Effect of Isolation Conditions on Diversity of Endolichenic Fungal Communities from a Foliose Lichen, Parmotrema tinctorum
Abstract
1. Introduction
2. Materials and Methods
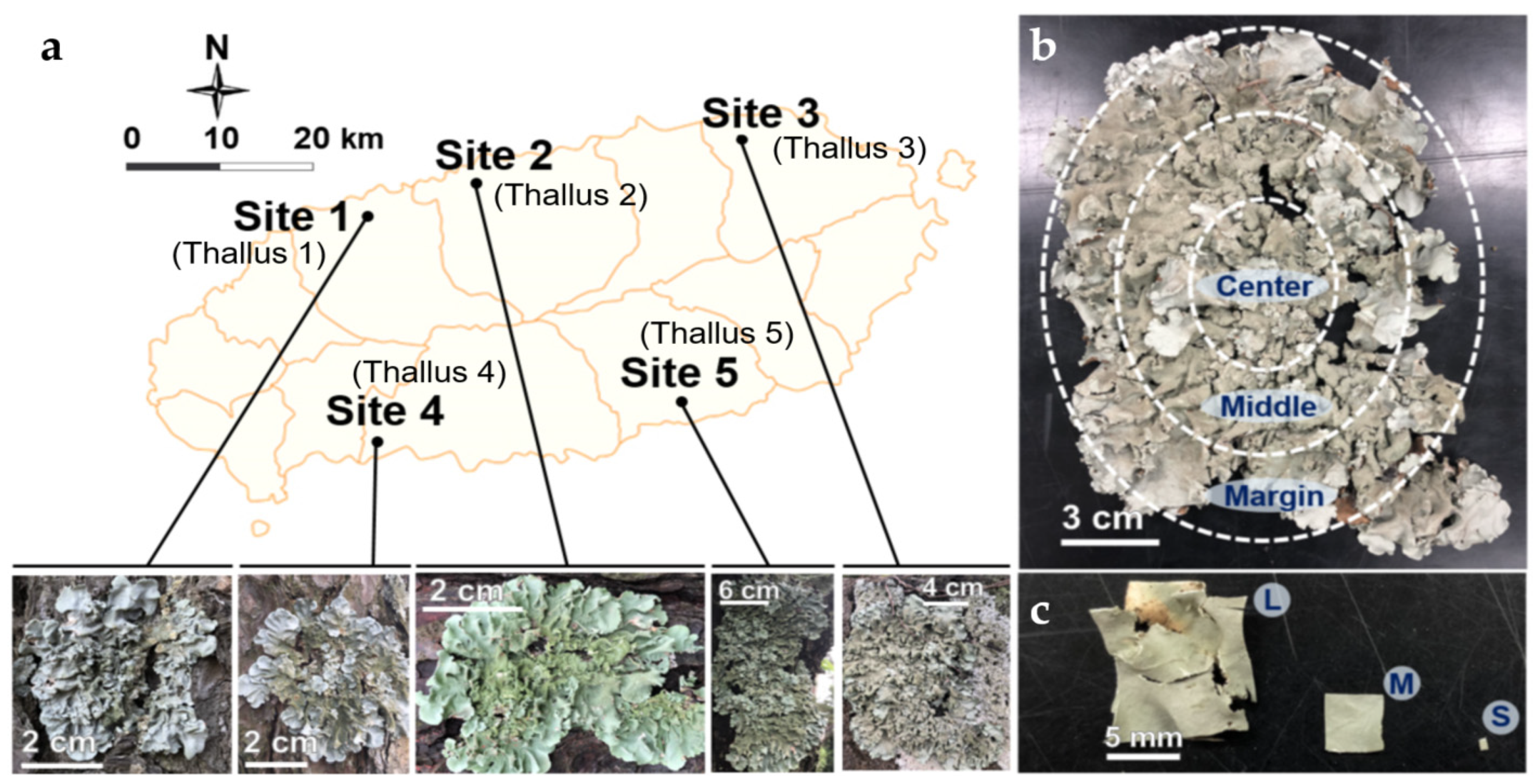
2.1. Lichen Sampling
2.2. Isolation of ELF: Modification of Four Variables
2.3. Molecular Identification
2.4. Statistics and Visualization
3. Results
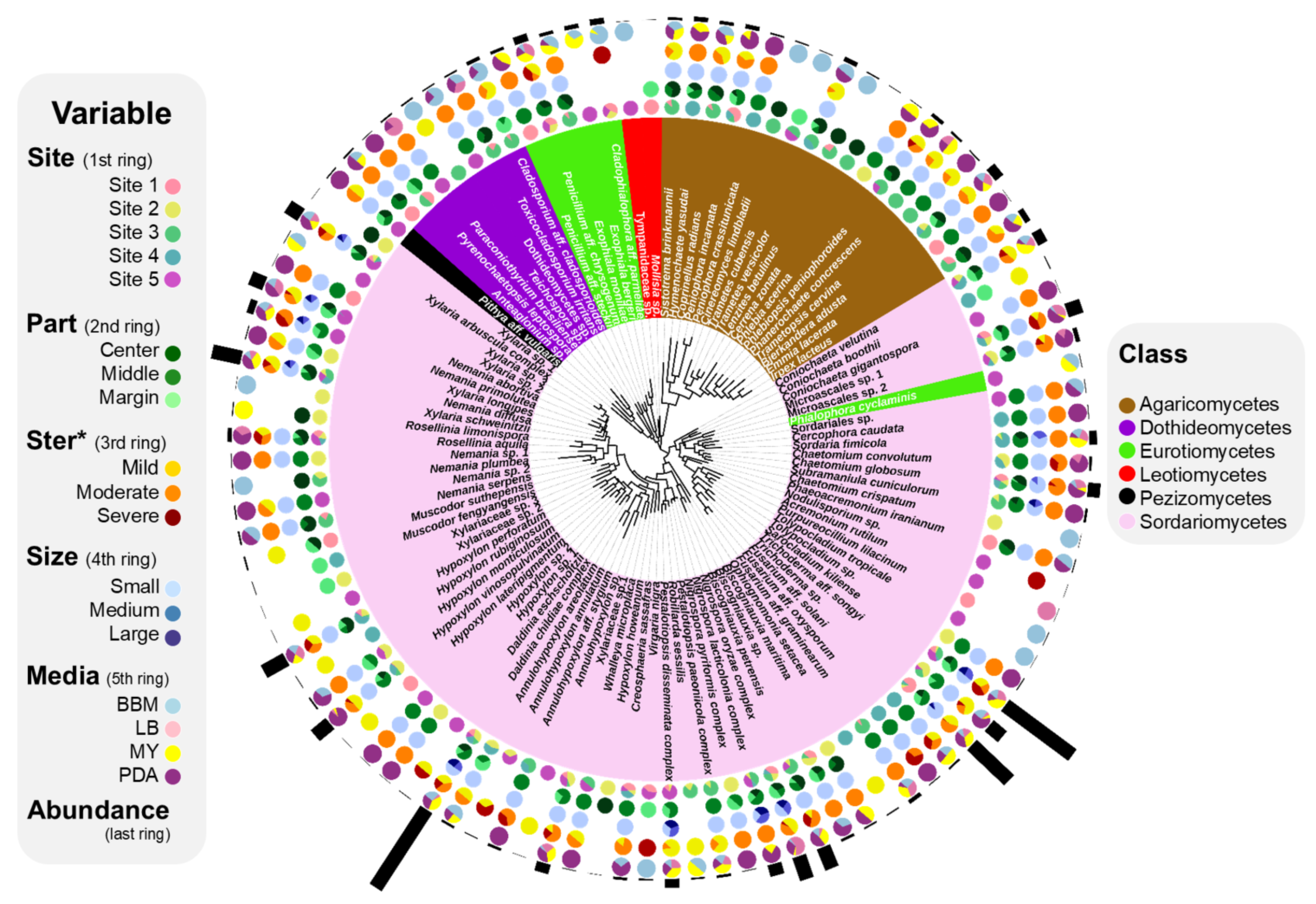
3.1. Composition of ELF Biota
3.2. Effect of Isolation Conditions on Compositions of ELF Communities
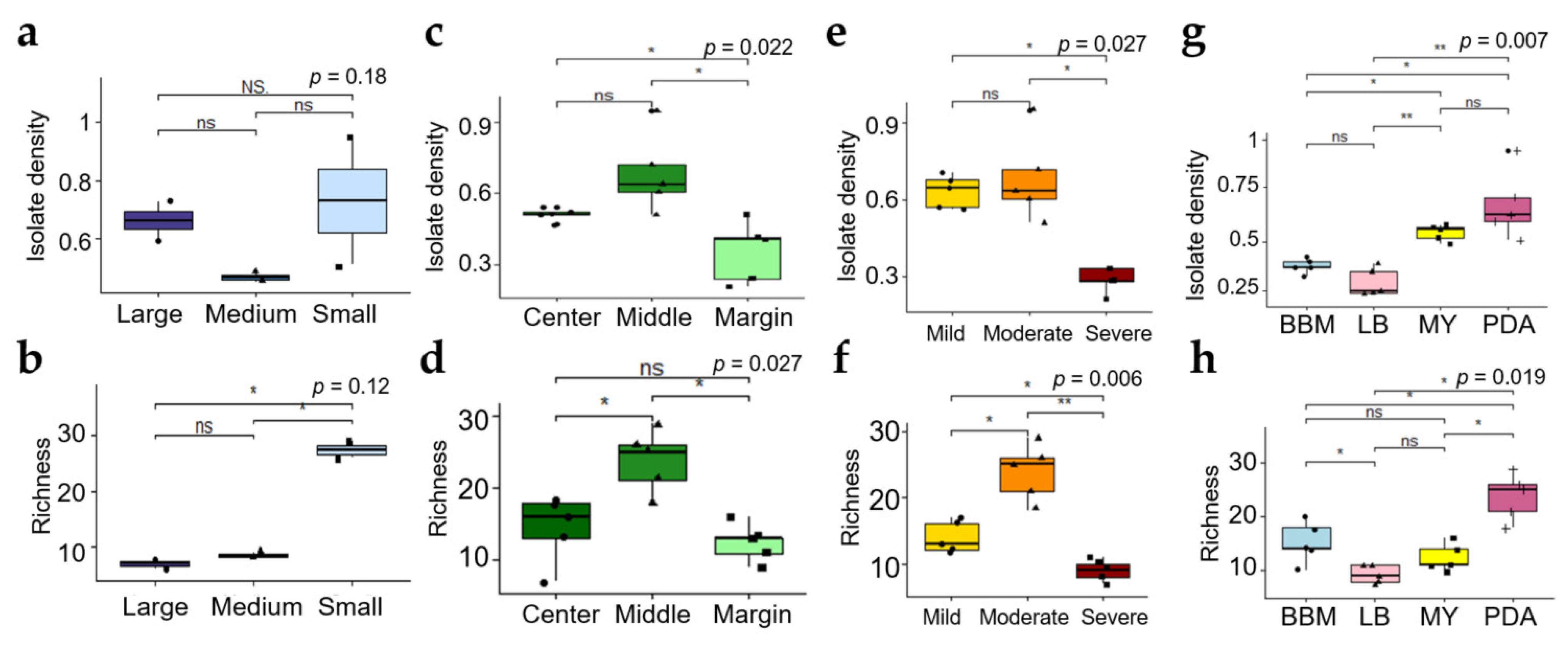
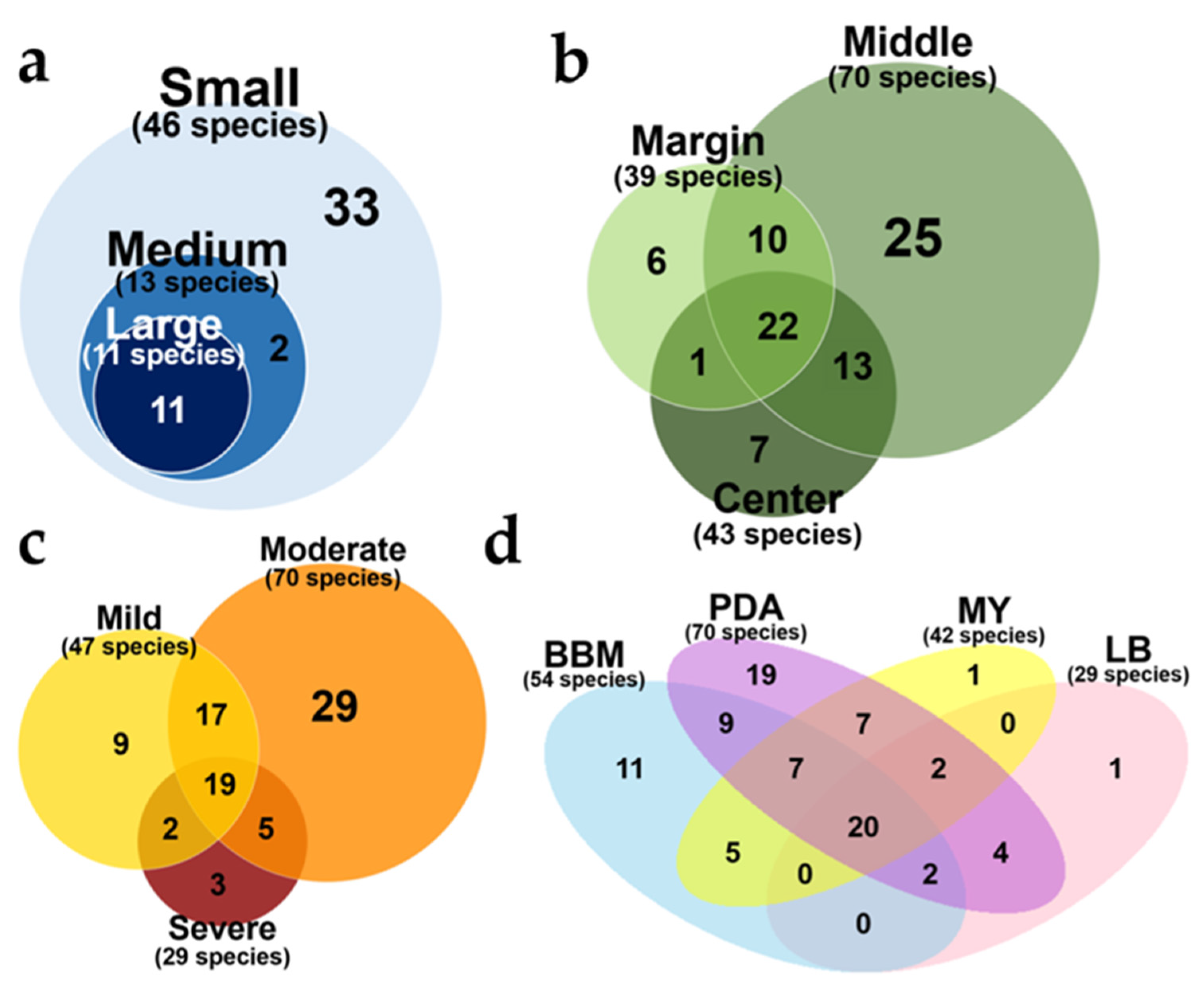
3.2.1. Effect of Size of Thallus Fragment on ELF Diversity
3.2.2. Effect of Thallus Age on the ELF Diversity
3.2.3. Effect of Severity of Thallus Surface Sterilization on ELF Diversity
3.2.4. Effect of the Medium Type on the ELF Diversity
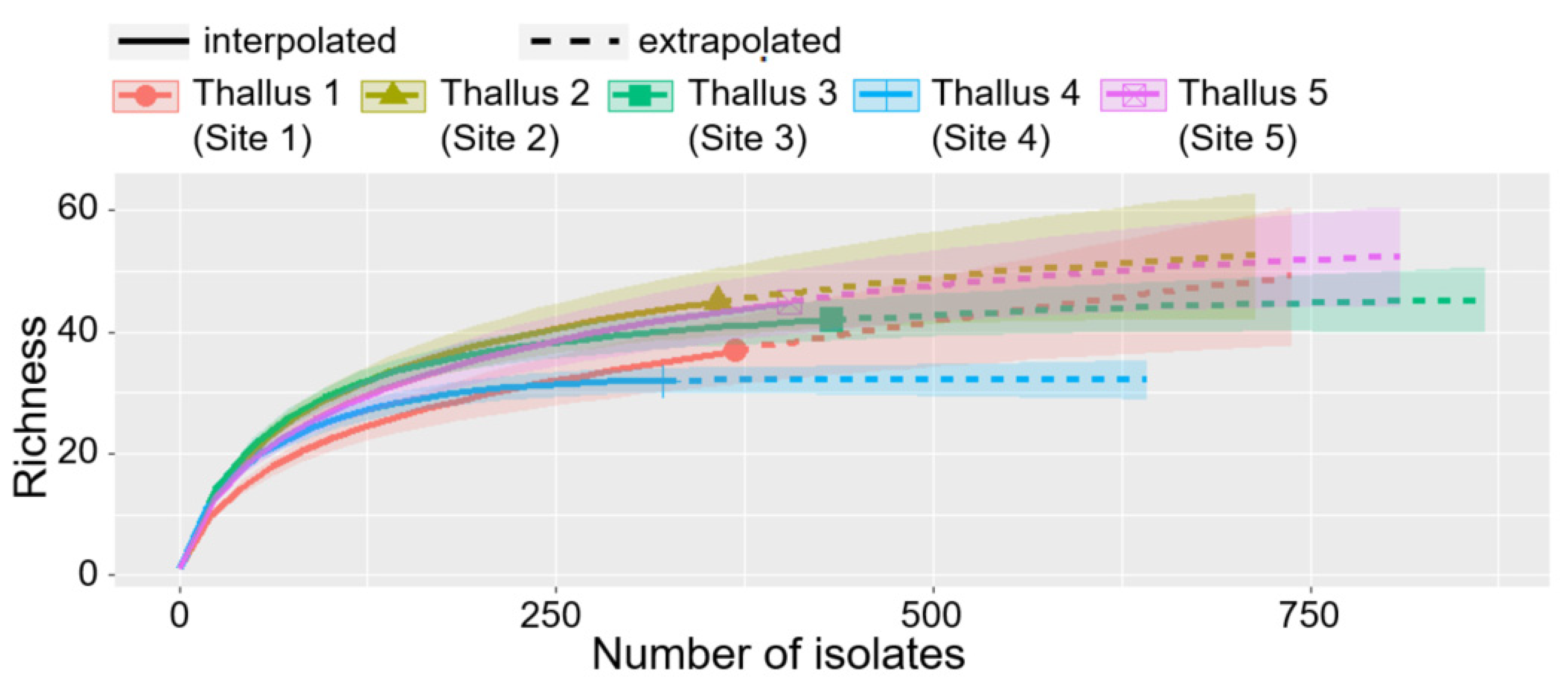
3.3. Comparison of ELF Communities among Five Individual Lichen Thalli
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmadjian, V. The Lichen Symbiosis; John Wiley & Sons: Hoboken, NJ, USA, 1993. [Google Scholar]
- Petrini, O. Fungal endophytes of tree leaves. In Microbial Ecology of Leaves; Springer: Berlin/Heidelberg, Germany, 1991; pp. 179–197. [Google Scholar]
- Arnold, A.E.; Miadlikowska, J.; Higgins, K.L.; Sarvate, S.D.; Gugger, P.; Way, A.; Hofstetter, V.; Kauff, F.; Lutzoni, F.A. Phylogenetic estimation of trophic transition networks for ascomycetous fungi: Are lichens cradles of symbiotrophic fungal diversification? Syst. Biol. 2009, 58, 283–297. [Google Scholar] [CrossRef]
- Suryanarayanan, T.S.; Thirunavukkarasu, N. Endolichenic fungi: The lesser known fungal associates of lichens. Mycology 2017, 8, 189–196. [Google Scholar] [CrossRef] [PubMed]
- U’Ren, J.M.; Lutzoni, F.; Miadlikowska, J.; Arnold, A.E. Community analysis reveals close affinities between endophytic and endolichenic fungi in mosses and lichens. Microb. Ecol. 2010, 60, 340–353. [Google Scholar] [CrossRef]
- U’Ren, J.M.; Lutzoni, F.; Miadlikowska, J.; Laetsch, A.D.; Arnold, A.E. Host and geographic structure of endophytic and endolichenic fungi at a continental scale. Am. J. Bot. 2012, 99, 898–914. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wei, X.-L.; Wei, Y.-Z.; Liu, H.-Y.; Yu, L.-Y. Diversity and distribution of cultured endolichenic fungi in the Ny-Ålesund Region, Svalbard (High Arctic). Extremophiles 2016, 20, 461–470. [Google Scholar] [CrossRef]
- Oh, S.-Y.; Yang, J.H.; Woo, J.-J.; Oh, S.-O.; Hur, J.-S. Diversity and Distribution Patterns of Endolichenic Fungi in Jeju Island, South Korea. Sustainability 2020, 12, 3769. [Google Scholar] [CrossRef]
- Wu, W.; Dai, H.; Bao, L.; Ren, B.; Lu, J.; Luo, Y.; Guo, L.; Zhang, L.; Liu, H. Isolation and structural elucidation of proline-containing cyclopentapeptides from an endolichenic Xylaria sp. J. Nat. Prod. 2011, 74, 1303–1308. [Google Scholar] [CrossRef]
- Samanthi, K.; Wickramaarachchi, S.; Wijeratne, E.; Paranagama, P. Two new antioxidant active polyketides from Penicillium citrinum, an endolichenic fungus isolated from Parmotrema species in Sri Lanka. J. Natl. Sci. Found. Sri Lanka 2015, 42, 119–126. [Google Scholar] [CrossRef]
- Li, G.; Wang, H.; Zhu, R.; Sun, L.; Wang, L.; Li, M.; Li, Y.; Liu, Y.; Zhao, Z.; Lou, H. Phaeosphaerins A–F, cytotoxic perylenequinones from an endolichenic fungus, Phaeosphaeria sp. J. Nat. Prod. 2012, 75, 142–147. [Google Scholar] [CrossRef]
- Ding, G.; Li, Y.; Fu, S.; Liu, S.; Wei, J.; Che, Y. Ambuic acid and torreyanic acid derivatives from the endolichenic fungus Pestalotiopsis sp. J. Nat. Prod. 2008, 72, 182–186. [Google Scholar] [CrossRef]
- Fernández-Mendoza, F.; Fleischhacker, A.; Kopun, T.; Grube, M.; Muggia, L. ITS1 metabarcoding highlights low specificity of lichen mycobiomes at a local scale. Mol. Ecol. 2017, 26, 4811–4830. [Google Scholar] [CrossRef] [PubMed]
- Muggia, L.; Kopun, T.; Grube, M. Effects of Growth Media on the Diversity of Culturable Fungi from Lichens. Molecules 2017, 22, 824. [Google Scholar] [CrossRef] [PubMed]
- Suryanarayanan, T.; Thirunavukkarasu, N.; Hariharan, G.; Balaji, P. Occurrence of non-obligate microfungi inside lichen thalli. Sydowia-Horn- 2005, 57, 120. [Google Scholar]
- Masumoto, H.; Degawa, Y. The effect of surface sterilization and the type of sterilizer on the genus composition of lichen-inhabiting fungi with notes on some frequently isolated genera. Mycoscience 2019, 60, 331–342. [Google Scholar] [CrossRef]
- Gomes, A.T.; Honda, N.K.; Roese, F.M.; Muzzi, R.M.; Marques, M.R. Bioactive derivatives obtained from lecanoric acid, a constituent of the lichen Parmotrema tinctorum (Nyl.) Hale (Parmeliaceae). Rev. Bras. De Farmacogn. 2002, 12, 74–75. [Google Scholar] [CrossRef][Green Version]
- Jayalal, U.; Divakar, P.K.; Joshi, S.; Oh, S.-O.; Koh, Y.J.; Hur, J.-S. The Lichen Genus Parmotrema in South Korea. Mycobiology 2013, 41, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Nam, S. An analysis of the effect of climate indicators on tourism demand: A case study of Jeju Island. J. Policy Res. Tour. Leis. Events 2020, 12, 185–196. [Google Scholar] [CrossRef]
- Won, J.-H.; Lee, J.-Y.; Kim, J.-W.; Koh, G.-W. Groundwater occurrence on Jeju Island, Korea. Hydrogeol. J. 2006, 14, 532–547. [Google Scholar] [CrossRef]
- Li, W.-C.; Zhou, J.; Guo, S.-Y.; Guo, L.-D. Endophytic fungi associated with lichens in Baihua mountain of Beijing, China. Fungal Divers 2007, 25, 69–80. [Google Scholar]
- Andreasen, A.A.; Stier, T. Anaerobic nutrition of Saccharomyces cerevisiae. I. Ergosterol requirement for growth in a defined medium. J. Cell. Comp. Physiol. 1953, 41, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Lwoff, A. Lysogeny. Bacteriol. Rev. 1953, 17, 269–337. [Google Scholar] [CrossRef] [PubMed]
- Bold, H.C. The morphology of Chlamydomonas chlamydogama, sp. nov. Bull. Torrey Botanical Club. 1949, 101–108. [Google Scholar] [CrossRef]
- Lacap, D.; Hyde, K.; Liew, E. An evaluation of the fungal’morphotype’concept based on ribosomal DNA sequences. Fungal Divers. 2003, 12, 53–66. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. Guide Methods Appl. 1990, 18, 315–322. [Google Scholar]
- Hall, T. BioEdit Version 7.0.0. 2004. Available online: www.mbio.ncsu.edu/BioEdit/bioedit.html (accessed on 1 March 2008).
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL): An online tool for phylogenetic tree display and annotation. Bioinformatics 2007, 23, 127–128. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2013. [Google Scholar]
- Hsieh, T.; Ma, K.; Chao, A. iNEXT: An R package for rarefaction and extrapolation of species diversity (H ill numbers). Methods Ecol. Evol. 2016, 7, 1451–1456. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; O’hara, R.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Vegan: Community Ecology Package. R Package Version 1.17-4. 2010. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 1 January 2011).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Breslow, N. A generalized Kruskal-Wallis test for comparing K samples subject to unequal patterns of censorship. Biometrika 1970, 57, 579–594. [Google Scholar] [CrossRef]
- Armstrong, R.A. When to use the B onferroni correction. Ophthalmic Physiol. Opt. 2014, 34, 502–508. [Google Scholar] [CrossRef]
- Gehan, E.A. A generalized Wilcoxon test for comparing arbitrarily singly-censored samples. Biometrika 1965, 52, 203–224. [Google Scholar] [CrossRef]
- Chen, H.; Boutros, P.C. VennDiagram: A package for the generation of highly-customizable Venn and Euler diagrams in R. BMC Bioinform. 2011, 12, 35. [Google Scholar] [CrossRef]
- Legendre, P.; Borcard, D.; Peres-Neto, P.R. Analyzing beta diversity: Partitioning the spatial variation of community composition data. Ecol. Monogr. 2005, 75, 435–450. [Google Scholar] [CrossRef]
- Suryanarayanan, T.S.; Govindarajulu, M.; Rajamani, T.; Tripathi, M.; Joshi, Y. Endolichenic fungi in lichens of Champawat district, Uttarakhand, northern India. Mycol. Prog. 2017, 16, 205–211. [Google Scholar] [CrossRef]
- Vinayaka, K.; Krishnamurthy, Y.; Banakar, S.; Kekuda, T.P. Association and variation of endophytic fungi among some macrolichens in central Western Ghats, Southern India. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 115–124. [Google Scholar] [CrossRef]
- Rajulu, M.B.G.; Thirunavukkarasu, N.; Kumar, S.S.; Kaur, T.; Reddy, M.S.; Suryanarayanan, T.S. Endolichenic fungal diversity associated with some lichens of the Western Ghats. Planta Med. 2020, 86, 960–966. [Google Scholar] [CrossRef]
- Nguyen, M.H.; Yong, J.H.; Sung, H.J.; Lee, J.K. Screening of Endophytic Fungal Isolates against Raffaelea quercus-mongolicae Causing Oak Wilt Disease in Korea. Mycobiology 2020, 48, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yang, C.; Meng, Q.; Liu, L.; Fu, S. A new alkanol from the endolichenic fungus Daldinia childiae. J. Chin. Chem. Soc. 2020, 68, 678681. [Google Scholar]
- Stadler, M.; Læssøe, T.; Fournier, J.; Decock, C.; Schmieschek, B.; Tichy, H.-V.; Peršoh, D. A polyphasic taxonomy of Daldinia (Xylariaceae). Stud. Mycology 2014, 77, 1–143. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, A.; Gené, J.; Sutton, D.; Madrid, H.; De Hoog, G.; Cano, J.; Decock, C.; Crous, P.W.; Guarro, J. Phylogeny of Sarocladium (Hypocreales). Persoonia: Mol. Phylogeny Evol. Fungi 2015, 34, 10. [Google Scholar] [CrossRef]
- Elad, Y.; Chet, I.; Henis, Y. A selective medium for improving quantitative isolation of Trichoderma spp. from soil. Phytoparasitica 1981, 9, 59–67. [Google Scholar] [CrossRef]
- Papavizas, G. Trichoderma and Gliocladium: Biology, ecology, and potential for biocontrol. Annu. Rev. Phytopathol. 1985, 23, 23–54. [Google Scholar] [CrossRef]
- Reino, J.L.; Guerrero, R.F.; Hernández-Galán, R.; Collado, I.G. Secondary metabolites from species of the biocontrol agent Trichoderma. Phytochem. Rev. 2008, 7, 89–123. [Google Scholar] [CrossRef]
- Kondratyuk, S.; Lőkös, L.; Halda, J.; Haji Moniri, M.; Farkas, E.; Park, J.; Mishra, G.K.; Nayaka, S.; Farkas, E.; Park, J.S.; et al. New and noteworthy lichen-forming and lichenicolous fungi 4. Acta Bot. Hung. 2016, 58, 75–136. [Google Scholar] [CrossRef]
- Lawrey, J.D.; Diederich, P. Lichenicolous fungi: Interactions, evolution, and biodiversity. Bryologist 2003, 106, 80–120. [Google Scholar] [CrossRef]
- Diederich, P.; Lawrey, J.D.; Ertz, D. The 2018 classification and checklist of lichenicolous fungi, with 2000 non-lichenized, obligately lichenicolous taxa. Bryologist 2018, 121, 340–426. [Google Scholar] [CrossRef]
- Harutyunyan, S.; Muggia, L.; Grube, M. Black fungi in lichens from seasonally arid habitats. Stud. Mycol. 2008, 61, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Boonpeng, C.; Polyiam, W.; Sriviboon, C.; Sangiamdee, D.; Watthana, S.; Nimis, P.L.; Boonpragob, K. Airborne trace elements near a petrochemical industrial complex in Thailand assessed by the lichen Parmotrema tinctorum (Despr. ex Nyl.) Hale. Environ. Sci. Pollut. Res. 2017, 24, 12393–12404. [Google Scholar] [CrossRef] [PubMed]
- Tanney, J.; Seifert, K. Mollisiaceae: An overlooked lineage of diverse endophytes. Stud. Mycol. 2020, 95, 293–380. [Google Scholar] [CrossRef]
- Checa, J.; Barrasa, J.; Moreno, G.; Fort, F.; Guarro, J. The genus Coniochaeta (Sacc.) Cooke (Coniochaetaceae, Ascomycotina) in Spain. Cryptog. Mycol. 1988, 9, 1–34. [Google Scholar]
- Wang, Y.; Zheng, Z.; Liu, S.; Zhang, H.; Li, E.; Guo, L.; Che, Y. Oxepinochromenones, furochromenone, and their putative precursors from the endolichenic fungus Coniochaeta sp. J. Nat. Prod. 2010, 73, 920–924. [Google Scholar] [CrossRef]
- Ezra, D.; Hess, W.; Strobel, G.A. New endophytic isolates of Muscodor albus, a volatile-antibiotic-producing fungus. Microbiology 2004, 150, 4023–4031. [Google Scholar] [CrossRef]
- Mitchell, A.M.; Strobel, G.A.; Moore, E.; Robison, R.; Sears, J. Volatile antimicrobials from Muscodor crispans, a novel endophytic fungus. Microbiology 2009, 156, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.H. A Monograph of the World Species of Hypoxylon; University of Georgia Press: Athens, GA, USA, 1961. [Google Scholar]
- Sánchez-Ballesteros, J.; González, V.; Salazar, O.; Acero, J.; Portal, M.A.; Julián, M.; Rubio, V.; Bills, G.F.; Polishook, J.D.; Platas, G.; et al. Phylogenetic study of Hypoxylon and related genera based on ribosomal ITS sequences. Mycologia 2000, 92, 964–977. [Google Scholar] [CrossRef]
- Badali, H.; Gueidan, C.; Najafzadeh, M.; Bonifaz, A.; van den Ende, A.G.; De Hoog, G. Biodiversity of the genus Cladophialophora. Stud. Mycol. 2008, 61, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, K.; Ishii, T.; Shiomi, K.; Ōmura, S.; Masuma, R. Virgaria boninensis, a new hyphomycete (Xylariaceae) from soils in the Bonin Islands, Japan. Mycoscience 2013, 54, 394–399. [Google Scholar] [CrossRef]
- Evans, H.C.; Holmes, K.A.; Thomas, S.E. Endophytes and mycoparasites associated with an indigenous forest tree, Theobroma gileri, in Ecuador and a preliminary assessment of their potential as biocontrol agents of cocoa diseases. Mycol. Prog. 2003, 2, 149–160. [Google Scholar] [CrossRef]
- Morrison-Gardiner, S. Dominant fungi from Australian coral reefs. Fungal Divers 2002, 9, 105–121. [Google Scholar]
- Osono, T. Endophytic and epiphytic phyllosphere fungi of Camellia japonica: Seasonal and leaf age-dependent variations. Mycologia 2008, 100, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-L. Two Pezizales from Taiwan. TAIWANIA-TAIPEI- 2001, 46, 238–245. [Google Scholar]
- Jayasiri, S.C.; Jones, E.G.; Kang, J.-C.; Promputtha, I.; Bahkali, A.H.; Hyde, K.D. A new species of genus Anteaglonium (Anteagloniaceae, Pleosporales) with its asexual morph. Phytotaxa 2016, 263, 233–244. [Google Scholar] [CrossRef]
- Jaklitsch, W.M.; Fournier, J.; Voglmayr, H. Two unusual new species of Pleosporales: Anteaglonium rubescens and Atrocalyx asturiensis. Sydowia 2018, 70, 129. [Google Scholar] [PubMed]
- Bills, G.F.; Foster, M.S. Formulae for selected materials used to isolate and study fungi and fungal allies. In Biodiversity of Fungi: Inventory and Monitoring Methods; Academic Press: Cambridge, MA, USA, 2004; pp. 595–618. [Google Scholar]
- Madrid, H.; Hernandez-Restrepo, M.; Gené, J.; Cano, J.; Guarro, J.; Silva, V. New and interesting chaetothyrialean fungi from Spain. Mycol. Prog. 2016, 151, 179–201. [Google Scholar] [CrossRef]
- Ilavarasi, A.; Mubarakali, D.; Praveenkumar, R.; Baldev, E.; Thajuddin, N. Optimization of various growth media to freshwater microalgae for biomass production. Biotechnology 2011, 10, 540–545. [Google Scholar] [CrossRef]
- Vicente, V.; Attili-Angelis, D.; Pie, M.; Queiroz-Telles, F.; Cruz, L.; Najafzadeh, M.J.; De Hoog, G.S.; Zhao, J.; Pizzirani-Kleiner, A. Environmental isolation of black yeast-like fungi involved in human infection. Stud. Mycol. 2008, 61, 137–144. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.H.; Oh, S.-Y.; Kim, W.; Woo, J.-J.; Kim, H.; Hur, J.-S. Effect of Isolation Conditions on Diversity of Endolichenic Fungal Communities from a Foliose Lichen, Parmotrema tinctorum. J. Fungi 2021, 7, 335. https://doi.org/10.3390/jof7050335
Yang JH, Oh S-Y, Kim W, Woo J-J, Kim H, Hur J-S. Effect of Isolation Conditions on Diversity of Endolichenic Fungal Communities from a Foliose Lichen, Parmotrema tinctorum. Journal of Fungi. 2021; 7(5):335. https://doi.org/10.3390/jof7050335
Chicago/Turabian StyleYang, Ji Ho, Seung-Yoon Oh, Wonyong Kim, Jung-Jae Woo, Hyeonjae Kim, and Jae-Seoun Hur. 2021. "Effect of Isolation Conditions on Diversity of Endolichenic Fungal Communities from a Foliose Lichen, Parmotrema tinctorum" Journal of Fungi 7, no. 5: 335. https://doi.org/10.3390/jof7050335
APA StyleYang, J. H., Oh, S.-Y., Kim, W., Woo, J.-J., Kim, H., & Hur, J.-S. (2021). Effect of Isolation Conditions on Diversity of Endolichenic Fungal Communities from a Foliose Lichen, Parmotrema tinctorum. Journal of Fungi, 7(5), 335. https://doi.org/10.3390/jof7050335






