Assessment of a New Copper-Based Formulation to Control Esca Disease in Field and Study of Its Impact on the Vine Microbiome, Vine Physiology and Enological Parameters of the Juice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Conditions
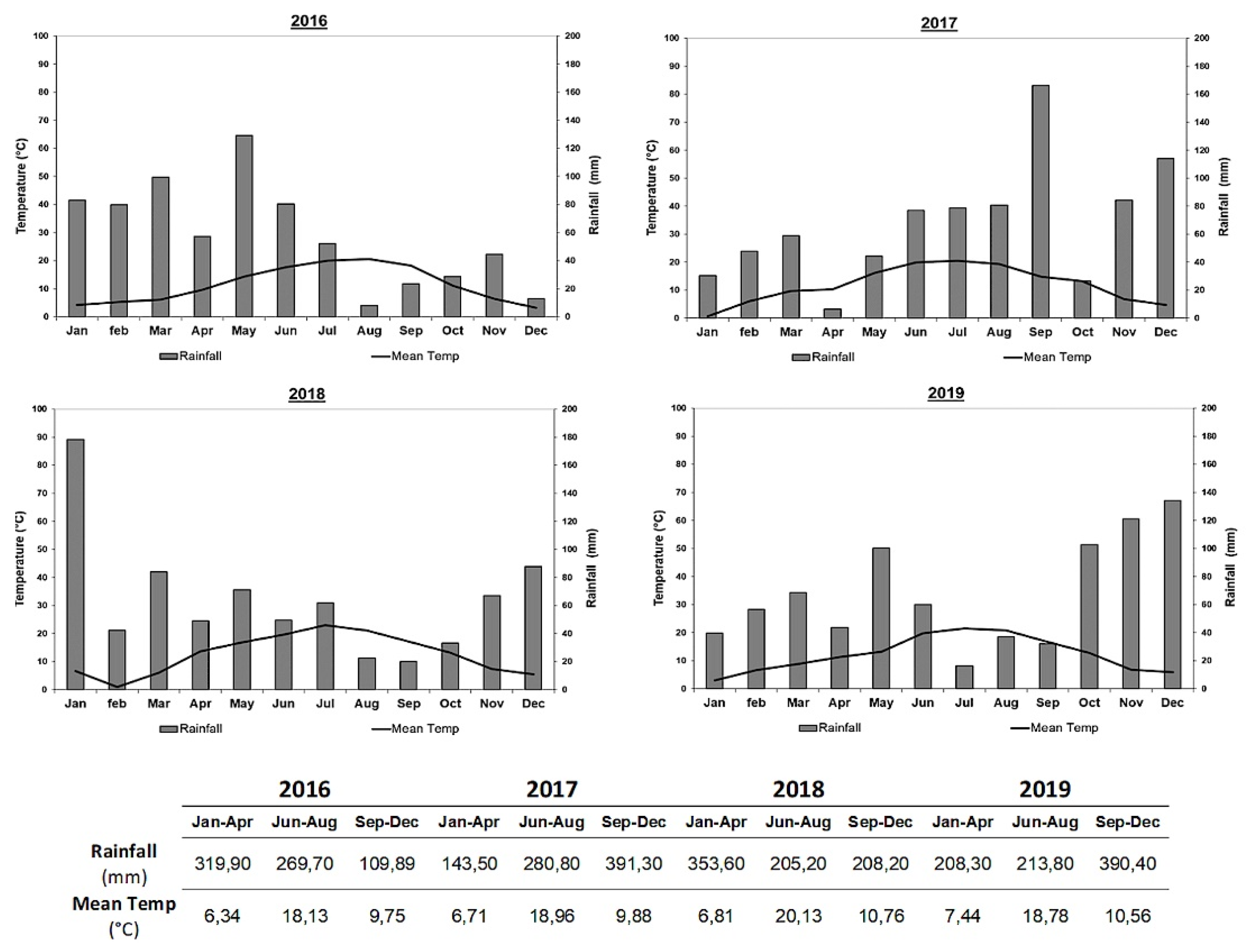
2.1.1. Plot
2.1.2. LC2017 Product Formulation and Field Treatments Schedule
2.1.3. Field Surveys and Esca Incidence Analysis
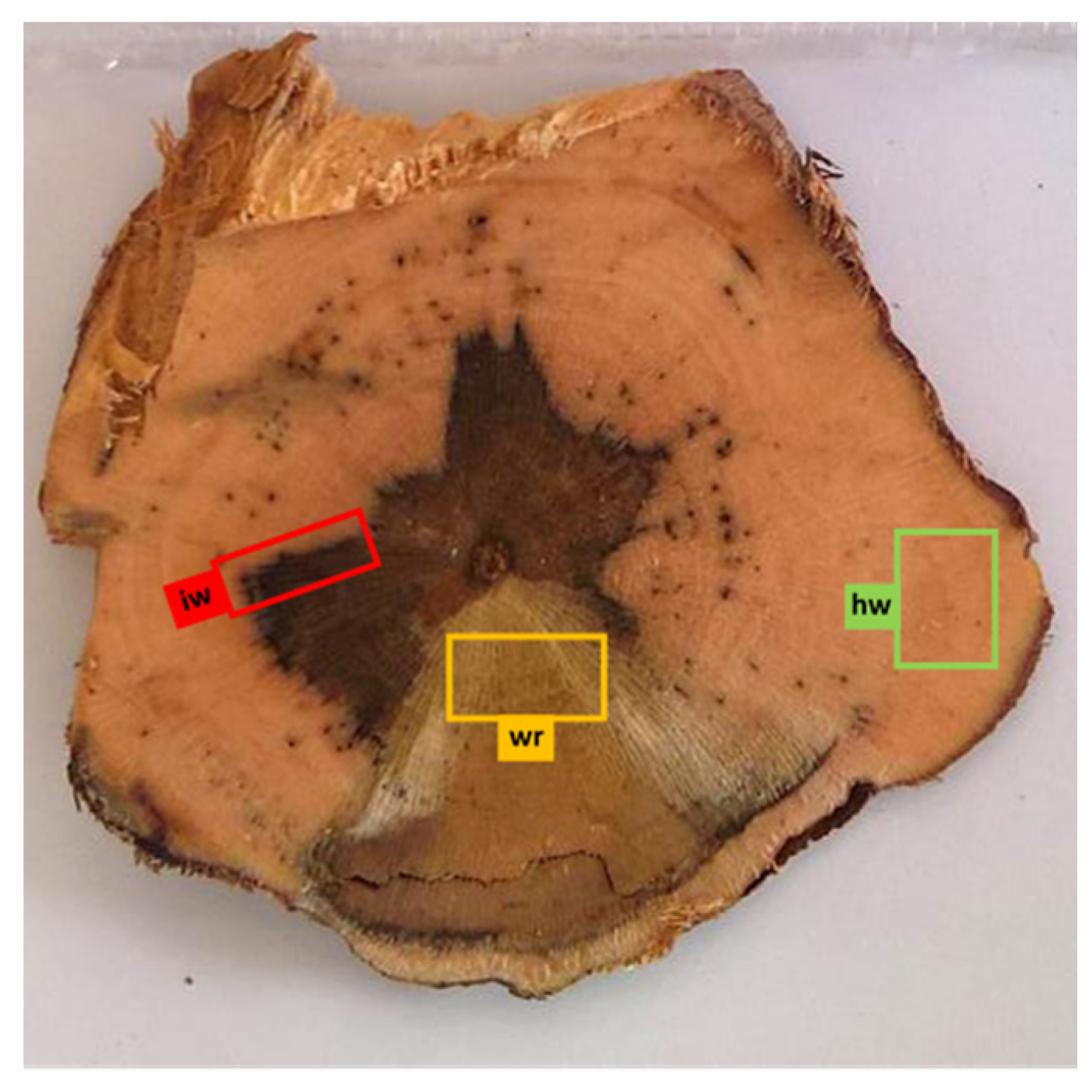
2.1.4. Whole Vines Sampling
2.2. Analysis of the Vine Microbiome
Subsamples’ Preparation
2.3. Metabolomic Analysis
2.4. Transcriptomic Analysis
2.5. Juice Analyses
3. Results
3.1. Effect of LC2017 Treatments on Esca Disease Symptoms Expression
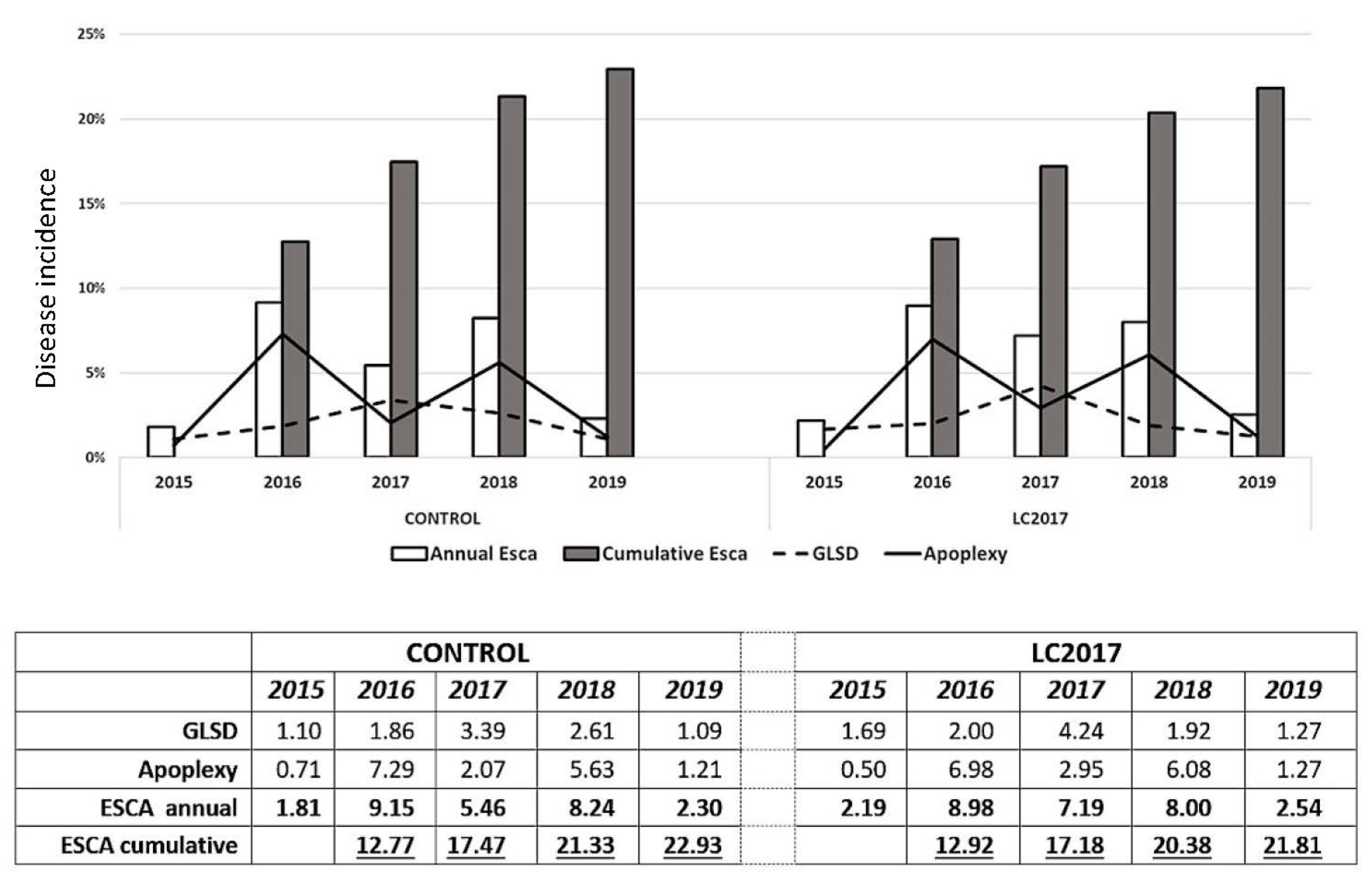
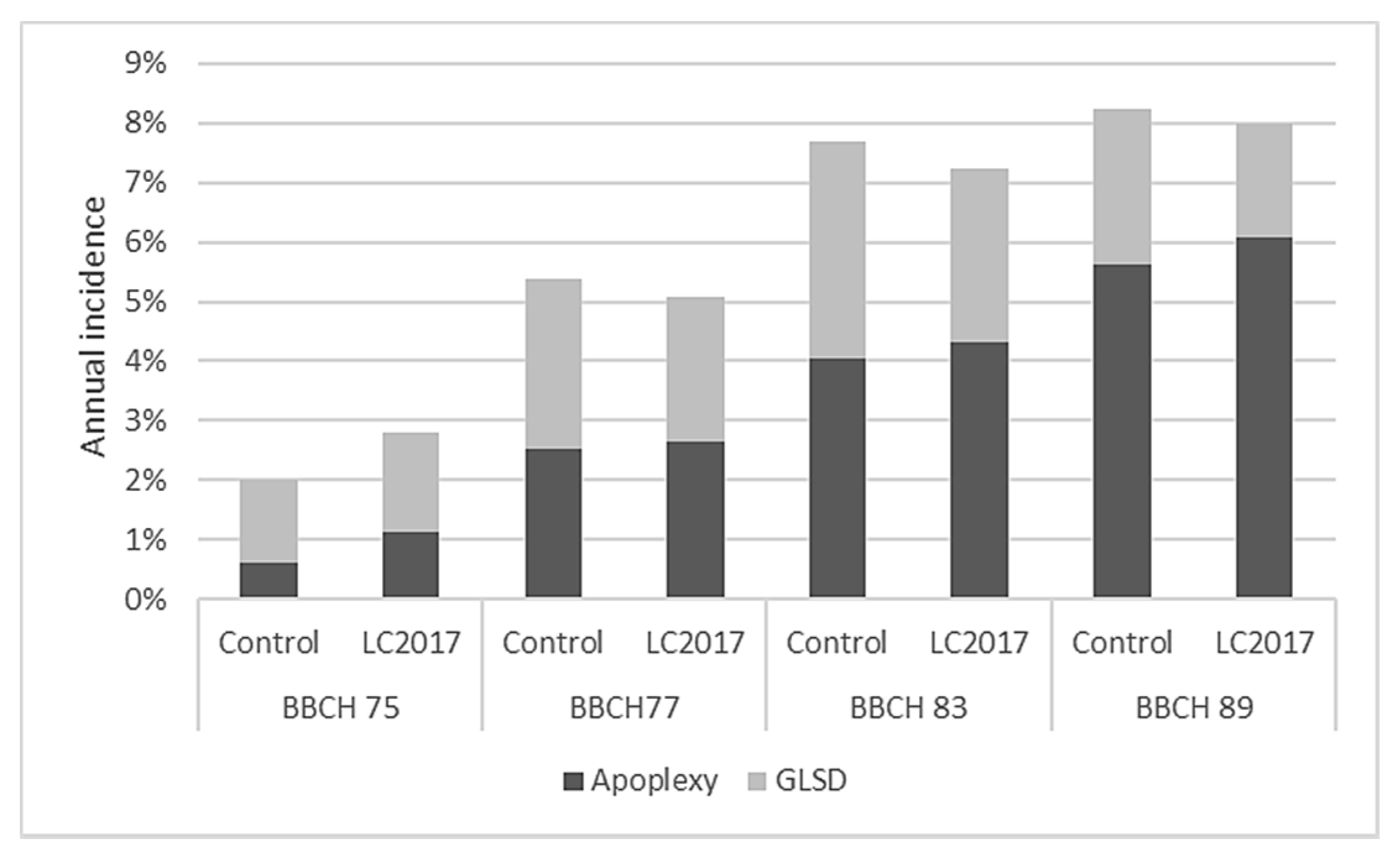
3.1.1. Annual Incidence
3.1.2. Cumulative Incidence from 2015 to 2019
3.1.3. Grapevine Leaf Stripe Disease (GLSD) vs. Apoplexy
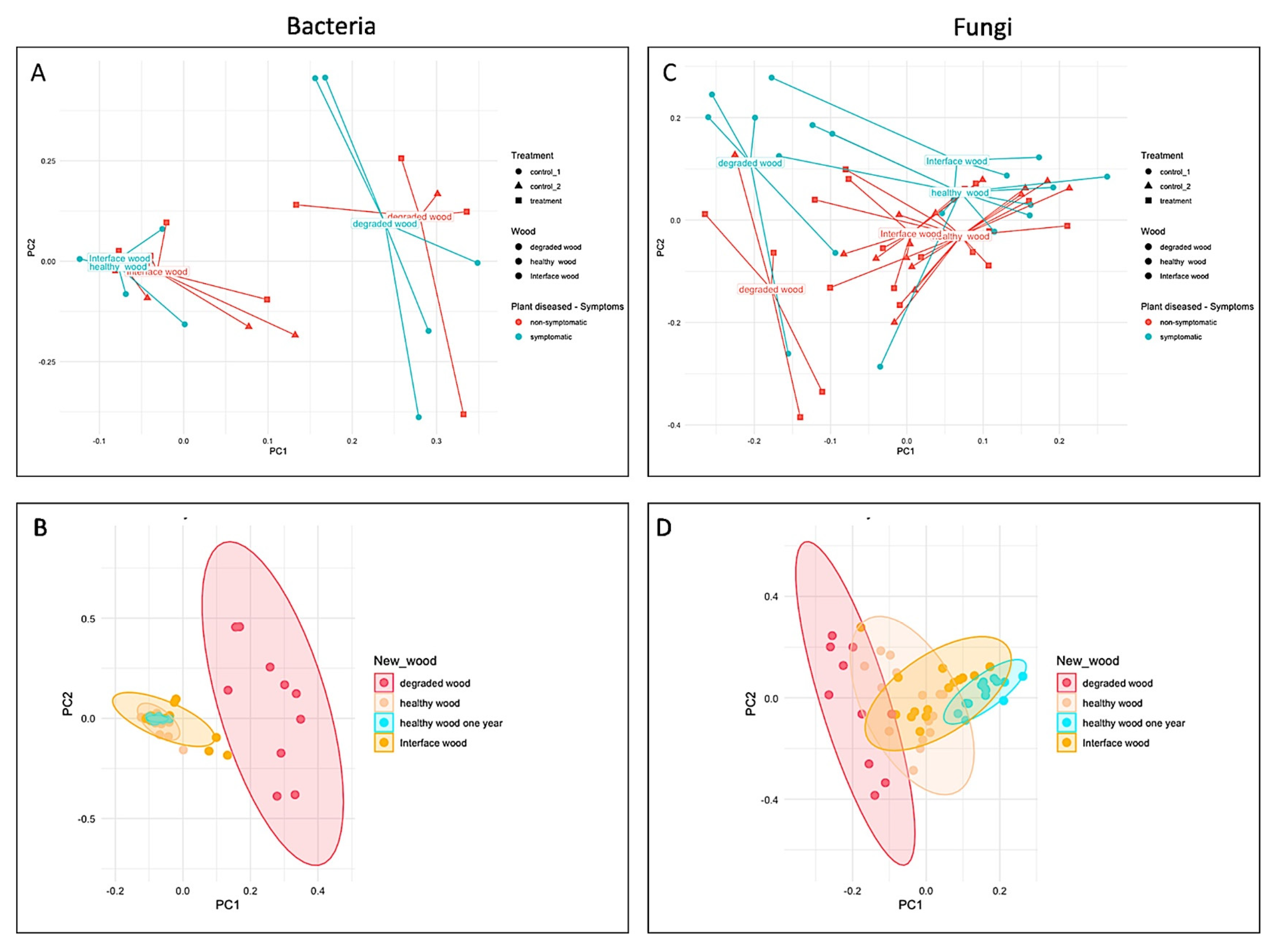
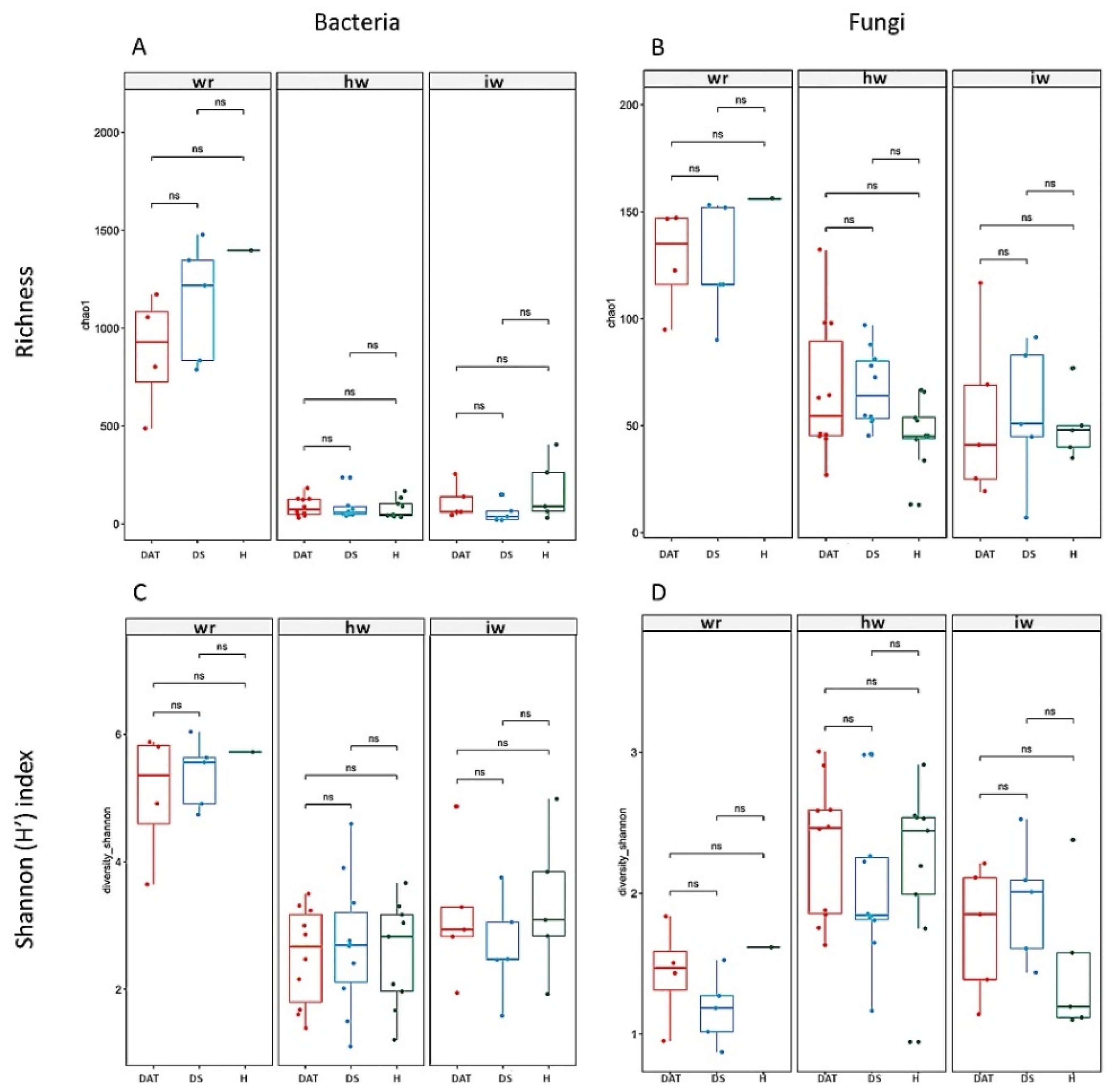
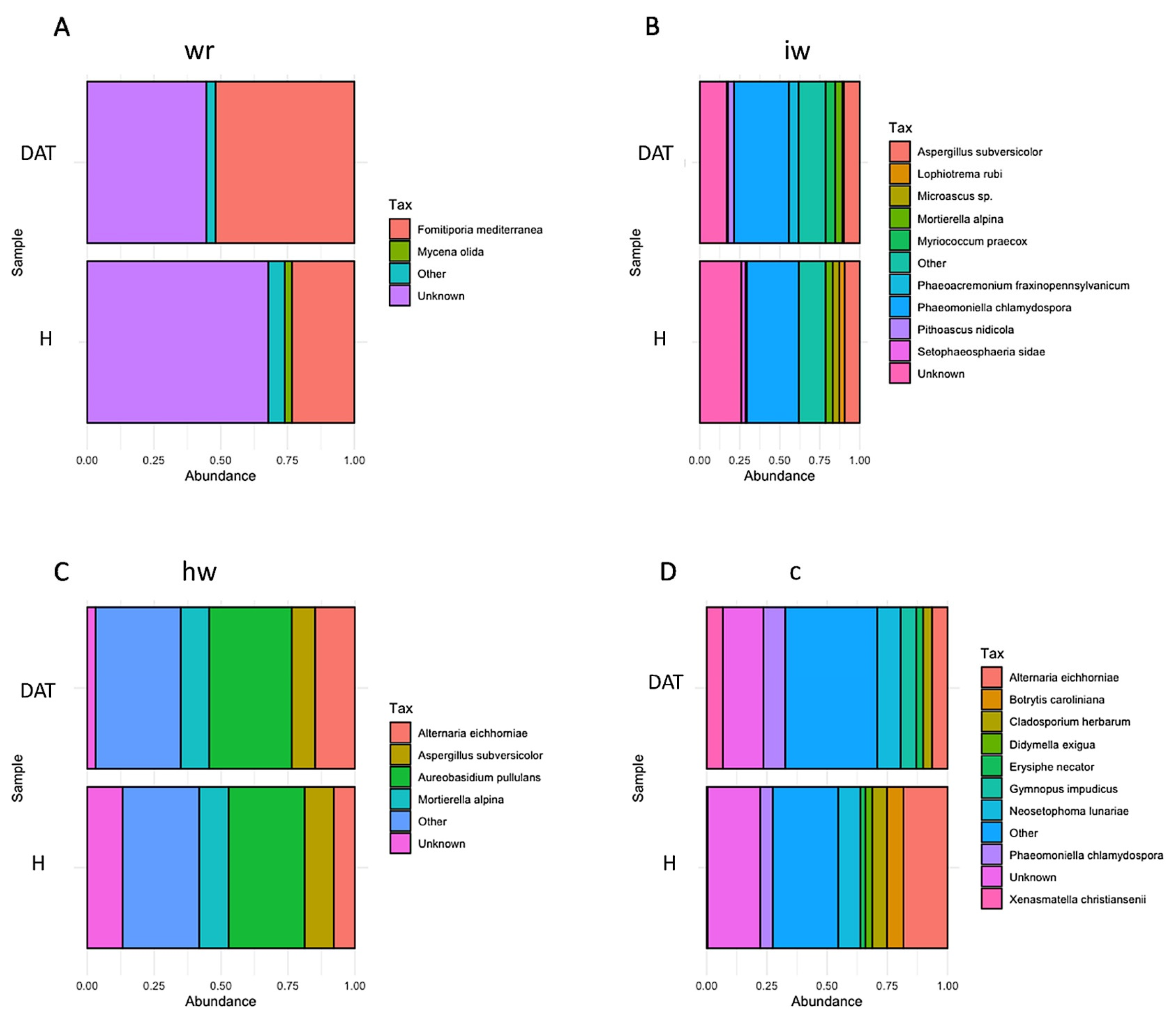
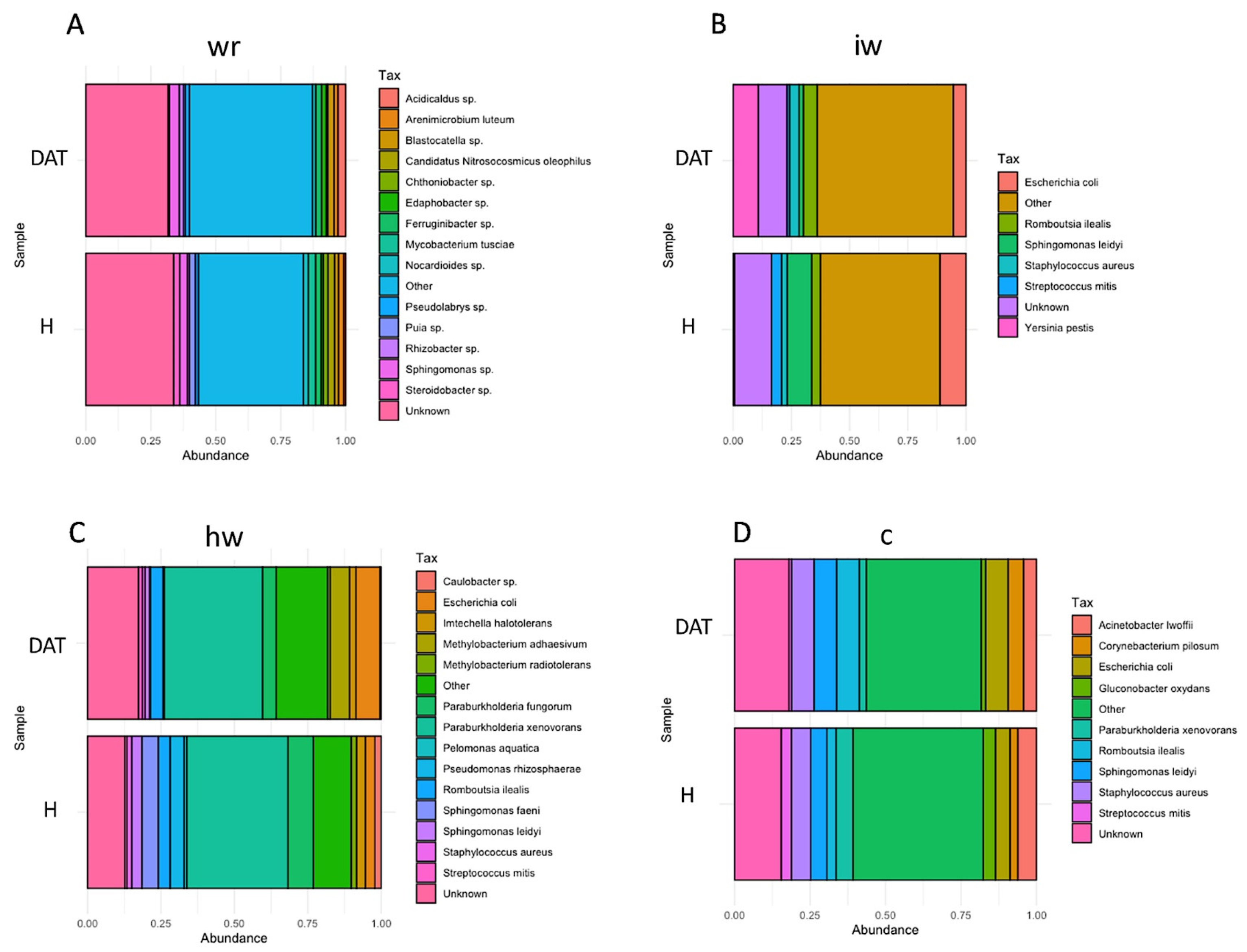
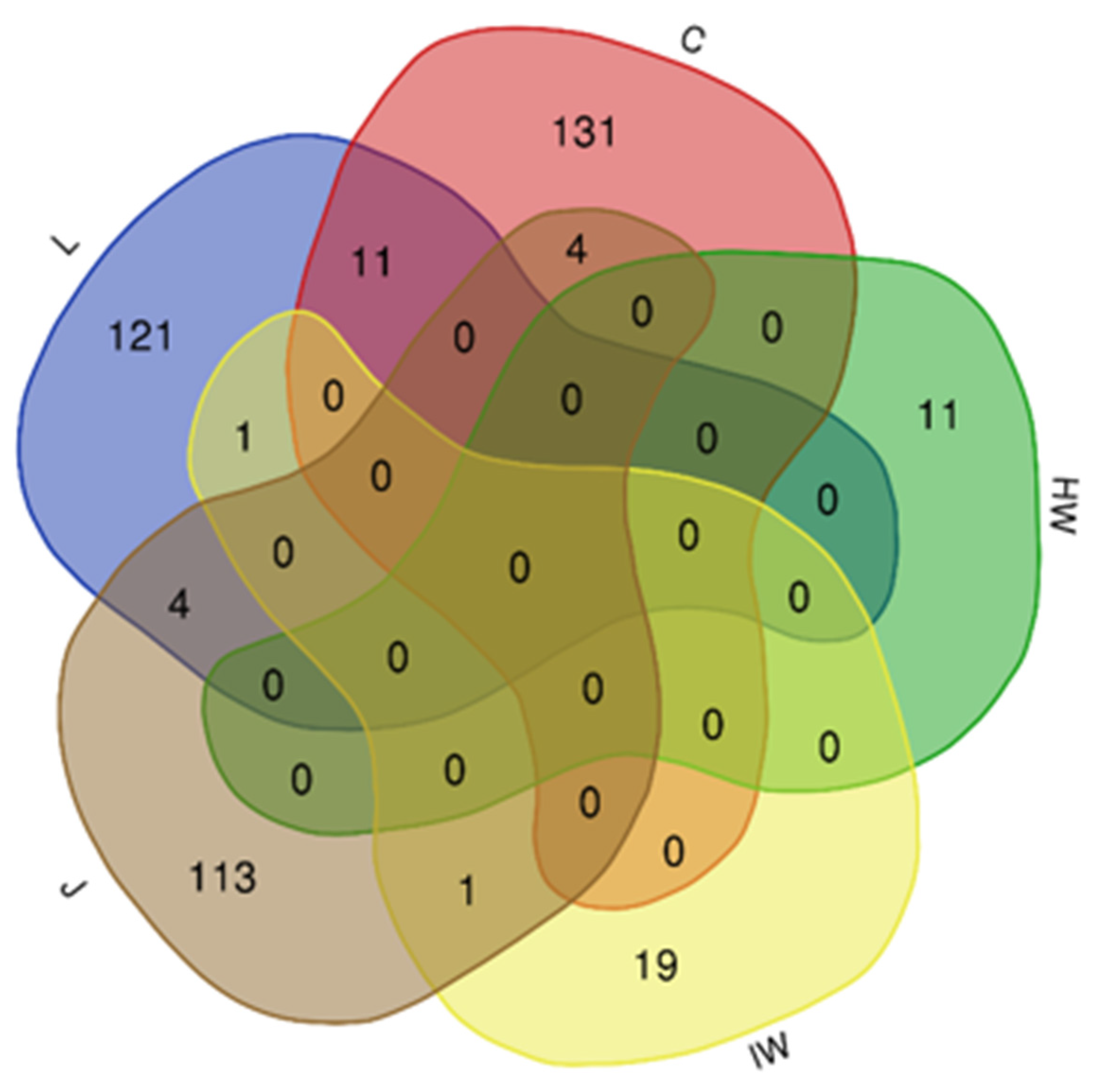
3.2. Impact of LC2017 on the Vine Microbiome
3.3. Impact of LC2017 Treatment on Plant Physiology
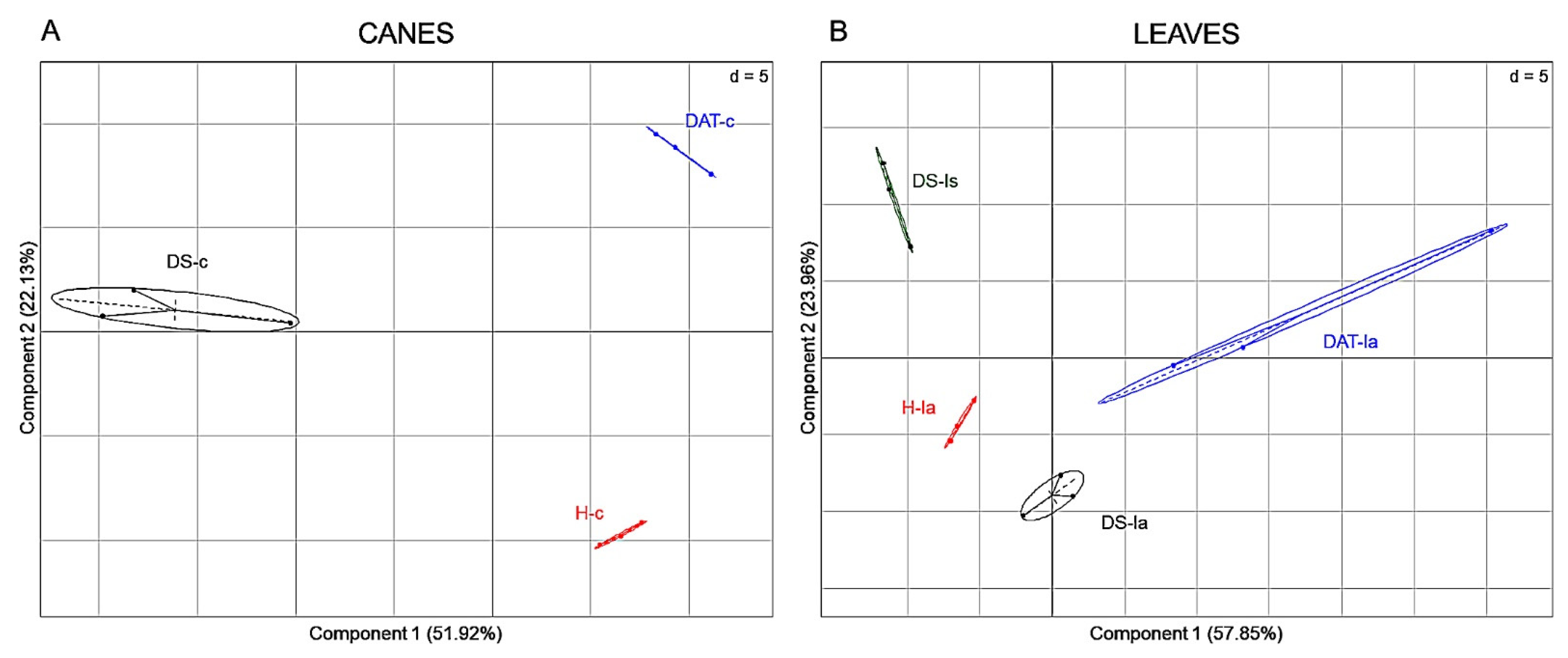
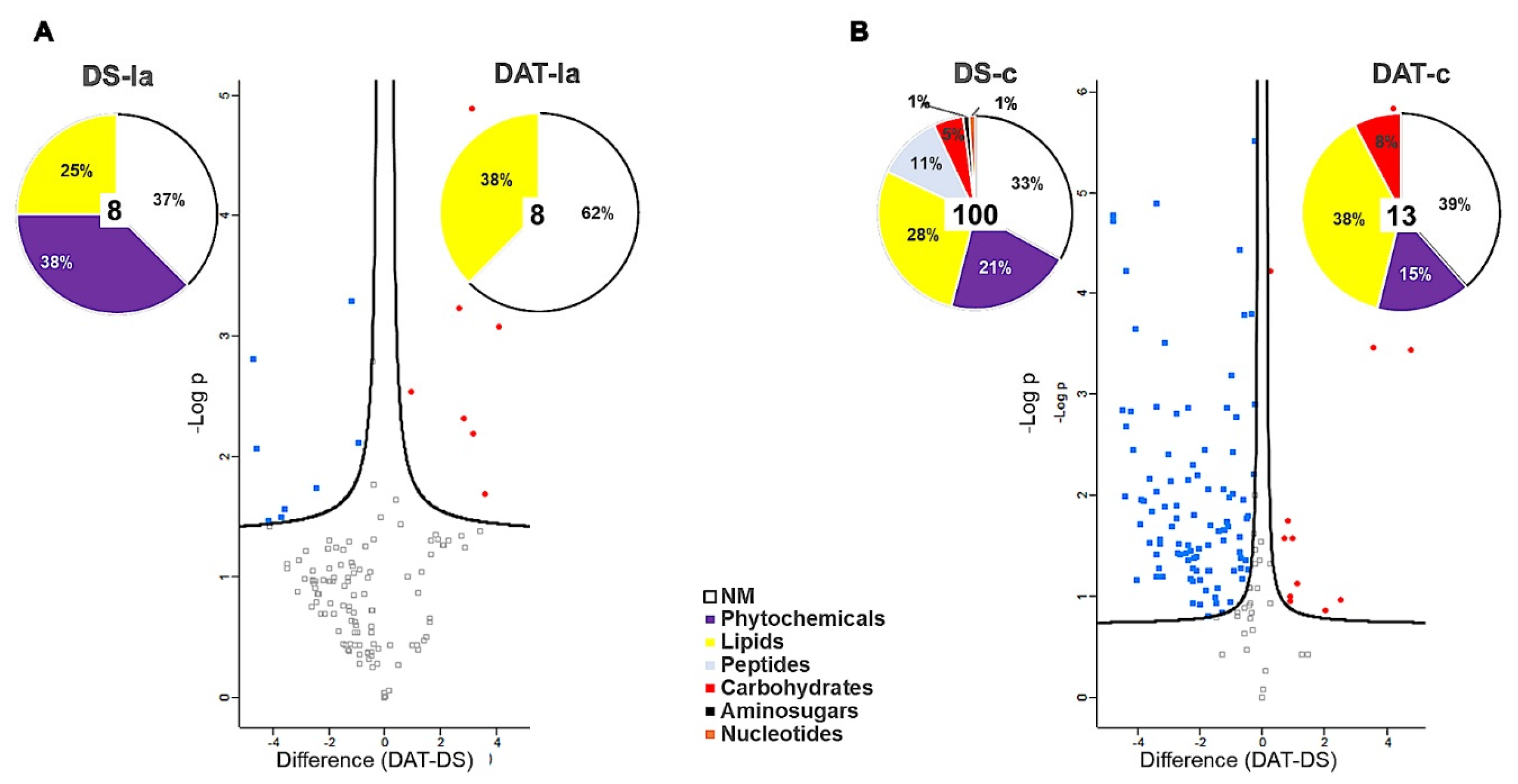
3.3.1. Metabolomic Analysis
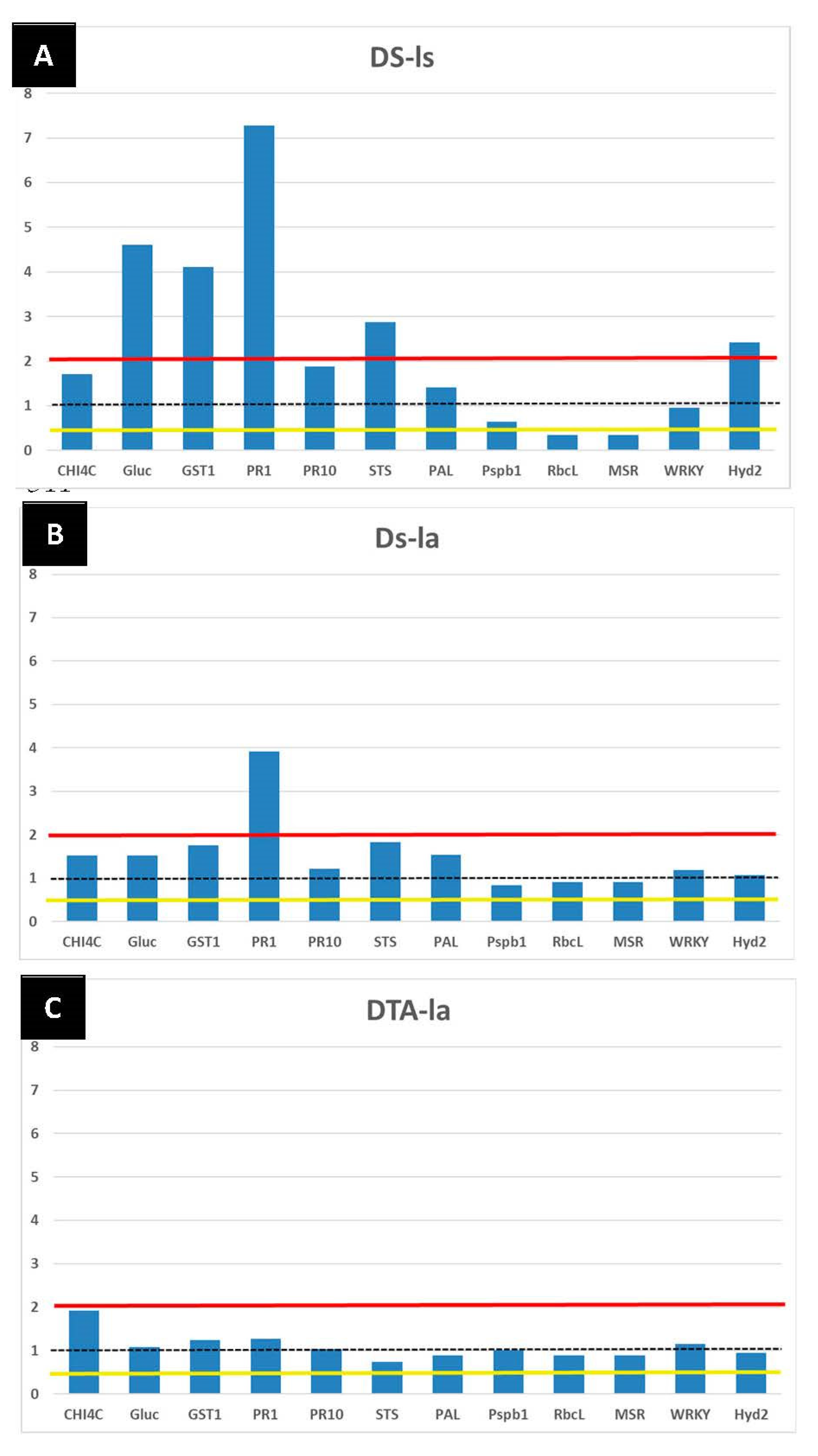
3.3.2. The LC2017 Effects on the Vine Defense Responses
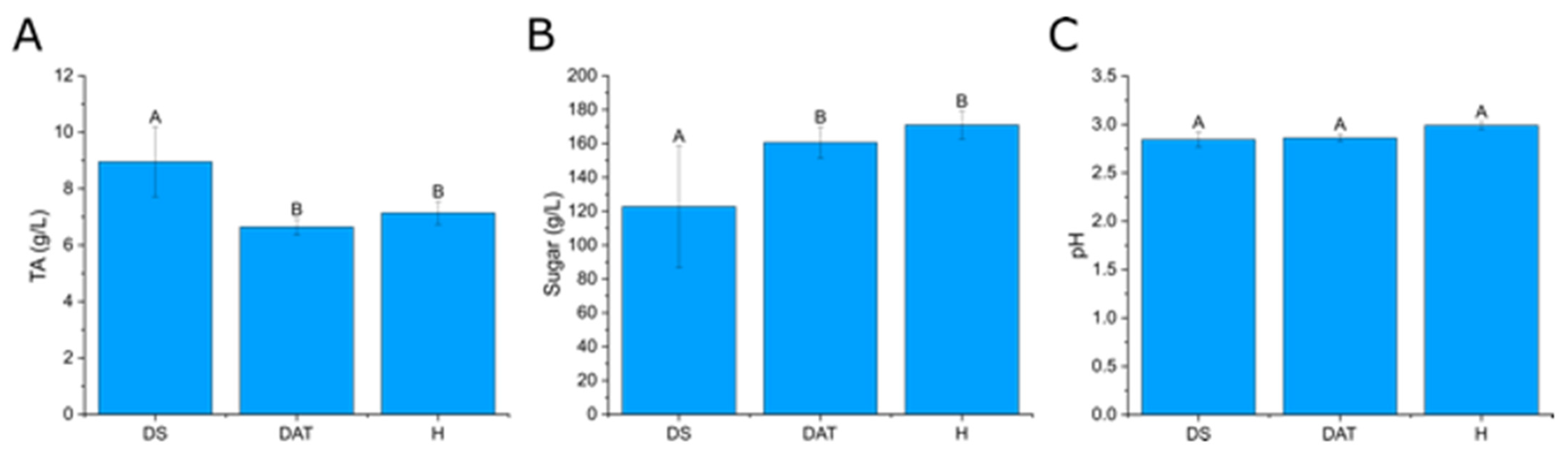
3.4. Impact of LC2017 on the Berry Juice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ayres, P. Alexis Millardet: France’s forgotten mycologist. Mycologist 2004, 18, 23–26. [Google Scholar] [CrossRef]
- Kovačič, G.R.; Lešnik, M.; Vršič, S. An overview of the copper situation and usage in viticulture. Bulg. J. Agric. Sci. 2013, 19, 50–59. [Google Scholar]
- Berkelmann-Löhnertz, B.; Klärner, S.; Baus, O.; Herrmann, G.; Flemming, B.; Keicher, R.; Schwarz, H.P.; Pfliehinger, M.; Löhnertz, O. Kupferreduzierung und Kupfersubstitution im Weinbau–zum Stand der Dinge aus Sicht der Forschung. In Reports from the Julius Kühn-Institut–Plant Protection in Organic Farming, Proceedings of the 11th Workshop in Berlin, Germany, 1 December 2011; Berichte aus dem Julius Kühn-Institut: Berlin, Germany, 2012; Volume 164, pp. 65–70. [Google Scholar]
- Leroux, P.; Clerjeau, M. Resistance of Botrytis cinerea Pers. and Plasmopara viticola (Berk.&Curt.) Berl. and de Toni to fungicides in French vineyards. Crop Prot. 1985, 4, 137–160. [Google Scholar]
- Brunetto, G.; Miotto, A.; Ceretta, C.A.; Schmitt, D.E.; Heinzen, J.; de Moraes, M.P.; Canton, L.; Tiecher, T.; Comin, J.; Girotto, E. Mobility of copper and zinc fractions in fungicide-amended vineyard sandy soils. Arch. Agron. Soil Sci. 2014, 60, 609–624. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Osdaghi, E.; Behlau, F.; Köhl, J.; Jones, J.B.; Aubertot, J.N. Thirteen decades of antimicrobial copper compounds applied in agriculture. A review. Agron. Sustain. Dev. 2018, 38, 28. [Google Scholar] [CrossRef] [Green Version]
- De la Fuente, M.; Fernandez-Calvino, D.; Tylkowski, B.; Montornes, J.M.; Olkiewics, M.; Pereira, R.; Cachada, A.; Caffi, T.; Fedele, G.; De Herralde, F. Alternatives to Cu applications in viticulture. How R&D projects can provide applied solutions, helping to establish legislation limits. In Grape and Wine; IntechOpen: London, UK, 2021. [Google Scholar]
- Cesco, S.; Pii, Y.; Borruso, L.; Orzes, G.; Lugli, P.; Mazzetto, F.; Genova, G.; Signorini, M.; Brunetto, G.; Terzano, R.; et al. A smart and sustainable future for viticulture is rooted in soil: How to face Cu toxicity. Appl. Sci. 2021, 11, 907. [Google Scholar] [CrossRef]
- La Torre, A.; Iovino, V.; Caradonia, F. Copper in plant protection: Current situation and prospects. Phytopathol. Mediterr. 2018, 57, 201–236. [Google Scholar] [CrossRef]
- Battiston, E.; Salvatici, M.C.; Lavacchi, A.; Gatti, A.; Di Marco, S.; Mugnai, L. Functionalisation of a nano-structured hydroxyapatite with copper(II) compounds as pesticide: In situ transmission electron microscopy and environmental scanning electron microscopy observations of treated Vitis vinifera L. leaves. Pest Manag. Sci. 2018, 74, 1903–1915. [Google Scholar] [CrossRef]
- Battiston, E.; Antonielli, L.; Di Marco, S.; Fontaine, F.; Mugnai, L. Innovative delivery of Cu(II) ions by a nanostructured hydroxyapatite: Potential application in planta to enhance the sustainable control of Plasmopara viticola. Phytopathology 2019, 109, 748–759. [Google Scholar] [CrossRef]
- Battiston, E.; Compant, S.; Antonelli, L.; Mondello, V.; Clément, C.; Simoni, A.; Di Marco, S.; Mugnai, L.; Fontaine, F. In planta activity of novel copper(II) based formulations to inhibit the Esca-associated fungus Phaeoacremonium minimum in grapevine propagation material. Front. Plant Sci. 2021, 12, 312. [Google Scholar] [CrossRef]
- Mondello, V.; Songy, A.; Battiston, E.; Pinto, C.; Coppin, C.; Trotel-Aziz, P.; Clément, C.; Mugnai, L.; Fontaine, F. Grapevine trunk diseases: A review of fifteen years of trials for their control with chemicals and biocontrol agents. Plant Dis. 2018, 102, 1189–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fontaine, F.; Gramaje, D.; Armengol, J.; Smart, R.; Nagy, Z.A.; Borgo, M.; Rego, C.; Corio-Costet, M.-F. Grapevine Trunk Diseases. A Review, 1st ed.; ©OIV Publications: Paris, France, 2016. [Google Scholar]
- Mondello, V.; Fernandez, O.; Guise, J.F.; Trotel-Aziz, P.; Fontaine, F. In planta activity of the novel copper product HA + Cu(II) based on a biocompatible drug delivery system on vine physiology and trials for the control of Botryosphaeria dieback. Front. Plant Sci. 2021, 12, 1761. [Google Scholar] [CrossRef] [PubMed]
- Reis, P.; Gaspar, A.; Alves, A.; Fontaine, F.; Rego, C. Combining an HA + Cu (II) site-targeted copper-based product with a pruning wound protection program to prevent infection with Lasiodiplodia spp. in grapevine. Plants 2021, 10, 2376. [Google Scholar] [CrossRef] [PubMed]
- Reis, P.; Magnin-Robert, M.; Nascimento, T.; Spagnolo, A.; Abou-Mansour, E.; Fioretti, C.; Clément, C.; Rego, C.; Fontaine, F. Reproducing botryosphaeria dieback foliar symptoms in a simple model system. Plant Dis. 2016, 100, 1071–1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roveri, N.; Cecchini, A.; Morselli, S.; Lelli, M.; Mercuri, R.; Manfredini, G.; Piva, M. Particule D’hydroxyapatite, Compositions à Base De Cette Particule, Utilisations Comme Vecteur De Molecules Bioactives. French Patent FR 3013182A1, 30 December 2016. [Google Scholar]
- Meier, U. Entwicklungsstadien Mono-Und Dikotyler Pflanzen. BBCH Monografie; BBCH Deutsch (openagrar.de), Verbundzentrale des GBV (VZG): Göttingen, Germany, 2001. [Google Scholar] [CrossRef]
- Becares, A.A.; Fernandez, A.F. Biome Makers Inc Microbiome Based Identification, Monitoring and Enhancement of Fermentation Processes and Products. French Patents WO2017096385A1, 8 June 2017. [Google Scholar]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [Green Version]
- Glöckner, F.O.; Yilmaz, P.; Quast, C.; Gerken, J.; Beccati, A.; Ciuprina, A.; Bruns, G.; Yarza, P.; Peplies, J.; Westram, R.; et al. 25 years of serving the community with ribosomal RNA gene reference databases and tools. J. Biotechnol. 2017, 261, 169–176. [Google Scholar] [CrossRef]
- Nilsson, R.H.; Larsson, K.H.; Taylor, A.F.S.; Bengtsson-Palme, J.; Jeppesen, T.S.; Schigel, D.; Kennedy, P.; Picard, K.; Glöckner, F.O.; Tedersoo, L.; et al. The UNITE database for molecular identification of fungi: Handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res. 2019, 47, D259–D264. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package, 2019. R package version 2.5-6. The Comprehensive R Archive Network. Available online: r-project.org (accessed on 28 August 2020).
- Rivas-Ubach, A.; Liu, Y.; Bianchi, T.S.; Tolić, N.; Jansson, C.; Paša-Tolić, L. Moving beyond the van Krevelen Diagram: A new stoichiometric approach for compound classification in organisms. Anal. Chem. 2018, 90, 6152–6160. [Google Scholar] [CrossRef]
- Magnin-Robert, M.; Letousey, P.; Spagnolo, A.; Rabenoelina, C.; Jacquens, L.; Mercier, L.; Clément, C.; Fontaine, F. Leaf stripe form of esca induces alteration of photosynthesis and defence reactions in pre- symptomatic leaves. Funct. Plant Biol. 2011, 38, 856–866. [Google Scholar] [CrossRef]
- Spagnolo, A.; Larignon, P.; Magnin-Robert, M.; Hovasse, A.; Cilindre, C.; Van Dorsselaer, A.; Clément, C.; Schaeffer-Reiss, C.; Fontaine, F. Flowering as the most highly sensitive period of grapevine (Vitis vinifera L. cv Mourvèdre) to the Botryosphaeria dieback agents Neofusicoccum parvum and Diplodia seriata infection. Int. J. Mol. Sci. 2014, 15, 9644–9669. [Google Scholar] [CrossRef]
- Spagnolo, A.; Mondello, V.; Larignon, P.; Villaume, S.; Rabenoelina, F.; Clément, C.; Fontaine, F. Defense Responses in Grapevine (cv. Mourvèdre) after Inoculation with the Botryosphaeria Dieback Pathogens Neofusicoccum parvum and Diplodia seriata and Their Relationship with Flowering. Int. J. Mol. Sci. 2017, 18, 393. [Google Scholar] [CrossRef] [Green Version]
- Magnin-Robert, M.; Spagnolo, A.; Dilezitoko, A.; Cilindre, C.; Mercier, L.; Schaeffer-Reiss, C.; Van Dorsselaer, A.; Clément, C.; Fontaine, F. Proteomic insights into changes in grapevine wood in response to Esca proper and apoplexy. Phytopathol. Mediterr. 2014, 53, 168–187. [Google Scholar] [CrossRef]
- Magnin-Robert, M.; Spagnolo, A.; Boulanger, A.; Joyeux, C.; Clément, C.; Abou-Mansour, E.; Fontaine, F. Changes in plant metabolism and accumulation of fungal metabolites in response to Esca proper and apoplexy expression in the whole grapevine. Phytopathology 2016, 106, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Trotel-Aziz, P.; Abou-Mansour, E.; Courteaux, B.; Rabenoelina, F.; Clément, C.; Fontaine, F.; Aziz, A. Bacillus subtilis PTA-271 Counteracts Botryosphaeria Dieback in Grapevine, Triggering Immune Responses and Detoxification of Fungal Phytotoxins. Front. Plant Sci. 2019, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Moretti, S.; Pacetti, A.; Pierron, R.; Kassemeyer, H.-H.; Fischer, M.; Péros, J.-P.; Perez-Gonzales, G.; Bieler, E.; Schilling, M.; Di Marco, S.; et al. Fomitiporia mediterranea M. Fisch., the historical Esca agent: A comprehensive review on the main grapevine wood rot agent in Europe. Phytopathol. Mediterr. 2021, 60, 351–379. [Google Scholar] [CrossRef]
- Di Marco, S.; Osti, F.; Mugnai, L. First studies on the potential of a copper formulation for the control of leaf stripe disease within esca complex in grapevine. Phytopathol. Mediterr. 2011, 50, 300–309. [Google Scholar]
- Bruez, E.; Vallance, J.; Gerbore, J.; Lecomte, P.; Da Costa, J.P.; Guerin-Dubrana, L. Analyses of the temporal dynamics of fungal communities colonizing the healthy wood tissues of esca leaf-symptomatic and asymptomatic vines. PLoS ONE 2014, 9, e0095928. [Google Scholar] [CrossRef]
- Bruez, E.; Haidar, R.; Alou, M.T.; Vallance, J.; Bertsch, C.; Mazet, F.; Fermaud, M.; Deschamps, A.; Guerin-Dubrana, L.; Compant, S.; et al. Bacteria in a wood fungal disease: Characterization of bacterial communities in wood tissues of esca-foliar symptomatic and asymptomatic grapevines. Front. Microbiol. 2015, 6, 1137. [Google Scholar] [CrossRef] [Green Version]
- Del Frari, G.; Gobbi, A.; Aggerbeck, M.R.; Oliveira, H.; Hansen, L.H.; Ferreira, R.B. Characterization of the wood mycobiome of Vitis vinifera in a vineyard affected by esca. Spatial distribution of fungal communities and their putative relation with leaf symptoms. Front. Plant Sci. 2019, 10, 910. [Google Scholar] [CrossRef] [Green Version]
- Niem, J.M.; Billones-Baaijens, R.; Stodart, B.; Savocchia, S. Diversity Profiling of Grapevine Microbial Endosphere and Antagonistic Potential of Endophytic Pseudomonas Against Grapevine Trunk Diseases. Front. Microbiol. 2020, 11, 477. [Google Scholar] [CrossRef]
- Andolfi, A.; Mugnai, L.; Luque, J.; Surico, G.; Cimmino, A.; Evidente, A. Phytotoxins produced by fungi associated with grapevine trunk diseases. Toxins 2011, 3, 1569–1605. [Google Scholar] [CrossRef] [Green Version]
- Abou-Mansour, E.; Débieux, J.L.; Ramírez-Suero, M.; Bénard-Gellon, M.; Magnin-Robert, M.; Spagnolo, A.; Chong, J.; Farine, S.; Bertsch, C.; L’Haridon, F.; et al. Phytotoxic metabolites from Neofusicoccum parvum, a pathogen of Botryosphaeria dieback of grapevine. Phytochemistry 2015, 115, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Xue, M.; Shen, Z.; Jia, X.; Hou, X.; Lai, D.; Zhou, L. Phytotoxic Secondary Metabolites from Fungi. Toxins 2021, 13, 261. [Google Scholar] [CrossRef]
- Munkvold, J.A.; Marois, J.J. Efficacy of natural epiphytes and colonizers of grapevine pruning wounds for biological control of Eutypa dieback. Phytopathology 1993, 83, 624–629. [Google Scholar] [CrossRef]
- Pinto, C.; Custódio, V.; Nunes, M.; Songy, A.; Rabenoelina, F.; Courteaux, B.; Clément, C.; Gomes, A.C.; Fontaine, F. Understand the Potential Role of Aureobasidium pullulans, a Resident Microorganism from Grapevine, to Prevent the Infection Caused by Diplodia seriata. Front. Microbiol. 2018, 9, 3047. [Google Scholar] [CrossRef] [PubMed]
- Pinto, C.; Gomes, A.C. Vitis vinifera microbiome: From basic research to technological development. BioControl 2016, 61, 243–256. [Google Scholar] [CrossRef]
- Pinto, C.; Pinho, D.; Sousa, S.; Pinheiro, M.; Egas, C.; Gomes, A.C. Unravelling the diversity of grapevine microbiome. PLoS ONE 2014, 9, e85622. [Google Scholar] [CrossRef] [Green Version]
- Bettenfeld, P.; Fontaine, F.; Trouvelot, S.; Fernandez, O.; Courty, P.E. Woody Plant Declines. What’s wrong with the microbiome? Trends Plant Sci. 2020, 25, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, R.L.; Pieterse, C.M.J.; Bakker, P.A.H.M. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef]
- Martins, V.; Teixeira, A.; Bassil, E.; Blumwald, E.; Gerós, H. Metabolic changes of Vitis vinifera berries and leaves exposed to Bordeaux mixture. Plant Physiol. Biochem. 2014, 82, 270–278. [Google Scholar] [CrossRef]
- Ramírez-Suero, M.; Bénard-Gellon, M.; Chong, J.; Laloue, H.; Stempien, E.; Abou-Mansour, E.; Fontaine, F.; Larignon, P.; Mazet-Kieffer, F.; Farine, S.; et al. Extracellular compounds produced by fungi associated with Botryosphaeria dieback induce differential defence gene expression patterns and necrosis in Vitis vinifera cv. Chardonnay Cells. Protoplasma 2014, 251, 1417–1426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallet, J.; Robert-Siegwald, G.; Guillier, C.; Godard, M.-L.; Bruez, E.; Adrian, M.; Trouvelot, S.; Jacquens, L.; Songy, A.; Clement, C.; et al. Sodium arsenite application on plants expressing grapevine trunk diseases-foliar symptoms: Impact on grapevine physiology. Abstracts of Oral and Poster Presentations Given at the 11th International Workshop on Grapevine Trunk Diseases, Penticton, BC, Canada, 7–12 July 2019. Phytopathol. Mediterr. 2019, 58, 572. [Google Scholar] [CrossRef]
- Lemaitre-Guillier, C.; Fontaine, F.; Roullier-Gall, C.; Harir, M.; Magnin-Robert, M.; Clément, C.; Trouvelot, S.; Gougeon, R.D.; Schmitt-Kopplin, P.; Adrian, M. Cultivar- and wood area-dependent metabolomic fingerprints of grapevine infected by Botryosphaeria dieback. Phytopathology 2020, 110, 1821–1837. [Google Scholar] [CrossRef] [PubMed]
- Coombe, B.G. Ripening berries—A critical issue. Aust. Vitic. 2001, 5, 28–33. [Google Scholar]
- Lorrain, B.; Ky, I.; Pasquier, G.; Jourdes, M.; Guérin-Dubrana, L.; Geny, L.; Rey, P.; Doneche, B.; Tesseidre, P.-L. Effect of Esca disease on the phenolic and sensory attributes of Cabernet Sauvignon grapes, musts and wines. Aust. J. Grape Wine R 2012, 18, 64–72. [Google Scholar] [CrossRef]
- Poitou, X.; Delière, L.; Pons, A.; Redon, P.; Thibon, C.; Darriet, P. Caractérisation de marqueurs volatils associés aux nuances végétales des vins rouges. Etude du lien avec la maturité et le pressurage. Partie 3/3: Paramètres environnementaux et composante aromatique végétale. Rev. Des Anol. Et Des Tech. Vitivinic. Et Anol. 2018, 166, 51–53. [Google Scholar]
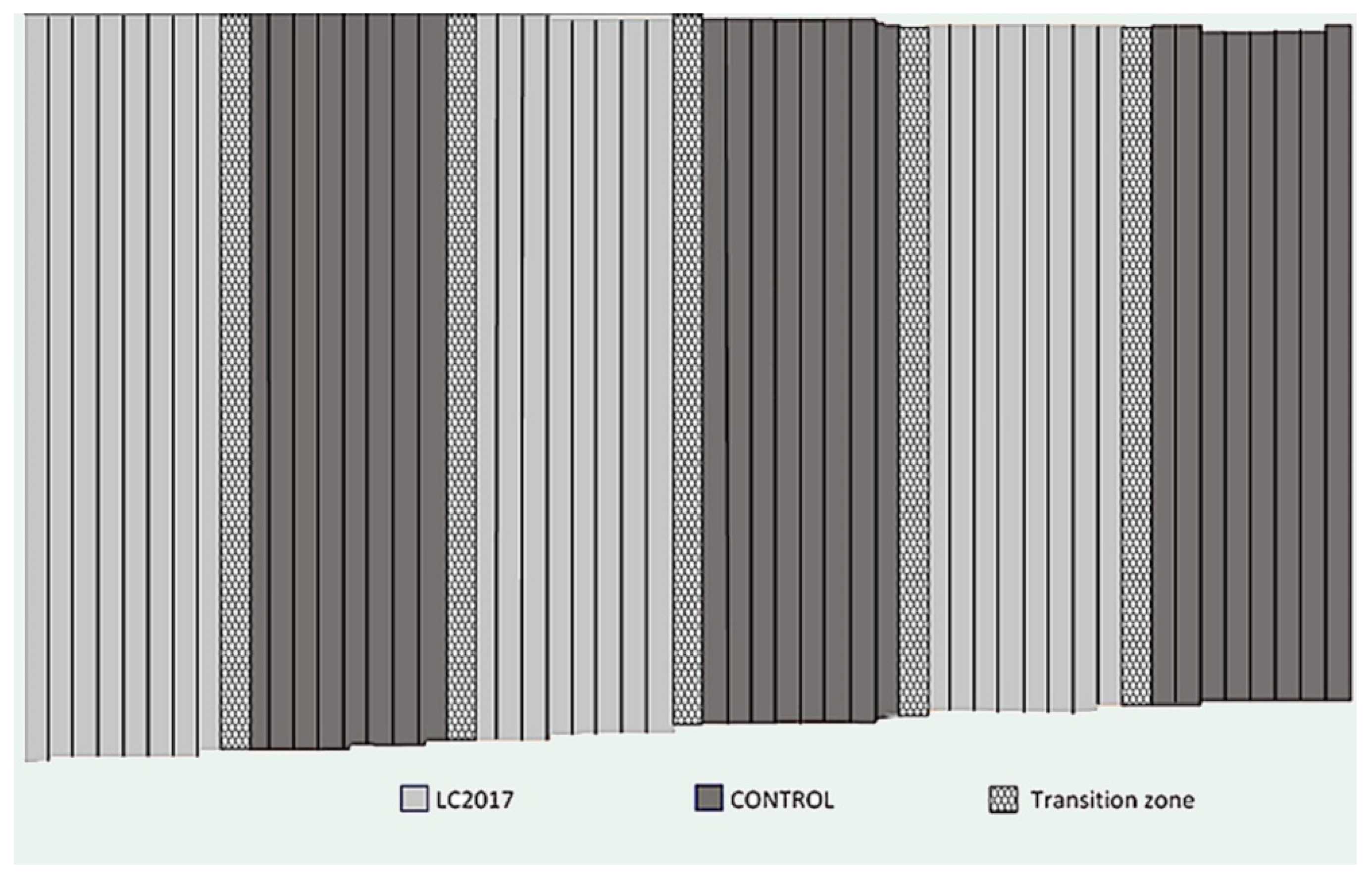

| 1st Treatment | 2nd Treatment | 3rd Treatment | 4th Treatment | 5th Treatment | |
|---|---|---|---|---|---|
| BBCH 00 | BBCH 14 | BBCH 73 | BBCH 81-83 | BBCH 97-99 | |
| LC2017 | 1.5 L·ha−1 | 1.5 L·ha−1 | 2 L·ha−1 | 2 L·ha−1 | 2 L·ha−1 |
| Water | 250 L·ha−1 | 250 L·ha−1 | 400 L·ha−1 | 400 L·ha−1 | 400 L·ha−1 |
| LC2017 final concentration | 0.6% | 0.6% | 0.5% | 0.5% | 0.5% |
| Vine Condition | Vines n. | Subsample | ||||||
|---|---|---|---|---|---|---|---|---|
| Leaves | Canes c | Grape Juice j | Healthy Wood hw | Wood Interaction Iw | White Rot Wr | |||
| Asymptomatic la | Symptomatic ls | |||||||
| Healthy not treated H | 5 | H-la | H-c | H-j | H-hw | H-iw | H-wr | |
| Me(3)–Tr(3) | Me(3)–Mi(5) | Me(5) | Me(3)–Mi(5) | Me(3)–Mi(5) | Mi(1) | |||
| Diseased symptomatic not treated DS | 5 | DS-la | DS-ls | DS-c | DS-j | DS-hw | DS-iw | DS-wr |
| Me(3)–Tr(3) | Me(3)–Tr(3) | Me(3)–Mi(5) | Me(5) | Me(3)–Mi(5) | Me(3)–Mi(5) | Mi(5) | ||
| Diseased asymptomatic treated DAT | 5 | DAT-la | DAT-c | DAT-j | DAT-hw | DAT-iw | DAT-wr | |
| Me(3)–Tr(3) | Me(3)–Mi(5) | Me(5) | Me(3)–Mi(5) | Me(3)–Mi(5) | Mi(4) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mondello, V.; Lemaître-Guillier, C.; Trotel-Aziz, P.; Gougeon, R.; Acedo, A.; Schmitt-Kopplin, P.; Adrian, M.; Pinto, C.; Fernandez, O.; Fontaine, F. Assessment of a New Copper-Based Formulation to Control Esca Disease in Field and Study of Its Impact on the Vine Microbiome, Vine Physiology and Enological Parameters of the Juice. J. Fungi 2022, 8, 151. https://doi.org/10.3390/jof8020151
Mondello V, Lemaître-Guillier C, Trotel-Aziz P, Gougeon R, Acedo A, Schmitt-Kopplin P, Adrian M, Pinto C, Fernandez O, Fontaine F. Assessment of a New Copper-Based Formulation to Control Esca Disease in Field and Study of Its Impact on the Vine Microbiome, Vine Physiology and Enological Parameters of the Juice. Journal of Fungi. 2022; 8(2):151. https://doi.org/10.3390/jof8020151
Chicago/Turabian StyleMondello, Vincenzo, Christelle Lemaître-Guillier, Patricia Trotel-Aziz, Régis Gougeon, Alberto Acedo, Philippe Schmitt-Kopplin, Marielle Adrian, Cátia Pinto, Olivier Fernandez, and Florence Fontaine. 2022. "Assessment of a New Copper-Based Formulation to Control Esca Disease in Field and Study of Its Impact on the Vine Microbiome, Vine Physiology and Enological Parameters of the Juice" Journal of Fungi 8, no. 2: 151. https://doi.org/10.3390/jof8020151
APA StyleMondello, V., Lemaître-Guillier, C., Trotel-Aziz, P., Gougeon, R., Acedo, A., Schmitt-Kopplin, P., Adrian, M., Pinto, C., Fernandez, O., & Fontaine, F. (2022). Assessment of a New Copper-Based Formulation to Control Esca Disease in Field and Study of Its Impact on the Vine Microbiome, Vine Physiology and Enological Parameters of the Juice. Journal of Fungi, 8(2), 151. https://doi.org/10.3390/jof8020151








