Use of Non-Saccharomyces Yeast Co-Fermentation with Saccharomyces cerevisiae to Improve the Polyphenol and Volatile Aroma Compound Contents in Nanfeng Tangerine Wines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tangerine Juice Preparation
2.2. Yeast Strains and Culture Media
2.3. Fermentation Process
2.4. Yeast Counting
2.5. Physicochemical Analysis
2.6. Flavonoid, Phenolic Acid and Organic Acid Compound Analysis
2.7. Electronic Nose
2.8. Volatile Aroma Composition Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Sugar Consumption Kinetics and Growth Kinetics of Yeast Strains during Fermentation
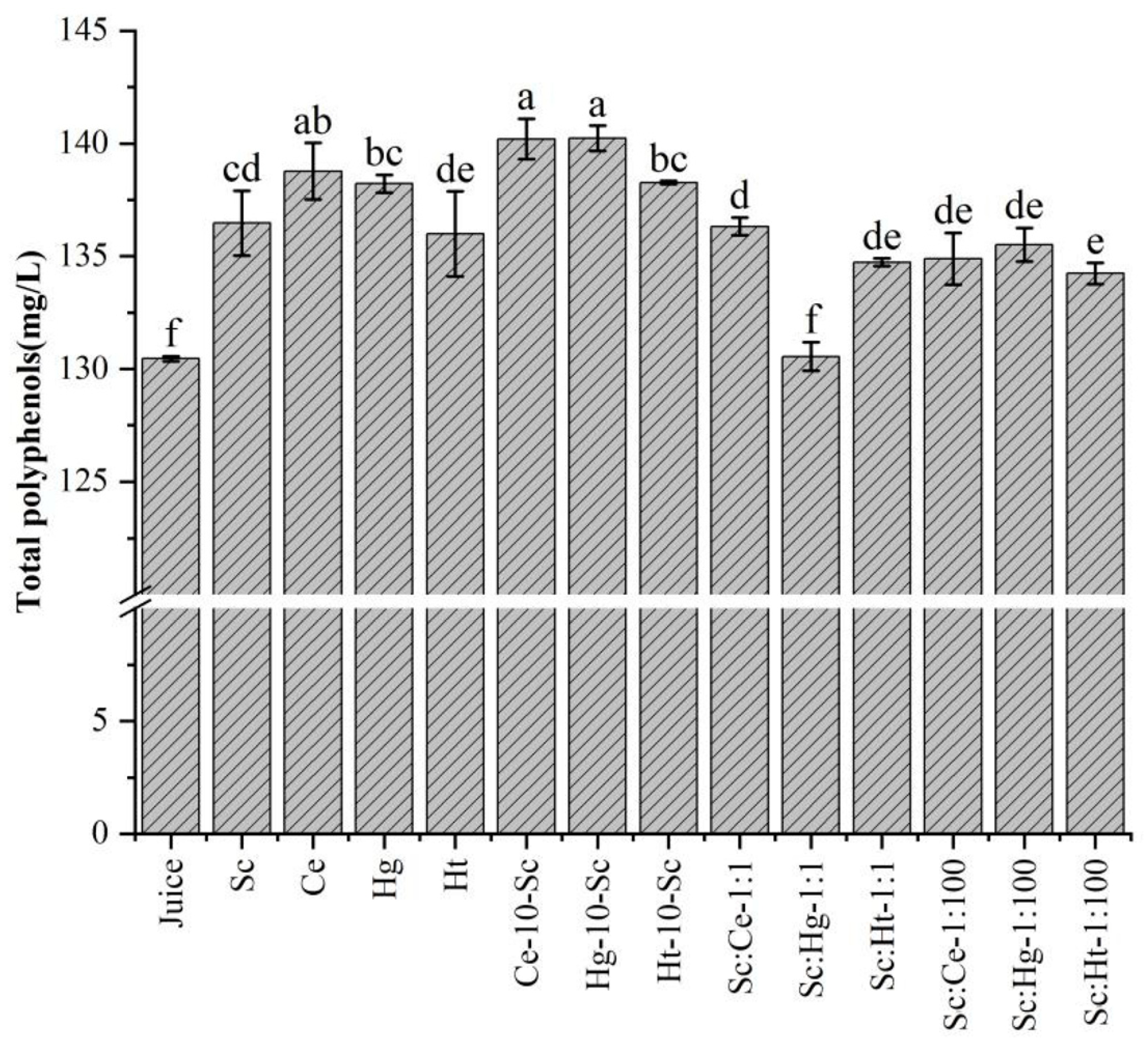
3.2. Polyphenols
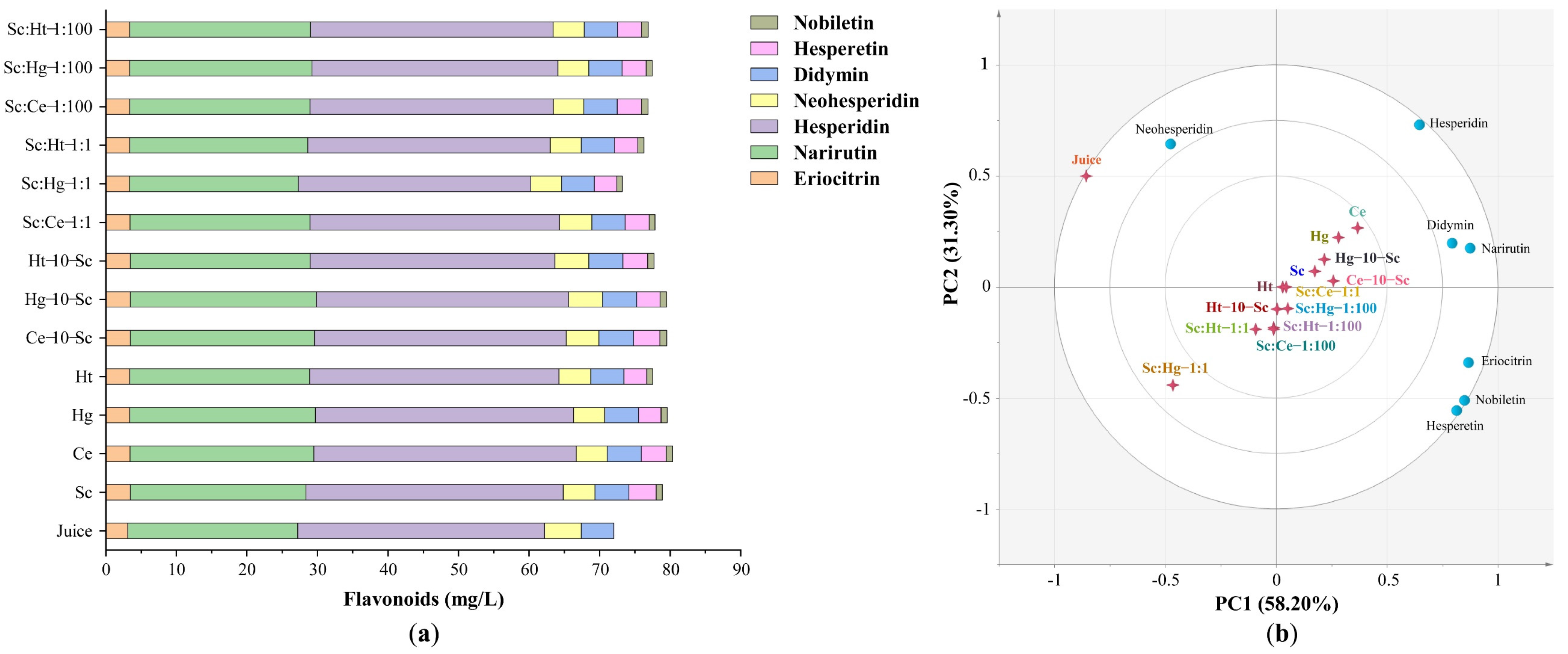
3.2.1. Flavonoids
3.2.2. Phenolic Acids
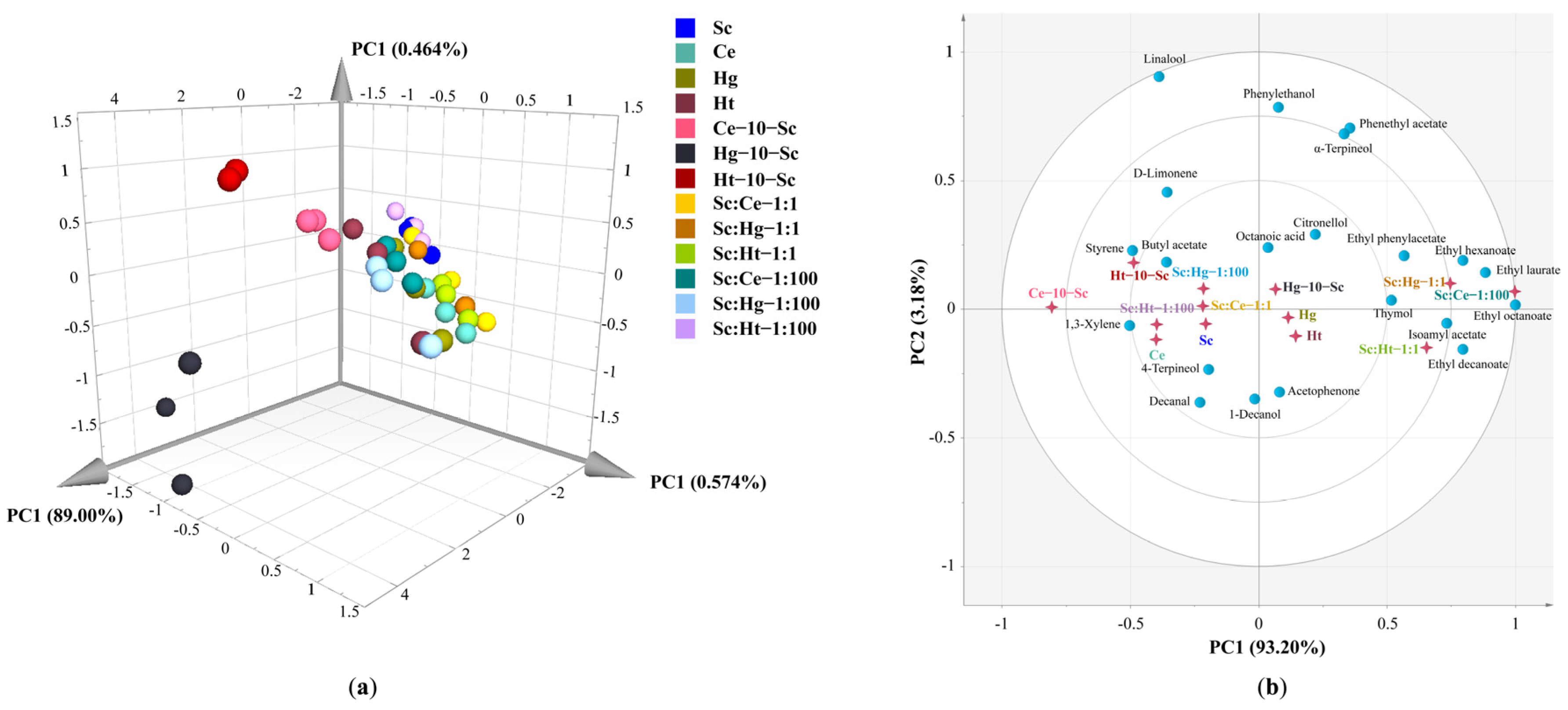
3.3. Volatile Aroma Compounds
3.4. Principal Component Analysis of Volatile Aroma Compounds (OAV ≥ 1) and Electronic Nose Data in Tangerine Wine
3.5. Other Metabolites
3.5.1. Ethanol
3.5.2. Organic Acids
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peng, X.; Wang, Y.; Tang, L.J.; Li, X.X.; Xiao, Y.W.; Zhang, Z.B.; Yan, R.M.; Yang, H.L.; Chang, J.; Zhu, B.; et al. Yeasts from Nanfeng mandarin plants: Occurrence, diversity and capability to produce indole-3-acetic acid. Biotechnol. Biotechnol. Equip. 2018, 32, 1496–1506. [Google Scholar] [CrossRef]
- Yi, F.P.; Jin, R.Y.; Sun, J.; Ma, B.D.; Bao, X.L. Evaluation of mechanical-pressed essential oil from Nanfeng mandarin (Citrus reticulata Blanco cv. Kinokuni) as a food preservative based on antimicrobial and antioxidant activities. LWT Food Sci. Technol. 2018, 95, 346–353. [Google Scholar] [CrossRef]
- Qiu, X.; Yu, L.; Wang, W.; Yan, R.; Zhang, Z.; Yang, H.; Zhu, D.; Zhu, B. Comparative evaluation of microbiota dynamics and metabolites correlation between spontaneous and inoculated fermentations of Nanfeng tangerine Wine. Front. Microbiol. 2021, 12, 649978. [Google Scholar] [CrossRef] [PubMed]
- Huynh, N.T.; Van Camp, J.; Smagghe, G.; Raes, K. Improved release and metabolism of flavonoids by steered fermentation processes: A review. Int. J. Mol. Sci. 2014, 15, 19369–19388. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Wang, J.; Ji, X.; Liu, R.; Chen, F.; Zhang, X. Selection of non-Saccharomyces yeasts for orange wine fermentation based on their enological traits and volatile compounds formation. J. Food Sci. Technol. 2018, 55, 4001–4012. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Liu, R.; Wang, X.; Zhang, X. The sensory quality improvement of citrus wine through co-fermentations with selected non-Saccharomyces yeast strains and Saccharomyces cerevisiae. Microorganisms 2020, 8, 323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domizio, P.; Romani, C.; Lencioni, L.; Comitini, F.; Gobbi, M.; Mannazzu, I.; Ciani, M. Outlining a future for non-Saccharomyces yeasts: Selection of putative spoilage wine strains to be used in association with Saccharomyces cerevisiae for grape juice fermentation. Int. J. Food Microbiol. 2011, 147, 170–180. [Google Scholar] [CrossRef]
- Liu, S.; Laaksonen, O.; Kortesniemi, M.; Kalpio, M.; Yang, B. Chemical composition of bilberry wine fermented with non-Saccharomyces yeasts (Torulaspora delbrueckii and Schizosaccharomyces pombe) and Saccharomyces cerevisiae in pure, sequential and mixed fermentations. Food. Chem. 2018, 266, 262–274. [Google Scholar] [CrossRef]
- Mateus, D.; Sousa, S.; Coimbra, C.; Frank, S.R.; Simões, J. Identification and characterization of non-Saccharomyces species isolated from port wine spontaneous fermentations. Foods 2020, 9, 120. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, I.; Ferreira, V. Modulating fermentative, varietal and aging aromas of wine using non-Saccharomyces yeasts in a sequential inoculation approach. Microorganisms 2019, 7, 164. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Zhang, Y.; Wang, Y.; Ju, H.; Niu, C.; Song, Z.; Yuan, Y.; Yue, T. Assessment of chemical composition and sensorial properties of ciders fermented with different non-Saccharomyces yeasts in pure and mixed fermentations. Int. J. Food Microbiol. 2020, 318, 108471. [Google Scholar] [CrossRef]
- Albergaria, H.; Arneborg, N. Dominance of Saccharomyces cerevisiae in alcoholic fermentation processes: Role of physiological fitness and microbial interactions. Appl. Microbiol. Biotechnol. 2016, 100, 2035–2046. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Mas, A.; Esteve-Zarzoso, B. The interaction between Saccharomyces cerevisiae and non-Saccharomyces yeast during alcoholic fermentation is species and strain specific. Front. Microbiol. 2016, 7, 502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escribano-Viana, R.; González-Arenzana, L.; Portu, J.; Garijo, P.; López-Alfaro, I.; López, R.; Santamaría, P.; Gutiérrez, A.R. Wine aroma evolution throughout alcoholic fermentation sequentially inoculated with non- Saccharomyces/Saccharomyces yeasts. Food Res. Int. 2018, 112, 17–24. [Google Scholar] [CrossRef]
- Malfeito-Ferreira, M. Two decades of "Horse Sweat" taint and Brettanomyces yeasts in wine: Where do we stand now? Beverages 2018, 4, 32. [Google Scholar] [CrossRef] [Green Version]
- Merín, M.G.; Morata de Ambrosini, V.I. Highly cold-active pectinases under wine-like conditions from non-Saccharomyces yeasts for enzymatic production during winemaking. Lett. Appl. Microbiol. 2015, 60, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Borren, E.; Tian, B. The important contribution of non-Saccharomyces yeasts to the aroma complexity of wine: A review. Foods 2020, 10, 13. [Google Scholar] [CrossRef]
- Lu, Y.; Voon, M.K.W.; Chua, J.Y.; Huang, D.; Lee, P.R.; Liu, S.Q. The effects of co- and sequential inoculation of Torulaspora delbrueckii and Pichia kluyveri on chemical compositions of durian wine. Appl. Microbiol. Biotechnol. 2017, 101, 7853–7863. [Google Scholar] [CrossRef]
- Arslan, E.; Çelik, Z.D.; Cabaroğlu, T. Effects of pure and mixed autochthonous Torulaspora delbrueckii and Saccharomyces cerevisiae on fermentation and volatile compounds of Narince wines. Foods 2018, 7, 147. [Google Scholar] [CrossRef] [Green Version]
- Petronilho, S.; Lopez, R.; Ferreira, V.; Coimbra, M.A.; Rocha, S.M. Revealing the usefulness of aroma networks toexplain wine aroma properties: A case study of portuguese wines. Molecules 2020, 25, 272. [Google Scholar] [CrossRef] [Green Version]
- Minnaar, P.P.; du Plessis, H.W.; Jolly, N.P.; van der Rijst, M.; du Toit, M. Non-Saccharomyces yeast and lactic acid bacteria in Co-inoculated fermentations with two Saccharomyces cerevisiae yeast strains: A strategy to improve the phenolic content of Syrah wine. Food Chem. X 2019, 4, 100070. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Mahato, N.; Cho, M.H.; Lee, Y.R. Converting citrus wastes into value-added products: Economic and environmently friendly approaches. Nutrition 2017, 34, 29–46. [Google Scholar] [CrossRef]
- Ferreira, M.C.B. Exploring the Non-Conventional Yeast Species Candida ethanolica for Biotechnological Applications: A Physiological and Genomic Analysis; Técnico Lisboa: Lisbon, Portugal, 2018. [Google Scholar]
- Albergaria, H.; Torrão, A.R.; Hogg, T.; Gírio, F.M. Physiological behaviour of Hanseniaspora guilliermondii in aerobic glucose-limited continuous cultures. FEMS Yeast Res. 2003, 3, 211–216. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, A.J.S. Physiological Features of Saccharomyces cerevisiae and Alternative Wine Yeast Species in Relation to Alcohol Level Reduction in Wine; Universidad de La Rioja: La Rioja, Spain, 2019. [Google Scholar]
- Forde, C.G.; Cox, A.; Williams, E.R.; Boss, P.K. Associations between the sensory attributes and volatile composition of Cabernet Sauvignon wines and the volatile composition of the grapes used for their production. J. Agric. Food Chem. 2011, 59, 2573–2583. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, Q.Q.; Xu, Y.H.; Li, A.H.; Tao, Y.S. Increased glycosidase activities improved the production of wine varietal odorants in mixed fermentation of P. fermentans and high antagonistic S. cerevisiae. Food Chem. 2020, 332, 127426. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Ye, H.; Wu, D.; Shi, H.; Zhou, X. Chemoenzymatic quantification for monitoring unpurified polysaccharide in rich medium. Appl. Microbiol. Biotechnol. 2019, 103, 7635–7645. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wu, Z.; Weng, P. Comparison of bayberry fermented wine aroma from different cultivars by GC-MS combined with electronic nose analysis. Food Sci. Nutr. 2020, 8, 830–840. [Google Scholar] [CrossRef] [PubMed]
- Martínez-García, R.; Moreno, J.; Bellincontro, A.; Centioni, L.; Puig-Pujol, A.; Peinado, R.A.; Mauricio, J.C.; García-Martínez, T. Using an electronic nose and volatilome analysis to differentiate sparkling wines obtained under different conditions of temperature, ageing time and yeast formats. Food Chem. 2021, 334, 127574. [Google Scholar] [CrossRef]
- Keyzers, R.A.; Boss, P.K. Changes in the volatile compound production of fermentations made from musts with increasing grape content. J. Agric. Food Chem. 2010, 58, 1153–1164. [Google Scholar] [CrossRef]
- Shi, J.; Wu, H.; Xiong, M.; Chen, Y.; Chen, J.; Zhou, B.; Wang, H.; Li, L.; Fu, X.; Bie, Z.; et al. Comparative analysis of volatile compounds in thirty nine melon cultivars by headspace solid-phase microextraction and gas chromatography-mass spectrometry. Food Chem. 2020, 316, 126342. [Google Scholar] [CrossRef]
- Gemert, L.J.V. Odour Thresholds; Oliemans, Punter & Partners BV: Zeist, The Netherlands, 2011. [Google Scholar]
- Grosch, W. Evaluation of the key odorants of foods by dilution experiments, aroma models and omission. Chem. Senses 2001, 26, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, L.; Xiao, Z.; Niu, Y. Characterization of the key aroma compounds in mulberry fruits by application of gas chromatography-olfactometry (GC-O), odor activity value (OAV), gas chromatography-mass spectrometry (GC-MS) and flame photometric detection (FPD). Food Chem. 2018, 245, 775–785. [Google Scholar] [CrossRef]
- Liu, J.; Arneborg, N.; Toldam-Andersen, T.B.; Petersen, M.A.; Bredie, W.L. Effect of sequential fermentations and grape cultivars on volatile compounds and sensory profiles of Danish wines. J. Sci. Food Agric. 2017, 97, 3594–3602. [Google Scholar] [CrossRef] [PubMed]
- Kemsawasd, V.; Branco, P.; Almeida, M.G.; Caldeira, J.; Albergaria, H.; Arneborg, N. Cell-to-cell contact and antimicrobial peptides play a combined role in the death of Lachanchea thermotolerans during mixed-culture alcoholic fermentation with Saccharomyces cerevisiae. FEMS Microbiol. Lett. 2015, 362, fnv103. [Google Scholar] [CrossRef]
- Sidari, R.; Caridi, A. Nutrient depletion modifies cell wall adsorption activity of wine yeast. World J. Microbiol. Biotechnol. 2016, 32, 89. [Google Scholar] [CrossRef] [PubMed]
- Seixas, I.; Barbosa, C.; Mendes-Faia, A.; Güldener, U.; Tenreiro, R.; Mendes-Ferreira, A.; Mira, N.P. Genome sequence of the non-conventional wine yeast Hanseniaspora guilliermondii UTAD222 unveils relevant traits of this species and of the Hanseniaspora genus in the context of wine fermentation. DNA Res. Int. J. Rapid Publ. Rep. Genes Genomes 2019, 26, 67–83. [Google Scholar] [CrossRef] [Green Version]
- Sujittra, S.; Vichitphan, K.; Han, J.; Vichitphan, S.; Swangkaew, J. Hanseniaspora thailandica BC9 β-glucosidase for the production of β-(D)-hexyl glucoside. J. Microbiol. Biotechnol. 2018, 28, 579–587. [Google Scholar] [CrossRef]
- Swangkeaw, J.; Vichitphan, S.; Butzke, C.E.; Vichitphan, K. Characterization of β-glucosidases from Hanseniaspora sp. and Pichia anomala with potentially aroma-enhancing capabilities in juice and wine. World J. Microbiol. Biotechnol. 2011, 27, 423–430. [Google Scholar] [CrossRef]
- Kanpiengjai, A.; Chui-Chai, N.; Chaikaew, S.; Khanongnuch, C. Distribution of tannin-‘tolerant yeasts isolated from Miang, a traditional fermented tea leaf (Camellia sinensis var. assamica) in northern Thailand. Int. J. Food Microbiol. 2016, 238, 121–131. [Google Scholar] [CrossRef]
- Slámová, K.; Kapešová, J.; Valentová, K. “Sweet Flavonoids”: Glycosidase-catalyzed modifications. Int. J. Mol. Sci. 2018, 19, 2126. [Google Scholar] [CrossRef] [Green Version]
- Parhiz, H.; Roohbakhsh, A.; Soltani, F.; Rezaee, R.; Iranshahi, M. Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: An updated review of their molecular mechanisms and experimental models. Phytother. Res. 2015, 29, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.W.; Wang, W.; Sheng, H.; Bai, Y.F.; Weng, Y.Y.; Fan, X.Y.; Zheng, F.; Zhu, X.T.; Xu, Z.C.; Zhang, F. Hesperetin, a SIRT1 activator, inhibits hepatic inflammation via AMPK/CREB pathway. Int. Immunopharmacol. 2020, 89, 107036. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, A.; Ohizumi, Y. Potential benefits of nobiletin, a citrus flavonoid, against Alzheimer’s disease and Parkinson’s disease. Int. J. Mol. Sci. 2019, 20, 3380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, X.; Zhao, S.; Ning, Z.; Zeng, H.; Shu, Y.; Tao, O.; Xiao, C.; Lu, C.; Liu, Y. Citrus fruits as a treasure trove of active natural metabolites that potentially provide benefits for human health. Chem. Cent. J. 2015, 9, 68. [Google Scholar] [CrossRef] [Green Version]
- Cameleyre, M.; Lytra, G.; Tempere, S.; Barbe, J.C. Olfactory impact of higher alcohols on red wine fruity ester aroma expression in model solution. J. Agric. Food Chem. 2015, 63, 9777–9788. [Google Scholar] [CrossRef]
- Liu, F.; Li, S.; Gao, J.; Cheng, K.; Yuan, F. Changes of terpenoids and other volatiles during alcoholic fermentation of blueberry wines made from two southern highbush cultivars. LWT-Food Sci. Technol. 2019, 109, 233–240. [Google Scholar] [CrossRef]
- Ayestarán, B.; Martínez-Lapuente, L.; Guadalupe, Z.; Canals, C.; Adell, E.; Vilanova, M. Effect of the winemaking process on the volatile composition and aromatic profile of Tempranillo Blanco wines. Food Chem. 2019, 276, 187–194. [Google Scholar] [CrossRef]
- Peng, C.T.; Wen, Y.; Tao, Y.S.; Lan, Y.Y. Modulating the formation of Meili wine aroma by prefermentative freezing process. J. Agric. Food Chem. 2013, 61, 1542–1553. [Google Scholar] [CrossRef]
- Ciani, M.; Morales, P.; Comitini, F.; Tronchoni, J.; Canonico, L.; Curiel, J.A.; Oro, L.; Rodrigues, A.J.; Gonzalez, R. Non-conventional yeast species for lowering ethanol content of wines. Front. Microbiol. 2016, 7, 642. [Google Scholar] [CrossRef] [Green Version]
- Maturano, Y.P.; Mestre, M.V.; Kuchen, B.; Toro, M.E.; Mercado, L.A.; Vazquez, F.; Combina, M. Optimization of fermentation-relevant factors: A strategy to reduce ethanol in red wine by sequential culture of native yeasts. Int. J. Food Microbiol. 2019, 289, 40–48. [Google Scholar] [CrossRef]
- Schelezki, O.J.; Smith, P.A.; Hranilovic, A.; Bindon, K.A.; Jeffery, D.W. Comparison of consecutive harvests versus blending treatments to produce lower alcohol wines from Cabernet Sauvignon grapes: Impact on polysaccharide and tannin content and composition. Food Chem. 2018, 244, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Canonico, L.; Comitini, F.; Oro, L.; Ciani, M. Sequential fermentation with selected immobilized non-Saccharomyces yeast for reduction of ethanol content in wine. Front. Microbiol. 2016, 7, 278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendes Ferreira, A.; Mendes-Faia, A. The role of yeasts and lactic acid bacteria on the metabolism of organic acids during winemaking. Foods 2020, 9, 1231. [Google Scholar] [CrossRef] [PubMed]
- Eldarov, M.A.; Mardanov, A.V. Metabolic engineering of wine strains of Saccharomyces cerevisiae. Genes 2020, 11, 964. [Google Scholar] [CrossRef] [PubMed]
- Wojdyło, A.; Samoticha, J.; Chmielewska, J. The influence of different strains of Oenococcus oeni malolactic bacteria on profile of organic acids and phenolic compounds of red wine cultivars Rondo and Regent growing in a cold region. J. Food Sci. 2020, 85, 1070–1081. [Google Scholar] [CrossRef]
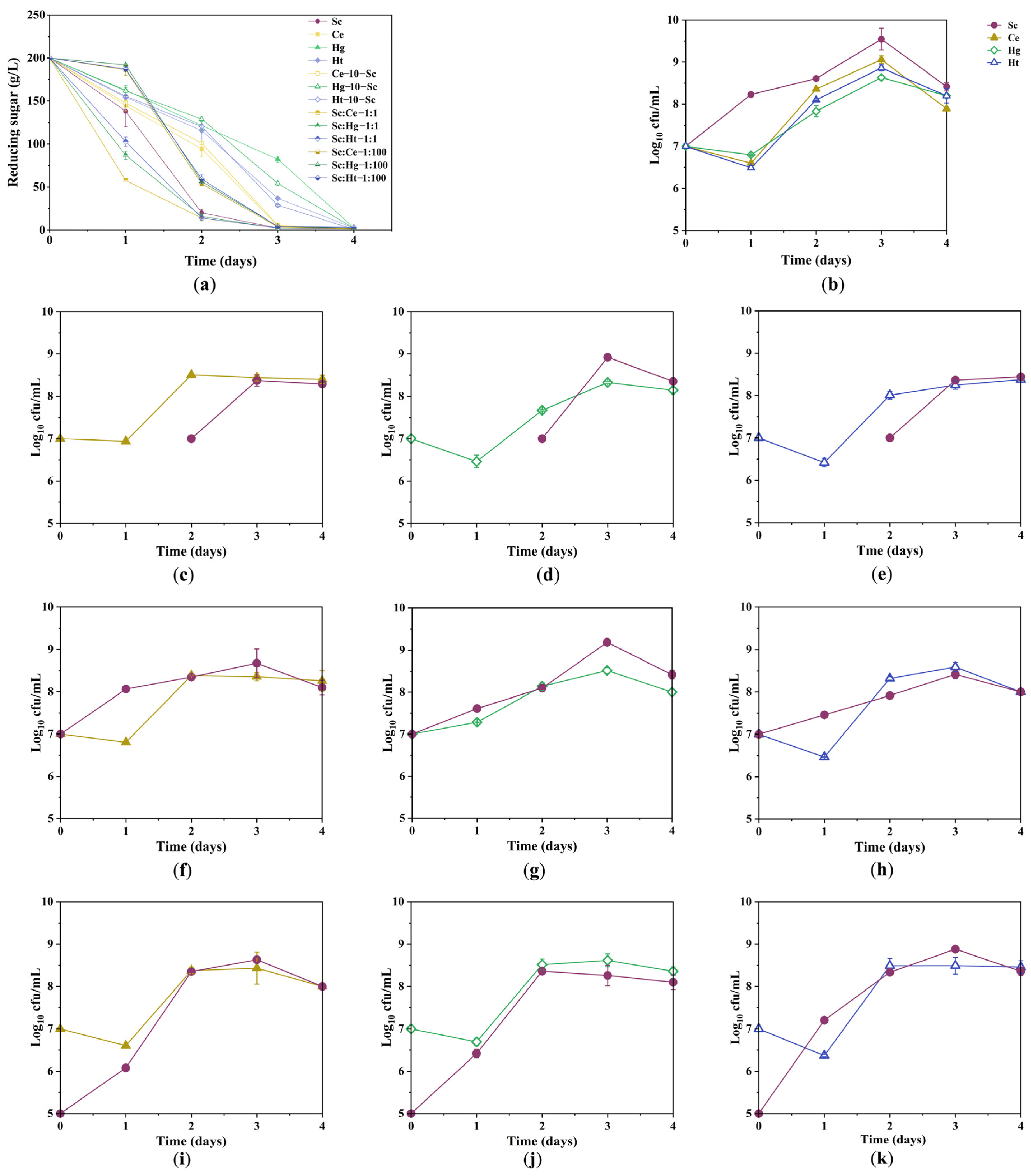


| Pure Fermentation | Sequential Fermentation | Mixed Fermentation (1:1) | Mixed Fermentation (1:100) |
|---|---|---|---|
| Sc | Ce-10-Sc | Sc:Ce-1:1 | Sc:Ce-1:100 |
| Ce | Hg-10-Sc | Sc:Hg-1:1 | Sc:Hg-1:100 |
| Hg | Ht-10-Sc | Sc:Ht-1:1 | Sc:Ht-1:100 |
| Ht |
| Time (min) | 0 | 2 | 10 | 12 | 15 | 20 | 25 | 30 |
|---|---|---|---|---|---|---|---|---|
| 0.3% acetic acid solution | 80 | 80 | 75 | 70 | 60 | 50 | 40 | 80 |
| Acetonitrile | 20 | 20 | 25 | 30 | 40 | 50 | 60 | 20 |
| Time (min) | 0 | 10 | 20 | 25 | 35 |
|---|---|---|---|---|---|
| 2% acetic acid solution | 85 | 80 | 80 | 70 | 85 |
| Methanol | 15 | 20 | 20 | 30 | 15 |
| Sensor Number | Sensor Name | Sensor Sensitive Compounds |
|---|---|---|
| 1 | T70/2 | Aromatic compounds |
| 2 | PA/2 | Ethanol, aromatic/organic amine |
| 3 | P30/1 | Hydrocarbons, ammonia, ethanol |
| 4 | P40/1 | Chlorine |
| 5 | LY2/AA | Ammonia/organic amine |
| 6 | LY2/gCT | Hydrogen sulfide |
| Ethanol (%) | Organic Acids (mg/L) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxalic Acid | Malic Acid | Vitamin C | Lactic Acid | Acetic Acid | Mleic Acid | Citric Acid | Succinic Acid | Fumaric Acid | Subtotal | ||
| Juice | - | 877.29 ± 3.14a | 103.07 ± 6.48f | 119.30 ± 1.52de | 517.54 ± 3.51h | 493.93 ± 45.52b | - | 8165.07 ± 11.51a | 249.76 ± 12.46g | 3.17 ± 0.09 | 10,529.12 ± 62.41f |
| Sc | 11.13 ± 0.02a | 536.36 ± 57.36bc | 230.77 ± 3.48b | 135.29 ± 2.03bc | 5949.32 ± 22.39g | 5084.68 ± 57.57a | - | 5789.19 ± 170.51cd | 1976.67 ± 15.41bc | - | 19,702.29 ± 285.60a |
| Ce | 9.39 ± 0.32b | 396.83 ± 44.51g | 228.65 ± 28.48b | 149.29 ± 9.05a | 8338.23 ± 81.41bc | - | - | 5795.31 ± 119.19cd | 1783.86 ± 149.45de | - | 16,692.17 ± 268.74cde |
| Hg | 8.06 ± 0.53cde | 516.91 ± 26.95cd | 274.74 ± 3.48a | 112.00 ± 15.40e | 7908.25 ± 19.94de | - | - | 5858.49 ± 4.67cd | 1566.96 ± 15.58f | - | 16,237.34 ± 58.42e |
| Ht | 7.79 ± 0.99de | 585.88 ± 30.59b | 263.85 ± 4.48a | 137.75 ± 3.72bc | 7947.0 ± 201.50de | - | - | 5820.35 ± 116.41cd | 1633.81 ± 144.50ef | - | 16,388.66 ± 321.33de |
| Ce-10-Sc | 10.74 ± 0.03a | 455.92 ± 26.31defg | 183.08 ± 1.48d | 135.44 ± 2.28bc | 8016.5 ± 211.11cde | - | - | 6372.43 ± 118.01b | 1935.11 ± 203.89bcd | - | 17,098.58 ± 272.01bc |
| Hg-10-Sc | 10.73 ± 0.08a | 414.59 ± 5.25efg | 176.40 ± 11.48d | 126.87 ± 1.55cd | 7739.8 ± 42.11ef | - | - | 6549.95 ± 45.93b | 2317.06 ± 17.34a | - | 17,324.69 ± 91.46b |
| Ht-10-Sc | 10.81 ± 0.03a | 463.41 ± 5.05def | 132.56 ± 0.48e | 140.64 ± 0.62ab | 7457.5 ± 86.81f | - | - | 6471.23 ± 19.22b | 2106.61 ± 35.22b | - | 16,772.02 ± 137.77cd |
| Sc:Ce-1:1 | 8.59 ± 0.14bcd | 464.27 ± 5.35def | 203.76 ± 4.48c | 133.99 ± 3.95bc | 8659.1 ± 204.95ab | - | - | 5679.36 ± 47.04d | 1774.93 ± 41.08de | - | 16,915.48 ± 223.27bc |
| Sc:Hg-1:1 | 8.30 ± 0.36cd | 504.22 ± 25.28cd | 184.10 ± 0.48d | 127.63 ± 1.69cd | 8566.4 ± 64.93ab | - | - | 5829.65 ± 31.74cd | 1704.18 ± 29.04ef | - | 16,916.20 ± 85.11bc |
| Sc:Ht-1:1 | 8.35 ± 0.43cd | 485.14 ± 58.24cd | 177.64 ± 2.48d | 141.88 ± 2.11ab | 8723.6 ± 438.83a | - | - | 5927.20 ± 149.75c | 1914.85 ± 89.35cd | - | 17,370.35 ± 468.01b |
| Sc:Ce-1:100 | 7.39 ± 0.57e | 410.27 ± 0.62fg | 140.13 ± 5.48e | 135.40 ± 3.57bc | 8103.1 ± 28.75cd | - | - | 5713.95 ± 24.61d | 1926.10 ± 15.77cd | - | 16,429.00 ± 54.33de |
| Sc:Hg-1:100 | 8.62 ± 0.26bcd | 476.75 ± 9.26cde | 132.96 ± 3.48e | 140.37 ± 4.55ab | 8061.8 ± 71.21cde | - | - | 5847.13 ± 9.44cd | 2007.81 ± 14.64bc | - | 16,666.88 ± 80.42cde |
| Sc:Ht-1:100 | 8.85 ± 0.28bc | 503.82 ± 32.29cd | 132.21 ± 0.48e | 135.65 ± 4.10bc | 8158.0 ± 164.32cd | - | - | 5918.53 ± 108.60c | 2062.12 ± 35.67bc | - | 16,910.34 ± 263.76bc |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, A.; Xiao, Y.; He, Z.; Liu, J.; Wang, Y.; Gao, B.; Chang, J.; Zhu, D. Use of Non-Saccharomyces Yeast Co-Fermentation with Saccharomyces cerevisiae to Improve the Polyphenol and Volatile Aroma Compound Contents in Nanfeng Tangerine Wines. J. Fungi 2022, 8, 128. https://doi.org/10.3390/jof8020128
Xu A, Xiao Y, He Z, Liu J, Wang Y, Gao B, Chang J, Zhu D. Use of Non-Saccharomyces Yeast Co-Fermentation with Saccharomyces cerevisiae to Improve the Polyphenol and Volatile Aroma Compound Contents in Nanfeng Tangerine Wines. Journal of Fungi. 2022; 8(2):128. https://doi.org/10.3390/jof8020128
Chicago/Turabian StyleXu, Ahui, Yiwen Xiao, Zhenyong He, Jiantao Liu, Ya Wang, Boliang Gao, Jun Chang, and Du Zhu. 2022. "Use of Non-Saccharomyces Yeast Co-Fermentation with Saccharomyces cerevisiae to Improve the Polyphenol and Volatile Aroma Compound Contents in Nanfeng Tangerine Wines" Journal of Fungi 8, no. 2: 128. https://doi.org/10.3390/jof8020128
APA StyleXu, A., Xiao, Y., He, Z., Liu, J., Wang, Y., Gao, B., Chang, J., & Zhu, D. (2022). Use of Non-Saccharomyces Yeast Co-Fermentation with Saccharomyces cerevisiae to Improve the Polyphenol and Volatile Aroma Compound Contents in Nanfeng Tangerine Wines. Journal of Fungi, 8(2), 128. https://doi.org/10.3390/jof8020128






