Proteomics of Two Thermotolerant Isolates of Trichoderma under High-Temperature Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains
2.2. Growth Conditions
2.3. Protein Extraction
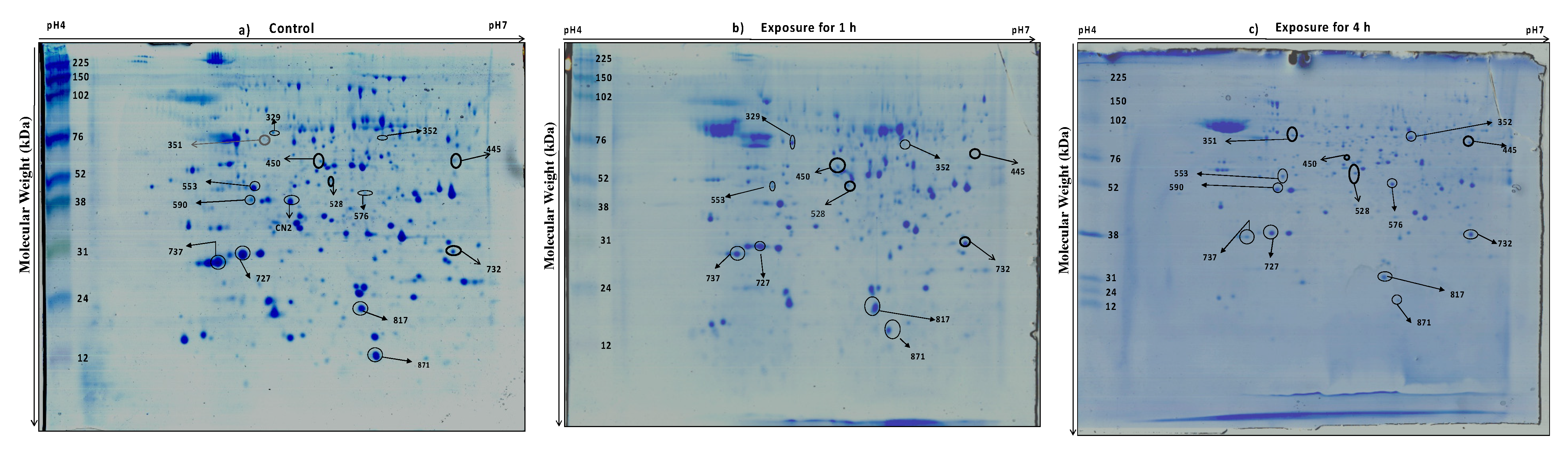
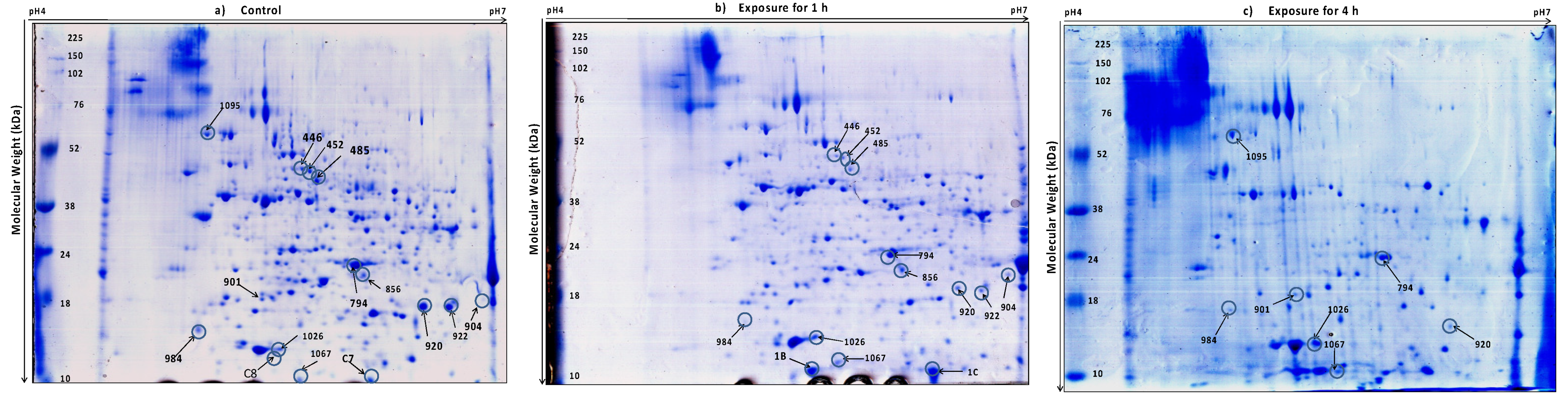
2.4. Two-Dimensional Electrophoresis (2DE) and Image Analysis
2.5. Mass Spectrometry and Protein Identification
3. Results and Discussion
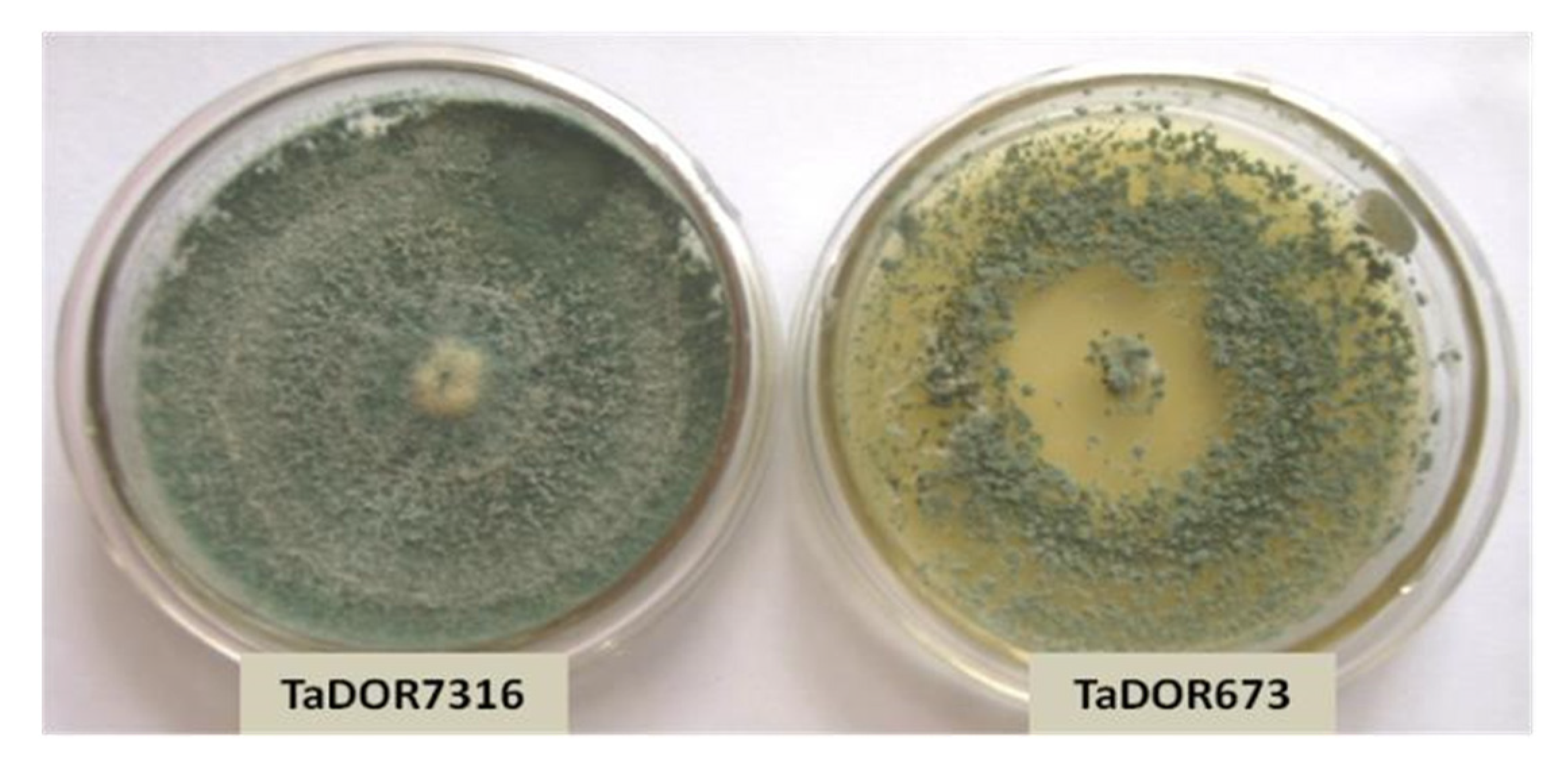
3.1. Morphological Differences of Thermotolerant Isolates
3.2. Protein Profiling of Thermotolerant Isolates
3.3. Differentially Expressed Proteins Common in the Two Trichoderma Strains
3.3.1. Proteins of Cell Wall Remodeling
3.3.2. Proteins of Carbohydrate Metabolism
3.3.3. Proteins of the Heat Shock Response (HSR) Pathway
3.3.4. Proteins of the Cell Signaling Pathway
3.3.5. mRNA Stability
3.4. Proteins Unique to TaDOR673
3.5. Proteins Unique to TaDOR7316
3.5.1. Unfolded Protein Response (UPR) and Protein Turnover
3.5.2. Vacuole Biogenesis and Autophagy
3.6. Other Proteins
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verma, M.; Brar, S.; Tyagi, R.; Surampalli, R.; Valero, J. Antagonistic fungi, Trichoderma spp.: Panoply of biological control. Biochem. Eng. J. 2007, 37, 1–20. [Google Scholar] [CrossRef]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Marra, R.; Woo, S.; Lorito, M. Trichoderma-plant-pathogen interatctions. Soil Biol. Biochem. 2008, 40, 1–10. [Google Scholar] [CrossRef]
- Benitez, T.; Rincon, A.M.; Limon, M.C.; Codon, A.C. Biocontrol mechanisms of Trichoderma strains. Int. Microbiol. 2004, 7, 249–260. [Google Scholar] [PubMed]
- Elad, Y. Biological control of foliar pathogens by means of Trichoderma harzianum and potential modes of action. Crop. Prot. 2000, 19, 709–714. [Google Scholar] [CrossRef]
- Zin, N.A.; Badaluddin, N.A. Biological functions of Trichoderma spp. for agriculture applications. Ann. Agric. Sci. 2020, 65, 68–178. [Google Scholar] [CrossRef]
- Hermosa, R.; Viterbo, A.; Chet, I.; Monte, E. Plant-beneficial effects of Trichoderma and of its genes. Microbiology 2012, 158, 17–25. [Google Scholar] [CrossRef]
- Luard, E.J.; Griffin, D.M. Effect of water potential on fungal growth and turgor. Trans. Br. Mycol. Soc. 1981, 76, 33–40. [Google Scholar] [CrossRef]
- Magan, N. Effects of water potential and temperature on spore germination and germ-tube growth in vitro and on straw leaf sheaths. Trans. Br. Mycol. Soc. 1988, 90, 97–107. [Google Scholar] [CrossRef]
- Knudsen, G.; Bin, L. Effects of temperature, soil moisture, and wheat bran on growth of Trichoderma harzianum from alginate pellets. Am. Phytopathol. Soc. 1990, 80, 724–727. [Google Scholar] [CrossRef]
- Katan, J. Solar heating (solarization) of soil for control of soil-borne pests. Ann. Rev. Phytopathol. 1981, 19, 211–236. [Google Scholar] [CrossRef]
- Tjamos, E.C.; Fravel, D.R. Detrimental effects of sublethal heating and Talaromyces flavus on microsclerotia of Verticillium dahliae. Phytopathology 1995, 85, 388–392. [Google Scholar] [CrossRef]
- Tjamos, E.C.; Paplomatas, E.J. Long-term effect of soil solarization on Verticillium wilt of artichokes in Greece. Can. J. Plant. Pathol. 1987, 9, 87. [Google Scholar]
- Kumar, G.P.; Ahmed, S.K.M.H.; Desai, S.; Amalraj, E.L.D.; Rasul, A. In vitro screening for abiotic stress tolerance in potent biocontrol and plant growth promoting strains of Pseudomonas and Bacillus spp. Int. J. Bacteriol. 2014, 2014, 195946. [Google Scholar]
- Alali, S.; Mereghetti, V.; Faoro, F.; Bocchi, S.; Al Azmeh, F.; Montagna, M. Thermotolerant isolates of Beauveria bassiana as potential control agent of insect pest in subtropical climates. PLoS ONE 2019, 14, e0211457. [Google Scholar] [CrossRef]
- Poosapati, S.; Ravulapalli, P.D.; Tippirishetty, N.; Vishwanathaswamy, D.K.; Chunduri, S. Selection of high temperature and salinity tolerant Trichoderma isolates with antagonistic activity against Sclerotium rolfsii. SpringerPlus 2014, 3, 641. [Google Scholar] [CrossRef]
- Shui, W.; Xiong, Y.; Xiao, W.; Qi, X.; Zhang, Y.; Lin, Y.; Guo, Y.; Zhang, Z.; Wang, Q.; Ma, Y. Understanding the mechanism of thermotolerance distinct from heat shock response through proteomic analysis of industrial strains of Saccharomyces cerevisiae. Mol. Cell Proteom. 2015, 14, 1885–1897. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.X.; Zhou, X.Z.; Meng, H.M.; Liu, Y.J.; Zhou, Q.; Huang, B. Comparative transcriptomic analysis of the heat stress response in the filamentous fungus Metarhizium anisopliae using RNA-Seq. Appl. Microbiol. Biotechnol. 2014, 98, 5589–5597. [Google Scholar] [CrossRef]
- Verghese, J.; Abrams, J.; Wang, Y.; Morano, K.A. Biology of the heat shock response and protein chaperones: Budding yeast (Saccharomyces cerevisiae) as a model system. Microbiol. Mol. Biol. Rev. 2012, 76, 115–158. [Google Scholar] [CrossRef]
- Tereshina, V.M. Thermotolerance in fungi: The role of heat shock proteins and trehalose. Microbiology 2005, 74, 247–257. [Google Scholar] [CrossRef]
- Montero-Barrientos, M.; Cardoza, R.E.; Gutiérrez, S.; Monte, E.; Hermosa, R. The heterologous overexpression of hsp23, a small heat-shock protein gene from Trichoderma virens, confers thermotolerance to T. harzianum. Curr. Genet. 2007, 52, 45–53. [Google Scholar] [CrossRef]
- Montero-Barrientos, M.; Hermosa, R.; Nicolas, C.; Cardoza, R.E.; Gutlerrez, S.; Monte, E. Over expression of a Trichoderma HSP70 gene increases fungal resistance to heat and other abiotic stresses. Fungal Genet. Biol. 2008, 45, 1506–1513. [Google Scholar] [CrossRef]
- Nicolas, C.; Hermosa, R.; Rubio, B.; Mukherjee, P.K.; Monte, E. Trichoderma genes in plants for stress tolerance- status and prospects. Plant. Sci. 2014, 228, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Jarana, J.; Sousa, S.; Gonza´lez, F.; Rey, M.; Llobell, A. ThHog1 controls the hyperosmotic stress response in Trichoderma harzianum. Microbiology 2006, 152, 1687–1700. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, M.T.; Khalawan, S.A.; Curran, B.P. Cellular lipid composition influences stress activation of the yeast general stress response element (STRE). Microbiology 2000, 146, 877–884. [Google Scholar] [CrossRef]
- Ram, A.F.; Arentshorst, M.; Damveld, R.A.; VanKuyk, P.A.; Klis, F.M.; den Hondel, C.A.V. The cell wall stress response in Aspergillus niger involves increased expression of the glutamine: Fructose-6-phosphate amidotransferase-encoding gene (gfaA) and increased deposition of chitin in the cell wall. Microbiology 2004, 150, 3315–3326. [Google Scholar] [CrossRef]
- Chen, S.; Tarsio, M.; Kane, P.M.; Greenberg, M.L. Cardiolipin mediates cross-talk between mitochondria and the vacuole. Mol. Biol. Cell 2008, 19, 5047–5058. [Google Scholar] [CrossRef]
- Konopka, J.B. N-acetylglucosamine (GlcNAc) functions in cell signaling. Scientifica 2012, 2012, 489208. [Google Scholar] [CrossRef]
- Yang, P.; Du, H.; Hoffman, C.S.; Marcus, S. The phospholipase B homolog Plb1 is a mediator of osmotic stress response and of nutrient-dependent repression of sexual differentiation in the fission yeast Schizosaccharomyces pombe. Mol. Genet. Genom. 2003, 269, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, N.; Latge, J.P.; Calderone, R. Signalling and oxidant adaptation in Candida albicans and Aspergillus fumigatus. Nat. Rev. Microbiol. 2006, 4, 435–444. [Google Scholar] [CrossRef]
- Chauhan, N.; Calderone, R. Two-component signal transduction proteins as potential drug targets in medically important fungi. Infect. Immun. 2008, 76, 4795–4803. [Google Scholar] [CrossRef]
- Colabardini, A.C.; Ries, L.N.A.; Brown, N.A.; Savoldi, M.; Dinamarco, T.M.; von Zeska, M.R.; Goldman, M.H.S.; Goldman, G.H. Protein Kinase C overexpression suppresses calcineurin-associated defects in Aspergillus nidulans and is involved in mitochondrial function. PLoS ONE 2014, 9, e104792. [Google Scholar] [CrossRef] [PubMed]
- Abrams, J.L.; Verghese, J.; Gibney, P.A.; Morano, K.A. Hierarchical functional specificity of cytosolic heat shock protein 70 (Hsp70) nucleotide exchange factors in yeast. J. Biol. Chem. 2014, 289, 13155–13167. [Google Scholar] [CrossRef] [PubMed]
- Gowda, N.K.; Kandasamy, G.; Froehlich, M.S.; Dohmen, R.J.; Andreasson, C. Hsp70 nucleotide exchange factor Fes1 is essential for ubiquitin-dependent degradation of misfolded cytosolic proteins. Proc. Natl. Acad. Sci. USA 2013, 110, 5975–5980. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Nandakumar, M.P.; Marten, M.R. Proteome map of Aspergillus nidulans during osmoadaptation. Fungal Genet. Biol. 2007, 44, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Stead, D.; Walker, J.; Selway, L.; Smith, D.A.; Brown, A.J.P.; Quinn, J. A proteomic analysis of the salt, cadmium and peroxide stress responses in Candida albicans and the role of the Hog1 stress-activated MAPK in regulating the stress-induced proteome. Proteomics 2009, 9, 4686–4703. [Google Scholar] [CrossRef]
- Imazu, H.; Sakurai, H. Saccharomyces cerevisiae heat shock transcription factor regulates cell wall remodeling in response to heat shock. Eukaryot. Cell 2005, 4, 1050–1056. [Google Scholar] [CrossRef]
- Mota, T.M.; Oshiquiri, L.H.; Lopes, É.C.V.; Barbosa, F.J.R.; Ulhoa, C.J.; Georg, R.C. Hsp genes are differentially expressed during Trichoderma asperellum self-recognition, mycoparasitism and thermal stress. Microbiol. Res. 2019, 227, 126296. [Google Scholar] [CrossRef]
- Dixit, P.; Mukherjee, P.K.; Ramachandran, V.; Eapen, S. Glutathione transferase from Trichoderma virens enhances cadmium tolerance without enhancing its accumulation in transgenic Nicotiana tobacum. PLoS ONE 2011, 6, e16360. [Google Scholar] [CrossRef]
- Hermosa, R.; Botella, L.; Keck, M.; Jimenez, J.A.; Montero Barrientos, M.; Arbona, V.; Gomez Cadenas, A.; Monte, E.; Nicolas, C. The over expression in Arabidopsis thaliana of a Trichoderma harzianum gene that modulates glucosidase activity, and enhances tolerance to salt and osmotic stresses. J. Plant. Physiol. 2011, 168, 1295–1302. [Google Scholar] [CrossRef]
- Kusch, H.; Engelmann, S.; Albrecht, D.; Morschhauser, J.; Hecker, M. Proteomic analysis of the oxidative stress response in Candida albicans. Proteomics 2007, 7, 666–697. [Google Scholar] [CrossRef]
- Liu, Y.; Solis, N.V.; Heilmann, C.J.; Phan, Q.T.; Mitchell, A.P.; Klis, F.M.; Filler, S.G. Role of retrograde trafficking in stress response, host cell interactions, and virulence of Candida albicans. Eukaryot. Cell 2014, 13, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, P.; Walker, J.; Selway, L.; Stead, D.; Yin, Z.; Enjalbert, B.; Weig, M.; Brown, A.J.P. Proteomic analysis of the pH response in the fungal pathogen Candida glabrata. Proteomics 2008, 8, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Rey, M.; Llobell, A.; Monte, E.; Scala, F.; Lorito, M. Genomics of Trichoderma. In Applied Mycology and Biotechnology, Fungal Genomics; Khachatourians, G.G., Arora, D.K., Eds.; Elsevier BV: Amsterdam, The Netherlands, 2004; Volume 4, pp. 225–248. [Google Scholar]
- Vizcaíno, J.A.; González, F.J.; Suárez, M.B.; Redondo, J.; Heinrich, J.; Delgado-Jarana, J.; Hermosa, R.; Gutiérrez, S.; Monte, E.; Llobell, A.; et al. Generation, annotation and analysis of ESTs from Trichoderma harzianum CECT 2413. BMC Genom. 2006, 7, 193. [Google Scholar] [CrossRef] [PubMed]
- Alfiky, A.; Weisskopf, L. Deciphering Trichoderma-Plant-Pathogen Interactions for Better Development of Biocontrol Applications. J. Fungi 2021, 7, 61. [Google Scholar] [CrossRef]
- Druzhinina, I.S.; Seidl-Seiboth, V.; Herrera-Estrella, A.; Horwitz, B.A.; Kenerley, C.M.; Monte, E.; Mukherjee, P.K.; Zeilinger, S.; Grigoriev, I.V.; Kubicek, C.P. Trichoderma: The genomics of opportunistic success. Nat. Rev. Microbiol. 2011, 9, 749–759. [Google Scholar] [CrossRef]
- Sharma, P.; Vignesh Kumar, P.; Ramesh, R.; Saravanan, K.; Deep, S.; Sharma, M.; Mahesh, S.; Dinesh, S. Biocontrol genes from Trichoderma species: A review. Afr. J. Biotechnol. 2011, 10, 19898–19907. [Google Scholar]
- Srivastava, M.; Shahid, M.; Pandey, S.; Singh, A.; Kumar, V.; Gupta, S.; Maurya, M. Trichoderma Genome to Genomics: A Review. J. Data Min. Genom. Proteom. 2014, 5, 162. [Google Scholar] [CrossRef]
- Atanasova, L.; Crom, S.L.; Gruber, S.; Coulpier, F.; Seidl-Seiboth, V.; Kubicek, C.P.; Druzhinina, I.S. Comparative transcriptomics reveals different strategies of Trichoderma mycoparasitism. BMC Genom. 2013, 14, 121. [Google Scholar] [CrossRef]
- Rawlings, N.D.; Barrett, A.J.; Bateman, A. MEROPS: The database of proteolytic enzymes, their substrates and inhibitors. Nucleic Acids Res. 2012, 40, D343–D350. [Google Scholar] [CrossRef]
- Suarez, M.B.; Vizcaino, J.A.; Llobell, A.; Monte, E. Characterization of genes encoding novel peptidases in the biocontrol fungus Trichoderma harzianum CECT 2413 using the TrichoEST functional genomics approach. Curr. Genet. 2007, 51, 331–342. [Google Scholar] [CrossRef]
- Poosapati, S.; Ravulapalli, P.D.; Tippirishetty, N. Morphological and biochemical characterization of thermotolerant Trichoderma. Int. J. Curr. Res. 2016, 8, 38668–38672. [Google Scholar]
- Jacobs, D.I.; van Rijssen, M.S.; van der Heijden, R.; Verpoorte, R. Sequential solubilization of proteins precipitated with trichloroacetic acid in acetone from cultured Catharanthus roseus cell yields 52% more spots after two-dimensional electrophoresis. Proteomics 2001, 1, 1345–1350. [Google Scholar] [CrossRef]
- Prieto, A.; Leal, J.A.; Poveda, A.; Jiménez-Barbero, J.; Gómez-Miranda, B.; Domenech, J.; Ahrazem, O.; Bernabé, M. Structure of complex cell wall polysaccharides isolated from Trichoderma and Hypocrea species. Carbohydr. Res. 1997, 304, 281–291. [Google Scholar] [CrossRef]
- Perlińska-Lenart, U.; Orłowski, J.; Laudy, A.E.; Zdebska, E.; Palamarczyk, G.; Kruszewska, J.S. Glycoprotein hypersecretion alters the cell wall in Trichoderma reesei strains expressing the Saccharomyces cerevisiae dolichylphosphate mannose synthase gene. Appl. Environ. Microbiol. 2006, 72, 7778–7784. [Google Scholar] [CrossRef][Green Version]
- De Nobel, J.G.; van den Ende, H.; Klis, F.M. Cell wall maintenance in fungi. Trends Microbiol. 2000, 8, 344–345. [Google Scholar] [CrossRef]
- Georg, R.C.; Gomes, S.L. Transcriptome analysis in response to heat shock and cadmium in the aquatic fungus Blastocladiella emersonii. Eukaryot. Cell 2007, 6, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, S.A.; Naseem, S.; Konopka, J.B.; Sil, A. N-acetylglucosamine (GlcNAc) triggers a rapid, temperature-responsive morphogenetic program in thermally dimorphic fungi. PLoS Genet. 2013, 9, e1003799. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Henquet, M.; Chen, Z.; Zhang, H.; Zhang, Y.; Ren, X.; van der Krol, S.; Gonneau, M.; Bosch, D.; Gong, Z. LEW3, encoding a putative alpha-1, 2-mannosyltransferase (ALG11) in N-linked glycoprotein, plays vital roles in cell-wall biosynthesis and the abiotic stress response in Arabidopsis thaliana. Plant. J. 2009, 60, 983–999. [Google Scholar] [CrossRef]
- Damveld, R.A.; Franken, A.; Arentshorst, M.; Punt, P.J.; Klis, F.M.; van den Hondel, C.A.M.J.J.; Ram, A.F.J. A novel screening method for cell wall mutants in Aspergillus niger identifies UDP-galactopyranose mutase as an important protein in fungal cell wall biosynthesis. Genetics 2008, 178, 873–881. [Google Scholar] [CrossRef]
- Zaccheo, O.; Dinsdale, D.; Meacock, P.A.; Glynn, P. Neuropathy target esterase and its yeast homolog degrade phosphatidylcholine to glycerophosphocholine in living cells. J. Biol. Chem. 2004, 279, 24024–24033. [Google Scholar] [CrossRef]
- Romantsov, T.; Guan, Z.; Wood, J.M. Cardiolipin and the osmotic stress responses of bacteria. Biochim. Biophys. Acta 2009, 1788, 2092–2100. [Google Scholar] [CrossRef]
- Feofilova, E.P.; Tereshina, V.M.; Khokhlova, N.S.; Memorskaya, A.S. Different mechanisms of the biochemical adaptation of mycelial fungi to temperature stress: Changes in the cytosol carbohydrate composition. Microbiology 2000, 69, 504–508. [Google Scholar] [CrossRef]
- Grossklaus, D.D.; Bailao, A.M.; Rezende, T.C.V.; Borges, C.L.; de Oliveira, M.A.P.; Parente, J.A.; Soares, C.M.D. Response to oxidative stress in Paracoccidioides yeast cells as determined by proteomic analysis. Microbes Infect. 2013, 15, 347–364. [Google Scholar] [CrossRef]
- Barelle, C.J.; Priest, C.L.; MacCallum, D.M.; Gow, N.A.; Odds, F.C.; Brown, A.J. Niche-specific regulation of central metabolic pathways in a fungal pathogen. Cell Microbiol. 2006, 8, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.; Hains, P.; Walsh, B.; Bergquist, P.; Nevalainen, H. Proteins associated with the cell envelope of Trichoderma reesei: A proteomic approach. Proteomics 2001, 1, 899–909. [Google Scholar] [CrossRef]
- Yamamoto, A.; Mizukami, Y.; Sakurai, H. Identification of a novel class of target genes and a novel type of binding sequence of heat shock transcription factor in Saccharomyces cerevisiae. J. Biol. Chem. 2005, 280, 11911–11919. [Google Scholar] [CrossRef] [PubMed]
- Desai, C.; Mavrianos, J.; Chauhan, N. Candida albicans SRR1, a putative two-component response regulator gene, is required for stress adaptation, morphogenesis, and virulence. Eukaryot. Cell 2011, 10, 1370–1374. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Perrino, B.A.; Soderling, T.R. Identification of an autoinhibitory domain in calcineurin. J. Biol. Chem. 1990, 265, 1924–1927. [Google Scholar] [CrossRef]
- Vinnemeier, J.; Hagemann, M. Identification of salt regulated genes in the genome of the cyanobacterium Synechocystis sp. strain PCC 6803 by subtractive RNA hybridization. Arch. Microbiol. 1999, 172, 377–386. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; He, Z.; Alm, E.J.; Arkin, A.P.; Baidoo, E.E.; Borglin, S.C.; Chen, W.; Hazen, T.C.; He, Q.; Holman, H.Y.; et al. Salt stress in Desulfovibrio vulgaris Hildenborough: An integrated genomics approach. J. Bacteriol. 2006, 188, 4068–4078. [Google Scholar] [CrossRef]
- Briolat, V.; Reysset, G. Identification of the Clostridium perfringens genes involved in the adaptive response to oxidative stress. J. Bacteriol. 2002, 184, 2333–2343. [Google Scholar] [CrossRef] [PubMed]
- Rocak, S.; Linder, P. DEAD-box proteins: The driving forces behind RNA metabolism. Nat. Rev. Mol. Cell Biol. 2004, 5, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Owttrim, G.W. RNA helicases and abiotic stress. Nucleic Acids Res. 2006, 34, 3220–3230. [Google Scholar] [CrossRef] [PubMed]
- Ambrus, A.M.; Frolov, M.V. The diverse roles of RNA helicases in RNAi. Cell Cycle 2009, 8, 3500–3505. [Google Scholar] [CrossRef]
- Linder, P.; Owttrim, G.W. Plant RNA helicases: Linking aberrant and silencing RNA. Trends Plant. Sci. 2009, 14, 344–352. [Google Scholar] [CrossRef]
- Jiao, X.; Xiang, S.; Oh, C.; Martin, C.E.; Tong, L.; Kiledjian, M. Identification of a quality control mechanism for mRNA 5′-end capping. Nature 2010, 467, 608–611. [Google Scholar] [CrossRef]
- Malerba, M.; Crosti, P.; Cerana, R. Effect of heat stress on actin cytoskeleton and endoplasmic reticulum of tobacco BY-2 cultured cells and its inhibition by Co2+. Protoplasma 2009, 239, 23–30. [Google Scholar] [CrossRef]
- Yang, J.; Everett, A.D. Hepatoma-derived growth factor binds DNA through the N-terminal PWWP domain. BMC Mol. Biol. 2007, 8, 101. [Google Scholar] [CrossRef]
- Hahn, J.S.; Hu, Z.; Thiele, D.J.; Iyer, V.R. Genome-wide analysis of the biology of stress responses through heat shock transcription factor. Mol. Cell Biol. 2004, 24, 5249–5256. [Google Scholar] [CrossRef]
- Pusztahelyi, T.; Klement, E.; Szajli, E.; Klem, J.; Miskei, M.; Karányi, Z.; Emri, T.; Kovács, S.; Orosz, G.; Kovács, K.L.; et al. Comparison of transcriptional and translational changes caused by long-term menadione exposure in Aspergillus nidulans. Fungal Genet. Biol. 2011, 48, 92–103. [Google Scholar] [CrossRef][Green Version]
- Druzhinina, I.S.; Shelest, E.; Kubicek, C.P. Novel traits of Trichoderma predicted through the analysis of its secretome. FEMS Microbiol. Lett. 2012, 337, 1–9. [Google Scholar] [CrossRef][Green Version]
- Hilt, W. Targets of programmed destruction: A primer to regulatory proteolysis in yeast. Cell Mol. Life Sci. 2004, 61, 1615–1632. [Google Scholar] [CrossRef]
- Mori, K.; Kawahara, T.; Yoshida, H.; Yanagi, H.; Yura, T. Signalling from endoplasmic reticulum to nucleus: Transcription factor with a basic-leucine zipper motif is required for the unfolded protein-response pathway. Genes Cells 1996, 1, 803–817. [Google Scholar] [CrossRef] [PubMed]
- Spear, E.D.; Ng, D.T.W. Stress tolerance of misfolded Carboxypeptidase Y requires maintenance of protein trafficking and degradative pathways. Mol. Biol. Cell 2003, 14, 2756–2767. [Google Scholar] [CrossRef]
- Yen, W.; Shintani, T.; Nair, U.; Cao, Y.; Richardson, B.C.; Li, Z.; Hughson, F.M.; Baba, M.; Klionsky, D.J. The conserved oligomeric Golgi complex is involved in double-membrane vesicle formation during autophagy. J. Cell Biol. 2010, 188, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Whyte, J.R.; Munro, S. The Sec34/35 Golgi transport complex is related to the exocyst, defining a family of complexes involved in multiple steps of membrane traffic. Dev. Cell 2001, 1, 527–537. [Google Scholar] [CrossRef]
- Ram, R.J.; Li, B.; Kaiser, C.A. Identification of Sec36p, Sec37p, and Sec38p: Components of yeast complex that contains Sec34p and Sec35p. Mol. Biol. Cell 2002, 13, 1484–1500. [Google Scholar] [CrossRef] [PubMed]
- Bruinsma, P.; Spelbrink, R.G.; Nothwehr, S.F. Retrograde transport of the mannosyltransferase Och1p to the early Golgi requires a component of the COG transport complex. J. Biol. Chem. 2004, 279, 39814–39823. [Google Scholar] [CrossRef]
- Oka, T.; Ungar, D.; Hughson, F.M.; Krieger, M. The COG and COPI complexes interact to control the abundance of GEARs, a subset of Golgi integral membrane proteins. Mol. Biol. Cell 2004, 15, 2423–2435. [Google Scholar] [CrossRef]
- Zolov, S.N.; Lupashin, V.V. Cog3p depletion blocks vesicle-mediated Golgi retrograde trafficking in HeLa cells. J. Cell Biol 2005, 168, 747–759. [Google Scholar] [CrossRef]
- Marcusson, E.G.; Horazdovsky, B.F.; Cereghino, J.L.; Gharakhanian, E.; Emr, S.D. The sorting receptor for yeast vacuolar carboxypeptidase Y is encoded by the VPS10 gene. Cell 1994, 77, 579–586. [Google Scholar] [CrossRef]
- Boreham, D.R.; Trivedi, A.; Weinberger, P.; Mitchel, R.E. The involvement of topoisomerases and DNA polymerase I in the mechanism of induced thermal and radiation resistance in yeast. Radiat. Res. 1990, 123, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Perumal, S.S.; Shanthi, P.; Sachdanandam, P. Augmented efficacy of tamoxifen in rat breast tumorigenesis when gavaged along with riboflavin, niacin, and CoQ10: Effect on lipid peroxidation and antioxidants in mitochondria. Chem. Biol. Interact. 2005, 152, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, M. Effects of vitamins on chromium (VI)-induced damage. Environ. Health Perspec. 1991, 92, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, B.A.; Gressel, J. Elevated riboflavin requirement for post photoinductive events in sporulation of a Trichoderma auxotroph. Plant. Physiol. 1983, 71, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, S.; Chandra, T.S. Effect of vitamin E and MD supplementation on riboflavin production and stress parameters in Ashbya gossypii. Proc. Chem. 2009, 44, 934–938. [Google Scholar]
- Khan, M.R.; Parveen, G.; Zaid, A.; Wani, S.H.; Jogaiah, S. 5-Potential of Trichoderma species in alleviating the adverse effects of biotic and abiotic stresses in plants. In Biocontrol Agents and Secondary Metabolites; Woodhead Publishing: Sawston, UK, 2021; pp. 85–112. [Google Scholar]

| Expression Pattern | Spot No a | Protein Identity b | Peptide Sequences Matched | Sequence ID c | Score d | Observed Mr (kDa) on the Gel | Theoretical Mr(kDa)/pI e | % Sequence Coverage f | Protein Fold Change g | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 h | 4 h | |||||||||
| Downregulated in 1 h and 4 h compared to control | 871 | heat shock protein |
| gi|294957635| | 35 | 12 | 8.5/9.4 | 43 | −4.7 | −16.48 |
| 727 | Hsp70 nucleotide exchange factor | LLQQLFGGGPDEPALMR | FES1_ASHGO | 12 | 31 | 32.06/5.07 | 5 | −1.07 | −1.5 | |
| 737 | ATP-dependent RNA helicase DBP7 | TLAYLLPIVNR | DBP7_MAGO7 | 20 | 30 | 89.4/9.23 | 1 | −2.0 | −3.9 | |
| 553 | Protein AIM7(Altered inheritance rate of mitochondria protein 7) | MLYAGALEMIR | AIM7_YEAST | 26 | 50 | 17.2/4.48 | 7 | −4.0 | −1.6 | |
| Unique to control and absent in treated samples | CN2 | Probable FAD synthase | LSNIPFVFVRPR | FAD1_SCHPO | 18 | 38 | 30.8/7.62 | 4 | nd | nd |
| Upregulated in 1 h compared to control and 4 h | 329 | Enolase | GNPTVEVDVVTETGLHR | ENO_ASPFU | 35 | 77 | 47.3/5.39 | 3 | 4.43 | nd |
| Upregulated in 1 h and downregulated in 4 h compared to control | 817 | ISWI one complex protein 4 | NEFITIFQSNNSLLLNFRILFNLR | IOC4_YEAST | 22 | 23 | 55.6/5.21 | 5 | 1.95 | −1.0 |
| Upregulated in 4 h compared to control | 576 | Uncharacterized protein UNK4.17 | YDTCIEVQADGYYLR | YEAH_SCHPO | 45 | 45 | 45.8/5.31 | 3 | nd | 7.28 |
| 590 | glucose n-acetyltransferase, putative; n acetylglucosaminyltransferase, | ITLKSAPLIK | gi|241951426 | 52 | 38 | 56.9/8.54 | 2 | nd | 3.0 | |
| 351 | Enolase |
| ENO_EMENI | 48 | 74 | 47.5/5.37 | 7 | nd | 4.05 | |
| Upregulated in 1 h and 4 h compared to control | 450 | predicted protein |
| gi|340521168 | 56 | 65 | 14.5/9.36 | 21 | 2.51 | 2.0 |
| 352 | Stress response regulator protein 1 | LTRPMVR | SRR1_LODEL | 19 | 76 | 40.1/5.85 | 1 | 1.2 | 10.2 | |
| 445 | Heat shock factor protein | SGSIQSSSDDK | HSF_YEAST | 25 | 60 | 93.2/5.2 | 1 | 1.3 | 1.5 | |
| 528 | Enolase |
| ENO_EMENI | 48 | 55 | 47.5/5.37 | 7 | 1.47 | 2.2 | |
| 732 | GDP-Man:Man (3) GlcNAc (2)-PP-Dol alpha-1,2-mannosyltransferase | LKISPNDCENGDGFLNEMSR | ALG11_CANAL | 31 | 31 | 71.2/8.68 | 3 | 2.43 | 3.42 | |
| Expression Pattern | Spot No a | Protein Identity b | Peptide Sequences Matched | Sequence ID c | Score d | Observed Mr (kDa) on the Gel | Theoretical Mr (kDa)/pI e | % Sequence Coverage f | Protein Fold Change g | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 h | 4 h | |||||||||
| Unique to 1 h treated samples | 1B | Conserved oligomeric Golgi complex subunit 6 |
| COG6_PICGU | 68 | 11 | 85.5/5.05 | 4 | - | nd |
| 1C | Hypothetical protein TRIATDRAFT_306007 | GPHLGQAFLPIFDVR | gi|358398918 | 56 | 10 | 20.6/5.61 | 8 | - | nd | |
| Downregulated in 1 h compared to control | 485 | UDP-galactopyranose mutase |
| gi|406861020 | 104 | 41 | 58.9/5.64 | 6 | −2.9 | nd |
| 446 | DNA polymerase epsilon catalytic subunit A |
| DPOE_SCHPO | 49 | 45 | 254.4/6.74 | 1 | −2.6 | nd | |
| 922 | Lysophospholi-pase NTE1 | AGNPVSSLVNILNLFTSANDNVTSPSR | NTE1_CANGA | 19 | 17 | 193.5/8.29 | 1 | −4.3 | nd | |
| 452 | GTP-cyclo-hydrolase II |
| gi|453083792 | 93 | 45 | 59.1/6.23 | 5 | −2.3 | nd | |
| Upregulated in 1 h and 4 h compared to control | 1026 | tRNA N6-adenosine threonylcarbamoyltransferase | MGKPLIALGLEGSANK | KAE1_SCHPO | 26 | 12 | 38.06/7.55 | 4 | 2.2 | 4.2 |
| 1067 | mRNA cap guanine-N7 methyltransferase | SNTTMENTSGSATPKPR | MCES_ASPFU | 26 | 11 | 75.2/7.59 | 2 | 8.19 | 7.7 | |
| Upregulated in 4 h compared to control and 1 h | 1095 | Kexin | STTTTSSTTTATTTSGGEGDQK | KEX2_CANAW | 25 | 60 | 105.4/4.86 | 2 | nd | 2.1 |
| Unique to control and absent in treated samples | C7 | Vacuolar protein sorting/targeting protein 10 | RIHLHSVTELNNVGRKIPGNTCK | VPS10_SORMK | 46 | 10 | 173.2/5.63 | 1 | nd | nd |
| C8 | Serine/threonine-protein phosphatase 2B catalytic subunit A1 | 1. TPISSAIASGSPGSPGTPTSPSIGGPPLTAWRPGHGR | PP2B1_CRYNH | 20 | 12 | 72.19/5.11 | 5 | nd | nd | |
| Downregulated in 4 h compared to control | 901 | Phosphatidylglycerophosphatase GEP4 | MNISGTLNTLR | GEP4_YEAST | 32 | 18 | 21.1/8.87 | 5 | nd | −1.14 |
| Upregulated in 1 h compared to control | 904 | Probable tripeptidyl-peptidase SED4 | ELYKMGNTFATKDPR | SED4_TRIVH | 37 | 17 | 65.6/5.87 | 2 | 2.4 | nd |
| 856 | autophagy-related protein 28 | DGLDEDPLSPAGSISKLKPR | gi|429854636 | 67 | 20 | 65.7/5.39 | 3 | 2.7 | nd | |
| Downregulated in 1 h and 4 h compared to control | 920 | Subtilisin-like protease 8 |
| SUB8_ARTOC | 183 | 18 | 52.6/5.90 | 7 | −4.18 | −7.8 |
| 984 | Putative ATP-dependent RNA helicase C550.03c | HMIMGPSSKLISQFRLTYNMILNLLR | SKI2_SCHPO | 37 | 15 | 138.6/6.30 | 2 | −6.2 | −3.7 | |
| 794 | Fructose-bisphosphate aldolase, class II |
| gi|346326492 | 81 | 21 | 39.1/5.71 | 11 | −2.1 | −3.0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poosapati, S.; Ravulapalli, P.D.; Viswanathaswamy, D.K.; Kannan, M. Proteomics of Two Thermotolerant Isolates of Trichoderma under High-Temperature Stress. J. Fungi 2021, 7, 1002. https://doi.org/10.3390/jof7121002
Poosapati S, Ravulapalli PD, Viswanathaswamy DK, Kannan M. Proteomics of Two Thermotolerant Isolates of Trichoderma under High-Temperature Stress. Journal of Fungi. 2021; 7(12):1002. https://doi.org/10.3390/jof7121002
Chicago/Turabian StylePoosapati, Sowmya, Prasad Durga Ravulapalli, Dinesh Kumar Viswanathaswamy, and Monica Kannan. 2021. "Proteomics of Two Thermotolerant Isolates of Trichoderma under High-Temperature Stress" Journal of Fungi 7, no. 12: 1002. https://doi.org/10.3390/jof7121002
APA StylePoosapati, S., Ravulapalli, P. D., Viswanathaswamy, D. K., & Kannan, M. (2021). Proteomics of Two Thermotolerant Isolates of Trichoderma under High-Temperature Stress. Journal of Fungi, 7(12), 1002. https://doi.org/10.3390/jof7121002






