A Real-Time Fluorescent Reverse Transcription Quantitative PCR Assay for Rapid Detection of Genetic Markers’ Expression Associated with Fusarium Wilt of Banana Biocontrol Activities in Bacillus
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strain and Bacillus Strains
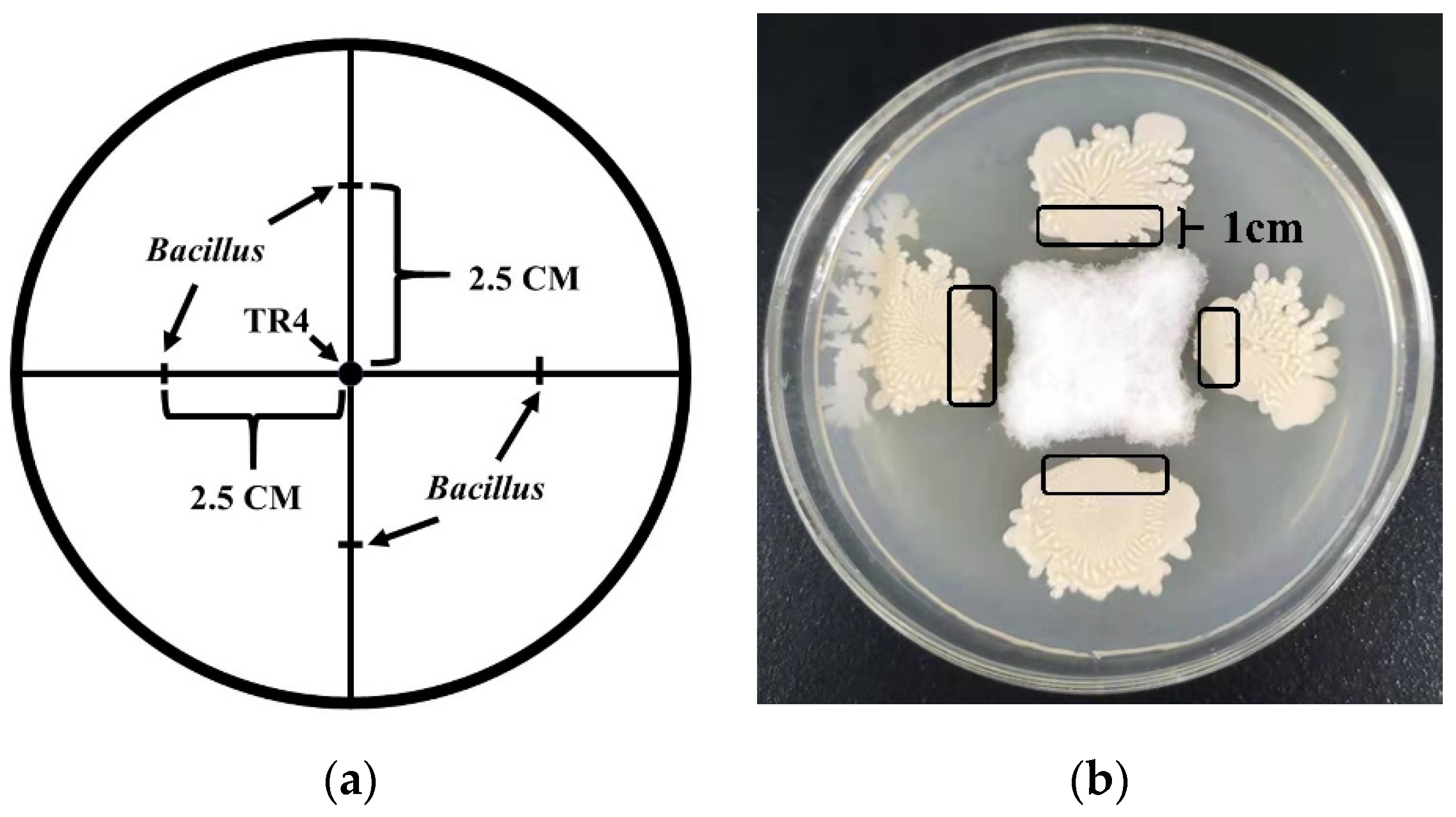
2.2. Dual-Culture Test
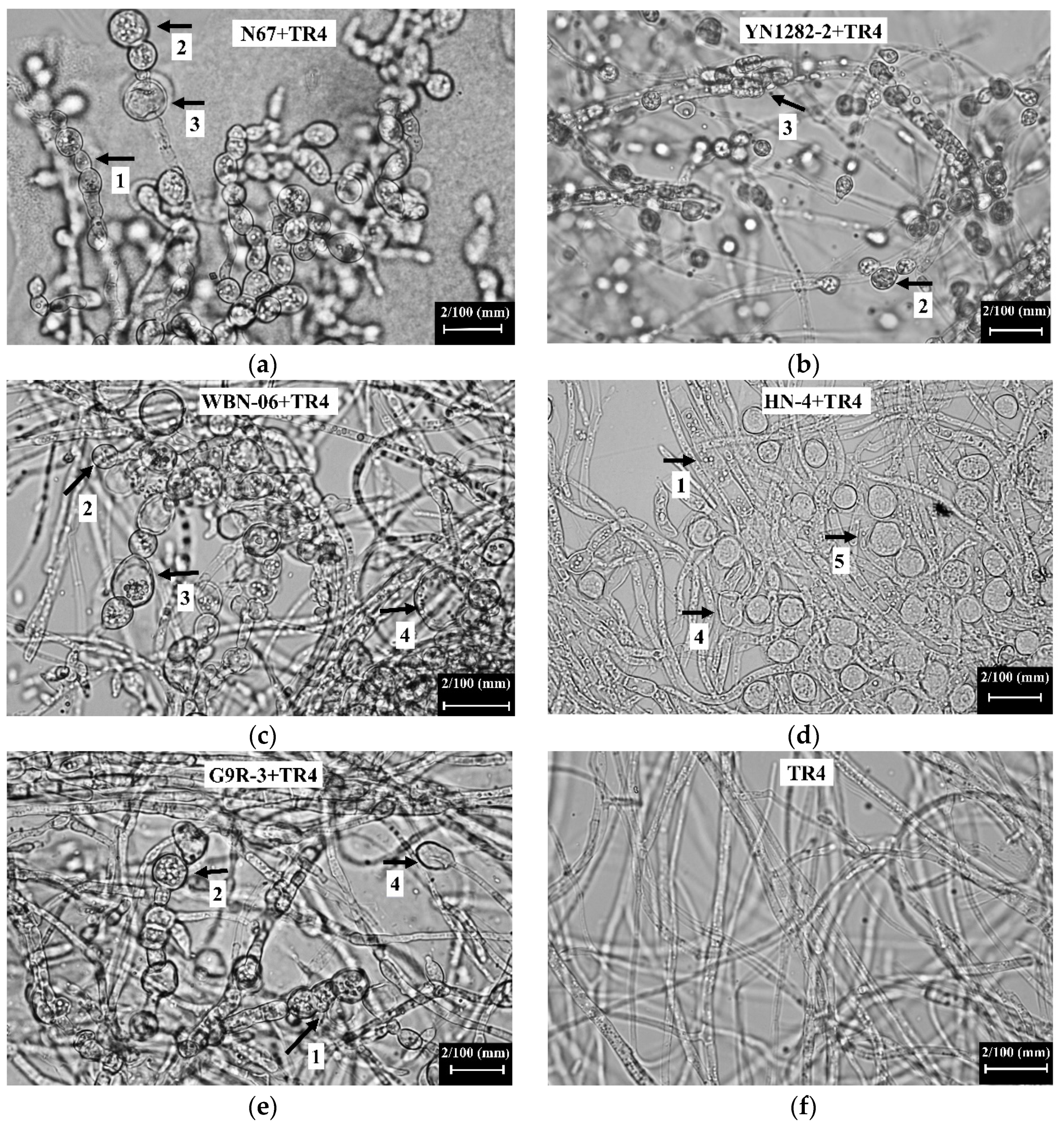
2.3. The Effects of Bacillus Strains N67, YN1282-2, WBN06, HN04 and G9R-3 on the Morphology of TR4 Mycelium
2.4. PCR Amplification of Biocontrol-Related Genes in Bacillus Strains
2.5. Comparison and Improvement of Bacillus RNA Extraction Methods and cDNA Synthesis
2.6. RT-qPCR Detection of Expression Levels of Genes Associated with TR4 Biocontrol Activities in Bacillus
2.7. Statistical Analyses
3. Results
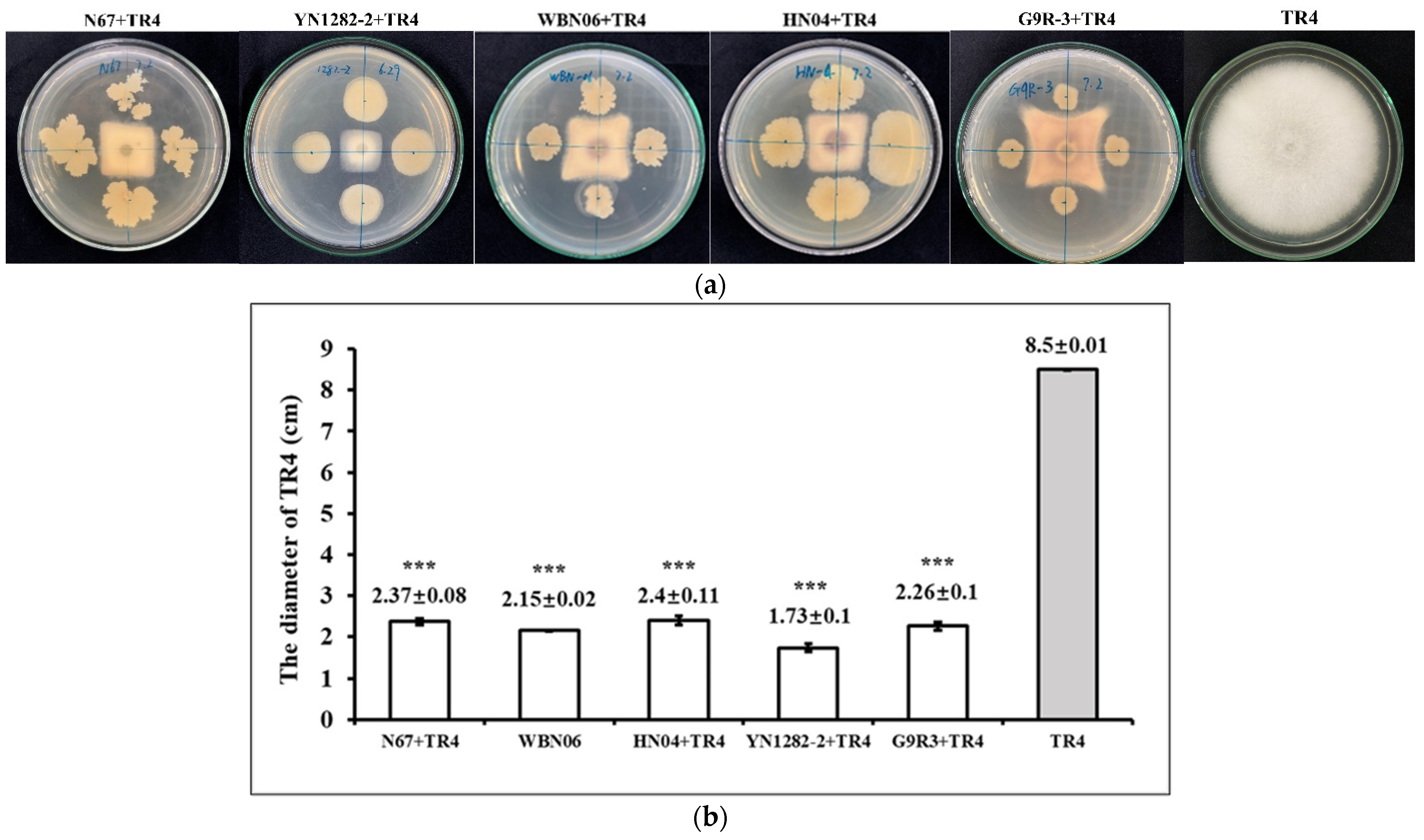
3.1. The Dual-Culture of Five Bacillus Strains against TR4
3.2. Bacillus Strains Inhibit the Growth of TR4 Hyphae and Causes Hyphae Malformation
3.3. PCR Amplification Results of Biocontrol Related Genes in Biocontrol Bacillus Strains
3.4. An Improved RNAiso Plus Method Suitable for Extraction of Total RNA from Bacillus Strains
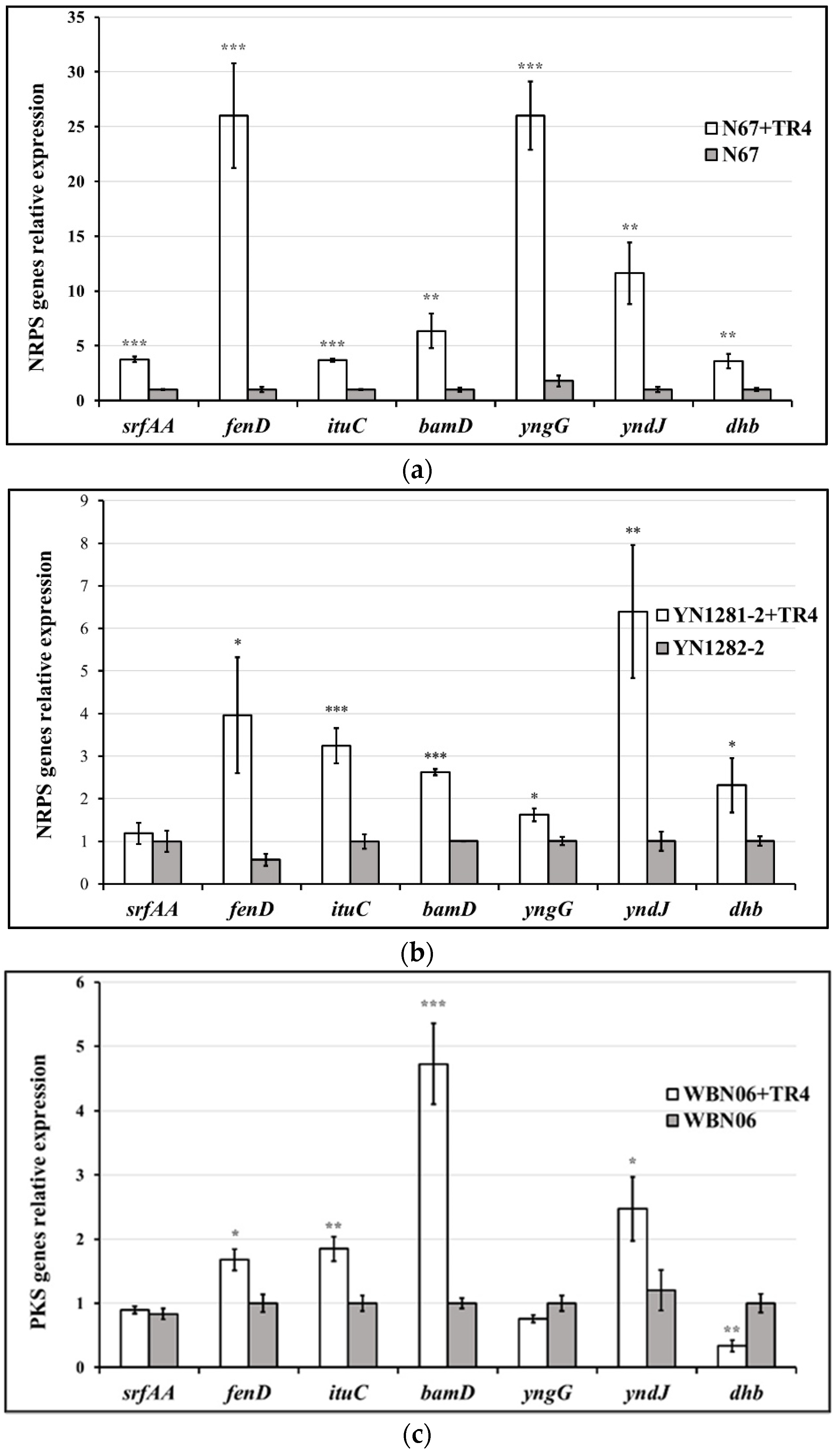
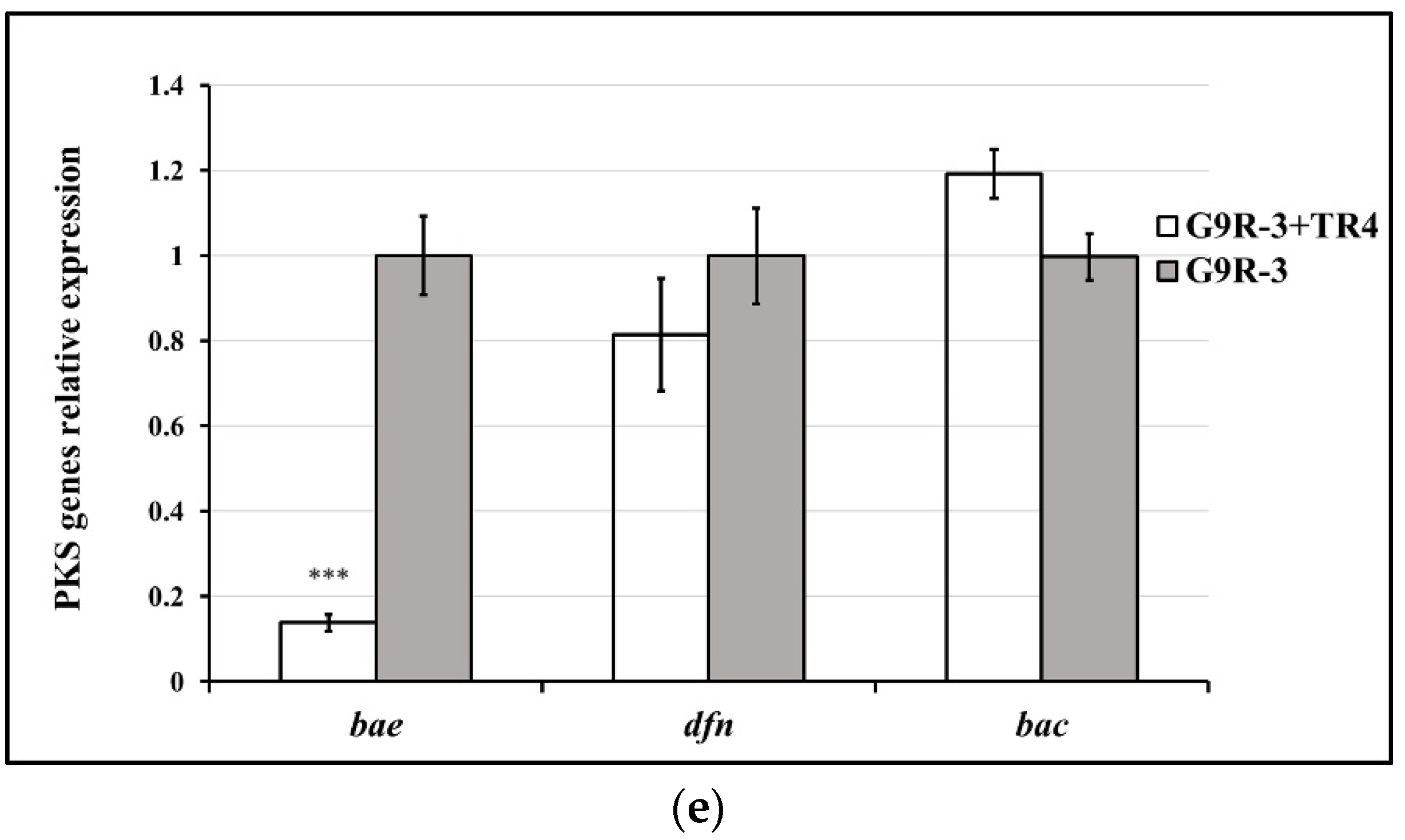
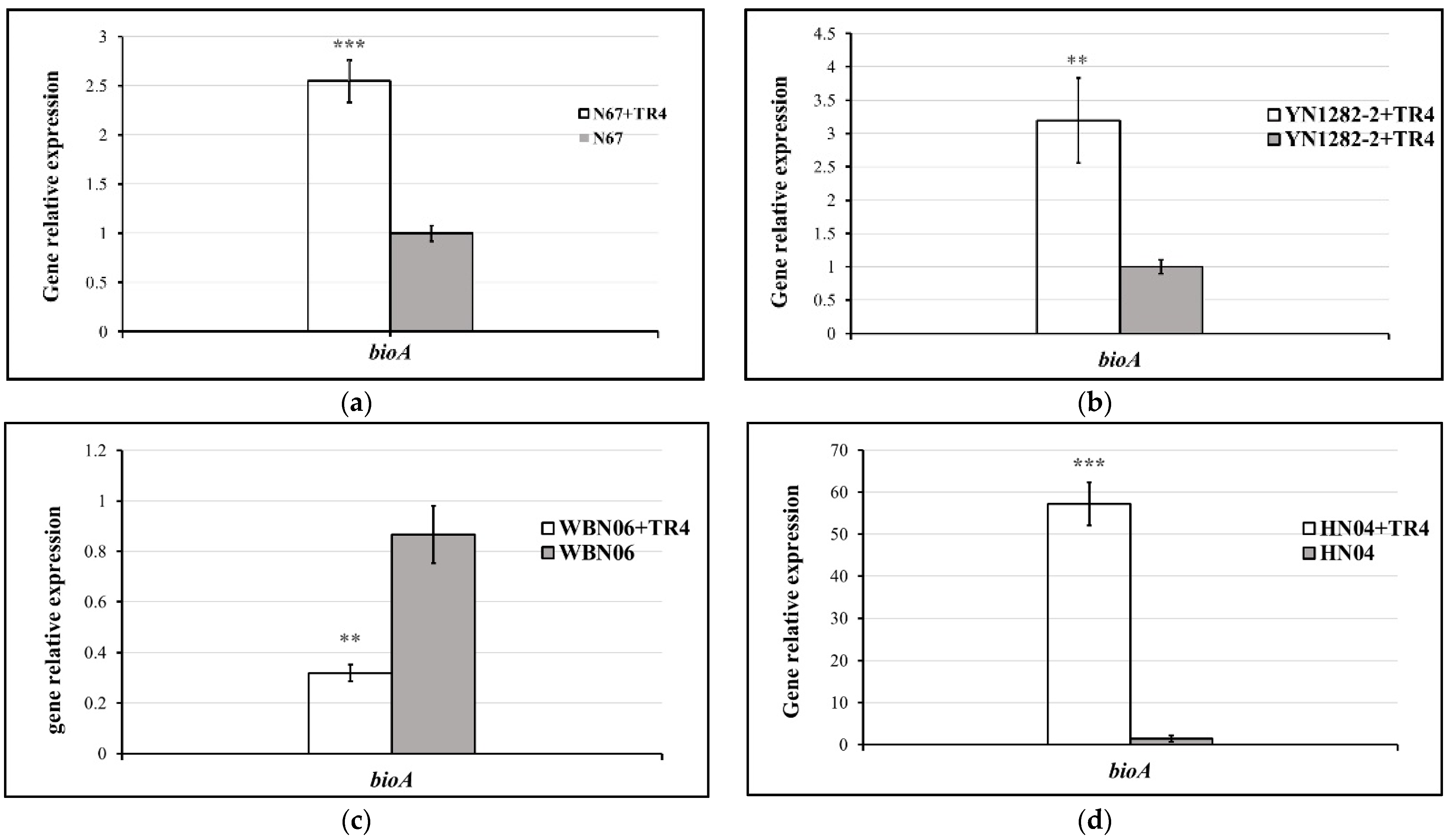
3.5. The Expression Levels of Biocontrol Related Genes in Bacillus Strains
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghag, S.B.; Shekhawat, U.K.; Ganapathi, T.R. Fusarium wilt of banana: Biology, epidemiology and management. Int. J. Pest Manag. 2015, 61, 250–263. [Google Scholar] [CrossRef]
- Nayar, N.M. The bananas: Botany, origin, dispersal. Hortic. Rev. 2010, 36, 117–164. [Google Scholar]
- Zheng, S.-J.; García-Bastidas, F.A.; Li, X.; Zeng, L.; Bai, T.; Xu, S.; Yin, K.; Li, H.; Fu, G.; Yu, Y.; et al. New Geographical Insights of the Latest Expansion of Fusarium oxysporum f.sp. cubense Tropical Race 4 Into the Greater Mekong Subregion. Front. Plant Sci. 2018, 9, 457. [Google Scholar] [CrossRef] [PubMed]
- Bubici, G.; Kaushal, M.; Prigigallo, M.I.; Gómez-Lama Cabanás, C.; Mercado-Blanco, J. Biological Control Agents against Fusarium Wilt of Banana. Front. Microbiol. 2019, 10, 616. [Google Scholar] [CrossRef]
- Compant, S.; Duffy, B.; Nowak, J.; Clément, C.; Barka, E.A. Use of plant growth-promoting bacteria for biocontrol of plant diseases: Principles, mechanisms of action, and future prospects. Appl. Env. Microbiol. 2005, 71, 4951–4959. [Google Scholar] [CrossRef] [PubMed]
- Card, S.; Johnson, L.; Teasdale, S.; Caradus, J. Deciphering endophyte behaviour: The link between endophyte biology and efficacious biological control agents. FEMS Microbiol. Ecol. 2016, 92, fiw114. [Google Scholar] [CrossRef]
- Nicholson, W.L.; Munakata, N.; Horneck, G.; Melosh, H.J.; Setlow, P. Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol. Mol. Biol. Rev. 2000, 64, 548–572. [Google Scholar] [CrossRef]
- Zhang, N.; Wu, K.; He, X.; Li, S.-Q.; Zhang, Z.-H.; Shen, B.; Yang, X.-M.; Zhang, R.-F.; Huang, Q.-W.; Shen, Q.-R. A new bioorganic fertilizer can effectively control banana wilt by strong colonization with Bacillus subtilis N11. Plant Soil 2011, 344, 87–97. [Google Scholar] [CrossRef]
- Aminian, H.; Irandegani, J. Biological control of Fusarium verticillioides agent of Fusarium wilt of banana by Pseudomonas fluorescens and Bacillus subtilis isolates. In Biological Control in Agriculture and Natural Resources; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Hadiwiyono, W.S. Endophytic Bacillus: The potentiality of antagonism to wilt pathogen and promoting growth to micro-plantlet of banana in vitro. Biomirror 2012, 3, 1–4. [Google Scholar]
- Wu, X.; Shan, Y.; Li, Y.; Li, Q.; Wu, C. The Soil Nutrient Environment Determines the Strategy by Which Bacillus velezensis HN03 Suppresses Fusarium wilt in Banana Plants. Front. Plant Sci. 2020, 11, 11. [Google Scholar] [CrossRef]
- Xue, C.; Penton, C.R.; Shen, Z.; Zhang, R.; Huang, Q.; Li, R.; Ruan, Y.; Shen, Q. Manipulating the banana rhizosphere microbiome for biological control of Panama disease. Sci. Rep. 2015, 5, 11124. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Li, B.; Zhang, N.; Waseem, R.; Shen, Q.; Huang, Q. Production of bacillomycin-and macrolactin-type antibiotics by Bacillus amyloliquefaciens NJN-6 for suppressing soilborne plant pathogens. J. Agric. Food Chem. 2012, 60, 2976–2981. [Google Scholar] [CrossRef]
- Wang, B.; Shen, Z.; Zhang, F.; Raza, W.; Yuan, J.; Huang, R.; Ruan, Y.; Li, R.; Shen, Q. Bacillus amyloliquefaciens Strain W19 can Promote Growth and Yield and Suppress Fusarium Wilt in Banana Under Greenhouse and Field Conditions. Pedosphere 2016, 26, 733–744. [Google Scholar] [CrossRef]
- Ongena, M.; Jourdan, E.; Adam, A.; Paquot, M.; Brans, A.; Joris, B.; Arpigny, J.-L.; Thonart, P. Surfactin and fengycin lipopeptides of Bacillus subtilis as elicitors of induced systemic resistance in plants. Env. Microbiol. 2007, 9, 1084–1090. [Google Scholar] [CrossRef]
- Peypoux, F.; Bonmatin, J.M.; Wallach, J. Recent trends in the biochemistry of surfactin. Appl. Microbiol. Biotechnol. 1999, 51, 553–563. [Google Scholar] [CrossRef]
- Cho, K.M.; Math, R.K.; Hong, S.Y.; Islam, S.M.A.; Mandanna, D.K.; Cho, J.J.; Yun, M.G.; Kim, J.M.; Yun, H.D. Iturin produced by Bacillus pumilus HY1 from Korean soybean sauce (kanjang) inhibits growth of aflatoxin producing fungi. Food Control. 2009, 20, 402–406. [Google Scholar] [CrossRef]
- Hsieh, F.-C.; Lin, T.-C.; Meng, M.; Kao, S.-S. Comparing Methods for Identifying Bacillus Strains Capable of Producing the Antifungal Lipopeptide Iturin A. Curr. Microbiol. 2008, 56, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.P.; Chen, C.L.; Chang, L.K.; Tschen, J.S.M.; Liu, S.T. Functional and transcriptional analyses of a fengycin synthetase gene, fenC, from Bacillus subtilis. J. Bacteriol. 1999, 181, 5060–5067. [Google Scholar] [CrossRef] [PubMed]
- Steller, S.; Vollenbroich, D.; Leenders, F.; Stein, T.; Conrad, B.; Hofemeister, J.; Vater, J. Structural and functional organization of the fengycin synthetase multienzyme system from Bacillus subtilis b213 and A1/3. Chem. Biol. 1999, 6, 31–41. [Google Scholar] [CrossRef]
- Joshi, R.; Gardener, M.S. Identification and Characterization of Novel Genetic Markers Associated with Biological Control Activities in Bacillus subtilis. Phytopathology 2006, 96, 145. [Google Scholar] [CrossRef]
- Leães, F.L.; Velho, R.V.; Caldas, D.G.G.; Ritter, A.C.; Tsai, S.M.; Brandelli, A. Expression of essential genes for biosynthesis of antimicrobial peptides of Bacillus is modulated by inactivated cells of target microorganisms. Res. Microbiol. 2016, 167, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yuan, T.; Wang, Y.; Zhang, D.; Bai, T.; Xu, S.; Wang, Y.; Tang, W.; Zheng, S.-J. Identification and evaluation of resistance to Fusarium oxysporum f. sp. cubense tropical race 4 in Musa acuminata Pahang. Euphytica 2018, 214, 106. [Google Scholar] [CrossRef]
- Li, B. The Antagonistic Mechanism of Bacillus amyloliquefaciens SQR9 against Soil-Borne Pathogens and In Situ Rhizosphere Antibiotic Gene Expression. Ph.D. Thesis, Nanjing Agriculture University, Nanjing, China, 2015. [Google Scholar]
- Mora, I.; Cabrefiga, J.; Montesinos, E. Antimicrobial peptide genes in Bacillus strains from plant environments. Int. Microbiol. 2011, 14, 213–223. [Google Scholar]
- Kang, X.X. Mechanism of Bacillus velezensis CC09 in Controlling Pathogen Gaeumannomyces Graminis var. tritici. Ph.D. Thesis, Nanjing Agriculture University, Nanjing, China, 2019. [Google Scholar]
- Maget-Dana, R.; Peypoux, F. Iturins, a special class of pore-forming lipopeptides: Biological and physicochemical properties. Toxicology 1994, 87, 151–174. [Google Scholar] [CrossRef]
- Stein, T. Bacillus subtilis antibiotics: Structures, syntheses and specific functions. Mol. Microbiol. 2005, 56, 845–857. [Google Scholar] [CrossRef] [PubMed]
- Hao, K.; He, P.; Blom, J.; Rueckert, C.; Mao, Z.; Wu, Y.; He, Y.; Borriss, R. The Genome of Plant Growth-Promoting Bacillus amyloliquefaciens subsp. plantarum Strain YAU B9601-Y2 Contains a Gene Cluster for Mersacidin Synthesis. J. Bacteriol. 2012, 194, 3264–3265. [Google Scholar] [CrossRef]
- Wang, S.-L.; Lin, T.Y.; Yen, Y.H.; Liao, H.F.; Chen, Y.J. Bioconversion of shellfish chitin wastes for the production of Bacillus subtilis W-118 chitinase. Carbohydr. Res. 2006, 341, 2507–2515. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.M.; Wang, J.P.; Xiao, R.F.; Liu, B.; Liu, X.G.; Ge, C.B.; Ruan, C.Q.; Zhu, Y.J. Structure identification of lipopeptide from Bacillus licheniformis FJAT-4 and its inhibitory effect on Fusarium oxysporum. Acta Microbiol. Sin. 2017, 12, 1924–1934. (in Chinese). [Google Scholar]
- Xu, Z.; Shao, J.; Li, B.; Yan, X.; Shen, Q.; Zhang, R. Contribution of Bacillomycin D in Bacillus amyloliquefaciens SQR9 to Antifungal Activity and Biofilm Formation. Appl. Env. Microbiol. 2013, 79, 808–815. [Google Scholar] [CrossRef]
- Bais, H.P.; Fall, R.; Vivanco, J.M. Biocontrol of against Infection of Arabidopsis Roots by Is Facilitated by Biofilm Formation and Surfactin Production. Acta Metall. Sinic 2004, 50, 409–414. [Google Scholar]
- Deleu, M.; Lorent, J.; Lins, L.; Brasseur, R.; Braun, N.; El Kirat, K.; Nylander, T.; Dufrêne, Y.F.; Mingeot-Leclercq, M.-P. Effects of surfactin on membrane models displaying lipid phase separation. Biochim. Biophys. Acta (Bba) Biomembr. 2013, 1828, 801–815. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.S.; Huang, S.; Fisher, S.; Pirnik, D.; Aklonis, C.; Dean, L.; Meyers, E.; Fernandes, P.; Mayerl, F. Bacillaene, a Novel Inhibitor of Procaryotic Protein Synthesis Produced by Bacillus subtilis: Production, Taxonomy, Isolation, Physico-chemical Characterization and Biological Activity. J. Antibiot. 1995, 48, 997–1003. [Google Scholar] [CrossRef]
- Wu, L.; Wu, H.; Chen, L.; Yu, X.; Borriss, R.; Gao, X. Difficidin and bacilysin from Bacillus amyloliquefaciens FZB42 have antibacterial activity against Xanthomonas oryzae rice pathogens. Sci. Rep. 2015, 5, 12975. [Google Scholar] [CrossRef]
- Sang, J.W.; Yang, Y.; Chen, Y.; Cai, J.; Lu, C.; Huang, G. Antibacterial activity analysis of lipopeptide and polyketide compounds produced by endophytic bacteria Bacillus amyloliquefaciens BEB17. Acta Phytopathol. Sinica 2018, 3, 402–412. (In Chinese) [Google Scholar]
- Bird, I.M. Extraction of RNA from Cells and Tissue. Mol. Cardiol. 2005, 108, 139–148. [Google Scholar]
- Chen, X.; Pan, Y.J.; Sun, X.H.; Zhao, Y. Progress of Extraction Methods for Bacteria Total RNA. Hunan Agric. Sci. 2010, 5, 9–11. (In Chinese) [Google Scholar]
- Slepecky, R.A.; Hemphill, H.E. The Genus Bacillus—Nonmedical. In The Prokaryotes; Springer: New York, NY, USA, 2011; Volume 4, pp. 530–562. [Google Scholar] [CrossRef]
- Znamenskaia, L.V.; Morozova, O.V.; Vershinina, I.V.; Krasnov, I.S.; Shul’Ga, A.; Leshchinskaia, I.B. Biosynthesis of extracellular guanyl-specific ribonuclease from Bacillus circulans. Микрoбиoлoгия 1999, 67, 619. [Google Scholar]
- Vanittanakom, N.; Loeffler, W.; Koch, U.; Jung, G. Fengycin-a novel antifungal lipopeptide antibiotic produced by Bacillus subtilis F-29-3. J. Antibiot. 1986, 39, 888–901. [Google Scholar] [CrossRef]
- He, P.; Li, S.; Xu, S.T.; Fan, H.C.; Wang, Y.F.; Zhou, W.; Fu, G.; Han, G.Y.; Wang, Y.Y.; Zheng, S.-J. Developing a reliable and efficient fluorescent transformation system for monitoring tritrophic biocontrol in-teractions between Bacillus spp., Foc TR4 and bananas. J. Fungi 2021, in press. [Google Scholar]




| Category | Metabolites | Synthesis Gene | Primers (5′-3′) | References |
|---|---|---|---|---|
| Non-ribosomal peptide synthetases (NRPS) | surfactin | srfAA | GAAAGAGCGGCTGCTGAAAC CCCAATATTGCCGCAATGAC | [21] |
| fengycin | fenD | CCTGCAGAAGGAGAAGTGAAG TGCTCATCGTCTTCCGTTTC | [21] | |
| iturin | ituC | TTCACTTTTGATCTGGCGAT CGT CCG GTA CAT TTT CAC | [21] | |
| bacillomycine D | bamD | AGTCTAAGTATTGGCGAAACGA ATTATGCTGAAAGTGAAGGGCG | [24] | |
| yngG | yngG | GAACTGTCCGAAACATGTCCG CTGAGCTCTTGAACGGTCCGG | [21] | |
| yndJ | yndJ | CAGAGCGACAGCAATCACAT TGAATTTCGGTCCGCTTATC | [21] | |
| bacillibactin | dhbA | TCGGATTTCTTTTGCCACTTGG ATAACGGGCGCTCCTTCGGTTC | [24] | |
| Polytide synthetases (PKS) | macrolactin | mln | GCTGATGAACTGATAACAACCG CATTACGAAGCAAAATAAAAAA | [24] |
| bacillaene | bae | TTGTGCGGTCGTGTATGAACAG TCGCCATCGAGATTAAGAAATT | [24] | |
| Difficidin | dfn | AGTAGTTTTTCTCATCGGTCTG GGCTCCTTATATTGGGGCATTC | [24] | |
| Bacilysin | bac | TGAAGGGACAAGTAGTGAGTAC GATAGGAGACGGGTGGGATA | [24] | |
| Ribosomal peptide synthetases (RPS) | Subtilosin | sboA | TCGGTTTGTAAACTTCAACTGC GTCCACTAGACAAGCGGCTC | [25] |
| Biotin | bioA | TTCCACGGCCATTCCTATAC TTTGTCCCCTTATCCTGCAC | [21] |
| Category | Metabolites | Synthesis Gene | Primers (5′-3′) | References |
|---|---|---|---|---|
| NRPS | surfactin | srfAA | AGCGTAAACGGCATTCAGGAG | This study |
| TATGAGACGGCAGTGTTTCGG | ||||
| fengycin | fenD | CATTTTAACCAGTCCGTCATGC | This study | |
| TCTTTTTTGCAGACAAGGCGC | ||||
| iturin | ituC | AACGAATACGGGCCTACAGAG | This study | |
| CTTCATGCTCTTATCCAGCACG | ||||
| bacillomycine D | bamD | ATTGGCGAAACGAAACATCTGC | This study | |
| AACATCTGATTGTGCTCACGTTC | ||||
| yngG | yngG | CAGAGCGACAGCAATCACATC | This study | |
| ATTGCTCGGCAGGATCATACG | ||||
| yndJ | yndJ | CAGAGCGACAGCAATCACATC | This study | |
| ATTGCTCGGCAGGATCATACG | ||||
| bacillibactin | dhb | CAGTGAAATCGAGCCGATCC | This study | |
| TCTGAAACGGCTTTACAGCATG | ||||
| PKS | macrolactin | mln | CTGATGAACTGATAACAACCGAG | This study |
| ACGTGCCGAAACAACGATTGG | ||||
| bacillaene | bae | TGT GCG GTC GTG TAT GAA CAG | This study | |
| AAC GGT CTG TAT AAA TGC CGA TG | ||||
| difficidin | dfn | TAT CTC AAT CGG ATC GCC GAG | This study | |
| ATA CGG TGC CTA ATC CGG AAG | ||||
| bacilysin | bac | TGAAGGGACAAGTAGTGAGTAC | This study | |
| AGGCACAATTGTGTATTCCAGC | ||||
| RPS | biotin | bioA | GTCGCCGAAAAATCAAAAACGG | This study |
| ACAAGCTCTATGCCGCACATG | ||||
| Housekeeping gene | RNA polymerase β subunit | RopB | AGTATCCCGTTGAAGAGTCAAAAGA | [26] |
| CAAGCTGAGATACGATAACACGTTC |
| Category | Metabolites | Synthesis Gene | N67 | WBN06 | HN04 | YN1282-2 | G9R-3 |
|---|---|---|---|---|---|---|---|
| NRPS | surfactin | srfAA | + | + | + | + | + |
| fengycin | fenD | + | + | + | + | + | |
| iturin | ituC | + | + | + | + | + | |
| bacillomycine D | bamD | + | + | + | + | + | |
| yngG | yngG | + | + | + | + | + | |
| yngJ | yndJ | + | + | + | + | + | |
| bacillibactin | dhb | + | + | + | + | + | |
| PKS | macrolactin | mln | − | + | − | + | − |
| bacillaene | bae | + | + | + | + | + | |
| difficidin | dfn | + | + | + | + | + | |
| bacilysin | bac | + | + | + | + | + | |
| RPS | subtilosin | sboA | − | − | − | − | − |
| biotin | bioA | + | + | + | + | + |
| Method | RNA Quality Determination | Bacillus Sample-1 | Bacillus Sample-2 | Bacillus Sample-3 |
|---|---|---|---|---|
| Mini BEST | A260/A280 | 1.87 | 1.64 | 1.77 |
| A260/A230 | 1.21 | 1.32 | 1.42 | |
| OD/(ng/μL) | 16.22 | 9.76 | 27.41 | |
| Eastep Super | A260/A280 | 2.13 | 2.44 | 1.68 |
| A260/A230 | 1.92 | 2.58 | 1.74 | |
| OD/(ng/μL) | 24.8 | 34.1 | 33.6 | |
| RNAiso Plus | A260/A280 | 2.43 | 2.17 | 1.86 |
| A260/A230 | 1.94 | 2.28 | 1.71 | |
| OD/(ng/μL) | 97.71 | 108.92 | 84.3 | |
| Modified RNAiso Plus | A260/A280 | 2.15 | 2.09 | 2.16 |
| A260/A230 | 2.19 | 2.23 | 2.17 | |
| OD/(ng/μL) | 534.17 | 648.45 | 549.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; He, P.; Fan, H.; Liu, L.; Yin, K.; Yang, B.; Li, Y.; Huang, S.-M.; Li, X.; Zheng, S.-J. A Real-Time Fluorescent Reverse Transcription Quantitative PCR Assay for Rapid Detection of Genetic Markers’ Expression Associated with Fusarium Wilt of Banana Biocontrol Activities in Bacillus. J. Fungi 2021, 7, 353. https://doi.org/10.3390/jof7050353
Li S, He P, Fan H, Liu L, Yin K, Yang B, Li Y, Huang S-M, Li X, Zheng S-J. A Real-Time Fluorescent Reverse Transcription Quantitative PCR Assay for Rapid Detection of Genetic Markers’ Expression Associated with Fusarium Wilt of Banana Biocontrol Activities in Bacillus. Journal of Fungi. 2021; 7(5):353. https://doi.org/10.3390/jof7050353
Chicago/Turabian StyleLi, Shu, Ping He, Huacai Fan, Lina Liu, Kesuo Yin, Baoming Yang, Yongping Li, Su-Mei Huang, Xundong Li, and Si-Jun Zheng. 2021. "A Real-Time Fluorescent Reverse Transcription Quantitative PCR Assay for Rapid Detection of Genetic Markers’ Expression Associated with Fusarium Wilt of Banana Biocontrol Activities in Bacillus" Journal of Fungi 7, no. 5: 353. https://doi.org/10.3390/jof7050353
APA StyleLi, S., He, P., Fan, H., Liu, L., Yin, K., Yang, B., Li, Y., Huang, S.-M., Li, X., & Zheng, S.-J. (2021). A Real-Time Fluorescent Reverse Transcription Quantitative PCR Assay for Rapid Detection of Genetic Markers’ Expression Associated with Fusarium Wilt of Banana Biocontrol Activities in Bacillus. Journal of Fungi, 7(5), 353. https://doi.org/10.3390/jof7050353






