Sesquiterpenoids and Xanthones from the Kiwifruit-Associated Fungus Bipolaris sp. and Their Anti-Pathogenic Microorganism Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Fermentation, Extraction, and Isolation
2.3. ECD Calculations
2.4. NMR Calculations
2.5. Antibacterial Activity Assay
2.6. Anti-Phytopathogens Assay
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dolly, S.; Kaur, J.; Bhadariya, V.; Sharma, K. Actinidia deliciosa (Kiwi fruit): A comprehensive review on the nutritional composition, health benefits, traditional utilization and commercialization. J. Food Process. Preserv. 2021, 45, e15588. [Google Scholar] [CrossRef]
- Wang, S.N.; Qiu, Y.; Zhu, F. Kiwifruit (Actinidia spp.): A review of chemical diversity and biological activities. Food Chem. 2021, 350, 128469. [Google Scholar] [CrossRef]
- Richardson, D.P.; Ansell, J.; Drummond, L.N. The nutritional and health attributes of kiwifruit: A review. Eur. J. Nutr. 2018, 57, 2659–2676. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, C.; Long, Y.; Wu, X.; Su, Y.; Lei, Y.; Ai, Q. Bioactivity and control efficacy of the novel antibiotic tetramycin against various kiwifruit diseases. Antibiotics 2021, 10, 289. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.H.; Jung, J.S.; Koh, Y.J. Occurrence and epidemics of bacterial canker of kiwifruit in Korea. Plant Pathol. J. 2017, 33, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, C.; Wu, X.; Long, Y.; Su, Y. Chitosan augments tetramycin against soft rot in kiwifruit and enhances its improvement for kiwifruit growth, quality and aroma. Biomolecules 2021, 11, 1257. [Google Scholar] [CrossRef]
- Lee, Y.S.; Han, H.S.; Kim, G.H.; Koh, Y.J.; Hur, J.S.; Jung, J.S. Causal agents of blossom blight of kiwifruit in Korea. Plant Pathol. J. 2009, 25, 220–224. [Google Scholar] [CrossRef][Green Version]
- Balestra, G.M.; Mazzaglia, A.; Rossetti, A. Outbreak of bacterial blossom blight caused by Pseudomonas viridiflava on Actinidia chinensis kiwifruit plants in Italy. Plant Dis. 2008, 92, 1707. [Google Scholar] [CrossRef]
- Jeong, I.H.; Lim, M.T.; Kim, G.H.; Han, T.W.; Kim, H.C.; Kim, M.J.; Park, H.S.; Shin, S.H.; Hur, J.S.; Shin, J.S.; et al. Incidences of leaf spots and blights on kiwifruit in Korea. Plant Pathol. J. 2008, 24, 125–130. [Google Scholar] [CrossRef]
- Li, H.H.; Tang, W.; Liu, K.; Zhang, L.; Tang, X.F.; Miao, M.; Liu, Y.S. First report of Fusarium fujikuroi causing brown leaf spot on kiwifruit. Plant Dis. 2020, 104, 1560. [Google Scholar] [CrossRef]
- Polat, Z.; Awan, Q.N.; Hussain, M.; Akgul, D.S. First report of Phytopythium vexans causing root and collar rot of kiwifruit in Turkey. Plant Dis. 2017, 101, 1058. [Google Scholar] [CrossRef]
- Wang, K.X.; Xie, Y.L.; Yuan, G.Q.; Li, Q.Q.; Lin, W. First report of root and collar rot caused by Phytopythium helicoides on kiwifruit (Actinidia chinensis). Plant Dis. 2015, 99, 725. [Google Scholar] [CrossRef]
- McCann, H.C.; Li, L.; Liu, Y.F.; Li, D.W.; Pan, H.; Zhong, C.H.; Rikkerink, E.H.A.; Templeton, M.D.; Straub, C.; Colombi, E.; et al. Origin and evolution of the kiwifruit canker pandemic. Genome Biol. Evol. 2017, 9, 932–944. [Google Scholar] [CrossRef]
- Vanneste, J.L. The scientific, economic, and social impacts of the New Zealand outbreak of bacterial canker of kiwifruit (Pseudomonas syringae pv. actinidiae). Annu. Rev. Phytopathol. 2017, 55, 377–399. [Google Scholar] [CrossRef]
- Wicaksono, W.A.; Jones, E.E.; Casonato, S.; Monk, J.; Ridgway, H.J. Biological control of Pseudomonas syringae pv. actinidiae (Psa), the causal agent of bacterial canker of kiwifruit, using endophytic bacteria recovered from a medicinal plant. Biol. Control 2018, 116, 103–112. [Google Scholar] [CrossRef]
- Scortichini, M. Aspects still to solve for the management of kiwifruit bacterial canker caused by Pseudomonas syringae pv. actinidiae biovar 3. Eur. J. Hortic. Sci. 2018, 83, 205–211. [Google Scholar] [CrossRef]
- Bardas, G.A.; Veloukas, T.; Koutita, O.; Karaoglanidis, G.S. Multiple resistance of botrytis cinerea from kiwifruit to SDHIs, QoIs and fungicides of other chemical groups. Pest Manag. Sci. 2010, 66, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Colombi, E.; Straub, C.; Kunzel, S.; Templeton, M.D.; McCann, H.C.; Rainey, P.B. Evolution of copper resistance in the kiwifruit pathogen Pseudomonas syringae pv. actinidiae through acquisition of integrative conjugative elements and plasmids. Environ. Microbiol. 2017, 19, 819–832. [Google Scholar] [CrossRef]
- Gupta, S.; Chaturvedi, P.; Kulkarni, M.G.; van Staden, J. A critical review on exploiting the pharmaceutical potential of plant endophytic fungi. Biotechnol. Adv. 2020, 39, 107462. [Google Scholar] [CrossRef]
- Kusari, S.; Hertweck, C.; Spitellert, M. Chemical ecology of endophytic fungi: Origins of secondary metabolites. Chem. Biol. 2012, 19, 792–798. [Google Scholar] [CrossRef]
- Strobel, G.; Daisy, B.; Castillo, U.; Harper, J. Natural products from endophytic microorganisms. J. Nat. Prod. 2004, 67, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Helaly, S.E.; Thongbai, B.; Stadler, M. Diversity of biologically active secondary metabolites from endophytic and saprotrophic fungi of the ascomycete order Xylariales. Nat. Prod. Rep. 2018, 35, 992–1014. [Google Scholar] [CrossRef]
- Zhang, J.Y.; He, J.; Li, Z.H.; Feng, T.; Liu, J.K. Zopfiellasins A–D, two pairs of epimeric cytochalasins from kiwi-associated fungus Zopfiella sp. and their antibacterial assessment. Molecules 2021, 26, 5611. [Google Scholar] [CrossRef]
- Yi, X.W.; He, J.; Sun, L.T.; Liu, J.K.; Wang, G.K.; Feng, T. 3-Decalinoyltetramic acids from kiwi-associated fungus Zopfiella sp. and their antibacterial activity against Pseudomonas syringae. RSC Adv. 2021, 11, 18827–18831. [Google Scholar] [CrossRef]
- Ma, J.T.; Du, J.X.; Zhang, Y.; Liu, J.K.; Feng, T.; He, J. Natural imidazole alkaloids as antibacterial agents against Pseudomonas syringae pv. actinidiae isolated from kiwi endophytic fungus Fusarium tricinctum. Fitoterapia 2022, 156, 105070. [Google Scholar] [CrossRef]
- Frisch, M.J.T.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision D. 01; Gaussian Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Shao, Y.; Molnar, L.F.; Jung, Y.; Kussmann, J.; Ochsenfeld, C.; Brown, S.T.; Gilbert, A.T.B.; Slipchenko, L.V.; Levchenko, S.V.; O’Neill, D.P.; et al. Advances in methods and algorithms in a modern quantum chemistry program package. Phys. Chem. Chem. Phys. 2006, 8, 3172–3191. [Google Scholar] [CrossRef] [PubMed]
- Hehre, W.J. A Guide to Molecular Mechanics and Quantum Chemical Calculations; Wavefunction Inc.: Irvine, CA, USA, 2003; Volume 51, pp. 1–812. [Google Scholar] [CrossRef]
- Bruhn, T.; Schaumlöffel, A.; Hemberger, Y.; Bringmann, G. SpecDis: Quantifying the comparison of calculated and experimental electronic circular dichroism spectra. Chirality 2013, 25, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Grimblat, N.; Zanardi, M.M.; Sarotti, A.M. Beyond DP4: An improved probability for the stereochemical assignment of isomeric compounds using quantum chemical calculations of NMR shifts. J. Org. Chem. 2015, 80, 12526–12534. [Google Scholar] [CrossRef] [PubMed]
- Nukina, M.; Hattori, H.; Marumo, S. Cis-Sativenediol, a plant growth promotor, produced by fungi. J. Am. Chem. Soc. 1975, 97, 2542–2543. [Google Scholar] [CrossRef]
- Osterhage, C.; König, G.M.; Höller, U.; Wright, A.D. Rare sesquiterpenes from the algicolous fungus Drechslera dematioidea. J. Nat. Prod. 2002, 65, 306–313. [Google Scholar] [CrossRef]
- Dorn, F.; Arigoni, D. Ein bicyclischer Abkömmling von (−) longifolen aus Helminthosporium sativum und H. victoriae. Experientia 1974, 30, 851–852. [Google Scholar] [CrossRef]
- Han, J.; Zhang, J.; Song, Z.; Liu, M.; Hu, J.; Hou, C.; Zhu, G.; Jiang, L.; Xia, X.; Quinn, R.; et al. Genome- and MS-based mining of antibacterial chlorinated chromones and xanthones from the phytopathogenic fungus Bipolaris sorokiniana strain 11134. Appl. Microbiol. Biotechnol. 2019, 103, 5167–5181. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Yang, M.S.; Wang, W.X.; Li, Z.H.; Elkhateeb, W.; Wen, T.C.; Ai, H.L.; Feng, T. Anti-phytopathogenic sesquiterpenoid-xanthone adducts from potato endophytic fungus Bipolaris eleusines. RSC Adv. 2019, 9, 128–131. [Google Scholar] [CrossRef]

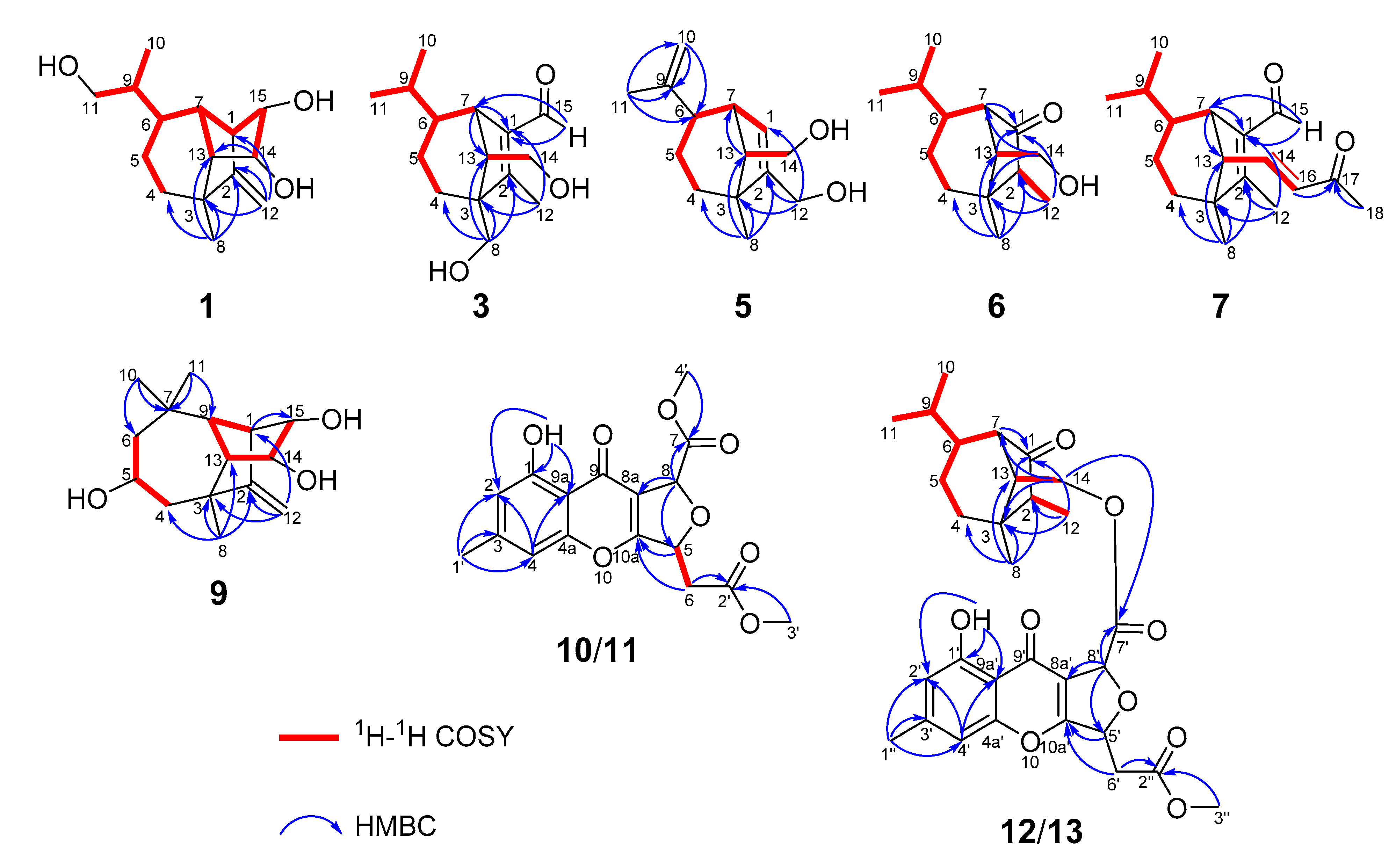
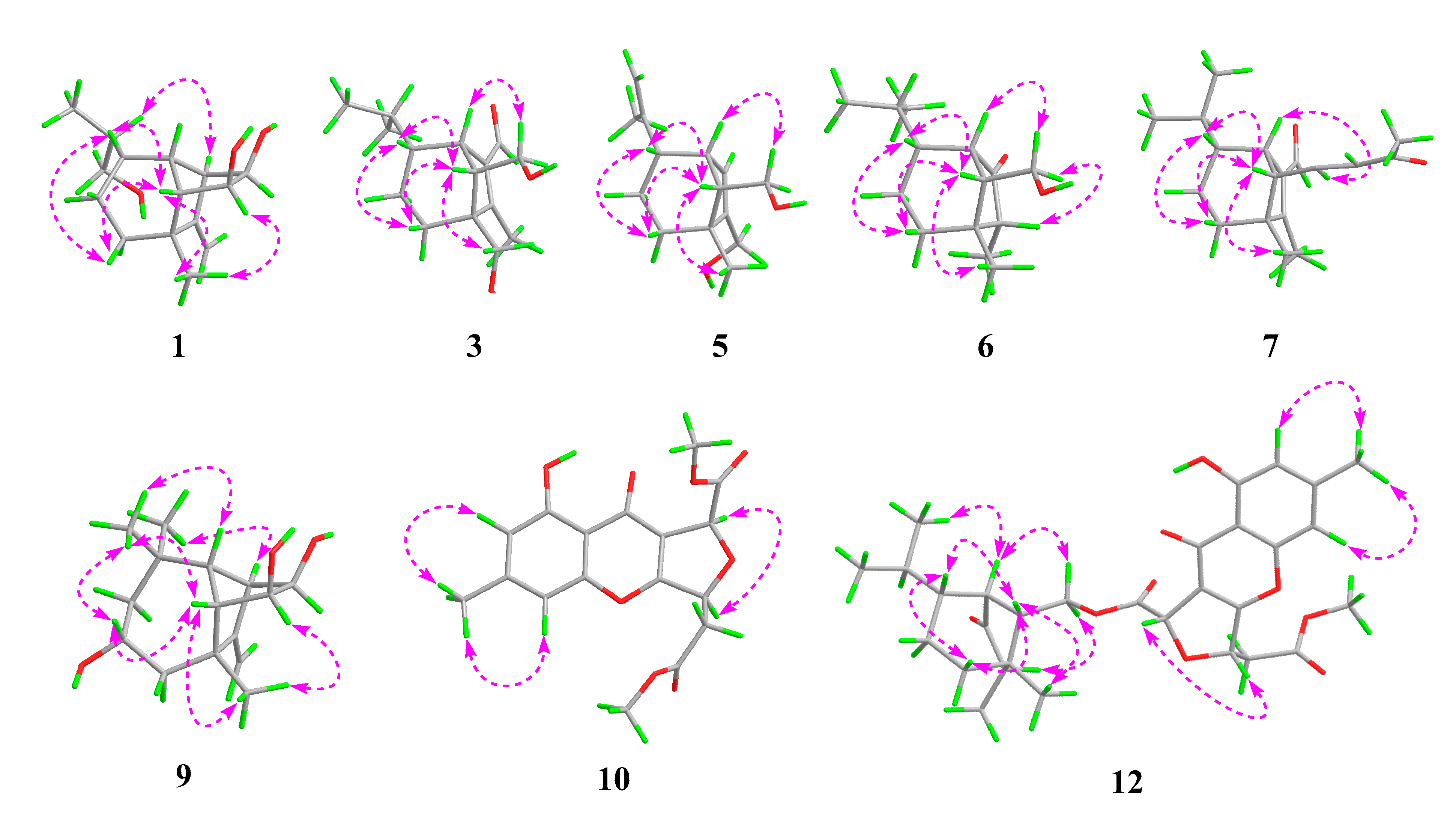

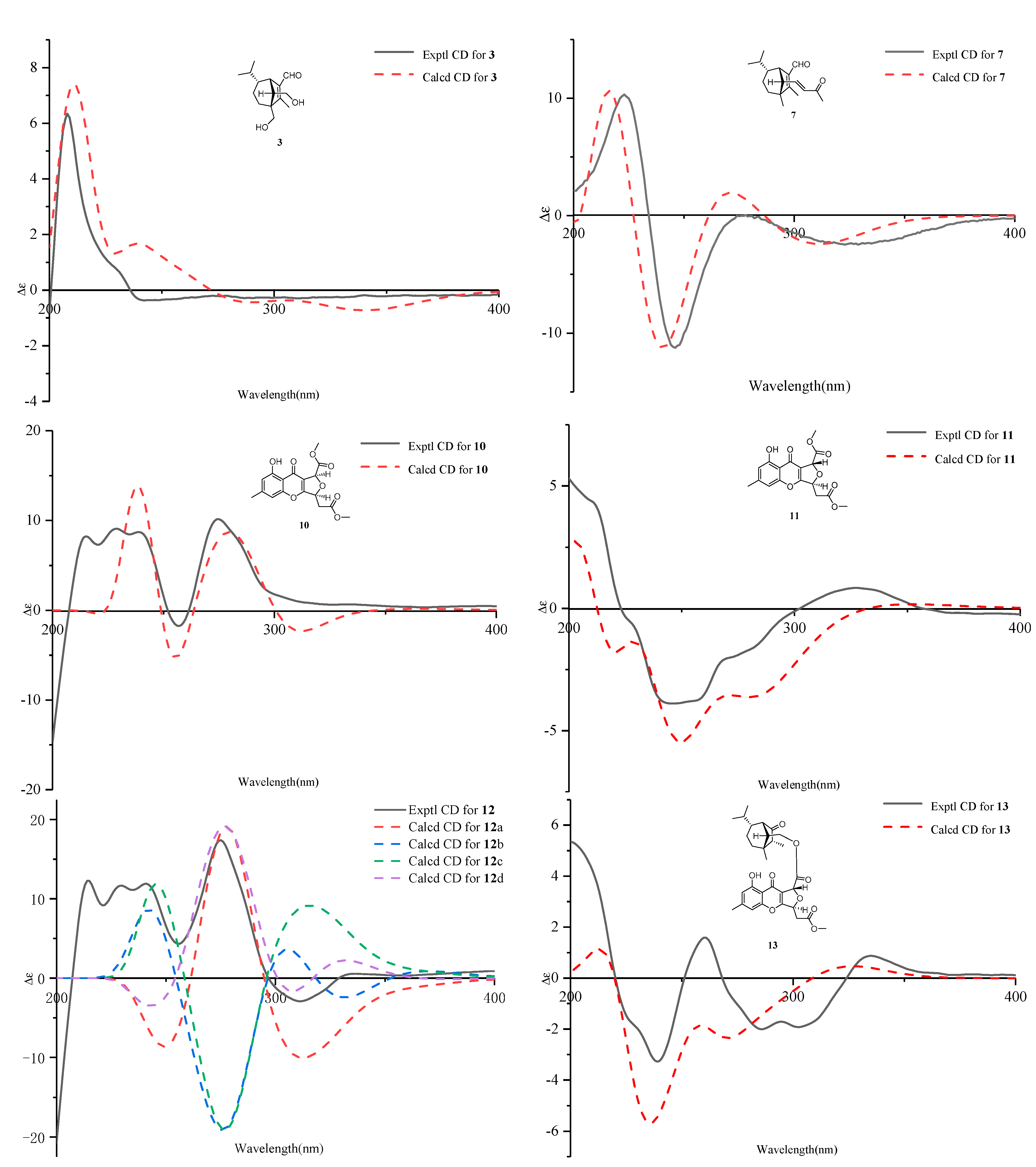
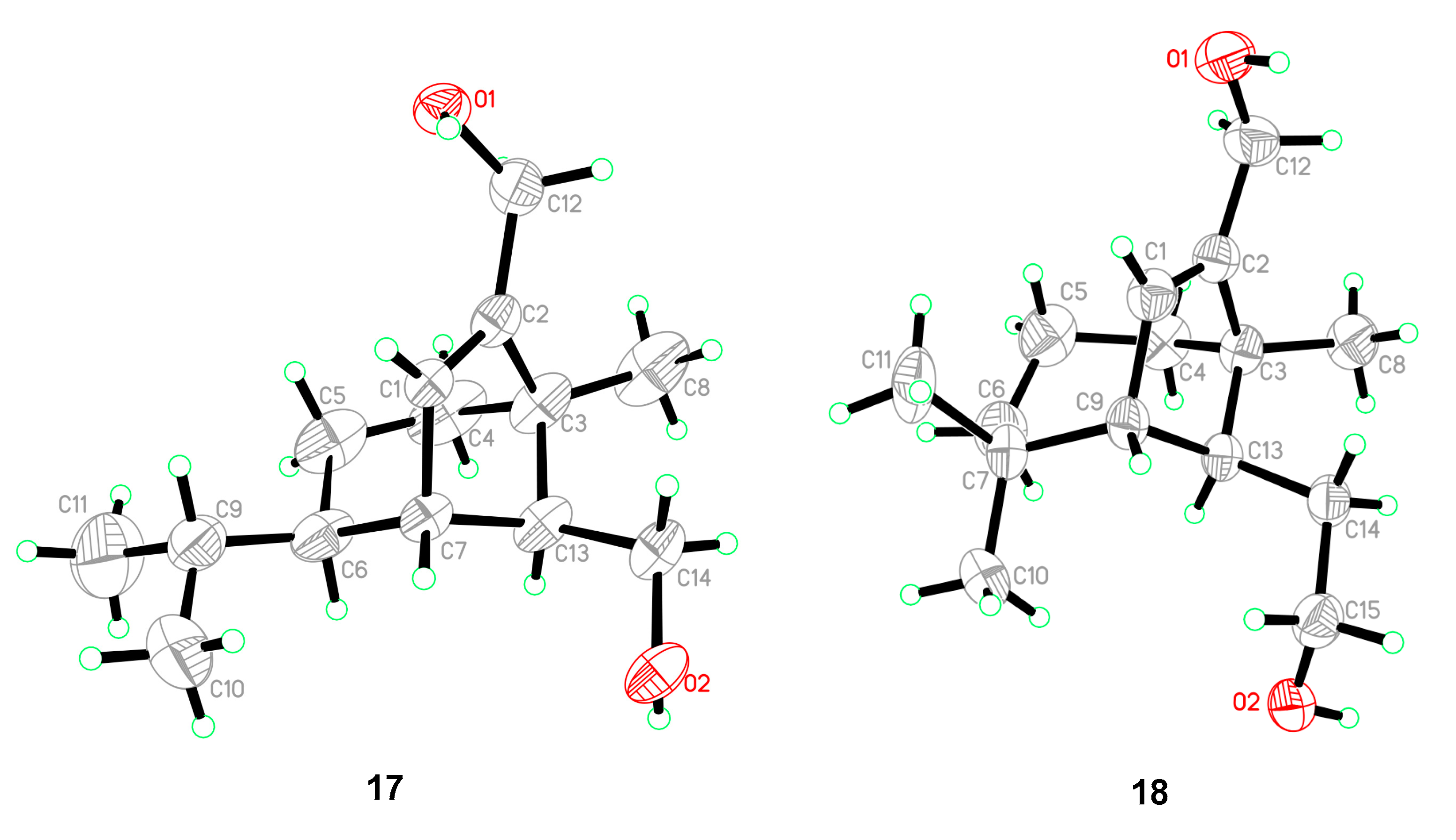
| No. | 1 a | 2 a | 3 b | |||
|---|---|---|---|---|---|---|
| δC, Type | δH (J in Hz) | δC, Type | δH (J in Hz) | δC, Type | δH (J in Hz) | |
| 1 | 54.3, CH | 2.71, s | 55.4, CH | 2.59, br s | 140,0, C | |
| 2 | 156.8, C | 155.7, C | 167.0, C | |||
| 3 | 43.0, C | 42.8, C | 58.0, C | |||
| 4a | 39.9, CH2 | 1.50, m | 36.7, CH2 | 1.23, m | 29.6, CH2 | 1.42, dd (13.2, 6.0) |
| 4b | 1.36, m | 1.74, m | 1.55, dd (12.8, 6.0) | |||
| 5a | 25.2, CH2 | 1.58, m | 32.3, CH2 | 1.44, m | 25.9, CH2 | 0.90, m |
| 5b | 1.24, m | 1.55, m | 1.80, m | |||
| 6 | 37.6, CH | 1.65, m | 73.7, C | 46.4, CH | 1.05, m | |
| 7 | 42.2, CH | 2.46, s | 47.8, CH | 2.44, br s | 42.7, CH | 3.06, br s |
| 8a | 20.8, CH3 | 1.05, s | 20.8, CH3 | 1.06, s | 64.6, CH2 | 3.63, d (11.6) |
| 8b | 3.71, d (11.6) | |||||
| 9 | 40.5, CH | 1.46, m | 36.9, CH | 1.57, m | 32.9, CH | 1.02, m |
| 10 | 15.4, CH3 | 0.92, d (6.8) | 16.2, CH3 | 0.88, d (6.9) | 21.1, CH3 | 0.78, d (6.4) |
| 11 | 66.9, CH2 | 3.64, overlap | 16.4, CH3 | 0.94, d (6.9) | 22.1, CH3 | 1.06, d (6.4) |
| 12a | 103.5, CH2 | 4.94, s | 105, CH2 | 4.69, s | 11.0, CH3 | 2.13, s |
| 12b | 4.62, s | 4.97, s | ||||
| 13 | 58.2, CH | 1.70, br s | 54.7, CH | 1.97, br s | 60.8, CH | 1.82, m |
| 14a | 69.6, CH | 4.02, d (5.9) | 69.5, CH | 4.07, d (6.1) | 62.9, CH2 | 3.34, dd (11.0, 6.8) |
| 14b | 3.61, dd (11.2, 6.8) | |||||
| 15 | 74.9, CH | 3.65, overlap | 74.8, CH | 3.68, d (6.1) | 190.0, CH | 10.02, s |
| No. | 4 b | 5 b | 6 a | |||
|---|---|---|---|---|---|---|
| δC, Type | δH (J in Hz) | δC, Type | δH (J in Hz) | δC, Type | δH (J in Hz) | |
| 1 | 140.5, C | 124.2, CH | 5.56, br s | 212.0, C | ||
| 2 | 170.4, C | 147.2, C | 50.7, CH | 2.10, m | ||
| 3 | 52.0, C | 47.7, C | 41.8, C | |||
| 4a | 32.4, CH2 | 1.38, m | 35.2, CH2 | 1.34, m | 36.1, CH2 | 1.44, m |
| 4b | 1.71, m | 1.41, dd (12.5, 5.2) | 1.66, dd (13.7,5.7) | |||
| 5a | 32.8, CH2 | 1.25, m | 26.0, CH2 | 1.56, m | 26.0, CH2 | 1.80, m |
| 5b | 1.61, m | 0.87, m | ||||
| 6 | 73.5, C | 45.2, CH | 2.03, m | 50.1, CH | 1.33, m | |
| 7 | 47.9, CH | 3.16, br s | 45.3, CH | 2.74, br s | 51.3, CH | 2.70, brs |
| 8 | 18.7, CH3 | 1.07, s | 18.9, CH3 | 0.99, s | 22.1, CH3 | 1.09, s |
| 9 | 37.2, CH | 1.28, m | 150.3, C | 29.9, CH | 1.55, m | |
| 10 | 17.1, CH3 | 1.02, d (6.6) | 109.2, CH2 | 4.69, d (5.1) | 20.3, CH3 | 1.03, d (6.5) |
| 11 | 16.4, CH3 | 0.80, d (6.6) | 22.7, CH3 | 1.74, s | 21.4, CH3 | 0.86, d (6.5) |
| 12 | 11.3, CH2 | 2.06, s | 59.8, CH2 | 4.06, m | 6.3, CH3 | 0.96, d (7.2) |
| 13 | 55.3, CH | 2.43, dd (9.1, 5.4) | 64.3, CH | 1.64, dd (9.6, 4.9) | 54.9, C | 1.72, dd (7.9, 5.0) |
| 14a | 62.3, CH2 | 3.19, dd (10.5, 9.1) | 62.5, CH2 | 3.38, m | 62.0, CH2 | 3.85, dd (10.7, 5.0) |
| 14b | 3.61, dd (10.5, 5.4) | 3.65, dd (10.5, 5.0) | 3.50, dd (10.7, 7.9) | |||
| 15 | 189.7, CH | 9.97, s | ||||
| No. | 7 a | 8 b | 9 b | |||
|---|---|---|---|---|---|---|
| δC, Type | δH (J in Hz) | δC, Type | δH (J in Hz) | δC, Type | δH (J in Hz) | |
| 1 | 137.5, C | 137.4, C | 57.5, CH | 2.54, br s | ||
| 2 | 165.3, C | 165.3, C | 163.7, C | |||
| 3 | 52.6, C | 52.5, C | 41.8, C | |||
| 4a | 33.7, CH2 | 1.41, dd (13.4, 5.9) | 33.6, CH2 | 1.40, dd (13.3, 5.9) | 53.2, CH2 | 1.66, dd (13.2, 10.4) |
| 4b | 1.50, dd (13.4, 6.4) | 1.48, dd (13.3, 6.5) | 2.10, dd (13.2, 10.4) | |||
| 5a | 25.2, CH2 | 0.91, m | 25.2, CH2 | 0.90, m | 67.0, CH | 3.84, m |
| 5b | 1.80, m | 1.78, m | ||||
| 6a | 44.3, CH | 1.06, m | 44.2, CH | 1.06, m | 47.2, CH2 | 1.21, m |
| 6b | 1.98, m | |||||
| 7 | 44.7, CH | 3.06, br s | 44.5, CH | 3.04, br s | 32.2, C | |
| 8 | 19.7, CH3 | 0.97, s | 19.6, CH3 | 0.96, s | 28.7, CH3 | 0.99, s |
| 9 | 31.6, CH | 1.03, m | 31.6, CH | 1.03, m | 55.0, CH | 2.02, br s |
| 10 | 21.7, CH3 | 1.06, d (5.9) | 21.7, CH3 | 1.04, d (5.8) | 30.3, CH3 | 1.09, s |
| 11 | 20.8, CH3 | 0.77, d (5.9) | 20.8, CH3 | 0.76, d (5.8) | 31.7, CH3 | 0.95, s |
| 12a | 11.0, CH3 | 2.06, s | 10.9, CH3 | 2.04, s | 103.9, CH2 | 4.75, br s |
| 12b | 4.97, br s | |||||
| 13 | 63.6, CH | 2.22, d (9.6) | 63.4, CH | 2.23, d (9.8) | 53.2, CH | 2.01, br s |
| 14 | 147.9, CH | 6.55, dd (15.9, 9.6) | 151.5, CH | 6.80, dd (15.4, 9.9) | 70.5, CH | 4.13, d (6.2) |
| 15 | 188.1, CH | 10.08, s | 188.1, CH | 10.05, s | 74.9, CH | 3.59, d (6.2) |
| 16 | 132.2, CH | 6.08, d (15.9) | 122.1, CH | 5.81, d (15.5) | ||
| 17 | 198.6, C | 171.1, C | ||||
| 18 | 27.5, CH3 | 2.20, s | ||||
| No. | 10 a | 11 a | ||
|---|---|---|---|---|
| δC, Type | δH (J in Hz) | δC, Type | δH (J in Hz) | |
| 1 | 161.1, C | 161.1, C | ||
| 2 | 113.7, CH | 6.68, s | 113.7, CH | 6.69, s |
| 3 | 147.7, C | 147.8, C | ||
| 4 | 108.1, CH | 6.75, s | 108.1, CH | 6.76, s |
| 4a | 157.4, C | 157.4, C | ||
| 5 | 78.2, CH | 5.73, ddd (6.6, 4.4, 3.9) | 78.6, CH | 5.62, ddd (8.4, 3.8, 1.7) |
| 6a | 37.7, CH2 | 3.01, dd (16.2, 4.4) | 39.3, CH2 | 3.10, dd (16.3, 8.4) |
| 6b | 2.85, dd (16.2, 6.6) | 2.99, dd (16.3, 3.8) | ||
| 7 | 170.0, C | 170.2, C | ||
| 8 | 79.4, CH | 5.64, d (3.9) | 79.8, CH | 5.63, d (1.7) |
| 8a | 114.7, C | 114.6, C | ||
| 9 | 178.3, C | 178.2, C | ||
| 9a | 109.0, C | 109.0, C | ||
| 10a | 167.7, C | 167.4, C | ||
| 1′ | 22.5, CH3 | 2.41, s | 22.5, CH3 | 2.42, s |
| 2′ | 169.5, C | 170.1, C | ||
| 3′ | 52.4, CH3 | 3.73, s | 52.5, CH3 | 3.78, s |
| 4′ | 53.0, CH3 | 3.81, s | 53.1, CH3 | 3.83, s |
| 1-OH | 12.06, s | 12.01, s | ||
| No. | 12 a | 13 a | ||
|---|---|---|---|---|
| δC, Type | δH (J in Hz) | δC, Type | δH (J in Hz) | |
| 1 | 221.6, C | 221.4, C | ||
| 2 | 50.6, CH | 2.16, m | 50.6, CH | 2.13, m |
| 3 | 42.1, C | 42.0, C | ||
| 4a | 36.0, CH2 | 1.45, m | 36.1, CH2 | 1.47, m |
| 4b | 1.66, m | 1.67, m | ||
| 5a | 26.0, CH2 | 0.84, m | 26.0, CH2 | 0.83, m |
| 5b | 1.78, m | 1.79, m | ||
| 6 | 50.2, CH | 1.29, m | 50.1, CH | 1.28, m |
| 7 | 51.5, CH | 2.56, br s | 51.5, CH | 2.62, br s |
| 8 | 22.1, CH3 | 1.08, s | 22.1, CH3 | 1.09, s |
| 9 | 29.9, CH | 1.41, m | 30.0, CH | 1.43, m |
| 10 | 20.4, CH3 | 0.77, d (6.6) | 20.4, CH3 | 0.78, d (6.7) |
| 11 | 21.3, CH3 | 0.89, d (6.4) | 21.3, CH3 | 0.92, d (6.5) |
| 12 | 6.5, CH3 | 0.95, d (7.2) | 6.5, CH3 | 0.96, d (7.2) |
| 13 | 51.6, CH | 1.90, m | 51.6, CH | 1.94, m |
| 14a | 65.3, CH2 | 4.05, dd (11.3, 5.1) | 65.5, CH2 | 4.04, dd (11.3, 5.2) |
| 14b | 4.35, dd (11.3, 5.1) | 4.37, dd (11.3, 5.2) | ||
| 1′ | 161.1, C | 161.1, C | ||
| 2′ | 113.6, CH | 6.67, s | 113.7, CH | 6.68, s |
| 3′ | 147.6, C | 147.8, C | ||
| 4′ | 108.1, CH | 6.75, s | 108.1, CH | 6.75, s |
| 4a′ | 157.3, C | 157.4, C | ||
| 5′ | 78.2, CH | 5.67, ddd (6.4, 4.3, 3.9) | 78.5, CH | 5.59, ddd (8.2, 3.9, 1.8) |
| 6′a | 37.7, CH2 | 2.99, dd (16.1, 4.3) | 39.2, CH2 | 3.07, dd (16.3, 8.2) |
| 6′b | 2.84, dd (16.1, 6.4) | 2.99, dd (16.3, 3.9) | ||
| 7′ | 169.5, C | 170.0, C | ||
| 8′ | 79.5, CH | 5.59, d (3.9) | 79.9, CH | 5.58, d (1.8) |
| 8a′ | 114.5, C | 114.5, C | ||
| 9′ | 178.2, C | 178.2, C | ||
| 9a′ | 109.0, C | 109.0, C | ||
| 10a′ | 167.7, C | 167.3, C | ||
| 1″ | 22.5, CH3 | 2.40, s | 22.5, CH3 | 2.41, s |
| 2″ | 169.4, C | 169.7, C | ||
| 3″ | 52.4, CH3 | 3.72, s | 52.5, CH3 | 3.72, s |
| 1′-OH | 12.06, s | 12.06, s | ||
| Compd | Psa | P. infestans | A. solani | R. solani | F. oxysporum |
|---|---|---|---|---|---|
| 3 | 256 | NA | 128 | 256 | NA |
| 4 | NA c | 128 | NA | NA | NA |
| 7 | 128 | NA | 64 | 128 | 256 |
| 8 | 256 | NA | 256 | NA | NA |
| 9 | NA | 128 | NA | NA | NA |
| 10 | 64 | 128 | NA | NA | NA |
| 11 | 128 | 64 | NA | NA | NA |
| 12 | 256 | 64 | NA | 64 | NA |
| 13 | 128 | 32 | NA | NA | NA |
| 14 | NA | NA | 8 | NA | 128 |
| 15 | 16 | NA | 16 | NA | NA |
| 16 | 128 | 128 | 128 | 256 | NA |
| Streptomycin b | 8 | − | − | − | − |
| Hygromycin B b | − | 8 | 4 | 16 | 32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.-J.; Jin, Y.-X.; Huang, S.-S.; He, J. Sesquiterpenoids and Xanthones from the Kiwifruit-Associated Fungus Bipolaris sp. and Their Anti-Pathogenic Microorganism Activity. J. Fungi 2022, 8, 9. https://doi.org/10.3390/jof8010009
Yu J-J, Jin Y-X, Huang S-S, He J. Sesquiterpenoids and Xanthones from the Kiwifruit-Associated Fungus Bipolaris sp. and Their Anti-Pathogenic Microorganism Activity. Journal of Fungi. 2022; 8(1):9. https://doi.org/10.3390/jof8010009
Chicago/Turabian StyleYu, Jun-Jie, Ying-Xue Jin, Shan-Shan Huang, and Juan He. 2022. "Sesquiterpenoids and Xanthones from the Kiwifruit-Associated Fungus Bipolaris sp. and Their Anti-Pathogenic Microorganism Activity" Journal of Fungi 8, no. 1: 9. https://doi.org/10.3390/jof8010009
APA StyleYu, J.-J., Jin, Y.-X., Huang, S.-S., & He, J. (2022). Sesquiterpenoids and Xanthones from the Kiwifruit-Associated Fungus Bipolaris sp. and Their Anti-Pathogenic Microorganism Activity. Journal of Fungi, 8(1), 9. https://doi.org/10.3390/jof8010009






