The Banana Root Endophytome: Differences between Mother Plants and Suckers and Evaluation of Selected Bacteria to Control Fusarium oxysporum f.sp. cubense
Abstract
1. Introduction
2. Materials and Methods
2.1. Banana Roots Sampling and Manipulation
2.2. Generation of a Collection of Culturable Bacteria and Fungi from the Banana Root Endosphere
2.3. Physical and Chemical Soil Analysis
2.4. DNA Extraction and Illumina Sequencing
2.5. Illumina Data Processing
2.6. Analysis of Alpha and Beta Diversities
2.7. Banana Root Endosphere Core Microbiome Construction
2.8. Endophytic Banana Root Microbiome Network Construction, Comparison, and Visualization
2.9. In Vitro Antagonism against Fusarium oxysporum f. sp. Cubense
2.10. Molecular Identification of Potential Biocontrol Agents
2.11. Phenotyping of Endophytic Bacteria with Biocontrol Potential
2.12. Assessment of Biocontrol Effectiveness against Fusarium oxysporum f. sp. Cubense STR4
2.13. Sequence Accession Numbers
3. Results
3.1. General Characteristics of Sequencing Datasets
3.2. Alpha and Beta Diversity
3.3. Differences in Taxonomical Profile of the Banana Root Endosphere among Islands and between Mother Plants and Suckers
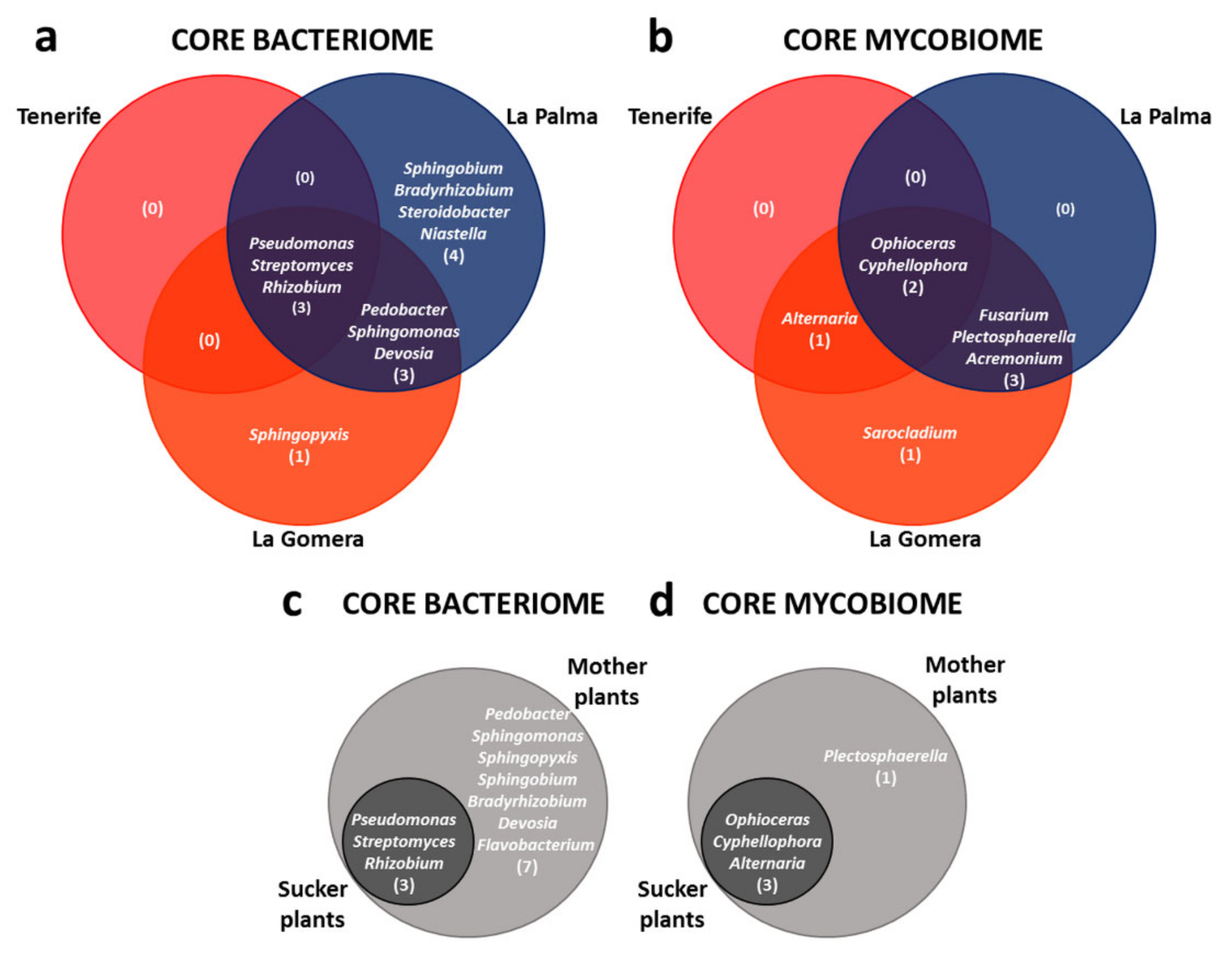
3.4. Defining the ‘Dwarf Cavendish’ Root Endosphere Core Microbiome
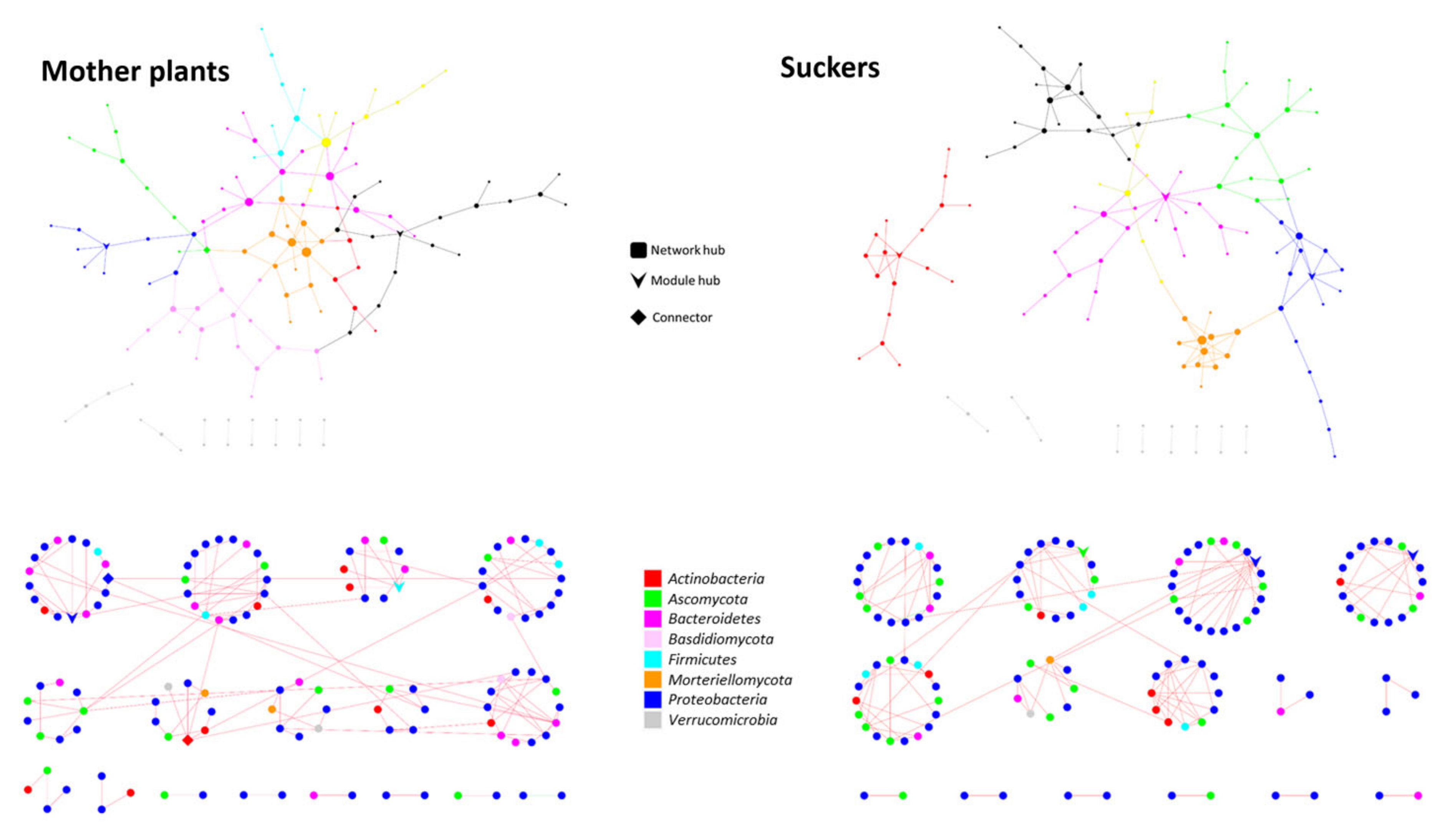
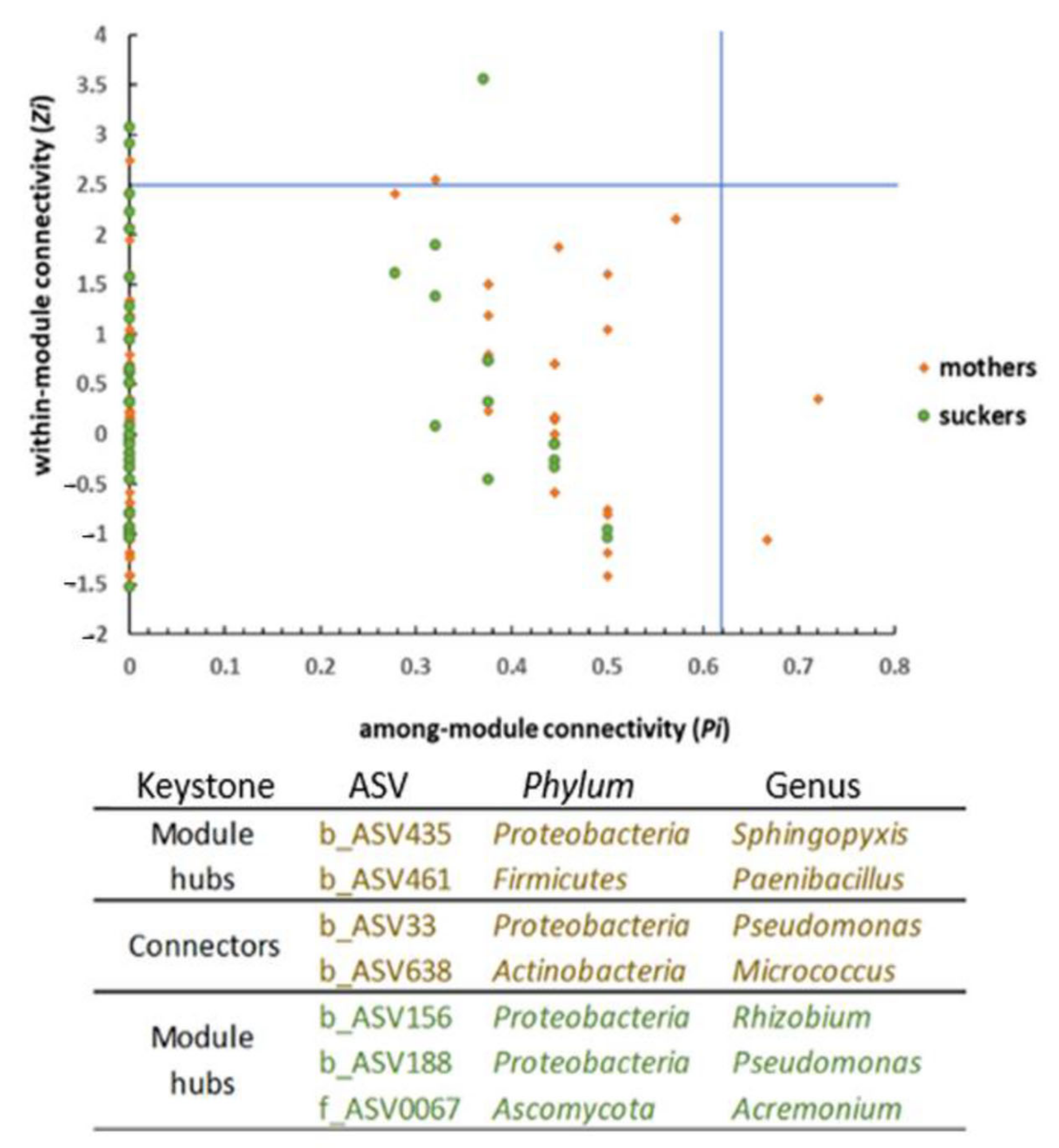
3.5. The Phenological Stage Influences the Co-Occurrence Networks Topology of the Banana Root Endosphere Microbial comMunities
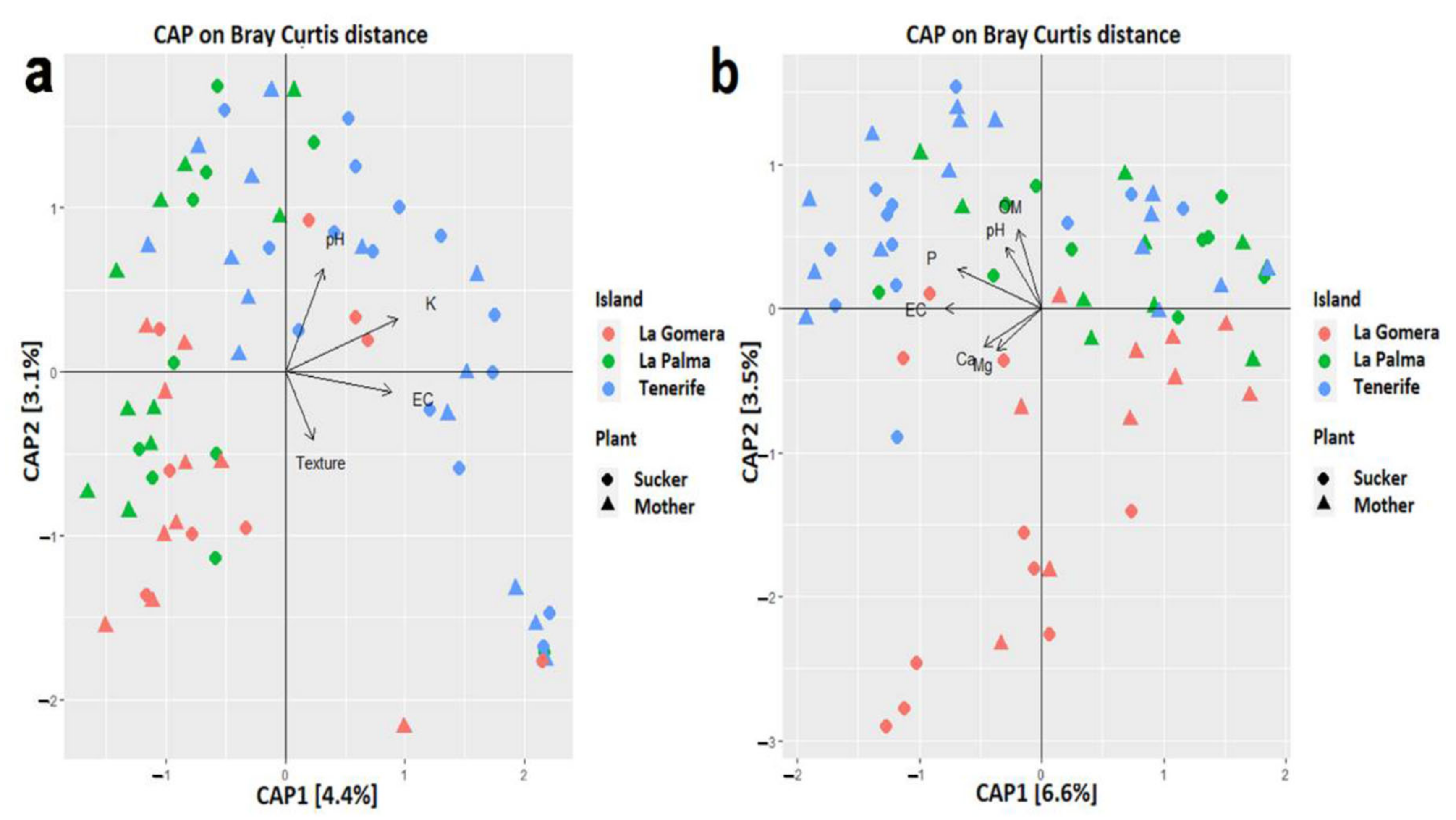
3.6. Scarce Influence of Soil Properties in the Banana Root Endophytome
3.7. The Banana Root Endosphere Is a Reservoir of Plant Growth Promoters and Antagonists against Fusarium oxysporum f.sp. cubense
3.8. Prevalence of Culturable Pseudomonas spp. in the Root Endosphere of ‘Dwarf Cavendish’ Grown in Canary Islands
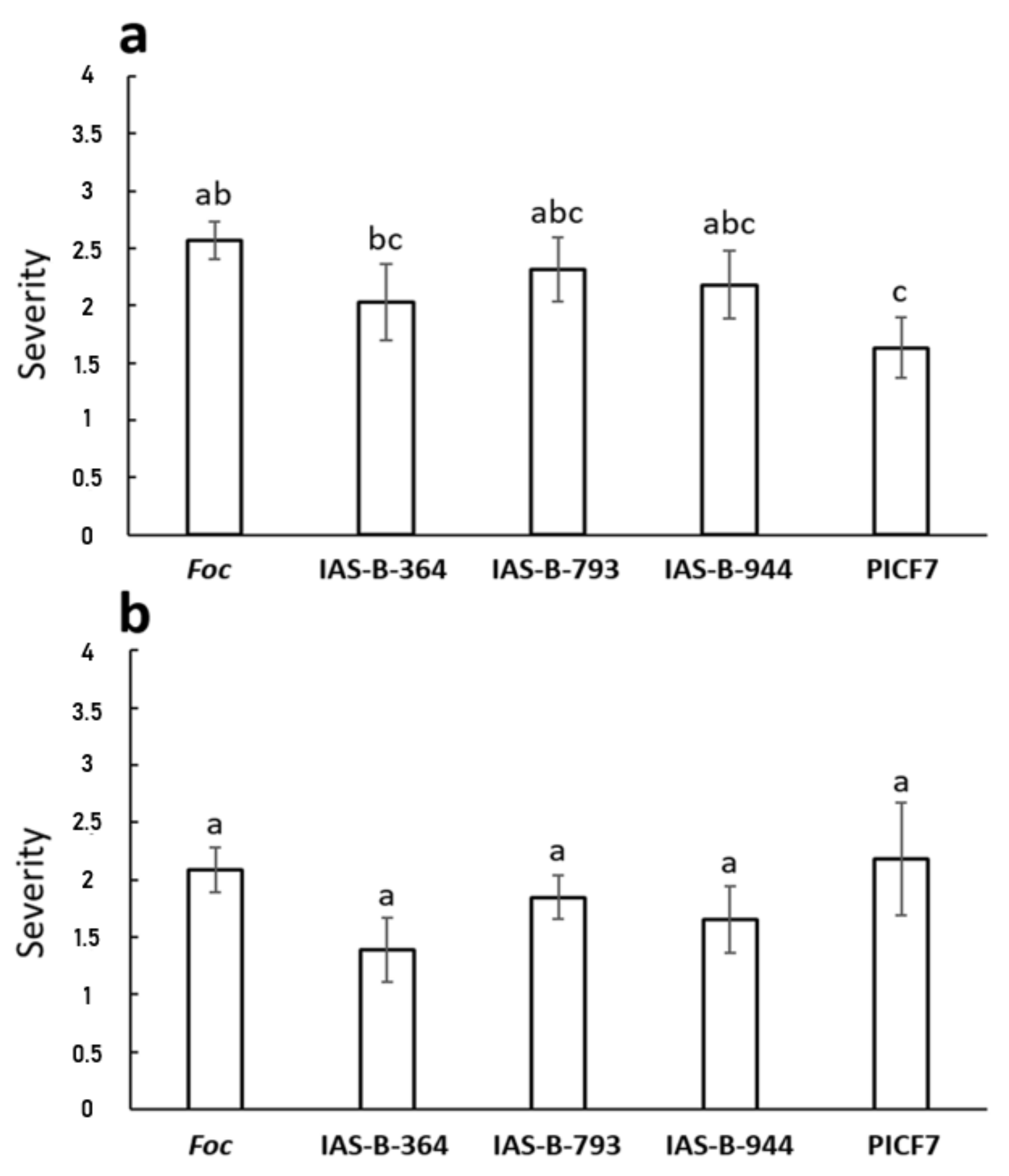
3.9. Biocontrol Performance of Selected Banana Root Endophytes against Fusarium Wilt
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Köberl, M.; Dita, M.; Martinuz, A.; Staver, C.; Berg, G. Members of Gammaproteobacteria as indicator species of healthy banana plants on Fusarium wilt-infested fields in Central America. Sci. Rep. 2017, 7, 45318. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, A.; Tan, H.; Cao, L.; Zhang, R. Engineering banana endosphere microbiome to improve Fusarium wilt resistance in banana. Microbiome 2019, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- FAO. 2019 Food Outlook-Biannual Report on Global Food Markets–November 2019. Rome. Available online: http://www.fao.org/3/ca6911en/ca6911en.pdf (accessed on 26 November 2020).
- Paparu, P.; Dubois, T.; Gold, C.S.; Niere, B.; Adipala, E.; Coyne, D. Screenhouse and field persistence of nonpathogenic endophytic Fusarium oxysporum in Musa tissue culture plants. Microb. Ecol. 2008, 55, 561–568. [Google Scholar] [CrossRef]
- Ploetz, R.C. Management of Fusarium wilt of banana: A review with special reference to tropical race 4. Crop. Prot. 2015, 73, 7–15. [Google Scholar] [CrossRef]
- Dita, M.; Barquero, M.; Heck, D.; Mizubuti, E.S.G.; Staver, C.P. Fusarium Wilt of Banana: Current knowledge on epidemiology and research needs toward sustainable disease management. Front. Plant Sci. 2018, 9, 1468. [Google Scholar] [CrossRef]
- Thangavelu, R.; Loganathan, M.; Arthee, R.; Prabakaran, M.; Uma, S. Fusarium wilt: A threat to banana cultivation and its management. CAB Rev. 2020, 15, 1–24. [Google Scholar] [CrossRef]
- Li, C.Y.; Chen, S.; Zuo, C.W.; Sun, Q.M.; Ye, Q.; Huang, B.Z. The use of GFP-transformed isolates to study infection of banana with Fusarium oxysporum f. sp. cubense race 4. Eur. J. Plant Pathol. 2011, 131, 327–340. [Google Scholar] [CrossRef]
- Siamak, S.B.; Zheng, S. Banana Fusarium wilt (Fusarium oxysporum f.sp. cubense) control and resistance, in the context of developing wilt-resistant bananas within sustainable production systems. Hortic. Plant J. 2018, 4, 208–218. [Google Scholar] [CrossRef]
- Waite, B.H.; Stover, R.H. Studies on fusarium wilt of bananas: VI. Variability and the cultivar concept in Fusarium oxysporum f. sp. cubense. Can. J. Bot. 1960, 38, 985–994. [Google Scholar] [CrossRef]
- Su, H.J.; Hwang, S.C.; Ko, W.H. Fusarial wilt of Cavendish bananas in Taiwan. Plant Dis. 1986, 70, 814–818. [Google Scholar] [CrossRef]
- Buddenhagen, I. Understanding strain diversity in Fusarium oxysporum f. sp. cubense and history of introduction of ‘tropical race 4′ to better manage banana production. Acta Hortic. 2009, 828, 193–204. [Google Scholar] [CrossRef]
- Maryani, N.; Lombard, L.; Poerba, Y.S.; Subandiyah, S.; Crous, P.W.; Kema, G.H.J. Phylogeny and genetic diversity of the banana Fusarium wilt pathogen Fusarium oxysporum f. sp. cubense in the Indonesian centre of origin. Stud. Mycol. 2019, 92, 155–194. [Google Scholar] [CrossRef] [PubMed]
- Bubici, G.; Kaushal, M.; Prigigallo, M.I.; Gómez-Lama Cabanás, C.; Mercado-Blanco, J. Biological control agents against Fusarium wilt of banana. Front. Microbiol. 2019, 10, 616. [Google Scholar] [CrossRef] [PubMed]
- Aguayo, J.; Cerf, I.; Folscher, A.B.; Fourrier-Jeandel, C.; Ioos, R.; Matthews, M.C.; Mostert, D.; Renault, C.; Wilson, V.; Viljoen, A. First report of Fusarium oxysporum f. sp. cubense tropical race 4 (TR4) causing banana wilt in the Island of Mayotte. Plant Dis. 2020, 5. [Google Scholar] [CrossRef]
- Li, C.Y.; Mostert, G.; Zuo, C.W.; Beukes, I.; Yang, Q.S.; Sheng, O.; Kuang, R.B.; Wei, Y.R.; Hu, C.H.; Rose, L.; et al. Diversity and distribution of the banana wilt pathogen Fusarium oxysporum f. sp. cubense in China. Fungal. Genom. Biol. 2013, 3, 2. [Google Scholar] [CrossRef]
- Mostert, D.; Molina, A.B.; Daniells, J.; Fourie, G.; Hermanto, C.; Chao, C.P.; Fabregar, E.; Sinohin, V.G.; Masdek, N.; Thangavelu, R.; et al. The distribution and host range of the banana Fusarium wilt fungus, Fusarium oxysporum f. sp. cubense, in Asia. PLoS ONE 2017, 12, e0181630. [Google Scholar] [CrossRef]
- García-Bastidas, F.A.; Quintero-Vargas, J.C.; Ayala-Vasquez, M.; Schermer, T.; Seidl, M.F.; Santos-Paiva, M.; Noguera, A.M.; Aguilera-Galvez, C.; Wittenberg, A.; Hofstede, R.; et al. First report of Fusarium wilt tropical race 4 in Cavendish bananas caused by Fusarium odoratissimum in Colombia. Plant Dis. 2020, 104, 994. [Google Scholar] [CrossRef]
- Dusunceli, F. Global Programme on Banana Fusarium Wilt Disease: Protecting Banana Production from the Disease with Focus on Tropical Race 4 (TR4); FAO: Rome, Italy, 2017; Available online: http://www.fao.org/3/a-i7921e.pdf (accessed on 26 November 2020).
- Zheng, S.-J.; García-Bastidas, F.A.; Li, X.; Zeng, L.; Bai, T.; Xu, S.; Yin, K.; Li, H.; Fu, G.; Yu, Y.; et al. New geographical insights of the latest expansion of Fusarium oxysporum f. sp. cubense tropical race 4 into the greater Mekong subregion. Front. Plant Sci. 2018, 9, 457. [Google Scholar] [CrossRef] [PubMed]
- Butler, D. Fungus threatens top banana. Nature 2013, 504, 195–196. [Google Scholar] [CrossRef]
- Gittleson, K. Battling to Save the World’s Bananas. 2018. Available online: https://www.bbc.com/news/business-42777803 (accessed on 26 November 2020).
- Dita, M.; Teixeira, L.A.J.; O’Neill, W.; Pattison, A.B.; Weinert, M.P.; Li, C.Y.; Zheng, S.J.; Staver, C.; Thangavelu, R.; Viljoen, A. Current state of Fusarium wilt of banana in the subtropics. Acta Hortic. 2020, 1272, 45–56. [Google Scholar] [CrossRef]
- Rodríguez Serrano, M. Mal de Panamá. Medidas de Control y Prevención. Technical Report. Agrocabildo, Servicio Técnico de Agricultura y Desarrollo Rural. 2012 Santa Cruz de Tenerife. Available online: http://www.agrocabildo.org/publica/Publicaciones/subt_443_mal_panama.pdf (accessed on 8 November 2018).
- Hennessy, C.; Walduck, G.; Daly, A.; Padovan, A. Weed hosts of Fusarium oxysporum f. sp. cubense tropical race 4 in northern Australia. Australas. Plant Pathol. 2005, 34, 115–117. [Google Scholar] [CrossRef]
- Berg, G.; Rybakova, D.; Grube, M.; Köberl, M. The plant microbiome explored: Implications for experimental botany. J. Exp. Bot. 2016, 67, 995–1002. [Google Scholar] [CrossRef]
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The rhizosphere microbiome: Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef]
- Berg, G.; Köberl, M.; Rybakova, D.; Muller, H.; Grosch, R.; Smalla, K. Plant microbial diversity is suggested as the key to future biocontrol and health trends. FEMS Microbiol. Ecol. 2017, 93, 5. [Google Scholar] [CrossRef] [PubMed]
- Köberl, M.; Dita, M.; Martinuz, A.; Staver, C.; Berg, G. Agroforestry leads to shifts within the gammaproteobacterial microbiome of banana plants cultivated in Central America. Front. Microbiol. 2015, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Jing, T.; Chen, Y.; Wang, F.; Qi, D.; Feng, R.; Xie, J.; Li, H. Deciphering microbial diversity associated with Fusarium wilt-diseased and disease-free banana rhizosphere soil. BMC Microbiol. 2019, 19, 161. [Google Scholar] [CrossRef]
- Kaushal, M.; Mahuku, G.; Swennen, R. Metagenomic insights of the root colonizing microbiome associated with symptomatic and non-symptomatic bananas in Fusarium wilt infected fields. Plants 2020, 9, 263. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, M.; Swennen, R.; Mahuku, G. Unlocking the microbiome communities of banana (Musa spp.) under disease stressed (Fusarium wilt) and non-stressed conditions. Microorganisms 2020, 8, 443. [Google Scholar] [CrossRef]
- Fernández-González, A.J.; Villadas, P.J.; Gómez-Lama Cabanás, C.; Valverde-Corredor, A.; Belaj, A.; Mercado-Blanco, J.; Fernández-López, M. Defining the root endosphere and rhizosphere microbiomes from the world olive germplasm collection. Sci. Rep. 2019, 9, 20423. [Google Scholar] [CrossRef]
- Fernández-González, A.J.; Cardoni, M.; Gómez-Lama Cabanás, C.; Valverde-Corredor, A.; Villadas, P.J.; Fernández-López, M.; Mercado-Blanco, J. Linking belowground microbial network changes to different tolerance level towards Verticillium wilt of olive. Microbiome 2020, 8, 11. [Google Scholar] [CrossRef]
- Pascale, A.; Proietti, S.; Pantelides, I.S.; Stringlis, I.A. Modulation of the root microbiome by plant molecules: The basis for targeted disease suppression and plant growth promotion. Front. Plant Sci. 2020, 10, 1741. [Google Scholar] [CrossRef]
- Saad, M.M.; Eida, A.A.; Hirt, H. Tailoring plant-associated microbial inoculants in agriculture: A roadmap for successful application. J. Exp. Bot. 2020, 71, 3878–3901. [Google Scholar] [CrossRef]
- Sekhar, A.C.; Thomas, P. Isolation and identification of shoot-tip associated endophytic bacteria from banana cv. Grand Naine and testing for antagonistic activity against Fusarium oxysporum f. sp. cubense. Am. J. Plant Sci. 2015, 6, 943–954. [Google Scholar] [CrossRef][Green Version]
- Proboningrum, A.; Hadiwiyono; Widono, S.; Sholahuddin. Effectivity and compatibility of Azotobacter and Bacillus for biological control agents of Fusarium wilt on banana seedlings. IOP Conf. Ser. Earth Environ. Sci. 2019, 250, 012003. [Google Scholar] [CrossRef]
- Bhende, S.; Kurien, S. Sucker production in banana: A review. J. Trop. Agric. 2015, 53, 97–106. [Google Scholar]
- Takahashi, S.; Tomita, J.; Nishioka, K.; Hisada, T.; Nishijima, M. Development of a prokaryotic universal primer for simultaneous analysis of bacteria and archaea using next-generation sequencing. PLoS ONE 2014, 9, 8. [Google Scholar] [CrossRef]
- Lundberg, D.S.; Yourstone, S.; Mieczkowski, P.; Jones, C.D.; Dangl, J.L. Practical innovations for high-throughput amplicon sequencing. Nat. Methods 2013, 10, 999–1002. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.D.; Lee, S.B.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA Genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academics Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Ihrmark, K.; Bödeker, I.T.M.; Cruz-Martinez, K.; Friberg, H.; Kubartova, A.; Schenck, J.; Strid, Y.; Stenlid, J.; Brandström-Durling, M.; Clemmensen, K.E.; et al. New primers to amplify the fungal ITS2 region–evaluation by 454-sequencing of artificial and natural communities. FEMS Microbiol. Ecol. 2012, 82, 666–677. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution simple inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://benjjneb.github.io/dada2/tutorial.html (accessed on 10 February 2020).
- Available online: https://benjjneb.github.io/dada2/ITS_workflow.html (accessed on 10 February 2020).
- Available online: https://doi.org/10.14806/ej.17.1.200 (accessed on 10 February 2020).
- Cole, J.R.; Wang, Q.; Fish, J.A.; Chai, B.; McGarrell, D.M.; Sun, Y.; Brown, C.T.; Porras-Alfaro, A.; Kuske, C.R.; Tiedje, J.M. Ribosomal Database Project: Data and tools for high throughput rRNA analysis. Nucleic Acids Res. 2014, 42, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://plutof.ut.ee/#/doi/10.15156/BIO/587478 (accessed on 18 February 2020).
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knigh, R.; Mills, D.A.; Caporaso, G. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 2013, 10, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, Third Edition, Sage Pubblication Inc. R pacage version 3.0-2.
- De Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research. R package Version 1.2-8. 2017. Available online: http://CRAN.R-project.org/package=agricolae (accessed on 18 February 2020).
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package, Version 2.5-3. 2018. Available online: http://CRAN.Rproject.org/package=vegan (accessed on 18 February 2020).
- Martínez-Arbizu, P. pairwiseAdonis: Pairwise Multilevel Comparison Using Adonis. R Package Version 0.3. 2019. Available online: https://github.com/pmartinezarbizu/pairwiseAdonis (accessed on 18 February 2020).
- McMurdie, P.J.; Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Parks, D.H.; Tyson, G.W.; Hugenholtz, P.; Beiko, R.G. STAMP: Statistical analysis of taxonomic and functional profiles. Bioinformatics 2014, 30, 3123–3124. [Google Scholar] [CrossRef]
- Hernandez-Agreda, A.; Gates, R.G.; Ainswoth, T.D. Defining the core microbiome in coral’s microbial soup. Trends Microbiol. 2017, 25, 125–140. [Google Scholar] [CrossRef]
- Available online: http://ieg4.rccc.ou.edu/mena/main.cgi (accessed on 20 February 2020).
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Deng, Y.; Jiang, Y.H.; Yang, Y.; He, Z.; Luo, F.; Zhou, J. Molecular ecological network analysis. BMC Bioinform. 2012, 13, 113. [Google Scholar] [CrossRef]
- Zhou, J.; Den, Y.; Luo, F.; He, Z.; Tu, Q.; Zhi, X. Functional molecular ecological networks. mBio 2010, 1, 4. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Deng, Y.; Luo, F.; He, Z.; Yang, Y. Phylogenetic molecular ecological network of soil microbial communities in response to elevated CO2. mBio 2011, 2, 4. [Google Scholar] [CrossRef]
- Gómez-Lama Cabanás, C.; Legarda, G.; Ruano-Rosa, D.; Pizarro-Tobías, P.; Valverde-Corredor, A.; Niqui, J.L.; Triviño, J.C.; Roca, A.; Mercado-Blanco, J. Indigenous Pseudomonas spp. strains from the olive (Olea europaea L.) rhizosphere as effective biocontrol agents against Verticillium dahliae: From the host roots to the bacterial genomes. Front. Microbiol. 2018, 9, 277. [Google Scholar] [CrossRef] [PubMed]
- Montes-Osuna, N.; Gómez-Lama Cabanás, C.; Valverde-Corredor, A.; Berendsen, R.L.; Prieto, P.; Mercado-Blanco, J. Assessing the involvement of selected phenotypes of Pseudomonas simiae PICF7 in olive root colonization and biological control of Verticillium dahliae. Plants 2021, 10, 412. [Google Scholar] [CrossRef]
- Ruano-Rosa, D.; Valverde-Corredor, A.; Gómez-Lama Cabanás, C.; Sesmero, R.; Mercado-Blanco, J. What lies beneath: Root-associated bacteria to improve the growth and health of olive trees. In Soil Biological Communities and Ecosystem Resilience, Sustainability in Plant and Crop Protection Series; Lukac, M., Grenni, P., Gamboni, M., Eds.; Springer: Cham, Switzerland, 2017; pp. 189–205. [Google Scholar] [CrossRef]
- Raja, H.A.; Miller, A.N.; Pearce, C.J.; Oberlies, N.H. Fungal identification using molecular tools: A primer for the natural products research community. J. Nat. Prod. 2017, 80, 756–770. [Google Scholar] [CrossRef]
- Truett, G.E.; Heeger, P.; Mynatt, R.L.; Truett, A.A.; Walker, J.A.; Warman, M.L. Preparation of PCR-quality mouse genomic DNA with hot sodium hydroxide and tris (HotSHOT). Biotechniques 2000, 29, 52–54. [Google Scholar] [CrossRef]
- Available online: http://www.ncbi.nlm.nih.gov/ (accessed on 1 October 2020).
- Gómez-Lama Cabanás, C.; Ruano-Rosa, D.; Legarda, G.; Pizarro-Tobías, P.; Valverde-Corredor, A.; Triviño, J.C.; Roca, A.; Mercado-Blanco, J. Bacillales members from the olive rhizosphere are effective biological control agents against the defoliating pathotype of Verticillium dahliae. Agriculture 2018, 8, 90. [Google Scholar] [CrossRef]
- Domínguez-Hernández, J.; Negrín, M.A.; Rodríguez, C.M. Soil potassium indices and clay-sized particles affecting banana-wilt expression caused by soil fungus in banana plantation development on transported volcanic soils. Commun. Soil Sci. Plant Anal. 2008, 39, 397–412. [Google Scholar] [CrossRef]
- López-Cepero, J.; Puerta, M.; Piedra Buena, A. Guía Para la Gestión Integrada de Plagas en Platanera; COPLACA: Santa Cruz de Tenerife, Spain, 2014; Volume 2, p. 44. [Google Scholar]
- Ploetz, R.C.; Kepler, A.K.; Daniells, J.; Nelson, S.C. Banana and plantain—An overview with emphasis on Pacific island cultivars Musaceae (banana family). In Species Profiles for Pacific Island Agroforestry; Elevitch, C.R., Ed.; Permanent Agriculture Resources: Holualoa, HI, USA, 2007. [Google Scholar]
- Rossmann, B.; Muller, H.; Smalla, K.; Mpiira, S.; Tumuhairwe, J.B.; Staver, C.; Berg, G. Banana associated microbial communities in Uganda are highly diverse but dominated by Enterobacteriaceae. Appl. Environ. Microbiol. 2012, 78, 4933–4941. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Penton, C.R.; Shen, Z.; Zhang, R.; Huang, Q.; Li, R.; Ruan, Y.; Shen, Q. Manipulating the banana rhizosphere microbiome for biological control of Panama disease. Sci. Rep. 2015, 5, 11124. [Google Scholar] [CrossRef]
- Thomas, P.; Swarna, G.K.; Patil, P.; Rawal, R.D. Ubiquitous presence of normally non-cultivable culturable endophytic bacteria in field shoot-tips of banana and their gradual activation to quiescent cultivable form in tissue cultures. Plant Cell Tissue Organ Cult. 2008, 93, 39–54. [Google Scholar] [CrossRef]
- Thomas, P.; Soly, T.A. Endophytic bacteria associated with growing shoot tips of banana (Musa sp.) cv. Grand Naine and the affinity of endophytes to the host. Microb. Ecol. 2009, 58, 952–964. [Google Scholar] [CrossRef] [PubMed]
- Rybakova, D.; Mancinelli, R.; Wikström, M.; Birch-Jensen, A.S.; Postma, J.; Ehlers, R.U.; Goerzt, S.; Berg, G. The structure of the Brassica napus seed microbiome is cultivar-dependent and affects the interactions of symbionts and pathogens. Microbiome 2017, 5, 104. [Google Scholar] [CrossRef]
- Yang, H.; Li, Y.; Xiao, Y.; Gu, Y.; Liu, H.; Liang, Y.; Liu, X.; Hu, J.; Meng, D.; Yin, H. An integrated insight into the relationship between soil microbial community and tobacco bacterial wilt disease. Front. Microbiol. 2017, 8, 2179. [Google Scholar] [CrossRef]
- Jiemeng, T.; Meng, D.; Qin, C.; Liu, X.; Liang, Y.; Xiao, Y.; Liu, Z.; Gu, Y.; Li, J.; Yin, H. Integrated network analysis reveals the importance of microbial interaction for maize growth. Appl. Microbiol. Biotechnol. 2018, 102, 3805–3818. [Google Scholar] [CrossRef]
- Delmas, E.; Besson, M.; Brice, M.; Burkle, L.A.; Dalla Riva, G.V.; Fortin, M.; Gravel, D.; Guimarães Jr, P.R.; Hembry, D.H.; Newman, E.A.; et al. Analysing ecological networks of species interactions. Biol. Rev. 2019, 94, 16–36. [Google Scholar] [CrossRef] [PubMed]
- Faust, K.; Lima-Mendez, G.; Lerat, J.-S.; Sathirapongsasuti, J.F.; Knight, R.; Hutternhower, C.; Lenaerts, T.; Raes, J. Cross-biome comparison of microbial association networks. Front. Microbiol. 2015, 6, 1200. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Wang, Y.; Ye, S.; Liu, S.; Stirling, E.; Gilbert, J.A.; Faust, K.; Knight, R.; Jansson, J.K.; Cardona, C.; et al. Earth microbial co-occurrence network reveals interconnection pattern across microbiomes. Microbiome 2020, 8, 82. [Google Scholar] [CrossRef]
- Faust, K.; Sathirapongsasuti, J.F.; Izard, J.; Segata, N.; Gevers, D.; Raes, J. Curtis Huttenhower. Microbial co-occurrence relationships in the human microbiome. PLoS Comput. Biol. 2012, 8, e1002606. [Google Scholar] [CrossRef] [PubMed]
- Mercado-Blanco, J.; Lugtenberg, B.J.J. Biotechnological applications of bacterial endophytes. Curr. Biotech. 2014, 3, 60–75. [Google Scholar] [CrossRef]
- Mercado-Blanco, J.; Rodríguez-Jurado, D.; Hervás, A.; Jiménez-Díaz, R.M. Suppression of Verticillium wilt in olive planting stocks by root-associated fluorescent Pseudomonas spp. Biol. Control 2004, 30, 474–486. [Google Scholar] [CrossRef]
- Sang, M.K.; Shrestha, A.; Kim, D.Y.; Park, K.; Pak, C.H.; Kim, K.D. Biocontrol of phytophthora blight and anthracnose in pepper by sequentially selected antagonistic rhizobacteria against Phytophthora capsici. Plant Pathol J. 2013, 29, 154–167. [Google Scholar] [CrossRef]
- Maldonado-González, M.M.; Bakker, P.A.; Prieto, P.; Mercado-Blanco, J. Arabidopsis thaliana as a tool to identify traits involved in Verticillium dahliae biocontrol by the olive root endophyte Pseudomonas fluorescens PICF7. Front. Microbiol. 2015, 6, 266. [Google Scholar] [CrossRef]
- Maldonado-González, M.M.; Schilirò, E.; Prieto, P.; Mercado-Blanco, J. Endophytic colonization and biocontrol performance of Pseudomonas fluorescens PICF7 in olive (Olea europaea L.) are determined neither by pyoverdine production nor swimming motility. Environ. Microbiol. 2015, 17, 3139–3153. [Google Scholar] [CrossRef]
- Tsolakidou, M.-D.; Stringlis, I.A.; Fanega-Sleziak, N.; Papageorgiou, S.; Tsalakou, A.; Pantelides, I.S. Rhizosphere-enriched microbes as a pool to design synthetic communities for reproducible beneficial outputs. FEMS Microbiol. Ecol. 2019, 95, 10. [Google Scholar] [CrossRef]
- Maldonado-González, M.M.; Prieto, P.; Ramos, C.; Mercado-Blanco, J. From the root to the stem: Interaction between the biocontrol root endophyte Pseudomonas fluorescens PICF7 and the pathogen Pseudomonas savastanoi NCPPB 3335 in olive knots. Microb. Biotechnol. 2013, 6, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Lama Cabanás, C.; Schilirò, E.; Valverde-Corredor, A.; Mercado-Blanco, J. The biocontrol endophytic bacterium Pseudomonas fluorescens PICF7 induces systemic defense responses in aerial tissues upon colonization of olive roots. Front. Microbiol. 2014, 5, 427. [Google Scholar] [CrossRef] [PubMed]
- Mercado-Blanco, J.; Alós, E.; Rey, M.D.; Prieto, P. Pseudomonas fluorescens PICF7 displays an endophytic lifestyle in cultivated cereals and enhances yield in barley. FEMS Microbiol. Ecol. 2016, 92, 8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mendes, R.; Kruijt, M.; de Bruijn, I.; Dekkers, E.; van der Voort, M.; Schneider, J.H.; Piceno, Y.M.; DeSantis, T.Z.; Andersen, G.Z.; Bakke, P.A.H.M.; et al. Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science 2011, 332, 1097–1100. [Google Scholar] [CrossRef]
- Wei, F.; Hu, X.; Xu, X. Dispersal of Bacillus subtilis and its effect on strawberry phyllosphere microbiota under open field and protection conditions. Sci. Rep. 2016, 6, 22611. [Google Scholar] [CrossRef] [PubMed]
- Orozco-Mosqueda, M.D.C.; Rocha-Granados, M.D.C.; Glick, B.R.; Santoyo, G. Microbiome engineering to improve biocontrol and plant growth-promoting mechanisms. Microbiol. Res. 2018, 208, 25–31. [Google Scholar] [CrossRef]
- Zhang, B.; Bai, Z.; Hoefel, D.; Tang, L.; Yang, Z.; Zhuang, G.; Yang, J.; Zhang, H. Assessing the impact of the biological control agent Bacillus thuringiensis on the indigenous microbial community within the pepper plant phyllosphere. FEMS Microbiol. Lett. 2008, 284, 102–108. [Google Scholar] [CrossRef]
- He, A.; Sun, J.; Wang, X.; Zou, L.; Fu, B.; Chen, J. Reprogrammed endophytic microbial community in maize stalk induced by Trichoderma asperellum biocontrol agent against Fusarium diseases and mycotoxin accumulation. Fungal Biol. 2019, 123, 448–455. [Google Scholar] [CrossRef] [PubMed]

| Farm Code | Farm Name | Island | Latitude (N) | Longitude (W) | Altitude (m.a.s.l.) | Management 1 | Fusarium Wilt Incidence (%) |
|---|---|---|---|---|---|---|---|
| F01 | Siverio | Tenerife | 28°10′06′′ | 16°26′13′′ | 37 | IPM | <5 |
| F02 | Temaso | Tenerife | 28°09′00′′ | 16°47′36′′ | 84 | IPM | <5 |
| F03 | Servicios Agrícolas Abdul | Tenerife | 28°11′02′′ | 16°47′01′′ | 326 | IPM | 25 |
| F04 | Malpaís-Colpon Agrícola | Tenerife | 28°22′36′′ | 16°44′08′′ | 22 | IPM | <5 |
| F05 * | Fco Pacheco, Arico | Tenerife | 28°10′57′′ | 16°27′00′′ | 189 | IPM | <5 |
| F06 * | La Caldera, Adeje | Tenerife | 28°04′34′′ | 16°43′08′′ | 113 | IPM | <5 |
| F07 * | Siso, Fuencaliente | La Palma | 28°28′36′′ | 17°51′58′′ | 29 | IPM | <5 |
| F08 * | Ortiz, Tijarafe/ | La Palma | 28°41′47′′ | 17°57′51′′ | 300 | IPM | 35 |
| F09 ** | Escuela Capataces | Tenerife | 28°29′46′′ | 16°25′15′′ | 299 | Organic | 0 |
| F10 * | Hermigua/ | La Gomera | 28°10′11′′ | 17°11′53′′ | 246 | Conventional | <5 |
| F11 * | David, San Sebastián/ | La Gomera | 28°06′31′′ | 17°08′32′′ | 82 | IPM | <5 |
| Richness (Observed ASV) | Shannon Index | ||||
|---|---|---|---|---|---|
| Dataset | Comparison | Bacteria | Fungi | Bacteria | Fungi |
| Tenerife | Farms | 0.090 | 0.207 | 0.538 | 0.098 |
| La Palma | Farms | 0.822 | 0.272 | 0.820 | 0.034 (F07 vs. F08) |
| La Gomera | Farms | 0.493 | 0.700 | 0.608 | 0.034 |
| Tenerife mothers | Farms | 0.223 | 0.316 | 0.509 | 0.049 (F05 vs. F06, F06 vs. F09) |
| Tenerife suckers | Farms | 0.225 | 0.304 | 0.904 | 0.908 |
| La Palma mothers | Farms | 0.107 | 0.256 | 0.190 | 0.117 |
| La Palma suckers | Farms | 0.538 | 0.256 | 0.665 | 0.075 |
| La Gomera mothers | Farms | 0.740 | 0.304 | 0.904 | 0.001 (F10 vs. F11) |
| La Gomera suckers | Farms | 0.538 | 0.601 | 0.190 | 0.117 |
| Mothers | Farms | 0.445 | 0.227 | 0.603 | 0.011 (F10 vs. F11, F06 vs. F10) |
| Suckers | Farms | 0.232 | 0.612 | 0.862 | 0.699 |
| F09/F09 * | Farm | 0.058 | 0.351 | 0.211 | 0.592 |
| F09/F09 mothers * | Farm | 0.878 | 0.949 | 0.354 | 0.248 |
| F09/F09 suckers * | Farm | 0.180 | 0.305 | 0.230 | 0.638 |
| Mothers | Islands | 0.418 | 0.612 | 0.466 | 0.699 |
| Suckers | Islands | 0.418 | 0.357 | 0.466 | 0.807 |
| Tenerife | Plants | 0.025 (Mothers vs. Suckers) | 0.822 | 0.021 (Mothers vs. Suckers) | 0.956 |
| La Palma | Plants | 0.228 | 0.426 | 0.085 | 0.069 |
| La Gomera | Plants | 0.061 | 0.705 | 0.013 (Mothers vs. Suckers) | 0.069 |
| F09/F09* | Plants | 0.204 | 0.535 | 0.118 | 0.420 |
| Weighted Unifrac | Bray–Curtis | ||
|---|---|---|---|
| Dataset | Comparison | Bacteria | Fungi |
| Tenerife | Farms | 0.025 (F05 vs. F06) (F09 vs. F05) (F09 vs. F06) | 0.001 (F05 vs. F06) (F05 vs. F09) (F06 vs. F09) |
| La Palma | Farms | 0.385 | 0.160 |
| La Gomera | Farms | 0.968 | 0.026 (F10 vs. F11) |
| Tenerife mothers | Farms | 0.116 | 0.027 (F05 vs. F06) (F05 vs. F09) (F06 vs. F09) |
| Tenerife suckers | Farms | 0.320 | 0.191 |
| La Palma mothers | Farms | 0.338 | 0.011 (F07 vs. F08) |
| La Palma suckers | Farms | 0.658 | 0.572 |
| La Gomera mothers | Farms | 0.320 | 0.122 |
| La Gomera suckers | Farms | 0.338 | 0.049 (F10 vs. F11) |
| Mothers | Farms | 0.009 (F05 vs. F11) (F07 vs. F05) (F07 vs. F06) (F09 vs. F07) (F10 vs. F05) (F10 vs. F06) (F10 vs. F07) | 0.002 (F05 vs. F11) (F07 vs. F10) |
| Suckers | Farms | 0.703 | 0.071 |
| F09/F09 * | Farm | 0.211 | 0.002 (F09 vs. F09) |
| F09/F09 mothers * | Farm | 0.485 | 0.060 |
| F09/F09 suckers * | Farm | 0.265 | 0.018 (F09 vs. F09) |
| Mothers | Islands | 0.700 | 0.050 (Tenerifevs. La Palma) (Tenerifevs. La Gomera) |
| Suckers | Islands | 0.561 | 0.001 (Tenerifevs. La Palma) (Tenerifevs. La Gomera) (La Palmavs. La Gomera) |
| Tenerife | Plants | 0.315 | 0.113 |
| La Palma | Plants | 0.170 | 0.811 |
| La Gomera | Plants | 0.350 | 0.029 (Mothers vs. Suckers) |
| F09/F09 * | Plants | 0.328 | 0.390 |
| Community | No. Of Original ASV | Similarity Threshold (St) | Total Nodes | Total Links | Percentage of Positive Edges (PEP) | R2 of Power- Law | Average Degree (avgK) | Avg Clustering Coefficient (avgCC) | Avg Path Distance (GD) | Modularity (M) |
|---|---|---|---|---|---|---|---|---|---|---|
| Mothers | 323 | 0.87 | 127 | 136 | 0.74% | 0.893 | 2.142 | 0.004 | 6.342 | 0.757 (17) |
| Suckers | 245 | 0.88 | 131 | 153 | 0.65% | 0.878 | 2.336 | 0.078 | 6.580 | 0.816 (15) |
| Phenotype | Number of Isolates | Percentage of Isolates (%) |
|---|---|---|
| Catalase | 118 | 96.7 |
| Phytase | 90 | 73.8 |
| Siderophores | 88 | 72.1 |
| Protease | 83 | 68 |
| Phosphatase | 75 | 61.5 |
| β-glucosydase | 49 | 40.2 |
| HCN | 49 | 40.2 |
| Butanediol | 26 | 21.3 |
| Amylase | 16 | 13.1 |
| Xylanase | 9 | 7.4 |
| ISOLATE | Molecular ID * | Island/Farm | In Vitro Antagonism against Foc | N° of PGP and Biocontrol Activities | |
|---|---|---|---|---|---|
| TR4 | STR4 | ||||
| IAS-B-197 | P. chlororaphis | Tenerife/Farm 011 | Yes | Yes | 6 |
| IAS-B-364 | P. chlororaphis | Tenerife/Farm F09 | Yes | Yes | 7 |
| IAS-B-481 | P. chlororaphis | Tenerife/Farm F02 | Yes | Yes | 7 |
| IAS-B-793 | Pseudomonas protegens | Tenerife/Farm F04 | Yes | Yes | 6 |
| IAS-B-931 | P. chlororaphis subsp. aurantiaca | La Palma/Farm F07 | Yes | Yes | 6 |
| IAS-B-944 | P. chlororaphis subp. aureofaciens | La Palma/Farm F07 | Yes | Yes | 5 |
| IAS-B-966 | P. chlororaphis subsp. piscium | La Palma/Farm F08 | Yes | Yes | 5 |
| IAS-B-1013 | P. chlororaphis | Tenerife/Farm F09 | Yes | Yes | 5 |
| IAS-B-1054 | P. chlororaphis aureofaciens | Tenerife/Farm F09 | Yes | Yes | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Lama Cabanás, C.; Fernández-González, A.J.; Cardoni, M.; Valverde-Corredor, A.; López-Cepero, J.; Fernández-López, M.; Mercado-Blanco, J. The Banana Root Endophytome: Differences between Mother Plants and Suckers and Evaluation of Selected Bacteria to Control Fusarium oxysporum f.sp. cubense. J. Fungi 2021, 7, 194. https://doi.org/10.3390/jof7030194
Gómez-Lama Cabanás C, Fernández-González AJ, Cardoni M, Valverde-Corredor A, López-Cepero J, Fernández-López M, Mercado-Blanco J. The Banana Root Endophytome: Differences between Mother Plants and Suckers and Evaluation of Selected Bacteria to Control Fusarium oxysporum f.sp. cubense. Journal of Fungi. 2021; 7(3):194. https://doi.org/10.3390/jof7030194
Chicago/Turabian StyleGómez-Lama Cabanás, Carmen, Antonio J. Fernández-González, Martina Cardoni, Antonio Valverde-Corredor, Javier López-Cepero, Manuel Fernández-López, and Jesús Mercado-Blanco. 2021. "The Banana Root Endophytome: Differences between Mother Plants and Suckers and Evaluation of Selected Bacteria to Control Fusarium oxysporum f.sp. cubense" Journal of Fungi 7, no. 3: 194. https://doi.org/10.3390/jof7030194
APA StyleGómez-Lama Cabanás, C., Fernández-González, A. J., Cardoni, M., Valverde-Corredor, A., López-Cepero, J., Fernández-López, M., & Mercado-Blanco, J. (2021). The Banana Root Endophytome: Differences between Mother Plants and Suckers and Evaluation of Selected Bacteria to Control Fusarium oxysporum f.sp. cubense. Journal of Fungi, 7(3), 194. https://doi.org/10.3390/jof7030194










