Cryptococcus neoformans Infection in the Central Nervous System: The Battle between Host and Pathogen
Abstract
1. Introduction
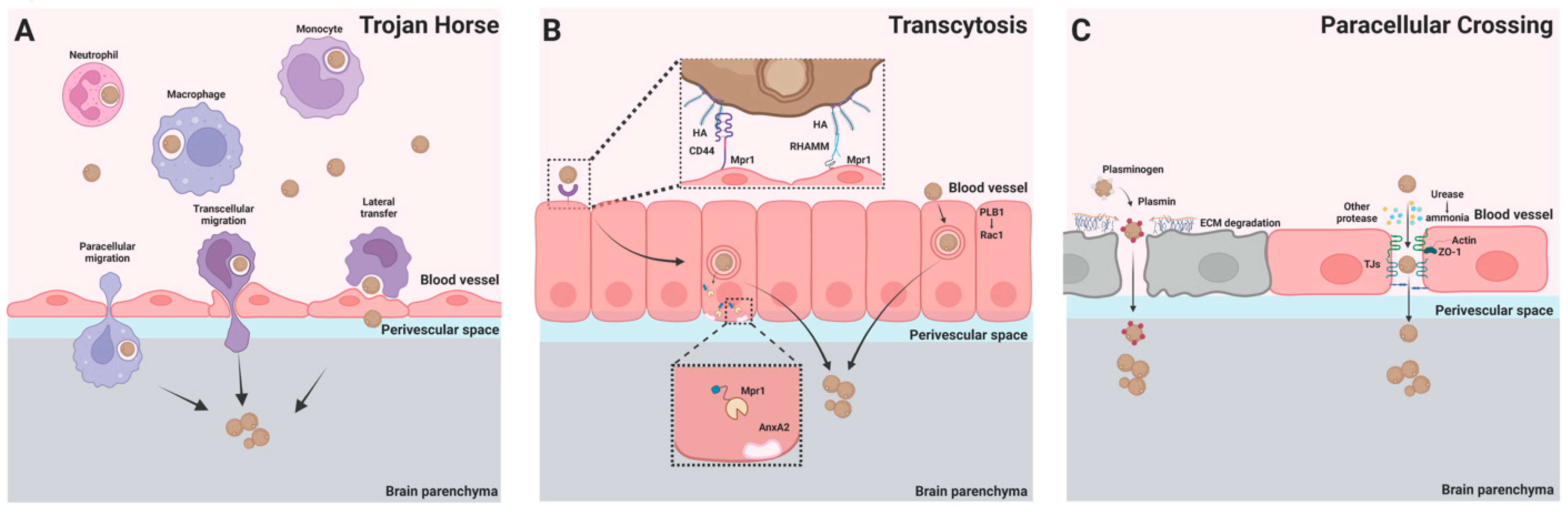
2. Invasion of C. neoformans into the Central Nervous System
2.1. Trojan Horse
2.2. Transcytosis
2.3. Paracellular Crossing
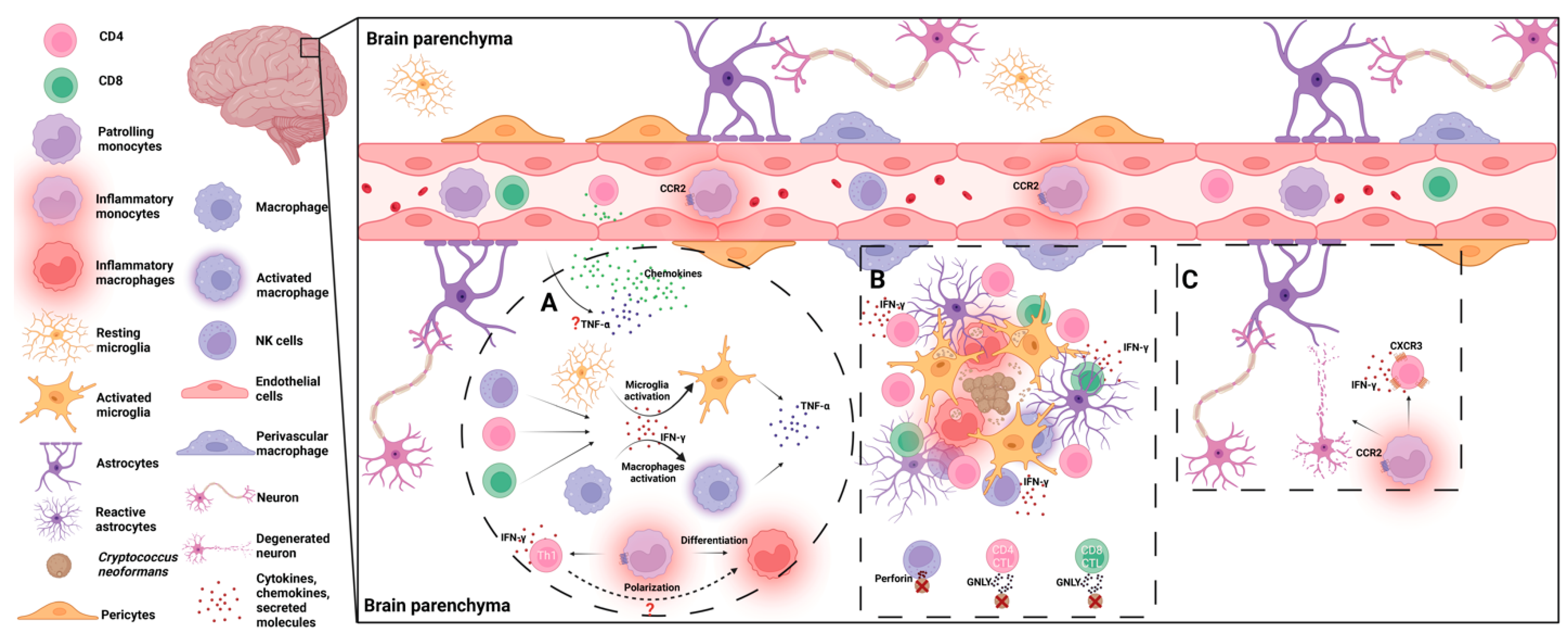
3. Host Immune Responses to C. neoformans in the Brain
3.1. Microglia
3.2. Inflammatory Monocytes/Macrophages
3.3. NK/NKT Cells
3.4. CD4/8+ T Cells
3.5. Proinflammatory Cytokines
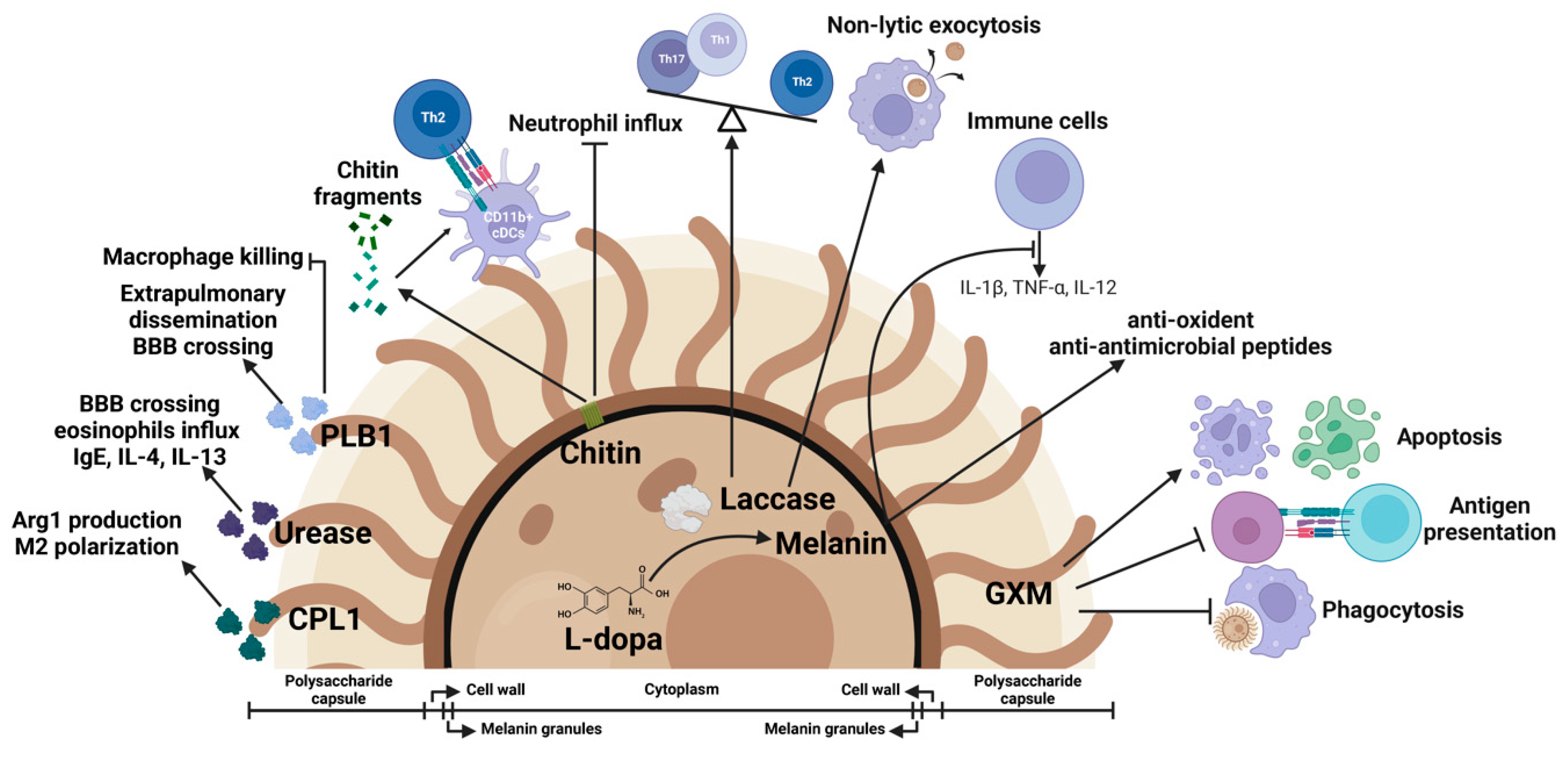
4. Fungal Virulence Factors as Immune Modulators
4.1. Polysaccharide Capsule
4.2. Laccase Activity and Melanin Formation
4.3. Phospholipase B1 Activity
4.4. Urease Activity
4.5. Chitin
4.6. CPL1
5. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Mitchell, T.G.; Perfect, J.R. Cryptococcosis in the Era of AIDS—100 Years after the Discovery of Cryptococcus neoformans. Clin. Microbiol. Rev. 1995, 8, 515–548. [Google Scholar] [CrossRef]
- Gottfredsson, M.; Perfect, J.R. Fungal Meningitis. Semin. Neurol. 2000, 20, 307–322. [Google Scholar] [CrossRef] [PubMed]
- Kwon-Chung, K.J.; Sorrell, T.C.; Dromer, F.; Fung, E.; Levitz, S.M. Cryptococcosis: Clinical and Biological Aspects. Med. Mycol. 2000, 38, 205–213. [Google Scholar] [CrossRef]
- Casadevall, A.; Coelho, C.; Alanio, A. Mechanisms of Cryptococcus neoformans-Mediated Host Damage. Front. Immunol. 2018, 9, 855. [Google Scholar] [CrossRef] [PubMed]
- Perfect, J.R.; Dismukes, W.E.; Dromer, F.; Goldman, D.L.; Graybill, J.R.; Hamill, R.J.; Harrison, T.S.; Larsen, R.A.; Lortholary, O.; Nguyen, M.H.; et al. Clinical Practice Guidelines for the Management of Cryptococcal Disease: 2010 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2010, 50, 291–322. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, T. Understanding and Managing the Adverse Effects of Antiretroviral Therapy. Antivir. Res. 2010, 85, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Montessori, V.; Press, N.; Harris, M.; Akagi, L.; Montaner, J.S.G. Adverse Effects of Antiretroviral Therapy for HIV Infection. CMAJ 2004, 170, 229–238. [Google Scholar] [PubMed]
- Rajasingham, R.; Smith, R.M.; Park, B.J.; Jarvis, J.N.; Govender, N.P.; Chiller, T.M.; Denning, D.W.; Loyse, A.; Boulware, D.R. Global Burden of Disease of HIV-Associated Cryptococcal Meningitis: An Updated Analysis. Lancet Infect. Dis. 2017, 17, 873–881. [Google Scholar] [CrossRef]
- Leopold Wager, C.M.; Hole, C.R.; Wozniak, K.L.; Wormley, F.L. Cryptococcus and Phagocytes: Complex Interactions That Influence Disease Outcome. Front. Microbiol. 2016, 7, 105. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.S.; Drummond, R.A. The Diverse Roles of Monocytes in Cryptococcosis. J. Fungi 2020, 6, 111. [Google Scholar] [CrossRef] [PubMed]
- Voelz, K.; May, R.C. Cryptococcal Interactions with the Host Immune System. Eukaryot. Cell 2010, 9, 835–846. [Google Scholar] [CrossRef] [PubMed]
- Olszewski, M.A.; Zhang, Y.; Huffnagle, G.B. Mechanisms of Cryptococcal Virulence and Persistence. Future Microbiol. 2010, 5, 1269–1288. [Google Scholar] [CrossRef]
- Mohamed, S.H.; Nyazika, T.K.; Ssebambulidde, K.; Lionakis, M.S.; Meya, D.B.; Drummond, R.A. Fungal CNS Infections in Africa: The Neuroimmunology of Cryptococcal Meningitis. Front. Immunol. 2022, 13, 804674. [Google Scholar] [CrossRef]
- Nelson, B.N.; Hawkins, A.N.; Wozniak, K.L. Pulmonary Macrophage and Dendritic Cell Responses to Cryptococcus neoformans. Front. Cell. Infect. Microbiol. 2020, 10, 37. [Google Scholar] [CrossRef]
- Doering, T.L. How Sweet It Is! Cell Wall Biogenesis and Polysaccharide Capsule Formation in Cryptococcus neoformans. Annu. Rev. Microbiol. 2009, 63, 223–247. [Google Scholar] [CrossRef] [PubMed]
- Casadevall, A.; Coelho, C.; Cordero, R.J.B.; Dragotakes, Q.; Jung, E.; Vij, R.; Wear, M.P. The Capsule of Cryptococcus neoformans. Virulence 2019, 10, 822–831. [Google Scholar] [CrossRef] [PubMed]
- Williamson, P.R. Laccase and Melanin in the Pathogenesis of Cryptococcus neoformans. Front. Biosci. 1997, 2, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Almeida, F.; Wolf, J.M.; Casadevall, A. Virulence-Associated Enzymes of Cryptococcus neoformans. Eukaryot. Cell 2015, 14, 1173–1185. [Google Scholar] [CrossRef] [PubMed]
- Dando, S.J.; Mackay-Sim, A.; Norton, R.; Currie, B.J.; St. John, J.A.; Ekberg, J.A.K.; Batzloff, M.; Ulett, G.C.; Beacham, I.R. Pathogens Penetrating the Central Nervous System: Infection Pathways and the Cellular and Molecular Mechanisms of Invasion. Clin. Microbiol. Rev. 2014, 27, 691–726. [Google Scholar] [CrossRef] [PubMed]
- Charlier, C.; Chrétien, F.; Baudrimont, M.; Mordelet, E.; Lortholary, O.; Dromer, F. Capsule Structure Changes Associated with Cryptococcus neoformans crossing of the Blood-Brain Barrier. Am. J. Pathol. 2005, 166, 421–432. [Google Scholar] [CrossRef]
- Chang, Y.C.; Stins, M.F.; McCaffery, M.J.; Miller, G.F.; Pare, D.R.; Dam, T.; Paul-Satyasee, M.; Kim, K.S.; Kwon-Chung, K.J. Cryptococcal Yeast Cells Invade the Central Nervous System via Transcellular Penetration of the Blood-Brain Barrier. Infect. Immun. 2004, 72, 4985–4995. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, K.P.; Pelargos, P.E.; Milton, C.K.; Peterson, J.E.G.; Bohnstedt, B. Cryptococcal Choroid Plexitis and Non-Communicating Hydrocephalus. Cureus 2020, 12, e8512. [Google Scholar] [CrossRef]
- Kumari, R.; Raval, M.; Dhun, A. Cryptococcal Choroid Plexitis: Rare Imaging Findings of Central Nervous System Cryptococcal Infection in an Immunocompetent Individual. Br. J. Radiol. 2010, 83, e14–e17. [Google Scholar] [CrossRef] [PubMed]
- Kovoor, J.M.E.; Mahadevan, A.; Narayan, J.P.; Govindappa, S.S.; Satishchandra, P.; Taly, A.V.; Shankar, S.K. Cryptococcal Choroid Plexitis as a Mass Lesion: MR Imaging and Histopathologic Correlation Case Report. Am. J. Neuroradiol. 2002, 23, 273–276. [Google Scholar]
- Hammoud, D.A.; Mahdi, E.; Panackal, A.A.; Wakim, P.; Sheikh, V.; Sereti, I.; Bielakova, B.; Bennett, J.E.; Williamson, P.R. Choroid Plexitis and Ependymitis by Magnetic Resonance Imaging Are Biomarkers of Neuronal Damage and Inflammation in HIV-Negative Cryptococcal Meningoencephalitis. Sci. Rep. 2017, 7, 9184. [Google Scholar] [CrossRef]
- Daneman, R.; Prat, A. The Blood–Brain Barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Calaruso, P.; Mody, C.H. Real-Time in Vivo Imaging of Fungal Migration to the Central Nervous System. Cell. Microbiol. 2012, 14, 1819–1827. [Google Scholar] [CrossRef] [PubMed]
- Kolaczkowska, E.; Kubes, P. Neutrophil Recruitment and Function in Health and Inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Li, S.S.; Zheng, C.; Jones, G.J.; Kim, K.S.; Zhou, H.; Kubes, P.; Mody, C.H. Real-Time Imaging of Trapping and Urease-Dependent Transmigration of Cryptococcus neoformans in Mouse Brain. J. Clin. Investig. 2010, 120, 1683–1693. [Google Scholar] [CrossRef] [PubMed]
- May, R.C.; Stone, N.R.H.; Wiesner, D.L.; Bicanic, T.; Nielsen, K. Cryptococcus: From Environmental Saprophyte to Global Pathogen. Nat. Rev. Microbiol. 2016, 14, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Strickland, A.B.; Shi, M. Mechanisms of Fungal Dissemination. Cell. Mol. Life Sci. 2021, 78, 3219–3238. [Google Scholar] [CrossRef]
- Feldmesser, M.; Kress, Y.; Novikoff, P.; Casadevall, A. Cryptococcus neoformans is a Facultative Intracellular Pathogen in Murine Pulmonary Infection. Infect. Immun. 2000, 68, 4225–4237. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.; Casadevall, A. Phagosome Extrusion and Host-Cell Survival after Cryptococcus neoformans Phagocytosis by Macrophages. Curr. Biol. 2006, 16, 2161–2165. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Croudace, J.E.; Lammas, D.A.; May, R.C. Expulsion of Live Pathogenic Yeast by Macrophages. Curr. Biol. 2006, 16, 2156–2160. [Google Scholar] [CrossRef]
- Nicola, A.M.; Robertson, E.J.; Albuquerque, P.; da Silveira Derengowski, L.; Casadevall, A. Nonlytic Exocytosis of Cryptococcus neoformans from Macrophages Occurs in Vivo and Is Influenced by Phagosomal PH. MBio 2011, 2, e00167-11. [Google Scholar] [CrossRef]
- Ma, H.; Croudace, J.E.; Lammas, D.A.; May, R.C. Direct Cell-to-Cell Spread of a Pathogenic Yeast. BMC Immunol. 2007, 8, 15. [Google Scholar] [CrossRef]
- Alvarez, M.; Casadevall, A. Cell-to-Cell Spread and Massive Vacuole Formation after Cryptococcus neoformans Infection of Murine Macrophages. BMC Immunol. 2007, 8, 16. [Google Scholar] [CrossRef]
- Chrétien, F.; Lortholary, O.; Kansau, I.; Neuville, S.; Gray, F.; Dromer, F. Pathogenesis of Cerebral Cryptococcus neoformans Infection after Fungemia. J. Infect. Dis. 2002, 186, 522–530. [Google Scholar] [CrossRef]
- Kaufman-Francis, K.; Djordjevic, J.T.; Juillard, P.G.; Lev, S.; Desmarini, D.; Grau, G.E.R.; Sorrell, T.C. The Early Innate Immune Response to, and Phagocyte-Dependent Entry of, Cryptococcus neoformans Map to the Perivascular Space of Cortical Post-Capillary Venules in Neurocryptococcosis. Am. J. Pathol. 2018, 188, 1653–1665. [Google Scholar] [CrossRef]
- Walsh, N.M.; Botts, M.R.; McDermott, A.J.; Ortiz, S.C.; Wüthrich, M.; Klein, B.; Hull, C.M. Infectious Particle Identity Determines Dissemination and Disease Outcome for the Inhaled Human Fungal Pathogen Cryptococcus. PLoS Pathog. 2019, 15, e1007777. [Google Scholar] [CrossRef]
- Kechichian, T.B.; Shea, J.; Del Poeta, M. Depletion of Alveolar Macrophages Decreases the Dissemination of a Glucosylceramide-Deficient Mutant of Cryptococcus neoformans in Immunodeficient Mice. Infect. Immun. 2007, 75, 4792–4798. [Google Scholar] [CrossRef] [PubMed]
- Charlier, C.; Nielsen, K.; Daou, S.; Brigitte, M.; Chretien, F.; Dromer, F. Evidence of a Role for Monocytes in Dissemination and Brain Invasion by Cryptococcus neoformans. Infect. Immun. 2009, 77, 120–127. [Google Scholar] [CrossRef]
- Santangelo, R.; Zoellner, H.; Sorrell, T.; Wilson, C.; Donald, C.; Djordjevic, J.; Shounan, Y.; Wright, L. Role of Extracellular Phospholipases and Mononuclear Phagocytes in Dissemination of Cryptococcosis in a Murine Model. Infect. Immun. 2004, 72, 2229–2239. [Google Scholar] [CrossRef]
- Santiago-Tirado, F.H.; Onken, M.D.; Cooper, J.A.; Klein, R.S.; Doering, T.L. Trojan Horse Transit Contributes to Blood-Brain Barrier Crossing of a Eukaryotic Pathogen. MBio 2017, 8, e02183-16. [Google Scholar] [CrossRef]
- Sorrell, T.C.; Juillard, P.G.; Djordjevic, J.T.; Kaufman-Francis, K.; Dietmann, A.; Milonig, A.; Combes, V.; Grau, G.E.R. Cryptococcal Transmigration across a Model Brain Blood-Barrier: Evidence of the Trojan Horse Mechanism and Differences between Cryptococcus neoformans var. grubii Strain H99 and Cryptococcus gattii Strain R265. Microbes Infect. 2016, 18, 57–67. [Google Scholar] [CrossRef]
- Sun, D.; Zhang, M.; Sun, P.; Liu, G.; Strickland, A.B.; Chen, Y.; Fu, Y.; Yosri, M.; Shi, M. VCAM1/VLA4 Interaction Mediates Ly6Clow Monocyte Recruitment to the Brain in a TNFR Signaling Dependent Manner during Fungal Infection. PLoS Pathog. 2020, 16, e1008361. [Google Scholar] [CrossRef] [PubMed]
- Panackal, A.A.; Wuest, S.C.; Lin, Y.C.; Wu, T.; Zhang, N.; Kosa, P.; Komori, M.; Blake, A.; Browne, S.K.; Rosen, L.B.; et al. Paradoxical Immune Responses in Non-HIV Cryptococcal Meningitis. PLoS Pathog. 2015, 11, e1004884. [Google Scholar] [CrossRef]
- Jarvis, J.N.; Meintjes, G.; Bicanic, T.; Buffa, V.; Hogan, L.; Mo, S.; Tomlinson, G.; Kropf, P.; Noursadeghi, M.; Harrison, T.S. Cerebrospinal Fluid Cytokine Profiles Predict Risk of Early Mortality and Immune Reconstitution Inflammatory Syndrome in HIV-Associated Cryptococcal Meningitis. PLoS Pathog. 2015, 11, e1004754. [Google Scholar] [CrossRef]
- Sabiiti, W.; Robertson, E.; Beale, M.A.; Johnston, S.A.; Brouwer, A.E.; Loyse, A.; Jarvis, J.N.; Gilbert, A.S.; Fisher, M.C.; Harrison, T.S.; et al. Efficient Phagocytosis and Laccase Activity Affect the Outcome of HIV-Associated Cryptococcosis. J Clin. Investig. 2014, 124, 2000–2008. [Google Scholar] [CrossRef]
- Li, H.; Han, X.; Du, W.; Meng, Y.; Li, Y.; Sun, T.; Liang, Q.; Li, C.; Suo, C.; Gao, X.; et al. Comparative miRNA Transcriptomics of Macaques and Mice Reveals MYOC Is an Inhibitor for Cryptococcus neoformans Invasion into the Brain. Emerg. Microbes Infect. 2022, 11, 1572–1585. [Google Scholar] [CrossRef]
- Jong, A.; Wu, C.H.; Chen, H.M.; Luo, F.; Kwon-Chung, K.J.; Chang, Y.C.; LaMunyon, C.W.; Plaas, A.; Huang, S.H. Identification and Characterization of CPS1 as a Hyaluronic Acid Synthase Contributing to the Pathogenesis of Cryptococcus neoformans Infection. Eukaryot. Cell 2007, 6, 1486–1496. [Google Scholar] [CrossRef] [PubMed]
- Jong, A.; Wu, C.H.; Shackleford, G.M.; Kwon-Chung, K.J.; Chang, Y.C.; Chen, H.M.; Ouyang, Y.; Huang, S.H. Involvement of Human CD44 during Cryptococcus neoformans Infection of Brain Microvascular Endothelial Cells. Cell. Microbiol. 2008, 10, 1313–1326. [Google Scholar] [CrossRef]
- Jong, A.; Wu, C.H.; Prasadarao, N.V.; Kwon-Chung, K.J.; Chang, Y.C.; Ouyang, Y.; Shackleford, G.M.; Huang, S.H. Invasion of Cryptococcus neoformans into Human Brain Microvascular Endothelial Cells Requires Protein Kinase C-αActivation. Cell. Microbiol. 2008, 10, 1854–1865. [Google Scholar] [CrossRef] [PubMed]
- Aaron, P.A.; Jamklang, M.; Uhrig, J.P.; Gelli, A. The Blood–Brain Barrier Internalises Cryptococcus neoformans via the EphA2-Tyrosine Kinase Receptor. Cell. Microbiol. 2018, 20, e12811. [Google Scholar] [CrossRef] [PubMed]
- Jong, A.; Wu, C.H.; Gonzales-Gomez, I.; Kwon-Chung, K.J.; Chang, Y.C.; Tseng, H.K.; Cho, W.L.; Huang, S.H. Hyaluronic Acid Receptor CD44 Deficiency Is Associated with Decreased Cryptococcus neoformans Brain Infection. J. Biol. Chem. 2012, 287, 15298–15306. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.B.; Kim, J.C.; Wang, Y.; Toffaletti, D.L.; Eugenin, E.; Perfect, J.R.; Kim, K.J.; Xue, C. Brain Inositol Is a Novel Stimulator for Promoting Cryptococcus Penetration of the Blood-Brain Barrier. PLoS Pathog. 2013, 9, e1003247. [Google Scholar] [CrossRef] [PubMed]
- Maruvada, R.; Zhu, L.; Pearce, D.; Zheng, Y.; Perfect, J.; Kwon-Chung, K.J.; Kim, K.S. Cryptococcus neoformans Phospholipase B1 Activates Host Cell Rac1 for Traversal across the Blood-Brain Barrier. Cell. Microbiol. 2012, 14, 1544–1553. [Google Scholar] [CrossRef]
- Vu, K.; Tham, R.; Uhrig, J.P.; Thompson, G.R.; Na Pombejra, S.; Jamklang, M.; Bautos, J.M.; Gelli, A. Invasion of the Central Nervous System by Cryptococcus neoformans Requires a Secreted Fungal Metalloprotease. MBio 2014, 5, e01101-14. [Google Scholar] [CrossRef]
- Na Pombejra, S.; Salemi, M.; Phinney, B.S.; Gelli, A. The Metalloprotease, Mpr1, Engages AnnexinA2 to Promote the Transcytosis of Fungal Cells across the Blood-Brain Barrier. Front. Cell. Infect. Microbiol. 2017, 7, 296. [Google Scholar] [CrossRef]
- Chen, Y.; Li, C.; Sun, D.; Strickland, A.B.; Liu, G.; Shi, M. Quantitative Analysis Reveals Internalisation of Cryptococcus neoformans by Brain Endothelial Cells in Vivo. Cell. Microbiol. 2021, 23, e13330. [Google Scholar] [CrossRef]
- Vu, K.; Eigenheer, R.A.; Phinney, B.S.; Gelli, A. Cryptococcus neoformans Promotes Its Transmigration into the Central Nervous System by Inducing Molecular and Cellular Changes in Brain Endothelial Cells. Infect. Immun. 2013, 81, 3139–3147. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.H.M.; Stins, M.F.; Huang, S.H.; Chen, Y.H.; Kwon-Chung, K.J.; Chang, Y.; Kim, K.S.; Suzuki, K.; Jong, A.Y. Cryptococcus neoformans Induces Alterations in the Cytoskeleton of Human Brain Microvascular Endothelial Cells. J. Med. Microbiol. 2003, 52, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.S.; Filler, S.G.; Alcouloumre, M.S.; Kozel, T.R.; Edwards, J.E.; Ghannoum, M.A. Adherence to and Damage of Endothelial Cells by Cryptococcus neoformans in Vitro: Role of the Capsule. Infect. Immun. 1995, 63, 4368–4374. [Google Scholar] [CrossRef]
- Cox, G.M.; Mukherjee, J.; Cole, G.T.; Casadevall, A.; Perfect, J.R. Urease as a Virulence Factor in Experimental Cryptococcosis. Infect. Immun. 2000, 68, 443–448. [Google Scholar] [CrossRef]
- Olszewski, M.A.; Noverr, M.C.; Chen, G.H.; Toews, G.B.; Cox, G.M.; Perfect, J.R.; Huffnagle, G.B. Urease Expression by Cryptococcus neoformans Promotes Microvascular Sequestration, Thereby Enhancing Central Nervous System Invasion. Am. J. Pathol. 2004, 164, 1761–1771. [Google Scholar] [CrossRef]
- Singh, A.; Panting, R.J.; Varma, A.; Saijo, T.; Waldron, K.J.; Jong, A.; Ngamskulrungroj, P.; Chang, Y.C.; Rutherford, J.C.; Kwon-Chung, K.J. Factors Required for Activation of Urease as a Virulence Determinant in Cryptococcus neoformans. MBio 2013, 4, e00220-13. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.Y.; Zhu, H.M.; Wu, J.H.; Wen, H.; Liu, C.J. Increased Permeability of Blood-Brain Barrier Is Mediated by Serine Protease during Cryptococcus Meningitis. J. Int. Med. Res. 2014, 42, 85–92. [Google Scholar] [CrossRef]
- Rodrigues, M.L.; Dos Reis, F.C.G.; Puccia, R.; Travassos, L.R.; Alviano, C.S. Cleavage of Human Fibronectin and Other Basement Membrane-Associated Proteins by a Cryptococcus neoformans Serine Proteinase. Microb. Pathog. 2003, 34, 65–71. [Google Scholar] [CrossRef]
- Stie, J.; Fox, D. Blood-Brain Barrier Invasion by Cryptococcus neoformans Is Enhanced by Functional Interactions with Plasmin. Microbiology 2012, 158, 240–258. [Google Scholar] [CrossRef]
- Stie, J.; Bruni, G.; Fox, D. Surface-Associated Plasminogen Binding of Cryptococcus neoformans Promotes Extracellular Matrix Invasion. PLoS ONE 2009, 4, e5780. [Google Scholar] [CrossRef]
- Ginhoux, F.; Guilliams, M. Tissue-Resident Macrophage Ontogeny and Homeostasis. Immunity 2016, 44, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Gomez Perdiguero, E.; Klapproth, K.; Schulz, C.; Busch, K.; Azzoni, E.; Crozet, L.; Garner, H.; Trouillet, C.; De Bruijn, M.F.; Geissmann, F.; et al. Tissue-Resident Macrophages Originate from Yolk-Sac-Derived Erythro-Myeloid Progenitors. Nature 2015, 518, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Guilliams, M.; Thierry, G.R.; Bonnardel, J.; Bajenoff, M. Establishment and Maintenance of the Macrophage Niche. Immunity 2020, 52, 434–451. [Google Scholar] [CrossRef]
- Nimmerjahn, A.; Kirchhoff, F.; Helmchen, F. Resting Microglial Cells Are Highly Dynamic Surveillants of Brain Parenchyma in Vivo. Science 2005, 308, 1314–1318. [Google Scholar] [CrossRef] [PubMed]
- Kreutzberg, G.W. Microglia: A Sensor for Pathological Events in the CNS. Trends Neurosci. 1996, 19, 312–318. [Google Scholar] [CrossRef]
- Kettenmann, H.; Hanisch, U.K.; Noda, M.; Verkhratsky, A. Physiology of Microglia. Physiol. Rev. 2011, 91, 461–553. [Google Scholar] [CrossRef]
- Nau, R.; Ribes, S.; Djukic, M.; Eiffert, H. Strategies to Increase the Activity of Microglia as Efficient Protectors of the Brain against Infections. Front. Cell. Neurosci. 2014, 8, 138. [Google Scholar] [CrossRef]
- Chhatbar, C.; Prinz, M. The Roles of Microglia in Viral Encephalitis: From Sensome to Therapeutic Targeting. Cell. Mol. Immunol. 2021, 18, 250–258. [Google Scholar] [CrossRef]
- Rock, R.B.; Gekker, G.; Hu, S.; Sheng, W.S.; Cheeran, M.; Lokensgard, J.R.; Peterson, P.K. Role of Microglia in Central Nervous System Infections. Clin. Microbiol. Rev. 2004, 17, 942. [Google Scholar] [CrossRef] [PubMed]
- Waltl, I.; Kalinke, U. Beneficial and Detrimental Functions of Microglia during Viral Encephalitis. Trends Neurosci. 2022, 45, 158–170. [Google Scholar] [CrossRef]
- Drummond, R.A.; Swamydas, M.; Oikonomou, V.; Zhai, B.; Dambuza, I.M.; Schaefer, B.C.; Bohrer, A.C.; Mayer-Barber, K.D.; Lira, S.A.; Iwakura, Y.; et al. CARD9+ Microglia Promote Antifungal Immunity via IL-1β- and CXCL1-Mediated Neutrophil Recruitment. Nat. Immunol. 2019, 20, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Tanaka, S.; Cox, D.; Lee, S.C. Fcγ Receptor Signaling in Primary Human Microglia: Differential Roles of PI-3K and Ras/ERK MAPK Pathways in Phagocytosis and Chemokine Induction. J. Leukoc. Biol. 2004, 75, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Redlich, S.; Ribes, S.; Schütze, S.; Eiffert, H.; Nau, R. Toll-Like Receptor Stimulation Increases Phagocytosis of Cryptococcus neoformans by Microglial Cells. J. Neuroinflamm. 2013, 10, 841. [Google Scholar] [CrossRef]
- Lipovsky, M.M.; Gekker, G.; Anderson, W.R.; Molitor, T.W.; Peterson, P.K.; Hoepelman, A.I.M. Phagocytosis of Nonopsonized Cryptococcus neoformans by Swine Microglia Involves CD14 Receptors. Clin. Immunol. Immunopathol. 1997, 84, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Kress, Y.; Dickson, D.W.; Casadevall, A. Human Microglia Mediate Anti-Cryptococcus neoformans Activity in the Presence of Specific Antibody. J. Neuroimmunol. 1995, 62, 43–52. [Google Scholar] [CrossRef]
- Barluzzi, R.; Brozzetti, A.; Delfino, D.; Bistoni, F.; Blasi, E. Role of the Capsule in Microglial Cell—Cryptococcus neoformans Interaction: Impairment of Antifungal Activity but Not of Secretory Functions. Med. Mycol. 1998, 36, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Casadevall, A.; Dickson, D.W. Immunohistochemical Localization of Capsular Polysaccharide Antigen in the Central Nervous System Cells in Cryptococcal Meningoencephalitis. Am. J. Pathol. 1996, 148, 1267–1274. [Google Scholar]
- Goldman, D.; Song, X.; Kitai, R.; Casadevall, A.; Zhao, M.L.; Lee, S.C. Cryptococcus neoformans Induces Macrophage Inflammatory Protein 1α (MIP-1α) and MIP-1β in Human Microglia: Role of Specific Antibody and Soluble Capsular Polysaccharide. Infect. Immun. 2001, 69, 1808–1815. [Google Scholar] [CrossRef]
- Blasi, E.; Barluzzi, R.; Mazzolla, R.; Tancini, B.; Saleppico, S.; Puliti, M.; Pitzurra, L.; Bistoni, F. Role of Nitric Oxide and Melanogenesis in the Accomplishment of Anticryptococcal Activity by the BV-2 Microglial Cell Line. J Neuroimmunol. 1995, 58, 111–116. [Google Scholar] [CrossRef]
- Adami, C.; Sorci, G.; Blasi, E.; Agneletti, A.L.; Bistoni, F.; Donato, R. S100b Expression in and Effects on Microglia. Glia 2001, 33, 131–142. [Google Scholar] [CrossRef]
- Saleppico, S.; Boelaert, J.R.; Salè, F.O.; Mazzolla, R.; Morucci, P.; Bistoni, F.; Blasi, E. Differential Effects of Iron Load on Basal and Interferon-Gamma plus Lipopolysaccharide Enhance Anticryptococcal Activity by the Murine Microglial Cell Line BV-2. J. Neuroimmunol. 1999, 93, 102–107. [Google Scholar] [CrossRef]
- Lipovsky, M.M.; Juliana, A.E.; Gekker, G.; Hu, S.; Hoepelman, A.I.M.; Peterson, P.K. Effect of Cytokines on Anticryptococcal Activity of Human Microglial Cells. Clin. Diagn. Lab. Immunol. 1998, 5, 410–411. [Google Scholar] [CrossRef]
- Lee, S.C.; Kress, Y.; Zhao, M.L.; Dickson, D.W.; Casadevall, A. Cryptococcus neoformans Survive and Replicate in Human Microglia. Lab. Investig. 1995, 73, 871–879. [Google Scholar]
- Aguirre, K.; Miller, S. MHC Class II-Positive Perivascular Microglial Cells Mediate Resistance to Cryptococcus neoformans Brain Infection. Glia 2002, 39, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Gault, R.A.; Kozel, T.R.; Murphy, W.J. Immunomodulation with CD40 Stimulation and Interleukin-2 Protects Mice from Disseminated Cryptococcosis. Infect. Immun. 2006, 74, 2161–2168. [Google Scholar] [CrossRef]
- Zhou, Q.; Gault, R.A.; Kozel, T.R.; Murphy, W.J. Protection from Direct Cerebral Cryptococcus Infection by Interferon-γ-Dependent Activation of Microglial Cells. J. Immunol. 2007, 178, 5753–5761. [Google Scholar] [CrossRef]
- Neal, L.M.; Xing, E.; Xu, J.; Kolbe, J.L.; Osterholzer, J.J.; Segal, B.M.; Williamson, P.R.; Olszewski, M.A. CD4+ T Cells Orchestrate Lethal Immune Pathology despite Fungal Clearance during Cryptococcus neoformans Meningoencephalitis. MBio 2017, 8, e01415-17. [Google Scholar] [CrossRef]
- Shi, C.; Pamer, E.G. Monocyte Recruitment during Infection and Inflammation. Nat. Rev. Immunol. 2011, 11, 762–774. [Google Scholar] [CrossRef] [PubMed]
- Geissmann, F.; Jung, S.; Littman, D.R. Blood Monocytes Consist of Two Principal Subsets with Distinct Migratory Properties. Immunity 2003, 19, 71–82. [Google Scholar] [CrossRef]
- Sunderkötter, C.; Nikolic, T.; Dillon, M.J.; van Rooijen, N.; Stehling, M.; Drevets, D.A.; Leenen, P.J.M. Subpopulations of Mouse Blood Monocytes Differ in Maturation Stage and Inflammatory Response. J. Immunol. 2004, 172, 4410–4417. [Google Scholar] [CrossRef]
- Serbina, N.V.; Pamer, E.G. Monocyte Emigration from Bone Marrow during Bacterial Infection Requires Signals Mediated by Chemokine Receptor CCR2. Nat. Immunol. 2006, 7, 311–317. [Google Scholar] [CrossRef]
- Espinosa, V.; Jhingran, A.; Dutta, O.; Kasahara, S.; Donnelly, R.; Du, P.; Rosenfeld, J.; Leiner, I.; Chen, C.C.; Ron, Y.; et al. Inflammatory Monocytes Orchestrate Innate Antifungal Immunity in the Lung. PLoS Pathog. 2014, 10, e1003940. [Google Scholar] [CrossRef] [PubMed]
- Hohl, T.M.; Rivera, A.; Lipuma, L.; Gallegos, A.; Shi, C.; Mack, M.; Pamer, E.G. Inflammatory Monocytes Facilitate Adaptive CD4 T Cell Responses during Respiratory Fungal Infection. Cell Host Microbe 2009, 6, 470–481. [Google Scholar] [CrossRef]
- Ngo, L.Y.; Kasahara, S.; Kumasaka, D.K.; Knoblaugh, S.E.; Jhingran, A.; Hohl, T.M. Inflammatory Monocytes Mediate Early and Organ-Specific Innate Defense during Systemic Candidiasis. J. Infect. Dis. 2014, 209, 109–119. [Google Scholar] [CrossRef]
- Wüthrich, M.; Ersland, K.; Sullivan, T.; Galles, K.; Klein, B.S. Fungi Subvert Vaccine T Cell Priming at the Respiratory Mucosa by Preventing Chemokine-Induced Influx of Inflammatory Monocytes. Immunity 2012, 36, 680–692. [Google Scholar] [CrossRef]
- Szymczak, W.A.; Deepe, G.S. The CCL7-CCL2-CCR2 Axis Regulates IL-4 Production in Lungs and Fungal Immunity. J. Immunol. 2009, 183, 1964–1974. [Google Scholar] [CrossRef] [PubMed]
- Traynor, T.R.; Herring, A.C.; Dorf, M.E.; Kuziel, W.A.; Toews, G.B.; Huffnagle, G.B. Differential Roles of CC Chemokine Ligand 2/Monocyte Chemotactic Protein-1 and CCR2 in the Development of T1 Immunity. J. Immunol. 2002, 168, 4659–4666. [Google Scholar] [CrossRef] [PubMed]
- Traynor, T.R.; Kuziel, W.A.; Toews, G.B.; Huffnagle, G.B. CCR2 Expression Determines T1 versus T2 Polarization during Pulmonary Cryptococcus neoformans Infection. J. Immunol. 2000, 164, 2021–2027. [Google Scholar] [CrossRef]
- Osterholzer, J.J.; Chen, G.H.; Olszewski, M.A.; Zhang, Y.M.; Curtis, J.L.; Huffnagle, G.B.; Toews, G.B. Chemokine Receptor 2-Mediated Accumulation of Fungicidal Exudate Macrophages in Mice That Clear Cryptococcal Lung Infection. Am. J. Pathol. 2011, 178, 198–211. [Google Scholar] [CrossRef]
- Osterholzer, J.J.; Curtis, J.L.; Polak, T.; Ames, T.; Chen, G.H.; McDonald, R.; Huffnagle, G.B.; Toews, G.B. CCR2 Mediates Conventional Dendritic Cell Recruitment and the Formation of Bronchovascular Mononuclear Cell Infiltrates in the Lungs of Mice Infected with Cryptococcus neoformans. J. Immunol. 2008, 181, 610–620. [Google Scholar] [CrossRef]
- Osterholzer, J.J.; Milam, J.E.; Chen, G.H.; Toews, G.B.; Huffnagle, G.B.; Olszewski, M.A. Role of Dendritic Cells and Alveolar Macrophages in Regulating Early Host Defense against Pulmonary Infection with Cryptococcus neoformans. Infect. Immun. 2009, 77, 3749–3758. [Google Scholar] [CrossRef] [PubMed]
- Hardison, S.E.; Herrera, G.; Young, M.L.; Hole, C.R.; Wozniak, K.L.; Wormley, F.L. Protective Immunity against Pulmonary Cryptococcosis Is Associated with STAT1-Mediated Classical Macrophage Activation. J. Immunol. 2012, 189, 4060–4068. [Google Scholar] [CrossRef] [PubMed]
- Leopold Wager, C.M.; Hole, C.R.; Campuzano, A.; Castro-Lopez, N.; Cai, H.; Van Dyke, M.C.; Wozniak, K.L.; Wang, Y.; Wormley, F.L. IFN-γ Immune Priming of Macrophages in Vivo Induces Prolonged STAT1 Binding and Protection against Cryptococcus neoformans. PLoS Pathog. 2018, 14, e1007358. [Google Scholar] [CrossRef] [PubMed]
- Leopold Wager, C.M.; Hole, C.R.; Wozniak, K.L.; Olszewski, M.A.; Mueller, M.; Wormley, F.L. STAT1 Signaling within Macrophages Is Required for Antifungal Activity against Cryptococcus neoformans. Infect Immun. 2015, 83, 4513–4527. [Google Scholar] [CrossRef] [PubMed]
- Masso-Silva, J.; Espinosa, V.; Liu, T.B.; Wang, Y.; Xue, C.; Rivera, A. The F-Box Protein Fbp1 Shapes the Immunogenic Potential of Cryptococcus neoformans. MBio 2018, 9, e01828-17. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, D.L.; Specht, C.A.; Lee, C.K.; Smith, K.D.; Mukaremera, L.; Lee, S.T.; Lee, C.G.; Elias, J.A.; Nielsen, J.N.; Boulware, D.R.; et al. Chitin Recognition via Chitotriosidase Promotes Pathologic Type-2 Helper T Cell Responses to Cryptococcal Infection. PLoS Pathog. 2015, 11, e1004701. [Google Scholar] [CrossRef]
- Heung, L.J.; Hohl, T.M. Inflammatory Monocytes Are Detrimental to the Host Immune Response during Acute Infection with Cryptococcus neoformans. PLoS Pathog. 2019, 15, e1007627. [Google Scholar] [CrossRef]
- Williams, J.L.; Holman, D.W.; Klein, R.S. Chemokines in the Balance: Maintenance of Homeostasis and Protection at CNS Barriers. Front. Cell. Neurosci. 2014, 8, 154. [Google Scholar] [CrossRef]
- Getts, D.R.; Terry, R.L.; Getts, M.T.; Müller, M.; Rana, S.; Shrestha, B.; Radford, J.; Van Rooijen, N.; Campbell, I.L.; King, N.J.C. Ly6c+ “Inflammatory Monocytes” are Microglial Precursors Recruited in a Pathogenic Manner in West Nile Virus Encephalitis. J. Exp. Med. 2008, 205, 2319–2337. [Google Scholar] [CrossRef]
- Huffnagle, G.B.; McNeil, L.K. Dissemination of C. neoformans to the Central Nervous System: Role of Chemokines, Th1 Immunity and Leukocyte Recruitment. J. Neurovirol. 1999, 5, 76–81. [Google Scholar] [CrossRef]
- Xu, J.; Ganguly, A.; Zhao, J.; Ivey, M.; Lopez, R.; Osterholzer, J.J.; Cho, C.S.; Olszewski, M.A. CCR2 Signaling Promotes Brain Infiltration of Inflammatory Monocytes and Contributes to Neuropathology during Cryptococcal Meningoencephalitis. MBio 2021, 12, e01076-21. [Google Scholar] [CrossRef] [PubMed]
- Sedgwick, A.J.; Ghazanfari, N.; Constantinescu, P.; Mantamadiotis, T.; Barrow, A.D. The Role of NK Cells and Innate Lymphoid Cells in Brain Cancer. Front. Immunol. 2020, 11, 1549. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, N.; Murphy, J.W. In Vitro Binding of Natural Killer Cells to Cryptococcus neoformans Targets. Infect. Immun. 1985, 50, 50–57. [Google Scholar] [CrossRef]
- Levitz, S.M.; Dupont, M.P.; Smail, E.H. Direct Activity of Human T Lymphocytes and Natural Killer Cells against Cryptococcus neoformans. Infect. Immun. 1994, 62, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Hidore, M.R.; Nabavi, N.; Sonleitner, F.; Murphy, J.W. Murine Natural Killer Cells Are Fungicidal to Cryptococcus neoformans. Infect. Immun. 1991, 59, 1747–1754. [Google Scholar] [CrossRef]
- Murphy, J.W.; Hidore, M.R.; Wong, S.C. Direct Interactions of Human Lymphocytes with the Yeast-Like Organism, Cryptococcus neoformans. J. Clin. Investig. 1993, 91, 1553–1566. [Google Scholar] [CrossRef]
- Hidore, M.R.; Mislan, T.W.; Murphy, J.W. Responses of Murine Natural Killer Cells to Binding of the Fungal Target Cryptococcus neoformans. Infect. Immun. 1991, 59, 1489–1499. [Google Scholar] [CrossRef]
- Ma, L.L.; Wang, C.L.C.; Neely, G.G.; Epelman, S.; Krensky, A.M.; Mody, C.H. NK Cells Use Perforin Rather than Granulysin for Anticryptococcal Activity. J. Immunol. 2004, 173, 3357–3365. [Google Scholar] [CrossRef]
- Wiseman, J.C.D.; Ma, L.L.; Marr, K.J.; Jones, G.J.; Mody, C.H. Perforin-Dependent Cryptococcal Microbicidal Activity in NK Cells Requires PI3K-Dependent ERK1/2 Signaling. J. Immunol. 2007, 178, 6456–6464. [Google Scholar] [CrossRef]
- Li, S.S.; Ogbomo, H.; Mansour, M.K.; Xiang, R.F.; Szabo, L.; Munro, F.; Mukherjee, P.; Mariuzza, R.A.; Amrein, M.; Vyas, J.M.; et al. Identification of the Fungal Ligand Triggering Cytotoxic PRR-Mediated NK Cell Killing of Cryptococcus and Candida. Nat. Commun. 2018, 9, 751. [Google Scholar] [CrossRef]
- Li, S.S.; Kyei, S.K.; Timm-Mccann, M.; Ogbomo, H.; Jones, G.J.; Shi, M.; Xiang, R.F.; Oykhman, P.; Huston, S.M.; Islam, A.; et al. The NK Receptor NKp30 Mediates Direct Fungal Recognition and Killing and Is Diminished in NK Cells from HIV-Infected Patients. Cell Host Microbe 2013, 14, 387–397. [Google Scholar] [CrossRef]
- Zhang, T.; Kawakami, K.; Qureshi, M.H.; Okamura, H.; Kurimoto, M.; Saito, A. Interleukin-12 (IL-12) and IL-18 Synergistically Induce the Fungicidal Activity of Murine Peritoneal Exudate Cells against Cryptococcus neoformans through Production of Gamma Interferon by Natural Killer Cells. Infect. Immun. 1997, 65, 3594–3599. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, K.; Koguchi, Y.; Qureshi, M.H.; Miyazato, A.; Yara, S.; Kinjo, Y.; Iwakura, Y.; Takeda, K.; Akira, S.; Kurimoto, M.; et al. IL-18 Contributes to Host Resistance against Infection with Cryptococcus neoformans in Mice with Defective IL-12 Synthesis through Induction of IFN-γ Production by NK Cells. J. Immunol. 2000, 165, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, K.; Koguchi, Y.; Qureshi, M.H.; Yara, S.; Kinjo, Y.; Uezu, K.; Saito, A. NK Cells Eliminate Cryptococcus neoformans by Potentiating the Fungicidal Activity of Macrophages Rather than by Directly Killing Them upon Stimulation with IL-12 and IL-18. Microbiol. Immunol. 2000, 44, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Mars, L.T.; Mas, M.; Beaudoin, L.; Bauer, J.; Leite-de-Moraes, M.; Lehuen, A.; Bureau, J.F.; Liblau, R.S. Invariant NKT Cells Regulate the CD8 T Cell Response during Theiler’s Virus Infection. PLoS ONE 2014, 9, e87717. [Google Scholar] [CrossRef]
- Mars, L.T.; Gautron, A.-S.; Novak, J.; Beaudoin, L.; Diana, J.; Liblau, R.S.; Lehuen, A. Invariant NKT Cells Regulate Experimental Autoimmune Encephalomyelitis and Infiltrate the Central Nervous System in a CD1d-Independent Manner. J. Immunol. 2008, 181, 2321–2329. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, K.; Kinjo, Y.; Uezu, K.; Yara, S.; Miyagi, K.; Koguchi, Y.; Nakayama, T.; Taniguchi, M.; Saito, A. Monocyte Chemoattractant Protein-1-Dependent Increase of Vα14 NKT Cells in Lungs and Their Roles in Th1 Response and Host Defense in Cryptococcal Infection. J. Immunol. 2001, 167, 6525–6532. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, K.; Kinjo, Y.; Yara, S.; Uezu, K.; Koguchi, Y.; Tohyama, M.; Azuma, M.; Takeda, K.; Akira, S.; Saito, A. Enhanced Gamma Interferon Production through Activation of Vα14+ Natural Killer T Cells by α-Galactosylceramide in Interleukin-18-Deficient Mice with Systemic Cryptococcosis. Infect. Immun. 2001, 69, 6643–6650. [Google Scholar] [CrossRef] [PubMed]
- Blackstock, R.; Murphy, J.W. Age-Related Resistance of C57BL/6 Mice to Cryptococcus neoformans Is Dependent on Maturation of NKT Cells. Infect. Immun. 2004, 72, 5175–5180. [Google Scholar] [CrossRef] [PubMed]
- Mirza, S.A.; Phelan, M.; Rimland, D.; Graviss, E.; Hamill, R.; Brandt, M.E.; Gardner, T.; Sattah, M.; De Leon, G.P.; Baughman, W.; et al. The Changing Epidemiology of Cryptococcosis: An Update from Population-Based Active Surveillance in 2 Large Metropolitan Areas, 1992–2000. Clin. Infect. Dis. 2003, 36, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Huffnagle, G.B.; Yates, J.L.; Lipscomb, M.F. Immunity to a Pulmonary Cryptococcus neoformans Infection Requires Both CD4+ and CD8+ T Cells. J. Exp. Med. 1991, 173, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Huffnagle, G.B.; Lipscomb, M.F.; Lovchik, J.A.; Hoag, K.A.; Street, N.E. The Role of CD4+ and CD8+ T Cells in the Protective Inflammatory Response to a Pulmonary Cryptococcal Infection. J. Leukoc. Biol. 1994, 55, 35–42. [Google Scholar] [CrossRef]
- Hill, J.O.; Harmsen, A.G. Intrapulmonary Growth and Dissemination of an Avirulent Strain of Cryptococcus neoformans in Mice Depleted of CD4+ or CD8+ T Cells. J. Exp. Med. 1991, 173, 755–758. [Google Scholar] [CrossRef] [PubMed]
- Mody, C.H.; Chen, G.H.; Jackson, C.; Curtis, J.L.; Toews, G.B. Depletion of Murine CD8+ T Cells in Vivo Decreases Pulmonary Clearance of a Moderately Virulent Strain of Cryptococcus neoformans. J. Lab. Clin. Med. 1993, 121, 765–773. [Google Scholar]
- Lindell, D.M.; Moore, T.A.; McDonald, R.A.; Toews, G.B.; Huffnagle, G.B. Generation of Antifungal Effector CD8+ T Cells in the Absence of CD4+ T Cells during Cryptococcus neoformans Infection. J. Immunol. 2005, 174, 7920–7928. [Google Scholar] [CrossRef]
- Xu, J.; Neal, L.M.; Ganguly, A.; Kolbe, J.L.; Hargarten, J.C.; Elsegeiny, W.; Hollingsworth, C.; He, X.; Ivey, M.; Lopez, R.; et al. Chemokine Receptor CXCR3 Is Required for Lethal Brain Pathology but Not Pathogen Clearance during Cryptococcal Meningoencephalitis. Sci. Adv. 2020, 6, 2502–2519. [Google Scholar] [CrossRef]
- Buchanan, K.L.; Doyle, H.A. Requirement for CD4+ T Lymphocytes in Host Resistance against Cryptococcus neoformans in the Central Nervous System of Immunized Mice. Infect. Immun. 2000, 68, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Uicker, W.; McCracken, J.P.; Buchanan, K.L. Role of CD4+ T Cells in a Protective Immune Response against Cryptococcus neoformans in the Central Nervous System. Med. Mycol. 2006, 44, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.L.; Spurrell, J.C.L.; Wang, J.F.; Neely, G.G.; Epelman, S.; Krensky, A.M.; Mody, C.H. CD8 T Cell-Mediated Killing of Cryptococcus neoformans Requires Granulysin and Is Dependent on CD4 T Cells and IL-15. J. Immunol. 2002, 169, 5787–5795. [Google Scholar] [CrossRef]
- Chun, F.Z.; Ling, L.M.; Jones, G.J.; Gill, M.J.; Krensky, A.M.; Kubes, P.; Mody, C.H. Cytotoxic CD4+ T Cells Use Granulysin to Kill Cryptococcus neoformans, and Activation of This Pathway Is Defective in HIV Patients. Blood 2007, 109, 2049–2057. [Google Scholar] [CrossRef]
- Zheng, C.F.; Jones, G.J.; Shi, M.; Wiseman, J.C.D.; Marr, K.J.; Berenger, B.M.; Huston, S.M.; Gill, M.J.; Krensky, A.M.; Kubes, P.; et al. Late Expression of Granulysin by Microbicidal CD4+ T Cells Requires PI3K- and STAT5-Dependent Expression of IL-2Rβ That Is Defective in HIV-Infected Patients. J. Immunol. 2008, 180, 7221–7229. [Google Scholar] [CrossRef]
- Chen, G.H.; McDonald, R.A.; Wells, J.C.; Huffnagle, G.B.; Lukacs, N.W.; Toews, G.B. The Gamma Interferon Receptor Is Required for the Protective Pulmonary Inflammatory Response to Cryptococcus neoformans. Infect. Immun. 2005, 73, 1788–1796. [Google Scholar] [CrossRef]
- Hardison, S.E.; Ravi, S.; Wozniak, K.L.; Young, M.L.; Olszewski, M.A.; Wormley, F.L. Pulmonary Infection with an Interferon-γ-Producing Cryptococcus neoformans Strain Results in Classical Macrophage Activation and Protection. Am. J. Pathol. 2010, 176, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, K.; Kohno, S.; Kadota, J.I.; Tohyama, M.; Teruya, K.; Kudeken, N.; Saito, A.; Hara, K. T Cell-Dependent Activation of Macrophages and Enhancement of Their Phagocytic Activity in the Lungs of Mice Inoculated with Heat-Killed Cryptococcus neoformans: Involvement of IFN-γ and Its Protective Effect against Cryptococcal Infection. Microbiol. Immunol. 1995, 39, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, Y.; Arora, S.; Erb-Downward, J.R.; McDonald, R.A.; Toews, G.B.; Huffnagle, G.B. Distinct Roles for IL-4 and IL-10 in Regulating T2 Immunity during Allergic Bronchopulmonary Mycosis. J. Immunol. 2005, 174, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Müller, U.; Stenzel, W.; Köhler, G.; Werner, C.; Polte, T.; Hansen, G.; Schütze, N.; Straubinger, R.K.; Blessing, M.; McKenzie, A.N.J.; et al. IL-13 Induces Disease-Promoting Type 2 Cytokines, Alternatively Activated Macrophages and Allergic Inflammation during Pulmonary Infection of Mice with Cryptococcus neoformans. J. Immunol. 2007, 179, 5367–5377. [Google Scholar] [CrossRef]
- Stenzel, W.; Müller, U.; Köhler, G.; Heppner, F.L.; Blessing, M.; McKenzie, A.N.J.; Brombacher, F.; Alber, G. IL-4/IL-13-Dependent Alternative Activation of Macrophages but Not Microglial Cells Is Associated with Uncontrolled Cerebral Cryptococcosis. Am. J. Pathol. 2009, 174, 486–496. [Google Scholar] [CrossRef]
- Huffnagle, G.B.; Boyd, M.B.; Street, N.E.; Lipscomb, M.F. IL-5 Is Required for Eosinophil Recruitment, Crystal Deposition, and Mononuclear Cell Recruitment during a Pulmonary Cryptococcus neoformans Infection in Genetically Susceptible Mice (C57BL/6). J. Immunol. 1998, 160, 2393–2400. [Google Scholar] [PubMed]
- Mody, C.H.; Tyler, C.L.; Sitrin, R.G.; Jackson, C.; Toews, G.B. Interferon-γ Activates Rat Alveolar Macrophages for Anticryptococcal Activity. Am. J. Respir. Cell Mol. Biol. 1991, 5, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Joly, V.; Saint Julien, L.; Carbon, C.; Yeni, P. In Vivo Activity of Interferon-γ in Combination with Amphotericin B in the Treatment of Experimental Cryptococcosis. J. Infect. Dis. 1994, 170, 1331–1334. [Google Scholar] [CrossRef]
- Flesch, I.E.; Schwamberger, G.; Kaufmann, S.H. Fungicidal Activity of IFN-Gamma-Activated Macrophages. Extracellular Killing of Cryptococcus neoformans. J. Immunol. 1989, 142, 3219–3224. [Google Scholar]
- Huffnagle, G.B. Role of Cytokines in T Cell Immunity to a Pulmonary Cryptococcus neoformans Infection. Neurosignals 1996, 5, 215–222. [Google Scholar] [CrossRef]
- Wormley, F.L.; Perfect, J.R.; Steele, C.; Cox, G.M. Protection against Cryptococcosis by Using a Murine Gamma Interferon-Producing Cryptococcus neoformans Strain. Infect. Immun. 2007, 75, 1453–1462. [Google Scholar] [CrossRef]
- Uicker, W.C.; Doyle, H.A.; McCracken, J.P.; Langlois, M.; Buchanan, K.L. Cytokine and Chemokine Expression in the Central Nervous System Associated with Protective Cell-Mediated Immunity against Cryptococcus neoformans. Med. Mycol. 2005, 43, 27–38. [Google Scholar] [CrossRef]
- Aguirre, K.; Havell, E.A.; Gibson, G.W.; Johnson, L.L. Role of Tumor Necrosis Factor and Gamma Interferon in Acquired Resistance to Cryptococcus neoformans in the Central Nervous System of Mice. Infect. Immun. 1995, 63, 1725–1731. [Google Scholar] [CrossRef]
- Lee, S.C.; Dickson, D.W.; Brosnan, C.F.; Casadevall, A. Human Astrocytes Inhibit Cryptococcus neoformans Growth by a Nitric Oxide-Mediated Mechanism. J. Exp. Med. 1994, 180, 365–369. [Google Scholar] [CrossRef]
- Jarvis, J.N.; Casazza, J.P.; Stone, H.H.; Meintjes, G.; Lawn, S.D.; Levitz, S.M.; Harrison, T.S.; Koup, R.A. The Phenotype of the Cryptococcus-Specific CD4+ Memory T-Cell Response Is Associated with Disease Severity and Outcome in HIV-Associated Cryptococcal Meningitis. J. Infect. Dis. 2013, 207, 1817–1828. [Google Scholar] [CrossRef]
- Jarvis, J.N.; Meintjes, G.; Rebe, K.; Williams, G.N.; Bicanic, T.; Williams, A.; Schutz, C.; Bekker, L.G.; Wood, R.; Harrison, T.S. Adjunctive Interferon-γ Immunotherapy for the Treatment of HIV-Associated Cryptococcal Meningitis: A Randomized Controlled Trial. AIDS 2012, 26, 1105–1113. [Google Scholar] [CrossRef]
- Pappas, P.G.; Bustamante, B.; Ticona, E.; Hamill, R.J.; Johnson, P.C.; Reboli, A.; Aberg, J.; Hasbun, R.; Hsu, H.H. Recombinant Interferon-γ1b as Adjunctive Therapy for AIDS-Related Acute Cryptococcal Meningitis. J. Infect. Dis. 2004, 189, 2185–2191. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, F.; Tompkins, K.C.; McNamara, A.; Jain, A.V.; Moore, B.B.; Toews, G.B.; Huffnagle, G.B.; Olszewski, M.A. Robust Th1 and Th17 Immunity Supports Pulmonary Clearance but Cannot Prevent Systemic Dissemination of Highly Virulent Cryptococcus neoformans H99. Am. J. Pathol. 2009, 175, 2489–2500. [Google Scholar] [CrossRef]
- Murdock, B.J.; Huffnagle, G.B.; Olszewski, M.A.; Osterholzer, J.J. Interleukin-17A Enhances Host Defense against Cryptococcal Lung Infection through Effects Mediated by Leukocyte Recruitment, Activation, and Gamma Interferon Production. Infect. Immun. 2014, 82, 937–948. [Google Scholar] [CrossRef] [PubMed]
- Sionov, E.; Mayer-Barber, K.D.; Chang, Y.C.; Kauffman, K.D.; Eckhaus, M.A.; Salazar, A.M.; Barber, D.L.; Kwon-Chung, K.J. Type I IFN Induction via Poly-ICLC Protects Mice against Cryptococcosis. PLoS Pathog. 2015, 11, e1005040. [Google Scholar] [CrossRef] [PubMed]
- Huffnagle, G.B.; Toews, G.B.; Burdick, M.D.; Boyd, M.B.; McAllister, K.S.; McDonald, R.A.; Kunkel, S.L.; Strieter, R.M. Afferent Phase Production of TNF-Alpha Is Required for the Development of Protective T Cell Immunity to Cryptococcus neoformans. J. Immunol. 1996, 157, 4529–4536. [Google Scholar] [PubMed]
- Herring, A.C.; Lee, J.; McDonald, R.A.; Toews, G.B.; Huffnagle, G.B. Induction of Interleukin-12 and Gamma Interferon Requires Tumor Necrosis Factor Alpha for Protective T1-Cell-Mediated Immunity to Pulmonary Cryptococcus neoformans Infection. Infect. Immun. 2002, 70, 2959–2964. [Google Scholar] [CrossRef]
- Xu, J.; Eastman, A.J.; Flaczyk, A.; Neal, L.M.; Zhao, G.; Carolan, J.; Malachowski, A.N.; Stolberg, V.R.; Yosri, M.; Chensue, S.W.; et al. Disruption of Early Tumor Necrosis Factor Alpha Signaling Prevents Classical Activation of Dendritic Cells in Lung-Associated Lymph Nodes and Development of Protective Immunity against Cryptococcal Infection. MBio 2016, 7, e00510-16. [Google Scholar] [CrossRef]
- Fa, Z.; Xu, J.; Yi, J.; Sang, J.; Pan, W.; Xie, Q.; Yang, R.; Fang, W.; Liao, W.; Olszewski, M.A. TNF-α-Producing Cryptococcus neoformans Exerts Protective Effects on Host Defenses in Murine Pulmonary Cryptococcosis. Front. Immunol. 2019, 10, 1725. [Google Scholar] [CrossRef]
- Vecchiarelli, A.; Pericolini, E.; Gabrielli, E.; Kenno, S.; Perito, S.; Cenci, E.; Monari, C. Elucidating the Immunological Function of the Cryptococcus neoformans Capsule. Future Microbiol. 2013, 8, 1107–1116. [Google Scholar] [CrossRef]
- Zaragoza, O.; Rodrigues, M.L.; De Jesus, M.; Frases, S.; Dadachova, E.; Casadevall, A. The Capsule of the Fungal Pathogen Cryptococcus neoformans. Adv. Appl. Microbiol. 2009, 68, 133–216. [Google Scholar] [CrossRef]
- Macura, N.; Zhang, T.; Casadevall, A. Dependence of Macrophage Phagocytic Efficacy on Antibody Concentration. Infect. Immun. 2007, 75, 1904–1915. [Google Scholar] [CrossRef]
- Vecchiarelli, A.; Retini, C.; Monari, C.; Tascini, C.; Bistoni, F.; Kozel, T.R. Purified Capsular Polysaccharide of Cryptococcus neoformans Induces Interleukin-10 Secretion by Human Monocytes. Infect. Immun. 1996, 64, 2846–2849. [Google Scholar] [CrossRef]
- Retini, C.; Vecchiarelli, A.; Monari, C.; Bistoni, F.; Kozel, T.R. Encapsulation of Cryptococcus neoformans with Glucuronoxylomannan Inhibits the Antigen-Presenting Capacity of Monocytes. Infect. Immun. 1998, 66, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Monari, C.; Bistoni, F.; Casadevall, A.; Pericolini, E.; Pietrella, D.; Kozel, T.R.; Vecchiarelli, A. Glucuronoxylomannan, a Microbial Compound, Regulates Expression of Costimulatory Molecules and Production of Cytokines in Macrophages. J. Infect. Dis. 2005, 191, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Monari, C.; Pericolini, E.; Bistoni, G.; Casadevall, A.; Kozel, T.R.; Vecchiarelli, A. Cryptococcus neoformans Capsular Glucuronoxylomannan Induces Expression of Fas Ligand in Macrophages. J. Immunol. 2005, 174, 3461–3468. [Google Scholar] [CrossRef] [PubMed]
- Villena, S.N.; Pinheiro, R.O.; Pinheiro, C.S.; Nunes, M.P.; Takiya, C.M.; Dosreis, G.A.; Previato, J.O.; Mendonça-Previato, L.; Freire-de-Lima, C.G. Capsular Polysaccharides Galactoxylomannan and Glucuronoxylomannan from Cryptococcus neoformans Induce Macrophage Apoptosis Mediated by Fas Ligand. Cell. Microbiol. 2008, 10, 1274–1285. [Google Scholar] [CrossRef]
- Chiapello, L.S.; Baronetti, J.L.; Garro, A.P.; Spesso, M.F.; Masih, D.T. Cryptococcus neoformans Glucuronoxylomannan Induces Macrophage Apoptosis Mediated by Nitric Oxide in a Caspase-Independent Pathway. Int. Immunol. 2008, 20, 1527–1541. [Google Scholar] [CrossRef]
- Vecchiarelli, A.; Pietrella, D.; Lupo, P.; Bistoni, F.; McFadden, D.C.; Casadevall, A. The Polysaccharide Capsule of Cryptococcus neoformans Interferes with Human Dendritic Cell Maturation and Activation. J. Leukoc. Biol. 2003, 74, 370–378. [Google Scholar] [CrossRef]
- Huston, S.M.; Ngamskulrungroj, P.; Xiang, R.F.; Ogbomo, H.; Stack, D.; Li, S.S.; Timm-McCann, M.; Kyei, S.K.; Oykhman, P.; Kwon-Chung, K.J.; et al. Cryptococcus gattii Capsule Blocks Surface Recognition Required for Dendritic Cell Maturation Independent of Internalization and Antigen Processing. J. Immunol. 2016, 196, 1259–1271. [Google Scholar] [CrossRef]
- Denham, S.T.; Verma, S.; Reynolds, R.C.; Worne, C.L.; Daugherty, J.M.; Lane, T.E.; Brown, J.C.S. Regulated Release of Cryptococcal Polysaccharide Drives Virulence and Suppresses Immune Cell Infiltration into the Central Nervous System. Infect. Immun. 2018, 86, e00662-17. [Google Scholar] [CrossRef]
- Robertson, E.J.; Najjuka, G.; Rolfes, M.A.; Akampurira, A.; Jain, N.; Anantharanjit, J.; Von Hohenberg, M.; Tassieri, M.; Carlsson, A.; Meya, D.B.; et al. Cryptococcus neoformans Ex Vivo Capsule Size Is Associated with Intracranial Pressure and Host Immune Response in HIV-Associated Cryptococcal Meningitis. J. Infect. Dis. 2014, 209, 74–82. [Google Scholar] [CrossRef]
- Jarvis, J.N.; Percival, A.; Bauman, S.; Pelfrey, J.; Meintjes, G.; Williams, G.N.; Longley, N.; Harrison, T.S.; Kozel, T.R. Evaluation of a Novel Point-of-Care Cryptococcal Antigen Test on Serum, Plasma, and Urine from Patients with HIV-Associated Cryptococcal Meningitis. Clin. Infect. Dis. 2011, 53, 1019–1023. [Google Scholar] [CrossRef]
- Scriven, J.E.; Graham, L.M.; Schutz, C.; Scriba, T.J.; Wilkinson, K.A.; Wilkinson, R.J.; Boulware, D.R.; Urban, B.C.; Lalloo, D.G.; Meintjes, G. A Glucuronoxylomannan-Associated Immune Signature, Characterized by Monocyte Deactivation and an Increased Interleukin 10 Level, Is a Predictor of Death in Cryptococcal Meningitis. J. Infect. Dis. 2016, 213, 1725–1734. [Google Scholar] [CrossRef] [PubMed]
- Salas, S.D.; Bennett, J.E.; Kwon-Chung, K.J.; Perfect, J.R.; Williamson, P.R. Effect of the Laccase Gene, CNLAC1, on Virulence of Cryptococcus neoformans. J. Exp. Med. 1996, 184, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Tewari, R.P.; Williamson, P.R. Laccase Protects Cryptococcus neoformans from Antifungal Activity of Alveolar Macrophages. Infect. Immun. 1999, 67, 6034–6039. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Williamson, P.R. Role of Laccase in the Biology and Virulence of Cryptococcus neoformans. FEMS Yeast Res. 2004, 5, 1–10. [Google Scholar] [CrossRef]
- Noverr, M.C.; Williamson, P.R.; Fajardo, R.S.; Huffnagle, G.B. CNLAC1 is Required for Extrapulmonary Dissemination of Cryptococcus neoformans but not Pulmonary Persistence. Infect. Immun. 2004, 72, 1693–1699. [Google Scholar] [CrossRef]
- Qiu, Y.; Davis, M.J.; Dayrit, J.K.; Hadd, Z.; Meister, D.L.; Osterholzer, J.J.; Williamson, P.R.; Olszewski, M.A. Immune Modulation Mediated by Cryptococcal Laccase Promotes Pulmonary Growth and Brain Dissemination of Virulent Cryptococcus neoformans in Mice. PLoS ONE 2012, 7, e47853. [Google Scholar] [CrossRef]
- Erb-Downward, J.R.; Noggle, R.M.; Williamson, P.R.; Huffnagle, G.B. The Role of Laccase in Prostaglandin Production by Cryptococcus neoformans. Mol. Microbiol. 2008, 68, 1428–1437. [Google Scholar] [CrossRef]
- Hansakon, A.; Ngamskulrungroj, P.; Angkasekwinai, P. Contribution of Laccase Expression to Immune Response against Cryptococcus gattii Infection. Infect. Immun. 2020, 88, e00712-19. [Google Scholar] [CrossRef]
- Frazão, S.D.O.; de Sousa, H.R.; da Silva, L.G.; Folha, J.D.S.; Gorgonha, K.C.d.M.; de Oliveira, G.P.; Felipe, M.S.S.; Silva-Pereira, I.; Casadevall, A.; Nicola, A.M.; et al. Laccase Affects the Rate of Cryptococcus neoformans Nonlytic Exocytosis from Macrophages. MBio 2020, 11, e02085-20. [Google Scholar] [CrossRef]
- Wang, Y.; Casadevall, A. Susceptibility of Melanized and Nonmelanized Cryptococcus neoformans to Nitrogen- and Oxygen-Derived Oxidants. Infect. Immun. 1994, 62, 3004–3007. [Google Scholar] [CrossRef]
- Jacobson, E.S.; Tinnell, S.B. Antioxidant Function of Fungal Melanin. J. Bacteriol. 1993, 175, 7102–7104. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Aisen, P.; Casadevall, A. Cryptococcus neoformans Melanin and Virulence: Mechanism of Action. Infect. Immun. 1995, 63, 3131–3136. [Google Scholar] [CrossRef] [PubMed]
- Mednick, A.J.; Nosanchuk, J.D.; Casadevall, A. Melanization of Cryptococcus neoformans Affects Lung Inflammatory Responses during Cryptococcal Infection. Infect. Immun. 2005, 73, 2012–2019. [Google Scholar] [CrossRef] [PubMed]
- Doering, T.L.; Nosanchuk, J.D.; Roberts, W.K.; Casadevall, A. Melanin as a Potential Cryptococcal Defence against Microbicidal Proteins. Med. Mycol. 1999, 37, 175–181. [Google Scholar] [CrossRef]
- Tajima, K.; Yamanaka, D.; Ishibashi, K.I.; Adachi, Y.; Ohno, N. Solubilized Melanin Suppresses Macrophage Function. FEBS Open Bio 2019, 9, 791–800. [Google Scholar] [CrossRef]
- Huffnagle, G.B.; Chen, G.H.; Curtis, J.L.; McDonald, R.A.; Strieter, R.M.; Toews, G.B. Down-Regulation of the Afferent Phase of T Cell-Mediated Pulmonary Inflammation and Immunity by a High Melanin-Producing Strain of Cryptococcus neoformans. J. Immunol. 1995, 155, 3507–3516. [Google Scholar]
- Barluzzi, R.; Brozzetti, A.; Mariucci, G.; Tantucci, M.; Neglia, R.G.; Bistoni, F.; Blasi, E. Establishment of Protective Immunity against Cerebral Cryptococcosis by Means of an Avirulent, Non Melanogenic Cryptococcus neoformans Strain. J. Neuroimmunol. 2000, 109, 75–86. [Google Scholar] [CrossRef]
- Chen, S.C.A.; Wright, L.C.; Santangelo, R.T.; Muller, M.; Moran, V.R.; Kuchel, P.W.; Sorrell, T.C. Identification of Extracellular Phospholipase B, Lysophospholipase, and Acyltransferase Produced by Cryptococcus neoformans. Infect. Immun. 1997, 65, 405–411. [Google Scholar] [CrossRef]
- Chen, S.C.A.; Muller, M.; Zhou, J.Z.; Wright, L.C.; Sorrell, T.C. Phospholipase Activity in Cryptococcus neoformans: A New Virulence Factor? J. Infect. Dis. 1997, 175, 414–420. [Google Scholar] [CrossRef]
- Djordjevic, J.T. Role of Phospholipases in Fungal Fitness, Pathogenicity, and Drug Development—Lessons from Cryptococcus neoformans. Front. Microbiol. 2010, 1, 125. [Google Scholar] [CrossRef]
- Chayakulkeeree, M.; Johnston, S.A.; Oei, J.B.; Lev, S.; Williamson, P.R.; Wilson, C.F.; Zuo, X.; Leal, A.L.; Vainstein, M.H.; Meyer, W.; et al. SEC14 is a Specific Requirement for Secretion of Phospholipase B1 and Pathogenicity of Cryptococcus neoformans. Mol. Microbiol. 2011, 80, 1088–1101. [Google Scholar] [CrossRef]
- Cox, G.M.; McDade, H.C.; Chen, S.C.A.; Tucker, S.C.; Gottfredsson, M.; Wright, L.C.; Sorrell, T.C.; Eidich, S.D.; Casadevall, A.; Ghannoum, M.A.; et al. Extracellular Phospholipase Activity Is a Virulence Factor for Cryptococcus neoformans. Mol. Microbiol. 2001, 39, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Noverr, M.C.; Cox, G.M.; Perfect, J.R.; Huffnagle, G.B. Role of PLB1 in Pulmonary Inflammation and Cryptococcal Eicosanoid Production. Infect. Immun. 2003, 71, 1538–1547. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.J.; Li, Z.; Hughes, W.S.; Djordjevic, J.T.; Nielsen, K.; May, R.C. Cryptococcal Phospholipase B1 Is Required for Intracellular Proliferation and Control of Titan Cell Morphology during Macrophage Infection. Infect. Immun. 2015, 83, 1296–1304. [Google Scholar] [CrossRef] [PubMed]
- Osterholzer, J.J.; Surana, R.; Milam, J.E.; Montano, G.T.; Chen, G.H.; Sonstein, J.; Curtis, J.L.; Huffnagle, G.B.; Toews, G.B.; Olszewski, M.A. Cryptococcal Urease Promotes the Accumulation of Immature Dendritic Cells and a Non-Protective T2 Immune Response within the Lung. Am. J. Pathol. 2009, 174, 932–943. [Google Scholar] [CrossRef] [PubMed]
- Banks, I.R.; Specht, C.A.; Donlin, M.J.; Gerik, K.J.; Levitz, S.M.; Lodge, J.K. A Chitin Synthase and Its Regulator Protein Are Critical for Chitosan Production and Growth of the Fungal Pathogen Cryptococcus neoformans. Eukaryot. Cell 2005, 4, 1902–1912. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.G.; Da Silva, C.A.; Lee, J.Y.; Hartl, D.; Elias, J.A. Chitin Regulation of Immune Responses: An Old Molecule with New Roles. Curr. Opin. Immunol. 2008, 20, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Gordon-Thomson, C.; Kumari, A.; Tomkins, L.; Holford, P.; Djordjevic, J.T.; Wright, L.C.; Sorrell, T.C.; Moore, G.P.M. Chitotriosidase and Gene Therapy for Fungal Infections. Cell. Mol. Life Sci. 2009, 66, 1116–1125. [Google Scholar] [CrossRef] [PubMed]
- Gorzelanny, C.; Pöppelmann, B.; Pappelbaum, K.; Moerschbacher, B.M.; Schneider, S.W. Human Macrophage Activation Triggered by Chitotriosidase-Mediated Chitin and Chitosan Degradation. Biomaterials 2010, 31, 8556–8563. [Google Scholar] [CrossRef] [PubMed]
- Hole, C.R.; Lam, W.C.; Upadhya, R.; Lodge, J.K. Cryptococcus neoformans Chitin Synthase 3 Plays a Critical Role in Dampening Host Inflammatory Responses. MBio 2020, 11, e03373-19. [Google Scholar] [CrossRef]
- Liu, O.W.; Chun, C.D.; Chow, E.D.; Chen, C.; Madhani, H.D.; Noble, S.M. Systematic Genetic Analysis of Virulence in the Human Fungal Pathogen Cryptococcus neoformans. Cell 2008, 135, 174–188. [Google Scholar] [CrossRef]
- Dang, E.V.; Lei, S.; Radkov, A.; Volk, R.F.; Zaro, B.W.; Madhani, H.D. Secreted Fungal Virulence Effector Triggers Allergic Inflammation via TLR4. Nature 2022, 608, 161–167. [Google Scholar] [CrossRef]
- Coker, R.J. Cryptococcal Infection in AIDS. Int. J. STD AIDS 1992, 3, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Mocsny, N. Cryptococcal Meningitis in Patients with AIDS. J. Neurosci. Nurs. 1992, 24, 265–268. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Shi, Z.W.; Strickland, A.B.; Shi, M. Cryptococcus neoformans Infection in the Central Nervous System: The Battle between Host and Pathogen. J. Fungi 2022, 8, 1069. https://doi.org/10.3390/jof8101069
Chen Y, Shi ZW, Strickland AB, Shi M. Cryptococcus neoformans Infection in the Central Nervous System: The Battle between Host and Pathogen. Journal of Fungi. 2022; 8(10):1069. https://doi.org/10.3390/jof8101069
Chicago/Turabian StyleChen, Yanli, Zoe W. Shi, Ashley B. Strickland, and Meiqing Shi. 2022. "Cryptococcus neoformans Infection in the Central Nervous System: The Battle between Host and Pathogen" Journal of Fungi 8, no. 10: 1069. https://doi.org/10.3390/jof8101069
APA StyleChen, Y., Shi, Z. W., Strickland, A. B., & Shi, M. (2022). Cryptococcus neoformans Infection in the Central Nervous System: The Battle between Host and Pathogen. Journal of Fungi, 8(10), 1069. https://doi.org/10.3390/jof8101069




