Identification of the Antidepressant Function of the Edible Mushroom Pleurotus eryngii
Abstract
1. Introduction
2. Materials and Methods
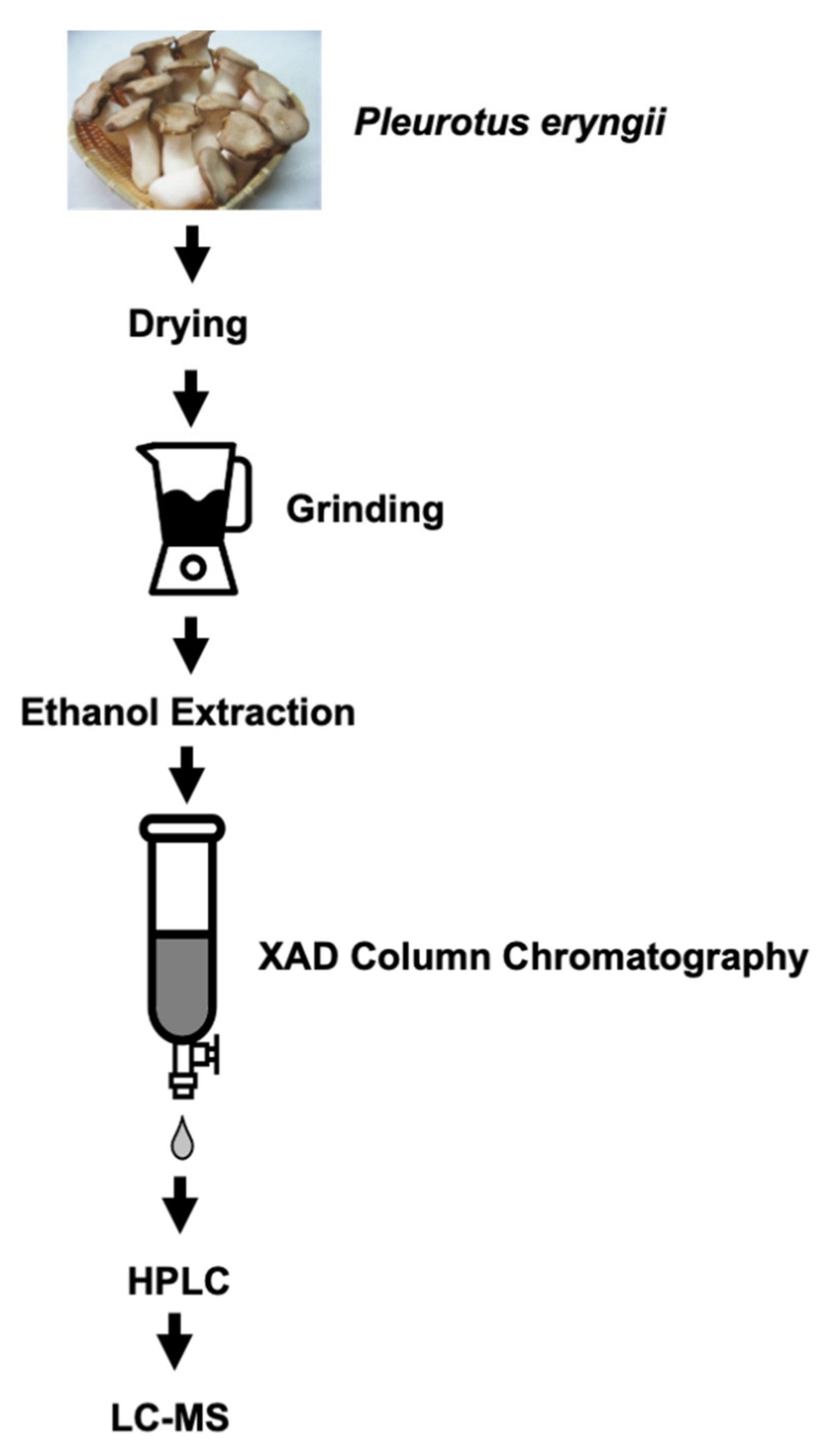
2.1. Ethanol Extraction of P. eryngii
2.2. HPLC Analysis of the Mixed Sample and Purification of Molecules with SSRI Functions
2.3. FIA-MS Analysis
2.4. Plate Assay
2.5. Yeast Strain, Media, and Growth Conditions
2.6. Cell Cultures
2.7. MTT Assay
2.8. Receptor Binding Assay
2.9. Forced Swimming Test
3. Results
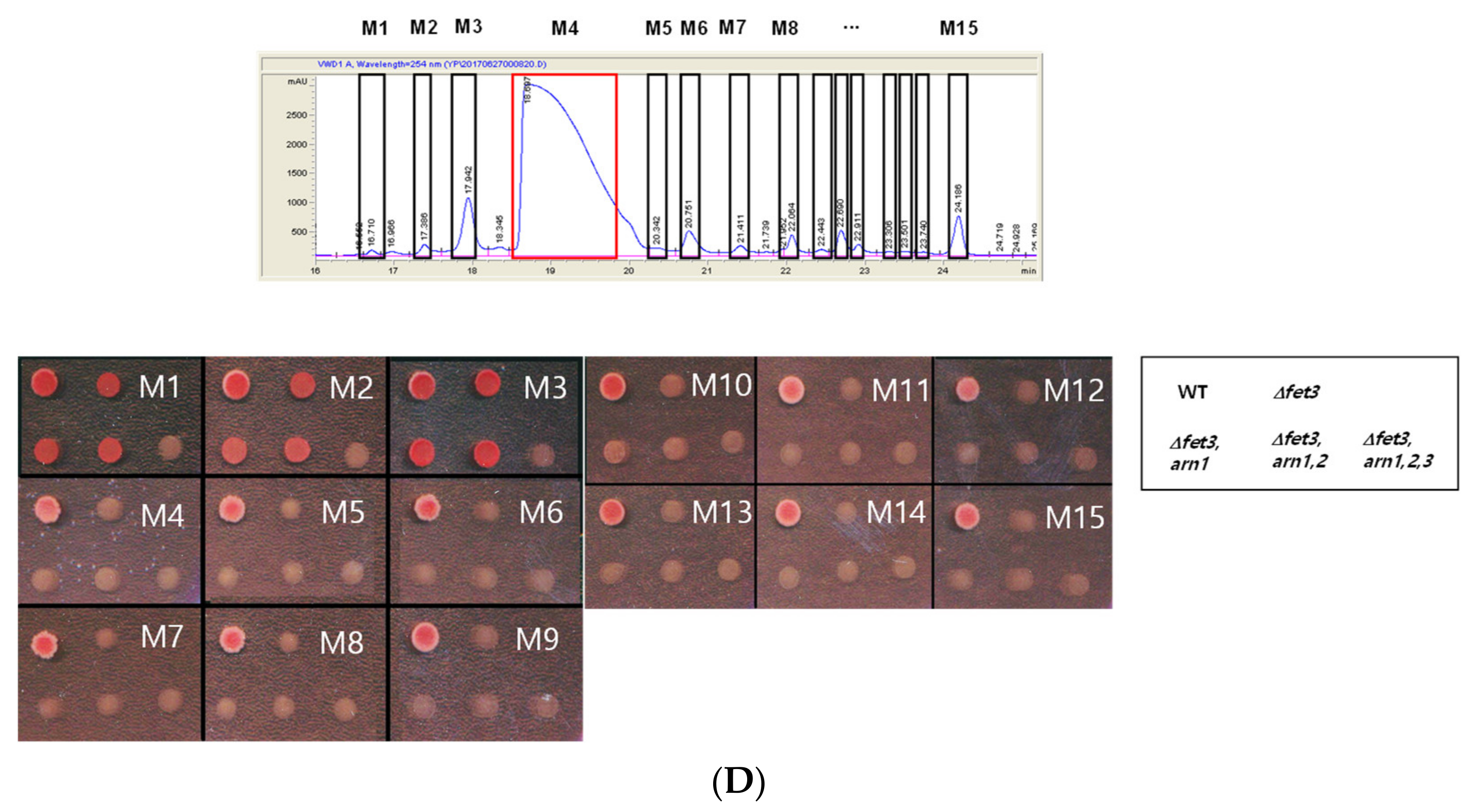
3.1. Isolation of Siderophores from P. eryngii
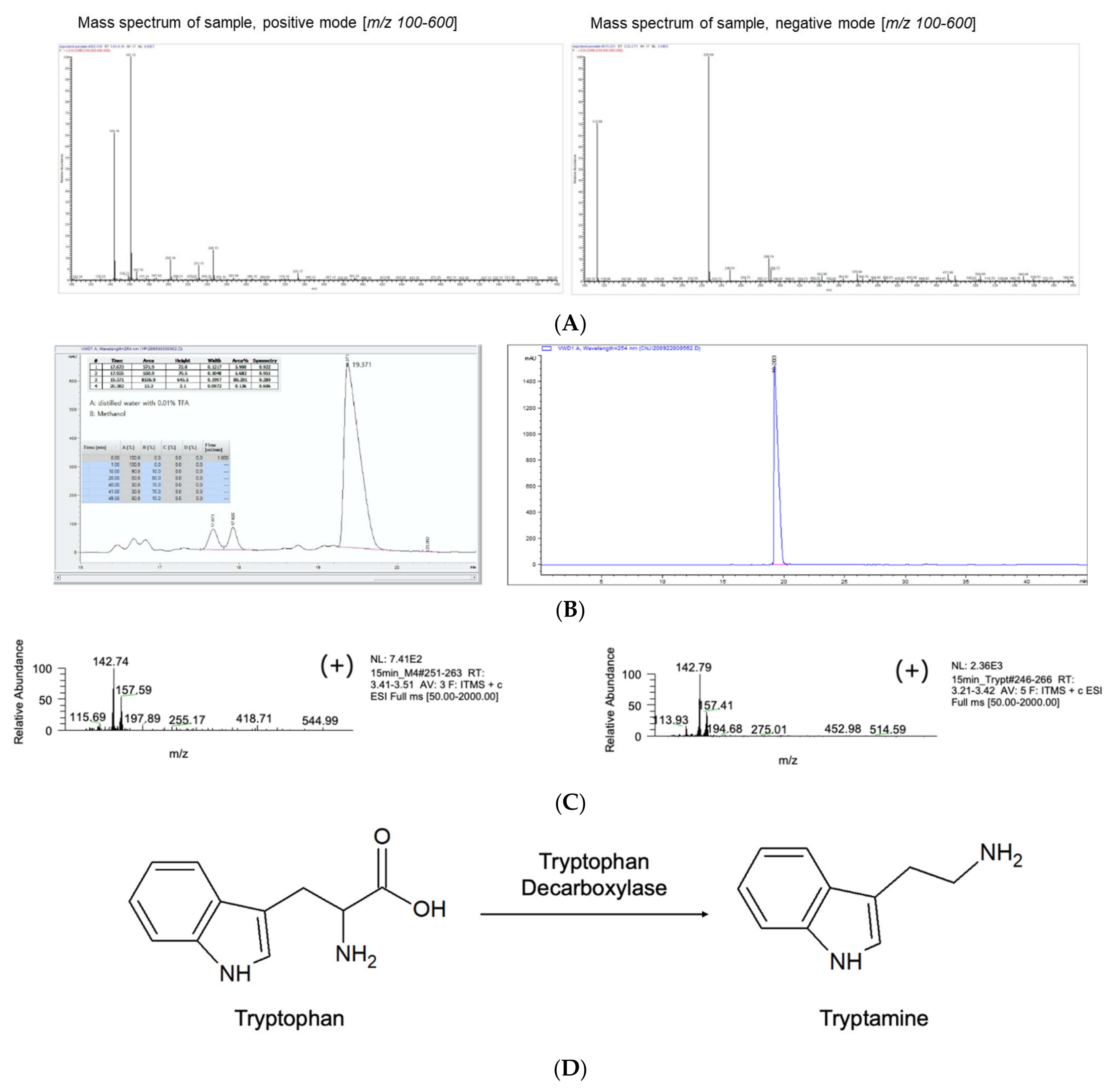
3.2. M4 Fraction of R2 Is Tryptamine
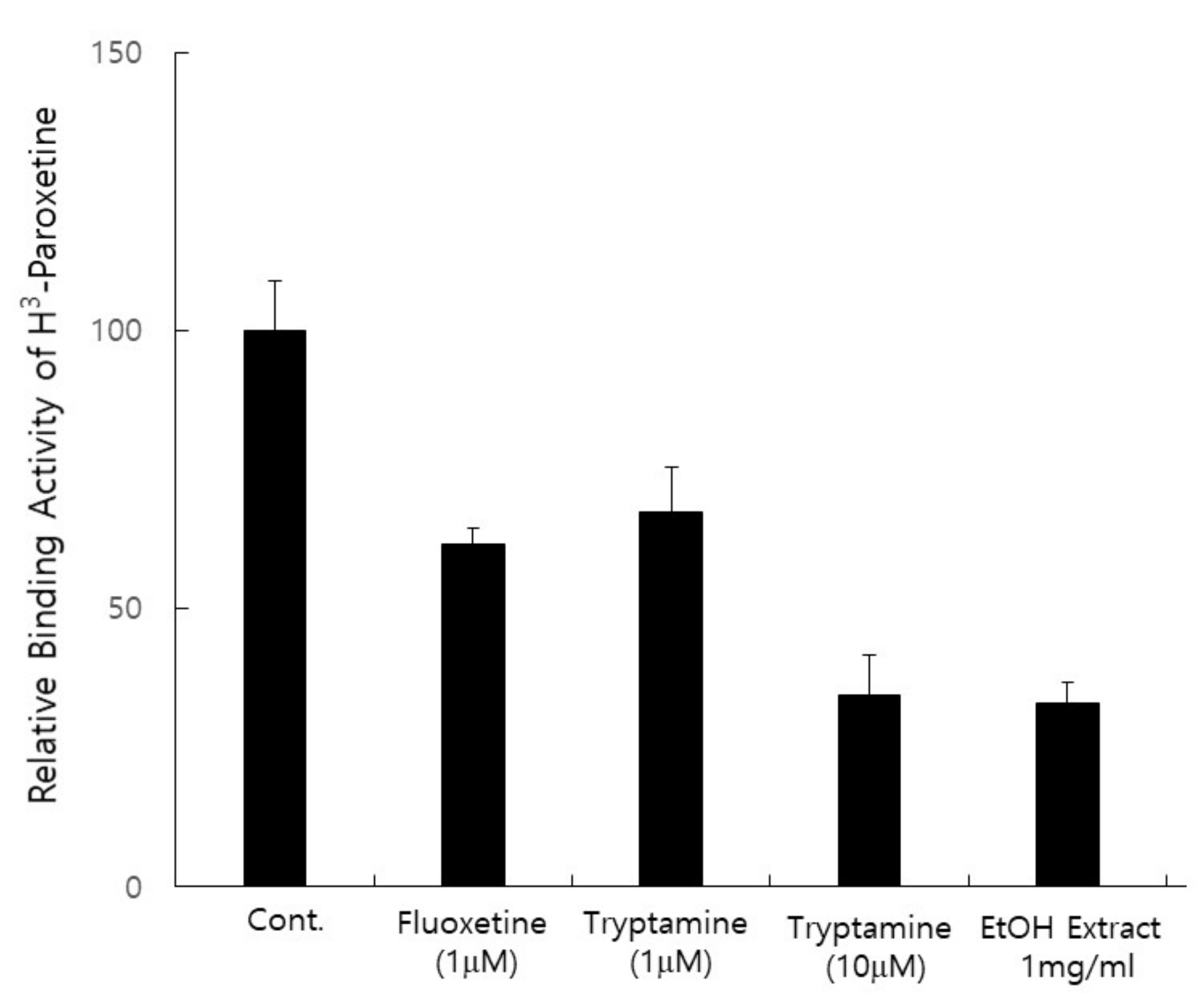
3.3. Extract of P. eryngii Has Antidepressant Activity
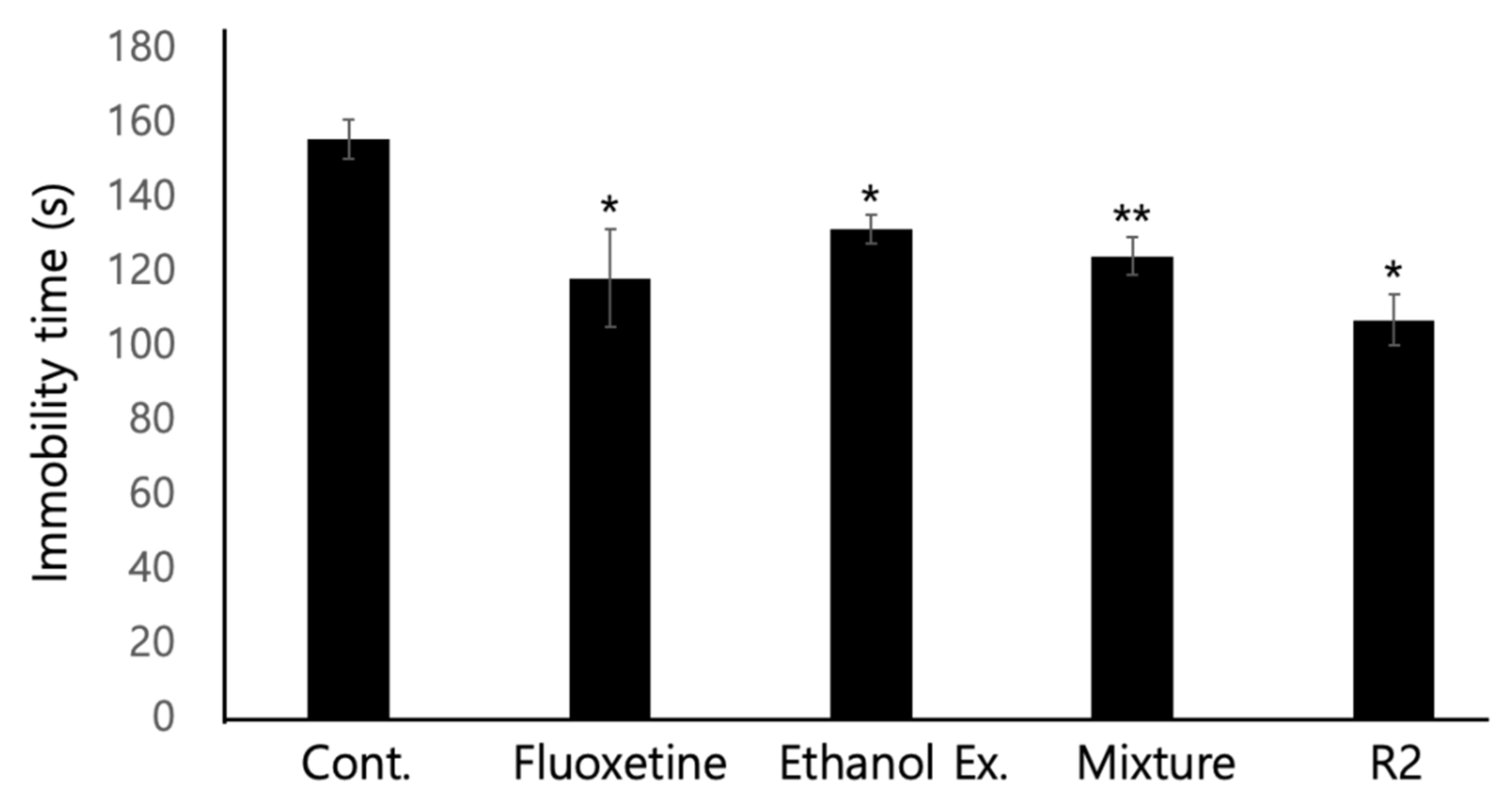
3.4. Forced Swimming Test (FST) Showed the SSRI Activity of the P. eryngii Extract
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davis, R.; Taylor, A.; Nally, R.; Benson, K.F.; Stamets, P.; Jensen, G.S. Differential Immune Activating, Anti-Inflammatory, and Regenerative Properties of the Aqueous, Ethanol, and Solid Fractions of a Medicinal Mushroom Blend. J. Inflamm. Res. 2020, 13, 117–131. [Google Scholar] [CrossRef]
- Kim, Y.H.; Jung, E.-G.; Han, K.-I.; Patnaik, B.B.; Kwon, H.-J.; Lee, H.-S.; Kim, W.J.; Han, M.-D. Immunomodulatory Effects of Extracellular b-Glucan Isolated from the King Oyster Mushroom Pleurotus eryngii (Agaricomycetes) and Its Sulfated Form on Signaling Molecules Involved in Innate Immunity. Int. J. Med. Mushrooms 2017, 19, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Rúbia, C.G.C.; Sandrina, A.H.; Maria, J.A.; Isabel, C.F.R.F. Bacterial Resistance: Antibiotics of Last Generation used in Clinical Practice and the Arise of Natural Products as New Therapeutic Alternatives. Curr. Pharm. Des. 2020, 26, 815–837. [Google Scholar]
- Pandya, U.; Dhuldhaj, U.; Sahay, N.S. Bioactive mushroom polysaccharides as antitumor: An overview. Nat. Prod. Res. 2019, 33, 2668–2680. [Google Scholar] [CrossRef] [PubMed]
- Geiger, H.A.; Wurst, M.G.; Daniels, R.N. DARK Classics in Chemical Neuroscience: Psilocybin. ACS Chem. Neurosci. 2018, 9, 2438–2447. [Google Scholar] [CrossRef]
- Griffiths, R.R.; Johnson, M.W.; Carducci, M.A.; Umbricht, A.; Richards, W.A.; Richards, B.D.; Cosimano, M.P.; Klinedinst, M.A. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized double-blind trial. J. Psychopharmacol. 2016, 30, 1181–1197. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Bolstridge, M.; Day, C.M.J.; Rucker, J.; Watts, R.; Erritzoe, D.E.; Kaelen, M.; Giribaldi, B.; Bloomfield, M.; Pilling, S.; et al. Psilocybin with psychological support for treatment-resistant depression: Six-month follow-up. Psychopharmacology 2018, 235, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.K.; Barrett, F.S.; May, D.G.; Cosimano, M.P.; Sepeda, N.D.; Johnson, M.W.; Finan, P.H.; Griffiths, R.R. Effects of Psilocybin-Assisted Therapy on Major Depressive Disorder: A Randomized Clinical Trial. JAMA Psychiatry 2020. [Google Scholar] [CrossRef] [PubMed]
- Gillman, P.K. Tricyclic antidepressant pharmacology and therapeutic drug interactions updated. Br. J. Pharmacol. 2007, 151, 737–748. [Google Scholar] [CrossRef]
- Malcomson, T.; Yelekci, K.; Borrello, M.T.; Ganesan, A.; Semina, E.; De Kimpe, N.; Mangelinckx, S.; Ramsay, R.R. cis-Cyclopropylamines as mechanism-based inhibitors of monoamine oxidases. FEBS J. 2015, 282, 3190–3198. [Google Scholar] [CrossRef]
- Lochmann, D.; Richardson, T. Selective Serotonin Reuptake Inhibitors. In Antidepressants: From Biogenic Amines to New Mechanisms of Action; Macaluso, M., Preskorn, S.H., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 135–144. [Google Scholar]
- Hancu, G.; Cârcu-Dobrin, M.; Budău, M.; Rusu, A. Analytical methodologies for the stereoselective determination of fluoxetine: An overview. Biomed. Chromatogr. 2018, 32, e4040. [Google Scholar] [CrossRef]
- Feighner, J.P.; Boyer, W.F. Selective Serotonin Re-uptake Inhibitors. Int. Clin. Psychopharmacol. 1991, 6, 126. [Google Scholar] [CrossRef]
- Stahl, S.M. Mechanism of action of serotonin selective reuptake inhibitors: Serotonin receptors and pathways mediate therapeutic effects and side effects. J. Affect. Disord. 1998, 51, 215–235. [Google Scholar] [CrossRef]
- Moore, R.T. Taxonomic Proposals for the Classification of Marine Yeasts and Other Yeast-like fungi including the Smuts [1980]. AGRIS 2013, 23, 361–373. [Google Scholar]
- Manzi, P.; Marconi, S.; Aguzzi, A.; Pizzoferrato, L. Commercial mushrooms: Nutritional quality and effect of cooking. Food Chem. 2004, 84, 201–206. [Google Scholar] [CrossRef]
- Manzi, P.; Gambelli, L.; Marconi, S.; Vivanti, V.; Pizzoferrato, L. Nutrients in edible mushrooms: An inter-species comparative study. Food Chem. 1999, 65, 477–482. [Google Scholar] [CrossRef]
- Baeza, E.; Dessay, C.; Wacrenier, N.; Marche, G.; Listrat, A. Effect of selection for improved body weight and composition on muscle and meat characteristics in Muscovy duck. Br. Poult. Sci. 2002, 43, 560–568. [Google Scholar] [CrossRef]
- Baeza, E.; Salichon, M.R.; Marche, G.; Wacrenier, N.; Dominguez, B.; Culioli, J. Effects of age and sex on the structural, chemical and technological characteristics of mule duck meat. Br. Poult. Sci. 2000, 41, 300–307. [Google Scholar] [CrossRef]
- Wang, H.; Ng, T.B. Pleureryn, a Novel Protease from Fresh Fruiting Bodies of the Edible Mushroom Pleurotus eryngii. Biochem. Biophys. Res. Commun. 2001, 289, 750–755. [Google Scholar] [CrossRef]
- Ng, T.B.; Wang, H.X. A novel ribonuclease from fruiting bodies of the common edible mushroom Pleurotus eryngii. Peptides 2004, 25, 1365–1368. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Kobayashi, C.; Tomita, T.; Inatomi, S.; Ikeda, M. Antiatherosclerotic effect of the edible mushrooms Pleurotus eryngii (Eringi), Grifola frondosa (Maitake), and Hypsizygus marmoreus (Bunashimeji) in apolipoprotein E–deficient mice. Nutr. Res. 2008, 28, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Ren, Z.; Zhang, J.; Song, X.; Gao, Z.; Jing, H.; Li, S.; Wang, S.; Jia, L. Antioxidant and anti-hyperlipidemic effects of mycelia zinc polysaccharides by Pleurotus eryngii var. tuoliensis. Int. J. Biol. Macromol. 2017, 95, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Krupodorova, T.; Rybalko, S.; Barshteyn, V. Antiviral activity of Basidiomycete mycelia against influenza type A (serotype H1N1) and herpes simplex virus type 2 in cell culture. Virol. Sin. 2014, 29, 284–290. [Google Scholar] [CrossRef]
- Wasser, S.P.; Weis, A.L. Medicinal Properties of Substances Occurring in Higher Basidiomycetes Mushrooms: Current Perspectives (Review). Int. J. Med. Mushrooms 1999, 1, 31–62. [Google Scholar] [CrossRef]
- Wang, H.; Ng, T.B. Isolation and characterization of velutin, a novel low-molecular-weight ribosome-inactivating protein from winter mushroom (Flammulina velutipes) fruiting bodies. Life Sci. 2001, 68, 2151–2158. [Google Scholar] [CrossRef]
- Wang, H.; Ng, T.B. Eryngin, a novel antifungal peptide from fruiting bodies of the edible mushroom Pleurotus eryngii. Peptides 2004, 25, 1–5. [Google Scholar] [CrossRef]
- Ngai, P.H.K.; Ng, T.B. A hemolysin from the mushroom Pleurotus eryngii. Appl. Microbiol. Biotechnol. 2006, 72, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.W.; Ferea, T.; Rashford, J.; Ardon, O.; Brown, P.O.; Botstein, D.; Kaplan, J.; Philpott, C.C. Desferrioxamine-mediated iron uptake in Saccharomyces cerevisiae-Evidence for two pathways of iron uptake. J. Biol. Chem. 2000, 275, 10709–10715. [Google Scholar] [CrossRef]
- Petit-Demouliere, B.; Chenu, F.; Bourin, M. Forced swimming test in mice: A review of antidepressant activity. Psychopharmacology 2005, 177, 245–255. [Google Scholar] [CrossRef]
- Parker, G.; Brotchie, H. Gender differences in depression. Int. Rev. Psychiatry 2010, 22, 429–436. [Google Scholar] [CrossRef]
- Ménard, C.; Hodes, G.E.; Russo, S.J. Pathogenesis of depression: Insights from human and rodent studies. Neuroscience 2016, 321, 138–162. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, J.; Plotsky, P.M.; Nemeroff, C.B.; Charney, D.S. Effects of early adverse experiences on brain structure and function: Clinical implications. Biol. Psychiatry 2000, 48, 778–790. [Google Scholar] [CrossRef]
- Ueda, N.; Yoshimura, R.; Shinkai, K.; Sakata, Y.; Nakamura, J. Higher Plasma 5-Hydroxyindoleacetic Acid Levels Are Associated with SSRI-Induced Nausea. Neuropsychobiology 2003, 48, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Yoshida, K.; Ito, K.; Sato, K.; Kamata, M.; Higuchi, H.; Shimizu, T.; Ito, K.; Inoue, K.; Tezuka, T.; et al. No association between the serotonergic polymorphisms and incidence of nausea induced by fluvoxamine treatment. Eur. Neuropsychopharmacol. 2002, 12, 477–481. [Google Scholar] [CrossRef]
- Brambilla, P.; Cipriani, A.; Hotopf, M.; Barbui, C.J.P. Side-effect profile of fluoxetine in comparison with other SSRIs, tricyclic and newer antidepressants: A meta-analysis of clinical trial data. Pharmacopsychiatry 2005, 38, 69–77. [Google Scholar] [CrossRef]
- Amminger, G.P.; Schäfer, M.R.; Papageorgiou, K.; Klier, C.M.; Cotton, S.M.; Harrigan, S.M.; Mackinnon, A.; McGorry, P.D.; Berger, G.E. Long-Chain ω-3 Fatty Acids for Indicated Prevention of Psychotic Disorders: A Randomized, Placebo-Controlled Trial. Arch. Gen. Psychiatry 2010, 67, 146–154. [Google Scholar] [CrossRef]
- Avalos, L.A.; Caan, B.; Nance, N.; Zhu, Y.; Li, D.-K.; Quesenberry, C.; Hyde, R.J.; Hedderson, M.M. Prenatal Depression and Diet Quality During Pregnancy. J. Acad. Nutr. Diet. 2020, 120, 972–984. [Google Scholar] [CrossRef]
- Muszyńska, B.; Łojewski, M.; Rojowski, J.; Opoka, W.; Sułkowska-Ziaja, K. Natural products of relevance in the prevention and supportive treatment of depression. Psychiatr. Polska 2015, 49, 435–453. [Google Scholar] [CrossRef]
- Matrisciano, F.; Pinna, G. PPAR and functional foods: Rationale for natural neurosteroid-based interventions for postpartum depression. Neurobiol. Stress 2020, 12, 100222. [Google Scholar] [CrossRef]
- Anjom-Shoae, J.; Sadeghi, O.; Keshteli, A.H.; Afshar, H.; Esmaillzadeh, A.; Adibi, P. Legume and nut consumption in relation to depression, anxiety and psychological distress in Iranian adults. Eur. J. Nutr. 2020, 59, 3635–3645. [Google Scholar] [CrossRef]
- Kargbo, R.B. Psilocybin Therapeutic Research: The Present and Future Paradigm. ACS Med. Chem. Lett. 2020, 11, 399–402. [Google Scholar] [CrossRef]
- Lenz, C.; Sherwood, A.; Kargbo, R.; Hoffmeister, D. Taking Different Roads: l-Tryptophan as the Origin of Psilocybe Natural Products. ChemPlusChem 2021, 86, 28–35. [Google Scholar] [CrossRef]
- Khan, M.Z.; Nawaz, W. The emerging roles of human trace amines and human trace amine-associated receptors (hTAARs) in central nervous system. Biomed. Pharmacother. 2016, 83, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Tronci, E.; Fidalgo, C.; Stancampiano, R.; Carta, M. Effect of selective and non-selective serotonin receptor activation on l-DOPA-induced therapeutic efficacy and dyskinesia in parkinsonian rats. Behav. Brain Res. 2015, 292, 300–304. [Google Scholar] [CrossRef]
- Blough, B.E.; Landavazo, A.; Partilla, J.S.; Decker, A.M.; Page, K.M.; Baumann, M.H.; Rothman, R.B. Alpha-ethyltryptamines as dual dopamine–serotonin releasers. Bioorganic Med. Chem. Lett. 2014, 24, 4754–4758. [Google Scholar] [CrossRef] [PubMed]
- Soria, V.; Martínez-Amorós, È.; Escaramís, G.; Valero, J.; Crespo, J.M.; Gutiérrez-Zotes, A.; Bayés, M.; Martorell, L.; Vilella, E.; Estivill, X.; et al. Resequencing and association analysis of arylalkylamine N-acetyltransferase (AANAT) gene and its contribution to major depression susceptibility. J. Pineal Res. 2010, 49, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Lemberger, L.; Axelrod, J.; Kopin, I.J. The Disposition and Metabolism of Tryptamine and the in vivo formation of 6-hydroxytryptamine in the rabbit. J. Pharmacol. Exp. Ther. 1971, 177, 169. [Google Scholar] [PubMed]
- Fricke, J.; Blei, F.; Hoffmeister, D. Enzymatic synthesis of psilocybin. Angew. Chem. Int. Ed. 2017, 56, 12352–12355. [Google Scholar] [CrossRef]
- Hartmann, A.; Singh, M.; Klingmüller, W. Isolation and characterization of Azospirillum mutants excreting high amounts of indoleacetic acid. Can. J. Microbiol. 1983, 29, 916–923. [Google Scholar] [CrossRef]
- Spaepen, S.; Vanderleyden, J.; Remans, R. Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol. Rev. 2007, 31, 425–448. [Google Scholar] [CrossRef]
- Qu, Y.; Safonova, O.; De Luca, V. Completion of the canonical pathway for assembly of anticancer drugs vincristine/vinblastine in Catharanthus roseus. Plant J. 2019, 97, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, Y.; Williams, B.B.; Battaglioli, E.J.; Whitaker, W.R.; Till, L.; Grover, M.; Linden, D.R.; Akiba, Y.; Kandimalla, K.K.; Zachos, N.C.; et al. Gut microbiota-produced tryptamine activates an epithelial G-protein-coupled receptor to increase colonic secretion. Cell Host Microbe 2018, 23, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Cameron, C.; Kelly, S.; Hsieh, S.-C.; Murphy, M.; Chen, L.; Kotb, A.; Peterson, J.; Coyle, D.; Skidmore, B.; Gomes, T.; et al. Triptans in the acute treatment of migraine: A systematic review and network meta-analysis. Headache J. Head Face Pain 2015, 55, 221–235. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, Y.-S.; Jang, S.; Lee, H.; Kang, S.; Seo, H.; Yeon, S.; Lee, D.; Yun, C.-W. Identification of the Antidepressant Function of the Edible Mushroom Pleurotus eryngii. J. Fungi 2021, 7, 190. https://doi.org/10.3390/jof7030190
Park Y-S, Jang S, Lee H, Kang S, Seo H, Yeon S, Lee D, Yun C-W. Identification of the Antidepressant Function of the Edible Mushroom Pleurotus eryngii. Journal of Fungi. 2021; 7(3):190. https://doi.org/10.3390/jof7030190
Chicago/Turabian StylePark, Yong-Sung, Subin Jang, Hyunkoo Lee, Suzie Kang, Hyewon Seo, Seoyeong Yeon, Dongho Lee, and Cheol-Won Yun. 2021. "Identification of the Antidepressant Function of the Edible Mushroom Pleurotus eryngii" Journal of Fungi 7, no. 3: 190. https://doi.org/10.3390/jof7030190
APA StylePark, Y.-S., Jang, S., Lee, H., Kang, S., Seo, H., Yeon, S., Lee, D., & Yun, C.-W. (2021). Identification of the Antidepressant Function of the Edible Mushroom Pleurotus eryngii. Journal of Fungi, 7(3), 190. https://doi.org/10.3390/jof7030190





