The Gβ-like Protein AfCpcB Affects Sexual Development, Response to Oxidative Stress and Phagocytosis by Alveolar Macrophages in Aspergillus fumigatus
Abstract
1. Introduction
2. Materials and Methods
2.1. The Strains, Media, and Growth Conditions
2.2. Generation of Strains
2.3. Sensitivity Test to Chemical Agents
2.4. Physiological Studies
2.5. Microscopy
2.6. RNA Isolation and Real-Time Quantitative PCR (RT-qPCR)
2.7. Phagocytosis Assay
2.8. β-glucan Analysis
2.9. Biofilm Formation Assay
2.10. Statistical Analysis
3. Results
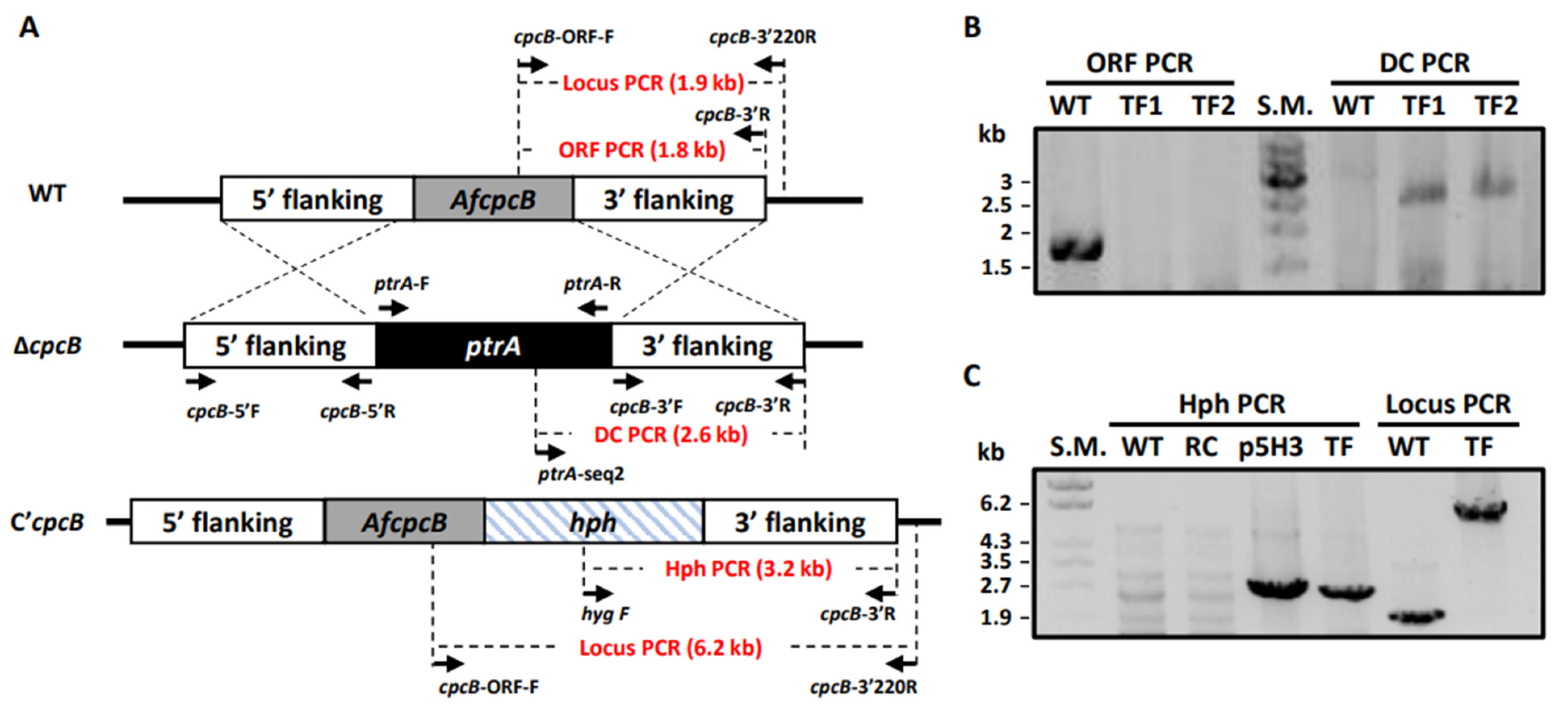
3.1. Targeted Deletion of AfcpcB
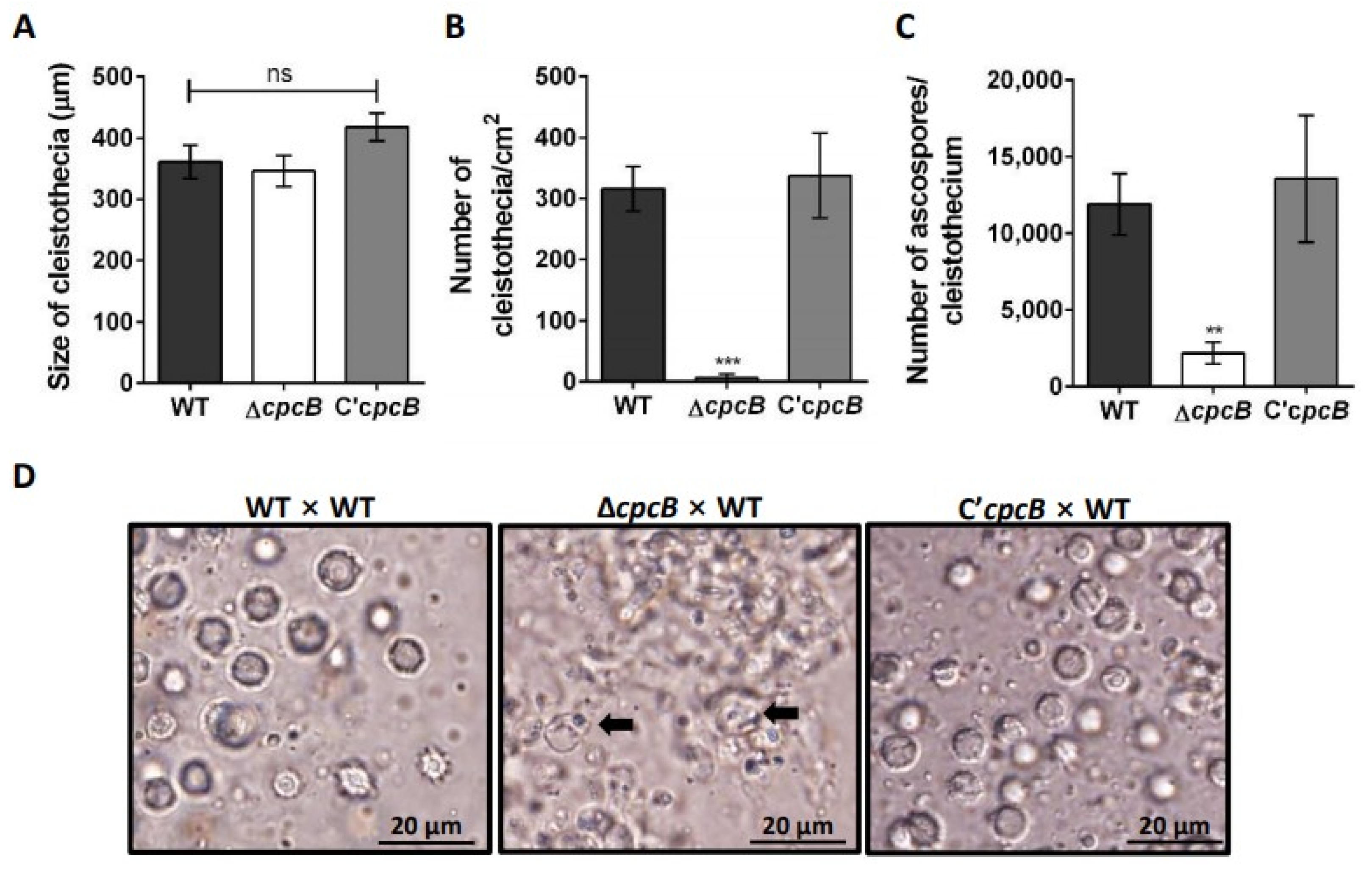
3.2. AfCpcB Is Required for Complete Sexual Development
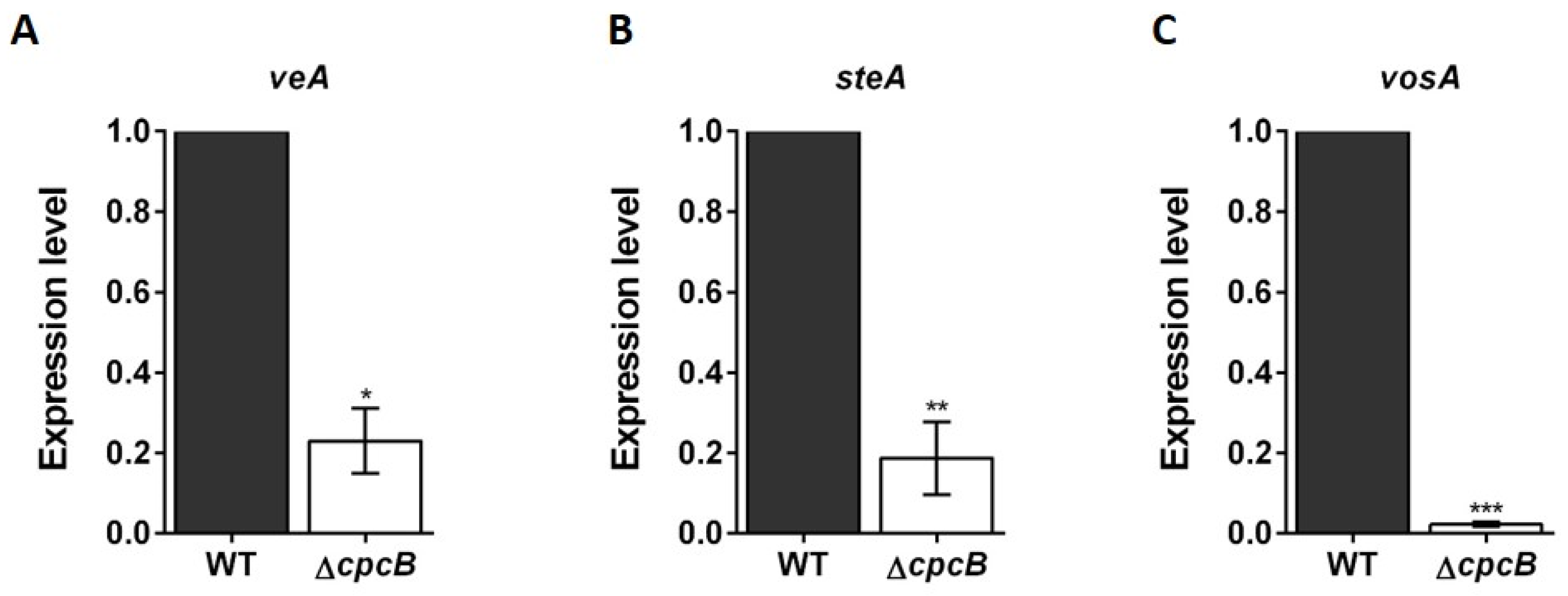
3.3. Transcript Expression Patterns in Cleistothecia
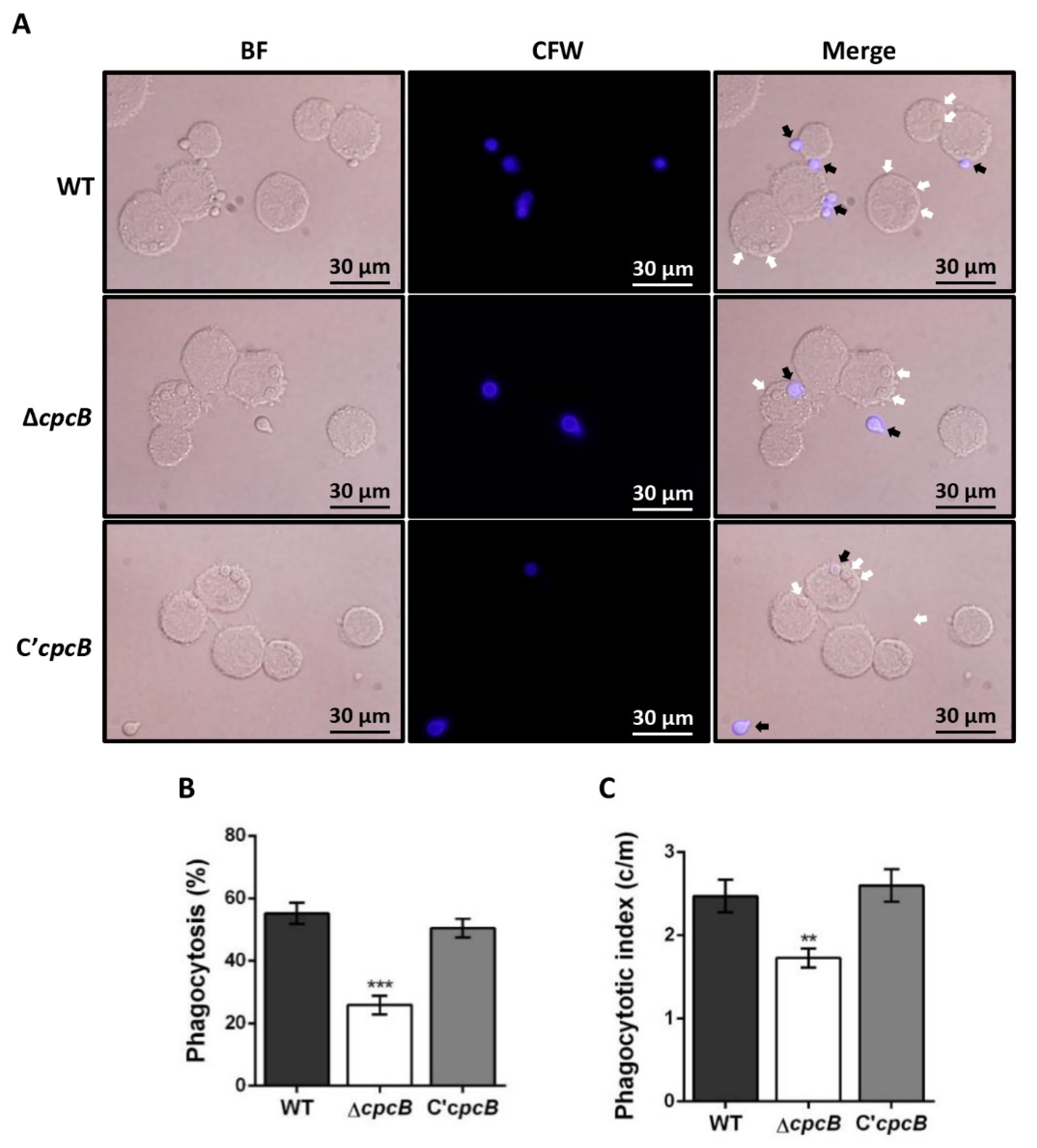
3.4. The ΔAfcpcB Mutant Is More Resistant to Phagocytosis by Alveolar Macrophages
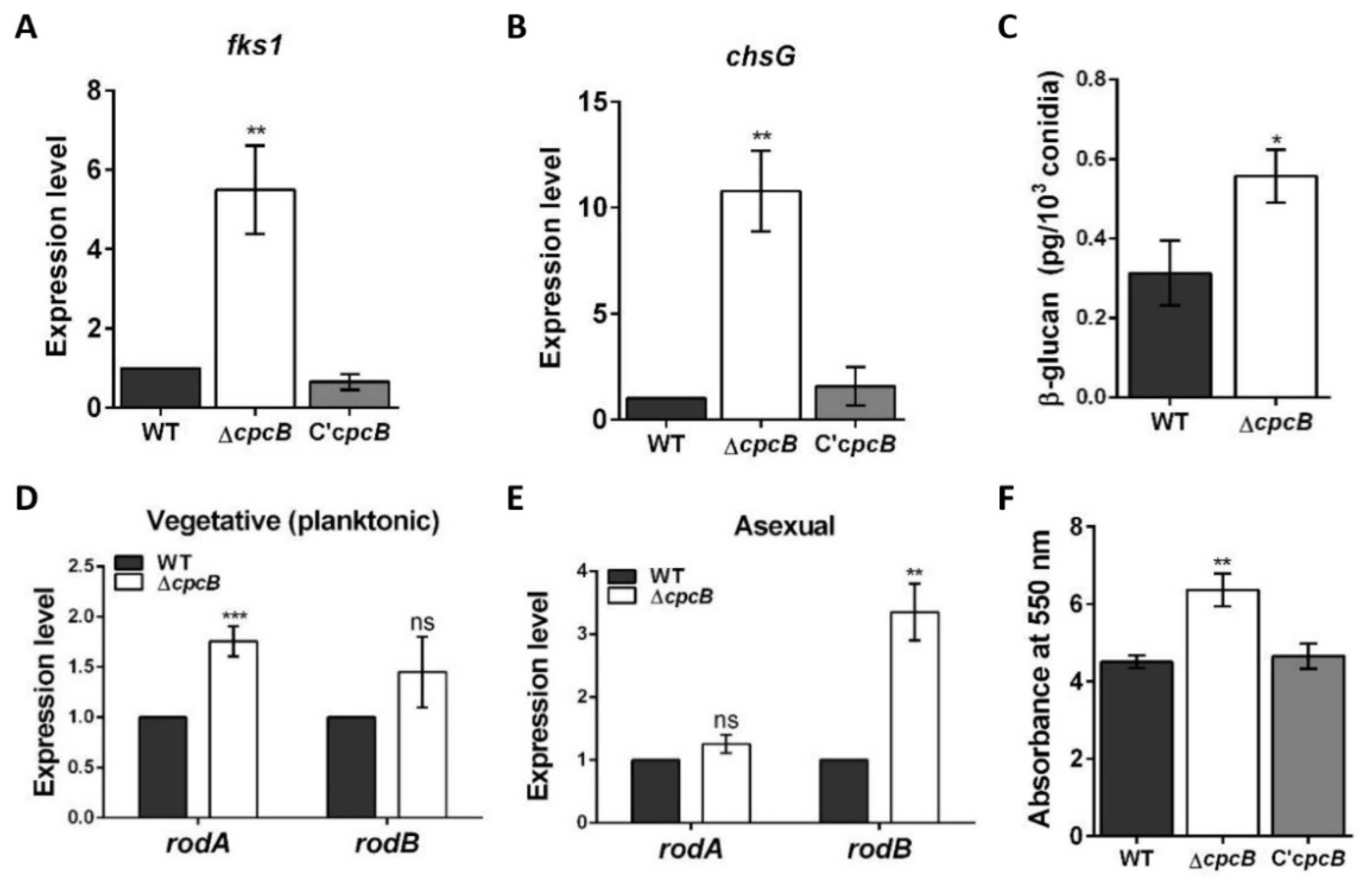
3.5. The AfcpcB Is Required for Biosynthesis of Cell Wall Components and Hydrophobin
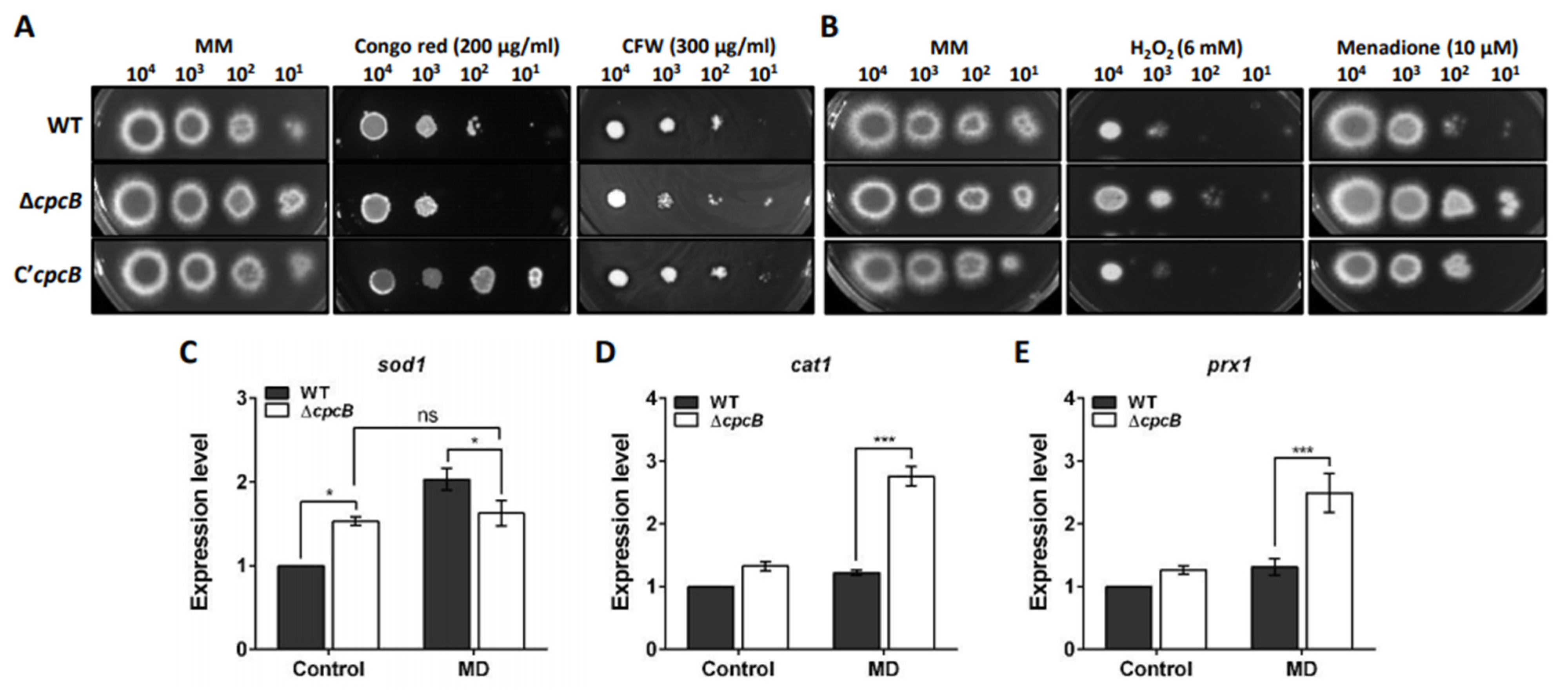
3.6. AfCpcB Functions in Response to Cell Wall and Oxidative Stress
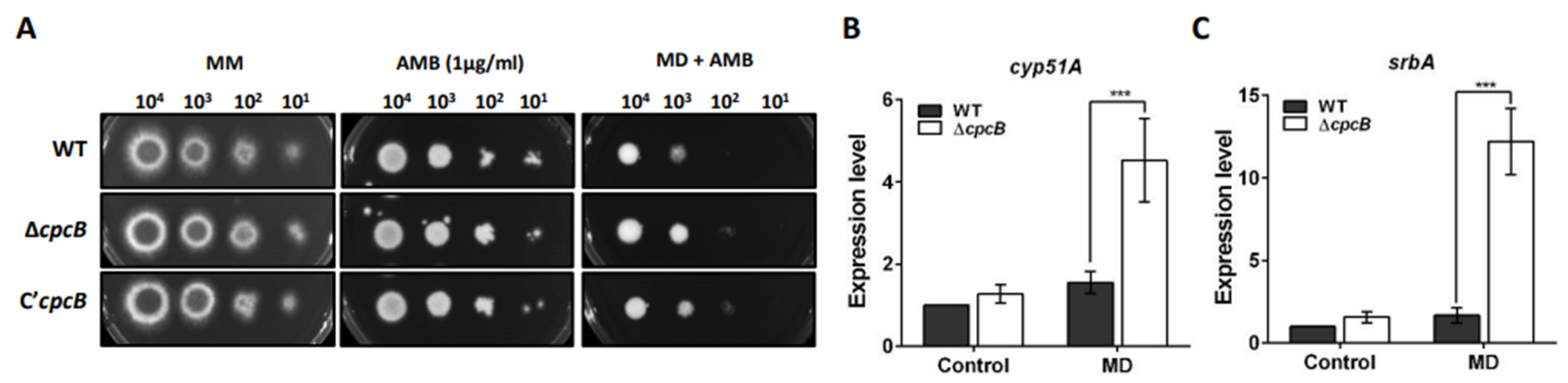
3.7. The ΔAfcpcB Strain under Oxidative Stress Showed Increased Expression of Ergosterol Biosynthesis-Related Genes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statements
Conflicts of Interest
References
- Richardson, M.; Bowyer, P.; Sabino, R. The human lung and Aspergillus: You are what you breathe in? Med. Mycol. 2019, 57, S145–S154. [Google Scholar] [CrossRef]
- Sakai, K.; Hiemori, K.; Tateno, H.; Hirabayashi, J.; Gonoi, T. Fucose-specific lectin of Aspergillus fumigatus: Binding properties and effects on immune response stimulation. Med. Mycol. 2019, 57, 71–83. [Google Scholar] [CrossRef]
- Abad, A.; Victoria Fernández-Molina, J.; Bikandi, J.; Ramírez, A.; Margareto, J.; Sendino, J.; Luis Hernando, F.; Pontón, J.; Garaizar, J.; Rementeria, A. What makes Aspergillus fumigatus a successful pathogen? Genes and molecules involved in invasive aspergillosis. Rev. Iberoam. Micol. 2010, 27, 155–182. [Google Scholar] [CrossRef]
- Pappas, P.G.; Alexander, B.D.; Andes, D.R.; Hadley, S.; Kauffman, C.A.; Freifeld, A.; Anaissie, E.J.; Brumble, L.M.; Herwaldt, L.; Ito, J.; et al. Invasive fungal infections among organ transplant recipients: Results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin. Infect. Dis. 2010, 50, 1101–1111. [Google Scholar] [CrossRef]
- Koehler, P.; Cornely, O.A.; Böttiger, B.W.; Dusse, F.; Eichenauer, D.A.; Fuchs, F.; Hallek, M.; Jung, N.; Klein, F.; Persigehl, T.; et al. COVID-19 associated pulmonary aspergillosis. Mycoses 2020, 63, 528–534. [Google Scholar] [CrossRef]
- Chan, J.F.; Lau, S.K.; Wong, S.C.; To, K.K.; So, S.Y.; Leung, S.S.; Chan, S.; Pang, C.; Xiao, C.; Hung, I.F.; et al. A 10-year study reveals clinical and laboratory evidence for the ‘semi-invasive’ properties of chronic pulmonary aspergillosis. Emerg. Microbes Infect. 2016, 5, 1–7. [Google Scholar] [CrossRef]
- Lafon, A.; Han, K.; Seo, J.; Yu, J. G-protein and cAMP-mediated signaling in aspergilli: A genomic perspective. Fungal Genet. Biol. 2019, 43, 490–502. [Google Scholar] [CrossRef]
- Cai, Z.D.; Chai, Y.F.; Zhang, C.Y.; Qiao, W.R.; Sang, H.; Lu, L. The Gβ-like protein CpcB is required for hyphal growth, conidiophore morphology and pathogenicity in Aspergillus fumigatus. Fungal Genet. Biol. 2015, 81, 120–131. [Google Scholar] [CrossRef]
- Cai, Z.; Chai, Y.; Zhang, C.; Feng, R.; Sang, H.; Lu, L. Molecular characterization of Gβ-like protein CpcB involved in antifungal drug susceptibility and virulence in A. fumigatus. Front. Microbiol. 2016, 7, 106. [Google Scholar] [CrossRef]
- Hoffmann, B.; Wanke, C.; LaPaglia, S.K.; Braus, G.H. c-Jun and RACK1 homologues regulate a control point for sexual development in Aspergillus nidulans. Mol. Microbiol. 2000, 37, 28–41. [Google Scholar] [CrossRef]
- Kong, Q.; Wang, L.; Liu, Z.; Kwon, N.; Kim, S.C.; Yu, J.H. Gβ-Like CpcB plays a crucial role for growth and development of Aspergillus nidulans and Aspergillus fumigatus. PLoS ONE 2013, 8, e70355. [Google Scholar] [CrossRef]
- O’Gorman, C.M.; Fuller, H.T.; Dyer, P.S. Discovery of a sexual cycle in the opportunistic fungal pathogen Aspergillus fumigatus. Nature 2009, 457, 471–474. [Google Scholar] [CrossRef]
- Sugui, J.A.; Losada, L.; Wang, W. Identification and characterization of an Aspergillus fumigatus supermater pair. MBio 2011, 2, e00234-11. [Google Scholar] [CrossRef]
- Lim, J.Y.; Park, H.M. Expression of sexual genes in Aspergillus fumigatus homogeneous culture produced by vegetative mass mating. J. Microbiol. 2019, 57, 1–6. [Google Scholar] [CrossRef]
- Bellocchio, S.; Bozza, S.; Montagnoli, C.; Perruccio, K.; Gaziano, R.; Pitzurra, L.; Romani, L. Immunity to Aspergillus fumigatus: The basis for immunotherapy and vaccination. Med. Mycol. 2005, 43, 181–188. [Google Scholar] [CrossRef]
- Akoumianaki, T.; Kyrmizi, I.; Valsecchi, I.; Beauvais, A.; Latge, J.P.; Chamilos, G. Aspergillus cell wall melanin blocks LC3-associated phagocytosis to promote pathogenicity. Cell Host Microbe 2016, 19, 79–90. [Google Scholar] [CrossRef]
- Hatinguais, R.; Willment, J.A.; Brown, G.D. PAMPs of the fungal cell wall and mammalian PRRs. Curr. Top. Microbiol. Immunol. 2020, 425, 187–223. [Google Scholar] [CrossRef]
- Heinekamp, T.; Thywißen, A.; Macheleidt, J.; Keller, S.; Valiante, V.; Brakhage, A.A. Aspergillus fumigatus melanins: Interference with the host endocytosis pathway and impact on virulence. Front. Microbiol. 2012, 3, 440. [Google Scholar] [CrossRef]
- Langfelder, K.; Streibel, M.; Jahn, B.; Haase, G.; Brakhage, A.A. Biosynthesis of fungal melanins and their importance for human pathogenic fungi. Fungal Genet. Biol. 2003, 38, 143–158. [Google Scholar] [CrossRef]
- Dagenais, T.R.T.T.; Keller, N.P. Pathogenesis of Aspergillus fumigatus in invasive aspergillosis. Clin. Microbiol. Rev. 2009, 22, 447–465. [Google Scholar] [CrossRef]
- Beauvais, A.; Latgé, J.P. Aspergillus biofilm in vitro and in vivo. Microbiol. Spectr. 2015, 3, 1–10. [Google Scholar] [CrossRef]
- Aimanianda, V.; Latgé, J.P. Fungal hydrophobins form a sheath preventing immune recognition of airborne conidia. Virulence 2010, 1, 185–187. [Google Scholar] [CrossRef]
- Matherne, G.P.; Headrick, J.P.; Coleman, S.D.; Berne, R.M. Interstitial transudate purines in normoxic and hypoxic immature and mature rabbit hearts. Pediatr. Res. 1990, 28, 348–353. [Google Scholar] [CrossRef]
- Fröhlich, S.; Boylan, J.; Mcloughlin, P. Hypoxia-induced inflammation in the lung: A potential therapeutic target in acute lung injury? Am. J. Respir. Cell Mol. Biol. 2013, 48, 271–279. [Google Scholar] [CrossRef]
- Willger, S.D.; Puttikamonkul, S.; Kim, K.H.; Burritt, J.B.; Grahl, N.; Metzler, L.J.; Barbuch, R.; Bard, M.; Lawrence, C.B.; Cramer, R.A. A sterol-regulatory element binding protein is required for cell polarity, hypoxia adaptation, azole drug resistance, and virulence in Aspergillus fumigatus. PLoS Pathog. 2008, 4, e1000200. [Google Scholar] [CrossRef]
- Han, K.H.; Lee, D.B.; Kim, J.H.; Kim, M.S.; Han, K.Y.; Kim, W.S.; Park, Y.S.; Kim, H.B.; Han, D.M. Environmental factors affecting development of Aspergillus nidulans. J. Microbiol. 2003, 41, 34–40. [Google Scholar]
- Zhang, J.; Snelders, E.E.; Zwaan, B.J.; Schoustra, S.E.; Kuijper, E.J.; Arendrup, M.C.; Melchers, W.J.G.; Verweij, P.E.; Debets, A.J.M. Relevance of heterokaryosis for adaptation and azole-resistance development in Aspergillus fumigatus. Proc. Biol. Sci. 2019, 286, 20182886. [Google Scholar] [CrossRef]
- Gintjee, T.J.; Donnelley, M.A.; Thompson, G.R. Aspiring antifungals: Review of current antifungal pipeline developments. J. Fungi 2020, 6, 28. [Google Scholar] [CrossRef]
- Mellado, E.; Diaz-Guerra, T.M.; Cuenca-Estrella, M.; Rodriguez-Tudela, J.L. Identification of two different 14-α sterol demethylase- related genes (cyp51A and cyp51B) in Aspergillus fumigatus and other Aspergillus species. J. Clin. Microbiol. 2001, 39, 2431–2438. [Google Scholar] [CrossRef]
- Van Rhijn, N.; Bromley, M.; Richardson, M.; Bowyer, P. CYP51 paralogue structure is associated with intrinsic azole resistance in fungi. MBio 2021, 12, e01945-21. [Google Scholar] [CrossRef]
- Zhang, J.; Snelders, E.; Zwaan, B.J.; Schoustra, S.E.; Meis, J.F.; Van Dijk, K.; Hagen, F.; Van Der Beek, M.T.; Kampinga, G.A.; Zoll, J.; et al. A novel environmental azole resistance mutation in Aspergillus fumigatus and a possible role of sexual reproduction in its emergence. MBio 2017, 8, e00791-17. [Google Scholar] [CrossRef]
- Verweij, P.E.; Zhang, J.; Debets, A.J.M.; Meis, J.F.; van de Veerdonk, F.L.; Schoustra, S.E.; Zwaan, B.J.; Melchers, W.J.G. In-host adaptation and acquired triazole resistance in Aspergillus fumigatus: A dilemma for clinical management. Lancet Infect. Dis. 2016, 16, e251–e260. [Google Scholar] [CrossRef]
- Käfer, E. Meiotic and mitotic recombination in Aspergillus and its chromosomal aberrations. Adv. Genet. 1977, 19, 33–131. [Google Scholar] [CrossRef]
- Oakley, B.R.; Szewczyk, E.; Nayak, T.; Oakley, C.E.; Edgerton, H.; Xiong, Y.; Taheri-Talesh, N.; Osmani, S.A. Fusion PCR and gene targeting in Aspergillus nidulans. Nat. Protoc. 2006, 1, 3111–3120. [Google Scholar] [CrossRef]
- Kubodera, T.; Yamashita, N.; Nishimura, A. Pyrithiamine resistance gene (ptrA) of Aspergillus oryzae: Cloning, characterization and application as a dominant selectable marker for transformation. Biosci. Biotechnol. Biochem. 2000, 64, 1416–1421. [Google Scholar] [CrossRef]
- Park, H.S.; Yu, J.H. Multi-copy genetic screen in Aspergillus nidulans. Methods Mol. Biol. 2012, 944, 183–190. [Google Scholar] [CrossRef]
- Lim, J.Y.; Kang, E.H.; Park, Y.H.; Kook, J.H.; Park, H.M. Survival factor SvfA plays multiple roles in differentiation and is essential for completion of sexual development in Aspergillus nidulans. Sci. Rep. 2020, 10, 5586. [Google Scholar] [CrossRef]
- Geoghegan, I.A.; Emes, R.D.; Archer, D.B.; Avery, S.V. Method for RNA extraction and transcriptomic analysis of single fungal spores. MethodsX 2020, 7, 50–55. [Google Scholar] [CrossRef]
- Park, D.S.; Yu, Y.M.; Kim, Y.J.; Maeng, P.J. Negative regulation of the vacuole-mediated resistance to K+ stress by a novel C2H2 zinc finger transcription factor encoded by aslA in Aspergillus nidulans. J. Microbiol. 2015, 53, 100–110. [Google Scholar] [CrossRef]
- Matsunaga, K.; Klein, T.W.; Friedman, H.; Yamamoto, Y. Alveolar macrophage cell line MH-S is valuable as an in vitro model for Legionella pneumophila infection. Am. J. Respir. Cell Mol. Biol. 2001, 24, 326–331. [Google Scholar] [CrossRef]
- Philippe, B.; Boleti, H.; Grenet, D.; Stern, M.; Latgé, J.P.; Latge, J.P. Phagocytosis and intracellular fate of Aspergillus fumigatus conidia in alveolar macrophages. Infect. Immun. 2003, 71, 891–903. [Google Scholar] [CrossRef]
- Rocha, M.C.; Fabri, J.H.T.M.; Franco de Godoy, K.; Alves de Castro, P.; Hori, J.I.; Ferreira da Cunha, A.; Arentshorst, M.; Ram, A.F.J.; van den Hondel, C.A.; Goldman, G.H.; et al. Aspergillus fumigatus MADS-Box transcription factor rlmA is required for regulation of the cell wall integrity and virulence. G3 2016, 6, 2983–3002. [Google Scholar] [CrossRef]
- Lim, J.Y.; Kim, Y.J.; Woo, S.A.; Jeong, J.W.; Lee, Y.R.; Kim, C.H.; Park, H.M. The LAMMER kinase, LkhA, affects Aspergillus fumigatus pathogenicity by modulating reproduction and biosynthesis of cell wall PAMPs. Front. Cell. Infect. Microbiol. 2021, 11, 756206. [Google Scholar] [CrossRef]
- Lohmar, J.M.; Puel, O.; Cary, J.W.; Calvo, A.M. The Aspergillus flavus rtfA gene regulates plant and animal pathogenesis and secondary metabolism. Appl. Environ. Microbiol. 2019, 85, e02446-18. [Google Scholar] [CrossRef]
- da Silva Ferreira, M.E.; Kress, M.; Savoldi, M.; Goldman, M.H.S.; Härtl, A.; Heinekamp, T.; Brakhage, A.A.; Goldman, G.H. The akuBKU80 mutant deficient for nonhomologous end joining is a powerful tool for analyzing pathogenicity in Aspergillus fumigatus. Eukaryot. Cell 2006, 5, 207–211. [Google Scholar] [CrossRef]
- Zhang, C.; Meng, X.; Wei, X.; Lu, L. Highly efficient CRISPR mutagenesis by microhomology-mediated end joining in Aspergillus fumigatus. Fungal Genet. Biol. 2016, 86, 47–57. [Google Scholar] [CrossRef]
- Bernstein, H.; Byers, G.S.; Michod, R.E. Evolution of sexual reproduction: Importance of DNA Repair, complementation, and variation. Am. Nat. 1981, 117, 537–549. [Google Scholar] [CrossRef]
- Dhingra, S.; Andes, D.; Calvoa, A.M. VeA regulates conidiation, gliotoxin production, and protease activity in the opportunistic human pathogen Aspergillus fumigatus. Eukaryot. Cell 2012, 11, 1531–1543. [Google Scholar] [CrossRef]
- Kim, H.S.; Han, K.Y.; Kim, K.J.; Han, D.M.; Jahng, K.Y.; Chae, K.S. The veA gene activates sexual development in Aspergillus nidulans. Fungal Genet. Biol. 2002, 37, 72–80. [Google Scholar] [CrossRef]
- Vallim, M.A.; Miller, K.Y.; Miller, B.L. Aspergillus SteA (sterile12-like) is a homeodomain-C2/H2-Zn+2 finger transcription factor required for sexual reproduction. Mol. Microbiol. 2000, 36, 290–301. [Google Scholar] [CrossRef]
- Ni, M.; Yu, J.H. A novel regulator couples sporogenesis and trehalose biogenesis in Aspergillus nidulans. PLoS ONE 2007, 2, e970. [Google Scholar] [CrossRef]
- Mellado, E.; Aufauvre-Brown, A.; Gow, N.A.R.; Holden, D.W. The Aspergillus fumigatus chsC and chsG genes encode Class III chitin synthases with different functions. Mol. Microbiol. 1996, 20, 667–679. [Google Scholar] [CrossRef]
- Gibbons, J.G.; Beauvais, A.; Beau, R.; McGary, K.L.; Latgé, J.P.; Rokas, A. Global transcriptome changes underlying colony growth in the opportunistic human pathogen Aspergillus fumigatus. Eukaryot. Cell 2012, 11, 68–78. [Google Scholar] [CrossRef]
- Valsecchi, I.; Dupres, V.; Stephen-Victor, E.; Guijarro, J.I.; Gibbons, J.; Beau, R.; Bayry, J.; Coppee, J.Y.; Lafont, F.; Latgé, J.P.; et al. Role of hydrophobins in Aspergillus fumigatus. J. Fungi 2018, 4, 2. [Google Scholar] [CrossRef]
- He, X.; Li, S.; Kaminskyj, S.G.W. Using Aspergillus nidulans to identify antifungal drug resistance mutations. Eukaryot. Cell 2014, 13, 288–294. [Google Scholar] [CrossRef]
- Valiante, V.; Macheleidt, J.; Föge, M.; Brakhage, A.A. The Aspergillus fumigatus cell wall integrity signalling pathway: Drug target, compensatory pathways and virulence. Front. Microbiol. 2015, 6, 1–12. [Google Scholar] [CrossRef]
- Roncero, C.; Durán, A. Effect of Calcofluor white and Congo red on fungal cell wall morphogenesis: In vivo activation of chitin polymerization. J. Bacteriol. 1985, 163, 1180–1185. [Google Scholar] [CrossRef]
- Liu, Z.; Raj, S.; van Rhijn, N.; Fraczek, M.; Michel, J.P.; Sismeiro, O.; Legendre, R.; Varet, H.; Fontaine, T.; Bromley, M.; et al. Functional genomic and biochemical analysis reveals pleiotropic effect of congo red on Aspergillus fumigatus. MBio 2021, 12, e00863-21. [Google Scholar] [CrossRef]
- Shin, K.S.; Yu, J.H. Expression and activity of catalases is differentially affected by GpaA (Ga) and FlbA (Regulator of G Protein Signaling) in Aspergillus fumigatus. Mycobiology 2013, 41, 145–148. [Google Scholar] [CrossRef][Green Version]
- Chi, M.H.; Craven, K.D. RacA-mediated ROS signaling is required for polarized cell differentiation in conidiogenesis of Aspergillus fumigatus. PLoS ONE 2016, 11, e0149548. [Google Scholar] [CrossRef][Green Version]
- Van De Veerdonk, F.L.; Gresnigt, M.S.; Romani, L.; Netea, M.G.; Latgé, J.P. Aspergillus fumigatus morphology and dynamic host interactions. Nat. Rev. Microbiol. 2017, 15, 661–674. [Google Scholar] [CrossRef]
- Calera, J.A.; Paris, S.; Monod, M.; Hamilton, A.J.; Debeaupuis, J.P.; Diaquin, M.; López-Medrano, R.; Leal, F.; Latgé, J.P. Cloning and disruption of the antigenic catalase gene of Aspergillus fumigatus. Infect. Immun. 1997, 65, 4718–4724. [Google Scholar] [CrossRef] [PubMed]
- Rocha, M.C.; de Godoy, K.F.; Bannitz-Fernandes, R.; Fabri, J.H.T.M.; Barbosa, M.M.F.; de Castro, P.A.; Almeida, F.; Goldman, G.H.; da Cunha, A.F.; Netto, L.E.S.; et al. Analyses of the three 1-Cys peroxiredoxins from Aspergillus fumigatus reveal that cytosolic Prx1 is central to H2O2 metabolism and virulence. Sci. Rep. 2018, 8, 12314. [Google Scholar] [CrossRef] [PubMed]
- Lambou, K.; Lamarre, C.; Beau, R.; Dufour, N.; Latge, J.P. Functional analysis of the superoxide dismutase family in Aspergillus fumigatus. Mol. Microbiol. 2010, 75, 910–923. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Ferreira, M.E.; Colombo, A.L.; Paulsen, I.; Ren, Q.; Wortman, J.; Huang, J.; Goldman, M.H.S.; Goldman, G.H. The ergosterol biosynthesis pathway, transporter genes, and azole resistance in Aspergillus fumigatus. Med. Mycol. 2005, 43, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Perlin, D.S.; Rautemaa-Richardson, R.; Alastruey-Izquierdo, A. The global problem of antifungal resistance: Prevalence, mechanisms, and management. Lancet Infect. Dis. 2017, 17, e383–e392. [Google Scholar] [CrossRef]
- Montañés, F.M.; Pascual-Ahuir, A.; Proft, M. Repression of ergosterol biosynthesis is essential for stress resistance and is mediated by the Hog1 MAP kinase and the Mot3 and Rox1 transcription factors. Mol. Microbiol. 2011, 79, 1008–1023. [Google Scholar] [CrossRef]
- Bertuzzi, M.; van Rhijn, N.; Krappmann, S.; Bowyer, P.; Bromley, M.J.; Bignell, E.M. On the lineage of Aspergillus fumigatus isolates in common laboratory use. Med. Mycol. 2021, 59, 7–13. [Google Scholar] [CrossRef]
- Paoletti, M.; Seymour, F.A.; Alcocer, M.J.C.; Kaur, N.; Calvo, A.M.; Archer, D.B.; Dyer, P.S. Mating type and the genetic basis of self-fertility in the model fungus Aspergillus nidulans. Curr. Biol. 2007, 17, 1384–1389. [Google Scholar] [CrossRef]
- Hoi, J.W.S.; Dumas, B. Ste12 and Ste12-like proteins, fungal transcription factors regulating development and pathogenicity. Eukaryot. Cell 2010, 9, 480–485. [Google Scholar]
- Li, D.; Bobrowicz, P.; Wilkinson, H.H.; Ebbole, D.J. A mitogen-activated protein kinase pathway essential for mating and contributing to vegetative growth in Neurospora crassa. Genetics 2005, 170, 1091–1104. [Google Scholar] [CrossRef]
- Nolting, N.; Pöggeler, S. A STE12 homologue of the homothallic ascomycete Sordaria macrospora interacts with the MADS box protein MCM1 and is required for ascosporogenesis. Mol. Microbiol. 2006, 62, 853–868. [Google Scholar] [CrossRef] [PubMed]
- Warris, A.; Ballou, E.R. Oxidative responses and fungal infection biology. Semin. Cell Dev. Biol. 2019, 89, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Loor, G.; Kondapalli, J.; Schriewer, J.M.; Chandel, N.S.; Vanden Hoek, T.L.; Schumacker, P.T. Menadione triggers cell death through ROS-dependent mechanisms involving PARP activation without requiring apoptosis. Free Radic. Biol. Med. 2010, 49, 1925–1936. [Google Scholar] [CrossRef] [PubMed]
- Zaccaria, M.; Ludovici, M.; Sanzani, S.M.; Ippolito, A.; Cigliano, R.A.; Sanseverino, W.; Scarpari, M.; Scala, V.; Fanelli, C.; Reverberi, M. Menadione-induced oxidative stress re-shapes the oxylipin profile of Aspergillus flavus and its lifestyle. Toxins 2015, 7, 4315–4329. [Google Scholar] [CrossRef] [PubMed]
- Tafforeau, L.; Le Blastier, S.; Bamps, S.; Dewez, M.; Vandenhaute, J.; Hermand, D. Repression of ergosterol level during oxidative stress by fission yeast F-box protein Pof14 independently of SCF. EMBO J. 2006, 25, 4547–4556. [Google Scholar] [CrossRef]
- Barker, B.M.; Kroll, K.; Vödisch, M.; Mazurie, A.; Kniemeyer, O.; Cramer, R.A. Transcriptomic and proteomic analyses of the Aspergillus fumigatus hypoxia response using an oxygen-controlled fermenter. BMC Genom. 2012, 13, 62. [Google Scholar] [CrossRef]
- Hagiwara, D.; Miura, D.; Shimizu, K.; Paul, S.; Ohba, A.; Gonoi, T.; Watanabe, A.; Kamei, K.; Shintani, T.; Moye-Rowley, W.S.; et al. A novel Zn2-Cys6 transcription factor AtrR plays a key role in an azole resistance mechanism of Aspergillus fumigatus by co-regulating cyp51A and cdr1B expressions. PLoS Pathog. 2017, 13, e1005775. [Google Scholar] [CrossRef] [PubMed]
- Gsaller, F.; Hortschansky, P.; Furukawa, T.; Carr, P.D.; Rash, B.; Capilla, J.; Müller, C.; Bracher, F.; Bowyer, P.; Haas, H.; et al. Sterol biosynthesis and azole tolerance is governed by the opposing actions of SrbA and the CCAAT binding complex. PLoS Pathog. 2016, 12, e1006096. [Google Scholar] [CrossRef]
- Chung, D.; Barker, B.M.; Carey, C.C.; Merriman, B.; Werner, E.R.; Lechner, B.E.; Dhingra, S.; Cheng, C.; Xu, W.; Blosser, S.J.; et al. ChIP-seq and in vivo transcriptome analyses of the Aspergillus fumigatus SREBP SrbA reveals a new regulator of the fungal hypoxia response and virulence. PLoS Pathog. 2014, 10, e1004487. [Google Scholar] [CrossRef]
- Dhingra, S.; Cramer, R.A. Regulation of sterol biosynthesis in the human fungal pathogen Aspergillus fumigatus: Opportunities for therapeutic development. Front. Microbiol. 2017, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Paris, S.; Debeaupuis, J.P.; Crameri, R.; Carey, M.; Charlès, F.; Prévost, M.C.; Schmitt, C.; Philippe, B.; Latgé, J.P. Conidial hydrophobins of Aspergillus fumigatus. Appl. Environ. Microbiol. 2003, 69, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, J.-Y.; Kim, Y.-J.; Park, H.-M. The Gβ-like Protein AfCpcB Affects Sexual Development, Response to Oxidative Stress and Phagocytosis by Alveolar Macrophages in Aspergillus fumigatus. J. Fungi 2022, 8, 56. https://doi.org/10.3390/jof8010056
Lim J-Y, Kim Y-J, Park H-M. The Gβ-like Protein AfCpcB Affects Sexual Development, Response to Oxidative Stress and Phagocytosis by Alveolar Macrophages in Aspergillus fumigatus. Journal of Fungi. 2022; 8(1):56. https://doi.org/10.3390/jof8010056
Chicago/Turabian StyleLim, Joo-Yeon, Yeon-Ju Kim, and Hee-Moon Park. 2022. "The Gβ-like Protein AfCpcB Affects Sexual Development, Response to Oxidative Stress and Phagocytosis by Alveolar Macrophages in Aspergillus fumigatus" Journal of Fungi 8, no. 1: 56. https://doi.org/10.3390/jof8010056
APA StyleLim, J.-Y., Kim, Y.-J., & Park, H.-M. (2022). The Gβ-like Protein AfCpcB Affects Sexual Development, Response to Oxidative Stress and Phagocytosis by Alveolar Macrophages in Aspergillus fumigatus. Journal of Fungi, 8(1), 56. https://doi.org/10.3390/jof8010056





