovative strategies adopted in Europe. Phytopathol. Mediterr. 2018, 57, 369–383. [Google Scholar] [CrossRef]
- Serra, S.; Mannoni, M.A.; Ligios, V. Studies on the susceptibility of pruning wounds to infection by fungi involved in grapevine wood diseases in Italy. Phytopathol. Mediterr. 2008, 47, 234–246. [Google Scholar] [CrossRef]
- Becker, A. Esca: Der Schnitt macht die Musik. Dtsch. Weinbau 2010, 1, 14–16. [Google Scholar]
- Travadon, R.; Lecomte, P.; Diarra, B.; Lawrence, D.P.; Renault, D.; Ojeda, H.; Rey, P.; Baumgartner, K. Grapevine pruning systems and cultivars influence the diversity of wood-colonizing fungi. Fungal Ecol. 2016, 24, 82–93. [Google Scholar] [CrossRef] [Green Version]
- Lecomte, P.; Diarra, B.; Carbonneau, A.; Rey, P.; Chevrier, C. Esca of grapevine and training practices in France: Results of a 10-year survey. Phytopathol. Mediterr. 2018, 57, 472–487. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Schulz, B.; Wanke, U.; Draeger, S.; Aust, H.-J. Endophytes from herbaceous plants and shrubs: Effectiveness of surface sterilization methods. Mycol. Res. 1993, 97, 1447–1450. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols, a Guide to Methods and Applications; Academic Press, Inc.: Cambridge, MA, USA, 1990; pp. 315–322. [Google Scholar]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [Green Version]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 17, 10. [Google Scholar] [CrossRef]
- Morgan, M.; Anders, S.; Lawrence, M.; Aboyoun, P.; Pagès, H.; Gentleman, R. ShortRead: A bioconductor package for input, quality assessment and exploration of high-throughput sequence data. Bioinformatics 2009, 25, 2607–2608. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, R.H.; Larsson, K.-H.; Taylor, A.F.S.; Bengtsson-Palme, J.; Jeppesen, T.S.; Schigel, D.; Kennedy, P.; Picard, K.; Glöckner, F.O.; Tedersoo, L.; et al. The UNITE database for molecular identification of fungi: Handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res. 2019, 47, D259–D264. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, G.F.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. 2020. Available online: http://vegan.r-forge.r-project.org/ (accessed on 25 January 2022).
- Sun, Q.; Rost, T.L.; Matthews, M.A. Wound-induced vascular occlusions in Vitis vinifera (Vitaceae): Tyloses in summer and gels in winter. Am. J. Bot. 2008, 95, 1498–1505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouadi, L.; Bruez, E.; Bastien, S.; Yacoub, A.; Coppin, C.; Guérin-Dubrana, L.; Fontaine, F.; Domec, J.-C.; Rey, P. Sap flow disruption in grapevine is the early signal predicting the structural, functional, and genetic responses to esca disease. Front. Plant Sci. 2021, 12, 695846. [Google Scholar] [CrossRef]
- Lorena, T.; Calamassi, R.; Mori, B.; Mugnai, L.; Surico, G. Phaeomoniella chlamydospora-grapevine interaction: Histochemical reactions to fungal infection. Phytopathol. Mediterr. 2001, 40, S400–S406. [Google Scholar]
- Bortolami, G.; Farolfi, E.; Badel, E.; Burlett, R.; Cochard, H.; Ferrer, N.; King, A.; Lamarque, L.J.; Lecomte, P.; Marchesseau-Marchal, M.; et al. Seasonal and long-term consequences of esca grapevine disease on stem xylem integrity. J. Exp. Bot. 2021, 72, 3914–3928. [Google Scholar] [CrossRef] [PubMed]
- Eskalen, A.; Feliciano, A.J.; Gubler, W.D. Susceptibility of grapevine pruning wounds and symptom development in response to infection by Phaeoacremonium aleophilum and Phaeomoniella chlamydospora. Plant Dis. 2007, 91, 1100–1104. [Google Scholar] [CrossRef] [Green Version]
- Rolshausen, P.E.; Úrbez-Torres, J.R.; Rooney-Latham, S.; Eskalen, A.; Smith, R.J.; Gubler, W.D. Evaluation of pruning wound susceptibility and protection against fungi associated with grapevine trunk diseases. Am. J. Enol. Vitic. 2010, 61, 113–119. [Google Scholar]
- Moretti, S.; Pacetti, A.; Pierron, R.; Kassemeyer, H.-H.; Fischer, M.; Péros, J.-P.; Perez-Gonzalez, G.; Bieler, E.; Schilling, M.; di Marco, S.; et al. Fomitiporia mediterranea M. Fisch., the historical Esca agent: A comprehensive review on the main grapevine wood rot agent in Europe. Phytopathol. Mediterr. 2021, 60, 351–379. [Google Scholar] [CrossRef]
- Armengol, J.; Vicent, A.; Torné, L.; Garcia-Figueres, F.; García-Jiménez, J. Fungi associated with esca and grapevine declines in Spain: A three-year survey. Phytopathol. Mediterr. 2001, 40, 325–329. [Google Scholar]
- Elena, G.; Bruez, E.; Rey, P.; Luque, J. Microbiota of grapevine woody tissues with or without esca-foliar symptoms in northeast Spain. Phytopathol. Mediterr. 2018, 57, 425–438. [Google Scholar] [CrossRef]
- Serra, S.; Borgo, M.; Zanzotto, A. Investigation into the presence of fungi associated with esca of young vines. Phytopathol. Mediterr. 2000, 39, 21–25. [Google Scholar]
- Pollastro, S.; Dongiovanni, C.; Abbatecola, A.; Faretra, F. Observations on the fungi associated with esca and on spatial distribution of esca-symptomatic plants in Apulian (Italy) vineyards. Phytopathol. Mediterr. 2000, 39, 206–210. [Google Scholar]
- Pacetti, A.; Moretti, S.; Pinto, C.; Compant, S.; Farine, S.; Bertsch, C.; Mugnai, L. Trunk surgery as a tool to reduce foliar symptoms in diseases of the esca complex and its influence on vine wood microbiota. J. Fungi 2021, 7, 521. [Google Scholar] [CrossRef] [PubMed]
- Bruez, E.; Larignon, P.; Bertsch, C.; Robert-Siegwald, G.; Lebrun, M.-H.; Rey, P.; Fontaine, F. Impacts of sodium arsenite on wood microbiota of esca-diseased grapevines. J. Fungi 2021, 7, 498. [Google Scholar] [CrossRef]
- Larignon, P.; Dubos, B. Preliminary studies on the biology of Phaeoacremonium. Phytopathol. Mediterr. 2000, 39, 184–189. [Google Scholar]
- Gramaje, D.; Armengol, J. Fungal trunk pathogens in the grapevine propagation process: Potential inoculum sources, detection, identification, and management strategies. Plant Dis. 2011, 95, 1040–1055. [Google Scholar] [CrossRef] [Green Version]
- Snelders, N.C.; Petti, G.C.; van den Berg, G.C.M.; Seidl, M.F.; Thomma, B.P.H.J. An ancient antimicrobial protein co-opted by a fungal plant pathogen for in planta mycobiome manipulation. Proc. Natl. Acad. Sci. USA 2021, 118, e2110968118. [Google Scholar] [CrossRef]
- Snelders, N.C.; Kettles, G.J.; Rudd, J.J.; Thomma, B.P.H.J. Plant pathogen effector proteins as manipulators of host microbiomes? Mol. Plant Pathol. 2018, 19, 257–259. [Google Scholar] [CrossRef] [Green Version]
- Hofstetter, V.; Buyck, B.; Croll, D.; Viret, O.; Couloux, A.; Gindro, K. What if esca disease of grapevine were not a fungal disease? Fungal Divers. 2012, 54, 51–67. [Google Scholar] [CrossRef] [Green Version]
- Bruez, E.; Vallance, J.; Gautier, A.; Laval, V.; Compant, S.; Maurer, W.; Sessitsch, A.; Lebrun, M.-H.; Rey, P. Major changes in grapevine wood microbiota are associated with the onset of esca, a devastating trunk disease. Environ. Microbiol. 2020, 22, 5189–5206. [Google Scholar] [CrossRef]
- Bruez, E.; Baumgartner, K.; Bastien, S.; Travadon, R.; Guérin-Dubrana, L.; Rey, P. Various fungal communities colonise the functional wood tissues of old grapevines externally free from grapevine trunk disease symptoms. Aust. J. Grape Wine Res. 2016, 22, 288–295. [Google Scholar] [CrossRef]
- Bruez, E.; Vallance, J.; Gerbore, J.; Lecomte, P.; Da Costa, J.-P.; Guerin-Dubrana, L.; Rey, P. Analyses of the temporal dynamics of fungal communities colonizing the healthy wood tissues of esca leaf-symptomatic and asymptomatic vines. PLoS ONE 2014, 9, e95928. [Google Scholar] [CrossRef] [PubMed]
- Kraus, C.; Voegele, R.T.; Fischer, M. Temporal development of the culturable, endophytic fungal community in healthy grapevine branches and occurrence of GTD-associated fungi. Microb. Ecol. 2019, 77, 866–876. [Google Scholar] [CrossRef] [PubMed]
- Rolshausen, P.E.; Baumgartner, K.; Travadon, R.; Fujiyoshi, P.; Pouzoulet, J.; Wilcox, W.F. Identification of Eutypa spp. causing Eutypa dieback of grapevine in Eastern North America. Plant Dis. 2014, 98, 483–491. [Google Scholar] [CrossRef] [Green Version]
- Úrbez-Torres, J.R.; Gubler, W.D. Pathogenicity of Botryosphaeriaceae species isolated from grapevine cankers in California. Plant Dis. 2009, 93, 584–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Billones-Baaijens, R.; Jones, E.E.; Ridgway, H.J.; Jaspers, M.V. Virulence affected by assay parameters during grapevine pathogenicity studies with Botryosphaeriaceae nursery isolates. Plant Pathol. 2013, 62, 1214–1225. [Google Scholar] [CrossRef] [Green Version]
- Carlucci, A.; Cibelli, F.; Lops, F.; Raimondo, M.L. Characterization of Botryosphaeriaceae species as causal agents of trunk diseases on grapevines. Plant Dis. 2015, 99, 1678–1688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Silva, M.A.; Correia, K.C.; Barbosa, M.A.G.; Câmara, M.P.S.; Gramaje, D.; Michereff, S.J. Characterization of Phaeoacremonium isolates associated with Petri disease of table grape in Northeastern Brazil, with description of Phaeoacremonium nordesticola sp. nov. Eur. J. Plant Pathol. 2017, 149, 695–709. [Google Scholar] [CrossRef]
- Mostert, L.; Halleen, F.; Fourie, P.H.; Crous, P.W. A review of Phaeoacremonium species involved in Petri disease and esca of grapevines. Phytopathol. Mediterr. 2006, 45, S12–S29. [Google Scholar]
- Travadon, R.; Lawrence, D.P.; Rooney-Latham, S.; Gubler, W.D.; Wilcox, W.F.; Rolshausen, P.E.; Baumgartner, K. Cadophora species associated with wood-decay of grapevine in North America. Fungal Biol. 2015, 119, 53–66. [Google Scholar] [CrossRef]
- Gramaje, D.; Mostert, L.; Armengol, J. Characterization of Cadophora luteo-olivacea and C. melinii isolates obtained from grapevines and environmental samples from grapevine nurseries in Spain. Phytopathol. Mediterr. 2011, 50, S112–S126. [Google Scholar]
- Úrbez-Torres, J.R.; Peduto, F.; Smith, R.J.; Gubler, W.D. Phomopsis Dieback: A grapevine trunk disease caused by Phomopsis viticola in California. Plant Dis. 2013, 97, 1571–1579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, A.J. Excoriose, cane blight and related diseases of grapevines: A taxonomic review of the pathogens. Phytopathol. Mediterr. 2000, 39, 341–356. [Google Scholar]
- Dissanayake, A.J.; Phillips, A.J.; Hyde, K.D.; Yan, J.T.; Li, X.H. The current status of species in Diaporthe. Mycosphere 2017, 8, 1106–1156. [Google Scholar] [CrossRef]
- Dai, Y.-C. Hymenochaetaceae (Basidiomycota) in China. Fungal Divers. 2010, 45, 131–343. [Google Scholar] [CrossRef]
- Zhou, L.-W.; Qin, W.-M. Phylogeny and taxonomy of the recently proposed genus Phellinopsis (Hymenochaetales, Basidiomycota). Mycologia 2013, 105, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Karácsony, Z.; Knapp, D.G.; Lengyel, S.; Kovács, G.M.; Váczy, K.Z. The fungus Kalmusia longispora is able to cause vascular necrosis on Vitis vinifera. PLoS ONE 2021, 16, e0258043. [Google Scholar] [CrossRef] [PubMed]
- Váczy, K.Z. First Report of Seimatosporium vitis associated with grapevine trunk disease symptoms in Hungary. Plant Dis. 2017, 101, 253. [Google Scholar] [CrossRef]
- Fischer, M. Grapevine trunk diseases in German viticulture. III. Biodiversity and spatial distribution of fungal pathogens in rootstock mother plants and possible relation to leaf symptoms. VITIS-J. Grapevine Res. 2019, 58, 141–149. [Google Scholar] [CrossRef]
- Lengyel, S.; Knapp, D.G.; Karácsony, Z.; Geml, J.; Tempfli, B.; Kovács, G.M.; Váczy, K.Z. Neofabraea kienholzii, A novel causal agent of grapevine trunk diseases in Hungary. Eur. J. Plant Pathol. 2020, 157, 975–984. [Google Scholar] [CrossRef]
- Cimmino, A.; Bahmani, Z.; Masi, M.; Abdollahzadeh, J.; Amini, J.; Tuzi, A.; Evidente, A. Phytotoxins produced by Didymella glomerata and Truncatella angustata, associated with grapevine trunk diseases (GTDs) in Iran. Nat. Prod. Res. 2021, 1–8. [Google Scholar] [CrossRef]
- Arzanlou, M.; Narmani, A.; Moshari, S.; Khodaei, S.; Babai-Ahari, A. Truncatella angustata associated with grapevine trunk disease in northern Iran. Arch. Phytopathol. Plant Prot. 2013, 46, 1168–1181. [Google Scholar] [CrossRef]
- Lawrence, D.P.; Travadon, R.; Baumgartner, K. Novel Seimatosporium species from grapevine in Northern California and their interactions with fungal pathogens Involved in the trunk-disease complex. Plant Dis. 2018, 102, 1081–1092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Z.-B.; Li, S.-D.; Ren, Q.; Xu, J.-L.; Lu, X.; Sun, M.-H. Biology and applications of Clonostachys rosea. J. Appl. Microbiol. 2020, 129, 486–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva-Valderrama, I.; Toapanta, D.; Miccono, M.d.L.A.; Lolas, M.; Díaz, G.A.; Cantu, D.; Castro, A. Biocontrol potential of grapevine endophytic and rhizospheric fungi against trunk pathogens. Front. Microbiol. 2020, 11, 614620. [Google Scholar] [CrossRef] [PubMed]




| Cultivar | Location | Berry Color | Rootstock | Year of Planting | Training System | Year of Conversion to SMPH | Plant Protection | Plants |
|---|---|---|---|---|---|---|---|---|
| ‘Dornfelder’ | 49°52′15.3″ N 8°14′03.3″ E | red | 5BB | 2003 | SMPH | 2013 | conventional | 2266 |
| SMPH | 2020 | conventional | 726 | |||||
| VSP | conventional | 1403 | ||||||
| ‘Müller-Thurgau’ | 49°51′40.3″ N 7°50′35.7″ E | white | SO4 | 1984 | SMPH | 2008 | organic | 357 |
| VSP | organic | 316 |
| ‘Dornfelder’ | ‘Müller-Thurgau’ | |||||
|---|---|---|---|---|---|---|
| Grapevine Property | VSP | SMPH | sig. | VSP | SMPH | sig. |
| number of pruning wounds on trunk | 17.3 ± 2.9 (n = 20) | 9.3 ± 2.8 (n = 20) | ** | 23.0 ± 6.3 (n = 20) | 13.3 ± 3.4 (n = 20) | ** |
| pruning wound size [mm2] | 16.1 ± 5.7 (n = 164) | 16.2 ± 6.4 (n = 89) | n.s. | 19.4 ± 10.3 (n = 178) | 18.2 ± 9.0 (n = 133) | n.s. |
| trunk height [cm] | 73.8 ± 5.6 (n = 20) | 74.5 ± 3.4 (n = 20) | n.s. | 83.4 ± 7.6 (n = 20) | 78.6 ± 6.0 (n = 20) | n.s. |
| circumference trunk head [cm] | 28.4 ± 5.0 (n = 20) | 23.8 ± 3.5 (n = 20) | * | 31.6 ± 5.1 (n = 20) | 27.7 ± 4.9 (n = 20) | * |
| circumference trunk center [cm] | 15.9 ± 1.6 (n = 20) | 15.4 ± 1.2 (n = 20) | n.s. | 17.0 ± 1.6 (n = 20) | 16.3 ± 3.0 (n = 20) | n.s. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kraus, C.; Rauch, C.; Kalvelage, E.M.; Behrens, F.H.; d’Aguiar, D.; Dubois, C.; Fischer, M. Minimal versus Intensive: How the Pruning Intensity Affects Occurrence of Grapevine Leaf Stripe Disease, Wood Integrity, and the Mycobiome in Grapevine Trunks. J. Fungi 2022, 8, 247. https://doi.org/10.3390/jof8030247
Kraus C, Rauch C, Kalvelage EM, Behrens FH, d’Aguiar D, Dubois C, Fischer M. Minimal versus Intensive: How the Pruning Intensity Affects Occurrence of Grapevine Leaf Stripe Disease, Wood Integrity, and the Mycobiome in Grapevine Trunks. Journal of Fungi. 2022; 8(3):247. https://doi.org/10.3390/jof8030247
Chicago/Turabian StyleKraus, Christian, Carolin Rauch, Elisa Maria Kalvelage, Falk Hubertus Behrens, Dagmar d’Aguiar, Cornelia Dubois, and Michael Fischer. 2022. "Minimal versus Intensive: How the Pruning Intensity Affects Occurrence of Grapevine Leaf Stripe Disease, Wood Integrity, and the Mycobiome in Grapevine Trunks" Journal of Fungi 8, no. 3: 247. https://doi.org/10.3390/jof8030247
APA StyleKraus, C., Rauch, C., Kalvelage, E. M., Behrens, F. H., d’Aguiar, D., Dubois, C., & Fischer, M. (2022). Minimal versus Intensive: How the Pruning Intensity Affects Occurrence of Grapevine Leaf Stripe Disease, Wood Integrity, and the Mycobiome in Grapevine Trunks. Journal of Fungi, 8(3), 247. https://doi.org/10.3390/jof8030247






