Antioxidant Properties and Industrial Uses of Edible Polyporales
Abstract
:1. Introduction
2. Biotechnological Importance of Polyporales
3. The Search for Novel Antioxidant Sources and Their Health Benefits
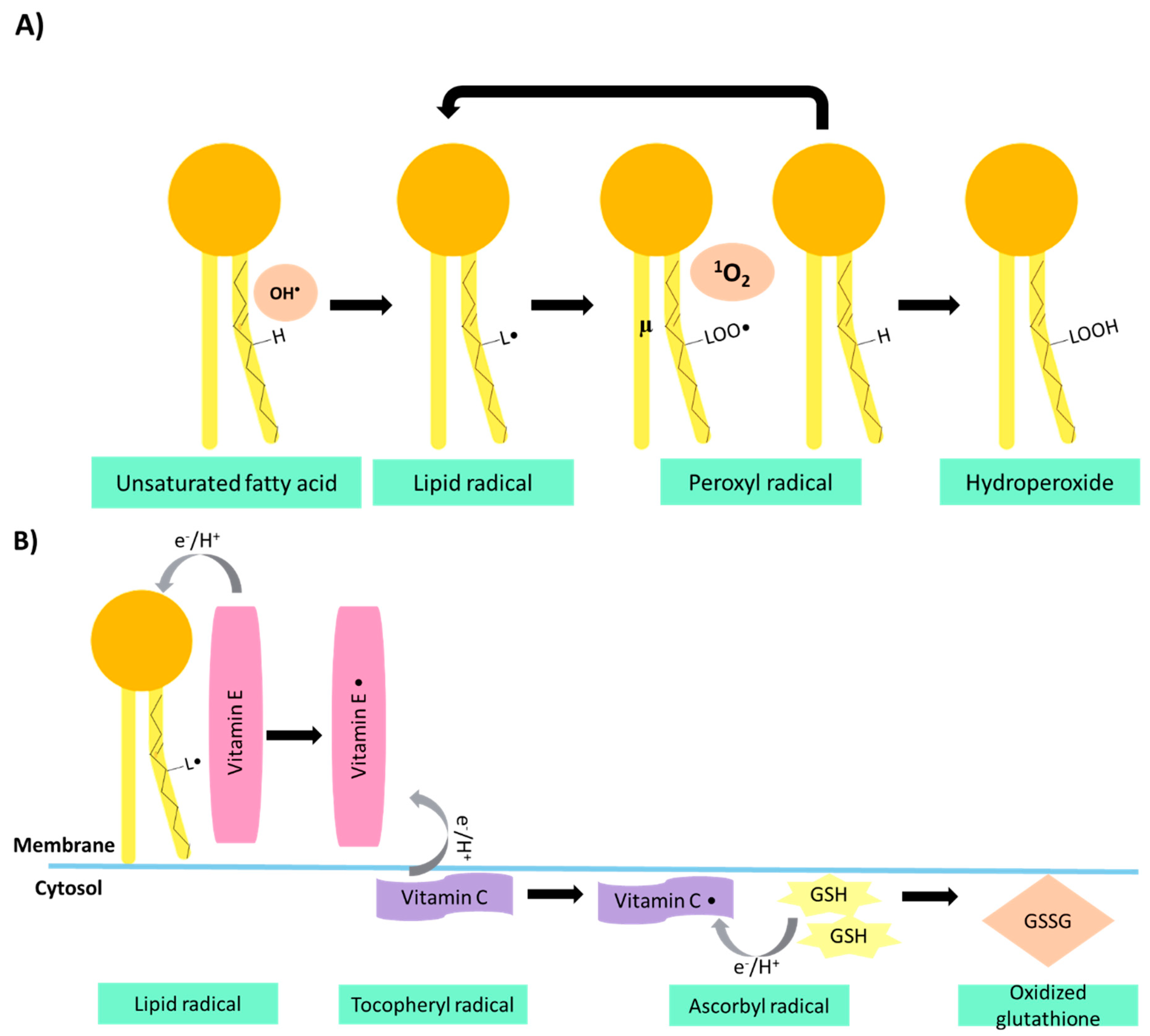
3.1. Vitamin Content: Tocopherols and Vitamin C
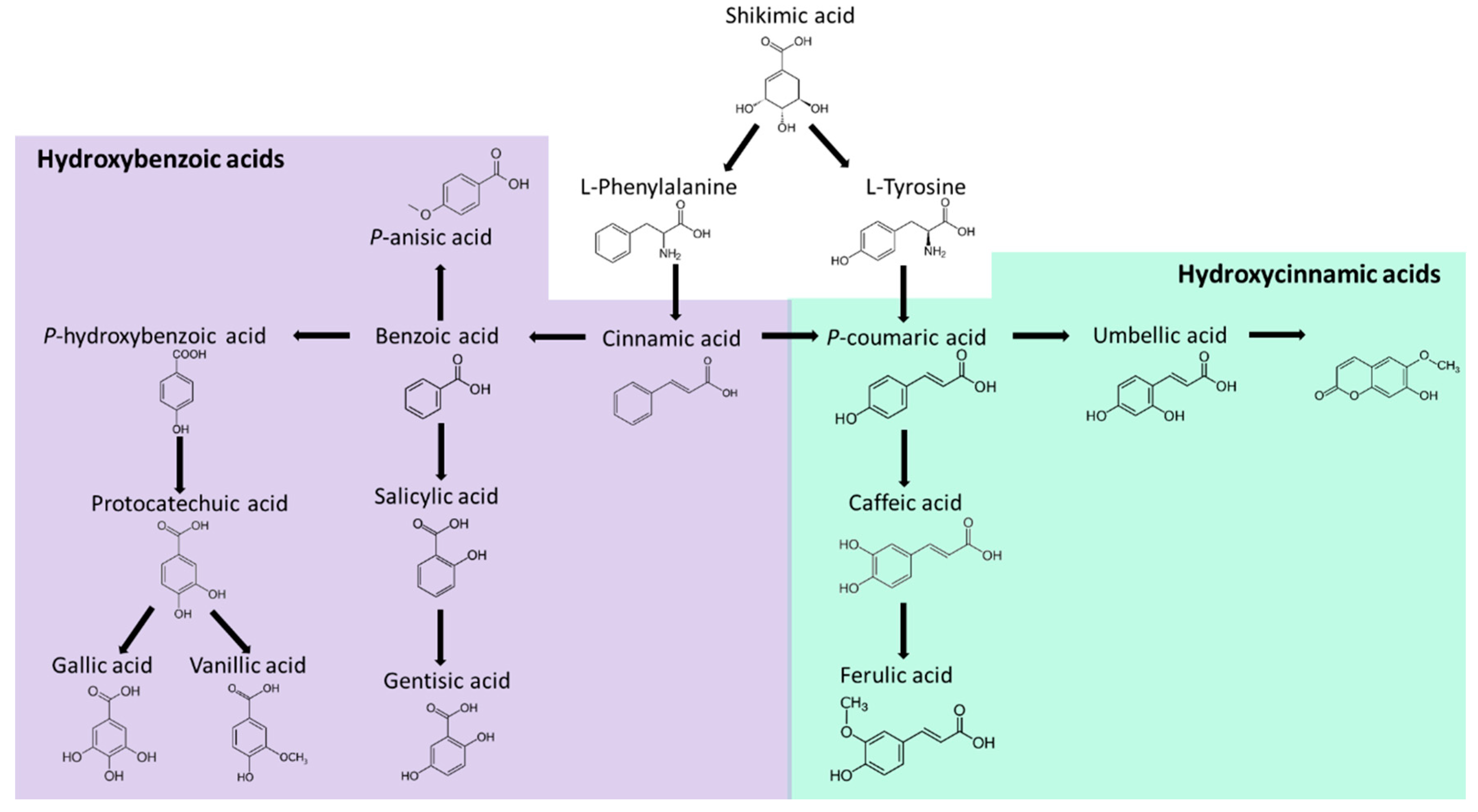
3.2. Phenolics Compounds
3.3. Ergosterol
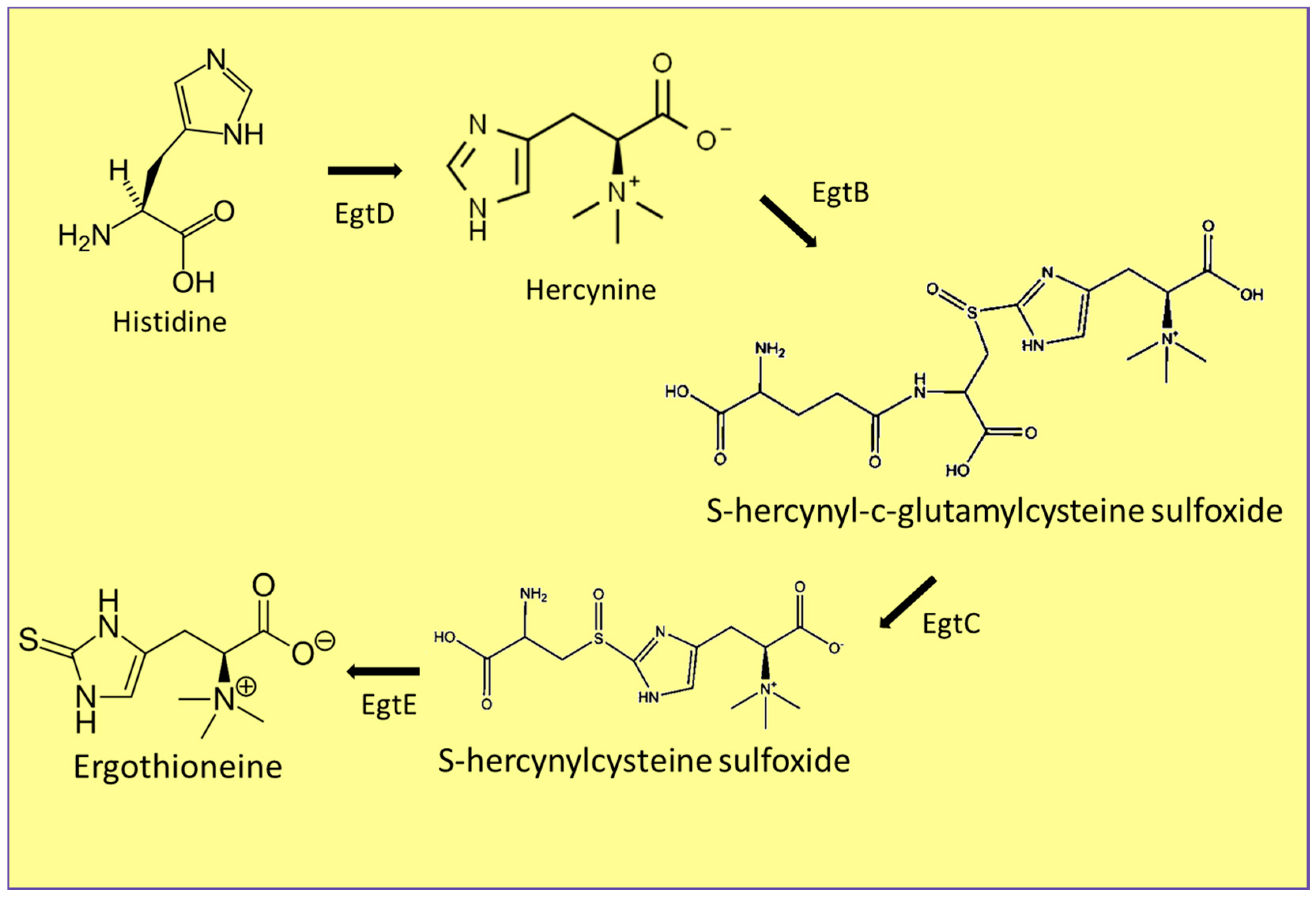
3.4. Ergothioneine
3.5. B-Glucans
4. Contribution of Individual Bioactive Compounds to the Total Antioxidant Capacity of Mushrooms
5. Conclusions and Future Trends
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cano-Estrada, A.; Romero-Bautista, L. Valor económico, nutricional y medicinal de hongos comestibles silvestres. Rev. Chil. Nutr. 2016, 43, 75–80. [Google Scholar] [CrossRef] [Green Version]
- Doskocil, I.; Havlik, J.; Verlotta, R.; Tauchen, J.; Vesela, L.; Macakova, K.; Opletal, L.; Kokoska, L.; Rada, V. In vitro immunomodulatory activity, cytotoxicity and chemistry of some central European polypores. Pharm. Biol. 2016, 54, 2369–2376. [Google Scholar] [CrossRef] [Green Version]
- Fuentes, Á.M. Un recurso alimentario de los grupos originarios y mestizos de méxico: Los hongos silvestres. An. Antropol. 2014, 48, 241–272. [Google Scholar] [CrossRef] [Green Version]
- Stojković, D.S.; Kovačević-Grujičić, N.; Reis, F.S.; Davidović, S.; Barros, L.; Popović, J.; Petrović, I.; Pavić, A.; Glamočlija, J.; Ćirić, A. Chemical composition of the mushroom Meripilus giganteus Karst. and bioactive properties of its methanolic extract. LWT-Food Sci. Technol. 2017, 79, 454–462. [Google Scholar] [CrossRef] [Green Version]
- Yildiz, O.; Can, Z.; Laghari, A.Q.; Şahin, H.; Malkoç, M. Wild Edible Mushrooms as a Natural Source of Phenolics and Antioxidants. J. Food Biochem. 2015, 39, 148–154. [Google Scholar] [CrossRef]
- Valverde, M.E.; Hernández-Pérez, T.; Paredes-López, O. Edible Mushrooms: Improving Human Health and Promoting Quality Life. Int. J. Microbiol. 2015, 2015, 1–14. [Google Scholar] [CrossRef]
- Heleno, S.A.; Ferreira, R.C.; Antonio, A.L.; Queiroz, M.J.R.P.; Barros, L.; Ferreira, I.C.F.R. Nutritional value, bioactive compounds and antioxidant properties of three edible mushrooms from Poland. Food Biosci. 2015, 11, 48–55. [Google Scholar] [CrossRef] [Green Version]
- Taofiq, O.; González-Paramás, A.M.; Martins, A.; Barreiro, M.F.; Ferreira, I.C. Mushrooms extracts and compounds in cosmetics, cosmeceuticals and nutricosmetics—A review. Ind. Crop. Prod. 2016, 90, 38–48. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Aguilar, G.A.; Castellá, L.M.; Flores, D.M.R.; Cabrera, A.G.; Tinoco, A.G. Antioxidantes en Alimentos y Salud, 1st ed.; Clave Editorial: Mexico City, Mexico, 2012. [Google Scholar]
- Ruan-Soto, F. 50 años de etnomicología en México. Lacandonia 2017, 1, 97–108. [Google Scholar]
- Guzmán, G. Las relaciones de los hongos sagrados con el hombre a través del tiempo. An. Antropol. 2016, 50, 134–147. [Google Scholar] [CrossRef] [Green Version]
- Comandini, O.; Rinaldi, A.C. Ethnomycology in Europe: The past, the present, and the future. In Mushrooms, Humans and Nature in a Changing World; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2020; pp. 341–364. [Google Scholar]
- Kamalebo, H.M.; Malale, H.N.S.W.; Ndabaga, C.M.; Degreef, J.; De Kesel, A. Uses and importance of wild fungi: Traditional knowledge from the Tshopo province in the Democratic Republic of the Congo. J. Ethnobiol. Ethnomedicine 2018, 14, 13. [Google Scholar] [CrossRef] [Green Version]
- Boa, E. Los Hongos Silvestres Comestibles, Perspectiva Global de su Uso e Importancia Para la Población; FAO: Roma, Italia, 2004; Available online: http://www.fao.org/3/y5489s/y5489s00.htm (accessed on 11 October 2020).
- Estrada-Martínez, E.; Guzmán, G.; Cibrián Tovar, D.; Ortega Paczka, R. Contribución al conocimiento etnomicológico de los hongos comestibles silvestres de mercados regionales y comunidades de la Sierra Nevada (México). Interciencia 2009, 34, 25–33. [Google Scholar]
- Royse, D.J.; Baars, J.; Tan, Q. Current Overview of Mushroom Production in the World. Edible Med. Mushrooms 2017, 5–13. [Google Scholar] [CrossRef]
- Li, Y. Present development situation and tendency of edible mushroom industry in China. Mushroom Sci. 2012, 18, 3–9. [Google Scholar]
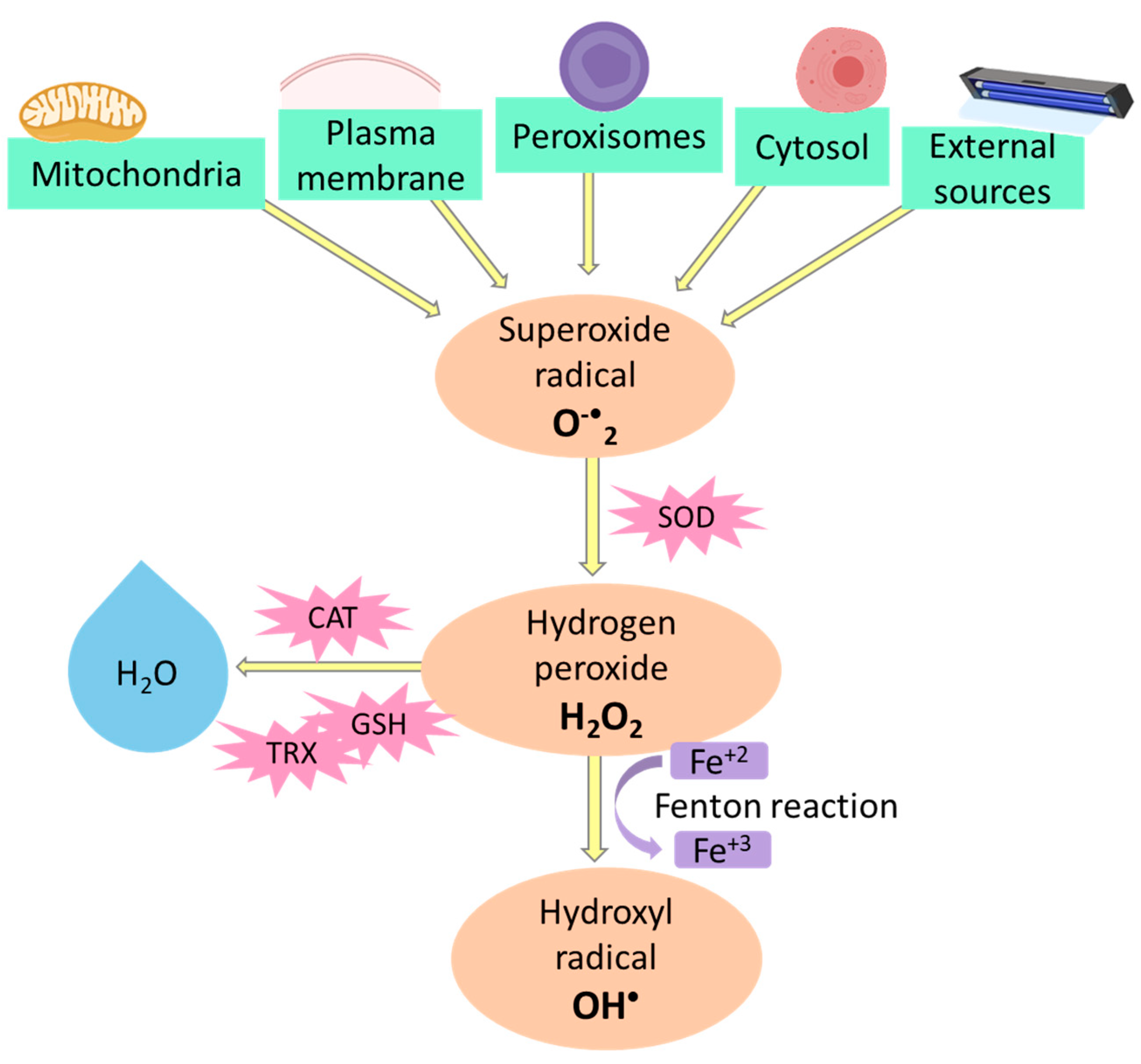
- Saavedra, O.M.; Vázquez, E.N.J.; Vargas, M.R.B.G.; Reyes, G.M.C. Radicales libres y su papel en las enfermedades crónico-degenerativas. Rev. Med. UV 2010, 10, 32–39. [Google Scholar]
- Sánchez, C. Reactive oxygen species and antioxidant properties from mushrooms. Synth. Syst. Biotechnol. 2017, 2, 13–22. [Google Scholar] [CrossRef]
- González-Torres, M.C.; Betancourt-Rule, M.; Ortiz-Muñiz, R. Daño oxidativo y antioxidantes. Bioquimia 2000, 25, 3–9. [Google Scholar]
- Kozarski, M.; Klaus, A.; Jakovljevic, D.; Todorovic, N.; Vunduk, J.; Petrović, P.; Niksic, M.; Vrvic, M.; Van Griensven, L. Antioxidants of edible mushrooms. Molecules 2015, 20, 19489–19525. [Google Scholar] [CrossRef] [Green Version]
- Rathore, H.; Prasad, S.; Sharma, S. Mushroom nutraceuticals for improved nutrition and better human health: A review. PharmaNutrition 2017, 5, 35–46. [Google Scholar] [CrossRef]
- Yuan, B.; Zhao, L.; Yang, W.; McClements, D.J.; Hu, Q. Enrichment of Bread with Nutraceutical-Rich Mushrooms: Impact of Auricularia auricula (Mushroom) Flour Upon Quality Attributes of Wheat Dough and Bread. J. Food Sci. 2017, 82, 2041–2050. [Google Scholar] [CrossRef]
- Azzi, A. Tocopherols, tocotrienols and tocomonoenols: Many similar molecules but only one vitamin E. Redox Biol. 2019, 26, 101259. [Google Scholar] [CrossRef]
- Sayago, A.; Marín, M.; Aparicio López, R.; Morales, M.T. Vitamina E y aceites vegetales. Grasas y Aceites 2007, 58, 74–86. [Google Scholar]
- Ferreira, L.C.; Barros, L.; Abreu, R.M.V. Antioxidants in Wild Mushrooms. Curr. Med. Chem. 2009, 16, 1543–1560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stojković, D.S.; Barros, L.; Calhelha, R.C.; Glamočlija, J.; Ćirić, A.; Van Griensven, L.J.; Soković, M.; Ferreira, I.C. A detailed comparative study between chemical and bioactive properties of Ganoderma lucidum from different origins. Int. J. Food Sci. Nutr. 2014, 65, 42–47. [Google Scholar] [CrossRef] [Green Version]
- Omar, N.A.M.; Abdullah, N.; Kuppusamy, U.R.; Abdulla, M.A.; Sabaratnam, V. Nutritional Composition, Antioxidant Activities, and Antiulcer Potential of Lentinus squarrosulus (Mont.) Mycelia Extract. Evid. Based Complement. Altern. Med. 2011, 2011, 1–8. [Google Scholar] [CrossRef]
- Mau, J.-L.; Lin, H.-C.; Chen, C.-C. Antioxidant Properties of Several Medicinal Mushrooms. J. Agric. Food Chem. 2002, 50, 6072–6077. [Google Scholar] [CrossRef]
- Fernandes, Â.; Petrović, J.; Stojković, D.; Barros, L.; Glamočlija, J.; Soković, M.; Martins, A.; Ferreira, I.C. Polyporus squamosus (Huds.) Fr from different origins: Chemical characterization, screening of the bioactive properties and specific antimicrobial effects against Pseudomonas aeruginosa. LWT Food Sci. Technol. 2016, 69, 91–97. [Google Scholar] [CrossRef] [Green Version]
- Petrović, J.; Stojković, D.; Reis, F.S.; Barros, L.; Glamočlija, J.; Ćirić, A.; Ferreira, I.C.F.R.; Soković, M. Study on chemical, bioactive and food preserving properties of Laetiporus sulphureus (Bull.: Fr.) Murr. Food Funct. 2014, 5, 1441–1451. [Google Scholar] [CrossRef] [Green Version]
- Mau, J.-L.; Chang, C.-N.; Huang, S.-J.; Chen, C.-C. Antioxidant properties of methanolic extracts from Grifola frondosa, Morchella esculenta and Termitomyces albuminosus mycelia. Food Chem. 2004, 87, 111–118. [Google Scholar] [CrossRef]
- Bouzgarrou, C. Pleurotus Species as a Source of Natural Preservatives: Mycelia Production to Obtain Tocopherols Used as Antioxidants in Yogurts. Ph.D. Thesis, Instituto Politécnico de Braganca, Braganzana, Portugal, 2017. [Google Scholar]
- Pinto, S.; Barros, L.; Sousa, M.J.; Ferreira, I.C. Chemical characterization and antioxidant properties of Lepista nuda fruiting bodies and mycelia obtained by in vitro culture: Effects of collection habitat and culture media. Food Res. Int. 2013, 51, 496–502. [Google Scholar] [CrossRef]
- Rana, M.; Dahot, M.U. Optimization of culture conditions to produce secondary metabolites by Pleurotus ostreatus. Pak. J. Biotechnol. 2017, 14, 251–256. [Google Scholar]
- Barros, L.; Correia, D.M.; Ferreira, I.C.; Baptista, P.; Santos-Buelga, C. Optimization of the determination of tocopherols in Agaricus sp. edible mushrooms by a normal phase liquid chromatographic method. Food Chem. 2008, 110, 1046–1050. [Google Scholar] [CrossRef] [PubMed]
- Arrozi, A.P.; Shukri, S.N.S.; Ngah, W.Z.W.; Yusof, Y.A.M.; Damanhuri, M.H.A.; Jaafar, F.; Makpol, S. Comparative Effects of Alpha- and Gamma-Tocopherol on Mitochondrial Functions in Alzheimer’s Disease In Vitro Model. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Kiyou, S.; Mizoguchi, Y. Natural Antioxidant for Food, Cosmetics and Pharmaceuticals—Contains Culture Medium of Mushroom Hypha and Antioxidant. Japan JP7059548-A, 1995. Available online: www.epo.org (accessed on 5 July 2020).
- Lee, S.-R.; Joo, W.-J.; Baek, Y.-U.; Lee, Y.-K.; Yu, S.-W.; Kim, Y.-R.; Chay, K.-O.; Cho, S.-H.; Rang, S.-O. Intracellular substrates of a heme-containing ascorbate oxidase in Pleurotus ostreatus. J. Microbiol. 2009, 47, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Froufe, H.; Abreu, R.; Ferreira, I. A QCAR model for predicting antioxidant activity of wild mushrooms. Sar. QSAR Environ. Res. 2009, 20, 579–590. [Google Scholar] [CrossRef]
- Gąsecka, M.; Siwulski, M.; Mleczek, M. Evaluation of bioactive compounds content and antioxidant properties of soil-growing and wood-growing edible mushrooms. J. Food Process. Preserv. 2017, 42, e13386. [Google Scholar] [CrossRef] [Green Version]
- Mattila, P.; Könkö, K.; Eurola, M.; Pihlava, J.-M.; Astola, J.; Vahteristo, L.; Hietaniemi, V.; Kumpulainen, J.; Valtonen, M.; Piironen, V. Contents of Vitamins, Mineral Elements, and Some Phenolic Compounds in Cultivated Mushrooms. J. Agric. Food Chem. 2001, 49, 2343–2348. [Google Scholar] [CrossRef] [PubMed]
- Baroja-Mazo, A.; del Valle, P.; Rúa, J.; de Cima, S.; Busto, F.; de Arriaga, D.; Smirnoff, N. Characterisation and biosynthesis of D-erythroascorbic acid in Phycomyces blakesleeanus. Fungal Genet. Biol. 2005, 42, 390–402. [Google Scholar] [CrossRef]
- Smirnoff, N. Ascorbic acid metabolism and functions: A comparison of plants and mammals. Free. Radic. Biol. Med. 2018, 122, 116–129. [Google Scholar] [CrossRef]
- Rózsa, S.; Măniuțiu, D.-N.; Poșta, G.; Gocan, T.-M.; Andreica, I.; Bogdan, I.; Rózsa, M.; Lazăr, V. Influence of the Culture Substrate on the Agaricus blazei Murrill Mushrooms Vitamins Content. Plants 2019, 8, 316. [Google Scholar] [CrossRef] [Green Version]
- Kuivanen, J.; Penttilä, M.; Richard, P. Metabolic engineering of the fungal D-galacturonate pathway for L-ascorbic acid production. Microb. Cell Factories 2015, 14, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Villagrán, M.; Muñoz, M.; Díaz, F.; Troncoso, C.; Celis-Morales, C.; Mardones, L. Una mirada actual de la vitamina C en salud y enfermedad. Rev. Chil. Nutr. 2019, 46, 800–808. [Google Scholar] [CrossRef] [Green Version]
- Kwak, J.Y.; Park, S.; Seok, J.K.; Liu, K.-H.; Boo, Y.C. Ascorbyl coumarates as multifunctional cosmeceutical agents that inhibit melanogenesis and enhance collagen synthesis. Arch. Derm. Res. 2015, 307, 635–643. [Google Scholar] [CrossRef]
- Heleno, S.A.; Martins, A.; Queiroz, M.J.R.; Ferreira, I.C. Bioactivity of phenolic acids: Metabolites versus parent compounds: A review. Food Chem. 2015, 173, 501–513. [Google Scholar] [CrossRef] [Green Version]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Puttaraju, N.G.; Venkateshaiah, S.U.; Dharmesh, S.M.; Urs, S.M.N.; Somasundaram, R. Antioxidant Activity of Indigenous Edible Mushrooms. J. Agric. Food Chem. 2006, 54, 9764–9772. [Google Scholar] [CrossRef]
- Cheung, L.; Cheung, P.C.; Ooi, V.E. Antioxidant activity and total phenolics of edible mushroom extracts. Food Chem. 2003, 81, 249–255. [Google Scholar] [CrossRef]
- Dubost, N.; Ou, B.; Beelman, R. Quantification of polyphenols and ergothioneine in cultivated mushrooms and correlation to total antioxidant capacity. Food Chem. 2007, 105, 727–735. [Google Scholar] [CrossRef]
- Kim, M.-Y.; Seguin, P.; Ahn, J.-K.; Kim, J.-J.; Chun, S.-C.; Kim, E.-H.; Seo, S.-H.; Kang, E.-Y.; Kim, S.-L.; Park, Y.-J.; et al. Phenolic Compound Concentration and Antioxidant Activities of Edible and Medicinal Mushrooms from Korea. J. Agric. Food Chem. 2008, 56, 7265–7270. [Google Scholar] [CrossRef] [PubMed]
- Valanciene, E.; Jonuskiene, I.; Syrpas, M.; Augustiniene, E.; Matulis, P.; Simonavicius, A.; Malys, N. Advances and Prospects of Phenolic Acids Production, Biorefinery and Analysis. Biomolecules 2020, 10, 874. [Google Scholar] [CrossRef]
- Sułkowska-Ziaja, K.; Muszynska, B.; Szewczyk, A. Antioxidant components of selected indigenous edible mushrooms of the obsolete order Aphyllophorales. Rev. Iberoam. De Micol. 2015, 32, 99–102. [Google Scholar] [CrossRef]
- Zhicai, Z.; Huihua, Z.; Xioaofeng, R.; Dezhong, X.; Hui, C.; Caiju, Z. Alimentos Funcionales Ganoderma Lucidum y Ginkgo y su Método de Preparaciçon. China CN103689557A, 27 September 2012. [Google Scholar]
- Jiao, Y.; Chen, Y. Application of Chaenomeles Speciosa Phenolic acid in Protection of Acute and Chronic Hypoxia Injury. China CN110664850A, 12 November 2019. Available online: https://patents.google.com/patent/CN110664850A/en?oq=CN110664850-A (accessed on 15 September 2020).
- Falcón García, G. Incremento del Contenido en Componentes Bio-Activos (ergosterol) en Hongos Comestibles Mediante Suplementación Dirigida del Medio de Cultivo y Compost. Aplicación al Caso del Champiñón (Agaricus bisporus). Ph.D. Thesis, Universidad de Sevilla, Seville, Spain, 2019. [Google Scholar]
- Medina, M.E.; Iuga, C.; Trigos, Á. Mechanism and kinetics of the oxidative damage to ergosterol induced by peroxyl radicals in lipid media: A theoretical quantum chemistry study. J. Phys. Org. Chem. 2015, 29, 196–203. [Google Scholar] [CrossRef]
- Villares, A.; García-Lafuente, A.; Guillamón, E.; Ramos, Á. Identification and quantification of ergosterol and phenolic compounds occurring in Tuber spp. truffles. J. Food Compos. Anal. 2012, 26, 177–182. [Google Scholar] [CrossRef]
- Rivera, A.; Nieto, I.J.; Valencia, M.A. Composición y cuantificación por cromatografía de gases acoplada a espectrometría de masas de la fracción esterólica de once hongos colombianos. Rev. Colomb. Química 2002, 31, 95–102. [Google Scholar]
- Parks, L.W.; Casey, W.M. Physiological implications of sterol biosynthesis in yeast. Annu. Rev. Microbiol. 1995, 49, 95–116. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, H.C.B.d.; Oliveira, S.C.B.d.; Santos, J.K.P. Flours Produced from Fungus Myceliated Grain. U.S. US2012/0231114A1, 17 September 2010. Available online: https://patents.google.com/patent/US20120231114A1/en?oq=US2012%2f0231114A1 (accessed on 12 October 2020).
- Iijima, T.; Takeda, K. Discoloration and Oxidation Inhibitor for Food Derived from Natural Product. Japan JP2009183266A, 2009. Available online: www.epo.org (accessed on 15 August 2020).
- Kalaras, M.D.; Richie, J.P.; Calcagnotto, A.; Beelman, R.B. Mushrooms: A rich source of the antioxidants ergothioneine and glutathione. Food Chem. 2017, 233, 429–433. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Srivastav, P.; Mishra, H. Optimization of process variables for supercritical fluid extraction of ergothioneine and polyphenols from Pleurotus ostreatus and correlation to free-radical scavenging activity. J. Supercrit. Fluids 2014, 95, 51–59. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Ho, K.-J.; Hsieh, Y.-J.; Wang, L.-T.; Mau, J.-L. Contents of lovastatin, γ-aminobutyric acid and ergothioneine in mushroom fruiting bodies and mycelia. LWT 2012, 47, 274–278. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, M.; Liu, Q.; Wang, X.; Zhao, R.; Geng, Y.; Wong, T.; Li, S. A comprehensive screening shows that ergothioneine is the most abundant antioxidant in the wild macrofungus Phylloporia ribis Ryvarden. J. Environ. Sci. Heal. Part C 2018, 36, 98–111. [Google Scholar] [CrossRef]
- Misson, L.; Burn, R.; Vit, A.; Hildesheim, J.; Beliaeva, M.A.; Blankenfeldt, W.; Seebeck, F.P. Inhibition and Regulation of the Ergothioneine Biosynthetic Methyltransferase EgtD. ACS Chem. Biol. 2018, 13, 1333–1342. [Google Scholar] [CrossRef]
- Yu, Y.-H.; Pan, H.-Y.; Guo, L.-Q.; Lin, J.-F.; Liao, H.-L.; Li, H.-Y. Successful biosynthesis of natural antioxidant ergothioneine in Saccharomyces cerevisiae required only two genes from Grifola frondosa. Microb. Cell Factories 2020, 19, 1–10. [Google Scholar] [CrossRef]
- Abiko, T.Y.M.; Nukina, M.; Suzuki, T. Composition Highly Containing Ergothioneine Extracted from Mushroom. Japan JP2009126863A, 2007. Available online: ww.epo.org (accessed on 7 June 2020).
- Hara, K.; Sasaki, E.; Takeda, Y. Food material derived from Pleurotus mycelium. Japan JP2020031597A, 2020. Available online: www.epo.org. (accessed on 7 June 2020).
- Bandara, A.R.; Rapior, S.; Bhat, D.J.; Kakumyan, P.; Chamyuang, S.; Xu, J.; Hyde, K.D. Polyporus umbellatus, an Edible-Medicinal Cultivated Mushroom with Multiple Developed Health-Care Products as Food, Medicine and Cosmetics: A Review. Cryptogam. Mycol. 2015, 36, 3–42. [Google Scholar] [CrossRef]
- Friedman, M. Mushroom Polysaccharides: Chemistry and Antiobesity, Antidiabetes, Anticancer, and Antibiotic Properties in Cells, Rodents, and Humans. Foods 2016, 5, 80. [Google Scholar] [CrossRef] [Green Version]
- Aguilar, G.A.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Álvarez-Parrilla, E.G.H.S. Los Alimentos Funcionales: Un Nuevo reto Para la Industria de Alimentos, 1st ed.; AGTeditor: Mexico City, Mexico, 2014; p. 687. [Google Scholar]
- Rossi, P.; Difrancia, R.; Quagliariello, V.; Savino, E.; Tralongo, P.; Randazzo, C.L.; Berretta, M. B-glucans from Grifola frondosa and Ganoderma lucidum in breast cancer: An example of complementary and integrative medicine. Oncotarget 2018, 9, 24837–24856. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y. Preparation method of bood-sugar-reducing and blood-lipid-reducing bread containing Hericium erinaceus glucan. China CN107518044A, 2017. Available online: https://patents.google.com/patent/CN107518044A/en?q=(B-glucans+extract+mushroom)&after=priority:20150101&language=ENGLISH&oq=(B-glucans+extract+mushroom)+after:priority:20150101+language:ENGLISH&page=2 (accessed on 6 May 2020).
- Li, W.Q.; Ding, G.; Wang, C.; Huang, Q.; Lin, F. Method for Preparing Nano-Membrane from Edible and Medical Fungi and use Thereof. China WO2015062156A1, 2013. Available online: https://patents.google.com/patent/WO2015062156A1/en?oq=WO2015062156A1 (accessed on 9 May 2020).
- Gardner, P.T.; White, T.A.; McPhail, D.B.; Duthie, G.G. The relative contributions of vitamin C, carotenoids and phenolics to the antioxidant potential of fruit juices. Food Chem. 2000, 68, 471–474. [Google Scholar] [CrossRef]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-Evans, C.; Davies, M.J.; Gopinathan, V.; Milner, A. A Novel Method for Measuring Antioxidant Capacity and its Application to Monitoring the Antioxidant Status in Premature Neonates. Clin. Sci. 1993, 84, 407–412. [Google Scholar] [CrossRef] [Green Version]
- Soobrattee, M.; Neergheen, V.; Luximon-Ramma, A.; Aruoma, O.; Bahorun, T. Phenolics as potential antioxidant therapeutic agents: Mechanism and actions. Mutat. Res. Mol. Mech. Mutagen. 2005, 579, 200–213. [Google Scholar] [CrossRef]
- Verma, B.; Hucl, P.; Chibbar, R. Phenolic acid composition and antioxidant capacity of acid and alkali hydrolysed wheat bran fractions. Food Chem. 2009, 116, 947–954. [Google Scholar] [CrossRef]
- Palafox-Carlos, H.; Yahia, E.; A Gonzalezaguilar, G. Identification and quantification of major phenolic compounds from mango (Mangifera indica, cv. Ataulfo) fruit by HPLC–DAD–MS/MS-ESI and their individual contribution to the antioxidant activity during ripening. Food Chem. 2012, 135, 105–111. [Google Scholar] [CrossRef]
- Kishk, Y.F.; Al-Sayed, H.M. Free-radical scavenging and antioxidative activities of some polysaccharides in emulsions. LWT 2007, 40, 270–277. [Google Scholar] [CrossRef]
- Lo, T.C.-T.; Chang, C.A.; Chiu, K.-H.; Tsay, P.-K.; Jen, J.-F. Correlation evaluation of antioxidant properties on the monosaccharide components and glycosyl linkages of polysaccharide with different measuring methods. Carbohydr. Polym. 2011, 86, 320–327. [Google Scholar] [CrossRef]
- Rojano, B.A. Oxidación de Lípidos y Antioxidantes; Universidad Nacional de Colombia: Bogota, Colombia, 1997. [Google Scholar]
| Tocopherol | Mushroom Species | Content µg/100g (dw) | Reference |
|---|---|---|---|
| α-tocopherol | M. giganteus | 3 | [4] |
| P. squamosus | 4 | [30] | |
| L. sulphureus | 109.25 | [31] | |
| G. lucidum | 15.02 | [27] | |
| G. frondosa | 50 | [32] | |
| β-tocopherol | M. giganteus | 9 | [4] |
| P. squamosus | 1960 | [30] | |
| δ-tocopherol | M. giganteus | 123 | [4] |
| L. sulphureus | 18.42 | [31] | |
| G. lucidum | 89.73 | [27] | |
| G. frondosa | 40 | [32] | |
| γ-tocopherol | M. giganteus | 77 | [4] |
| P. squamosus | N.D. | [30] | |
| L. sulphureus | 62.07 | [31] | |
| G. frondosa | 50 | [32] |
| Mushroom | Identified Phenolic Compound | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | Reference | |
| G. lucidum | 301.42 | 522.14 | 72.55 | 3.74 | 1596.01 | 233.68 | 138.64 | 59.16 | 148.96 | - | - | - | [5] |
| 1.9 | N.D. | 1.4 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | 0.8 | 1.8 | 2.6 | [54] | |
| M. giganteus | - | 1.01 | - | - | - | - | 2.42 | - | 0.34 | - | - | - | [4] |
| S. crispa | 96 | 34 | N.D. | N.D. | N.D. | 5 | 37 | N.D. | N.D. | 19 | 66 | 24 | [54] |
| 0.08 | - | - | - | - | - | - | - | - | 1.25 | - | - | [51] | |
| N.D. | - | N.D. | - | - | N.D. | N.D. | N.D. | - | 0.35 | - | N.D. | [41] | |
| L. sajor caju | 0.03 | - | - | - | 0.04 | 0.04 | - | - | N.D. | 1.51 | - | - | [51] |
| L. squarrulosus | 0.4 | - | - | - | - | - | - | - | 0.08 | 1.92 | - | - | [51] |
| P. squamosus | 0.035 | - | N.D. | - | - | 0.012 | 0.019 | N.D. | - | 0.052 | - | N.D. | [41] |
| G. frondosa | N.D. | - | 0.018 | - | - | N.D. | N.D. | N.D. | - | N.D. | - | N.D. | [41] |
| Compound | TEAC (µmol TE/L) | References |
|---|---|---|
| Vitamin C | 1000 | [81] |
| α-tocopherol | 970 | [82] |
| Ergothionein | 870 | [83] |
| Rosmarinic acid | 4500 | [83] |
| Gallic acid | 3620 | [83] |
| Gentisic acid | 0.48 | [84] |
| 4-hydroxybenzoic acid | 130 | [83] |
| Caffeic acid | 1300 | [81] |
| Vanillin | 0.335 | [84] |
| Ferulic acid | 980 | [83] |
| Salicylic acid | 3 | [84] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quintero-Cabello, K.P.; Lugo-Flores, M.A.; Rivera-Palafox, P.; Silva-Espinoza, B.A.; González-Aguilar, G.A.; Esqueda, M.; Gaitán-Hernández, R.; Ayala-Zavala, J.F. Antioxidant Properties and Industrial Uses of Edible Polyporales. J. Fungi 2021, 7, 196. https://doi.org/10.3390/jof7030196
Quintero-Cabello KP, Lugo-Flores MA, Rivera-Palafox P, Silva-Espinoza BA, González-Aguilar GA, Esqueda M, Gaitán-Hernández R, Ayala-Zavala JF. Antioxidant Properties and Industrial Uses of Edible Polyporales. Journal of Fungi. 2021; 7(3):196. https://doi.org/10.3390/jof7030196
Chicago/Turabian StyleQuintero-Cabello, Karen P., Marco A. Lugo-Flores, Patricia Rivera-Palafox, Brenda A. Silva-Espinoza, Gustavo A. González-Aguilar, Martín Esqueda, Rigoberto Gaitán-Hernández, and J. Fernando Ayala-Zavala. 2021. "Antioxidant Properties and Industrial Uses of Edible Polyporales" Journal of Fungi 7, no. 3: 196. https://doi.org/10.3390/jof7030196






