Evaluation of Four Indigenous Non-Saccharomyces Yeasts Isolated from the Shangri-La Wine Region (China) for Their Fermentation Performances and Aroma Compositions in Synthetic Grape Juice Fermentation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganisms
2.2. Fermentations in Synthetic Grape Juice
2.3. Oenological Parameters Analysis
2.4. Volatile Aroma Compounds Analysis
2.5. Odor Activity Values (OAVs) and Aroma Series Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Fermentation Kinetics and Oenological Parameters Analysis
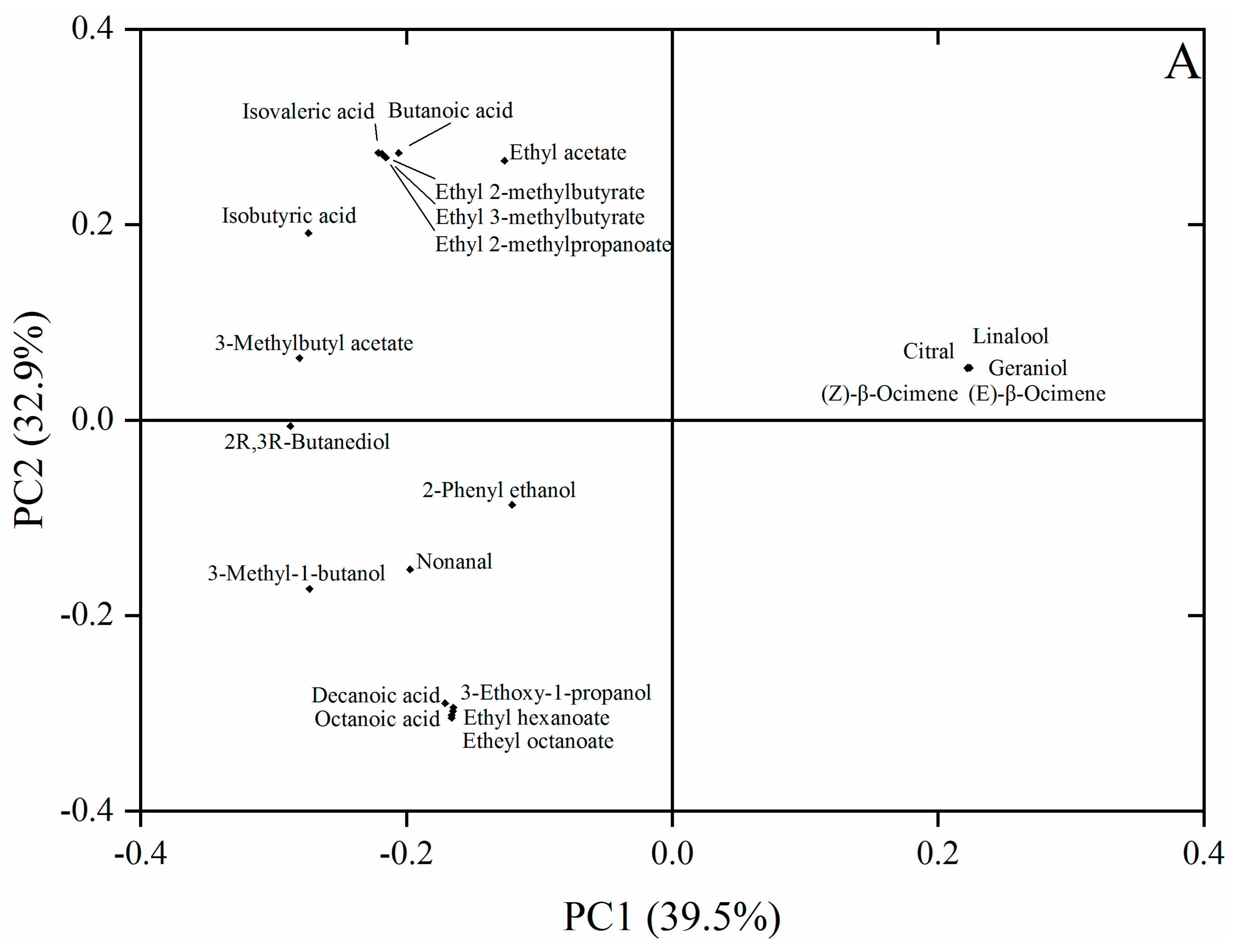
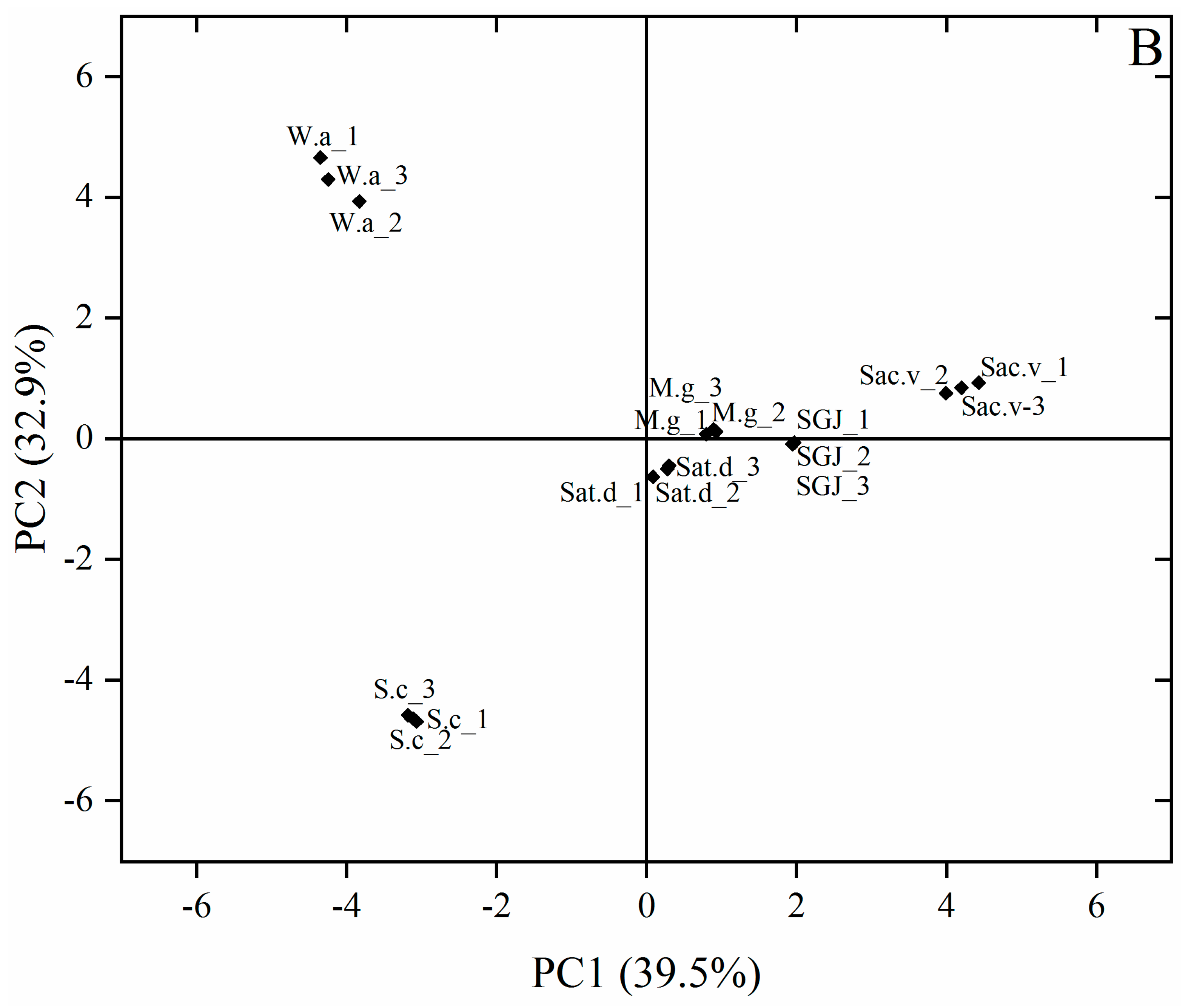
3.2. Volatile Aroma Compounds Analysis
| No. | RI a | Compounds b | Concentrations (μg/L) | Odor Threshold (μg/L) c | Odor Description d | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| M. guilliermondii (AD-58) | Sat. diversa (BZL-11) | Sac. vini (BZL-28) | W. anomalus (DR-110) | S. cerevisiae (EC1118) | SGJ (Uninoculated) | |||||
| 1 | 1036 | 1-Propanol A | 8408.27 ± 197.62 c | 2284.48 ± 57.42 d | 759.35 ± 34.64 d | 18,813.98 ± 1067.56 b | 42,118.28 ± 2014.65 a | nd | 306,000 [29,30] | Alcohol, ripe fruit [30] |
| 2 | 1085 | 2-Methyl-1-propanol A | 16,932.19 ± 561.49 d | 26,174.41 ± 675.13 c | tr | 31,727.84 ± 1142.44 b | 37,657.46 ± 2447.08 a | nd | 40,000 [46] | Alcohol, solvent [30] |
| 3 | 1142 | Butanol A | nd | nd | nd | 168.30 ± 9.33 | nd | nd | 150,000 [29,30] | Medicinal, phenolic [30] |
| 4 | 1213 | 3-Methyl-1-butanol A | 21,680.11 ± 530.17 c | 129,185.54 ± 8171.46 b | 5572.99 ± 496.70 c | 130,776.34 ± 2122.26 b | 250,240.90 ± 13,174.17 a | nd | 30,000 [46] | Alcohol, nail polish [30] |
| 5 | 1254 | 3-Methyl-3-buten-1-ol A | 25.44 ± 3.09 b | 25.42 ± 4.88 b | nd | 50.95 ± 5.72 a | nd | nd | 600 [24] | Alcohol, solvent * |
| 6 | 1333 | 3-Methyl-1-pentanol A | nd | 32.15 ± 3.63 b | nd | 22.03 ± 0.54 c | 62.44 ± 3.57 a | nd | 1000 [29,30] | Green, solvent [30] |
| 7 | 1386 | 3-Ethoxy-1-propanol A | tr | nd | nd | tr | 528.73 ± 20.45 | nd | 100 [29,30] | Fruity [30] |
| 8 | 1498 | 2-Ethyl-1-hexanol A | 5.80 ± 0.12 b | 8.41 ± 0.93 a | 2.16 ± 0.07 c | 5.95 ± 0.03 b | 4.82 ± 0.07 bc | 2.33 ± 0.09 | 8000 [24] | Waxy, soapy * |
| 9 | 1527 | 2-Nonanol C | nd | nd | 8.01 ± 0.24 b | nd | 11.52 ± 0.27 a | nd | NF | Green [26] |
| 10 | 1566 | Octanol A | nd | nd | nd | 5.62 ± 0.15 a | 5.31 ± 0.25 a | 4.37 ± 0.03 | 800 [29,30] | Lemon, jasmine [30] |
| 11 | 1669 | Nonanol A | 7.04 ± 0.05 b | nd | 7.42 ± 0.10 a | 7.52 ± 0.05 a | nd | nd | 600 [24] | Fruity, sweet [26] |
| 12 | 1730 | 3-Methylthio-1-propanol A | nd | 472.82 ± 57.90 b | nd | 497.08 ± 15.85 b | 730.88 ± 14.66 a | nd | 1000 [47] | Cooked potato, garlic [30] |
| 13 | 1891 | Benzyl alcohol A | tr | 136.39 ± 12.53 b | 240.85 ± 24.83 a | nd | nd | nd | 900,000 [29,30] | Toasted [30] |
| 14 | 1928 | 2-Phenylethanol A | 3743.17 ± 137.52 d | 63,510.12 ± 2979.73 a | 242.53 ± 51.51 e | 17,405.83 ± 404.72 c | 26,692.65 ± 439.28 b | nd | 10,000 [46] | Roses [30] |
| ∑ Higher alcohols | 50,802.02 ± 1261.85 d | 221,829.74 ± 11,647.04 b | 6833.32 ± 583.81 e | 199,481.44 ± 3552.16 c | 358,053.00 ± 13,985.63 a | 6.70 ± 0.12 | ||||
| 1 | 1549 | 2R,3R-Butanediol C | 537,151.25 ± 44,873.06 b | 231,750.33 ± 12,660.86 c | nd | 622,811.33 ± 29,652.85 a | 576,699.62 ± 24,216.94 ab | nd | 150,000 [29,30] | Fruity [30] |
| 2 | 1585 | 2R,3S-Butanediol A | 55,984.92 ± 4112.28 b | 25,422.53 ± 839.23 c | nd | 130,128.37 ± 7072.80 a | 122,089.94 ± 4230.99 a | nd | 150,000 [29,30] | Fruity [30] |
| ∑ Polyols | 593,136.16 ± 48,953.50 b | 257,172.86 ± 13,477.34 c | 752,939.70 ± 36590.38 a | 698,789.56 ± 26,073.88 a | ||||||
| 1 | 933 | Ethyl acetate A | 6706.40 ± 319.00 d | 1735.61 ± 234.45 d | 74,106.81 ± 2945.79 b | 143,023.77 ± 4894.98 a | 19,384.40 ± 886.41 c | tr | 7500 [46] | Pineapple, varnish, balsamic [30] |
| 2 | 985 | Propyl acetate A | tr | nd | 13.05 ± 0.88 b | 108.02 ± 2.57 a | nd | nd | 4700 [29,30] | Celery [29] |
| 3 | 1118 | 3-Methylbutyl acetate A | 50.89 ± 3.62 c | 14.22 ± 3.52 c | 106.22 ± 27.76 c | 507.00 ± 25.80 a | 376.16 ± 22.71 b | nd | 30 [46] | Fruity, sweet [30] |
| 4 | 1764 | Geranyl acetate A | nd | nd | 620.86 ± 42.94 | nd | nd | nd | NF | Roses, lavender * |
| 5 | 1829 | 2-Phenylethyl acetate A | 17.54 ± 0.35 d | 68.34 ± 4.10 b | nd | 42.13 ± 2.73 c | 135.35 ± 4.60 a | nd | 250 [46] | Fruity [30] |
| ∑ Acetate esters | 6774.82 ± 315.20 d | 1818.17 ± 233.44 e | 74,846.94 ± 2948.03 b | 143,680.92 ± 4871.63 a | 19,895.90 ± 877.40 c | |||||
| 1 | 974 | Ethyl propaonate A | nd | nd | tr | 280.82 ± 23.68 b | 352.76 ± 19.68 a | nd | 1800 [29,30] | Apple, banana [30] |
| 2 | 979 | Ethyl 2-methylpropanoate A | nd | nd | nd | 318.47 ± 69.69 | nd | nd | 15 [47] | Fruity [30] |
| 3 | 1048 | Ethyl 2-methylbutyrate A | nd | nd | nd | 126.49 ± 12.26 | nd | nd | 18 [47] | Fruity [26] |
| 4 | 1063 | Ethyl 3-methylbutyrate A | nd | nd | nd | 4.37 ± 1.17 | nd | nd | 3 [47] | Fruity [26] |
| 5 | 1239 | Ethyl hexanoate A | nd | 12.05 ± 0.51 b | nd | 10.99 ± 0.35 b | 435.23 ± 49.59 a | nd | 5 [46] | Green apple, banana [30] |
| 6 | 1444 | Ethyl octanoate A | nd | 30.89 ± 4.34 b | nd | nd | 217.02 ± 8.37 a | nd | 2 [46] | Fruity, sweet [30] |
| 7 | 1649 | Ethyl decanoate A | nd | 37.03 ± 3.82 b | nd | nd | 142.44 ± 14.40 a | nd | 200 [47] | Fruity, rose, waxy [26] |
| 8 | 1797 | Ethyl phenylacetate A | nd | 24.80 ± 1.02 a | nd | 17.69 ± 0.13 c | 22.38 ± 0.54 b | nd | 73 [48,49] | Honey * |
| 9 | 1854 | Ethyl dodecanoate A | 19.14 ± 0.11 c | 22.01 ± 1.09 b | nd | 19.41 ± 0.16 c | 36.95 ± 1.30 a | nd | 1500 [24] | Fruity, floral, sweet, cream [26] |
| 10 | 2053 | Ethyl tetradecanoate D | 9.58 ± 1.40 b | 37.07 ± 1.82 a | nd | 41.89 ± 3.66 a | nd | nd | 2000 [24] | Mild waxy, soapy [26] |
| 11 | 2243 | Ethyl hexadecanoate A | nd | 51.60 ± 3.00 | nd | nd | nd | nd | 1500 [24] | Fruity, sweet, fatty [26] |
| ∑ Fatty acid ethyl esters | 28.72 ± 1.46 d | 215.46 ± 8.03 c | 883.13 ± 95.74 b | 1206.77 ± 30.13 a | ||||||
| 1 | 1182 | 2-Methylpropyl 2-methylbutanoate D | nd | nd | nd | 31.68 ± 6.59 | nd | nd | NF | NF |
| 2 | 1196 | 3-Methylbutyl propionate A | nd | nd | nd | 8.88 ± 0.58 b | 10.72 ± 0.29 a | nd | NF | Fruity * |
| 3 | 1202 | 3-Methylbutyl 2-methylpropanoate D | nd | nd | nd | 176.61 ± 20.70 | nd | nd | NF | NF |
| 4 | 1285 | 3-Methylbutyl 2-methylbutanoate D | nd | nd | nd | 28.42 ± 5.80 | nd | nd | NF | NF |
| 5 | 1288 | 2-Methylbutyl 2-methylbutanoate D | nd | nd | nd | 15.39 ± 3.97 | nd | nd | NF | NF |
| ∑ other esters | 260.98 ± 24.34 a | 10.72 ± 0.29 b | ||||||||
| 1 | 1163 | β-Myrcene A | nd | nd | 68.38 ± 6.19 | nd | nd | nd | 100 [49] | Lemon, pine * |
| 2 | 1207 | D-Limonene A | nd | nd | 22.70 ± 1.33 | nd | nd | nd | 200 [49] | Citrus, floral, green [28] |
| 3 | 1238 | (Z)-β-Ocimene B | nd | nd | 111.25 ± 10.62 | nd | nd | nd | 34 [49] | Fruity [28] |
| 4 | 1256 | (E)-β-Ocimene B | nd | nd | 175.79 ± 18.76 | nd | nd | nd | 34 [49] | Fruity [28] |
| 5 | 1554 | Linalool A | nd | nd | 28.75 ± 2.09 | nd | nd | nd | 15 [46] | Citrus, floral [30] |
| 6 | 1711 | α-Terpineol A | 10.20 ± 0.01 b | 10.28 ± 0.20 b | 9.50 ± 0.02 a | nd | nd | nd | 250 [47] | Floral [26] |
| 7 | 1745 | Citral A | nd | nd | 375.83 ± 28.54 | nd | nd | nd | 85.3 [50] | Citrus * |
| 8 | 1774 | Citronellol A | 10.47 ± 0.29 b | nd | 87.28 ± 3.96 a | nd | 17.89 ± 0.58 b | nd | 100 [46] | Rose [30] |
| 9 | 1810 | Nerol A | 15.74 ± 0.10 b | 15.89 ± 0.03 b | 33.30 ± 2.71 a | nd | nd | nd | 700 [48,51] | Floral [25] |
| 10 | 1858 | Geraniol A | 19.35 ± 0.22 b | 21.38 ± 0.17 b | 1936.43 ± 192.70 a | nd | nd | nd | 30 [46] | Citrus, geranium [25] |
| ∑ Terpenes | 55.77 ± 0.60 b | 47.55 ± 0.35 b | 2849.23 ± 230.50 a | 17.89 ± 0.58 b | ||||||
| 1 | 1577 | Isobutyric acid A | 747.25 ± 19.58 e | 2775.14 ± 171.63 c | 1029.00 ± 22.92 d | 15,624.12 ± 558.72 a | 4988.69 ± 437.36 b | nd | 2300 [47] | Fatty, rancid [30] |
| 2 | 1638 | Butanoic acid A | 173.67 ± 2.98 b | 180.24 ± 8.29 b | nd | 476.02 ± 10.62 a | nd | nd | 173 [47] | Cheese, rancid [30] |
| 3 | 1682 | Isovaleric acid A | nd | 519.01 ± 31.16 b | nd | 5579.41 ± 173.14 a | nd | nd | 33.4 [47] | Rancid [30] |
| 4 | 2071 | Octanoic acid A | nd | 107.13 ± 4.38 b | nd | nd | 1078.91 ± 40.80 a | nd | 500 [47] | Cheese, fatty, rancid [30] |
| 5 | 2265 | Decanoic acid D | nd | 76.66 ± 12.37 b | nd | nd | 1613.53 ± 137.53 a | nd | 1000 [47] | Fatty, rancid [30] |
| ∑ Fatty acids | 920.91 ± 21.53 d | 3658.18 ± 207.93 c | 1029.00 ± 22.92 d | 21679.20 ± 429.27 a | 7681.12 ± 277.69 b | |||||
| 1 | 1010 | 4-Methyl-2-pentanone D | 234.09 ± 9.33 c | 237.13 ± 7.41 c | 380.54 ± 4.54 b | 211.62 ± 4.12 d | 402.13 ± 4.83 a | nd | NF | NF |
| 2 | 1130 | 3-Penten-2-one D | nd | 10.73 ± 0.36 | nd | nd | nd | nd | NF | NF |
| 3 | 1187 | 5-Methyl-2-hexanone D | nd | nd | nd | nd | 34.00 ± 1.42 | 0.60 | NF | NF |
| 4 | 1299 | Acetoin A | 3480.97 ± 395.35 b | 25524.16 ± 3141.09 a | nd | 3911.90 ± 632.45 b | tr | nd | 150,000 [29,30] | Cream, butter [30] |
| 5 | 1344 | 6-Methyl-5-hepten-2-one A | 1.72 ± 0.01 b | 2.25 ± 0.14 a | nd | nd | nd | nd | NF | Fruity * |
| 6 | 1402 | Nonanal A | 13.48 ± 0.19 c | 13.85 ± 0.57 bc | 13.98 ± 0.21 bc | 14.20 ± 0.12 b | 15.12 ± 0.21 a | 13.10 ± 0.05 | 15 [23] | Green [28] |
| 7 | 1396 | 2-Nonanone D | nd | nd | nd | nd | 194.55 ± 22.07 | nd | NF | NF |
| 8 | 1535 | Benzaldehyde A | 20.81 ± 4.66 d | 85.19 ± 9.90 b | 59.36 ± 7.83 c | 22.40 ± 1.76 d | 133.71 ± 2.68 a | 10.54 ± 0.55 | 2000 [29,30] | Almond [31] |
| ∑ Carbonyl compounds | 3751.07 ± 399.99 b | 25,873.31 ± 3145.56 c | 453.89 ± 11.52 d | 4160.11 ± 631.12 b | 779.51 ± 24.21 c | 24.23 ± 0.60 | ||||
| 1 | 1101 | 1-(1-ethoxyethoxy)-pentane D | 11.46 ± 0.53 c | 832.32 ± 85.35 a | nd | 215.96 ± 14.16 b | 288.58 ± 44.78 b | nd | NF | NF |
| 2 | 1643 | γ-Butyrolactone D | nd | 26.95 ± 2.07 | nd | nd | nd | nd | 20,000 [29,30] | Caramel, sweet [29] |
| ∑ Other compounds | 11.46 ± 0.53 c | 859.27 ± 87.25 a | 215.96 ± 14.16 b | 288.58 ± 44.78 b | ||||||
| ∑ All volatile aroma compounds | 655,480.59 ± 50,073.53 b | 511,474.53 ± 28,041.48 c | 86,012.36 ± 2168.69 d | 1,123,301.80 ± 30,072.42 a | 1,086,723.08 ± 39,911.55 a | |||||
3.3. PCA Analysis of Key Aroma Compounds
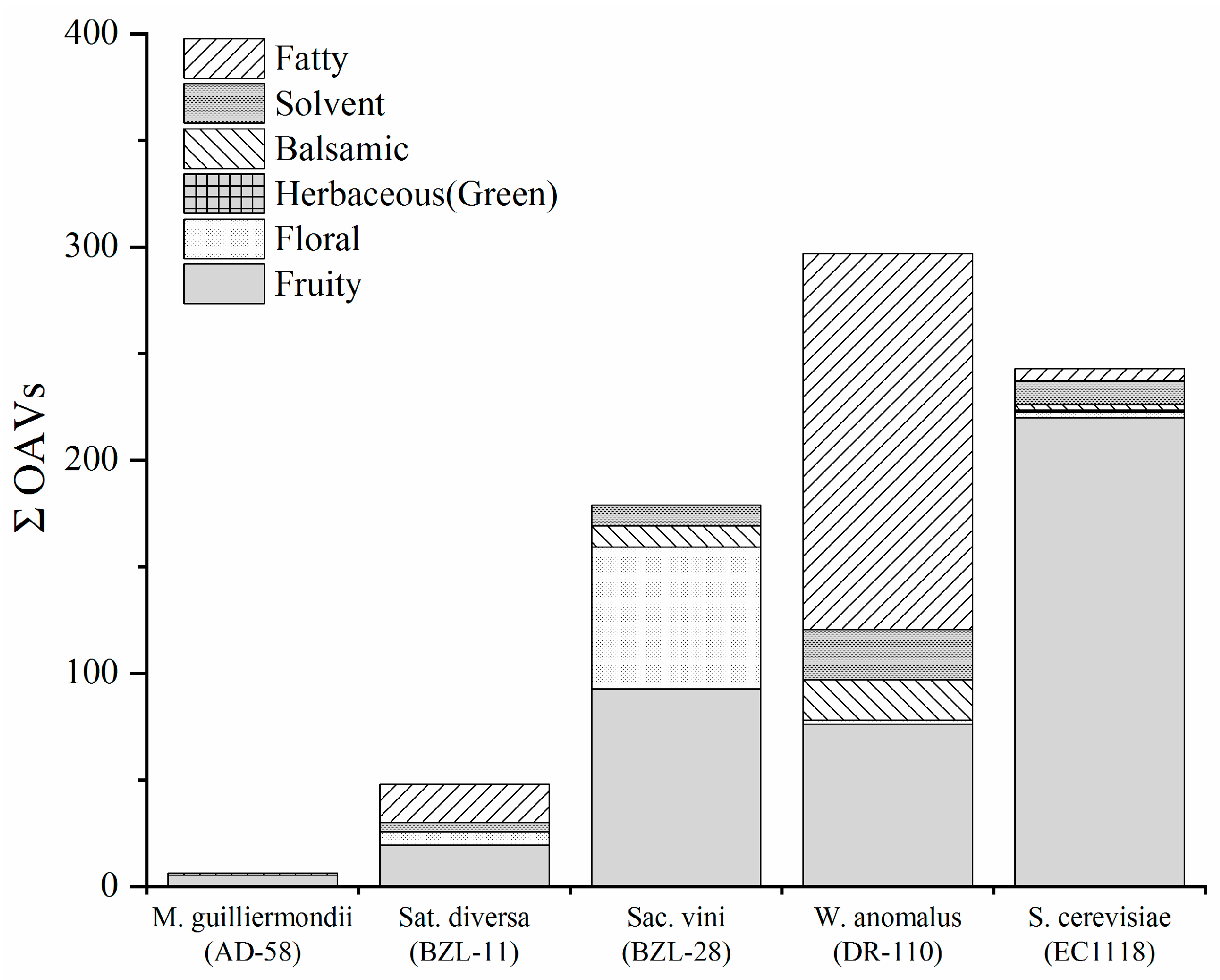
3.4. Aroma Profile Analysis of Resultant Fermented Media
| No. | Compounds | M. guilliermondii(AD-58) | Sat. diversa (BZL-11) | Sac. vini (BZL-28) | W. anomalus (DR-110) | S. cerevisiae (EC1118) | SGJ (Uninoculated) | Aroma Series |
|---|---|---|---|---|---|---|---|---|
| 1 | 3-Methyl-1-butanol | 0.72 ± 0.02c | 4.31 ± 0.27b | 0.19 ± 0.02d | 4.36 ± 0.07b | 8.34 ± 0.44a | - | Solvent [30] |
| 2 | 3-Ethoxy-1-propanol | - | - | - | - | 5.29 ± 0.20 | - | Fruity [30] |
| 3 | 2-Phenylethanol | 0.37 ± 0.01d | 6.35 ± 0.30a | 0.02 ± 0.01e | 1.74 ± 0.04c | 2.67 ± 0.04b | - | Floral [30] |
| 4 | 2R,3R-Butanediol | 3.58 ± 0.30c | 1.55 ± 0.08d | 4.15 ± 0.20a | 3.84 ± 0.16b | - | Fruity [30] | |
| 5 | Ethyl acetate | 0.89 ± 0.04d | 0.23 ± 0.03e | 9.88 ± 0.39b | 19.07 ± 0.65a | 2.58 ± 0.12c | - | Fruity, Balsamic, Solvent [30] |
| 6 | 3-Methylbutyl acetate | 1.70 ± 0.12d | 0.47 ± 0.12e | 3.54 ± 0.93c | 16.90 ± 0.86a | 12.54 ± 0.76b | - | Fruity [30] |
| 7 | Ethyl 2-methylpropanoate | - | - | - | 25.43 ± 4.65 | - | - | Fruity [30] |
| 8 | Ethyl 2-methylbutyrate | - | - | - | 7.03 ± 0.68 | - | - | Fruity [26] |
| 9 | Ethyl 3-methylbutyrate | - | - | - | 1.45 ± 0.39 | - | - | Fruity [26] |
| 10 | Ethyl hexanoate | - | 2.41 ± 0.10b | - | 2.20 ± 0.07b | 87.05 ± 9.92a | - | Fruity [30] |
| 11 | Etheyl octanoate | - | 15.45 ± 2.17b | - | - | 108.51 ± 4.18a | - | Fruity [30] |
| 12 | (Z)-β-Ocimene | - | - | 3.27 ± 0.31 | - | - | - | Fruity [27] |
| 13 | (E)-β-Ocimene | - | - | 5.17 ± 0.55 | - | - | - | Fruity [27] |
| 14 | Linalool | - | - | 1.92 ± 0.14 | - | - | - | Fruity, Floral [30] |
| 15 | Citral | - | - | 4.41 ± 0.33 | - | - | - | Fruity * |
| 16 | Geraniol | 0.65 ± 0.01b | 0.71 ± 0.01b | 64.55 ± 6.42a | - | - | - | Fruity, Floral [25] |
| 17 | Isobutyric acid | 0.32 ± 0.01d | 1.21 ± 0.07c | 0.45 ± 0.01d | 6.79 ± 0.24a | 2.17 ± 0.19b | - | Fatty [30] |
| 18 | Butanoic acid | 1.00 ± 0.02b | 1.04 ± 0.05b | - | 2.75 ± 0.06a | - | - | Fatty [30] |
| 19 | Isovaleric acid | - | 15.54 ± 0.93b | - | 167.05 ± 5.18a | - | - | Fatty [30] |
| 20 | Octanoic acid | - | 0.21 ± 0.01b | - | - | 2.16 ± 0.08a | - | Fatty [30] |
| 21 | Decanoic acid | - | 0.08 ± 0.01b | - | - | 1.61 ± 0.14a | - | Fatty [30] |
| 22 | Nonanal | 0.90 ± 0.01cd | 0.92 ± 0.04bc | 0.93 ± 0.01bc | 0.95 ± 0.01b | 1.01 ± 0.01a | 0.87 ± 0.00d | Herbaceous (Green) [28] |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fugelsang, K.C.; Edwards, C.G. Grape and wine microorganisms. In Wine Microbiology, 2nd ed.; Springer: New York, NY, USA, 2007; pp. 3–61. [Google Scholar]
- Padilla, B.; Gil, J.V.; Manzanares, P. Past and future of non-Saccharomyces yeasts: From spoilage microorganisms to biotechnological tools for improving wine aroma complexity. Front. Microbiol. 2016, 7, 411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canonico, L.; Comitini, F.; Oro, L.; Ciani, M. Sequential fermentation with selected immobilized non-Saccharomyces yeast for reduction of ethanol content in wine. Front. Microbiol. 2016, 7, 278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gobbi, M.; De Vero, L.; Solieri, L.; Comitini, F.; Oro, L.; Giudici, P.; Ciani, M. Fermentative aptitude of non-Saccharomyces wine yeast for reduction in the ethanol content in wine. Eur. Food Res. Technol. 2014, 239, 41–48. [Google Scholar] [CrossRef]
- del Fresno, J.M.; Morata, A.; Loira, I.; Bañuelos, M.A.; Escott, C.; Benito, S.; Chamorro, C.G.; Suárez-Lepe, J.A. Use of non-Saccharomyces in single-culture, mixed and sequential fermentation to improve red wine quality. Eur. Food Res. Technol. 2017, 243, 2175–2185. [Google Scholar] [CrossRef]
- del Mónaco, S.M.; Barda, N.; Rubio, N.C.; Caballero, A. Selection and characterization of a Patagonian Pichia kudriavzevii for wine deacidification. J. Appl. Microbiol. 2014, 117, 451–464. [Google Scholar] [CrossRef]
- Gobbi, M.; Comitini, F.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Lachancea thermotolerans and Saccharomyces cerevisiae in simultaneous and sequential co-fermentation: A strategy to enhance acidity and improve the overall quality of wine. Food Microbiol. 2013, 33, 271–281. [Google Scholar] [CrossRef]
- Merín, M.G.; de Ambrosini, V.I.M. Highly cold-active pectinases under wine-like conditions from non-Saccharomyces yeasts for enzymatic production during winemaking. Lett. Appl. Microbiol. 2015, 60, 467–474. [Google Scholar] [CrossRef]
- Escribano, R.; González-Arenzana, L.; Portu, J.; Garijo, P.; López-Alfaro, I.; López, R.; Santamaría, P.; Gutiérrez, A.R. Wine aromatic compound production and fermentative behaviour within different non-Saccharomyces species and clones. J. Appl. Microbiol. 2018, 124, 1521–1531. [Google Scholar] [CrossRef]
- Domizio, P.; Romani, C.; Lencioni, L.; Comitini, F.; Ciani, M.; Mannazzu, I.; Ciani, M. Outlining a future for non-Saccharomyces yeasts: Selection of putative spoilage wine strains to be used in association with Saccharomyces cerevisiae for grape juice fermentation. Int. J. Food Microbiol. 2011, 147, 170–180. [Google Scholar] [CrossRef]
- Larroque, M.N.; Carrau, F.; Faria, L.; Boido, E.; Medina, K. Effect of Saccharomyces and non-Saccharomyces native yeasts on beer aroma compounds. Int. J. Food Microbiol. 2021, 312, 108373. [Google Scholar] [CrossRef]
- Sadineni, V.; Kondapalli, N.; Obulam, V.S.R. Effect of co-fermentation with Saccharomyces cerevisiae and Torulaspora delbrueckii or Metschnikowia pulcherrima on the aroma and sensory properties of mango wine. Ann. Microbiol. 2012, 62, 1353–1360. [Google Scholar] [CrossRef]
- Wei, J.P.; Zhang, Y.X.; Wang, Y.W.; Ju, H.M.; Niu, C.; Song, Z.H.; Yuan, Y.H.; Yue, T.L. Assessment of chemical composition and sensorial properties of ciders fermented with different non-Saccharomyces yeasts in pure and mixed fermentations. Int. J. Food Microbiol. 2019, 318, 108471. [Google Scholar] [CrossRef]
- Hu, K.; Qin, Y.; Tao, Y.S.; Zhu, X.L.; Ullah, N. Potential of glycosidase from non-Saccharomyces isolates for enhancement of wine aroma. J. Food Sci. 2016, 81, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Mateo, J.J.; Peris, L.; Ibañez, C.; Maicas, S. Characterization of glycolytic activities from non-Saccharomyces yeasts isolated from Bobal musts. J. Ind. Microbiol. Biotechnol. 2011, 38, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Palmeri, R.; Spagna, G. β-Glucosidase in cellular and acellular form for winemaking application. Enzym. Microb. Technol. 2007, 40, 382–389. [Google Scholar] [CrossRef]
- Fernández-González, M.; Stefano, R.D.; Briones, A. Hydrolysis and transformation of terpene glycosides from muscat must by different yeast species. Food Microbiol. 2003, 20, 35–41. [Google Scholar] [CrossRef]
- Hu, K.; Zhu, X.L.; Mu, H.; Ma, Y.; Ullah, N.; Tao, Y.S. A novel extracellular glycosidase activity from Rhodotorula mucilaginosa: Its application potential in wine aroma enhancement. Lett. Appl. Microbiol. 2016, 62, 169–176. [Google Scholar] [CrossRef]
- Barata, A.; Malfeito-Ferreira, M.; Loureiro, V. The microbial ecology of wine grape berries. Int. J. Food Microbiol. 2012, 153, 243–259. [Google Scholar] [CrossRef]
- Morgan, H.H.; du Toit, M.; Setati, M.E. The grapevine and wine microbiome: Insights from high-throughput amplicon sequencing. Front. Microbiol. 2017, 8, 820. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Sun, Q.Y.; Zhu, S.S.; Du, F.; Mao, R.Z.; Liu, L.J.; Tian, B.; Zhu, Y.F. Biodiversity of non-Saccharomyces yeasts associated with spontaneous fermentation of Cabernet Sauvignon wines from Shangri-La wine region, China. Sci. Rep. 2021, 11, 5150. [Google Scholar] [CrossRef]
- Zhang, M.X.; Pan, Q.H.; Yan, G.L.; Duan, C.Q. Using headspace solid phase micro-extraction for analysis of aromatic compounds during alcoholic fermentation of red wine. Food Chem. 2011, 125, 743–749. [Google Scholar] [CrossRef]
- Culleré, L.; Escudero, A.; Cacho, J.; Ferreira, V. Gas chromatography-olfactometry and chemical quantitative study of the aroma of six premium quality Spanish aged red wines. J. Agric. Food Chem. 2004, 52, 1653–1660. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.S.; Zhang, L. Intensity prediction of typical aroma characters of cabernet sauvignon wine in Changli County (China). LWT-Food Sci. Technol. 2010, 43, 1550–1556. [Google Scholar] [CrossRef]
- Souid, I.; Hassene, Z.; Palomo, E.S.; Perez-Coello, M.S.; Ghorbel, A. Varietal aroma compounds of Vitis vinifera cv. Khamri grown in Tunisia. J. Food Qual. 2007, 30, 718–730. [Google Scholar] [CrossRef]
- Wang, X.C.; Li, A.H.; Dizy, M.; Ullah, N.; Sun, W.X.; Tao, Y.S. Evaluation of aroma enhancement for “Ecolly” dry white wines by mixed inoculation of selected Rhodotorula mucilaginosa and Saccharomyces cerevisiae. Food Chem. 2017, 228, 550–559. [Google Scholar] [CrossRef] [PubMed]
- de Sousa Galvão, M.; Narain, N.; dos Santos, M.S.P.; Nunes, M.L. Volatile compounds and descriptive odor attributes in umbu (Spondias tuberosa) fruits during maturation. Food Res. Int. 2011, 44, 1919–1926. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, Z.W. Volatile compounds of young wines from cabernet sauvignon, cabernet gernischet and chardonnay varieties grown in the loess plateau region of China. Molecules 2010, 15, 9184–9196. [Google Scholar] [CrossRef] [Green Version]
- Peinado, R.A.; Moreno, J.; Bueno, J.E.; Moreno, J.A.; Mauricio, J.C. Comparative study of aromatic compounds in two young white wines subjected to pre-fermentative cryomaceration. Food Chem. 2004, 84, 585–590. [Google Scholar] [CrossRef]
- Peinado, R.A.; Mauricio, J.C.; Moreno, J. Aromatic series in sherry wines with gluconic acid subjected to different biological aging conditions by Saccharomyces cerevisiae var. capensis. Food Chem. 2006, 94, 232–239. [Google Scholar] [CrossRef]
- Sadoudi, M.; Tourdot-Maréchal, R.; Rousseaux, S.; Steyer, D.; Gallardo-Chacon, J.J.; Ballester, J.; Vichi, S.; Guérin-Schneider, R.; Caixach, J.; Alexandrea, H. Yeast-yeast interactions revealed by aromatic profile analysis of Sauvignon Blanc wine fermented by single or co-culture of non-Saccharomyces and Saccharomyces yeasts. Food Microbiol. 2012, 32, 243–253. [Google Scholar] [CrossRef]
- Sun, S.Y.; Gong, H.S.; Jiang, X.M.; Zhao, Y.P. Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae on alcoholic fermentation behaviour and wine aroma of cherry wines. Food Microbiol. 2014, 44, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, L.M.; Vega-Lopez, G.A.; de Ullivarri, M.F.; Raya, R.R. Population and oenological characteristics of non-Saccharomyces yeasts associated with grapes of Northwestern Argentina. Arch. Microbiol. 2019, 201, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Ciani, M.; Ferraro, L. Role of oxygen on acetic acid production by Brettanomyces/Dekkera in winemaking. J. Sci. Food Agric. 1997, 75, 489–495. [Google Scholar] [CrossRef]
- Swiegers, J.H.; Pretorius, I.S. Yeast modulation of wine flavor. Adv. Appl. Microbiol. 2005, 57, 131–175. [Google Scholar] [PubMed]
- Romano, P.; Brandolini, V.; Ansaloni, C.; Menziani, E. The production of 2,3-butanediol as a differentiating character in wine yeasts. World J. Microbiol. Biotechnol. 1998, 14, 649–653. [Google Scholar] [CrossRef]
- Sumby, K.M.; Grbin, P.R.; Jiranek, V. Microbial modulation of aromatic esters in wine: Current knowledge and future prospects. Food Chem. 2010, 121, 1–16. [Google Scholar] [CrossRef]
- Ye, M.Q.; Yue, T.L.; Yuan, Y.H. Effects of sequential mixed cultures of Wickerhamomyces anomalus and Saccharomyces cerevisiae on apple cider fermentation. FEMS Yeast Res. 2014, 14, 873–882. [Google Scholar]
- Synos, K.; Reynolds, A.G.; Bowen, A.J. Effect of yeast strain on aroma compounds in Cabernet franc icewines. LWT-Food Sci. Technol. 2015, 64, 227–235. [Google Scholar] [CrossRef]
- Carrau, F.M.; Medina, K.; Boido, E.; Farina, L.; Gaggero, C.; Dellacassa, E.; Versini, G.; Henschke, P.A. De novo synthesis of monoterpenes by Saccharomyces cerevisiae wine yeasts. FEMS Microbiol. Lett. 2005, 243, 107–115. [Google Scholar] [CrossRef] [Green Version]
- Hock, R.; Benda, I.; Schreier, P. Formation of terpenes by yeasts during alcoholic fermentation. Z. Für Lebensm.-Unters. Und–Forsch. 1984, 179, 450–452. [Google Scholar] [CrossRef]
- Wu, Q.; Zhu, W.A.; Wang, W.; Xu, Y. Effect of yeast species on the terpenoids profile of Chinese light-style liquor. Food Chem. 2015, 168, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, C.E.; Litov, R.E.; Thormar, H. Antimicrobial activity of lipids added to human milk, infant formula, and bovine milk. J. Nutr. Biochem. 1995, 6, 362–366. [Google Scholar] [CrossRef]
- Ogbolu, D.O.; Oni, A.A.; Daini, O.A.; Oloko, A.P. In vitro antimicrobial properties of coconut oil on Candida Species in Ibadan, Nigeria. J. Med. Food 2007, 10, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Parfene, G.; Horincar, V.; Tyagi, A.K.; Malik, A.; Bahrim, G. Production of medium chain saturated fatty acids with enhanced antimicrobial activity from crude coconut fat by solid state cultivation of Yarrowia lipolytica. Food Chem. 2013, 136, 1345–1349. [Google Scholar] [CrossRef] [PubMed]
- Guth, H. Quantitation and sensory studies of character impact odorants of different white wine varieties. J. Agric. Food Chem. 1997, 45, 3027–3032. [Google Scholar] [CrossRef]
- Ferreira, V.; López, R.; Cacho, J.F. Quantitative determination of the odorants of young red wines from different grape varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Tat, L.; Comuzzo, P.; Battistutta, F.; Zironi, R. Sweet-like off-flavor in Aglianico del vulture wine: Ethyl phenylacetate as the mainly involved compound. J. Agric. Food Chem. 2007, 55, 5205–5212. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Boonbumrung, S.; Yoshizawa, T.; Varanyanond, W. The volatile constituents in the peel and pulp of a green Thai mango, Khieo sawoei cultivar (Mangifera indica L.). Food Sci. Technol. Res. 2001, 7, 72–77. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, E.M.; Dennison, R.A.; Dougherty, R.H.; Shaw, P.E. Flavor and odor thresholds in water of selected orange juice components. J. Agric. Food Chem. 1978, 26, 187–191. [Google Scholar] [CrossRef]
- Ferreira, V.; Ardanuy, M.; López, R.; Cacho, J.F. Relationship between flavor dilution values and odor unit values in hydroalcoholic solutions: Role of volatility and a practical rule for its estimation. J. Agric. Food Chem. 1998, 46, 4341–4346. [Google Scholar] [CrossRef]
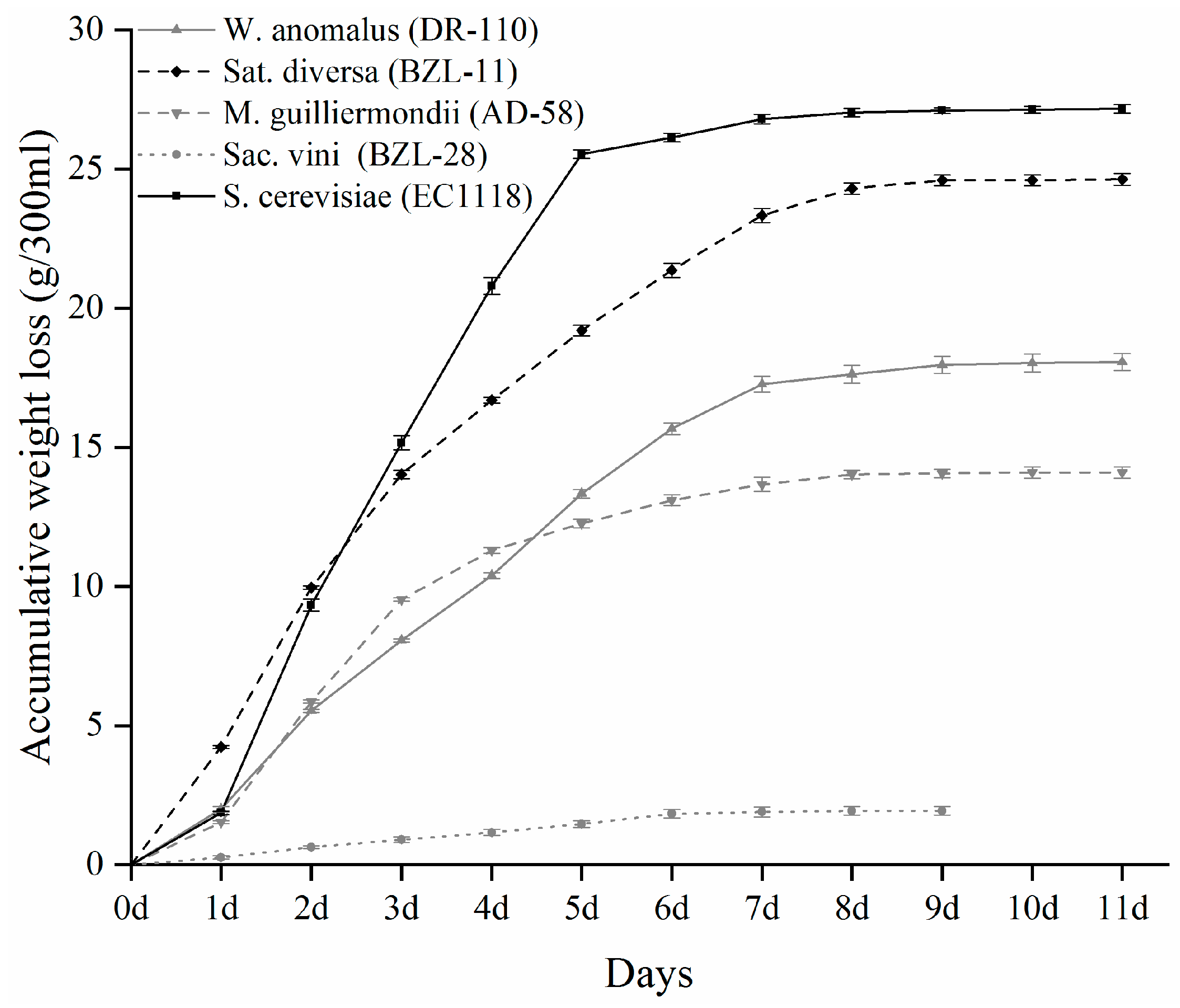
| M. guilliermondii (AD-58) | Sat. diversa (BZL-11) | Sac. vini (BZL-28) | W. anomalus (DR-110) | S. cerevisiae (EC1118) | SGJ (Uninoculated) | |
|---|---|---|---|---|---|---|
| Reducing (residual) sugar (g/L) | 88.06 ± 2.19b | 16.35 ± 1.12d | 167.28 ± 1.44a | 57.73 ± 1.53c | 0.75 ± 0.01e | 173.66 ± 0.87 |
| Alcohol content (% v/v) | 4.40 ± 0.08d | 8.41 ± 0.02b | 0.06 ± 0.02e | 5.39 ± 0.03c | 9.54 ± 0.05a | 0.01 ± 0.01 |
| pH (20 °C) | 3.42 ± 0.01b | 3.31 ± 0.02c | 3.50 ± 0.02a | 3.32 ± 0.01c | 3.26 ± 0.02d | 3.50 ± 0.02 |
| Total acidity (g/L as tartatic acid) | 5.86 ± 0.04a | 5.19 ± 0.06b | 3.28 ± 0.04d | 5.81 ± 0.06a | 4.93 ± 0.04c | 2.69 ± 0.02 |
| Volatile acidity (g/L as accetic acid) | 1.96 ± 0.02a | 0.79 ± 0.02b | 0.44 ± 0.00c | 1.97 ± 0.06a | 0.25 ± 0.02d | 0.06 ± 0.00 |
| Reducing sugar consumption (g/L) | 85.59 ± 2.19d | 157.31 ± 1.12b | 6.37 ± 1.44e | 115.93 ± 1.53c | 172.91 ± 0.01a | - |
| Sugars used for 1% ethanol production (g) | 19.45 | 18.70 | 106.17 | 21.51 | 18.12 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Sun, Q.; Tian, B.; Zhu, S.; Du, F.; Mao, R.; Li, S.; Liu, L.; Zhu, Y. Evaluation of Four Indigenous Non-Saccharomyces Yeasts Isolated from the Shangri-La Wine Region (China) for Their Fermentation Performances and Aroma Compositions in Synthetic Grape Juice Fermentation. J. Fungi 2022, 8, 146. https://doi.org/10.3390/jof8020146
Zhao Y, Sun Q, Tian B, Zhu S, Du F, Mao R, Li S, Liu L, Zhu Y. Evaluation of Four Indigenous Non-Saccharomyces Yeasts Isolated from the Shangri-La Wine Region (China) for Their Fermentation Performances and Aroma Compositions in Synthetic Grape Juice Fermentation. Journal of Fungi. 2022; 8(2):146. https://doi.org/10.3390/jof8020146
Chicago/Turabian StyleZhao, Yue, Qingyang Sun, Bin Tian, Shusheng Zhu, Fei Du, Ruzhi Mao, Su Li, Lijing Liu, and Yifan Zhu. 2022. "Evaluation of Four Indigenous Non-Saccharomyces Yeasts Isolated from the Shangri-La Wine Region (China) for Their Fermentation Performances and Aroma Compositions in Synthetic Grape Juice Fermentation" Journal of Fungi 8, no. 2: 146. https://doi.org/10.3390/jof8020146
APA StyleZhao, Y., Sun, Q., Tian, B., Zhu, S., Du, F., Mao, R., Li, S., Liu, L., & Zhu, Y. (2022). Evaluation of Four Indigenous Non-Saccharomyces Yeasts Isolated from the Shangri-La Wine Region (China) for Their Fermentation Performances and Aroma Compositions in Synthetic Grape Juice Fermentation. Journal of Fungi, 8(2), 146. https://doi.org/10.3390/jof8020146






