Compatibility of Beauveria bassiana and a Plant Secondary Metabolite: A Novel Modeling Approach to Invade Host Defense for Effective Control of Oligonychus afrasiaticus (McGregor) on Date Palms
Abstract
1. Introduction
2. Materials and Methods
2.1. Microbial Culture
2.2. Oligonychus afrasiaticus
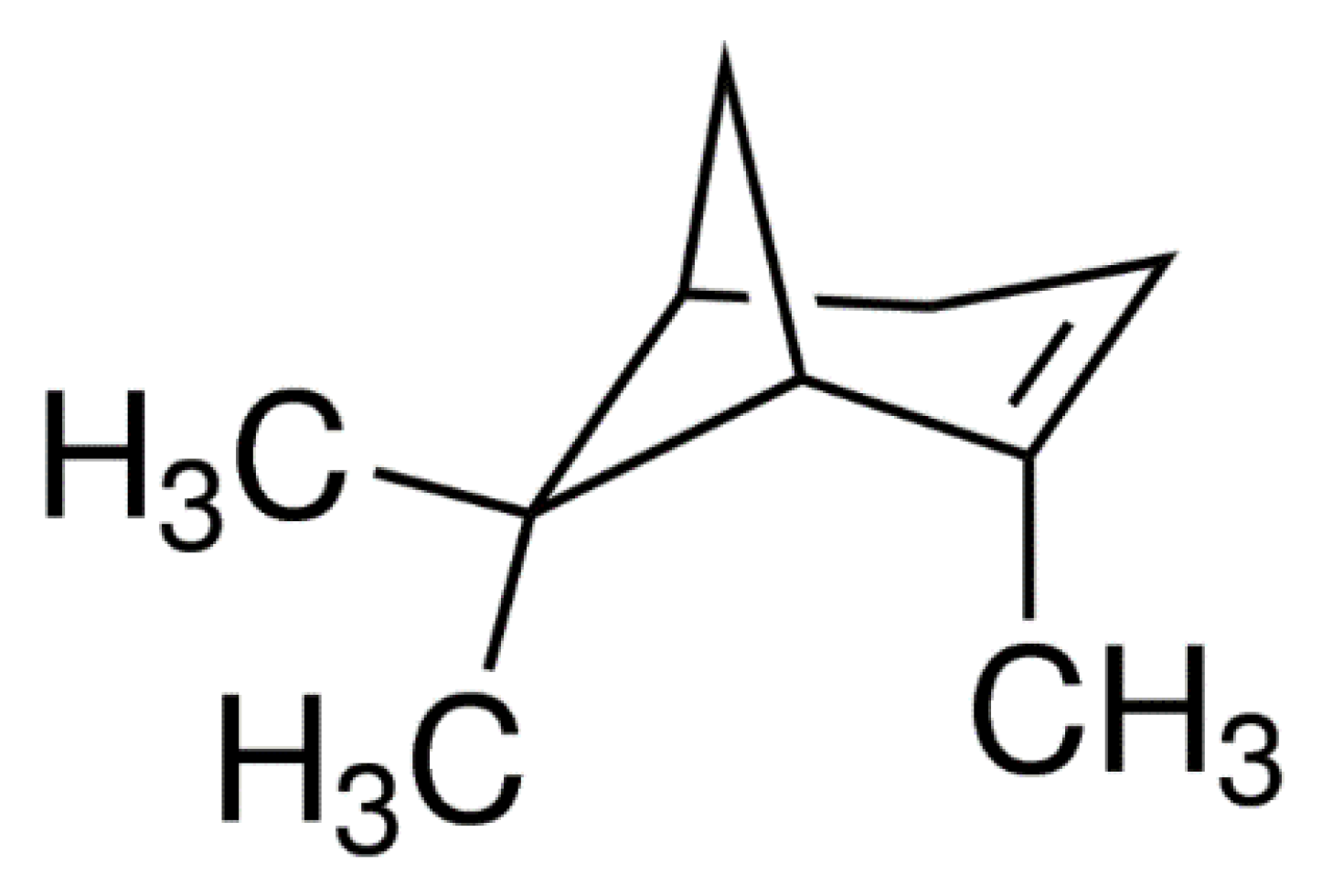
2.3. (+)-α-Pinene
2.4. Fungus-Toxin Compatibility Assays
2.4.1. Germination (GR)
2.4.2. Vegetative Growth (VG)
2.4.3. Conidiation (SP)
2.5. Fungus-Toxin Synergism Bioassays
2.6. Response of Fungus-Toxin Interactions on the Target Host Defense-Related Enzymatic Regulation
3. Results
3.1. Pathogen-Toxin Compatibility Response Analysis
3.2. Date Palm Dust Mites Mortality Response against Pathogen-Toxin Interaction
3.3. Defense-Related Enzymatic Activities Analysis
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Food and Agricultural Commodities Production. Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 21 February 2021).
- Hussain, A.; Rizwan-ul-haq, M.; AlJabr, A.M.; Al-Ayedh, H. Lethality of Sesquiterpenes Reprogramming Red Palm Weevil Detoxification Mechanism for Natural Novel Biopesticide Development. Molecules 2019, 24, 1648. [Google Scholar] [CrossRef]
- Hussain, A.; AlJabr, A.M.; Al-Ayedh, H. Development-Disrupting Chitin Synthesis Inhibitor, Novaluron, Reprogramming the Chitin Degradation Mechanism of Red Palm Weevils. Molecules 2019, 24, 4304. [Google Scholar] [CrossRef]
- Ehsine, M.; Belkadhi, M.S.; Chaieb, M. Seasonal and nocturnal activities of the Rhinoceros Borer (Coleoptera: Scarabaeidae) in the North Saharan Oases Ecosystems. J. Insect Sci. 2014, 14, 256. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hussain, A.; Rizwan-ul-haq, M.; AlJabr, A.M.; Al-Ayedh, H. Host-pathogen interaction for screening potential of Metarhizium anisopliae isolates against the date-palm dust mite, Oligonychus afrasiaticus (McGregor) (Acari: Tetranychidae). Egypt. J. Biol. Pest Control 2019, 29, 1–6. [Google Scholar] [CrossRef]
- Hussain, A.; AlJabr, A.M. Potential synergy between spores of Metarhizium anisopliae and plant secondary metabolite, 1-chlorooctadecane for effective natural acaricide development. Molecules 2020, 25, 1900. [Google Scholar] [CrossRef]
- Coudin, R.; Galves, F. Biologie de l’acarien du palmier dattier Oligonychus afrasiaticus (MacGregor) en Mauritanie. Fruits 1976, 31, 543–550. [Google Scholar]
- Ba-Angood, S.A.; Bass’haih, G.S. A study on the effect of date palm dust mite Oligonychus afrasiaticus (McGregor) (Acarina: Tetranychidae) on the physiochemical characters of three different date varieties in Wadi Hadhramout, Yemen. Arab J. Plant Prot. 2000, 18, 82–85. [Google Scholar]
- Elwan, A.A. Survey of the insect and mite pests associated with date palm trees in Al-Dakhliya region, Sultanate of Oman. Egypt. J. Agric. Res. 2000, 78, 653–664. [Google Scholar]
- Palevsky, E.; Ucko, O.; Peles, S.; Yablonski, S.; Gerson, U. Evaluation of control measures for Oligonychus afrasiaticus infesting date palm cultivars in the Southern Arava Valley of Israel. Crop. Prot. 2004, 23, 387–392. [Google Scholar] [CrossRef]
- Ben Chaabane, S.; Chermiti, B. Characteristics of date fruit and its influence on population dynamics of Oligonychus afrasiaticus in the southern of Tunisia. Acarologia 2009, 49, 29–37. [Google Scholar]
- Chaaban, S.B.; Brahim, C.; Serge, K. Effects of host plants on distribution, abundance, developmental time and life table parameters of Oligonychus afrasiaticus (McGregor) (Acari: Tetranychidae). Papéis Avulsos Zool. 2012, 52, 121–133. [Google Scholar] [CrossRef]
- Yousof, D.E.; Mahmoud, M.E.E. Distribution of date palm dust mite Oligonychus afrasiaticus Meg., (Acari: Tetranychidae) in northern state in Sudan and its impact on productivity of fruits of date. Persian Gulf Crop Prot. 2013, 2, 54–59. [Google Scholar]
- Latifian, M. Integrated Pest Management of Date Palm Fruit Pests: A Review. J. Entomol. 2017, 14, 112–121. [Google Scholar] [CrossRef]
- Edongali, E.; Kerra, H.; Gashira, B. Distribution and control of date mite (Oligonychus afrasiaticus) McGregor in Libya. Arab Near East Plant Prot. Newsl. 1988, 7, 1–25. [Google Scholar]
- Talhouk, A.S. On the management of the date palm and its arthropod enemies in the Arabian Peninsula. J. Appl. Entomol. 1991, 111, 514–520. [Google Scholar] [CrossRef]
- Al-Doghairi, M.A. Effect of eight acaricides against the Date Dust Mite, Oligonychus afrasiaticus (Mcgregor) (Acari: Tetranychidae). Pak. J. Biol. Sci. 2004, 7, 1168–1171. [Google Scholar] [CrossRef]
- Blumberg, D. Review: Date palm arthropod pests and their management in Israel. Phytoparasitica 2008, 36, 411–448. [Google Scholar] [CrossRef]
- Chaaban, S.B.; Chermiti, B.; Kreiter, S. Comparative demography of the spider mite, Oligonychus afrasiaticus, on four date palm varieties in Southwestern Tunisia. J. Insect Sci. 2011, 11, 136. [Google Scholar] [CrossRef]
- Kim, J.J.; Jeong, G.; Han, J.H.; Lee, S. Biological control of aphid using fungal culture and culture filtrates of Beauveria bassiana. Mycobiology 2013, 41, 221–224. [Google Scholar] [CrossRef]
- Doberski, J.W. Comparative laboratory studies on three fungal pathogens of the elm bark beetle Scolytus scolytus: Pathogenicity of Beauveria bassiana, Metarhizium anisopliae, and Paecilomyces farinosus to larvae and adults of S. scolytus. J. Invertebr. Pathol. 1981, 37, 188–194. [Google Scholar] [CrossRef]
- Lord, J.C. Enhanced efficacy of Beauveria bassiana for red flour beetle with reduced moisture. J. Econ. Entomol. 2007, 100, 1071–1074. [Google Scholar] [CrossRef]
- C, M.M.; Lakshmi, K.A.; Devi, K.U. Laboratory evaluation of the pathogenicity of three isolates of the entomopathogenic fungus Beauveria bassiana (Bals.) Vuillemin on the American cockroach (Periplaneta americana). Biocontrol Sci. Technol. 1999, 9, 29–33. [Google Scholar]
- Inglis, G.; Johnson, D.; Goettel, M. Effects of temperature and sunlight on mycosis (Beauveria bassiana) (Hyphomycetes: Sympodulosporae) of Grasshoppers under field conditions. Environ. Entomol. 1997, 26, 400–409. [Google Scholar] [CrossRef]
- Hussain, A.; Tian, M.Y.; He, Y.R.; Ahmed, S. Entomopathogenic fungi disturbed the larval growth and feeding performance of Ocinara varians (Lepidoptera: Bombycidae) larvae. Insect Sci. 2009, 16, 511–517. [Google Scholar] [CrossRef]
- Vandenberg, J.D.; Shelton, A.M.; Wilsey, W.T.; Ramos, M. Assessment of Beauveria bassiana sprays for control of Diamondback moth (Lepidoptera: Plutellidae) on crucifers. J. Econ. Entomol. 1998, 91, 624–630. [Google Scholar] [CrossRef]
- Hussain, A.; Tian, M.-Y.; Wen, S.-Y. Exploring the caste-specific multi-layer defense mechanism of Formosan Subterranean Termites, Coptotermes formosanus Shiraki. Int. J. Mol. Sci. 2017, 18, 2694. [Google Scholar] [CrossRef] [PubMed]
- Murphy, B.C.; Morisawa, T.A.; Newman, J.P.; Tjosvold, S.A.; Parrella, M.P. Fungal pathogen controls thrips in greenhouse flowers. Calif. Agric. 1998, 52, 32–36. [Google Scholar] [CrossRef]
- Hussain, A.; Rizwan-ul-Haq, M.; Al-Ayedh, H.; Ahmed, S.; Al-Jabr, A.M. Effect of Beauveria bassiana infection on the feeding performance and antioxidant defence of red palm weevil, Rhynchophorus ferrugineus. BioControl 2015, 60, 849–859. [Google Scholar] [CrossRef]
- Kim, C.-S.; Lee, J.-B.; Kim, B.-S.; Nam, Y.-H.; Shin, K.-S.; Kim, J.-W.; Kim, J.-E.; Kwon, G.-S. A Technique for the prevention of Greenhouse Whitefly (Trialeurodes vaporariorum) using the entomopathogenic fungus Beauveria bassiana M130. J. Microbiol. Biotechnol. 2014, 24, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Dambolena, J.S.; Zunino, M.P.; Herrera, J.M.; Pizzolitto, R.P.; Areco, V.A.; Zygadlo, J.A. Terpenes: Natural Products for Controlling Insects of Importance to Human Health—A Structure-Activity Relationship Study. Psyche 2016, 2016, 1–17. [Google Scholar] [CrossRef]
- Langsi, J.D.; Nukenine, E.N.; Oumarou, K.M.; Moktar, H.; Fokunang, C.N.; Mbata, G.N. Evaluation of the insecticidal activities of α-Pinene and 3-Carene on Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae). Insects 2020, 11, 540. [Google Scholar] [CrossRef]
- Usha Pathipati, R.; Madhusudhana Murthy, J.; Sreedhar, B. Dynamic adsorption of α-pinene and linalool on silica nanoparticles for enhanced antifeedant activity against agricultural pests. J. Pest. Sci. 2014, 87, 191–200. [Google Scholar] [CrossRef]
- Gonçalves Diniz, A.; Barbosa, L.F.S.; Santos, A.C.S.; Oliveira, N.T.; da Costa, A.F.; Carneiro-Leão, M.P.; Tiago, P.V. Bio-insecticide effect of isolates of Fusarium caatingaense (Sordariomycetes: Hypocreales) combined to botanical extracts against Dactylopius opuntiae (Hemiptera: Dactylopiidae). Biocontrol Sci. Technol. 2020, 384–395. [Google Scholar] [CrossRef]
- Hernández, M.M.; Martínez-Villar, E.; Peace, C.; Pérez-Moreno, I.; Marco, V. Compatibility of the entomopathogenic fungus Beauveria bassiana with flufenoxuron and azadirachtin against Tetranychus urticae. Exp. Appl. Acarol. 2012, 58, 395–405. [Google Scholar] [CrossRef]
- Akbar, W.; Lord, J.C.; Nechols, J.R.; Loughin, T.M. Efficacy of Beauveria bassiana for red flour beetle when applied with plant essential oils or in mineral oil and organosilicone carriers. J. Econ. Entomol. 2005, 98, 683–688. [Google Scholar] [CrossRef] [PubMed]
- AlJabr, A.; Hussain, A.; Rizwan-ul-haq, M. Toxin-Pathogen synergy reshaping detoxification and antioxidant defense mechanism of Oligonychus afrasiaticus (McGregor). Molecules 2018, 23, 1978. [Google Scholar] [CrossRef]
- Schumacher, V.; Poehling, H.-M. In vitro effect of pesticides on the germination, vegetative growth, and conidial production of two strains of Metarhizium anisopliae. Fungal Biol. 2012, 116, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Statistix Statistix 8.1 Tallahassee, FL: Analytical Software 2003. Available online: https://www.amazon.com/Statistix-Analytical-Software-Users-Manual/dp/1881789063 (accessed on 25 April 2021).
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- StatsDirect StatsDirect Statistical Software; England StatsDirect Ltd.: Merseyside, UK, 2013; Available online: http://www.statsdirect.com (accessed on 25 April 2021).
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Hussain, A.; Rizwan-ul-Haq, M.; AlJabr, A.M.; Al-Ayedh, H. Evaluation of host–pathogen interactions for selection of entomopathogenic fungal isolates against Oligonychus afrasiaticus (McGregor). BioControl 2020, 65, 185–195. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Hussain, A.; Rizwan-ul-Haq, M.; Al-Ayedh, H.; AlJabr, A. Susceptibility and immune defence mechanisms of Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae) against entomopathogenic fungal infections. Int. J. Mol. Sci. 2016, 17, 1518. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Hussain, A.; Guan, Z.; Wang, D.; Jaleel, W.; Lyu, L.; He, Y. Unraveling the mode of action of Cordyceps fumosorosea: Potential biocontrol agent against Plutella xylostella (Lepidoptera: Plutellidae). Insects 2021, 12, 179. [Google Scholar] [CrossRef]
- Hussain, A.; Tian, M.-Y.; Wen, S.-Y. Proteomic analysis of Formosan Subterranean Termites during exposure to entomopathogenic fungi. Curr. Proteom. 2018, 15, 229–240. [Google Scholar] [CrossRef]
- Hussain, A. Reprogramming the virulence: Insect defense molecules navigating the epigenetic landscape of Metarhizium robertsii. Virulence 2018, 9, 447–449. [Google Scholar] [CrossRef]
- Wraight, S.P.; Ramos, M.E. Synergistic interaction between Beauveria bassiana and Bacillus thuringiensis tenebrionis-based biopesticides applied against field populations of Colorado potato beetle larvae. J. Invertebr. Pathol. 2005, 90, 139–150. [Google Scholar] [CrossRef]
- Hussain, A.; Ali, M.W.; AlJabr, A.M.; Al-Kahtani, S.N. Insights into the Gryllus bimaculatus immune-related transcriptomic profiling to combat naturally invading pathogens. J. Fungi 2020, 6, 232. [Google Scholar] [CrossRef]
- Dannon, H.F.; Dannon, A.E.; Douro-Kpindou, O.K.; Zinsou, A.V.; Houndete, A.T.; Toffa-Mehinto, J.; Elegbede, I.A.T.M.; Olou, B.D.; Tamo, M. Toward the efficient use of Beauveria bassiana in integrated cotton insect pest management. J. Cott. Res. 2020, 3, 1–21. [Google Scholar]
- Al-mazra’awi, M.S.; Al-Abbadi, A.; Shatnawi, M.A.; Ateyyat, M. Effect of application method on the interaction between Beauveria bassiana and neem tree extract when combined for Thrips tabaci(Thysanoptera: Thripidae) control. J. Food Agric. Environ. 2009, 7, 869–873. [Google Scholar]
- Dampc, J.; Kula-Maximenko, M.; Molon, M.; Durak, R. Enzymatic defense response of apple aphid Aphis pomi to increased temperature. Insects 2020, 11, 436. [Google Scholar] [CrossRef]
- AlJabr, A.M.; Hussain, A.; Rizwan-ul-Haq, M.; Al-Ayedh, H. Toxicity of Plant Secondary Metabolites Modulating Detoxification Genes Expression for Natural Red Palm Weevil Pesticide Development. Molecules 2017, 22, 169. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Rizwan-ul-Haq, M.; Al-Ayedh, H.; Aljabr, A.M. Toxicity and detoxification mechanism of black pepper and its major constituent in controlling Rhynchophorus ferrugineus Olivier (Curculionidae: Coleoptera). Neotrop. Entomol. 2017, 46, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Ma, C.; Luo, J.; Niu, L.; Hua, H.; Zhang, S.; Cui, J. Potential of Cucurbitacin B and Epigallocatechin Gallate as Biopesticides against Aphis gossypii. Insects 2021, 12, 32. [Google Scholar] [CrossRef] [PubMed]
- Badawy, M.E.I.; Nasr, H.M.; Rabea, E.I. Toxicity and biochemical changes in the honey bee Apis mellifera exposed to four insecticides under laboratory conditions. Apidologie 2015, 46, 177–193. [Google Scholar] [CrossRef]
- Zibaee, A.; Bandani, A.R. A study on the toxicity of a medicinal plant, Artemisia annua L. (Asteracea) extracts to the Sunn pest, Eurygaster integriceps Puton (Hemiptera: Scutelleridae). J. Plant Prot. Res. 2010, 50, 79–85. [Google Scholar] [CrossRef]
| Biological Index 1 | Criterion | Classification |
|---|---|---|
| Conidia vs. (+)-α-Pinene | >66 | Compatible |
| 42–66 | Moderately Toxic | |
| <42 | Toxic |
| (+)-α-Pinene (mg/mL). | Scheme I | Scheme II | Scheme III | Scheme IV | Conidia (mg/mL) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Share of Category Weights | Share of Category Weights | Share of Category Weights | Share of Category Weights | ||||||
| 20% (+)-α-Pinene: 80% Conidia | 40% (+)-α-Pinene: 60% Conidia | 60% (+)-α-Pinene: 40% Conidia | 80% (+)-α-Pinene: 20% Conidia | ||||||
| (+)-α-Pinene (mg/mL) | Conidia mg/mL | (+)-α-Pinene (mg/mL) | Conidia mg/mL | (+)-α-Pinene (mg/mL) | Conidia mg/mL | (+)-α-Pinene (mg/mL) | Conidia mg/mL | ||
| 0.7 | 0.14 | 3.2 | 0.28 | 2.4 | 0.42 | 1.6 | 0.56 | 0.8 | 4 |
| 1.4 | 0.28 | 6.4 | 0.56 | 4.8 | 0.84 | 3.2 | 1.12 | 1.6 | 8 |
| 2.1 | 0.42 | 9.6 | 0.84 | 7.2 | 1.26 | 4.8 | 1.68 | 2.4 | 12 |
| 2.8 | 0.56 | 12.8 | 1.12 | 9.6 | 1.68 | 6.4 | 2.24 | 3.2 | 16 |
| 3.5 | 0.70 | 16.0 | 1.40 | 12.0 | 2.10 | 8.0 | 2.80 | 4.0 | 20 |
| Treatments (mg/mL) | Germination (%) * | Vegetative Growth (mm) * | Conidiation (×107 Conidia/mL) * | Biological Index | Classification |
|---|---|---|---|---|---|
| Control | 98.90 ± 0.50 a | 86.60 ± 1.98 a | 7.90 ± 0.66 a | - | - |
| 0.7 | 98.00 ± 0.96 ab | 85.40 ± 2.32 ab | 7.20 ± 0.59 ab | 95 | Compatibility |
| 1.4 | 97.60 ± 0.76 ab | 83.10 ± 1.69 ab | 6.70 ± 0.54 abc | 91 | Compatibility |
| 2.1 | 96.40 ± 0.80 bc | 80.40 ± 1.83 bc | 6.40 ± 0.56 abc | 88 | Compatibility |
| 2.8 | 95.90 ± 0.98 bc | 76.80 ± 2.06 cd | 5.80 ± 0.47 bc | 83 | Compatibility |
| 3.5 | 95.20 ± 1.19 c | 74.20 ±1.69 d | 5.30 ± 0.30 c | 79 | Compatibility |
| Treatments | Post-Exposure Duration | ||
|---|---|---|---|
| 2 d | 4 d | 6 d | |
| (+)-α-Pinene | (%) | (%) | (%) |
| 0.7 mg/mL | 08.40 ± 0.75 i | 12.40 ± 0.75 h | 24.40 ± 1.33 fg |
| 1.4 mg/mL | 12.40 ± 0.98 h | 21.60 ± 1.33 g | 32.40 ± 2.14 e |
| 2.1 mg/mL | 23.60 ± 1.72 g | 28.80 ± 2.06 ef | 49.20 ± 2.87 c |
| 2.8 mg/mL | 27.60 ± 1.72 ef | 42.40 ± 1.83 d | 63.20 ± 2.33 b |
| 3.5 mg/mL | 31.60 ± 1.94 e | 56.80 ± 2.15 b | 79.60 ± 2.48 a |
| Scheme I: 20% (+)-α-Pinene: 80% Conidia | |||
| 0.14 mg/mL (+)-α-Pinene + 3.2 mg/mL conidia | 19.60 ± 1.17 k | 36.40 ± 1.94 h | 66.40 ± 1.72 e |
| 0.28 mg/mL (+)-α-Pinene + 6.4 mg/mL conidia | 24.40 ± 1.17 j | 43.20 ± 2.06 g | 72.40 ± 2.99 d |
| 0.42 mg/mL (+)-α-Pinene + 9.6 mg/mL conidia | 28.00 ± 1.10 i | 54.40 ± 1.60 f | 77.60 ± 2.86 c |
| 0.56 mg/mL (+)-α-Pinene + 12.8 mg/mL conidia | 29.20 ± 1.74 i | 65.60 ± 3.06 e | 83.60 ± 2.79 b |
| 0.70 mg/mL (+)-α-Pinene + 16.0 mg/mL conidia | 32.80 ± 2.15 h | 76.40 ± 2.48 cd | 87.60 ± 2.71 a |
| Scheme II: 40% (+)-α-Pinene: 60% Conidia | |||
| 0.28 mg/mL (+)-α-Pinene + 2.4 mg/mL conidia | 21.60 ± 1.17 l | 41.60 ± 1.72 h | 70.40 ± 2.14 f |
| 0.56 mg/mL (+)-α-Pinene + 4.8 mg/mL conidia | 23.60 ± 1.60 l | 43.20 ± 2.06 h | 80.80 ± 2.42 de |
| 0.84 mg/mL (+)-α-Pinene + 7.2 mg/mL conidia | 28.40 ± 1.47 k | 61.20 ± 2.15 g | 84.40 ± 2.14 c |
| 1.12 mg/mL (+)-α-Pinene + 9.6 mg/mL conidia | 31.60 ± 1.72 j | 77.60 ± 2.48 e | 89.60 ± 2.14 b |
| 1.40 mg/mL (+)-α-Pinene + 12.0 mg/mL conidia | 34.80 ± 1.62 i | 81.60 ± 2.56 cd | 92.80 ± 1.85 a |
| Scheme III: 60% (+)-α-Pinene: 40% Conidia | |||
| 0.42 mg/mL (+)-α-Pinene + 1.6 mg/mL conidia | 23.60 ± 1.17 j | 46.60 ± 2.14 e | 77.20 ± 1.85 c |
| 0.84 mg/mL (+)-α-Pinene + 3.2 mg/mL conidia | 27.60 ± 1.47 i | 71.20 ± 2.58 d | 87.60 ± 2.32 b |
| 1.26 mg/mL (+)-α-Pinene + 4.8 mg/mL conidia | 34.40 ± 1.60 h | 87.20 ± 2.58 b | 96.40 ± 0.98 a |
| 1.68 mg/mL (+)-α-Pinene + 6.4 mg/mL conidia | 38.40 ± 1.47 g | 97.60 ± 0.75 a | 98.80 ± 0.49 a |
| 2.10 mg/mL (+)-α-Pinene + 8.0 mg/mL conidia | 40.40 ± 1.60 f | 98.80 ± 0.49 a | 99.20 ± 0.49 a |
| Scheme IV: 80% (+)-α-Pinene: 20% Conidia | |||
| 0.56 mg/mL (+)-α-Pinene + 0.8 mg/mL conidia | 24.40 ± 1.60 h | 51.60 ± 1.72 d | 80.80 ± 1.85 c |
| 1.12 mg/mL (+)-α-Pinene + 1.6 mg/mL conidia | 29.60 ± 1.60 g | 88.00 ± 3.46 b | 96.80 ± 0.80 a |
| 1.68 mg/mL (+)-α-Pinene + 2.4 mg/mL conidia | 36.00 ± 1.67 f | 94.80 ± 2.15 a | 97.20 ± 1.02 a |
| 2.24 mg/mL (+)-α-Pinene + 3.2 mg/mL conidia | 42.80 ± 1.32 e | 98.80 ± 0.80 a | 98.80 ± 0.80 a |
| 2.80 mg/mL (+)-α-Pinene + 4.0 mg/mL conidia | 43.20 ± 1.85 e | 99.20 ± 0.49 a | 99.60 ± 0.40 a |
| B. bassiana ARSEF 8465 Conidia | |||
| 4 mg/mL | 6.40 ± 0.75 j | 15.60 ± 1.17 hi | 24.40 ± 1.72 ef |
| 8 mg/mL | 12.80 ± 1.02 i | 20.80 ± 1.02 fg | 31.60 ± 1.17 d |
| 12 mg/mL | 19.20 ± 1.02 gh | 32.40 ± 3.97 d | 42.40 ± 1.72 c |
| 16 mg/mL | 22.40 ± 1.47 f | 44.40 ± 1.72 c | 52.80 ± 2.33 b |
| 20 mg/mL | 26.40 ± 1.33 de | 56.40 ± 2.79 b | 69.60 ± 2.79 a |
| Schemes | * LC50 (mg/mL) | Joint Toxicity | Interaction ** |
|---|---|---|---|
| Scheme IV: 80% (+)-α-Pinene: 20% B. bassiana ARSEF 8465 Conidia | |||
| 0.56 mg/mL (+)-α-Pinene + 0.8 mg/mL conidia | 1.32 (1.09 to 1.51) | 755 | Synergistic |
| 1.12 mg/mL (+)-α-Pinene + 1.6 mg/mL conidia | |||
| 1.68 mg/mL (+)-α-Pinene + 2.4 mg/mL conidia | |||
| 2.24 mg/mL (+)-α-Pinene + 3.2 mg/mL conidia | |||
| 2.80 mg/mL (+)-α-Pinene + 4.0 mg/mL conidia | |||
| Scheme III: 60% (+)-α-Pinene: 40% B. bassiana ARSEF 8465 Conidia | |||
| 0.42 mg/mL (+)-α-Pinene + 1.6 mg/mL conidia | 2.40 (2.02 to 2.74) | 280 | Synergistic |
| 0.84 mg/mL (+)-α-Pinene + 3.2 mg/mL conidia | |||
| 1.26 mg/mL (+)-α-Pinene + 4.8 mg/mL conidia | |||
| 1.68 mg/mL (+)-α-Pinene + 6.4 mg/mL conidia | |||
| 2.10 mg/mL (+)-α-Pinene + 8.0 mg/mL conidia | |||
| Scheme II: 40% (+)-α-Pinene: 60% B. bassiana ARSEF 8465 Conidia | |||
| 0.28 mg/mL (+)-α-Pinene + 2.4 mg/mL conidia | 4.20 (3.16 to 5.07) | 121 | Synergistic |
| 0.56 mg/mL (+)-α-Pinene + 4.8 mg/mL conidia | |||
| 0.84 mg/mL (+)-α-Pinene + 7.2 mg/mL conidia | |||
| 1.12 mg/mL (+)-α-Pinene + 9.6 mg/mL conidia | |||
| 1.40 mg/mL (+)-α-Pinene + 12.0 mg/mL conidia | |||
| Scheme I: 20% (+)-α-Pinene: 80% B. bassiana ARSEF 8465 Conidia | |||
| 0.14 mg/mL (+)-α-Pinene + 3.2 mg/mL conidia | 7.06 (5.63 to 8.44) | 58 | Antagonistic |
| 0.28 mg/mL (+)-α-Pinene + 6.4 mg/mL conidia | |||
| 0.42 mg/mL (+)-α-Pinene + 9.6 mg/mL conidia | |||
| 0.56 mg/mL (+)-α-Pinene + 12.8 mg/mL conidia | |||
| 0.70 mg/mL (+)-α-Pinene + 16.0 mg/mL conidia | |||
| Treatments | Relative Enzymatic Activities (%) | |||
|---|---|---|---|---|
| CAT | SOD | GST | AchE | |
| B. bassiana ARSEF 8465 Conidia | ||||
| 4 mg/mL | 2.67 ± 0.23 n | 9.67 ± 0.25 opq | 7.69 ± 0.74 no | 1.18 ± 0.29 m |
| 8 mg/mL | 4.45 ± 0.28 n | 11.53 ± 0.21 no | 11.66 ± 0.93 klm | 2.62 ± 0.37 jklm |
| 12 mg/mL | 8.33 ± 0.98 m | 15.20 ± 0.20 lm | 13.49 ± 1.24 ijk | 3.92 ± 0.48 hij |
| 16 mg/mL | 9.06 ± 0.71 m | 17.87 ± 1.42 k | 15.38 ± 1.97 hij | 5.44 ± 0.65 fgh |
| 20 mg/mL | 10.44 ± 0.69 m | 19.13 ± 0.60 k | 21.87 ± 0.34 g | 12.98 ± 1.52 d |
| (+)-α-Pinene | ||||
| 0.7 mg/mL | 19.55 ± 0.83 k | 1.24 ± 0.13 u | 9.04 ± 0.45 mn | 2.14 ± 0.35 klm |
| 1.4 mg/mL | 23.27 ± 0.44 j | 1.79 ± 0.15 tu | 16.02 ± 1.59 hi | 3.58 ± 0.30 ijk |
| 2.1 mg/mL | 28.07 ± 0.62 i | 7.09 ± 1.22 qr | 21.87 ± 0.34 g | 5.92 ± 0.78 fg |
| 2.8 mg/mL | 35.71 ± 1.04 h | 9.67 ± 0.25 opq | 26.78 ± 0.32 f | 9.11 ± 0.48 e |
| 3.5 mg/mL | 36.46 ± 1.04 h | 14.17 ± 1.62 mn | 39.24 ± 1.20 d | 14.62 ± 1.08 d |
| Scheme I: 20% (+)-α-Pinene: 80% Conidia | ||||
| 0.14 mg/mL (+)-α-Pinene + 3.2 mg/mL conidia | 10.85 ± 1.99 m | 3.33 ± 0.55 stu | 4.59 ± 0.88 o | 1.59 ± 0.36 lm |
| 0.28 mg/mL (+)-α-Pinene + 6.4 mg/mL conidia | 14.80 ± 1.08 l | 4.37 ± 0.96 st | 7.40 ± 0.96 no | 2.18 ± 0.27 jklm |
| 0.42 mg/mL (+)-α-Pinene + 9.6 mg/mL conidia | 23.81 ± 0.91 j | 5.45 ± 0.57 rs | 10.08 ± 0.85 lmn | 3.56 ± 0.47 ijk |
| 0.56 mg/mL (+)-α-Pinene + 12.8 mg/mL conidia | 27.79 ± 0.73 i | 7.64 ± 1.19 pqr | 13.24 ± 0.87 ijk | 4.86 ± 0.59 ghi |
| 0.70 mg/mL (+)-α-Pinene + 16.0 mg/mL conidia | 29.12 ± 0.50 i | 9.32 ± 0.31 opq | 17.59 ± 1.01 h | 6.03 ± 0.31 fg |
| Scheme II: 40% (+)-α-Pinene: 60% Conidia | ||||
| 0.28 mg/mL (+)-α-Pinene + 2.4 mg/mL conidia | 43.08 ± 0.82 g | 9.79 ± 0.26 op | 13.05 ± 0.98 ijkl | 3.16 ± 0.63 ijkl |
| 0.56 mg/mL (+)-α-Pinene + 4.8 mg/mL conidia | 44.12 ± 0.96 g | 20.10 ± 0.24 k | 15.59 ± 1.28 hij | 7.13 ± 0.46 f |
| 0.84 mg/mL (+)-α-Pinene + 7.2 mg/mL conidia | 49.70 ± 0.63 f | 24.09 ± 0.50 j | 24.73 ± 1.12 fg | 9.04 ± 0.53 e |
| 1.12 mg/mL (+)-α-Pinene + 9.6 mg/mL conidia | 58.19 ± 0.81 d | 27.30 ± 0.69 i | 35.61 ± 2.84 e | 13.41 ± 0.83 d |
| 1.40 mg/mL (+)-α-Pinene + 12.0 mg/mL conidia | 63.86 ± 1.89 c | 39.06 ± 0.56 g | 44.30 ± 2.42 c | 19.61 ± 1.10 c |
| Scheme III: 60% (+)-α-Pinene: 40% Conidia | ||||
| 0.42 mg/mL (+)-α-Pinene + 1.6 mg/mL conidia | 44.12 ± 0.96 g | 17.44 ± 0.32 kl | 12.67 ± 0.81 jkl | 6.49 ± 0.36 fg |
| 0.84 mg/mL (+)-α-Pinene + 3.2 mg/mL conidia | 49.89 ± 1.62 f | 26.54 ± 0.72 ij | 18.31 ± 0.86 h | 7.15 ± 0.44 f |
| 1.26 mg/mL (+)-α-Pinene + 4.8 mg/mL conidia | 57.30 ± 1.11 d | 35.71 ± 1.04 h | 27.93 ± 1.40 f | 9.90 ± 0.70 e |
| 1.68 mg/mL (+)-α-Pinene + 6.4 mg/mL conidia | 68.07 ± 0.98 b | 49.70 ± 0.63 e | 39.68 ± 1.47 d | 14.68 ± 0.76 d |
| 2.10 mg/mL (+)-α-Pinene + 8.0 mg/mL conidia | 71.84 ± 2.05 a | 57.47 ± 1.02 c | 53.18 ± 1.46 b | 22.85 ± 0.88 b |
| Scheme IV: 80% (+)-α-Pinene: 20% Conidia | ||||
| 0.56 mg/mL (+)-α-Pinene + 0.8 mg/mL conidia | 53.89 ± 1.11 e | 25.73 ± 1.83 ij | 17.59 ± 1.01 h | 9.71 ± 0.54 e |
| 1.12 mg/mL (+)-α-Pinene + 1.6 mg/mL conidia | 57.13 ± 1.21 d | 43.08 ± 0.82 f | 25.29 ± 1.53 f | 13.11 ± 0.74 d |
| 1.68 mg/mL (+)-α-Pinene + 2.4 mg/mL conidia | 66.27 ± 1.19 bc | 52.42 ± 0.57 d | 40.33 ± 1.30 d | 19.89 ± 0.99 c |
| 2.24 mg/mL (+)-α-Pinene + 3.2 mg/mL conidia | 66.61 ± 1.20 bc | 63.89 ± 1.88 b | 53.91 ± 1.29 b | 22.20 ± 0.64 b |
| 2.80 mg/mL (+)-α-Pinene + 4.0 mg/mL conidia | 72.36 ± 1.89 a | 75.22 ± 2.02 a | 61.09 ± 0.89 a | 30.07 ± 1.09 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, A. Compatibility of Beauveria bassiana and a Plant Secondary Metabolite: A Novel Modeling Approach to Invade Host Defense for Effective Control of Oligonychus afrasiaticus (McGregor) on Date Palms. J. Fungi 2021, 7, 334. https://doi.org/10.3390/jof7050334
Hussain A. Compatibility of Beauveria bassiana and a Plant Secondary Metabolite: A Novel Modeling Approach to Invade Host Defense for Effective Control of Oligonychus afrasiaticus (McGregor) on Date Palms. Journal of Fungi. 2021; 7(5):334. https://doi.org/10.3390/jof7050334
Chicago/Turabian StyleHussain, Abid. 2021. "Compatibility of Beauveria bassiana and a Plant Secondary Metabolite: A Novel Modeling Approach to Invade Host Defense for Effective Control of Oligonychus afrasiaticus (McGregor) on Date Palms" Journal of Fungi 7, no. 5: 334. https://doi.org/10.3390/jof7050334
APA StyleHussain, A. (2021). Compatibility of Beauveria bassiana and a Plant Secondary Metabolite: A Novel Modeling Approach to Invade Host Defense for Effective Control of Oligonychus afrasiaticus (McGregor) on Date Palms. Journal of Fungi, 7(5), 334. https://doi.org/10.3390/jof7050334






