Phosphorus Starvation- and Zinc Excess-Induced Astragalus sinicus AsZIP2 Zinc Transporter Is Suppressed by Arbuscular Mycorrhizal Symbiosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant and AM Fungus Materials and Growth Conditions
2.2. Phosphate and Zinc Treatments
2.3. AsZIP2 Gene Cloning and Sequencing
2.4. Gene Expression Analysis
2.5. Plasmid Constructs
2.6. Plant Transformation
2.7. Microscopy
2.8. GUS Expression Analysis
2.9. Yeast Transformation, Growth and Zn Uptake Assay
2.10. Mycorrhizal Colonization Analysis
2.11. Elemental Analysis
2.12. In Silico Analysis
2.13. Phylogenetic Analysis
2.14. Statistical Analysis
2.15. Accession Numbers
3. Results
3.1. Zn Concentration Is Over-Accumulated in Pi-Starved Astragalus sinicus, yet Reduced by Arbuscular Mycorrhizal Colonization
3.2. Identification of the AsZIP2 Gene in A. sinicus
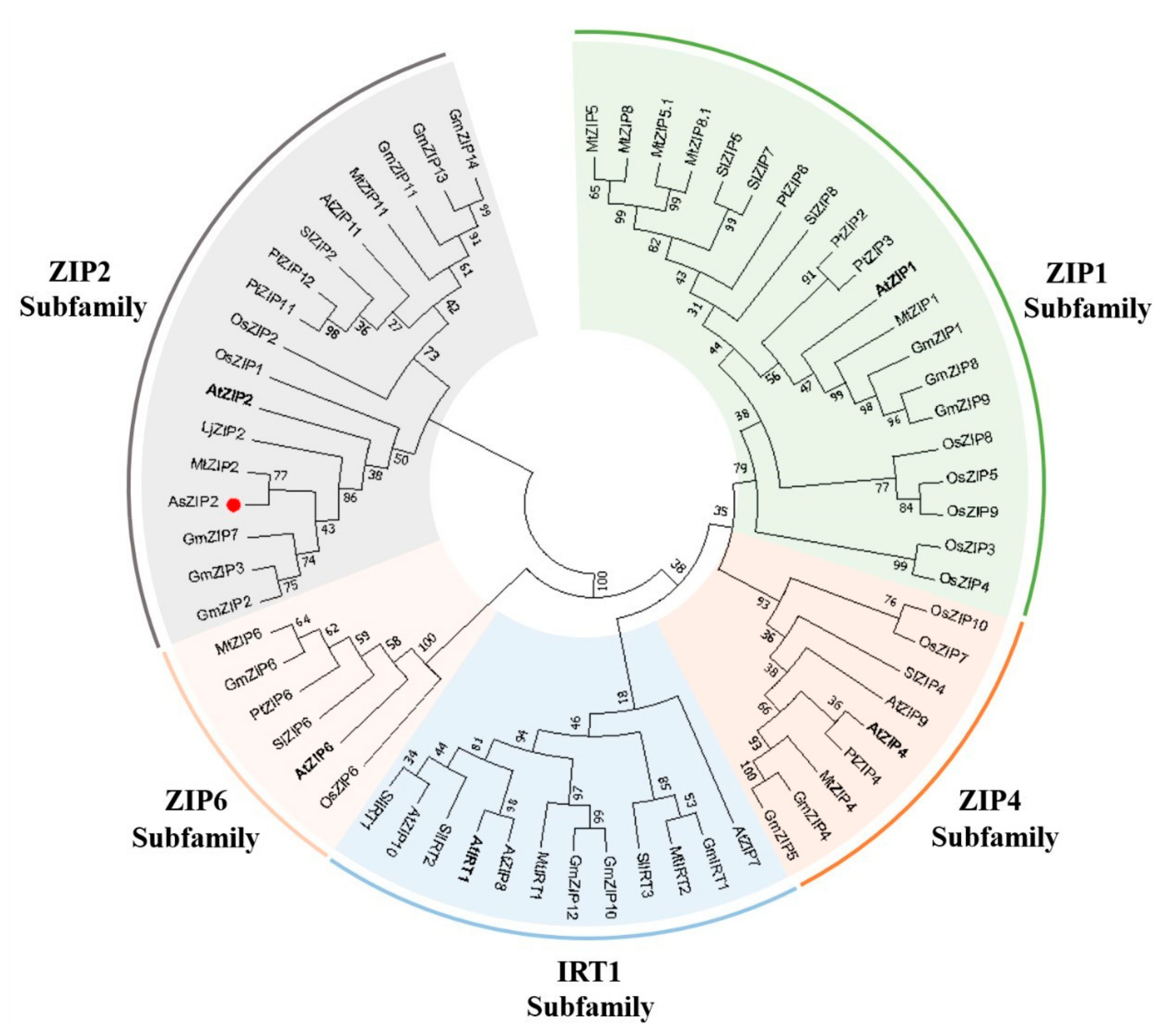
3.3. ZIP2 Zinc Transporters Are Conserved across Dicotyledons and Monocotyledons
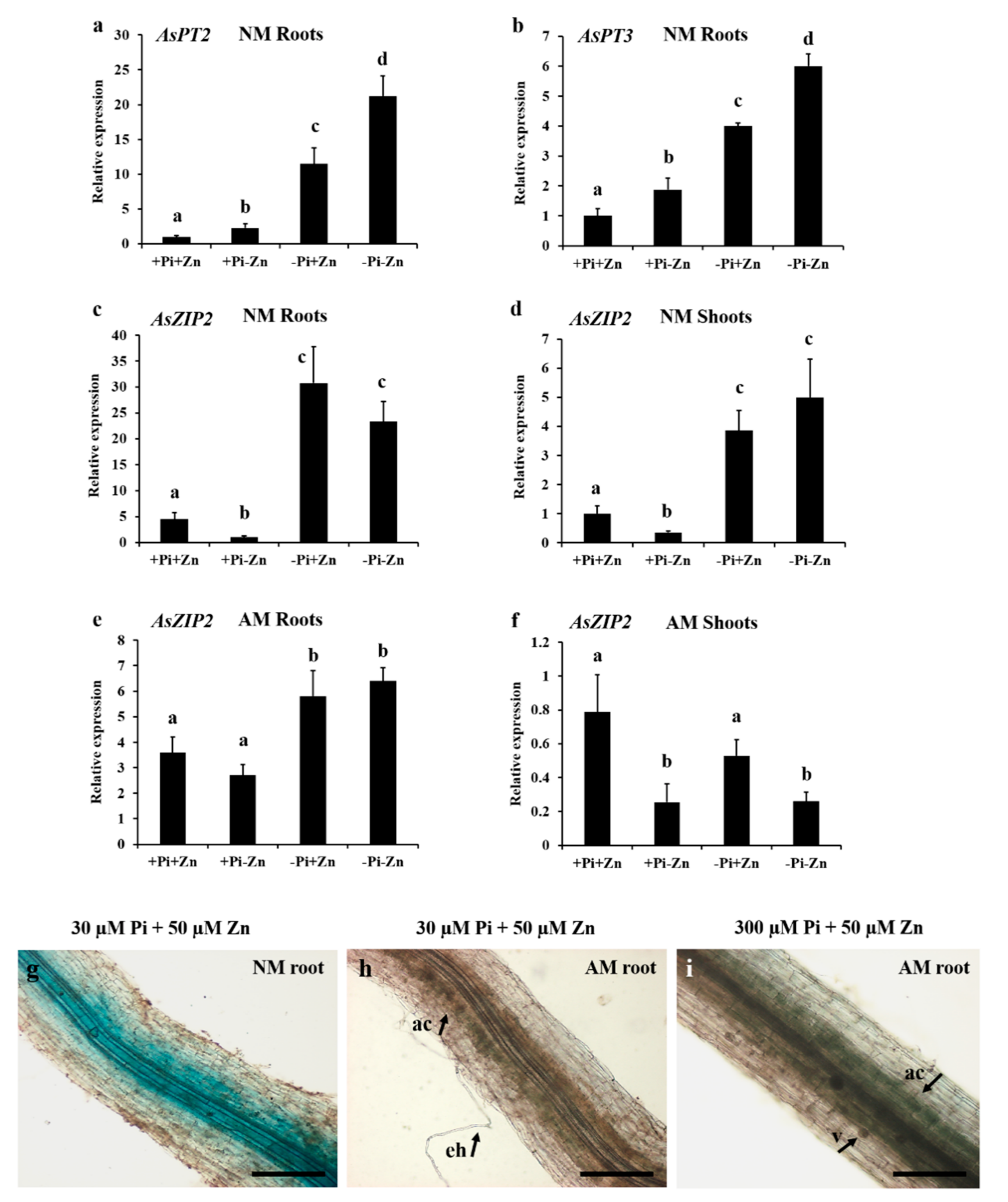
3.4. The A. sinicus AsZIP2 Gene Is Highly Expressed in Response to Pi Starvation
3.5. High Zn Supply Induces AsZIP2 Expression
3.6. Expression Analyses of AsZIP2 Involved in Pi and Zn Interaction in A. sinicus
3.7. Arbuscular Mycorrhization Represses AsZIP2 Expression in a Pi-Dependent Manner
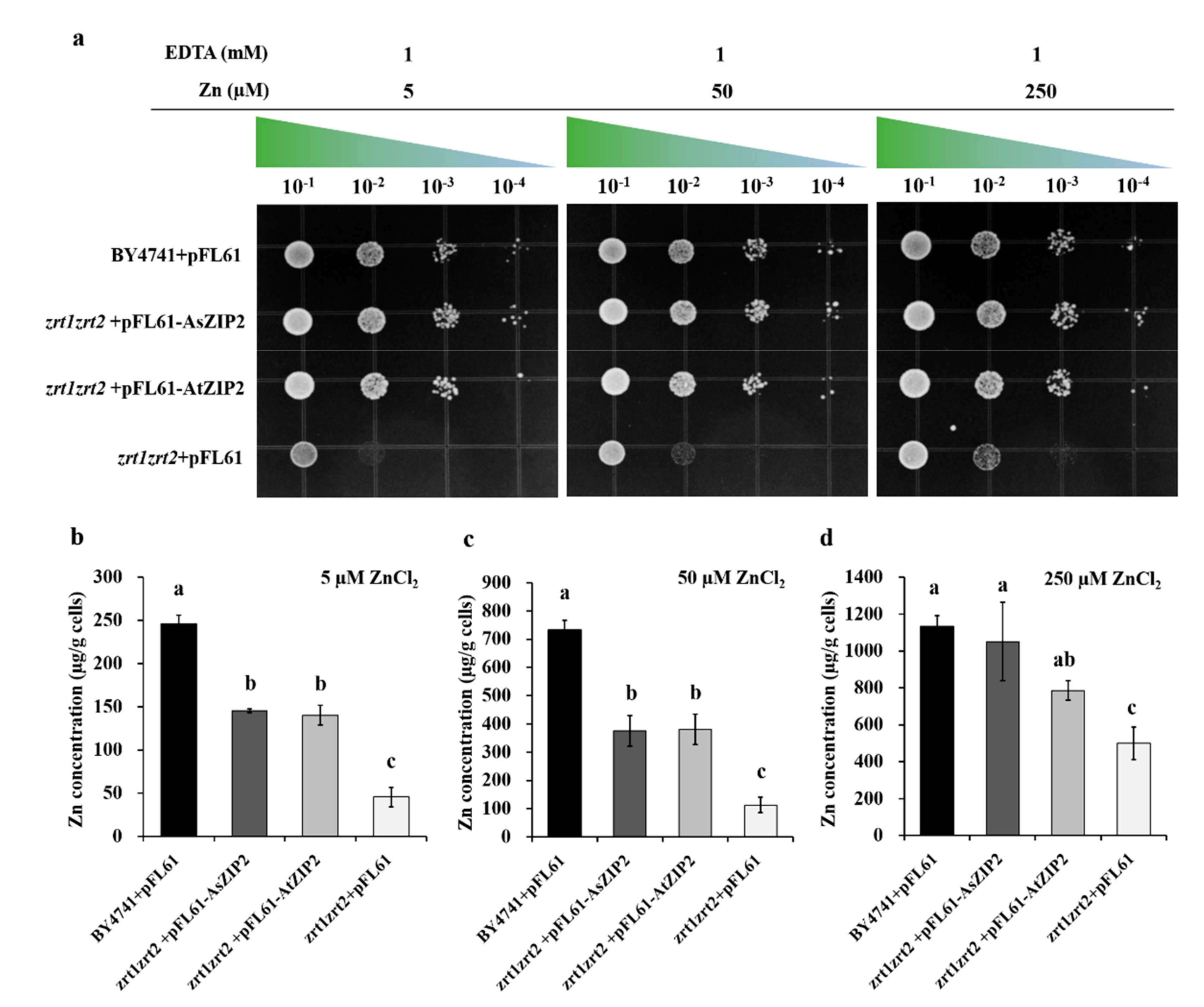
3.8. The A. sinicus AsZIP2 Transporter Behaves as a Zinc Transporter in Yeast
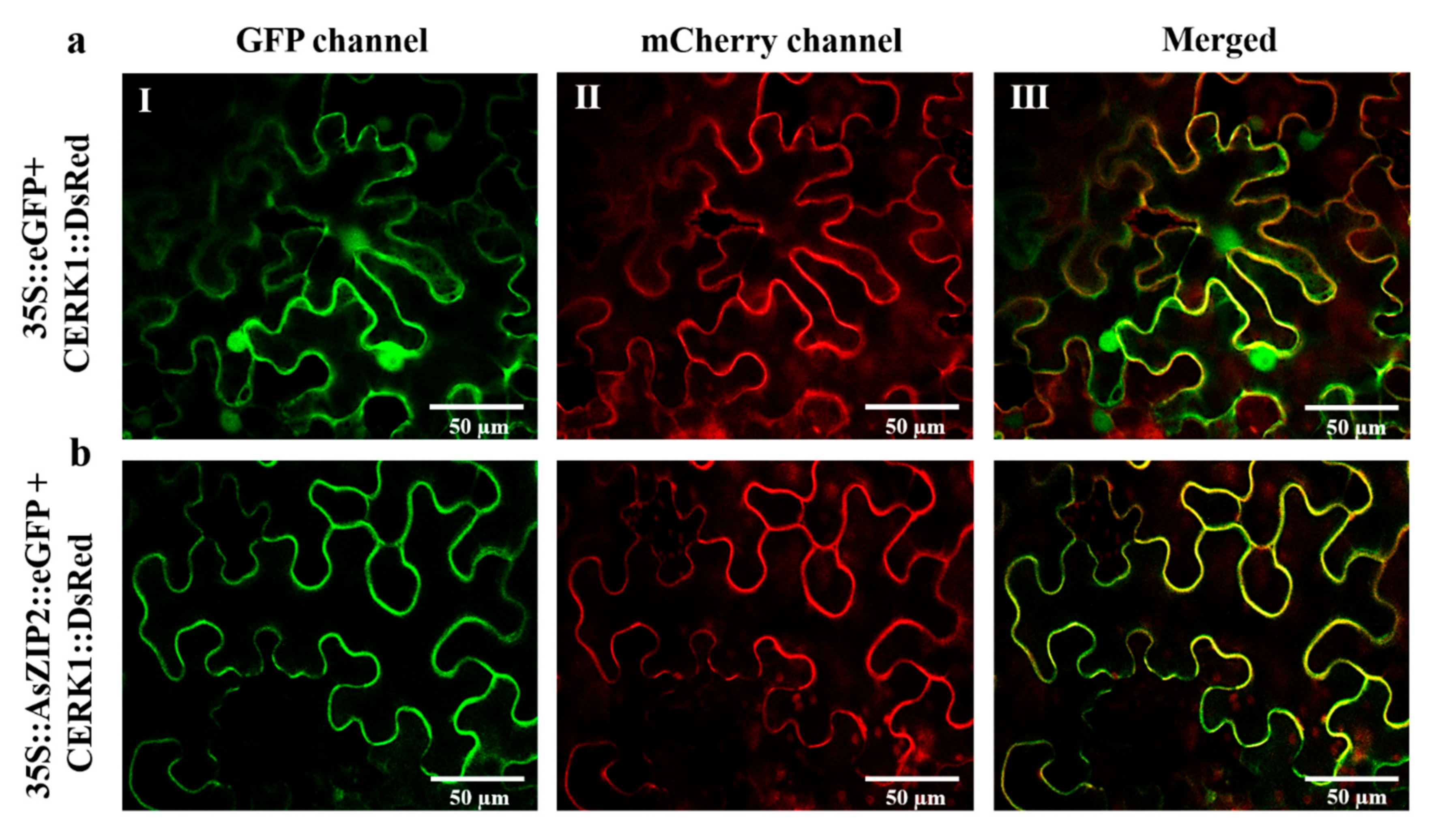
3.9. The A. sinicus AsZIP2 Gene Encodes a Plasma Membrane-Localized Transporter
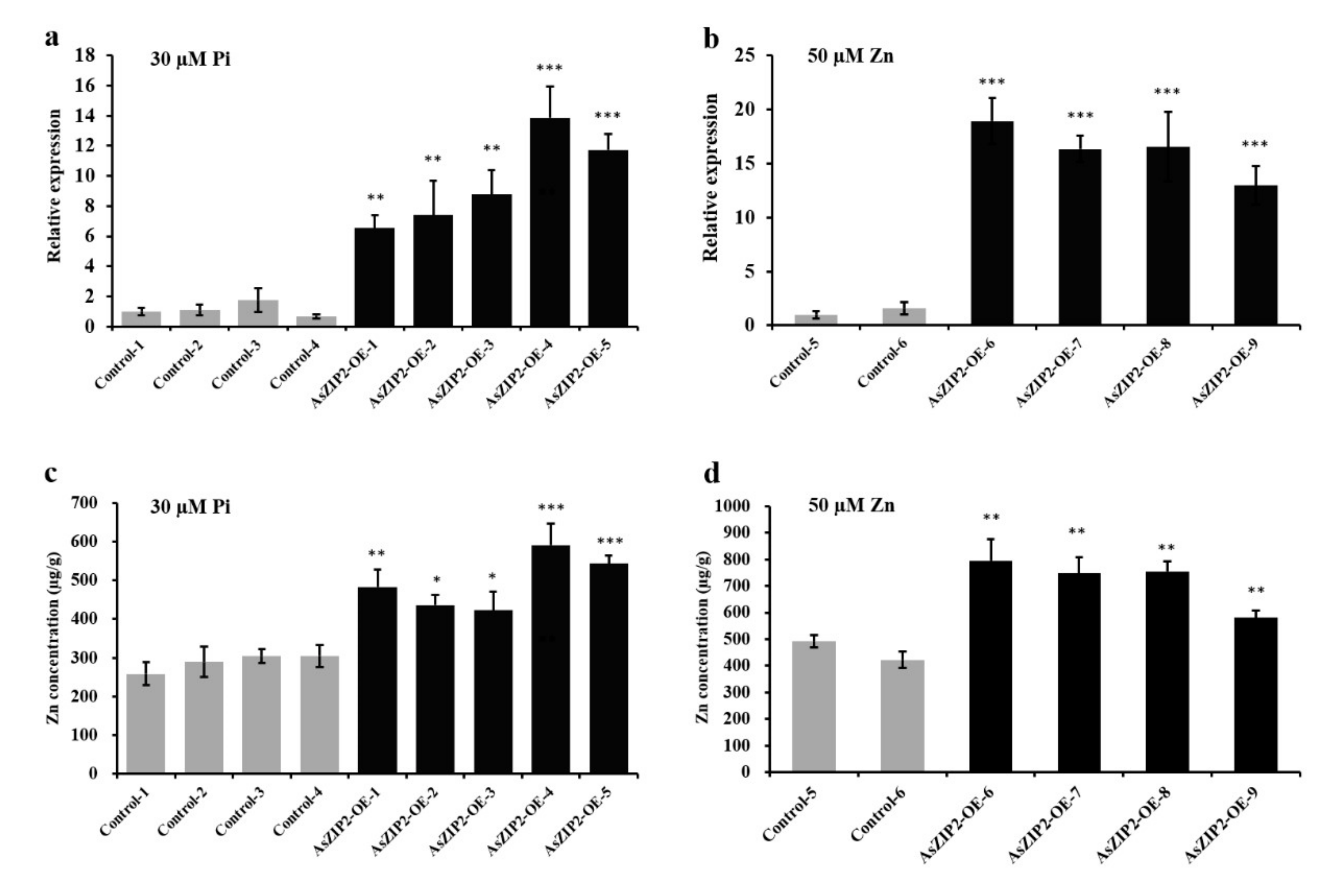
3.10. Overexpression of AsZIP2 Results in Increased Zn Concentration in A. sinicus Roots under Pi Starvation or High Zn Condition
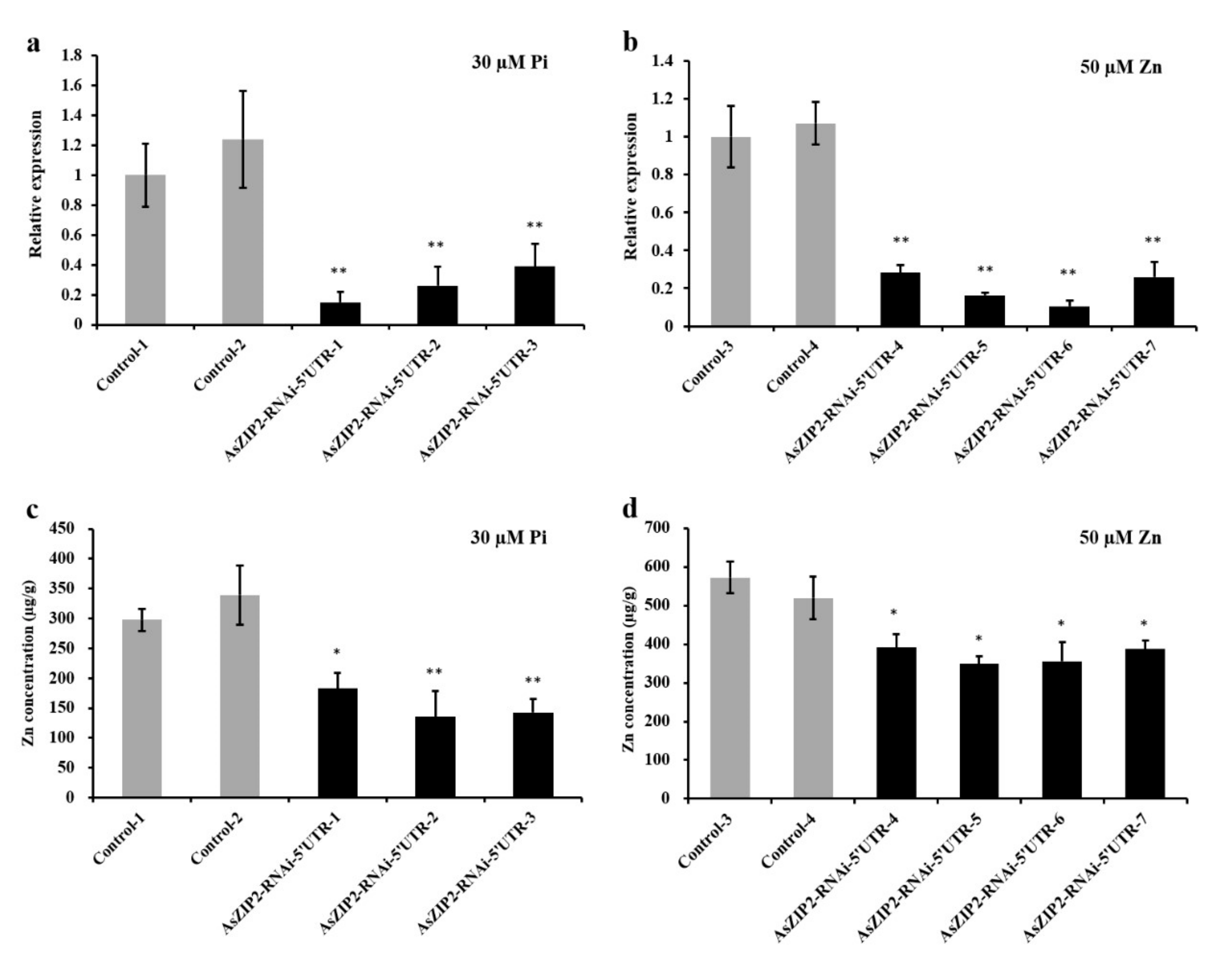
3.11. Loss of AsZIP2 Function Leads to Reduced Zn Concentration in Roots of A. sinicus under Low Pi or Excessive Zn Condition
4. Discussion
4.1. Pi Starvation-Induced AsZIP2 Is Involved in the Pi–Zn Interaction in A. sinicus
4.2. AsZIP2 Transport Zn in Roots under Pi Starvation or Zn Excess Resulting in Plant Zn Over-Accumulation
4.3. AM Contributes to Plant Tolerance to Zinc by Suppressing AsZIP2 Expression
4.4. The Proposed Working Model in Which AsZIP2 Is Inhibited by Pi and AM Symbiosis
5. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krishna, T.P.A.; Maharajan, T.; Roch, G.V.; Ignacimuthu, S.; Ceasar, S.A. Structure, Function, Regulation and Phylogenetic Relationship of ZIP Family Transporters of Plants. Front. Plant Sci. 2020, 11, 662. [Google Scholar] [CrossRef]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants; Academic Press: Cambridge, MA, USA, 1995. [Google Scholar] [CrossRef]
- Cakmak, I. Possible roles of zinc in protecting plant cells from damage by reactive oxygen species. New Phytol. 2000, 146, 185–205. [Google Scholar] [CrossRef] [PubMed]
- Marichali, A.; Dallali, S.; Ouerghemmi, S.; Sebei, H.; Hosni, K. Germination, morpho-physiological and biochemical responses of coriander (Coriandrum sativum L.) to zinc excess. Ind. Crop. Prod. 2014, 55, 248–257. [Google Scholar] [CrossRef]
- Ermolin, M.; Fedyunina, N.; Katasonova, O. Mobility and fate of cerium dioxide, zinc oxide, and copper nanoparticles in agricultural soil at sequential wetting-drying cycles. Materials 2019, 12, 1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Z.; Liang, X.; Pendlowski, H.; Hillier, S.; Suntornvongsagul, K.; Sihanonth, P.; Gadd, G.M. Fungal biotransformation of zinc silicate and sulfide mineral ores. Environ. Microbiol. 2013, 15, 2173–2186. [Google Scholar] [CrossRef] [PubMed]
- Hacisalihoglu, G.; Hart, J.J.; Kochian, L. High- and Low-Affinity Zinc Transport Systems and Their Possible Role in Zinc Efficiency in Bread Wheat. Plant Physiol. 2001, 125, 456–463. [Google Scholar] [CrossRef] [Green Version]
- Sperotto, R.A.; Ricachenevsky, F.K.; Williams, L.E.; Vasconcelos, M.W.; Menguer, P.K. From soil to seed: Micronutrient movement into and within the plant. Front. Plant Sci. 2014, 5, 438. [Google Scholar] [CrossRef]
- Abreu, I.; Saéz, Á.; Castro-Rodríguez, R.; Escudero, V.; Rodríguez-Haas, B.; Senovilla, M.; Larue, C.; Grolimund, D.; Tejada-Jiménez, M.; Imperial, J.; et al. Medicago truncatulaZinc-Iron Permease6 provides zinc to rhizobia-infected nodule cells. Plant Cell Environ. 2017, 40, 2706–2719. [Google Scholar] [CrossRef]
- Moreau, S.; Thomson, R.; Kaiser, B.; Trevaskis, B.; Guerinot, M.L.; Udvardi, M.; Puppo, A.; Day, D. GmZIP1 Encodes a Symbiosis-specific Zinc Transporter in Soybean. J. Biol. Chem. 2002, 277, 4738–4746. [Google Scholar] [CrossRef] [Green Version]
- Cavagnaro, T.R. The role of arbuscular mycorrhizas in improving plant zinc nutrition under low soil zinc concentrations: A review. Plant Soil 2008, 304, 315–325. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: London, UK, 2008. [Google Scholar]
- Liu, X.; Wang, F.; Shi, Z.; Tong, R.; Shi, X. Bioavailability of Zn in ZnO nanoparticle-spiked soil and the implications to maize plants. J. Nanopart. Res. 2015, 17, 175. [Google Scholar] [CrossRef]
- Kumari, M.; Khan, S.S.; Pakrashi, S.; Mukherjee, A.; Chandrasekaran, N. Cytogenetic and genotoxic effects of zinc oxide nanoparticles on root cells of Allium cepa. J. Hazard. Mater. 2011, 190, 613–621. [Google Scholar] [CrossRef]
- Priester, J.H.; Ge, Y.; Mielke, R.E.; Horst, A.M.; Moritz, S.C.; Espinosa, K.; Gelb, J.; Walker, S.L.; Nisbet, R.M.; An, Y.-J.; et al. Soybean susceptibility to manufactured nanomaterials with evidence for food quality and soil fertility interruption. Proc. Natl. Acad. Sci. USA 2012, 109, E2451–E2456. [Google Scholar] [CrossRef] [Green Version]
- Brune, A.; Urbach, W.; Dietz, K.-J. Compartmentation and transport of zinc in barley primary leaves as basic mechanisms involved in zinc tolerance. Plant Cell Environ. 1994, 17, 153–162. [Google Scholar] [CrossRef]
- Grotz, N.; Guerinot, M.L. Molecular aspects of Cu, Fe and Zn homeostasis in plants. Biochim. Biophys. Acta (BBA)-Bioenerg. 2006, 1763, 595–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menguer, P.K.; Farthing, E.; Peaston, K.A.; Ricachenevsky, F.K.; Fett, J.P.; Williams, L.E. Functional analysis of the rice vacuolar zinc transporter OsMTP1. J. Exp. Bot. 2013, 64, 2871–2883. [Google Scholar] [CrossRef] [Green Version]
- Vera-Estrella, R.; Gómez-Méndez, M.F.; Amezcua-Romero, J.C.; Barkla, B.; Rosas-Santiago, P.; Pantoja, O. Cadmium and zinc activate adaptive mechanisms in Nicotiana tabacum similar to those observed in metal tolerant plants. Planta 2017, 246, 433–451. [Google Scholar] [CrossRef] [PubMed]
- Ferrol, N.; Tamayo, E.; Vargas, P. The heavy metal paradox in arbuscular mycorrhizas: From mechanisms to biotechnological applications. J. Exp. Bot. 2016, 67, 6253–6265. [Google Scholar] [CrossRef] [Green Version]
- Kozak, K.; Papierniak-Wygladala, A.; Barabasz, A.; Kendziorek, M.; Palusińska, M.; Williams, L.E.; Antosiewicz, D.M. NtZIP11, a new Zn transporter specifically upregulated in tobacco leaves by toxic Zn level. Environ. Exp. Bot. 2018, 157, 69–78. [Google Scholar] [CrossRef] [Green Version]
- Tiong, J.; McDonald, G.K.; Genc, Y.; Pedas, P.; Hayes, J.E.; Toubia, J.; Langridge, P.; Huang, C.Y. H v ZIP 7 mediates zinc accumulation in barley (Hordeum vulgare) at moderately high zinc supply. New Phytol. 2013, 201, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Liang, H.; Yang, S.; Boch, A.; Clemens, S.; Chen, C.; Wu, J.; Huang, J.; Yeh, K. Arabidopsis IRT3 is a zinc-regulated and plasma membrane localized zinc/iron transporter. New Phytol. 2009, 182, 392–404. [Google Scholar] [CrossRef]
- Lanquar, V.; Lelièvre, F.; Barbier-Brygoo, H.; Thomine, S. Regulation and function of AtNRAMP4 metal transporter protein. Soil Sci. Plant Nutr. 2004, 50, 1141–1150. [Google Scholar] [CrossRef]
- Gaitán-Solís, E.; Taylor, N.J.; Siritunga, D.; Stevens, W.; Schachtman, D.P. Overexpression of the transporters AtZIP1 and AtMTP1 in cassava changes zinc accumulation and partitioning. Front. Plant Sci. 2015, 6, 492. [Google Scholar] [CrossRef] [Green Version]
- Krämer, U.; Talke, I.N.; Hanikenne, M. Transition metal transport. FEBS Lett. 2007, 581, 2263–2272. [Google Scholar] [CrossRef] [PubMed]
- Colangelo, E.P.; Guerinot, M.L. Put the metal to the petal: Metal uptake and transport throughout plants. Curr. Opin. Plant Biol. 2006, 9, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Olsen, L.I.; Palmgren, M.G. Many rivers to cross: The journey of zinc from soil to seed. Front. Plant Sci. 2014, 5, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.-F.; Aarts, M.G.M. The molecular mechanism of zinc and cadmium stress response in plants. Experientia 2012, 69, 3187–3206. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, S.; Krämer, U. The zinc homeostasis network of land plants. Biochim. Biophys. Acta (BBA)-Bioenerg. 2012, 1823, 1553–1567. [Google Scholar] [CrossRef]
- Palmer, C.; Guerinot, M.L. Facing the challenges of Cu, Fe and Zn homeostasis in plants. Nat. Chem. Biol. 2009, 5, 333–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milner, M.; Seamon, J.; Craft, E.; Kochian, L. Transport properties of members of the ZIP family in plants and their role in Zn and Mn homeostasis. J. Exp. Bot. 2012, 64, 369–381. [Google Scholar] [CrossRef] [Green Version]
- Kumar, L.; Meena, N.L.; Singh, U. Zinc Transporter: Mechanism for Improving Zn Availability. In Biofortification of Food Crops; Singh, U., Praharaj, C., Singh, S., Singh, N., Eds.; Springer: New Delhi, India, 2016; pp. 129–146. [Google Scholar] [CrossRef]
- Eide, D.J. The Zip Family of Zinc Transporters. In Zinc Finger Proteins. Molecular Biology Intelligence Unit; Iuchi, S., Kuldell, N., Eds.; Springer: Boston, MA, USA, 2005; pp. 261–264. [Google Scholar] [CrossRef]
- Eide, D.J. Zinc transporters and the cellular trafficking of zinc. Biochim. Biophys. Acta (BBA)-Bioenerg. 2006, 1763, 711–722. [Google Scholar] [CrossRef] [Green Version]
- Hussain, D.; Haydon, M.; Wang, Y.; Wong, E.; Sherson, S.M.; Young, J.; Camakaris, J.; Harper, J.F.; Cobbett, C.S. P-Type ATPase Heavy Metal Transporters with Roles in Essential Zinc Homeostasis in Arabidopsis. Plant Cell 2004, 16, 1327–1339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanikenne, M.; Talke, I.N.; Haydon, M.; Lanz, C.; Nolte, A.; Motte, P.; Kroymann, J.; Weigel, D.; Kraemer, U. Evolution of metal hyperaccumulation required cis-regulatory changes and triplication of HMA4. Nature 2008, 453, 391–395. [Google Scholar] [CrossRef]
- Narayanan, N.N.; Vasconcelos, M.W.; Grusak, M.A. Expression profiling of Oryza sativa metal homeostasis genes in different rice cultivars using a cDNA macroarray. Plant Physiol. Biochem. 2007, 45, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.R.; Feng, Y.; Chao, Y.E. Genomic analysis and expression pattern of OsZIP1, OsZIP3, and OsZIP4 in two rice (Oryza sativa L.) genotypes with different zinc efficiency. Russ. J. Plant Physiol. 2008, 55, 400–409. [Google Scholar] [CrossRef]
- López-Millán, A.-F.; Ellis, D.R.; Grusak, M.A. Identification and Characterization of Several New Members of the ZIP Family of Metal Ion Transporters in Medicago Truncatula. Plant Mol. Biol. 2004, 54, 583–596. [Google Scholar] [CrossRef]
- Li, S.; Zhou, X.; Huang, Y.; Zhu, L.; Zhang, S.; Zhao, Y.; Guo, J.; Chen, J.; Chen, R. Identification and characterization of the zinc-regulated transporters, iron-regulated transporter-like protein (ZIP) gene family in maize. BMC Plant Biol. 2013, 13, 114. [Google Scholar] [CrossRef] [Green Version]
- Kavitha, P.; Kuruvilla, S.; Mathew, M. Functional characterization of a transition metal ion transporter, OsZIP6 from rice (Oryza sativa L.). Plant Physiol. Biochem. 2015, 97, 165–174. [Google Scholar] [CrossRef]
- Jain, A.; Sinilal, B.; Dhandapani, G.; Meagher, R.B.; Sahi, S.V. Effects of Deficiency and Excess of Zinc on Morphophysiological Traits and Spatiotemporal Regulation of Zinc-Responsive Genes Reveal Incidence of Cross Talk between Micro- and Macronutrients. Environ. Sci. Technol. 2013, 47, 5327–5335. [Google Scholar] [CrossRef]
- Nazri, A.Z.; Griffin, J.H.; Peaston, K.A.; Alexander-Webber, D.G.; Williams, L.E. F-group bZIPs in barley-a role in Zn deficiency. Plant Cell Environ. 2017, 40, 2754–2770. [Google Scholar] [CrossRef] [Green Version]
- Burleigh, S.H.; Kristensen, B.K.; Bechmann, I.E. A plasma membrane zinc transporter from Medicago truncatula is up-regulated in roots by Zn fertilization, yet down-regulated by arbuscular mycorrhizal colonization. Plant Mol. Biol. 2003, 52, 1077–1088. [Google Scholar] [CrossRef]
- Wintz, H.; Fox, T.; Wu, Y.-Y.; Feng, V.; Chen, W.; Chang, H.-S.; Zhu, T.; Vulpe, C. Expression Profiles of Arabidopsis thaliana in Mineral Deficiencies Reveal Novel Transporters Involved in Metal Homeostasis. J. Biol. Chem. 2003, 278, 47644–47653. [Google Scholar] [CrossRef] [Green Version]
- Papierniak, A.; Kozak, K.; Kendziorek, M.; Barabasz, A.; Palusińska, M.; Tiuryn, J.; Paterczyk, B.; Williams, L.E.; Antosiewicz, D.M. Contribution of NtZIP1-Like to the Regulation of Zn Homeostasis. Front. Plant Sci. 2018, 9, 185. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Barker, S.J.; Langridge, P.; Smith, F.W.; Graham, R.D. Zinc Deficiency Up-Regulates Expression of High-Affinity Phosphate Transporter Genes in Both Phosphate-Sufficient and -Deficient Barley Roots. Plant Physiol. 2000, 124, 415–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouain, N.; Kisko, M.; Rouached, A.; Dauzat, M.; Lacombe, B.; Belgaroui, N.; Ghnaya, T.; Davidian, J.-C.; Berthomieu, P.; Abdelly, C.; et al. Phosphate/Zinc Interaction Analysis in Two Lettuce Varieties Reveals Contrasting Effects on Biomass, Photosynthesis, and Dynamics of Pi Transport. BioMed Res. Int. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Khan, G.A.; Bouraine, S.; Wege, S.; Li, Y.; De Carbonnel, M.; Berthomieu, P.; Poirier, Y.; Rouached, H. Coordination between zinc and phosphate homeostasis involves the transcription factor PHR1, the phosphate exporter PHO1, and its homologue PHO1; H3 in Arabidopsis. J. Exp. Bot. 2014, 65, 871–884. [Google Scholar] [CrossRef] [Green Version]
- Briat, J.-F.; Rouached, H.; Tissot, N.; Gaymard, F.; Dubos, C. Integration of P, S, Fe, and Zn nutrition signals in Arabidopsis thaliana: Potential involvement of PHOSPHATE STARVATION RESPONSE 1 (PHR1). Front. Plant Sci. 2015, 6, 290. [Google Scholar] [CrossRef] [Green Version]
- Xie, X.; Hu, W.; Fan, X.; Chen, H.; Tang, M. Interactions between Phosphorus, Zinc, and Iron Homeostasis in Nonmycorrhizal and Mycorrhizal Plants. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef]
- Ding, J.; Liu, L.; Wang, C.; Shi, L.; Xu, F.; Cai, H. High level of zinc triggers phosphorus starvation by inhibiting root-to-shoot translocation and preferential distribution of phosphorus in rice plants. Environ. Pollut. 2021, 277, 116778. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, S.; Mohapatra, T. Interaction between Macro- and Micro-Nutrients in Plants. Front. Plant Sci. 2021, 12, 753. [Google Scholar] [CrossRef] [PubMed]
- Bouain, N.; Krouk, G.; Lacombe, B.; Rouached, H. Getting to the Root of Plant Mineral Nutrition: Combinatorial Nutrient Stresses Reveal Emergent Properties. Trends Plant Sci. 2019, 24, 542–552. [Google Scholar] [CrossRef]
- Bouain, N.; Shahzad, Z.; Rouached, A.; Khan, G.A.; Berthomieu, P.; Abdelly, C.; Poirier, Y.; Rouached, H. Phosphate and zinc transport and signalling in plants: Toward a better understanding of their homeostasis interaction. J. Exp. Bot. 2014, 65, 5725–5741. [Google Scholar] [CrossRef] [Green Version]
- Kisko, M.; Bouain, N.; Rouached, A.; Choudhary, S.P.; Rouached, H. Molecular mechanisms of phosphate and zinc signalling crosstalk in plants: Phosphate and zinc loading into root xylem in Arabidopsis. Environ. Exp. Bot. 2015, 114, 57–64. [Google Scholar] [CrossRef]
- Kisko, M.; Bouain, N.; Safi, A.; Medici, A.; Akkers, R.C.; Secco, D.; Fouret, G.; Krouk, G.; Aarts, M.G.; Busch, W.; et al. LPCAT1 controls phosphate homeostasis in a zinc-dependent manner. eLife 2018, 7, e32077. [Google Scholar] [CrossRef]
- Genre, A.; Lanfranco, L.; Perotto, S.; Bonfante, P. Unique and common traits in mycorrhizal symbioses. Nat. Rev. Genet. 2020, 18, 1–12. [Google Scholar] [CrossRef]
- Bonfante, P.; Venice, F. Mucoromycota: Going to the roots of plant-interacting fungi. Fungal Biol. Rev. 2020, 34, 100–113. [Google Scholar] [CrossRef]
- Bonfante, P. The future has roots in the past: The ideas and scientists that shaped mycorrhizal research. New Phytol. 2018, 220, 982–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; Wang, W.; Xie, Q.; Liu, N.; Liu, L.; Wang, D.; Zhang, X.; Yang, C.; Chen, X.; Tang, D.; et al. Plants transfer lipids to sustain colonization by mutualistic mycorrhizal and parasitic fungi. Science 2017, 356, 1172–1175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, J.; Zeng, T.; Ji, C.; De Graaf, S.; Zheng, Z.; Xiao, T.T.; Deng, X.; Xiao, S.; Bisseling, T.; Limpens, E.; et al. A Medicago truncatula SWEET transporter implicated in arbuscule maintenance during arbuscular mycorrhizal symbiosis. New Phytol. 2019, 224, 396–408. [Google Scholar] [CrossRef] [PubMed]
- Javot, H.; Penmetsa, R.V.; Terzaghi, N.; Cook, D.R.; Harrison, M.J. A Medicago truncatula phosphate transporter indispensable for the arbuscular mycorrhizal symbiosis. Proc. Natl. Acad. Sci. USA 2007, 104, 1720–1725. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.-Y.; Grønlund, M.; Jakobsen, I.; Grotemeyer, M.S.; Rentsch, D.; Miyao, A.; Hirochika, H.; Kumar, C.S.; Sundaresan, V.; Salamin, N.; et al. Nonredundant Regulation of Rice Arbuscular Mycorrhizal Symbiosis by Two Members of the PHOSPHATE TRANSPORTER1 Gene Family. Plant Cell 2012, 24, 4236–4251. [Google Scholar] [CrossRef] [Green Version]
- Guether, M.; Neuhäuser, B.; Balestrini, R.; Dynowski, M.; Ludewig, U.; Bonfante, P. A Mycorrhizal-Specific Ammonium Transporter from Lotus japonicus Acquires Nitrogen Released by Arbuscular Mycorrhizal Fungi. Plant Physiol. 2009, 150, 73–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Chen, A.; Xie, K.; Yang, X.; Luo, Z.; Chen, J.; Zeng, D.; Ren, Y.; Yang, C.; Wang, L.; et al. Functional analysis of the OsNPF4.5 nitrate transporter reveals a conserved mycorrhizal pathway of nitrogen acquisition in plants. Proc. Natl. Acad. Sci. USA 2020, 117, 16649–16659. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, J.; Liu, J.; Cui, M.; Huang, Y.; Tian, Y.; Chen, A.; Xu, G. The Potassium Transporter SlHAK10 Is Involved in Mycorrhizal Potassium Uptake. Plant Physiol. 2019, 180, 465–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giovannetti, M.; Tolosano, M.; Volpe, V.; Kopriva, S.; Bonfante, P. Identification and functional characterization of a sulfate transporter induced by both sulfur starvation and mycorrhiza formation in L otus japonicus. New Phytol. 2014, 204, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Li, X.; Tao, H.; Christie, P.; Wong, M. The role of arbuscular mycorrhiza in zinc uptake by red clover growing in a calcareous soil spiked with various quantities of zinc. Chemosphere 2002, 50, 839–846. [Google Scholar] [CrossRef]
- González-Guerrero, M.; Azcon, C.; Mooney, M.; Valderas, A.; MacDiarmid, C.W.; Eide, D.J.; Ferrol, N. Characterization of a Glomus intraradices gene encoding a putative Zn transporter of the cation diffusion facilitator family. Fungal Genet. Biol. 2005, 42, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Guerrero, M.; Escudero, V.; Saez, A.; Tejada-Jiménez, M. Transition Metal Transport in Plants and Associated Endosymbionts: Arbuscular Mycorrhizal Fungi and Rhizobia. Front. Plant Sci. 2016, 7, 1088. [Google Scholar] [CrossRef] [Green Version]
- Tamayo, E.; Gã3Mez-Gallego, T.; Azcã3N-Aguilar, C.; Eferrol, N. Genome-wide analysis of copper, iron and zinc transporters in the arbuscular mycorrhizal fungus Rhizophagus irregularis. Front. Plant Sci. 2014, 5, 547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamayo, E.; Knight, S.; Valderas, A.; Dancis, A.; Ferrol, N. The arbuscular mycorrhizal fungus Rhizophagus irregularis uses a reductive iron assimilation pathway for high-affinity iron uptake. Environ. Microbiol. 2018, 20, 1857–1872. [Google Scholar] [CrossRef]
- Gómez-Gallego, T.; Benabdellah, K.; Merlos, M.A.; Jiménez-Jiménez, A.M.; Alcon, C.; Berthomieu, P.; Ferrol, N. The Rhizophagus irregularis Genome Encodes Two CTR Copper Transporters That Mediate Cu Import into the Cytosol and a CTR-Like Protein Likely Involved in Copper Tolerance. Front. Plant Sci. 2019, 10, 604. [Google Scholar] [CrossRef] [Green Version]
- Yao, Q.; Yang, R.; Long, L.; Zhu, H. Phosphate application enhances the resistance of arbuscular mycorrhizae in clover plants to cadmium via polyphosphate accumulation in fungal hyphae. Environ. Exp. Bot. 2014, 108, 63–70. [Google Scholar] [CrossRef]
- Cavagnaro, T.R.; Dickson, S.; Smith, F.A. Arbuscular mycorrhizas modify plant responses to soil zinc addition. Plant Soil 2009, 329, 307–313. [Google Scholar] [CrossRef]
- Watts-Williams, S.J.; Patti, A.F.; Cavagnaro, T.R. Arbuscular mycorrhizas are beneficial under both deficient and toxic soil zinc conditions. Plant Soil 2013, 371, 299–312. [Google Scholar] [CrossRef]
- Wang, F.; Liu, X.; Shi, Z.; Tong, R.; Adams, C.A.; Shi, X. Arbuscular mycorrhizae alleviate negative effects of zinc oxide nanoparticle and zinc accumulation in maize plants—A soil microcosm experiment. Chemosphere 2016, 147, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Jing, X.; Adams, C.A.; Shi, Z.; Sun, Y. Decreased ZnO nanoparticle phytotoxicity to maize by arbuscular mycorrhizal fungus and organic phosphorus. Environ. Sci. Pollut. Res. 2018, 25, 23736–23747. [Google Scholar] [CrossRef] [PubMed]
- Watts-Williams, S.J.; Tyerman, S.; Cavagnaro, T. The dual benefit of arbuscular mycorrhizal fungi under soil zinc deficiency and toxicity: Linking plant physiology and gene expression. Plant Soil 2017, 420, 375–388. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, D.; Liu, Y.; Cui, Z.; Chen, X.; Zou, C. Zinc uptake and accumulation in winter wheat relative to changes in root morphology and mycorrhizal colonization following varying phosphorus application on calcareous soil. Field Crop. Res. 2016, 197, 74–82. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, X.-X.; Liu, Y.-M.; Liu, D.-Y.; Chen, X.-P.; Zou, C.-Q. Zinc uptake by roots and accumulation in maize plants as affected by phosphorus application and arbuscular mycorrhizal colonization. Plant Soil 2017, 413, 59–71. [Google Scholar] [CrossRef]
- Watts-Williams, S.; Cavagnaro, T. Nutrient interactions and arbuscular mycorrhizas: A meta-analysis of a mycorrhiza-defective mutant and wild-type tomato genotype pair. Plant Soil 2014, 384, 79–92. [Google Scholar] [CrossRef]
- Ova, E.A.; Kutman, U.B.; Ozturk, L.; Cakmak, I. High phosphorus supply reduced zinc concentration of wheat in native soil but not in autoclaved soil or nutrient solution. Plant Soil 2015, 393, 147–162. [Google Scholar] [CrossRef]
- Nafady, N.A.; Elgharably, A. Mycorrhizal symbiosis and phosphorus fertilization effects on Zea mays growth and heavy metals uptake. Int. J. Phytoremediation 2018, 20, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Che, X.; Lai, W.; Wang, S.; Hu, W.; Chen, H.; Zhao, B.; Tang, M.; Xie, X. The auxin-inducible phosphate transporter AsPT5 mediates phosphate transport and is indispensable for arbuscule formation in Chinese milk vetch at moderately high phosphate supply. Environ. Microbiol. 2020, 22, 2053–2079. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, E.J. Sand and Water Culture Methods Used in the Study of Plant Nutrition; Farnham Royal: England, UK, 1966; Volume 22, pp. 315–709. [Google Scholar]
- Scotto–Lavino, E.; Du, G.; A Frohman, M. 5′ end cDNA amplification using classic RACE. Nat. Protoc. 2006, 1, 2555–2562. [Google Scholar] [CrossRef]
- Scotto–Lavino, E.; Du, G.; A Frohman, M. 3′ End cDNA amplification using classic RACE. Nat. Protoc. 2006, 1, 2742–2745. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-G.; Chen, Y. High-efficiency thermal asymmetric interlaced PCR for amplification of unknown flanking sequences. BioTechniques 2007, 43, 649–656. [Google Scholar] [CrossRef]
- Sambrook, J.; Russell, D.W. Inverse PCR. Cold Spring Harb. Protoc. 2006, 2, 3487. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Limpens, E.; Ramos, J.; Franken, C.; Raz, V.; Compaan, B.; Franssen, H.; Bisseling, T.; Geurts, R. RNA interference in Agrobacterium rhizogenes-transformed roots of Arabidopsis and Medicago truncatula. J. Exp. Bot. 2004, 55, 983–992. [Google Scholar] [CrossRef]
- Volpe, V.; Dell’Aglio, E.; Giovannetti, M.; Ruberti, C.; Costa, A.; Genre, A.; Guether, M.; Bonfante, P. An AM-induced,MYB-family gene ofLotus japonicus(LjMAMI) affects root growth in an AM-independent manner. Plant J. 2012, 73, 442–455. [Google Scholar] [CrossRef]
- Miya, A.; Albert, P.; Shinya, T.; Desaki, Y.; Ichimura, K.; Shirasu, K.; Narusaka, Y.; Kawakami, N.; Kaku, H.; Shibuya, N. CERK1, a LysM receptor kinase, is essential for chitin elicitor signaling in Arabidopsis. Proc. Natl. Acad. Sci. USA 2007, 104, 19613–19618. [Google Scholar] [CrossRef] [Green Version]
- Pan, Q.; Cui, B.; Deng, F.; Quan, J.; Loake, G.; Shan, W. RTP 1 encodes a novel endoplasmic reticulum ( ER )-localized protein in Arabidopsis and negatively regulates resistance against biotrophic pathogens. New Phytol. 2015, 209, 1641–1654. [Google Scholar] [CrossRef]
- Cho, H.-J.; Widholm, J.M. Improved shoot regeneration protocol for hairy roots of the legume Astragalus sinicus. Plant Cell, Tissue Organ Cult. (PCTOC) 2002, 69, 259–269. [Google Scholar] [CrossRef]
- Zhao, H.; Eide, D. The Gene Encodes the Low Affinity Zinc Transporter in Saccharomyces cerevisiae. J. Biol. Chem. 1996, 271, 23203–23210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grotz, N.; Fox, T.; Connolly, E.; Park, W.; Guerinot, M.L.; Eide, D. Identification of a family of zinc transporter genes from Arabidopsis that respond to zinc deficiency. Proc. Natl. Acad. Sci. USA 1998, 95, 7220–7224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minet, M.; Dufour, M.-E.; Lacroute, F. Complementation of Saccharomyces cerevisiae auxotrophic mutants by Arabidopsis thaliana cDNAs. Plant J. 1992, 2, 417–422. [Google Scholar] [CrossRef]
- Gietz, R.D.; Schiestl, R.H. Applications of high efficiency lithium acetate transformation of intact yeast cells using single-stranded nucleic acids as carrier. Yeast 1991, 7, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Trouvelot, A.; Kough, J.L.; Gianinazzi-Pearson, V. Mesure du Taux de Mycorhization VA d’un Système Radiculaire. In Recherche de Methods d’estimation Ayant une Signification Fonctionnelle. Proceedings of the 1st ESM; Gianinazzi-Pearson, V., Gianinazzi, S., Eds.; INRA Press: Paris, France, 1986; Volume V, pp. 217–221. [Google Scholar]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, X.; Huang, W.; Liu, F.; Tang, N.; Liu, Y.; Lin, H.; Zhao, B. Functional analysis of the novel mycorrhiza-specific phosphate transporter A s PT 1 and PHT 1 family from A stragalus sinicus during the arbuscular mycorrhizal symbiosis. New Phytol. 2013, 198, 836–852. [Google Scholar] [CrossRef]
- Eng, B.; Guerinot, M.; Eide, D.; Saier, M. Sequence Analyses and Phylogenetic Characterization of the ZIP Family of Metal Ion Transport Proteins. J. Membr. Biol. 1998, 166, 1–7. [Google Scholar] [CrossRef]
- Guerinot, M.L. The ZIP family of metal transporters. Biochim. Biophys. Acta (BBA)-Biomembr. 2000, 1465, 190–198. [Google Scholar] [CrossRef] [Green Version]
- Ruan, W.; Guo, M.; Cai, L.; Hu, H.; Li, C.; Liu, Y.; Wu, Z.; Mao, C.; Yi, K.; Wu, P.; et al. Genetic manipulation of a high-affinity PHR1 target cis-element to improve phosphorous uptake in Oryza sativa L. Plant Mol. Biol. 2015, 87, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Remy, E.; Cabrito, T.R.; Baster, P.; Batista, R.A.; Teixeira, M.C.; Friml, J.; Sa-Correia, I.; Duque, P. A Major Facilitator Superfamily Transporter Plays a Dual Role in Polar Auxin Transport and Drought Stress Tolerance in Arabidopsis. Plant Cell 2013, 25, 901–926. [Google Scholar] [CrossRef] [Green Version]
- Nelson, B.K.; Cai, X.; Nebenführ, A. A multicolored set of in vivo organelle markers for co-localization studies in Arabidopsis and other plants. Plant J. 2007, 51, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Liu, C.; Wang, Y.; Liu, X.; Li, F.; Zhang, G.; Li, X. Heavy metal contamination in soils and vegetables near an e-waste processing site, south China. J. Hazard. Mater. 2011, 186, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.-J.; Kwon, Y.K.; Yu, S.; Lee, P.-K.; Park, H.-S.; Song, N. Assessment of Zn pollution sources and apportionment in agricultural soils impacted by a Zn smelter in South Korea. J. Hazard. Mater. 2018, 364, 475–487. [Google Scholar] [CrossRef]
- Zeng, H.; Wu, H.; Yan, F.; Yi, K.; Zhu, Y. Molecular regulation of zinc deficiency responses in plants. J. Plant Physiol. 2021, 261, 153419. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Zhang, X.; Ding, M.; Zhu, Y. Integrated analyses of miRNAome and transcriptome reveal zinc deficiency responses in rice seedlings. BMC Plant Biol. 2019, 19, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Dong, C.; He, F.; Berkowitz, O.; Liu, J.; Cao, P.; Tang, M.; Shi, H.; Wang, W.; Li, Q.; Shen, Z.; et al. Alternative Splicing Plays a Critical Role in Maintaining Mineral Nutrient Homeostasis in Rice (Oryza sativa). Plant Cell 2018, 30, 2267–2285. [Google Scholar] [CrossRef] [Green Version]
- Migocka, M.; Małas, K.; Maciaszczyk-Dziubinska, E.; Posyniak, E.; Migdal, I.; Szczech, P. Cucumber Golgi protein CsMTP5 forms a Zn-transporting heterodimer with high molecular mass protein CsMTP12. Plant Sci. 2018, 277, 196–206. [Google Scholar] [CrossRef]
- Dubeaux, G.; Neveu, J.; Zelazny, E.; Vert, G. Metal Sensing by the IRT1 Transporter-Receptor Orchestrates Its Own Degradation and Plant Metal Nutrition. Mol. Cell 2018, 69, 953–964.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Remy, E.; Cabrito, T.R.; Batista, R.A.; Hussein, M.; Teixeira, M.C.; Athanasiadis, A.; Sa-Correia, I.; Duque, P. Intron Retention in the 5′UTR of the Novel ZIF2 Transporter Enhances Translation to Promote Zinc Tolerance in Arabidopsis. PLoS Genet. 2014, 10, e1004375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshinari, A.; Takano, J. Insights into the Mechanisms Underlying Boron Homeostasis in Plants. Front. Plant. Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broadley, M.R.; White, P.J.; Hammond, J.P.; Zelko, I.; Lux, A. Zinc in plants: Tansley review. New Phytol. 2007, 173, 677–702. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, X.; Fan, X.; Chen, H.; Tang, M. Phosphorus Starvation- and Zinc Excess-Induced Astragalus sinicus AsZIP2 Zinc Transporter Is Suppressed by Arbuscular Mycorrhizal Symbiosis. J. Fungi 2021, 7, 892. https://doi.org/10.3390/jof7110892
Xie X, Fan X, Chen H, Tang M. Phosphorus Starvation- and Zinc Excess-Induced Astragalus sinicus AsZIP2 Zinc Transporter Is Suppressed by Arbuscular Mycorrhizal Symbiosis. Journal of Fungi. 2021; 7(11):892. https://doi.org/10.3390/jof7110892
Chicago/Turabian StyleXie, Xianan, Xiaoning Fan, Hui Chen, and Ming Tang. 2021. "Phosphorus Starvation- and Zinc Excess-Induced Astragalus sinicus AsZIP2 Zinc Transporter Is Suppressed by Arbuscular Mycorrhizal Symbiosis" Journal of Fungi 7, no. 11: 892. https://doi.org/10.3390/jof7110892
APA StyleXie, X., Fan, X., Chen, H., & Tang, M. (2021). Phosphorus Starvation- and Zinc Excess-Induced Astragalus sinicus AsZIP2 Zinc Transporter Is Suppressed by Arbuscular Mycorrhizal Symbiosis. Journal of Fungi, 7(11), 892. https://doi.org/10.3390/jof7110892





