The Importance of Nitric Oxide as the Molecular Basis of the Hydrogen Gas Fumigation-Induced Alleviation of Cd Stress on Ganoderma lucidum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Hydrogen-Rich Water (HRW)
2.3. Fungal Materials, Growth Condition, and Screening of Cd Inhibitory Concentrations
2.4. Experimental Design: Response of G. lucidum to Different Concentrations of H2 Fumigation
2.5. Determination of Intracellular Cadmium Content
2.6. Effect of H2 Fumigation on Reactive Oxygen Species (ROS) and TBARS Contents
2.7. Measurement of the Antioxidant Enzyme Activities
2.8. Detection of Endogenous H2
2.9. Detection of Nitrate Reductase (NR) Activity
2.10. Detection of Nitric Oxide by Confocal Laser Microscopy and Spectrophotometry
2.11. Measurement of Proline, Cysteine, and Glutathione Contents
2.12. Determination of Gene Expression in G. lucidum
2.13. Statistical Analysis
3. Results
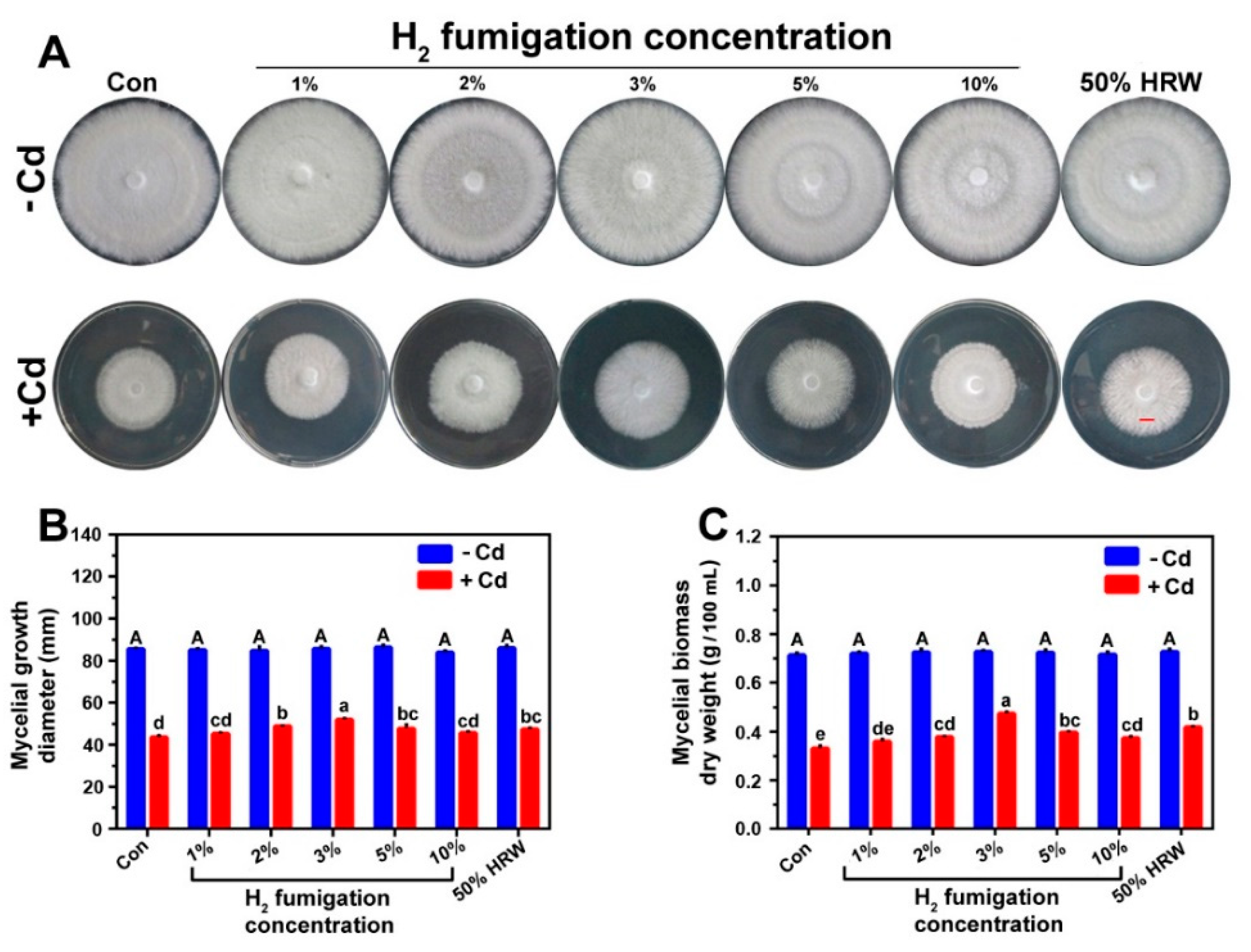
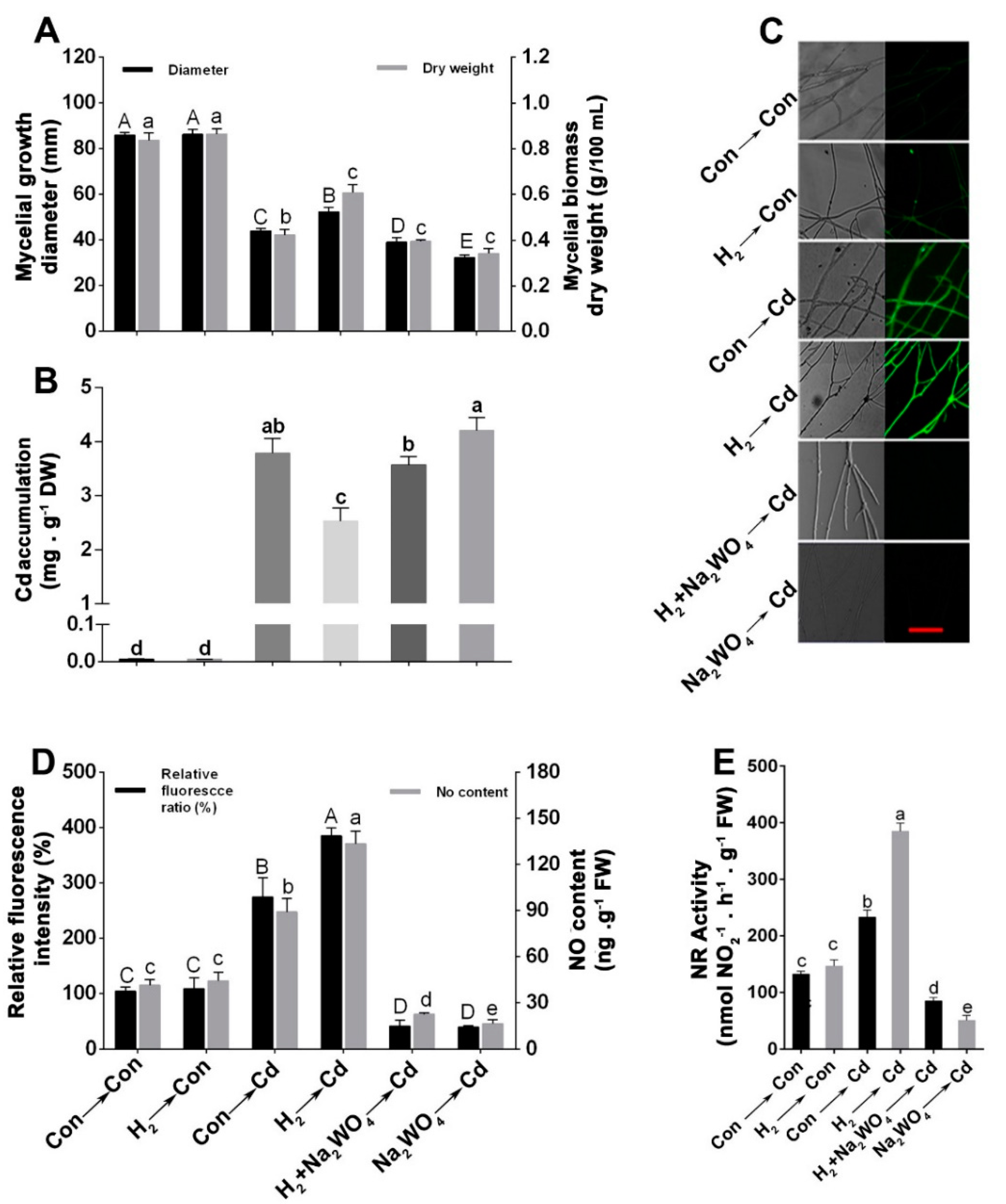
3.1. H2 Fumigation Alleviated Cd-Induced Inhibition of Mycelial Growth Diameter and Biomass Dry Weight in Ganoderma lucidum Mycelia
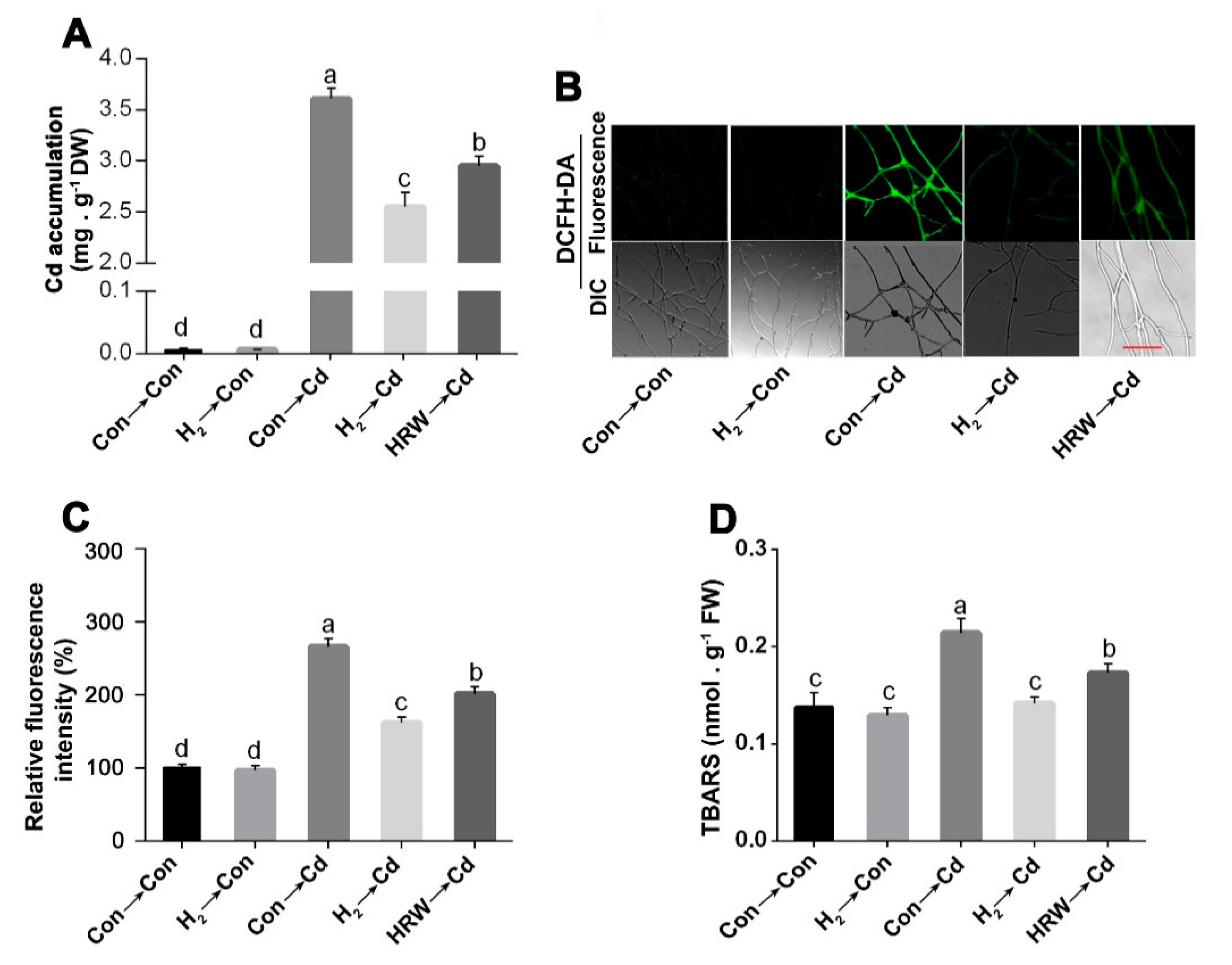
3.2. H2 Fumigation Reduced Cd Accumulation, Decreased the Production of ROS and Alleviated Lipid Peroxidation in G. lucidum under Cd Stress
3.3. H2 Fumigation Enhanced the Antioxidant Enzymes’ Activities and Their Corresponding Transcripts under Oxidative Stress Caused by Cd
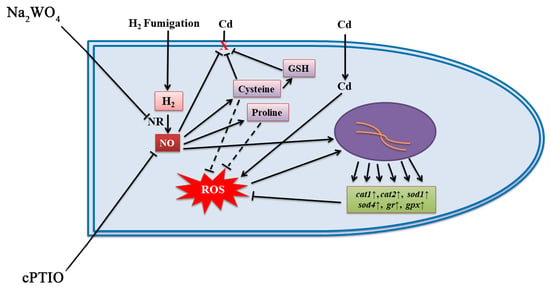
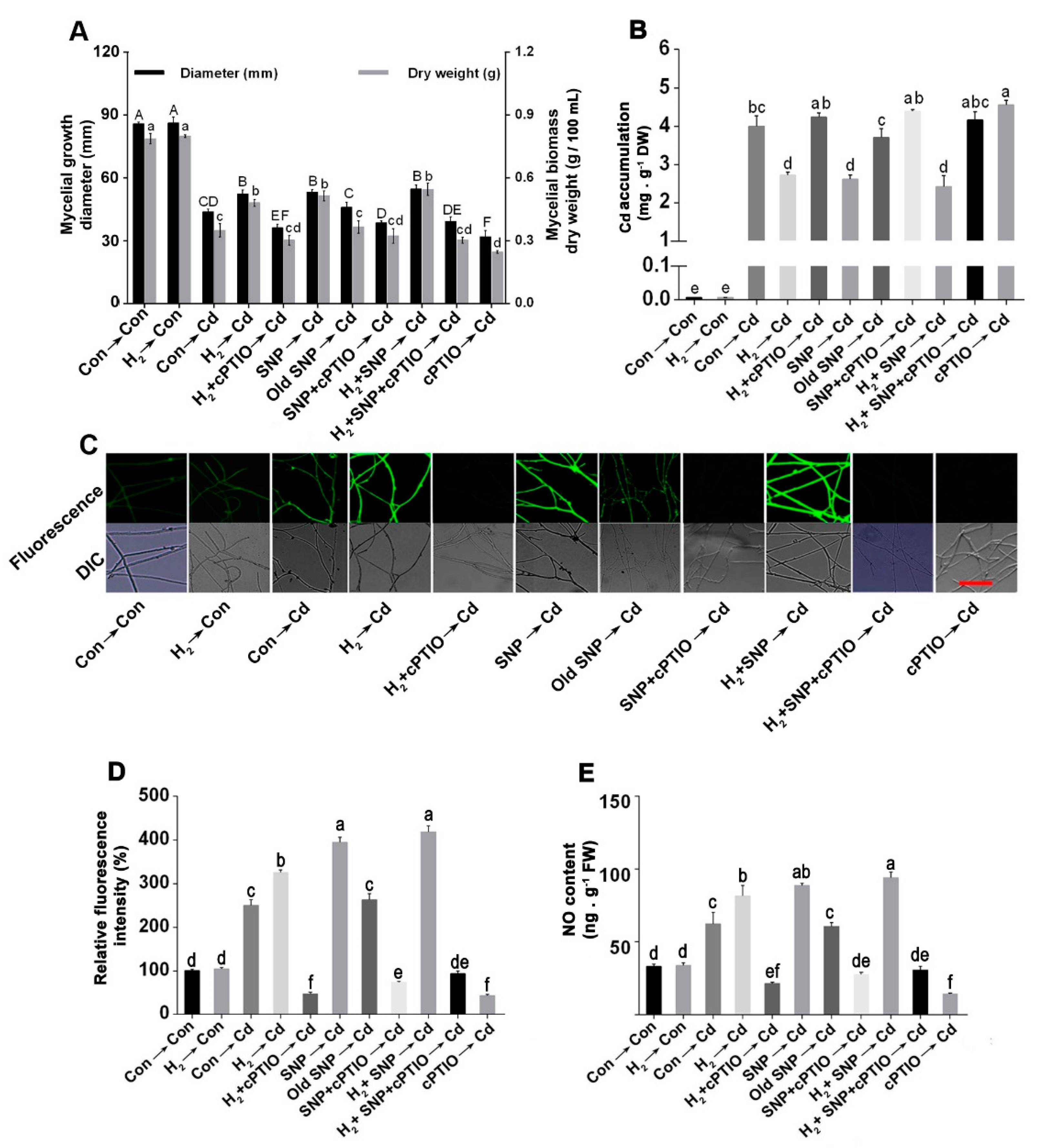
3.4. Endogenous NO Was Involved in the H2-Induced Cd Stress Tolerance
3.5. H2-Induced Alleviation of the Cd-Caused Mycelial Growth Inhibition and Excess Cd Accumulation Was Reversed by the NO Scavenger, cPTIO
3.6. The H2-Induced Cd Tolerance Was Linked to the NR-Dependent NO Formation
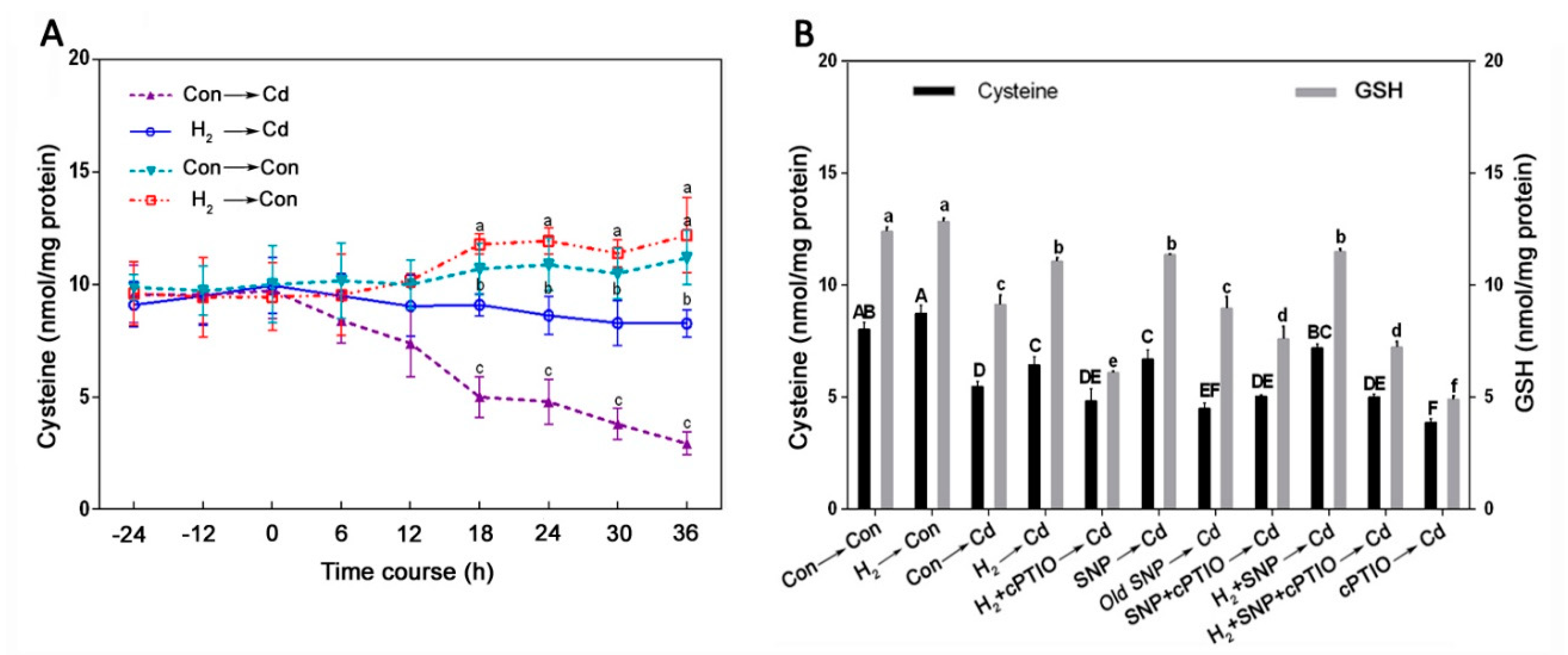
3.7. Cysteine Was Involved in the H2-Induced, NO-Mediated Cd Tolerance
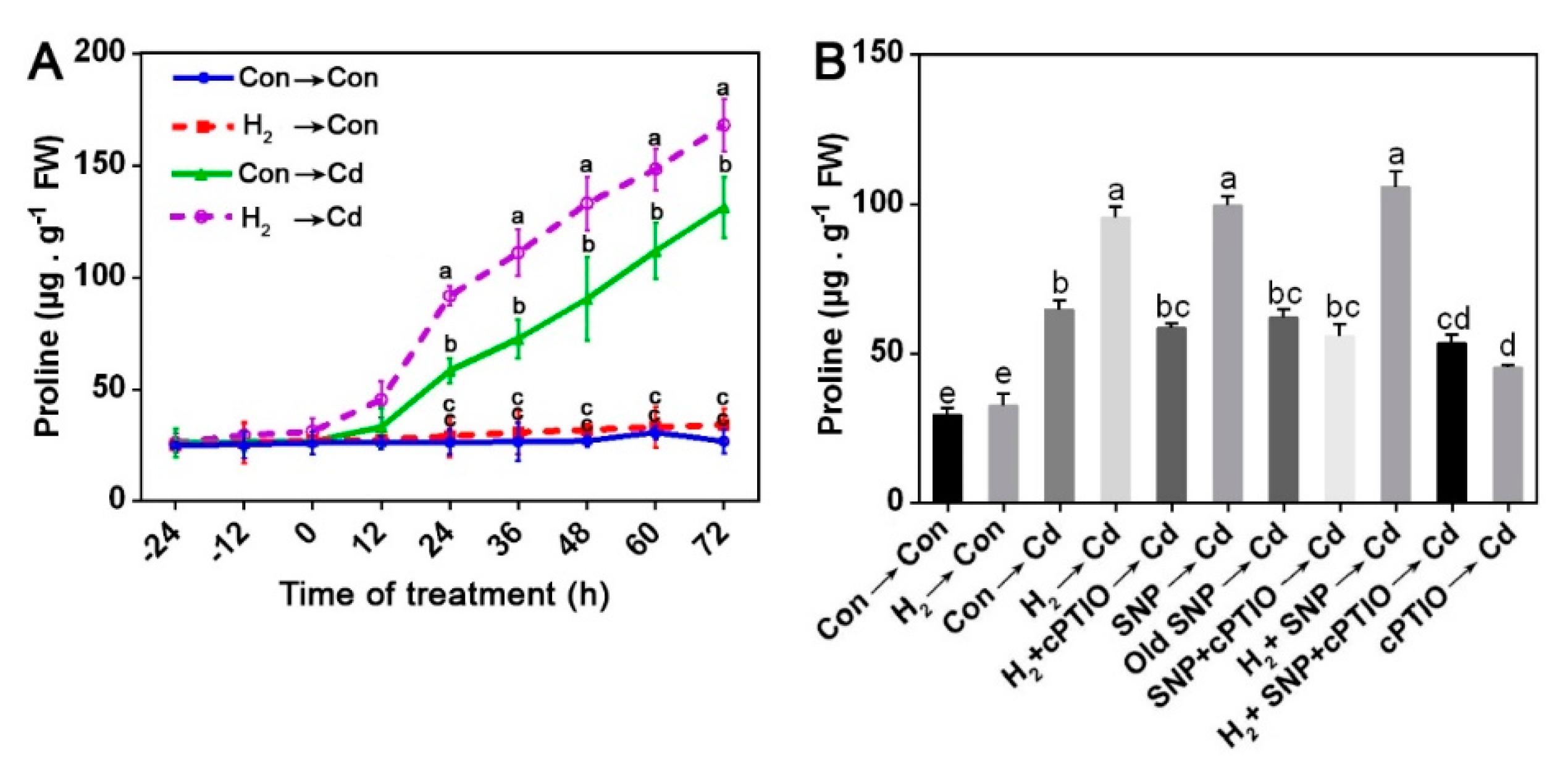
3.8. H2-Enhanced Proline Content Was Susceptible to cPTIO
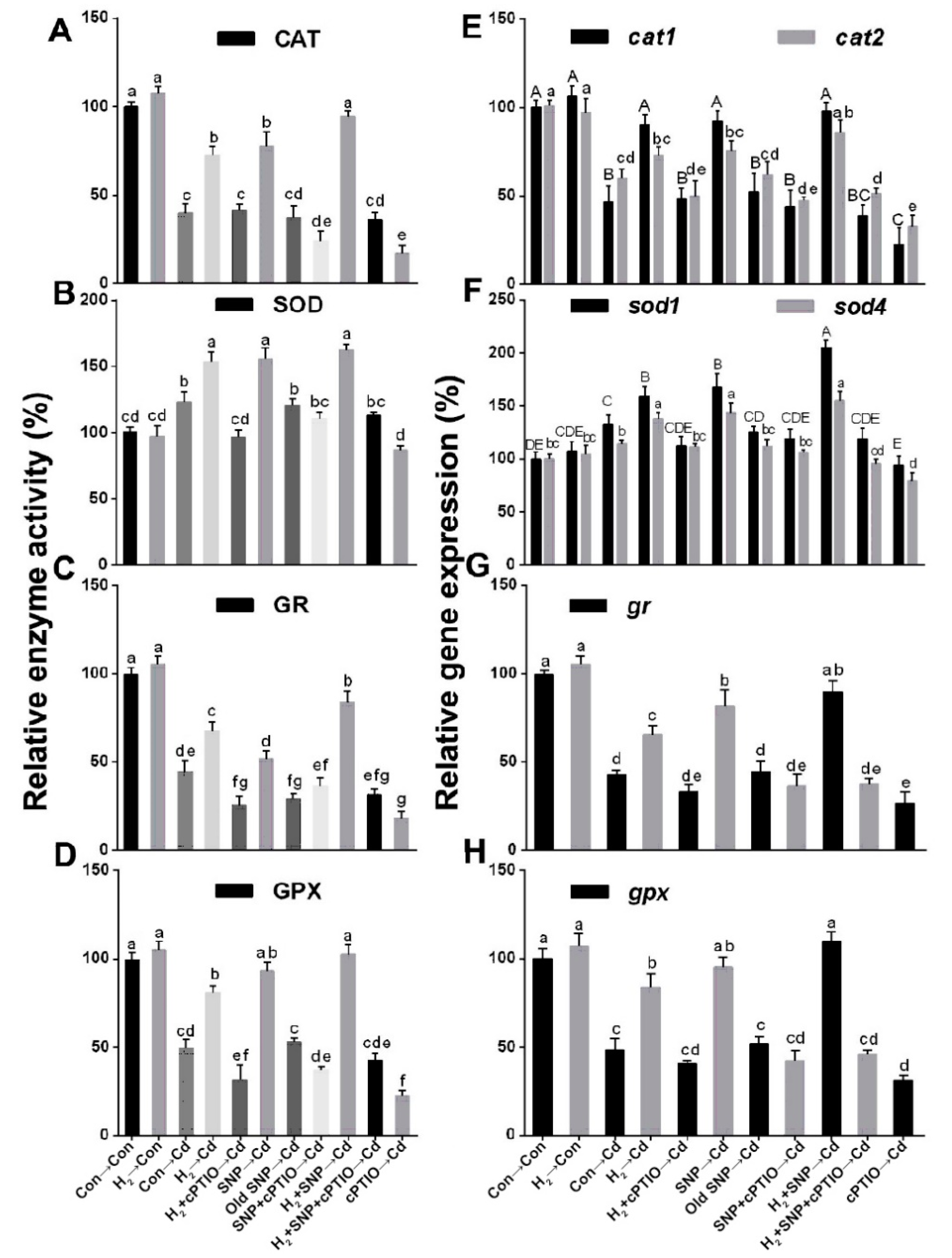
3.9. H2-Induced Reestablishment of Redox Homeostasis Was Sensitive to cPTIO
3.10. The NO Scavenger cPTIO Reversed the H2-Induced Regulation of the Activities and Related Transcripts of the Antioxidant Enzymes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jia, J.; Zhang, X.; Hu, Y.-S.; Wu, Y.; Wang, Q.-Z.; Li, N.-N.; Guo, Q.-C.; Dong, X.-C. Evaluation of in vivo antioxidant activities of Ganoderma lucidum polysaccharides in STZ-diabetic rats. Food Chem. 2009, 115, 32–36. [Google Scholar] [CrossRef]
- Wang, D.; Liu, Y.; Tan, X.; Liu, H.; Zeng, G.; Hu, X.; Jian, H.; Gu, Y. Effect of exogenous nitric oxide on antioxidative system and S-nitrosylation in leaves of Boehmeria nivea (L.) Gaud under cadmium stress. Environ. Sci. Pollut. Res. 2015, 22, 3489–3497. [Google Scholar] [CrossRef] [PubMed]
- Hart, J.J.; Welch, R.M.; Norvell, W.A.; Sullivan, L.A.; Kochian, L. Characterization of Cadmium Binding, Uptake, and Translocation in Intact Seedlings of Bread and Durum Wheat Cultivars. Plant Physiol. 1998, 116, 1413–1420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- di Toppi, L.S.; Gabbrielli, R. Response to cadmium in higher plants. Environ. Exp. Bot. 1999, 41, 105–130. [Google Scholar] [CrossRef]
- Deng, X.; Xia, Y.; Hu, W.; Zhang, H.; Shen, Z. Cadmium-induced oxidative damage and protective effects of N-acetyl-l-cysteine against cadmium toxicity in Solanum nigrum L. J. Hazard. Mater. 2010, 180, 722–729. [Google Scholar] [CrossRef]
- DalCorso, G.; Farinati, S.; Furini, A. Regulatory networks of cadmium stress in plants. Plant Signal. Behav. 2010, 5, 663–667. [Google Scholar] [CrossRef]
- Zhang, R.; Li, X.; Fadel, J. Oyster mushroom cultivation with rice and wheat straw. Bioresour. Technol. 2002, 82, 277–284. [Google Scholar] [CrossRef]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.-I.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef]
- Fukuda, K.-I.; Asoh, S.; Ishikawa, M.; Yamamoto, Y.; Ohsawa, I.; Ohta, S. Inhalation of hydrogen gas suppresses hepatic injury caused by ischemia/reperfusion through reducing oxidative stress. Biochem. Biophys. Res. Commun. 2007, 361, 670–674. [Google Scholar] [CrossRef]
- Nakashima-Kamimura, N.; Mori, T.; Ohsawa, I.; Asoh, S.; Ohta, S. Molecular hydrogen alleviates nephrotoxicity induced by an anti-cancer drug cisplatin without compromising anti-tumor activity in mice. Cancer Chemother. Pharmacol. 2009, 64, 753–761. [Google Scholar] [CrossRef]
- Jin, Q.; Zhu, K.; Cui, W.; Li, L.; Shen, W. Hydrogen-Modulated Stomatal Sensitivity to Abscisic Acid and Drought Tolerance Via the Regulation of Apoplastic pH in Medicago sativa. J. Plant Growth Regul. 2016, 35, 565–573. [Google Scholar] [CrossRef]
- Cui, W.; Gao, C.; Fang, P.; Lin, G.; Shen, W. Alleviation of cadmium toxicity in Medicago sativa by hydrogen-rich water. J. Hazard. Mater. 2013, 260, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Su, N.; Cai, J.; Shen, Z.; Cui, J. Hydrogen-rich water enhances cadmium tolerance in Chinese cabbage by reducing cadmium uptake and increasing antioxidant capacities. J. Plant Physiol. 2015, 175, 174–182. [Google Scholar] [CrossRef]
- Wu, X.; Zhu, Z.B.; Chen, J.H.; Huang, Y.F.; Liu, Z.L.; Zou, J.W.; Chen, Y.H.; Na Su, N.; Cui, J. Transcriptome analysis revealed pivotal transporters involved in the reduction of cadmium accumulation in pak choi (Brassica chinensis L.) by exogenous hydrogen-rich water. Chemosphere 2019, 216, 684–697. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Su, N.; Yue, X.; Fang, B.; Zou, J.; Chen, Y.; Shen, Z.; Cui, J. IRT1 and ZIP2 were involved in exogenous hydrogen-rich water-reduced cadmium accumulation in Brassica chinensis and Arabidopsis thaliana. J. Hazard. Mater. 2021, 407, 124599. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Cao, H.; Fang, W.; Pan, J.; Chen, J.; Zhang, J.; Shen, W. Linking hydrogen-enhanced rice aluminum tolerance with the reestablishment of GA/ABA balance and miRNA-modulated gene expression: A case study on germination. Ecotoxicol. Environ. Saf. 2017, 145, 303–312. [Google Scholar] [CrossRef]
- Cao, Z.; Duan, X.; Yao, P.; Cui, W.; Cheng, D.; Zhang, J.; Jin, Q.; Chen, J.; Dai, T.; Shen, W. Hydrogen Gas Is Involved in Auxin-Induced Lateral Root Formation by Modulating Nitric Oxide Synthesis. Int. J. Mol. Sci. 2017, 18, 2084. [Google Scholar] [CrossRef]
- Zhu, Y.; Liao, W.; Wang, M.; Niu, L.; Xu, Q.; Jin, X. Nitric oxide is required for hydrogen gas-induced adventitious root formation in cucumber. J. Plant Physiol. 2016, 195, 50–58. [Google Scholar] [CrossRef]
- Li, C.; Gong, T.; Bian, B.; Liao, W. Roles of hydrogen gas in plants: A review. Funct. Plant Biol. 2018, 45, 783–792. [Google Scholar] [CrossRef]
- Ren, A.; Liu, R.; Miao, Z.-G.; Zhang, X.; Cao, P.-F.; Chen, T.-X.; Li, C.-Y.; Shi, L.; Jiang, A.-L.; Zhao, M.-W. Hydrogen-rich water regulates effects of ROS balance on morphology, growth and secondary metabolism via glutathione peroxidase inGanoderma lucidum. Environ. Microbiol. 2017, 19, 566–583. [Google Scholar] [CrossRef]
- Zhang, J.; Hao, H.; Chen, M.; Wang, H.; Feng, Z.; Chen, H. Hydrogen-rich water alleviates the toxicities of different stresses to mycelial growth in Hypsizygus marmoreus. AMB Express 2017, 7, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Zhang, J.; Hao, H.; Feng, Z.; Chen, M.; Wang, H.; Ye, M. Hydrogen-rich water increases postharvest quality by enhancing antioxidant capacity in Hypsizygus marmoreus. AMB Express 2017, 7, 221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, H.; Zhao, S.; Li, P.; Shen, W. Hydrogen gas prolongs the shelf life of kiwifruit by decreasing ethylene biosynthesis. Postharvest Biol. Technol. 2018, 135, 123–130. [Google Scholar] [CrossRef]
- Alwazeer, D.; Tan, K.; Örs, B. Reducing atmosphere packaging as a novel alternative technique for extending shelf life of fresh cheese. J. Food Sci. Technol. 2020, 57, 3013–3023. [Google Scholar] [CrossRef]
- Alwazeer, D.; Delbeau, C.; Divies, C.; Cachon, R. Use of redox potential modification by gas improves microbial quality, color retention, and ascorbic acid stability of pasteurized orange juice. Int. J. Food Microbiol. 2003, 89, 21–29. [Google Scholar] [CrossRef]
- Alwazeer, D.; Örs, B. Reducing atmosphere drying as a novel drying technique for preserving the sensorial and nutritional notes of foods. J. Food Sci. Technol. 2019, 56, 3790–3800. [Google Scholar] [CrossRef]
- Dong, Z.; Wu, L.; Kettlewell, B.; Caldwell, C.D.; Layzell, D.B. Hydrogen fertilization of soils—is this a benefit of legumes in rotation? Plant Cell Environ. 2003, 26, 1875–1879. [Google Scholar] [CrossRef]
- Stein, S.; Selesi, D.; Schilling, R.; Pattis, I.; Schmid, M.; Hartmann, A. Microbial activity and bacterial composition of H2-treated soils with net CO2 fixation. Soil Biol. Biochem. 2005, 37, 1938–1945. [Google Scholar] [CrossRef]
- Liu, H.; Wang, W.; Cao, G.; Tang, M. Effect of hydrogen on microbial population and enzyme activity in Robinia pseudoacacia rhizosphere soil. Chin. J. Appl. Environ. Biol. 2010, 16, 515–518. [Google Scholar] [CrossRef]
- Li, L.; Lou, W.; Kong, L.; Shen, W. Hydrogen Commonly Applicable from Medicine to Agriculture: From Molecular Mechanisms to the Field. Curr. Pharm. Des. 2021, 27, 747–759. [Google Scholar] [CrossRef]
- Kapoor, P.; Sharma, B.M. Studies on different growth parameters of Ganoderma lucidum. Int. J. Sci. Environ. Tech. 2014, 3, 1515–1524. [Google Scholar]
- Tang, W.; Kuehn, T.H.; Simcik, M.F. Effects of Temperature, Humidity and Air Flow on Fungal Growth Rate on Loaded Ventilation Filters. J. Occup. Environ. Hyg. 2015, 12, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.H. Clinical Applications of Magnesium Hydride. In Magnesium Alloys—Selected Issue; Tański, T., Borek, W., Król, M., Eds.; IntechOpen: London, UK, 2018; pp. 115–128. [Google Scholar] [CrossRef]
- Yü, Y.; Yang, Z.; Guo, K.; Li, Z.; Zhou, H.; Wei, Y.; Li, J.; Zhang, X.; Harvey, P.; Yang, H. Oxidative Damage Induced by Heat Stress Could be Relieved by Nitric Oxide in Trichoderma harzianum LTR-2. Curr. Microbiol. 2015, 70, 618–622. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Huang, C.; Chen, Q.; Zou, Y.; Zhang, J. Nitric oxide alleviates heat stress-induced oxidative damage in Pleurotus eryngii var. tuoliensis. Fungal Genet. Biol. 2012, 49, 15–20. [Google Scholar] [CrossRef]
- Chiang, K.T.; Shinyashiki, M.; Switzer, C.H.; Valentine, J.S.; Gralla, E.B.; Thiele, D.J.; Fukuto, J.M. Effects of Nitric Oxide on the Copper-Responsive Transcription Factor Ace1 in Saccharomyces cerevisiae: Cytotoxic and Cytoprotective Actions of Nitric Oxide. Arch. Biochem. Biophys. 2000, 377, 296–303. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Zhu, T.; Yang, T.; Yang, Z.; Ren, A.; Shi, L.; Zhu, J.; Yu, H.; Zhao, M. Nitric oxide regulates ganoderic acid biosynthesis by the S-nitrosylation of aconitase under heat stress in Ganoderma lucidum. Environ. Microbiol. 2021, 23, 682–695. [Google Scholar] [CrossRef]
- Xie, Y.; Mao, Y.; Zhang, W.; Lai, D.; Wang, Q.; Shen, W. Reactive Oxygen Species-Dependent Nitric Oxide Production Contributes to Hydrogen-Promoted Stomatal Closure in Arabidopsis. Plant Physiol. 2014, 165, 759–773. [Google Scholar] [CrossRef] [Green Version]
- Su, N.; Wu, Q.; Chen, H.; Huang, Y.; Zhu, Z.; Chen, Y.; Cui, J. Hydrogen gas alleviates toxic effects of cadmium in Brassica campestris seedlings through up-regulation of the antioxidant capacities: Possible involvement of nitric oxide. Environ. Pollut. 2019, 251, 45–55. [Google Scholar] [CrossRef]
- Wang, Y.; Lv, P.; Konga, L.; Shenac, W.; Hebc, Q. Nanomaterial-mediated sustainable hydrogen supply induces lateral root formation via nitrate reductase-dependent nitric oxide. Chem. Eng. J. 2021, 405, 126905. [Google Scholar] [CrossRef]
- Liu, R.; Shi, L.; Zhu, T.; Yang, T.; Ren, A.; Zhu, J.; Zhao, M.-W. Cross Talk between Nitric Oxide and Calcium-Calmodulin Regulates Ganoderic Acid Biosynthesis in Ganoderma lucidum under Heat Stress. Appl. Environ. Microbiol. 2018, 84. [Google Scholar] [CrossRef] [Green Version]
- Chamizo-Ampudia, A.; Sanz-Luque, E.; Llamas, A.; Galvan, A.; Fernandez, E.P.-A. Nitrate Reductase Regulates Plant Nitric Oxide Homeostasis. Trends Plant Sci. 2017, 22, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Marcos, A.T.; Ramos, M.S.; Marcos, J.F.; Carmona, L.; Strauss, J.; Cánovas, D. Nitric oxide synthesis by nitrate reductase is regulated during development in A. spergillus. Mol. Microbiol. 2016, 99, 15–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, H.; Wang, X.; Dou, Y.; Liu, D.; Si, W.; Fang, H.; Zhao, C.; Chen, S.; Xi, J.; Li, J. Hydrogen sulfide-cysteine cycle system enhances cadmium tolerance through alleviating cadmium-induced oxidative stress and ion toxicity in Arabidopsis roots. Sci. Rep. 2016, 6, 39702. [Google Scholar] [CrossRef]
- Yamauchi, A.; Bloom, E.T. Requirement of thiol compounds as reducing agents for IL-2-mediated induction of LAK activity and proliferation of human NK cells. J. Immunol. 1993, 151, 5535–5544. [Google Scholar] [PubMed]
- Yamauchi, A.; Bloom, E.T. Control of Cell Cycle Progression in Human Natural Killer Cells Through Redox Regulation of Expression and Phosphorylation of Retinoblastoma Gene Product Protein. Blood 1997, 89, 4092–4099. [Google Scholar] [CrossRef] [PubMed]
- Bannai, S. Transport of cystine and cysteine in mammalian cells. Biochim. Biophys. Acta Rev. Biomembr. 1984, 779, 289–306. [Google Scholar] [CrossRef]
- Lu, S.C. Regulation of hepatic glutathione synthesis: Current concepts and controversies. FASEB J. 1999, 13, 1169–1183. [Google Scholar] [CrossRef] [Green Version]
- Cobbett, C.; Goldsbrough, P. PHYTOCHELATINS AND METALLOTHIONEINS: Roles in Heavy Metal Detoxification and Homeostasis. Annu. Rev. Plant Biol. 2002, 53, 159–182. [Google Scholar] [CrossRef] [Green Version]
- Fang, H.; Liu, Z.; Jin, Z.; Zhang, L.; Liu, D.; Pei, Y. An emphasis of hydrogen sulfide-cysteine cycle on enhancing the tolerance to chromium stress in Arabidopsis. Environ. Pollut. 2016, 213, 870–877. [Google Scholar] [CrossRef]
- Alamri, S.; Ali, H.M.; Khan, M.I.R.; Singh, V.P.; Siddiqui, M.H. Exogenous nitric oxide requires endogenous hydrogen sulfide to induce the resilience through sulfur assimilation in tomato seedlings under hexavalent chromium toxicity. Plant Physiol. Biochem. 2020, 155, 20–34. [Google Scholar] [CrossRef]
- Maresca, B.; Lambowitz, A.M.; Kumar, V.B.; Grant, G.A.; Kobayashi, G.S.; Medoff, G. Role of cysteine in regulating morphogenesis and mitochondrial activity in the dimorphic fungus Histoplasma capsulatum. Proc. Natl. Acad. Sci. USA 1981, 78, 4596–4600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Yin, C.; Ren, J.; Li, C. Effect of osmotic stress and sodium nitroprusside pretreatment on proline metabolism of wheat seedlings. Biol. Plant. 2007, 51, 386–390. [Google Scholar] [CrossRef]
- Shi, F.M.; Wu, R.W.; Wang, C.; Li, J. Nitrate reductase-dependent NO production is critical for Arabidopsis roots response to ABA. Life Sci. J. 2013, 10, 297–303. [Google Scholar]
- Guo, S.; Yao, Y.; Zuo, L.; Shi, W.; Gao, N.; Xu, H. Enhancement of tolerance ofGanoderma lucidumto cadmium by nitric oxide. J. Basic Microbiol. 2015, 56, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Bright, J.; Desikan, R.; Hancock, J.T.; Weir, I.S.; Neill, S.J. ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J. 2006, 45, 113–122. [Google Scholar] [CrossRef]
- Zhao, M.-G.; Chen, L.; Zhang, L.-L.; Zhang, W.-H. Nitric Reductase-Dependent Nitric Oxide Production Is Involved in Cold Acclimation and Freezing Tolerance in Arabidopsis. Plant Physiol. 2009, 151, 755–767. [Google Scholar] [CrossRef] [Green Version]
- Qi, F.; Xiang, Z.; Kou, N.; Cui, W.; Xu, D.; Wang, R.; Zhu, D.; Shen, W. Nitric oxide is involved in methane-induced adventitious root formation in cucumber. Physiol. Plant. 2016, 159, 366–377. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Cui, W.; Zhu, K.; Xie, Y.; Zhang, C.; Shen, W. Hydrogen-rich water alleviates aluminum-induced inhibition of root elongation in alfalfa via decreasing nitric oxide production. J. Hazard. Mater. 2014, 267, 40–47. [Google Scholar] [CrossRef]
- Su, J.; Nie, Y.; Zhao, G.; Cheng, D.; Wang, R.; Chen, J.; Zhang, S.; Shen, W. Endogenous hydrogen gas delays petal senescence and extends the vase life of lisianthus cut flowers. Postharvest Biol. Technol. 2019, 147, 148–155. [Google Scholar] [CrossRef]
- Mu, D.; Li, C.; Zhang, X.; Li, X.; Shi, L.; Ren, A.; Zhao, M. Functions of the nicotinamide adenine dinucleotide phosphate oxidase family in Ganoderma lucidum: An essential role in ganoderic acid biosynthesis regulation, hyphal branching, fruiting body development, and oxidative-stress resistance. Environ. Microbiol. 2013, 16, 1709–1728. [Google Scholar] [CrossRef] [PubMed]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. i. kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Kula, I.; Solak, M.H.; Uğurlu, M.; Işıloğlu, M.; Arslan, Y. Determination of Mercury, Cadmium, Lead, Zinc, Selenium and Iron by ICP-OES in Mushroom Samples from Around Thermal Power Plant in Muğla, Turkey. Bull. Environ. Contam. Toxicol. 2011, 87, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Hammond, J.B.W.; Kruger, N.J. The Bradford Method for Protein Quantitation. In New Protein Techniques; Springer: Berlin/Heidelberg, Germany, 1988; Volume 3, pp. 25–32. [Google Scholar]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide Dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Garrett, R.H.; Cove, D.J. Formation of NADPH-nitrate reductase activity in vitro from Aspergillus nidulans niaD and cnx mu-tants. Mol. Gen. Genet. 1976, 149, 179–186. [Google Scholar] [CrossRef]
- Planchet, E.; Verdu, I.; Delahaie, J.; Cukier, C.; Girard, C.; Morere-Le Paven, M.-C.; Limami, A.M. Abscisic acid-induced nitric oxide and proline accumulation in independent pathways under water-deficit stress during seedling establishment in Medicago truncatula. J. Exp. Bot. 2014, 65, 2161–2170. [Google Scholar] [CrossRef] [Green Version]
- Reed, D.; Babson, J.; Beatty, P.; Brodie, A.; Ellis, W.; Potter, D. High-performance liquid chromatography analysis of nanomole levels of glutathione, glutathione disulfide, and related thiols and disulfides. Anal. Biochem. 1980, 106, 55–62. [Google Scholar] [CrossRef]
- Mu, D.; Shi, L.; Ren, A.; Li, M.; Wu, F.; Jiang, A.; Zhao, M. The Development and Application of a Multiple Gene Co-Silencing System Using Endogenous URA3 as a Reporter Gene in Ganoderma lucidum. PLoS ONE 2012, 7, e43737. [Google Scholar] [CrossRef]
- Khan, M.N.; Mobin, M.; Abbas, Z.K.; Siddiqui, M.H. Nitric oxide-induced synthesis of hydrogen sulfide alleviates osmotic stress in wheat seedlings through sustaining antioxidant enzymes, osmolyte accumulation and cysteine homeostasis. Nitric Oxide 2017, 68, 91–102. [Google Scholar] [CrossRef]
- Siripornadulsil, S.; Traina, S.; Verma, D.P.S.; Sayre, R.T. Molecular Mechanisms of Proline-Mediated Tolerance to Toxic Heavy Metals in Transgenic Microalgae. Plant Cell 2002, 14, 2837–2847. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Li, Q.; Wang, Q.; Lu, D.; Zhang, H.; Wang, J.; Fu, R. Transcriptional profiling provides new insights into the role of nitric oxide in enhancing Ganoderma oregonense resistance to heat stress. Sci. Rep. 2017, 7, 15694. [Google Scholar] [CrossRef] [Green Version]
- Itoh, T.; Hamada, N.; Terazawa, R.; Ito, M.; Ohno, K.; Ichihara, M.; Nozawa, Y.; Ito, M. Molecular hydrogen inhibits lipopolysaccharide/interferon γ-induced nitric oxide production through modulation of signal transduction in macrophages. Biochem. Biophys. Res. Commun. 2011, 411, 143–149. [Google Scholar] [CrossRef]
- Chen, H.; Hai, H.; Wang, H.; Wang, Q.; Chen, M.; Feng, Z.; Ye, M.; Zhang, J. Hydrogen-rich water mediates redox regulation of the antioxidant system, mycelial regeneration and fruiting body development in Hypsizygus marmoreus. Fungal Biol. 2018, 122, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Noda, T.; Iwakiri, R.; Fujimoto, K.; Rhoads, C.A.; Aw, T.Y. Exogenous cysteine and cystine promote cell proliferation in CaCo-2 cells. Cell Prolif. 2002, 35, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Zhang, Y.; Huang, S.; Yang, Y.; Gu, C. Effects of exogenous glutathione and cysteine on growth, lead accumulation, and tolerance of Iris lactea var. chinensis. Environ. Sci. Pollut. Res. 2014, 22, 2808–2816. [Google Scholar] [CrossRef] [PubMed]
- Terzi, H.; Yıldız, M. Proteomic analysis reveals the role of exogenous cysteine in alleviating chromium stress in maize seedlings. Ecotoxicol. Environ. Saf. 2021, 209, 111784. [Google Scholar] [CrossRef]
- Mendoza-Cózatl, D.; Loza-Tavera, H.; Hernández-Navarro, A.; Moreno-Sanchez, R. Sulfur assimilation and glutathione metabolism under cadmium stress in yeast, protists and plants. FEMS Microbiol. Rev. 2005, 29, 653–671. [Google Scholar] [CrossRef] [Green Version]
- Khedr, A.H.A.; Abbas, M.A.; Wahid, A.A.A.; Quick, W.P.; Abogadallah, G.M. Proline induces the expression of salt-stress-responsive proteins and may improve the adaptation of Pancratium maritimum L. to salt-stress. J. Exp. Bot. 2003, 54. [Google Scholar] [CrossRef] [Green Version]
- Kaur, G.; Asthir, B. Proline: A key player in plant abiotic stress tolerance. Biol. Plant. 2015, 59, 609–619. [Google Scholar] [CrossRef]
- Chen, C.; Dickman, M.B. From The Cover: Proline suppresses apoptosis in the fungal pathogen Colletotrichum trifolii. Proc. Natl. Acad. Sci. USA 2005, 102, 3459–3464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.P.; Mehta, S.K.; Liu, Z.P.; Yang, Z.M. Copper-Induced Proline Synthesis is Associated with Nitric Oxide Generation in Chlamydomonas reinhardtii. Plant Cell Physiol. 2008, 49, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Zhang, Y.; Nie, Y.; Cheng, D.; Wang, R.; Hu, H.; Chen, J.; Zhang, J.; Du, Y.; Shen, W. Hydrogen-induced osmotic tolerance is associated with nitric oxide-mediated proline accumulation and reestablishment of redox balance in alfalfa seedlings. Environ. Exp. Bot. 2018, 147, 249–260. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdalmegeed, D.; Zhao, G.; Cheng, P.; Bhat, J.A.; Khattak, W.A.; Ali, M.G.; Alnadari, F.; Ali, I.; Ali, Q.; Korma, S.A.; et al. The Importance of Nitric Oxide as the Molecular Basis of the Hydrogen Gas Fumigation-Induced Alleviation of Cd Stress on Ganoderma lucidum. J. Fungi 2022, 8, 10. https://doi.org/10.3390/jof8010010
Abdalmegeed D, Zhao G, Cheng P, Bhat JA, Khattak WA, Ali MG, Alnadari F, Ali I, Ali Q, Korma SA, et al. The Importance of Nitric Oxide as the Molecular Basis of the Hydrogen Gas Fumigation-Induced Alleviation of Cd Stress on Ganoderma lucidum. Journal of Fungi. 2022; 8(1):10. https://doi.org/10.3390/jof8010010
Chicago/Turabian StyleAbdalmegeed, Dyaaaldin, Gan Zhao, Pengfei Cheng, Javaid A. Bhat, Wajid Ali Khattak, Mostafa G. Ali, Fawze Alnadari, Ilyas Ali, Qurban Ali, Sameh A. Korma, and et al. 2022. "The Importance of Nitric Oxide as the Molecular Basis of the Hydrogen Gas Fumigation-Induced Alleviation of Cd Stress on Ganoderma lucidum" Journal of Fungi 8, no. 1: 10. https://doi.org/10.3390/jof8010010
APA StyleAbdalmegeed, D., Zhao, G., Cheng, P., Bhat, J. A., Khattak, W. A., Ali, M. G., Alnadari, F., Ali, I., Ali, Q., Korma, S. A., Mahmoud, Y. A.-G., Abd Elnabi, M. K., Cui, W., & Shen, W. (2022). The Importance of Nitric Oxide as the Molecular Basis of the Hydrogen Gas Fumigation-Induced Alleviation of Cd Stress on Ganoderma lucidum. Journal of Fungi, 8(1), 10. https://doi.org/10.3390/jof8010010