The Molecular Mechanism of Fludioxonil Action Is Different to Osmotic Stress Sensing
Abstract
1. Introduction
2. Material and Methods
2.1. Strains and Culture Conditions
2.2. Identification of and Sequence Analysis in Magnaporthe oryzae
2.3. Construction of Vectors for Genetic Manipulation
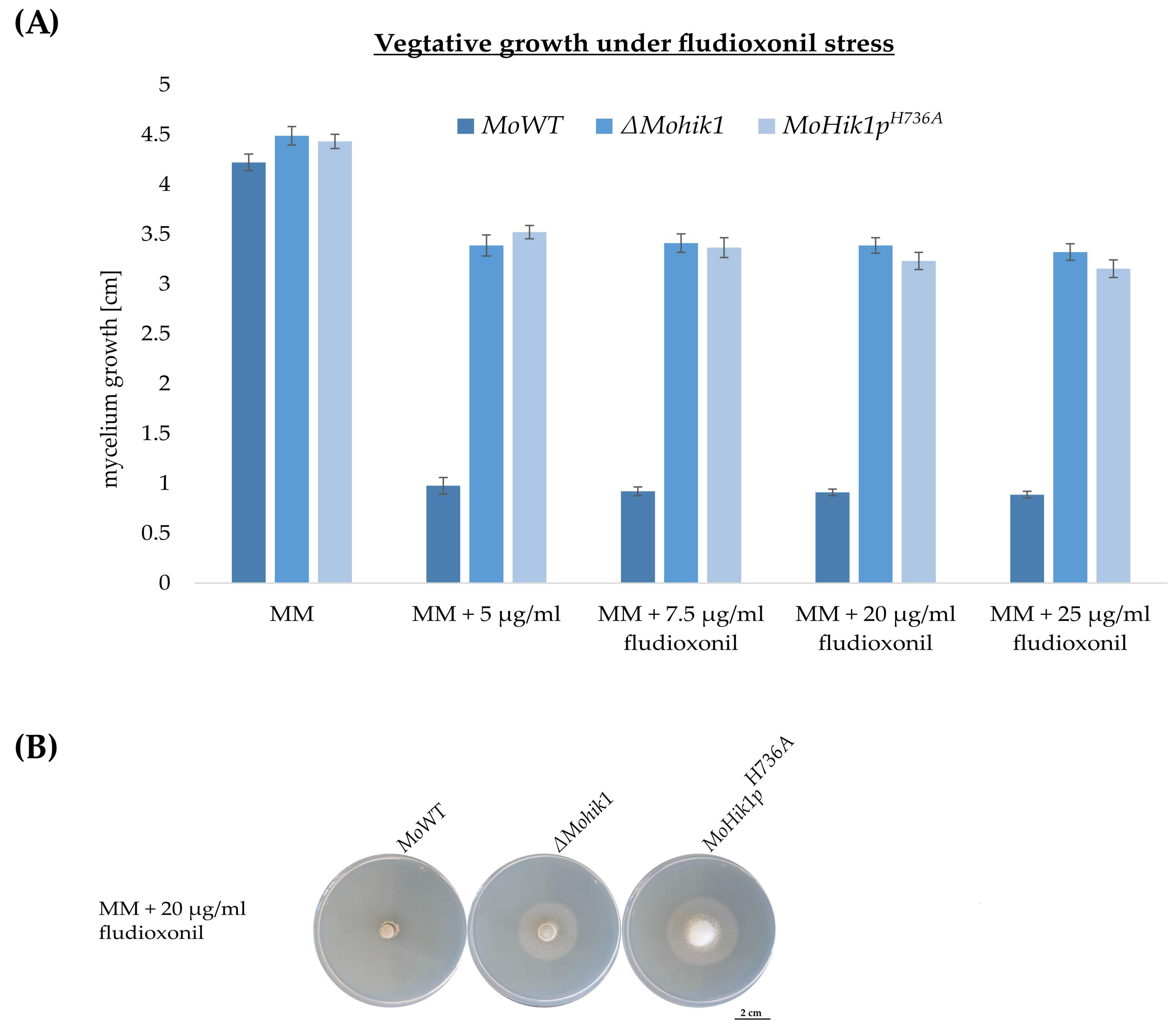
2.4. Vegetative Growth Assays
2.5. Germination Assay
3. Results
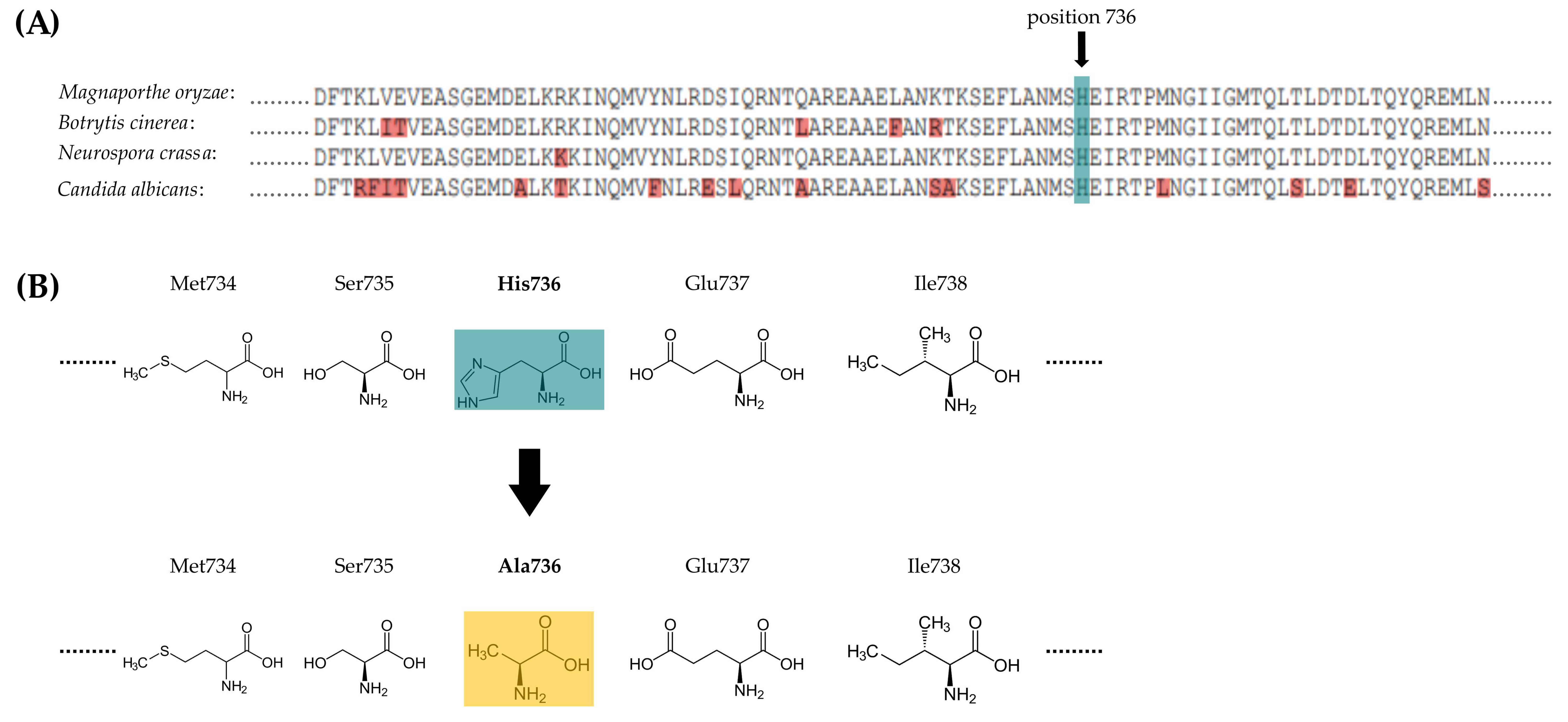
3.1. Manipulation of H736 within the Sensor Histidine Kinase MoHik1p
3.2. Histidine H736 Is a Prerequisite for Fludioxonil Action but Not Essential for Osmosensing
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nabi, S.; Nabi, W.H.; Raja, W.; Dar, M.S.; Kirmani, S.; Magray, M.M. New generation fungicides in disease management of horticultural crops. Indian Hortic. J. 2017, 7, 1–7. [Google Scholar]
- Gehmann, K.; Nyfeler, R.; Leadbeater, A.J.; Nevill, D.J.; Sozzi, D. CGA 173506: A new phenylpyrrole fungicide for broad-spectrum disease control. Bright Crop. Prot. Conf. Pests Dis. 1990, 2, 399–406. [Google Scholar]
- Kim, J.H.; Campbell, B.C.; Mahoney, N.; Chan, K.L.; Molyneux, R.J.; May, G.S. Enhancement of fludioxonil fungicidal activity by disrupting cellular glutathione homeostasis with 2,5-Dihydroxybenzoic acid. FEMS Microbiol. Lett. 2007, 270, 284–290. [Google Scholar] [CrossRef]
- Yoshimi, A.; Kojima, K.; Takano, Y.; Tanaka, C. Group III histidine kinase is a positive regulator of Hog1-type mitogen-activated protein kinase in filamentous fungi. Eukaryot. Cell 2005, 4, 1820–1828. [Google Scholar] [CrossRef]
- Jacob, S.; Brandhorst, T.T. The mechanism of phenylpyrrole fungicides cannot be explained by a stearic effect upon triososephosphate isomerase alone. OSF Prepr. 2020. [Google Scholar] [CrossRef]
- Jacob, S.; Foster, A.J.; Yemelin, A.; Thines, E. Histidine kinases mediate differentiation, stress response, and pathogenicity in Magnaporthe oryzae. Microbiologyopen 2014, 3, 668–687. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Foster, A.J.; Yemelin, A.; Thines, E. High osmolarity glycerol (HOG) signalling in Magnaporthe Oryzae: Identification of MoYPD1 and its role in osmoregulation, fungicide action, and pathogenicity. Fungal. Biol. 2015, 119, 580–594. [Google Scholar] [CrossRef] [PubMed]
- Lawry, S.; Tebbets, B.; Kean, I.; Stewart, D.; Hetelle, J.; Klein, B.S. Fludioxonil induces Drk1, a fungal group III hybrid histidine kinase, to dephosphorylate its downstream target, Ypd1. Am. Soc. Microbiol. 2017, 61, e01414–e01416. [Google Scholar]
- Brandhorst, T.T.; Klein, B.S. Uncertainty surrounding the mechanism and safety of the post-harvest fungicide fludioxonil. Food Chem. Toxicol. 2019, 123, 561–565. [Google Scholar] [CrossRef]
- Jacob, S.; Thines, E. Multistep phosphorelay in fungi: The enigma of multiple signals and a limited number of signaling pathways. Mycol. Prog. 2017, 16, 1007–1013. [Google Scholar] [CrossRef]
- Catlett, N.L.; Yoder, O.C.; Turgeon, B.G. Whole-genome analysis of two-component signal transduction genes in fungal pathogens. Eukaryot. Cell 2003, 2, 1151–1161. [Google Scholar] [CrossRef]
- West, A.H.; Stock, A.M. Histidine kinases and response regulator proteins in two-component signaling systems. Trends Biochem. Sci. 2001, 26, 369–376. [Google Scholar] [CrossRef]
- Espinasse, A.; Wen, X.; Goodpaster, J.D.; Carlson, E.E. Mechanistic studies of bioorthogonal ATP analogues for assessment of histidine kinase autophosphorylation. ACS Chem. Biol. 2020, 15, 1252–1260. [Google Scholar] [CrossRef]
- Lapek, J.D.; Tombline, G.; Friedman, A.E. Mass spectrometry detection of histidine phosphorylation on NM23-H1. J. Proteome Res. 2011, 10, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Oslund, R.C.; Kee, J.-M.; Couvillon, A.D.; Bhatia, V.N.; Perlman, D.H.; Muir, T.W. A phosphohistidine proteomics strategy based on elucidation of a unique gas-phase phosphopeptide fragmentation mechanism. J. Am. Chem. Soc. 2014, 136, 12899–12911. [Google Scholar] [CrossRef]
- Green, M.R.; Sambrook, J. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2012. [Google Scholar]
- Gibson, D.G.; Young, L.; Chuang, R.-Y.; Venter, J.C.; Hutchison, C.A.; Smith, H.O. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 2009, 6, 343–345. [Google Scholar] [CrossRef]
- de Groot, M.J.A.; Bundock, P.; Hooykaas, P.J.; Beijersbergen, A.G.M. Agrobacterium tumefaciens-mediated transformation of filamentous fungi. Nat. Biotechnol. 1998, 16, 839–842. [Google Scholar] [CrossRef]
- Rho, H.S.; Kang, S.; Lee, Y.H. Agrobacterium tumefaciens-mediated transformation of the plant pathogenic fungus, Magnaporthe grisea. Mol. Cells 2001, 12, 407–411. [Google Scholar] [PubMed]
- Odenbach, D.; Breth, B.; Thines, E.; Weber, R.W.S.; Anke, H.; Foster, A.J. The transcription factor Con7p is a central regulator of infection-related morphogenesis in the rice blast fungus Magnaporthe grisea. Mol. Microbiol. 2007, 64, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Schüffler, A.; Thines, E. Hog1p Activation by Marasmic Acid through Inhibition of the Histidine Kinase Sln1p. Pest. Manag. Sci. 2016, 72, 1268–1274. [Google Scholar] [CrossRef] [PubMed]
- Kilani, J.; Fillinger, S. Phenylpyrroles: 30 years, two molecules and (nearly) no resistance. Front. Microbiol. 2016, 7, 2014. [Google Scholar] [CrossRef]
- Bohnert, S.; Heck, L.; Gruber, C.; Neumann, H.; Distler, U.; Tenzer, S.; Yemelin, A.; Thines, E.; Jacob, S. Fungicide resistance towards fludioxonil conferred by overexpression of the phosphatase gene Mo. PTP 2 in Magnaporthe oryzae. Mol. Microbiol. 2019, 111, 662–667. [Google Scholar] [CrossRef]
- Lew, R.R. Turgor and net ion flux responses to activation of the osmotic MAP kinase cascade by fludioxonil in the filamentous fungus Neurospora Crassa. Fungal. Genet. Biol. 2010, 47, 721–726. [Google Scholar] [CrossRef] [PubMed]
- El-Mowafy, M.; Bahgat, M.M.; Bilitewski, U. Deletion of the HAMP domains from the histidine kinase CaNik1p of Candida albicans or treatment with fungicides activates the MAP kinase Hog1p in S. cerevisiae transformants. BMC Microbiol. 2013, 13, 1–12. [Google Scholar] [CrossRef]
- Robinson, V.L.; Buckler, D.R.; Stock, A.M. A tale of two components: A novel kinase and a regulatory switch. Nat. Struct. Biol. 2000, 7, 626–633. [Google Scholar] [CrossRef]
- Wilke, K.E.; Francis, S.; Carlson, E.E. Activity-based probe for histidine kinase signaling. J. Am. Chem. Soc. 2012, 134, 9150–9153. [Google Scholar] [CrossRef] [PubMed]
- Casino, P.; Rubio, V.; Marina, A. Structural insight into partner specificity and phosphoryl transfer in two-component signal transduction. Cell 2009, 139, 325–336. [Google Scholar] [CrossRef]
- Chase, O.M.; Espinasse, A.; Wilke, K.E.; Carlson, E.E. Exploration of the effects of γ-phosphate-modified ATP analogues on histidine kinase autophosphorylation. Biochemistry 2018, 57, 4368–4373. [Google Scholar] [CrossRef] [PubMed]
- Willett, J.W.; Crosson, S. Atypical modes of bacterial histidine kinase signaling. Mol. Microbiol. 2017, 103, 197–202. [Google Scholar] [CrossRef]
- Zschiedrich, C.P.; Keidel, V.; Szurmant, H. Molecular mechanisms of two-component signal transduction. J. Mol. Biol. 2016, 428, 3752–3775. [Google Scholar] [CrossRef]
- Bhate, M.P.; Molnar, K.S.; Goulian, M.; DeGrado, W.F. Signal transduction in histidine kinases: Insights from new structures. Structure 2015, 23, 981–994. [Google Scholar] [CrossRef] [PubMed]
- Diensthuber, R.P.; Bommer, M.; Gleichmann, T.; Möglich, A. Full-length structure of a sensor histidine kinase pinpoints coaxial coiled coils as signal transducers and modulators. Structure 2013, 21, 1127–1136. [Google Scholar] [CrossRef]
- Moore, J.O.; Hendrickson, W.A. An asymmetry-to-symmetry switch in signal transmission by the histidine kinase receptor for TMAO. Structure 2012, 20, 729–741. [Google Scholar] [CrossRef] [PubMed]
- Dubey, B.N.; Agustoni, E.; Böhm, R.; Kaczmarczyk, A.; Mangia, F.; von Arx, C.; Jenal, U.; Hiller, S.; Plaza-Menacho, I.; Schirmer, T. Hybrid histidine kinase activation by cyclic Di-GMP-mediated domain liberation. Proc. Natl. Acad. Sci. USA 2020, 117, 1000–1008. [Google Scholar] [CrossRef]
- Bohnert, S.; Neumann, H.; Thines, E.; Jacob, S. Visualizing fungicide action: An in vivo tool for rapid validation of fungicides with target location HOG pathway. Pest. Manag. Sci. 2019, 75, 772–778. [Google Scholar] [CrossRef] [PubMed]
- Bohnert, S.; Antelo, L.; Grünewald, C.; Yemelin, A.; Andresen, K.; Jacob, S. Rapid adaptation of signaling networks in the fungal pathogen Magnaporthe oryzae. BMC Genom. 2019, 20, 763. [Google Scholar] [CrossRef]

| Name | Sequence (5′→3′) |
|---|---|
| SJ-945 (HIK1prom-H736-for) | ctggctggtggcaggatatattgtggtgtaaacaaGAAGAAAAGAAGAAAAAGCACCAGGTAATTAATC |
| SJ-946 (HIK1prom-H736-rev) | gggtgtgcggatttccgcGGACATGTTAGCGAGGAACTCCGAC |
| SJ-947 (HIK1H736-flank-for) | ttcctcgctaacatgtccgcgGAAATCCGCACACCCATGAACGGTA |
| SJ-948 (HIK1H736-flank-rev) | agtgctccttcaatatcaTTTGCCCTCTACGTATATCATACTTCTACAGAGGTATATAGTTG |
| SJ-949 (trpC + BAR-for) | atatacgtagagggcaaaTGATATTGAAGGAGCACTTTTTGGGC |
| SJ-950 (trpC + BAR-rev) | ctaataaacgctcttttctcttaggtttacctgcaCTAAATCTCGGTGACGGGCAGGACC |
| SJ-1815 (PCR 1 for H736) | GGGCGATGGAAGGAGATTAC |
| SJ-1816 (PCR 1 rev H736) | CGCTTAGCCTCAATCTTTGAC |
| SJ-1817 (PCR 2 for H736) | ACGAGTCGCAAAAGATGTAG |
| SJ-1818 (PCR 2 rev H736) | CGTCGATAATGGTGAGCAG |
| SJ-1819 (Seq-primer) | GCTAACCTAATCCGCAGACG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bersching, K.; Jacob, S. The Molecular Mechanism of Fludioxonil Action Is Different to Osmotic Stress Sensing. J. Fungi 2021, 7, 393. https://doi.org/10.3390/jof7050393
Bersching K, Jacob S. The Molecular Mechanism of Fludioxonil Action Is Different to Osmotic Stress Sensing. Journal of Fungi. 2021; 7(5):393. https://doi.org/10.3390/jof7050393
Chicago/Turabian StyleBersching, Katharina, and Stefan Jacob. 2021. "The Molecular Mechanism of Fludioxonil Action Is Different to Osmotic Stress Sensing" Journal of Fungi 7, no. 5: 393. https://doi.org/10.3390/jof7050393
APA StyleBersching, K., & Jacob, S. (2021). The Molecular Mechanism of Fludioxonil Action Is Different to Osmotic Stress Sensing. Journal of Fungi, 7(5), 393. https://doi.org/10.3390/jof7050393






