Distribution and Genetic Diversity of the Amphibian Chytrid in Japan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Swab Samples from Wild Amphibians in Japan
2.2. Nested PCR Assay
2.3. Phylogenetic Analysis of the ITS Gene
3. Results
3.1. Infection Status and Haplotype Variation of Bd in Japan
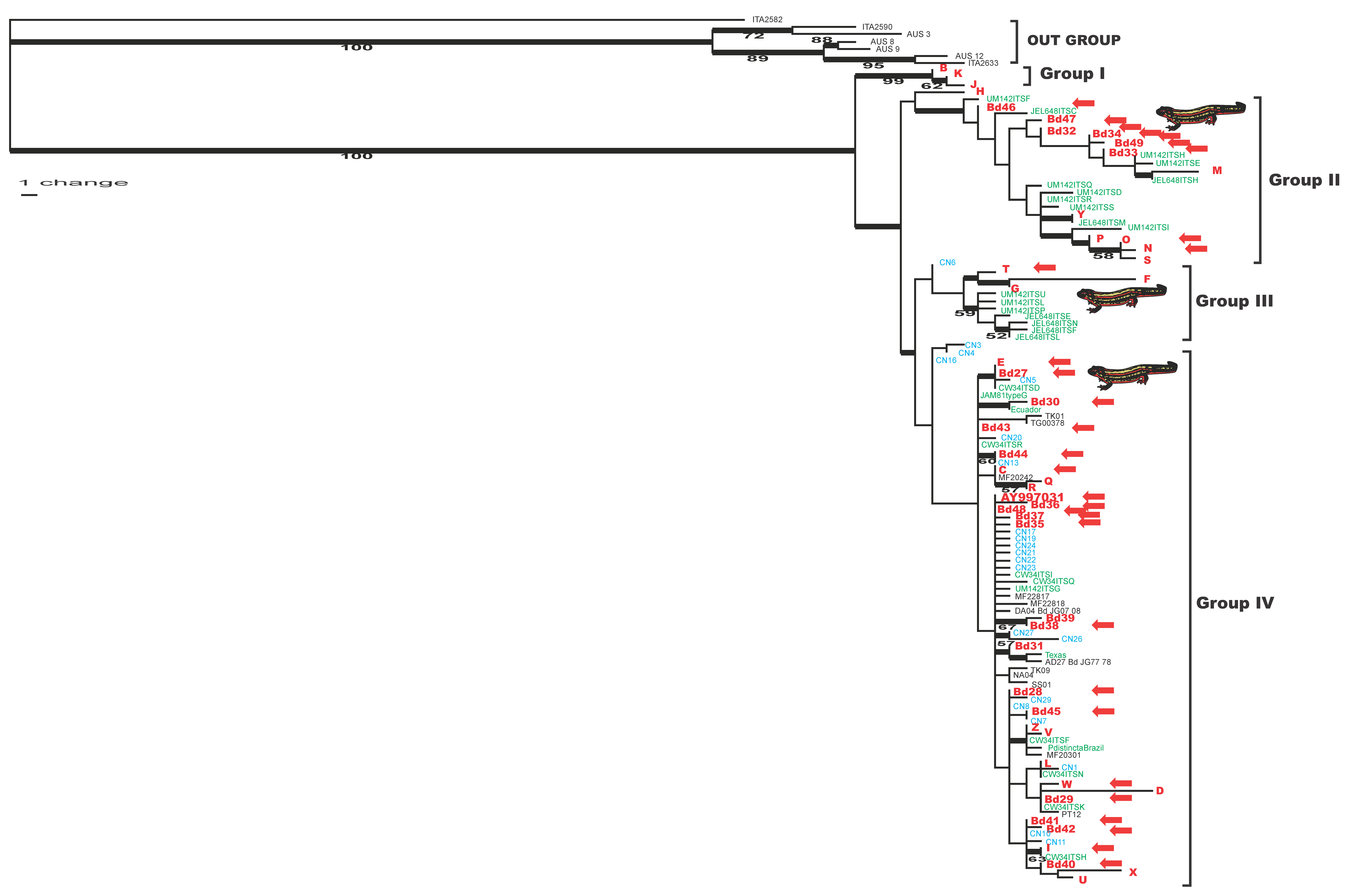
3.2. Phylogenetic Analysis of ITS-DNA Haplotypes in Bd
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berger, L.; Speare, R.; Daszak, P.; Green, D.E.; Cunningham, A.A.; Goggin, C.L.; Slocombe, R.; Ragan, M.A.; Hyatt, A.D.; McDonald, K.R.; et al. Chytridiomycosis causes amphibian mortality associated with population declines in the rainforests of Australia and Central America. Proc. Natl. Acad. Sci. USA 1998, 95, 9031–9036. [Google Scholar] [CrossRef] [Green Version]
- Lips, K.R. Mass mortality and population declines of anurans at an upland site in western Panama. Conserv. Biol. 1999, 13, 117–125. [Google Scholar] [CrossRef]
- Pessier, A.P.; Nichols, D.K.; Longcore, J.E.; Fuller, M.S. Cutaneous chytridiomycosis in poison dart frogs (Dendrobates spp.) and White’s tree frogs (Litoria caerulea). J. Vet. Diag. Investig. 1999, 11, 194–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosch, J.; Martínez-Solano, I.; García-París, M. Evidence of chytrid fungus infection involved in the decline of the common midwife toad (Alytes obstetricans) in protected areas of central Spain. Biol. Conserv. 2001, 97, 331–337. [Google Scholar] [CrossRef]
- Bradley, G.A.; Rosen, P.C.; Sredl, M.J.; Jones, T.R.; Longcore, J.E. Chytridiomycosis in native Arizona frogs. J. Wildl. Dis. 2002, 38, 206–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, D.E.; Converse, K.A.; Schrader, A.K. Epizootiology of sixty-four amphibian morbidity and mortality events in the USA, 1996–2001. Ann. N. Y. Acad. Sci. 2002, 969, 323–339. [Google Scholar] [CrossRef] [PubMed]
- Ron, S.R.; Duellman, W.E.; Coloma, L.A.; Bustamante, M.R. Population decline of the Jambato toad Atelopus ignescens (Anura: Bufonidae) in the Andes of Ecuador. J. Herpetol. 2003, 37, 116–126. [Google Scholar] [CrossRef]
- Weldon, C.; Du Preez, L.H.; Hyatt, A.D.; Muller, R.; Speare, R. Origin of the amphibian chytrid fungus. Emerg. Infect. Dis. 2004, 10, 2100–2105. [Google Scholar] [CrossRef] [PubMed]
- Scheele, B.C.; Pasmans, F.; Skerratt, L.F.; Berger, L.; Martel, A.; Beukema, W.; Acevedo, A.A.; Burrowes, P.A.; Carvalho, T.; Catenazzi, A.; et al. Amphibian fungal panzootic causes catastrophic and ongoing loss of biodiversity. Science 2019, 363, 1459–1463. [Google Scholar] [CrossRef] [PubMed]
- Daszak, P.; Berger, L.; Cunningham, A.A.; Hyatt, A.D.; Green, D.E.; Speare, R. Emerging infectiousdiseases & amphibian population declines. Emerg. Infect. Dis. 1999, 5, 735–748. [Google Scholar]
- Lips, K.R.; Brem, F.; Brenes, R.; Reeve, J.D.; Alford, R.A.; Voyles, J.; Carey, C.; Livo, L.; Pessier, A.P.; Collins, J.P. Emerging infectious disease and the loss of biodiversity in a Neotropical amphibian community. Proc. Natl Acad. Sci. USA 2006, 103, 3165–3170. [Google Scholar] [CrossRef] [Green Version]
- Morehouse, E.A.; James, T.Y.; Ganley, A.R.D.; Vilgalys, R.; Berger, L.; Murphy, P.J.; Longcore, J.E. Multilocus sequence typing suggests the chytrid pathogen of amphibians is a recently emerged clone. Mol. Ecol. 2003, 12, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.A.; Vredenberg, V.T.; Rachowicz, L.J.; Knapp, R.A.; Stice, M.J.; Tunstall, T.; Bingham, R.E.; Parker, J.M.; Longcore, J.E.; Moritz, C.; et al. Population genetics of the frog killing fungus Batrachochytrium dendrobatidis. Proc. Nat. Acad. Sci. USA 2007, 104, 13845–13850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, T.Y.; Litvintseva, A.P.; Vilgalys, R.; Morgan, J.A.T.; Taylor, J.W.; Fisher, M.C.; Berger, L.; Weldon, C.; du Preez, L.; Longcore, J.E. Rapid global expansion of the fungal disease chytridiomycosis into declining and healthy amphibian populations. PLoS Pathog. 2009, 5, e1000458. [Google Scholar] [CrossRef] [Green Version]
- Velo-Anton, G.; Rodriguez, D.; Savage, A.E.; Parra-Olea, G.; Lips, K.R.; Zamudio, K.R. Amphibian-killing fungus loses genetic diversity as it spreads across the New World. Biol. Conserv. 2012, 146, 213–218. [Google Scholar] [CrossRef]
- Daszak, P.; Cunningham, A.A.; Hyatt, A.D. Emerging infectious diseases of wildlife—Threats to biodiversity and human health. Science 2000, 287, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Blaustein, A.R.; Kiesecker, J.M. Complexity in conservation: Lessons from the global decline of amphibian populations. Ecol. Lett. 2002, 5, 597–608. [Google Scholar] [CrossRef] [Green Version]
- Pounds, J.A.; Bustamante, M.R.; Coloma, L.A.; Consuegra, J.A.; Fogden, M.P.; Foster, P.N.; La Marca, E.; Masters, K.L.; Merino-Viteri, A.; Puschendorf, R.; et al. Widespread amphibian extinctions from epi- demic disease driven by global warming. Nature 2006, 439, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Rachowicz, L.J.; Hero, J.M.; Alford, R.A.; Taylor, J.W.; Morgan, J.A.T.; Vredenburg, V.T.; Collins, J.P.; Briggs, C.J. The novel and endemic pathogen hypotheses: Competing explanations for the origin of emerging infectious diseases of wildlife. Conserv. Biol. 2005, 19, 1441–1448. [Google Scholar] [CrossRef]
- Soto-Azat, C.; Clarke, B.T.; Poynton, J.C.; Cunningham, A.A. Widespread historical presence of Batrachochytrium dendrobatidis in African pipid frogs. Divers. Distrib. 2010, 16, 126–131. [Google Scholar] [CrossRef]
- Vredenburg, V.T.; Felt, S.A.; Morgan, E.C.; McNally, S.V.G.; Wilson, S.; Green, S.L. Prevalence of Batrachochytrium dendrobatidis in Xenopus Collected in Africa (1871–2000) and in California (2001–2010). PLoS ONE 2013, 8, e63791. [Google Scholar] [CrossRef]
- Rodriguez, D.; Becker, C.G.; Pupin, N.C.; Haddad, C.F.B.; Zamudio, K.R. Long-term endemism of two highly divergent lineages of the amphibian-killing fungus in the Atlantic Forest of Brazil. Mol. Ecol. 2014, 23, 774–787. [Google Scholar] [CrossRef]
- Talley, B.L.; Muletz, C.R.; Vredenburg, V.T.; Fleischer, R.C.; Lips, K.R. A century of Batrachochytrium dendrobatidis in Illinois amphibians (1888–1989). Biol. Conserv. 2015, 182, 254–261. [Google Scholar] [CrossRef]
- Basanta, M.D.; Byrne, A.Q.; Rosenblum, E.B.; Piovia-Scott, J.; Parra-Olea, G. Early presence of Batrachochytrium dendrobatidis in Mexico with a contemporary dominance of the global panzootic lineage. Mol. Ecol. 2021, 30, 424–437. [Google Scholar] [CrossRef]
- Farrer, R.A.; Weinert, L.A.; Bielby, J.; Garner, T.W.J.; Balloux, F.; Clare, F.; Bosch, J.; Cunningham, A.A.; Weldon, C.; du Preez, L.H.; et al. Multiple emergences of genetically diverse amphibian-infecting chytrids include a globalized hypervirulent recombinant lineage. Proc. Nat. Acad. Sci. USA 2011, 108, 18732–18736. [Google Scholar] [CrossRef] [Green Version]
- Schloegel, L.M.; Toledo, L.F.; Longcore, J.E.; Greenspan, S.E.; Vieira, C.A.; Lee, M. Novel, pan-zootic and hybrid genotypes of amphibian chytridiomycosis associated with the bullfrog trade. Mol. Ecol. 2012, 21, 5162–5177. [Google Scholar] [CrossRef] [Green Version]
- Rosenblum, E.B.; Jamesb, T.Y.; Zamudioc, K.R.; Poortena, T.J.; Ilutc, D.; Rodriguezc, D.; Eastmand, J.M.; Richards-Hrdlickae, K.; Jonesond, S.; Jenkinson, T.S.; et al. Complex history of the amphibian-killing chytrid fungus revealed with genome resequencing data. Proc. Natl. Acad. Sci. USA 2013, 110, 9385–9390. [Google Scholar] [CrossRef] [Green Version]
- O’Hanlon, S.J.; Rieux, A.; Farrer, R.A.; Rosa, G.M.; Waldman, B.; Bataille, A.; Kosch, T.A.; Murray, K.A.; Brankovics, B.; Fumagalli, M.; et al. Recent Asian origin of chytrid fungi causing global amphibian declines. Science 2018, 360, 621–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byrne, A.Q.; Vredenburg, V.T.; Martel, A.; Pasmans, F.; Belle, R.C.; Blackburn, D.C.; Bletzh, M.C.; Boschi, J.; Briggs, C.J.; Brownl, R.M.; et al. Cryptic diversity of a widespread global pathogen reveals expanded threats to amphibian conservation. Proc. Natl Acad. Sci. USA 2019, 116, 20382–20387. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.J.; Waldman, B. Ancestral chytrid pathogen remains hypervirulent following its long coevolution with amphibian hosts. Proc. Biol. Sci. 2019, 286, 20190833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Une, Y.; Kadekaru, S.; Tamukai, K.; Goka, K.; Kuroki, T. First report of spontaneous chytridiomycosis in frogs in Asia. Dis. Aquat. Org. 2008, 82, 157–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goka, K.; Une, Y.; Kuroki, T.; Suzuki, K.; Nakahara, M.; Kobayashi, A.; Yokoyama, J.; Mizutani, T.; Hyatt, A.D. Amphibian chytridiomycosis in Japan: Distribution, haplotypes, and possible entry into Japan. Mol. Ecol. 2009, 18, 4757–4774. [Google Scholar] [CrossRef]
- Annis, S.L.; Dastoor, F.P.; Ziel, H.; Daszak, P.; Longcore, J.E. A DNA-based assay identifies Batrachochytrium dendrobatidis in amphibians. J. Wild. Dis. 2004, 40, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.-M.; Liu, X.; Fisher, M.; Garner, T.W.J.; Li, Y. Global and endemic Asian lineages of the emerging pathogenic fungus Batrachochytrium dendrobatidis widely infect amphibians in China. Divers. Distrib. 2012, 18, 307–318. [Google Scholar] [CrossRef]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL_x windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 24, 4876–4882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swofford, D.L. PAUP*: Phylogenetic Analysis Using Parsimony (*and Other Methods), 4th ed.; Sinauer Associates: Sunderland, MA, USA, 1998. [Google Scholar]
- Maddison, W.P. (Squared-change parsimony reconstructions of ancestral states for continuous-valued characters on a phylogenetic tree. Syst. Biol. 1991, 40, 304–314. [Google Scholar] [CrossRef]
- Bataille, A.; Fong, J.J.; Cha, M.; Wogan, G.O.U.; Baek, H.J.; Lee, H.; MI-SOOK Min, M.-S.; Waldman, B. Genetic evidence for a high diversity and wide distribution of endemic strains of the pathogenic chytrid fungus Batrachochytrium dendrobatidis in wild Asian amphibians. Mol. Ecol. 2013, 22, 4196–4209. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Fisher, M.C.; Garner, T.W.J. Chytrid fungi and global amphibian declines. Nat. Rev. Microbiol. 2020, 18, 332–343. [Google Scholar] [CrossRef]
- Nishida, M. Japanese Immigration to Brazil. Latin Am. Hist. 2007. [Google Scholar] [CrossRef]
- Takagi, K. A Brief Historical Review on the Development of Japanese Community in Brazil. Glob. Commun. Study 2017, 5, 7–31. (In Japanese) [Google Scholar]
- Inger, R.F. Preliminary survey of the amphibians of the Riukiu islands. Fieldiana Zool. 1947, 32, 296–352. [Google Scholar]
- Tominaga, A.; Ota, T.; Matsui, M. Phylogeny and phylogeography of the sword-tailed newt, Cynops ensicauda (Amphibia: Caudata), as revealed by nucleotide sequences of mitochondrial DNA. Mol. Phylogenet. Evol. 2010, 54, 910–921. [Google Scholar] [CrossRef]
- Ikehara, S. Islands of valuable animals: Fauna of the Ryukyu Archipelago. In Nature of Japan, 8th ed.; Southern Islands; Nakamura, K., Ujiie, H., Ikehara, S., Tagawa, H., Hori, N., Eds.; Iwanami-Shoten: Tokyo, Japan, 1996; pp. 149–160. (In Japanese) [Google Scholar]
- Ota, H. Geographic patterns of endemism and speciation in amphibians and reptiles of the Ryukyu Archipelago, Japan, with special reference to their paleogeographical implications. Res. Popul. Ecol. 1998, 40, 189–204. [Google Scholar] [CrossRef]
- Ota, H. The current geographic faunal pattern of reptiles and amphibians of the Ryukyu Archipelago and adjacent regions. Tropics 2000, 10, 51–62. [Google Scholar] [CrossRef]
- Garner, T.W.J.; Perkins, M.W.; Govindarajulu, P.; Seglie, D.; Walker, S.; Cunningham, A.A.; Fisher, M.C. The emerging amphibian pathogen Batrachochytrium dendrobatidis globally infects introduced populations of the North American bullfrog, Rana catesbeiana. Biol. Lett. 2006, 2, 455–459. [Google Scholar] [CrossRef] [Green Version]
- Schloegel, L.M.; Picco, A.; Kilpatrick, A.M.; Davies, A.J.; Hyatt, A.; Daszak, P. Magnitude of the US trade in amphibians and presence of presence of Batrachochytrium dendrobatidis and ranavirus infection in imported North American bullfrogs (Rana catesbeiana). Biol. Conserv. 2009, 142, 1420–1426. [Google Scholar] [CrossRef]
- Jenkinson, T.S.; Betancourt-Román, C.M.; Lambertini, C.; Valencia-Aguilar, A.; Rodriguez, D.; Nunes-de-Almeida, C.H.L.; Ruggeri, J.; Belasen, A.M.; da Silva Leite, D.; Zamudio, K.R.; et al. Amphibian-killing chytrid in Brazil comprises both locally endemic and globally expanding populations. Mol. Ecol. 2016, 25, 2978–2996. [Google Scholar] [CrossRef]
- Fisher, M.C.; Garner, T.W.J. The relationship between the emergence of Batrachochytrium dendrobatidis, the international trade in amphibians and introduced amphibian species. Fungal Biol. Rev. 2007, 21, 2–9. [Google Scholar] [CrossRef]

| Distribution | Species | Number of Animals Tested | Number of Animals Infected | Prevalence of Infection |
|---|---|---|---|---|
| Honshu Is. | Andrias japonicus | 46 | 23 | 0.500 |
| Honshu, Shikoku, Kyushu and South-Western Is. | Fejervarya limnocharis | 987 | 6 | 0.006 |
| Hokkaido, Honshu, Shikoku and Kyushu Is. | Dryophytes japonicus | 700 | 1 | 0.001 |
| Honshu, Shikoku, and Kyushu Is. | Pelophylax nigromaculatus | 656 | 2 | 0.003 |
| Honshu Is. | Pelophylax porosus | 188 | 3 | 0.016 |
| Honshu, Shikoku, and Kyushu Is. | Glandirana rugosa | 346 | 2 | 0.006 |
| Amami Is. (endemic) | Buergeria japonica | 71 | 1 | 0.014 |
| Cynops ensicauda ensicauda * | 134 | 6 | 0.045 | |
| Okinawa Is. (endemic) | Cynops ensicauda popei * | 137 | 87 | 0.635 |
| Odorrana narina | 11 | 1 | 0.091 | |
| Alien species | Aquarana catesbeiana | 260 | 67 | 0.258 |
| Xenopus laevis | 24 | 8 | 0.333 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goka, K.; Yokoyama, J.; Tominaga, A. Distribution and Genetic Diversity of the Amphibian Chytrid in Japan. J. Fungi 2021, 7, 522. https://doi.org/10.3390/jof7070522
Goka K, Yokoyama J, Tominaga A. Distribution and Genetic Diversity of the Amphibian Chytrid in Japan. Journal of Fungi. 2021; 7(7):522. https://doi.org/10.3390/jof7070522
Chicago/Turabian StyleGoka, Koichi, Jun Yokoyama, and Atsushi Tominaga. 2021. "Distribution and Genetic Diversity of the Amphibian Chytrid in Japan" Journal of Fungi 7, no. 7: 522. https://doi.org/10.3390/jof7070522
APA StyleGoka, K., Yokoyama, J., & Tominaga, A. (2021). Distribution and Genetic Diversity of the Amphibian Chytrid in Japan. Journal of Fungi, 7(7), 522. https://doi.org/10.3390/jof7070522







