The Roles of Chromatin Accessibility in Regulating the Candida albicans White-Opaque Phenotypic Switch
Abstract
1. Introduction
2. Regulation of White-Opaque Switching by “Writers”
2.1. Regulation of White-Opaque Switching by the NuA4 Histone Acetyltransferase Yng2
2.2. Regulation of White-Opaque Switching by the Histone Acetyltransferase Rtt109
2.3. Regulation of White-Opaque Switching by the Histone Acetyltransferase Hat1
2.4. Regulation of White-Opaque Switching by the Histone Acetyltransferase Nat4
2.5. Regulation of White-Opaque Switching by the Histone Methyltransferase Set1
3. Regulation of White-Opaque Switching by “Erasers”
4. Potential Roles of “Readers” in Regulating White-Opaque Switching
5. Regulation of White-Opaque Switching by Chromatin Remodeling Complexes
Regulation of White-Opaque Switching by the SWR1 Chromatin Remodeling Complex
6. Regulation of White-Opaque Switching by Histone Chaperone Complexes
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zordan, R.E.; Miller, M.G.; Galgoczy, D.J.; Tuch, B.B.; Johnson, A.D. Interlocking transcriptional feedback loops control white-opaque switching in Candida albicans. PLoS Biol. 2007, 5, 2166–2176. [Google Scholar] [CrossRef]
- Zordan, R.E.; Galgoczy, D.J.; Johnson, A.D. Epigenetic properties of white-opaque switching in Candida albicans are based on a self-sustaining transcriptional feedback loop. Proc. Natl. Acad. Sci. USA 2006, 103, 12807–12812. [Google Scholar] [CrossRef]
- Tuch, B.B.; Mitrovich, Q.M.; Homann, O.R.; Hernday, A.D.; Monighetti, C.K.; de La Vega, F.M.; Johnson, A.D. The transcriptomes of two heritable cell types illuminate the circuit governing their differentiation. PLoS Genet. 2010, 6, e1001070. [Google Scholar] [CrossRef]
- Hernday, A.D.; Lohse, M.B.; Fordyce, P.M.; Nobile, C.J.; Derisi, J.L.; Johnson, A.D. Structure of the transcriptional network controlling white-opaque switching in Candida albicans. Mol. Microbiol. 2013, 90, 22–35. [Google Scholar] [CrossRef]
- Guan, Z.; Liu, H. Overlapping functions between SWR1 deletion and H3K56 acetylation in Candida albicans. Eukaryot. Cell 2015, 14, 578–587. [Google Scholar] [CrossRef]
- Anderson, M.Z.; Porman, A.M.; Wang, N.; Mancera, E.; Huang, D.; Cuomo, C.A.; Bennett, R.J. A multistate toggle switch defines fungal cell fates and is regulated by synergistic genetic cues. PLoS Genet. 2016, 12, e1006353. [Google Scholar] [CrossRef] [PubMed]
- Frazer, C.; Staples, M.I.; Kim, Y.; Hirakawa, M.; Dowell, M.A.; Johnson, N.V.; Hernday, A.D.; Ryan, V.H.; Fawzi, N.L.; Finkelstein, I.J.; et al. Epigenetic cell fate in Candida albicans is controlled by transcription factor condensates acting at super-enhancer-like elements. Nat. Microbiol. 2020, 5, 1374–1389. [Google Scholar] [CrossRef] [PubMed]
- Takagi, J.; Singh-Babak, S.D.; Lohse, M.B.; Dalal, C.K.; Johnson, A.D. Candida albicans white and opaque cells exhibit distinct spectra of organ colonization in mouse models of infection. PLoS ONE 2019, 14, e0218037. [Google Scholar] [CrossRef] [PubMed]
- Lohse, M.B.; Ene, I.V.; Craik, V.B.; Hernday, A.D.; Mancera, E.; Morschhäuser, J.; Bennett, R.J.; Johnson, A.D. Systematic genetic screen for transcriptional regulators of the Candida albicans white-opaque switch. Genetics 2016, 203, 1679–1692. [Google Scholar] [CrossRef][Green Version]
- Solis, N.V.; Park, Y.-N.; Swidergall, M.; Daniels, K.J.; Filler, S.G.; Soll, D.R. Candida albicans white-opaque switching influences virulence but not mating during oropharyngeal candidiasis. Infect. Immun. 2018, 86, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Craik, V.B.; Johnson, A.D.; Lohse, M.B. Sensitivity of white and opaque Candida albicans cells to antifungal drugs. Antimicrob. Agents Chemother. 2017, 61, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Pande, K.; Chen, C.; Noble, S.M. Passage through the mammalian gut triggers a phenotypic switch that promotes Candida albicans commensalism. Nat. Genet. 2013, 45, 1088–1091. [Google Scholar] [CrossRef] [PubMed]
- Lohse, M.B.; Johnson, A.D. Differential phagocytosis of white versus opaque Candida albicans by Drosophila and mouse phagocytes. PLoS ONE 2008, 3, e1473. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.G.; Johnson, A.D. White-opaque switching in Candida albicans is controlled by mating-type locus homeodomain proteins and allows efficient mating. Cell 2002, 110, 293–302. [Google Scholar] [CrossRef]
- Sasse, C.; Hasenberg, M.; Weyler, M.; Gunzer, M.; Morschhäuser, J. White-opaque switching of Candida albicans allows immune evasion in an environment-dependent fashion. Eukaryot. Cell 2013, 12, 50–58. [Google Scholar] [CrossRef]
- Alby, K.; Bennett, R.J. Phenotypic switching is sensitive to multiple inputs in a pathogenic fungus. Mol. Biol. Cell 2009, 2, 509–511. [Google Scholar] [CrossRef]
- Huang, G.; Srikantha, T.; Sahni, N.; Yi, S.; Soll, D.R. CO2 regulates white-to-opaque switching in Candida albicans. Curr. Biol. 2009, 19, 330–334. [Google Scholar] [CrossRef]
- Huang, G.; Yi, S.; Sahni, N.; Daniels, K.J.; Srikantha, T.; Soll, D.R. N-acetylglucosamine induces white to opaque switching, a mating prerequisite in Candida albicans. PLoS Pathog. 2010, 6, e1000806. [Google Scholar] [CrossRef]
- Morrow, B.; Anderson, J.; Wilson, J.; Soll, D.R. Bidirectional stimulation of the white-opaque transition of Candida albicans by ultraviolet irradiation. J. Gen. Microbiol. 1989, 135, 1201–1208. [Google Scholar] [CrossRef]
- Ramírez-Zavala, B.; Reuß, O.; Park, Y.N.; Ohlsen, K.; Morschhäuser, J. Environmental induction of white-opaque switching in Candida albicans. PLoS Pathog. 2008, 4, e1000089. [Google Scholar] [CrossRef]
- Alby, K.; Bennett, R.J. Stress-Induced Phenotypic Switching in Candida albicans. Mol. Biol. Cell 2009, 20, 3178–3191. [Google Scholar] [CrossRef]
- Lohse, M.B.; Hernday, A.D.; Fordyce, P.M.; Noiman, L.; Sorrells, T.R.; Hanson-Smith, V.; Nobile, C.J.; DeRisi, J.L.; Johnson, A.D. Identification and characterization of a previously undescribed family of sequence-specific DNA-binding domains. Proc. Natl. Acad. Sci. USA 2013, 110, 7660–7665. [Google Scholar] [CrossRef] [PubMed]
- Lohse, M.B.; Johnson, A.D. Identification and characterization of Wor4, a new transcriptional regulator of white-opaque switching. G3 Genes Genomes Genet. 2016, 6, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, J.S.; Liu, H. Regulation of white and opaque cell-type formation in Candida albicans by Rtt109 and Hst3. Mol. Microbiol. 2011, 81, 1078–1091. [Google Scholar] [CrossRef] [PubMed]
- Hernday, A.D.; Lohse, M.B.; Nobile, C.J.; Noiman, L.; Laksana, C.N.; Johnson, A.D. Ssn6 defines a new level of regulation of white-opaque switching in Candida albicans and is required for the stochasticity of the switch. MBio 2016, 7, e01565-15. [Google Scholar] [CrossRef] [PubMed]
- Alkafeef, S.S.; Yu, C.; Huang, L.; Liu, H. Wor1 establishes opaque cell fate through inhibition of the general co-repressor Tup1 in Candida albicans. PLoS Genet. 2018, 14, e1007176. [Google Scholar] [CrossRef]
- Srikantha, T.; Borneman, A.R.; Daniels, K.J.; Pujol, C.; Wu, W.; Seringhaus, M.R.; Gerstein, M.; Yi, S.; Snyder, M.; Soll, D.R. TOS9 regulates white-opaque switching in Candida albicans. Eukaryot. Cell 2006, 5, 1674–1687. [Google Scholar] [CrossRef]
- Neph, S.; Stergachis, A.B.; Reynolds, A.; Sandstrom, R.; Borenstein, E.; Stamatoyannopoulos, J.A. Circuitry and dynamics of human transcription factor regulatory networks. Cell 2012, 150, 1274–1286. [Google Scholar] [CrossRef]
- Loh, Y.-H.; Wu, Q.; Chew, J.-L.; Vega, V.B.; Zhang, W.; Chen, X.; Bourque, G.; George, J.; Leong, B.; Liu, J.; et al. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat. Genet. 2006, 38, 431–440. [Google Scholar] [CrossRef]
- Chen, X.; Xu, H.; Yuan, P.; Fang, F.; Huss, M.; Vega, V.B.; Wong, E.; Orlov, Y.L.; Zhang, W.; Jiang, J.; et al. Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell 2008, 133, 1106–1117. [Google Scholar] [CrossRef]
- Shannon, P. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Askew, C.; Sellam, A.; Epp, E.; Mallick, J.; Hogues, H.; Mullick, A.; Nantel, A.; Whiteway, M. The zinc cluster transcription factor Ahr1p directs Mcm1p regulation of Candida albicans adhesion. Mol. Microbiol. 2011, 79, 940–953. [Google Scholar] [CrossRef]
- Brown, D.H., Jr.; Giusani, A.D.; Chen, X.; Kumamoto, C.A. Filamentous growth of Candida albicans in response to physical environmental cues and its regulation by the unique CZF1 gene. Mol. Microbiol. 1999, 34, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Doedt, T.; Krishnamurthy, S.; Bockmühl, D.P.; Tebarth, B.; Stempel, C.; Russell, C.L.; Brown, A.J.P.; Ernst, J.F. APSES proteins regulate morphogenesis and metabolism in Candida albicans. Mol. Biol. Cell 2004, 15, 3167–3180. [Google Scholar] [CrossRef] [PubMed]
- Korting, H.C.; Hube, B.; Oberbauer, S.; Januschke, E.; Hamm, G.; Albrecht, A.; Borelli, C.; Schaller, M. Reduced expression of the hyphal-independent Candida albicans proteinase genes SAP1 and SAP3 in the efg1 mutant is associated with attenuated virulence during infection of oral epithelium. J. Med. Microbiol. 2003, 52, 623–632. [Google Scholar] [CrossRef]
- Hwang, C.S.; Oh, J.H.; Huh, W.K.; Yim, H.S.; Kang, S.O. Ssn6, an important factor of morphological conversion and virulence in Candida albicans. Mol. Microbiol. 2003, 47, 1029–1043. [Google Scholar] [CrossRef]
- García-Sánchez, S.; Mavor, A.L.; Russell, C.L.; Argimon, S.; Dennison, P.; Enjalbert, B.; Brown, A.J.P. Global roles of Ssn6 in Tup1- and Nrg1-dependent gene regulation in the fungal pathogen, Candida albicans. Mol. Biol. Cell 2005, 16, 2913–2925. [Google Scholar] [CrossRef]
- Li, F.; Palecek, S.P. Identification of Candida albicans genes that induce Saccharomyces cerevisiae cell adhesion and morphogenesis. Biotechnol. Prog. 2005, 21, 1601–1609. [Google Scholar] [CrossRef]
- Singh, R.P.; Prasad, H.K.; Sinha, I.; Agarwal, N.; Natarajan, K. Cap2-HAP complex is a critical transcriptional regulator that has dual but contrasting roles in regulation of iron homeostasis in Candida albicans. J. Biol. Chem. 2011, 286, 25154–25170. [Google Scholar] [CrossRef]
- Fu, Y.; Filler, S.G.; Spellberg, B.J.; Fonzi, W.; Ibrahim, A.S.; Kanbe, T.; Ghannoum, M.A.; Edwards, J.E. Cloning and characterization of CAD1/AAF1, a gene from Candida albicans that induces adherence to endothelial cells after expression in Saccharomyces cerevisiae. Infect. Immun. 1998, 66, 2078–2084. [Google Scholar] [CrossRef]
- Liang, Y.; Wei, D.; Wang, H.; Xu, N.; Zhang, B.; Xing, L.; Li, M. Role of Candida albicans Aft2p transcription factor in ferric reductase activity, morphogenesis and virulence. Microbiology 2010, 156, 2912–2919. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Cheng, X.; Yu, Q.; Qian, K.; Ding, X.; Liu, R.; Zhang, B.; Xing, L.; Li, M. Aft2, a novel transcription regulator, is required for iron metabolism, oxidative stress, surface adhesion and hyphal development in Candida albicans. PLoS ONE 2013, 8, e62367. [Google Scholar] [CrossRef] [PubMed]
- Nobile, C.J.; Mitchell, A.P. Regulation of cell-surface genes and biofilm formation by the C. albicans transcription factor Bcr1p. Curr. Biol. 2005, 15, 1150–1155. [Google Scholar] [CrossRef]
- Coste, A.T.; Ramsdale, M.; Ischer, F.; Sanglard, D. Divergent functions of three Candida albicans zinc-cluster transcription factors (CTA4, ASG1 and CTF1) complementing pleiotropic drug resistance in Saccharomyces cerevisiae. Microbiology 2008, 154, 1491–1501. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mulhern, S.M.; Logue, M.E.; Butler, G. Candida albicans transcription factor Ace2 regulates metabolism and is required for filamentation in hypoxic conditions. Eukaryot. Cell 2006, 5, 2001–2013. [Google Scholar] [CrossRef]
- Inglis, D.O.; Johnson, A.D. Ash1 Protein, an asymmetrically localized transcriptional regulator, controls filamentous growth and virulence of Candida albicans. Mol. Cell. Biol. 2002, 22, 8669–8680. [Google Scholar] [CrossRef]
- Wangsanut, T.; Ghosh, A.K.; Metzger, P.G.; Fonzi, W.A.; Rolfes, R.J. Grf10 and Bas1 regulate transcription of adenylate and one-carbon biosynthesis genes and affect virulence in the human fungal pathogen Candida albicans. mSphere 2017, 2, e00161-17. [Google Scholar] [CrossRef]
- Nobile, C.J.; Fox, E.P.; Nett, J.E.; Sorrells, T.R.; Mitrovich, Q.M.; Hernday, A.D.; Tuch, B.B.; Andes, D.R.; Johnson, A.D. A recently evolved transcriptional network controls biofilm development in Candida albicans. Cell 2012, 148, 126–138. [Google Scholar] [CrossRef]
- Srikantha, T.; Daniels, K.J.; Pujol, C.; Kim, E.; Soll, D.R. Identification of genes upregulated by the transcription factor Bcr1 that are involved in impermeability, impenetrability, and drug resistance of Candida albicans a/α biofilms. Eukaryot. Cell 2013, 12, 875–888. [Google Scholar] [CrossRef]
- Nobile, C.J.; Andes, D.R.; Nett, J.E.; Smith, F.J.; Yue, F.; Phan, Q.T.; Edwards, J.E.; Filler, S.G.; Mitchell, A.P. Critical role of Bcr1-dependent adhesins in C. albicans biofilm formation in vitro and in vivo. PLoS Pathog. 2006, 2, e63. [Google Scholar] [CrossRef]
- Lu, Y.; Su, C.; Liu, H. A GATA transcription factor recruits Hda1 in response to reduced Tor1 signaling to establish a hyphal chromatin state in Candida albicans. PLoS Pathog. 2012, 8, e1002663. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Guan, G.; Xie, J.; Sun, Y.; Tong, Y.; Zhang, L.; Huang, G. Roles of Candida albicans Gat2, a GATA-type zinc finger transcription factor, in biofilm formation, filamentous growth and virulence. PLoS ONE 2012, 7, e29707. [Google Scholar] [CrossRef] [PubMed]
- Alarco, A.-M.; Raymond, M. The bZip transcription factor Cap1p is involved in multidrug resistance and oxidative stress response in Candida albicans. J. Bacteriol. 1999, 181, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; De Micheli, M.; Coleman, S.T.; Sanglard, D.; Moye-Rowley, W.S. Analysis of the oxidative stress regulation of the Candida albicans transcription factor, Cap1p. Mol. Microbiol. 2002, 36, 618–629. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, Y.-Y.; Jia, X.-M.; Cao, Y.-B.; Gao, P.-H.; Fu, X.-P.; Ying, K.; Chen, W.-S.; Jiang, Y.-Y. Cap1p is involved in multiple pathways of oxidative stress response in Candida albicans. Free Radic. Biol. Med. 2006, 40, 1201–1209. [Google Scholar] [CrossRef]
- Dai, B.-D.; Wang, Y.; Zhao, L.-X.; Li, D.-D.; Li, M.-B.; Cao, Y.-B.; Jiang, Y.-Y. Cap1p attenuates the apoptosis of Candida albicans. FEBS J. 2013, 280, 2633–2643. [Google Scholar] [CrossRef]
- Kelly, J.; Rowan, R.; Mccann, M.; Kavanagh, K. Exposure to caspofungin activates Cap and Hog pathways in Candida albicans. Med. Mycol. 2009, 47, 697–706. [Google Scholar] [CrossRef]
- Bruno, V.M.; Kalachikov, S.; Subaran, R.; Nobile, C.J.; Kyratsous, C.; Mitchell, A.P. Control of the C. albicans cell wall damage response by transcriptional regulator Cas5. PLoS Pathog. 2006, 2, e21. [Google Scholar] [CrossRef]
- Xie, J.L.; Qin, L.; Miao, Z.; Grys, B.T.; Diaz, J.D.L.C.; Ting, K.; Krieger, J.R.; Tong, J.; Tan, K.; Leach, M.D.; et al. The Candida albicans transcription factor Cas5 couples stress responses, drug resistance and cell cycle regulation. Nat. Commun. 2017, 8, 499. [Google Scholar] [CrossRef]
- Chamilos, G.; Nobile, C.J.; Bruno, V.M.; Lewis, R.E.; Mitchell, A.P.; Kontoyiannis, D.P. Candida albicans Cas5, a regulator of cell wall integrity, is required for virulence in murine and Toll mutant fly models. J. Infect. Dis. 2009, 200, 152–157. [Google Scholar] [CrossRef]
- Liu, H.; Kohler, J.; Fink, G. Suppression of hyphal formation in Candida albicans by mutation of a STE12 homolog. Science 1994, 266, 1723–1726. [Google Scholar] [CrossRef] [PubMed]
- Malathi, K.; Ganesan, K.; Datta, A. Identification of a putative transcription factor in Candida albicans that can complement the mating defect of Saccharomyces cerevisiae ste12 mutants. J. Biol. Chem. 1994, 269, 22945–22951. [Google Scholar] [CrossRef]
- Chen, J.; Chen, J.; Lane, S.; Liu, H. A conserved mitogen-activated protein kinase pathway is required for mating in Candida albicans. Mol. Microbiol. 2002, 46, 1335–1344. [Google Scholar] [CrossRef]
- Magee, B.B.; Legrand, M.; Alarco, A.-M.; Raymond, M.; Magee, P.T. Many of the genes required for mating in Saccharomyces cerevisiae are also required for mating in Candida albicans. Mol. Microbiol. 2002, 46, 1345–1351. [Google Scholar] [CrossRef] [PubMed]
- Lane, S.; Zhou, S.; Pan, T.; Dai, Q.; Liu, H. The basic helix-loop-helix transcription factor Cph2 regulates hyphal development in Candida albicans partly via Tec1. Mol. Cell. Biol. 2001, 21, 6418–6428. [Google Scholar] [CrossRef] [PubMed]
- Karababa, M.; Valentino, E.; Pardini, G.; Coste, A.T.; Bille, J.; Sanglard, D. CRZ1, a target of the calcineurin pathway in Candida albicans. Mol. Microbiol. 2006, 59, 1429–1451. [Google Scholar] [CrossRef]
- Onyewu, C.; Wormley, F.L.; Perfect, J.R.; Heitman, J. The calcineurin target, Crz1, functions in azole tolerance but is not required for virulence of Candida albicans. Infect. Immun. 2004, 72, 7330–7333. [Google Scholar] [CrossRef]
- Santos, M.; de Larrinoa, I.F. Functional characterization of the Candida albicans CRZ1 gene encoding a calcineurin-regulated transcription factor. Curr. Genet. 2005, 48, 88–100. [Google Scholar] [CrossRef]
- Wang, H.; Liang, Y.; Zhang, B.; Zheng, W.; Xing, L.; Li, M. Alkaline stress triggers an immediate calcium fluctuation in Candida albicans mediated by Rim101p and Crz1p transcription factors. FEMS Yeast Res. 2011, 11, 430–439. [Google Scholar] [CrossRef]
- Hameed, S.; Dhamgaye, S.; Singh, A.; Goswami, S.K.; Prasad, R. Calcineurin signaling and membrane lipid homeostasis regulates iron mediated multidrug resistance mechanisms in Candida albicans. PLoS ONE 2011, 6, e18684. [Google Scholar] [CrossRef]
- Kim, W.-I.; Lee, W.-B.; Song, K.; Kim, J. Identification of a putative DEAD-box RNA helicase and a zinc-finger protein in Candida albicans by functional complementation of the S. cerevisiae rok1 mutation. Yeast 2000, 16, 401–409. [Google Scholar] [CrossRef]
- Kim, M.J.; Kil, M.; Jung, J.H.; Kim, J. Roles of zinc-responsive transcription factor Csr1 in filamentous growth of the pathogenic yeast Candida albicans. J. Microbiol. Biotechnol. 2008, 18, 242–247. [Google Scholar] [PubMed]
- Chiranand, W.; McLeod, I.; Zhou, H.; Lynn, J.J.; Vega, L.A.; Myers, H.; Yates, J.R.; Lorenz, M.C.; Gustin, M.C. CTA4 transcription factor mediates induction of nitrosative stress response in Candida albicans. Eukaryot. Cell 2008, 7, 268–278. [Google Scholar] [CrossRef]
- Buchman, C.; Skroch, P.; Welch, J.; Fogel, S.; Karin, M. The CUP2 gene product, regulator of yeast metallothionein expression, is a copper-activated DNA-binding protein. Mol. Cell. Biol. 1989, 9, 4091–4095. [Google Scholar] [CrossRef] [PubMed]
- Nantel, A.; Dignard, D.; Bachewich, C.; Harcus, D.; Marcil, A.; Bouin, A.P.; Sensen, C.W.; Hogues, H.; Van het Hoog, M.; Gordon, P.; et al. Transcription profiling of Candida albicans cells undergoing the yeast-to-hyphal transition. Mol. Biol. Cell 2002, 13, 3452–3465. [Google Scholar] [CrossRef]
- Finkel, J.S.; Xu, W.; Huang, D.; Hill, E.M.; Desai, J.V.; Woolford, C.A.; Nett, J.E.; Taff, H.; Norice, C.T.; Andes, D.R.; et al. Portrait of Candida albicans adherence regulators. PLoS Pathog. 2012, 8, e1002525. [Google Scholar] [CrossRef] [PubMed]
- Khamooshi, K.; Sikorski, P.; Sun, N.; Calderone, R.; Li, D. The Rbf1, Hfl1 and Dbp4 of Candida albicans regulate common as well as transcription factor-specific mitochondrial and other cell activities. BMC Genom. 2014, 15, 56. [Google Scholar] [CrossRef]
- Talibi, D.; Raymond, M. Isolation of a putative Candida albicans transcriptional regulator involved in pleiotropic drug resistance by functional complementation of a pdr1 pdr3 Mutation in Saccharomyces cerevisiae. J. Bacteriol. 1999, 181, 231–240. [Google Scholar] [CrossRef]
- Uhl, M.A. Haploinsufficiency-based large-scale forward genetic analysis of filamentous growth in the diploid human fungal pathogen C.albicans. EMBO J. 2003, 22, 2668–2678. [Google Scholar] [CrossRef]
- Cao, F.; Lane, S.; Raniga, P.P.; Lu, Y.; Zhou, Z.; Ramon, K.; Chen, J.; Liu, H. The Flo8 transcription factor is essential for hyphal development and virulence in Candida albicans. Mol. Biol. Cell 2006, 17, 295–307. [Google Scholar] [CrossRef]
- Du, H.; Guan, G.; Xie, J.; Cottier, F.; Sun, Y.; Jia, W.; Mühlschlegel, F.A.; Huang, G. The transcription factor Flo8 mediates CO2 sensing in the human fungal pathogen Candida albicans. Mol. Biol. Cell 2012, 23, 2692–2701. [Google Scholar] [CrossRef] [PubMed]
- Askew, C.; Sellam, A.; Epp, E.; Hogues, H.; Mullick, A.; Nantel, A.; Whiteway, M. Transcriptional regulation of carbohydrate metabolism in the human pathogen Candida albicans. PLoS Pathog. 2009, 5, e1000612. [Google Scholar] [CrossRef] [PubMed]
- Homann, O.R.; Dea, J.; Noble, S.M.; Johnson, A.D. A phenotypic profile of the Candida albicans regulatory network. PLoS Genet. 2009, 5, e1000783. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Wangsanut, T.; Fonzi, W.A.; Rolfes, R.J. The GRF10 homeobox gene regulates filamentous growth in human fungal pathogen Candida albicans. FEMS Yeast Res. 2015, 15, fov093. [Google Scholar] [CrossRef] [PubMed]
- Baek, Y.-U.; Li, M.; Davis, D.A. Candida albicans ferric reductases are differentially regulated in response to distinct forms of iron limitation by the Rim101 and CBF transcription factors. Eukaryot. Cell 2008, 7, 1168–1179. [Google Scholar] [CrossRef]
- Bensen, E.S.; Filler, S.G.; Berman, J. A forkhead transcription factor is important for true hyphal as well as yeast morphogenesis in Candida albicans. Eukaryot. Cell 2002, 1, 787–798. [Google Scholar] [CrossRef]
- Araki, H.; Hamatake, R.K.; Morrison, A.; Johnson, A.L.; Johnston, L.H.; Sugino, A. Cloning DPB3, the gene encoding the third subunit of DNA polymerase II of Saccharomyces cerevisiae. Nucleic Acids Res. 1991, 19, 4867–4872. [Google Scholar] [CrossRef][Green Version]
- Hoppen, J.; Dietz, M.; Warsow, G.; Rohde, R.; Schüller, H.J. Ribosomal protein genes in the yeast Candida albicans may be activated by a heterodimeric transcription factor related to Ino2 and Ino4 from S. cerevisiae. Mol. Genet. Genom. 2007, 278, 317–330. [Google Scholar] [CrossRef]
- Enjalbert, B.; Smith, D.A.; Cornell, M.J.; Alam, I.; Nicholls, S.; Brown, A.J.P.; Quinn, J. Role of the Hog1 stress-activated protein kinase in the global transcriptional response to stress in the fungal pathogen Candida albicans. Mol. Biol. Cell 2006, 17, 1018–1032. [Google Scholar] [CrossRef]
- Lockhart, S.R.; Zhao, R.; Daniels, K.J.; Soll, D.R. Alpha-pheromone-induced “shmooing” and gene regulation require white-opaque switching during Candida albicans mating. Eukaryot. Cell 2003, 2, 847–855. [Google Scholar] [CrossRef]
- Bennett, R.J.; Uhl, M.A.; Miller, M.G.; Johnson, A.D. Identification and characterization of a Candida albicans mating pheromone. Mol. Cell. Biol. 2003, 23, 8189–8201. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.-H.; Nie, X.-Y.; Chen, J.-Y. CaMac1, a Candida albicans copper ion-sensing transcription factor, promotes filamentous and invasive growth in Saccharomyces cerevisiae. Acta Biochim. Biophys. Sin. 2006, 38, 213–217. [Google Scholar] [CrossRef]
- Lagree, K.; Woolford, C.A.; Huang, M.Y.; May, G.; McManus, C.J.; Solis, N.V.; Filler, S.G.; Mitchell, A.P. Roles of Candida albicans Mig1 and Mig2 in glucose repression, pathogenicity traits, and SNF1 essentiality. PLOS Genet. 2020, 16, e1008582. [Google Scholar] [CrossRef] [PubMed]
- Murad, A.M.A.; D’Enfert, C.; Gaillardin, C.; Tournu, H.; Tekaia, F.; Talibi, D.; Marechal, D.; Marchais, V.; Cottin, J.; Brown, A.J.P. Transcript profiling in Candida albicans reveals new cellular functions for the transcriptional repressors CaTup1, CaMig1 and CaNrg1. Mol. Microbiol. 2001, 42, 981–993. [Google Scholar] [CrossRef] [PubMed]
- Zaragoza, O.; Rodríguez, C.; Gancedo, C. Isolation of the MIG1 gene from Candida albicans and effects of its disruption on catabolite repression. J. Bacteriol. 2000, 182, 320–326. [Google Scholar] [CrossRef]
- Chen, C.-G.; Yang, Y.-L.; Shih, H.-I.; Su, C.-L.; Lo, H.-J. CaNdt80 is involved in drug resistance in Candida albicans by regulating CDR1. Antimicrob. Agents Chemother. 2004, 48, 4505–4512. [Google Scholar] [CrossRef]
- Heyken, W.-T.; Wagner, C.; Wittmann, J.; Albrecht, A.; Schüller, H.-J. Negative regulation of phospholipid biosynthesis in Saccharomyces cerevisiae by a Candida albicans orthologue of OPI1. Yeast 2003, 20, 1177–1188. [Google Scholar] [CrossRef]
- Chen, Y.-L.; de Bernardis, F.; Yu, S.-J.; Sandini, S.; Kauffman, S.; Tams, R.N.; Bethea, E.; Reynolds, T.B. Candida albicans OPI1 regulates filamentous growth and virulence in vaginal infections, but not inositol biosynthesis. PLoS ONE 2015, 10, e0116974. [Google Scholar] [CrossRef]
- Biswas, K.; Rieger, K.-J.; Morschhäuser, J. Functional analysis of CaRAP1, encoding the repressor/activator protein 1 of Candida albicans. Gene 2003, 307, 151–158. [Google Scholar] [CrossRef]
- Yu, E.Y.; Yen, W.-F.; Steinberg-Neifach, O.; Lue, N.F. Rap1 in Candida albicans: An unusual structural organization and a critical function in suppressing telomere recombination. Mol. Cell. Biol. 2010, 30, 1254–1268. [Google Scholar] [CrossRef]
- Uemura, H.; Watanabe-Yoshida, M.; Ishii, N.; Shinzato, T.; Haw, R.; Aoki, Y. Isolation and characterization of Candida albicans homologue of RAP1, a repressor and activator protein gene in Saccharomyces cerevisiae. Yeast 2004, 21, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ishii, N.; Yamamoto, M.; Yoshihara, F.; Arisawa, M.; Aoki, Y. Biochemical and genetic characterization of Rbf1p, a putative transcription factor of Candida albicans. Microbiology 1997, 143, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Cottier, F.; Raymond, M.; Kurzai, O.; Bolstad, M.; Leewattanapasuk, W.; Jiménez-López, C.; Lorenz, M.C.; Sanglard, D.; Váchová, L.; Pavelka, N.; et al. The bZIP transcription factor Rca1p is a central regulator of a novel CO2 sensing pathway in yeast. PLoS Pathog. 2012, 8, e1002485. [Google Scholar] [CrossRef] [PubMed]
- Vandeputte, P.; Pradervand, S.; Ischer, F.; Coste, A.T.; Ferrari, S.; Harshman, K.; Sanglard, D. Identification and functional characterization of Rca1, a transcription factor involved in both antifungal susceptibility and host response in Candida albicans. Eukaryot. Cell 2012, 11, 916–931. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-G.; Yang, Y.-L.; Tseng, K.-Y.; Shih, H.-I.; Liou, C.-H.; Lin, C.-C.; Lo, H.-J. Rep1p negatively regulating MDR1 efflux pump involved in drug resistance in Candida albicans. Fungal Genet. Biol. 2009, 46, 714–720. [Google Scholar] [CrossRef]
- Kadosh, D.; Johnson, A.D. Rfg1, a protein related to the Saccharomyces cerevisiae hypoxic regulator Rox1, controls filamentous growth and virulence in Candida albicans. Mol. Cell. Biol. 2001, 21, 2496–2505. [Google Scholar] [CrossRef]
- Khalaf, R.A.; Zitomer, R.S. The DNA binding protein Rfg1 is a repressor of filamentation in Candida albicans. Genetics 2001, 157, 1503–1512. [Google Scholar]
- Cleary, I.A.; Mulabagal, P.; Reinhard, S.M.; Yadev, N.P.; Murdoch, C.; Thornhill, M.H.; Lazzell, A.L.; Monteagudo, C.; Thomas, D.P.; Saville, S.P. Pseudohyphal regulation by the transcription factor Rfg1p in Candida albicans. Eukaryot. Cell 2010, 9, 1363–1373. [Google Scholar] [CrossRef]
- Hao, B.; Clancy, C.J.; Cheng, S.; Raman, S.B.; Iczkowski, K.A.; Nguyen, M.H. Candida albicans RFX2 encodes a DNA binding protein involved in dna damage responses, morphogenesis, and virulence. Eukaryot. Cell 2009, 8, 627–639. [Google Scholar] [CrossRef]
- Rogers, P.D.; Barker, K.S. Genome-wide expression profile analysis reveals coordinately regulated genes associated with stepwise acquisition of azole resistance in Candida albicans clinical isolates. Antimicrob. Agents Chemother. 2003, 47, 1220–1227. [Google Scholar] [CrossRef]
- Mannhaupt, G.; Schnall, R.; Karpov, V.; Vetter, I.; Feldmann, H. Rpn4p acts as a transcription factor by binding to PACE, a nonamer box found upstream of 26S proteasomal and other genes in yeast. FEBS Lett. 1999, 450, 27–34. [Google Scholar] [CrossRef]
- Xie, Y.; Varshavsky, A. RPN4 is a ligand, substrate, and transcriptional regulator of the 26S proteasome: A negative feedback circuit. Proc. Natl. Acad. Sci. USA 2001, 98, 3056–3061. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Velásquez, S.D.; Tint, S.H.; del Olmo Toledo, V.; Torsin, S.; De, S.; Pérez, J.C. The regulatory proteins Rtg1/3 govern sphingolipid homeostasis in the human-associated yeast Candida albicans. Cell Rep. 2020, 30, 620–629. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Wendland, J. Candida albicans Sfl1 suppresses flocculation and filamentation. Eukaryot. Cell 2007, 6, 1736–1744. [Google Scholar] [CrossRef]
- Li, Y.; Su, C.; Mao, X.; Cao, F.; Chen, J. Roles of Candida albicans Sfl1 in hyphal development. Eukaryot. Cell 2007, 6, 2112–2121. [Google Scholar] [CrossRef]
- Singh, P.; Chauhan, N.; Ghosh, A.; Dixon, F.; Calderone, R. SKN7 of Candida albicans: Mutant construction and phenotype analysis. Infect. Immun. 2004, 72, 2390–2394. [Google Scholar] [CrossRef]
- Rauceo, J.M.; Blankenship, J.R.; Fanning, S.; Hamaker, J.J.; Deneault, J.-S.; Smith, F.J.; Nantel, A.; Mitchell, A.P. Regulation of the Candida albicans cell wall damage response by transcription factor Sko1 and PAS kinase Psk1. Mol. Biol. Cell 2008, 19, 2741–2751. [Google Scholar] [CrossRef]
- Alonso-Monge, R.; Román, E.; Arana, D.M.; Prieto, D.; Urrialde, V.; Nombela, C.; Pla, J. The Sko1 protein represses the yeast-to-hypha transition and regulates the oxidative stress response in Candida albicans. Fungal Genet. Biol. 2010, 47, 587–601. [Google Scholar] [CrossRef]
- Heredia, M.Y.; Ikeh, M.A.C.; Gunasekaran, D.; Conrad, K.A.; Filimonava, S.; Marotta, D.H.; Nobile, C.J.; Rauceo, J.M. An expanded cell wall damage signaling network is comprised of the transcription factors Rlm1 and Sko1 in Candida albicans. PLOS Genet. 2020, 16, e1008908. [Google Scholar] [CrossRef]
- Martínez, P.; Ljungdahl, P.O. Divergence of Stp1 and Stp2 transcription factors in Candida albicans places virulence factors required for proper nutrient acquisition under amino acid control. Mol. Cell. Biol. 2005, 25, 9435–9446. [Google Scholar] [CrossRef]
- Hussein, B.; Huang, H.; Glory, A.; Osmani, A.; Kaminskyj, S.; Nantel, A.; Bachewich, C. G1/S transcription factor orthologues Swi4p and Swi6p are important but not essential for cell proliferation and influence hyphal development in the fungal pathogen Candida albicans. Eukaryot. Cell 2011, 10, 384–397. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, M.; Thompson, D.S.; Lazzell, A.; Carlisle, P.L.; Pierce, C.; Monteagudo, C.; López-Ribot, J.L.; Kadosh, D. UME6, a novel filament-specific regulator of Candida albicans hyphal extension and virulence. Mol. Biol. Cell 2008, 19, 1354–1365. [Google Scholar] [CrossRef] [PubMed]
- Childers, D.S.; Mundodi, V.; Banerjee, M.; Kadosh, D. A 5’ UTR-mediated translational efficiency mechanism inhibits the Candida albicans morphological transition. Mol. Microbiol. 2014, 92, 570–585. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Su, C.; Ray, S.; Yuan, Y.; Liu, H. CO2 signaling through the Ptc2-Ssn3 axis governs sustained hyphal development of Candida albicans by reducing Ume6 phosphorylation and degradation. MBio 2019, 10, e02320-18. [Google Scholar] [CrossRef] [PubMed]
- Zeidler, U.; Lettner, T.; Lassnig, C.; Müller, M.; Lajko, R.; Hintner, H.; Breitenbach, M.; Bito, A. UME6 is a crucial downstream target of other transcriptional regulators of true hyphal development in Candida albicans. FEMS Yeast Res. 2009, 9, 126–142. [Google Scholar] [CrossRef]
- Silver, P.M.; Oliver, B.G.; White, T.C. Role of Candida albicans transcription factor upc2p in drug resistance and sterol metabolism. Eukaryot. Cell 2004, 3, 1391–1397. [Google Scholar] [CrossRef]
- Hoot, S.J.; Oliver, B.G.; White, T.C. Candida albicans UPC2 is transcriptionally induced in response to antifungal drugs and anaerobicity through Upc2p-dependent and -independent mechanisms. Microbiology 2008, 154, 2748–2756. [Google Scholar] [CrossRef]
- MacPherson, S.; Akache, B.; Weber, S.; De Deken, X.; Raymond, M.; Turcotte, B. Candida albicans zinc cluster protein Upc2p confers resistance to antifungal drugs and is an activator of ergosterol biosynthetic genes. Antimicrob. Agents Chemother. 2005, 49, 1745–1752. [Google Scholar] [CrossRef]
- Hoot, S.J.; Brown, R.P.; Oliver, B.G.; White, T.C. The UPC2 promoter in Candida albicans contains two cis-acting elements that bind directly to Upc2p, resulting in transcriptional autoregulation. Eukaryot. Cell 2010, 9, 1354–1362. [Google Scholar] [CrossRef]
- Lebel, K.; MacPherson, S.; Turcotte, B. New tools for phenotypic analysis in Candida albicans: The WAR1 gene confers resistance to sorbate. Yeast 2006, 23, 249–259. [Google Scholar] [CrossRef]
- Nett, J.E.; Lepak, A.J.; Marchillo, K.; Andes, D.R. Time course global gene expression analysis of an in vivo Candida biofilm. J. Infect. Dis. 2009, 200, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Hennicke, F.; Grumbt, M.; Lermann, U.; Ueberschaar, N.; Palige, K.; Böttcher, B.; Jacobsen, I.D.; Staib, C.; Morschhäuser, J.; Monod, M.; et al. Factors supporting cysteine tolerance and sulfite production in Candida albicans. Eukaryot. Cell 2013, 12, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Chebaro, Y.; Lorenz, M.; Fa, A.; Zheng, R.; Gustin, M. Adaptation of Candida albicans to reactive sulfur species. Genetics 2017, 206, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Pande, K.; French, S.D.; Tuch, B.B.; Noble, S.M. An iron homeostasis regulatory circuit with reciprocal roles in Candida albicans commensalism and pathogenesis. Cell Host Microbe 2011, 10, 118–135. [Google Scholar] [CrossRef] [PubMed]
- Hnisz, D.; Sehwarzmüller, T.; Kuchler, K. Transcriptional loops meet chromatin: A dual-layer network controls white-opaque switching in Candida albicans. Mol. Microbiol. 2009, 74, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Jenull, S.; Tscherner, M.; Kuchler, K. Roles in regulating the white-opaque switch in the fungal pathogen Candida albicans. MBio 2016, 7, e01807-16. [Google Scholar] [CrossRef] [PubMed]
- Srikantha, T.; Tsai, L.; Daniels, K.; Klar, A.J.S.; Soll, D.R. The histone deacetylase genes HDA1 and RPD3 play distinct roles in regulation of high-frequency phenotypic switching in Candida albicans. J. Bacteriol. 2001, 183, 4614–4625. [Google Scholar] [CrossRef]
- Klar, A.J.S.; Srikantha, T.; Soll, D.R. A histone deacetylation inhibitor and mutant promote colony-type switching of the human pathogen Candida albicans. Genetics 2001, 158, 919–924. [Google Scholar]
- Stevenson, J.S.; Liu, H. Nucleosome assembly factors CAF-1 and HIR modulate epigenetic switching frequencies in an H3K56 acetylation-associated manner in Candida albicans. Eukaryot. Cell 2013, 12, 591–603. [Google Scholar] [CrossRef]
- Tscherner, M.; Stappler, E.; Hnisz, D.; Kuchler, K. The histone acetyltransferase Hat1 facilitates DNA damage repair and morphogenesis in Candida albicans. Mol. Microbiol. 2012, 86, 1197–1214. [Google Scholar] [CrossRef]
- Klemm, S.L.; Shipony, Z.; Greenleaf, W.J. Chromatin accessibility and the regulatory epigenome. Nat. Rev. Genet. 2019, 20, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Williamson, W.D.; Pinto, I. Histones and genome integrity. Front. Biosci. 2012, 17, 984–995. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, M.; Daujat, S.; Schneider, R. Lateral thinking: How histone modifications regulate gene expression. Trends Genet. 2016, 32, 42–56. [Google Scholar] [CrossRef] [PubMed]
- Tessarz, P.; Kouzarides, T. Histone core modifications regulating nucleosome structure and dynamics. Nat. Rev. Mol. Cell Biol. 2014, 15, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Badeaux, A.I.; Shi, Y. Emerging roles for chromatin as a signal integration and storage platform. Nat. Rev. Mol. Cell Biol. 2013, 14, 211–224. [Google Scholar] [CrossRef]
- Rando, O.J.; Winston, F. Chromatin and transcription in yeast. Genetics 2012, 190, 351–387. [Google Scholar] [CrossRef]
- Kurdistani, S.K.; Grunstein, M. Histone acetylation and deacetylation in yeast. Nat. Rev. Mol. Cell Biol. 2003, 4, 276–284. [Google Scholar] [CrossRef]
- Jaiswal, D.; Turniansky, R.; Green, E.M. Choose your own adventure: The role of histone modifications in yeast cell fate. J. Mol. Biol. 2017, 429, 1946–1957. [Google Scholar] [CrossRef]
- Sheikh, B.N.; Akhtar, A. The many lives of KATs—detectors, integrators and modulators of the cellular environment. Nat. Rev. Genet. 2019, 20, 7–23. [Google Scholar] [CrossRef]
- Marmorstein, R.; Trievel, R.C. Histone modifying enzymes: Structures, mechanisms, and specificities. Biochim. Biophys. Acta Gene Regul. Mech. 2009, 1789, 58–68. [Google Scholar] [CrossRef]
- Marmorstein, R.; Zhou, M.M. Writers and readers of histone acetylation: Structure, mechanism, and inhibition. Cold Spring Harb. Perspect. Biol. 2014, 6, a018762. [Google Scholar] [CrossRef] [PubMed]
- Gurard-Levin, Z.A.; Quivy, J.-P.; Almouzni, G. Histone chaperones: Assisting histone traffic and nucleosome dynamics. Annu. Rev. Biochem. 2014, 83, 487–517. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.D.; Vishnoi, N.; Prochasson, P. A global requirement for the HIR complex in the assembly of chromatin. Biochim. Biophys. Acta Gene Regul. Mech. 2012, 1819, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Clapier, C.R.; Iwasa, J.; Cairns, B.R.; Peterson, C.L. Mechanisms of action and regulation of ATP-dependent chromatin-remodelling complexes. Nat. Rev. Mol. Cell Biol. 2017, 18, 407–422. [Google Scholar] [CrossRef]
- Venkatesh, S.; Workman, J.L. Histone exchange, chromatin structure and the regulation of transcription. Nat. Rev. Mol. Cell Biol. 2015, 16, 178–189. [Google Scholar] [CrossRef]
- Pérez-Martín, J.; Uría, J.A.; Johnson, A.D. Phenotypic switching in Candida albicans is controlled by a SIR2 gene. EMBO J. 1999, 18, 2580–2592. [Google Scholar] [CrossRef]
- Stevenson, J.; Krycer, J.R.; Phan, L.; Brown, A.J. A practical comparison of ligation-independent cloning techniques. PLoS ONE 2013, 8, e83888. [Google Scholar] [CrossRef]
- Peterson, M.R.; Price, R.J.; Gourlay, S.; May, A.; Tullet, J.; Buscaino, A. The fungal-specific Hda2 and Hda3 proteins regulate morphological switches in the human fungal pathogen Candida albicans. bioRxiv 2018, 340364. [Google Scholar] [CrossRef]
- Kročová, E.; Neradová, S.; Kupcik, R.; Janovská, S.; Bílková, Z.; Heidingsfeld, O. PHO15 genes of Candida albicans and Candida parapsilosis encode HAD-Type phosphatases dephosphorylating 2-phosphoglycolate. FEMS Yeast Res. 2019, 19, foy112. [Google Scholar] [CrossRef]
- Collard, F.; Baldin, F.; Gerin, I.; Bolsée, J.; Noël, G.; Graff, J.; Veiga-da-Cunha, M.; Stroobant, V.; Vertommen, D.; Houddane, A.; et al. A conserved phosphatase destroys toxic glycolytic side products in mammals and yeast. Nat. Chem. Biol. 2016, 12, 601–607. [Google Scholar] [CrossRef]
- Roth, S.Y.; Denu, J.M.; Allis, C.D. Histone acetyltransferases. Annu. Rev. Biochem. 2001, 70, 81–120. [Google Scholar] [CrossRef] [PubMed]
- Jagannath, A.; Wood, M.J.A. Efg1-mediated recruitment of NuA4 to promoters is required for hypha-specific Swi/Snf binding and activation in Candida albicans. Mol. Biol. Cell 2009, 20, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Mizuguchi, G.; Shen, X.; Landry, J.; Wu, W.H.; Sen, S.; Wu, C. ATP-driven exchange of histone h2az variant catalyzed by SWR1 chromatin remodeling complex. Science 2004, 303, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhu, W.; Chang, P.; Wu, H.; Liu, H.; Chen, J. Merge and separation of NuA4 and SWR1 complexes control cell fate plasticity in Candida albicans. Cell Discov. 2018, 4, 45. [Google Scholar] [CrossRef]
- Masumoto, H.; Hawke, D.; Kobayashi, R.; Verreault, A. A role for cell-cycle-regulated histone H3 lysine 56 acetylation in the DNA damage response. Nature 2005, 436, 294–298. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, K.; Grunstein, M. Acetylation in histone H3 globular domain regulates gene expression in yeast. Cell 2005, 121, 375–385. [Google Scholar] [CrossRef]
- Voichek, Y.; Mittelman, K.; Gordon, Y.; Bar-Ziv, R.; Lifshitz Smit, D.; Shenhav, R.; Barkai, N. Epigenetic control of expression homeostasis during replication is stabilized by the replication checkpoint. Mol. Cell 2018, 70, 1121–1133. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, Q.; Zhang, K.; Xie, W.; Grunstein, M. Sir2 deacetylates histone H3 lysine 56 to regulate telomeric heterochromatin structure in yeast. Mol. Cell 2007, 27, 890–900. [Google Scholar] [CrossRef]
- Värv, S.; Kristjuhan, K.; Peil, K.; Lõoke, M.; Mahlakõiv, T.; Paapsi, K.; Kristjuhan, A. Acetylation of H3 K56 is required for RNA polymerase II transcript elongation through heterochromatin in yeast. Mol. Cell. Biol. 2010, 30, 1467–1477. [Google Scholar] [CrossRef]
- Hu, G.; Cui, K.; Northrup, D.; Liu, C.; Wang, C.; Tang, Q.; Ge, K.; Levens, D.; Crane-Robinson, C.; Zhao, K. H2A.Z facilitates access of active and repressive complexes to chromatin in embryonic stem cell self-renewal and differentiation. Cell Stem Cell 2013, 12, 180–192. [Google Scholar] [CrossRef]
- Haigney, A.; Ricketts, M.D.; Marmorstein, R. Dissecting the molecular roles of histone chaperones in histone acetylation by type B histone acetyltransferases (HAT-B). J. Biol. Chem. 2015, 290, 30648–30657. [Google Scholar] [CrossRef] [PubMed]
- Song, O.K.; Wang, X.; Waterborg, J.H.; Sternglanz, R. An Nα-acetyltransferase responsible for acetylation of the N-terminal residues of histones H4 and H2A. J. Biol. Chem. 2003, 278, 38109–38112. [Google Scholar] [CrossRef] [PubMed]
- Ree, R.; Varland, S.; Arnesen, T. Spotlight on protein N-terminal acetylation. Exp. Mol. Med. 2018, 50, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Polevoda, B.; Hoskins, J.; Sherman, F. Properties of Nat4, an Nα-Acetyltransferase of Saccharomyces cerevisiae that modifies N termini of histones H2A and H4. Mol. Cell. Biol. 2009, 29, 2913–2924. [Google Scholar] [CrossRef] [PubMed]
- Freitag, M. Histone methylation by SET domain proteins in fungi. Annu. Rev. Microbiol. 2017, 36, 413–439. [Google Scholar] [CrossRef]
- Raman, S.B.; Nguyen, M.H.; Zhang, Z.; Cheng, S.; Jia, H.Y.; Weisner, N.; Iczkowski, K.; Clancy, C.J. Candida albicans SET1 encodes a histone 3 lysine 4 methyltransferase that contributes to the pathogenesis of invasive candidiasis. Mol. Microbiol. 2006, 60, 697–709. [Google Scholar] [CrossRef]
- Nislow, C.; Ray, E.; Pillus, L. SET1, a yeast member of the Trithorax family, functions in transcriptional silencing and diverse cellular processes. Mol. Biol. Cell 1997, 8, 2421–2436. [Google Scholar] [CrossRef]
- Downs, J.A.; Allard, S.; Jobin-Robitaille, O.; Javaheri, A.; Auger, A.; Bouchard, N.; Kron, S.J.; Jackson, S.P.; Côté, J. Binding of chromatin-modifying activities to phosphorylated histone H2A at DNA damage sites. Mol. Cell 2004, 16, 979–990. [Google Scholar] [CrossRef]
- Hnisz, D.; Bardet, A.F.; Nobile, C.J.; Petryshyn, A.; Glaser, W.; Schöck, U.; Stark, A.; Kuchler, K. A histone deacetylase adjusts transcription kinetics at coding sequences during Candida albicans morphogenesis. PLoS Genet. 2012, 8, e1003118. [Google Scholar] [CrossRef]
- Hnisz, D.; Majer, O.; Frohner, I.E.; Komnenovic, V.; Kuchler, K. The SET3/HOS2 histone deacetylase complex attenuates CAMP/pka signaling to regulate morphogenesis and virulence of Candida albicans. PLoS Pathog. 2010, 6, e1000889. [Google Scholar] [CrossRef]
- Kornitzer, D. Regulation of Candida albicans hyphal morphogenesis by endogenous signals. J. Fungi 2019, 5, 21. [Google Scholar] [CrossRef]
- Bockmühl, D.P.; Krishnamurthy, S.; Gerads, M.; Sonneborn, A.; Ernst, J.F. Distinct and redundant roles of the two protein kinase A isoforms Tpk1p and Tpk2p in morphogenesis and growth of Candida albicans. Mol. Microbiol. 2001, 42, 1243–1257. [Google Scholar] [CrossRef] [PubMed]
- Bockmüh, D.P.; Ernst, J.F. A potential phosphorylation site for an A-Type kinase in the Efgl regulator protein contributes to hyphal morphogenesis of Candida albicans. Genetics 2001, 157, 1523–1530. [Google Scholar]
- Pijnappel, W.P.; Schaft, D.; Roguev, A.; Shevchenko, A.; Tekotte, H.; Wilm, M.; Rigaut, G.; Séraphin, B.; Aasland, R.; Francis Stewart, A. The S. cerevisiae SET3 complex includes two histone deacetylases, Hos2 and Hst1, and is a meiotic-specific repressor of the sporulation gene program. Genes Dev. 2001, 15, 2991–3004. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Pierce, M.; Gailus-Durner, V.; Wagner, M.; Winter, E.; Vershon, A.K. Sum1 and Hst1 repress middle sporulation-specific gene expression during mitosis in Saccharomyces cerevisiae. EMBO J. 1999, 18, 6448–6454. [Google Scholar] [CrossRef]
- Orta-Zavalza, E.; Guerrero-Serrano, G.; Gutiérrez-Escobedo, G.; Cañas-Villamar, I.; Juárez-Cepeda, J.; Castaño, I.; De Las Peñas, A. Local silencing controls the oxidative stress response and the multidrug resistance in Candida glabrata. Mol. Microbiol. 2013, 88, 1135–1148. [Google Scholar] [CrossRef]
- Lu, Y.; Su, C.; Wang, A.; Liu, H. Hyphal development in Candida albicans requires two temporally linked changes in promoter chromatin for initiation and maintenance. PLoS Biol. 2011, 9, e1001105. [Google Scholar] [CrossRef]
- Sanchez, R.; Zhou, M.-M. The role of human bromodomains in chromatin biology and gene transcription. Curr. Opin. Drug Discov. Dev. 2009, 12, 659–665. [Google Scholar]
- Dhalluin, C.; Carlson, J.E.; Zeng, L.; He, C.; Aggarwal, A.K.; Zhou, M.M. Structure and ligand of a histone acetyltransferase bromodomain. Nature 1999, 399, 491–496. [Google Scholar] [CrossRef]
- Simithy, J.; Sidoli, S.; Yuan, Z.F.; Coradin, M.; Bhanu, N.V.; Marchione, D.M.; Klein, B.J.; Bazilevsky, G.A.; McCullough, C.E.; Magin, R.S.; et al. Characterization of histone acylations links chromatin modifications with metabolism. Nat. Commun. 2017, 8, 1–13. [Google Scholar] [CrossRef]
- Wang, Q.; Verma, J.; Vidan, N.; Wang, Y.; Tucey, T.M.; Lo, T.L.; Harrison, P.F.; See, M.; Swaminathan, A.; Kuchler, K.; et al. The YEATS domain histone crotonylation readers control virulence-related biology of a major human pathogen. Cell Rep. 2020, 31, 107528. [Google Scholar] [CrossRef] [PubMed]
- Mietton, F.; Ferri, E.; Champleboux, M.; Zala, N.; Maubon, D.; Zhou, Y.; Harbut, M.; Spittler, D.; Garnaud, C.; Courçon, M.; et al. Selective BET bromodomain inhibition as an antifungal therapeutic strategy. Nat. Commun. 2017, 8, 15482. [Google Scholar] [CrossRef] [PubMed]
- Yap, K.L.; Zhou, M.-M. Keeping it in the family: Diverse histone recognition by conserved structural folds. Crit. Rev. Biochem. Mol. Biol. 2010, 45, 488–505. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Musselman, C.A.; Lalonde, M.-E.; Côté, J.; Kutateladze, T.G. Perceiving the epigenetic landscape through histone readers. Nat. Struct. Mol. Biol. 2012, 19, 1218–1227. [Google Scholar] [CrossRef] [PubMed]
- Taverna, S.D.; Li, H.; Ruthenburg, A.J.; Allis, C.D.; Patel, D.J. How chromatin-binding modules interpret histone modifications: Lessons from professional pocket pickers. Nat. Struct. Mol. Biol. 2007, 14, 1025–1040. [Google Scholar] [CrossRef]
- Andrews, F.H.; Strahl, B.D.; Kutateladze, T.G. Insights into newly discovered marks and readers of epigenetic information. Nat. Chem. Biol. 2016, 12, 662–668. [Google Scholar] [CrossRef]
- Buschbeck, M.; Hake, S.B. Variants of core histones and their roles in cell fate decisions, development and cancer. Nat. Rev. Mol. Cell Biol. 2017, 18, 299–314. [Google Scholar] [CrossRef]
- Xu, Q.; Xie, W. Epigenome in early mammalian development: Inheritance, reprogramming and establishment. Trends Cell Biol. 2018, 28, 237–253. [Google Scholar] [CrossRef]
- Alabert, C.; Groth, A. Chromatin replication and epigenome maintenance. Nat. Rev. Mol. Cell Biol. 2012, 13, 153–167. [Google Scholar] [CrossRef]
- Serra-Cardona, A.; Zhang, Z. Replication-coupled nucleosome assembly in the passage of epigenetic information and cell identity. Trends Biochem. Sci. 2018, 43, 136–148. [Google Scholar] [CrossRef]
- Kaufman, P.D.; Rando, O.J. Chromatin as a potential carrier of heritable information. Curr. Opin. Cell Biol. 2010, 22, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Swygert, S.G.; Peterson, C.L. Chromatin dynamics: Interplay between remodeling enzymes and histone modifications. Biochim. Biophys. Acta Gene Regul. Mech. 2014, 1839, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Luk, E.; Ranjan, A.; FitzGerald, P.C.; Mizuguchi, G.; Huang, Y.; Wei, D.; Wu, C. Stepwise histone replacement by SWR1 requires dual activation with histone H2A.Z and canonical nucleosome. Cell 2010, 143, 725–736. [Google Scholar] [CrossRef]
- Zlatanova, J.; Thakar, A. H2A.Z: View from the top. Structure 2008, 16, 166–179. [Google Scholar] [CrossRef] [PubMed]
- Creyghton, M.P.; Markoulaki, S.; Levine, S.S.; Hanna, J.; Lodato, M.A.; Sha, K.; Young, R.A.; Jaenisch, R.; Boyer, L.A. H2AZ is enriched at polycomb complex target genes in es cells and is necessary for lineage commitment. Cell 2008, 135, 649–661. [Google Scholar] [CrossRef]
- Raisner, R.M.; Hartley, P.D.; Meneghini, M.D.; Bao, M.Z.; Liu, C.L.; Schreiber, S.L.; Rando, O.J.; Madhani, H.D. Histone variant H2A.Z Marks the 5′ ends of both active and inactive genes in euchromatin. Cell 2005, 123, 233–248. [Google Scholar] [CrossRef]
- Jackson, J.D.; Gorovsky, M.A. Histone H2A.Z has a conserved function that is distinct from that of the major H2A sequence variants. Nucleic Acids Res. 2000, 28, 3811–3816. [Google Scholar] [CrossRef]
- Liu, X.; Li, B. GorovskyMA Essential and nonessential histone H2A variants in Tetrahymena thermophila. Mol. Cell. Biol. 1996, 16, 4305–4311. [Google Scholar] [CrossRef]
- Brunelle, M.; Nordell Markovits, A.; Rodrigue, S.; Lupien, M.; Jacques, P.É.; Gévry, N. The histone variant H2A.Z is an important regulator of enhancer activity. Nucleic Acids Res. 2015, 43, 9742–9756. [Google Scholar] [CrossRef]
- Altaf, M.; Auger, A.; Monnet-Saksouk, J.; Brodeur, J.; Piquet, S.; Cramet, M.; Bouchard, N.; Lacoste, N.; Utley, R.T.; Gaudreau, L.; et al. NuA4-dependent acetylation of nucleosomal histones H4 and H2A directly stimulates incorporation of H2A.Z by the SWR1 complex. J. Biol. Chem. 2010, 285, 15966–15977. [Google Scholar] [CrossRef]
- Watanabe, S.; Radman-Livaja, M.; Rando, O.J.; Peterson, C.L. A histone acetylation switch regulates H2A.Z deposition by the SWR-C remodeling enzyme. Science 2013, 340, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Rufiange, A.; Jacques, P.É.; Bhat, W.; Robert, F.; Nourani, A. Genome-wide replication-independent histone H3 exchange occurs predominantly at promoters and implicates H3 K56 acetylation and Asf1. Mol. Cell 2007, 27, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Elsässer, S.J.; D’Arcy, S. Towards a mechanism for histone chaperones. Biochim. Biophys. Acta Gene Regul. Mech. 2012, 1819, 211–221. [Google Scholar] [CrossRef]
- Kaufman, P.D.; Kobayashi, R.; Stillman, B. Ultraviolet radiation sensitivity and reduction of telomeric silencing in Saccharomyces cerevisiae cells lacking chromatin assembly factor-I. Genes Dev. 1997, 11, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Stillman, B. Chromatin assembly during SV40 DNA replication in vitro. Cell 1986, 45, 555–565. [Google Scholar] [CrossRef]
- Green, E.M.; Antczak, A.J.; Bailey, A.O.; Franco, A.A.; Wu, K.J.; Yates, J.R.; Kaufman, P.D. Replication-independent histone deposition by the HIR complex and Asf1. Curr. Biol. 2005, 15, 2044–2049. [Google Scholar] [CrossRef]
- Prochasson, P.; Florens, L.; Swanson, S.K.; Washburn, M.P.; Workman, J.L. The HIR corepressor complex binds to nucleosomes generating a distinct protein/DNA complex resistant to remodeling by SWI/SNF. Genes Dev. 2005, 19, 2534–2539. [Google Scholar] [CrossRef]
- Houlard, M.; Berlivet, S.; Probst, A.V.; Quivy, J.-P.; Héry, P.; Almouzni, G.; Gérard, M. CAF-1 is essential for heterochromatin organization in pluripotent embryonic cells. PLoS Genet. 2006, 2, e181. [Google Scholar] [CrossRef]
- Roberts, C.; Sutherland, H.F.; Farmer, H.; Kimber, W.; Halford, S.; Carey, A.; Brickman, J.M.; Wynshaw-Boris, A.; Scambler, P.J. Targeted mutagenesis of the Hira gene results in gastrulation defects and patterning abnormalities of mesoendodermal derivatives prior to early embryonic lethality. Mol. Cell. Biol. 2002, 22, 2318–2328. [Google Scholar] [CrossRef]
- Ray-Gallet, D.; Woolfe, A.; Vassias, I.; Pellentz, C.; Lacoste, N.; Puri, A.; Schultz, D.C.; Pchelintsev, N.A.; Adams, P.D.; Jansen, L.E.T.; et al. Dynamics of histone H3 deposition in vivo reveal a nucleosome gap-filling mechanism for H3.3 to maintain chromatin integrity. Mol. Cell 2011, 44, 928–941. [Google Scholar] [CrossRef]
- Zaret, K.S.; Mango, S.E. Pioneer transcription factors, chromatin dynamics, and cell fate control. Curr. Opin. Genet. Dev. 2016, 37, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Schulz, V.P.; Yan, H.; Lezon-Geyda, K.; An, X.; Hale, J.; Hillyer, C.D.; Mohandas, N.; Gallagher, P.G. A unique epigenomic landscape defines human erythropoiesis. Cell Rep. 2019, 28, 2996–3009. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, J.; Yang, X.; Zhou, C.; Guo, J.; Wu, C.; Qin, Y.; Guo, L.; He, J.; Yu, S.; et al. Chromatin accessibility dynamics during iPSC reprogramming. Cell Stem Cell 2017, 21, 819–833. [Google Scholar] [CrossRef] [PubMed]
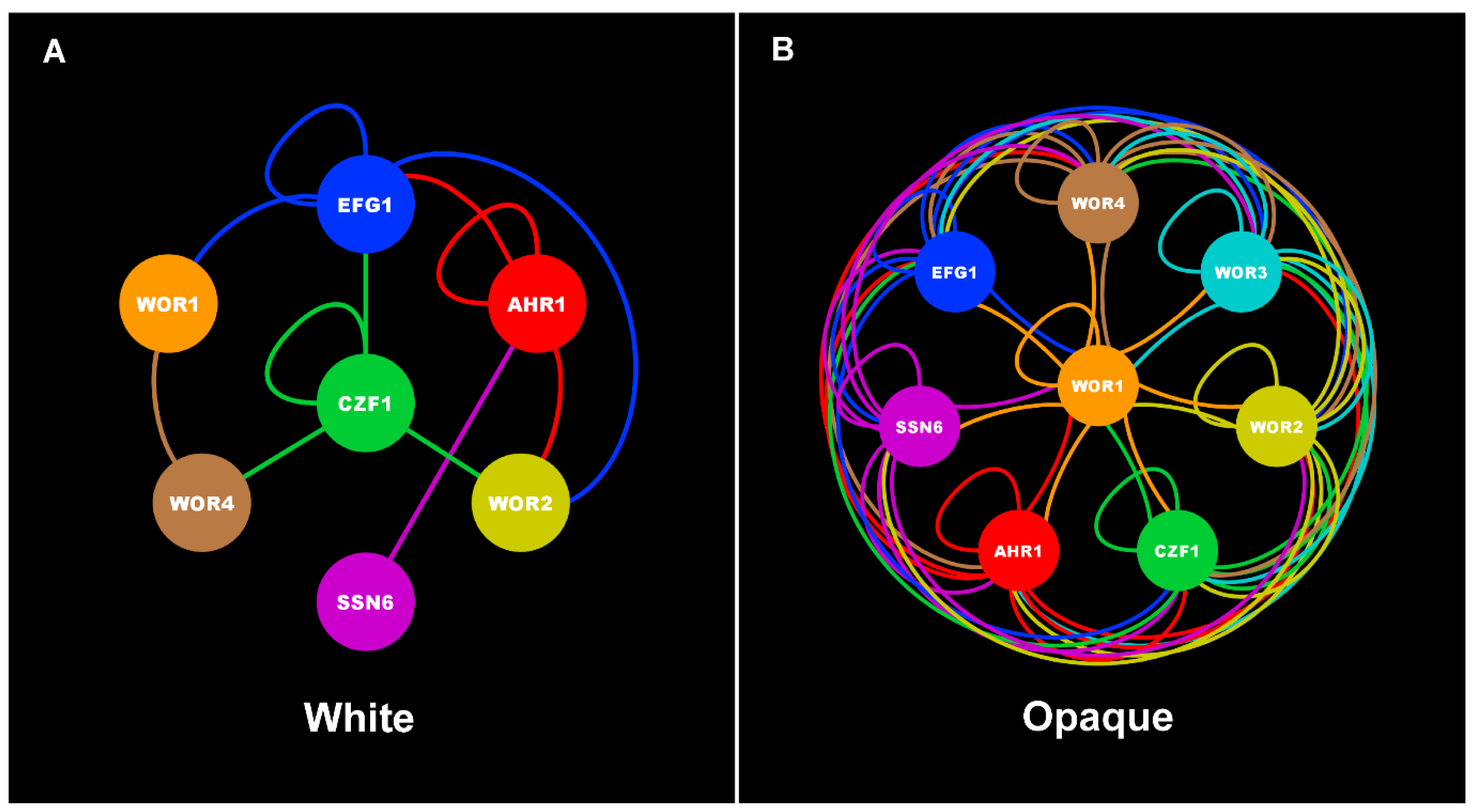
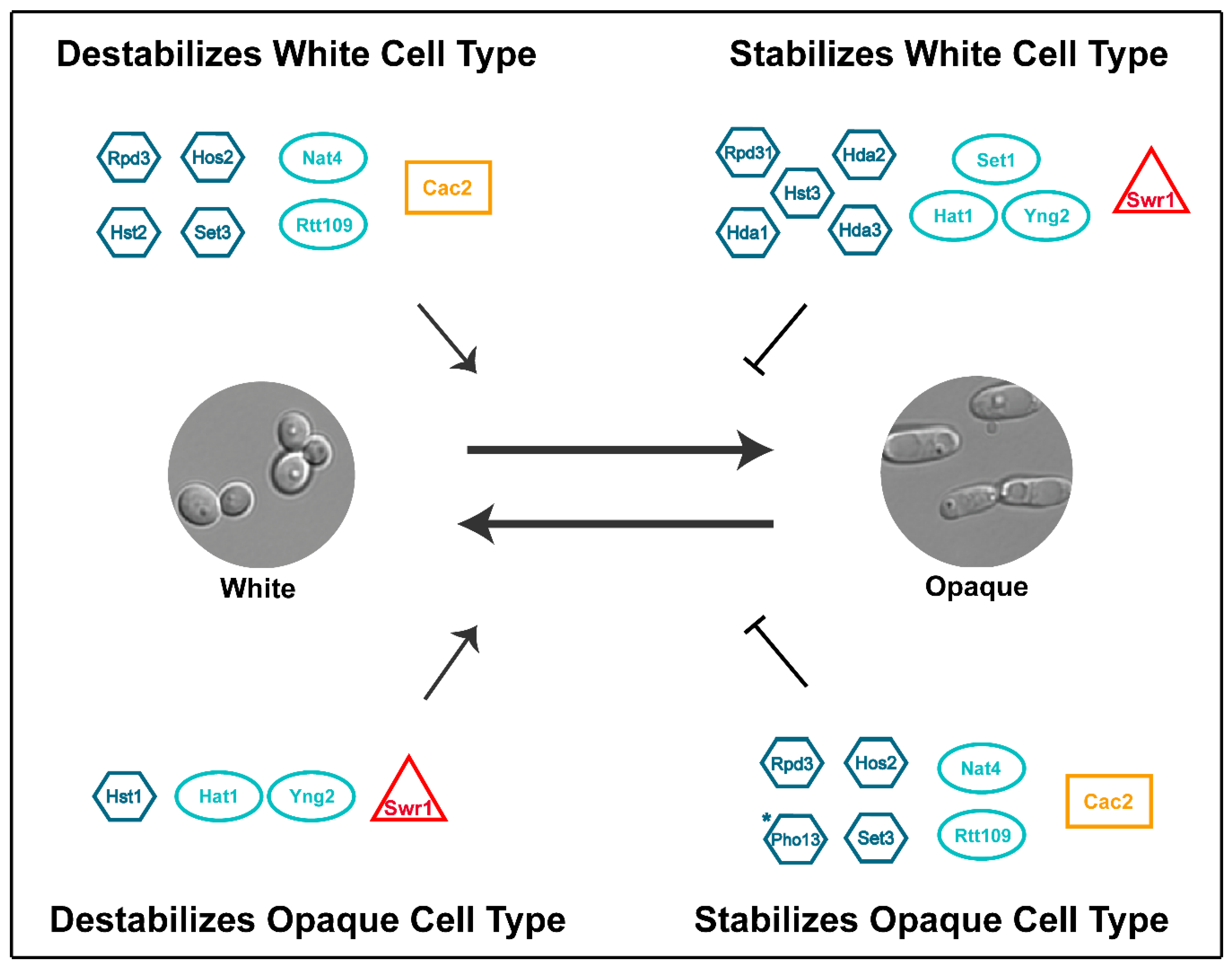
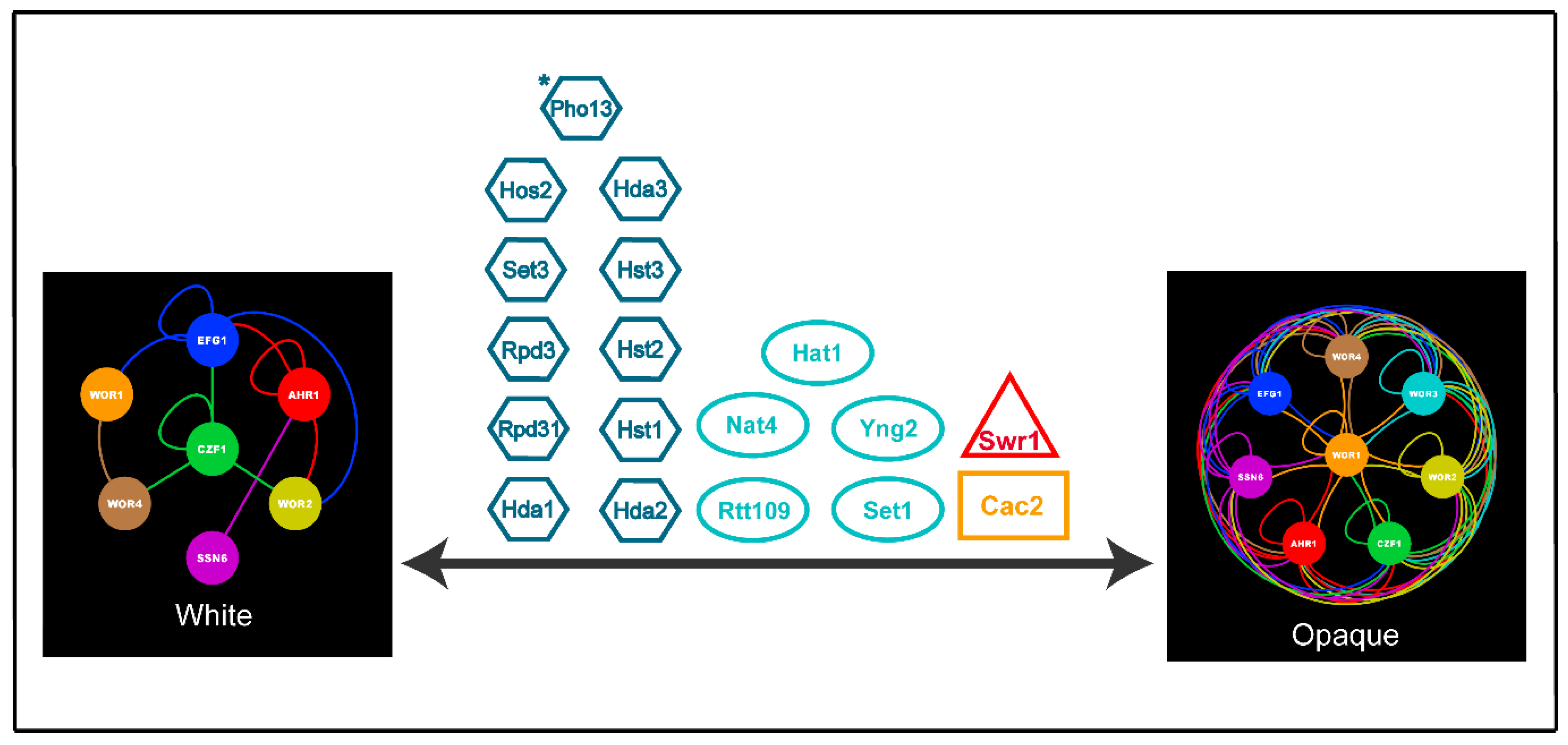
| Core White-Opaque Transcriptional Regulators | |||||
| Gene Name | Orf19 # | Known Effect on White-Opaque Switch in Mutant Strain | |||
| White to Opaque 1 | Opaque to White 1 | Other Functions | References | ||
| AHR1 | Orf19.7381 | 1.96 | 0.13 | Adherence | [32] |
| CZF1 | Orf19.3127 | 0.05 | 0.06 | Filamentation | [33] |
| EFG1 | Orf19.610 | 24.0 | <0.02 | Filamentation, Metabolism | [34,35] |
| SSN6 * | Orf19.6798 | N/A OP | <0.04 | Filamentation | [36,37] |
| WOR1 | Orf19.4884 | >0.05 | N/A WH | Adherence | [38] |
| WOR2 | Orf19.5992 | >0.03 | N/A WH | Iron homeostasis | [39] |
| WOR3 | Orf19.467 | 0.42 | 0.26 | ||
| WOR4 | Orf19.6713 | >0.08 | N/A WH | ||
| Auxiliary White-Opaque Transcriptional Regulators | |||||
| Gene Name | Orf19 # | Known Effect on White-Opaque Switch in Mutant Strain | |||
| White to Opaque 1 | Opaque to White 1 | Other Functions | References | ||
| AAF1 | Orf19.7436 | 0.88 | 0.38 | Adherence | [40] |
| AFT2 | Orf19.2272 | 0.36 | 0.59 | Iron metabolism, Stress response, Adherence | [41,42] |
| ARG81 | Orf19.4766 | 1.75 | 0.44 | Adherence | [43] |
| ASG1 | Orf19.166 | 0.05 | 0.05 | Filamentation | [44] |
| ASH1 | Orf19.5343 | 0.83 | 0.04 | Filamentation, Metabolism | [45,46] |
| BAS1 | Orf19.6874 | 1.49 | 1.15 | Filamentation | [47] |
| BCR1 | Orf19.723 | 2.21 | N/A WH | Adherence, Biofilm formation, Drug resistance | [43,48,49,50] |
| BRG1 | Orf19.4056 | 1.87 | 0.66 | Filamentation, Biofilm formation | [48,51,52] |
| CAP1 | Orf19.1623 | 0.67 | 0.23 | Drug resistance, Stress response, Apoptosis | [53,54,55,56,57] |
| CAS5 | Orf19.4670 | 1.45 | 0.43 | Drug resistance, Stress response, Cell cycle | [58,59,60] |
| CPH1 | Orf19.4433 | 0.46 | 0.39 | Filamentation, Mating | [61,62,63,64] |
| CPH2 | Orf19.1187 | 0.44 | 0.70 | Filamentation | [65] |
| CRZ1 | Orf19.7359 | 1.86 | 0.18 | Drug resistance, Stress response, Calcineurin pathway | [66,67,68,69,70] |
| CSR1 | Orf19.3794 | 1.02 | 2.56 | Zinc ion homeostasis, Filamentation | [71,72] |
| CTA4 | Orf19.7374 | 0.88 | 0.17 | Stress response, Drug resistance | [44,73] |
| CTA7 | Orf19.4288 | 2.37 | 0.48 | ||
| CUP2 | Orf19.5001 | 0.81 | 0.63 | Stress response | [74] |
| CUP9 | Orf19.6514 | 4.74 | 0.07 | Filamentation | [75] |
| DAL81 | Orf19.3252 | 0.16 | 0.57 | Adherence | [76] |
| DPB4 | Orf19.2088 | 0.32 | 0.41 | Filamentation | [77] |
| ECM22 | Orf19.2623 | 1.31 | 0.46 | ||
| EFH1 | Orf19.5498 | 1.69 | 0.61 | Metabolism | [34] |
| FCR1 | Orf19.6817 | 0.52 | 0.63 | Drug resistance | [78] |
| FGR15 | Orf19.2054 | 0.06 | 4.78 | Filamentation | [79] |
| FLO8 | Orf19.1093 | <0.04 | N/A WH | Filamentation, CO2 sensing | [80,81] |
| GAL4 | Orf19.5338 | <0.04 | 0.77 | Metabolism | [82] |
| GIS2 | Orf19.3182 | 0.86 | 0.11 | Drug resistance | [83] |
| GRF10 | Orf19.4000 | 1.37 | 0.14 | Filamentation, Metabolism | [47,84] |
| GZF3 | Orf19.2842 | 0.08 | 1.70 | Stress response | [83] |
| HAP2 | Orf19.1228 | <0.04 | 0.62 | Iron homeostasis | [39] |
| HAP3 | Orf19.4647 | 0.34 | 1.30 | Stress response | [39] |
| HAP31 | Orf19.517 | 0.04 | 0.79 | Stress response, Drug resistance | [39,83] |
| HAP41 | Orf19.740 | 0.10 | 1.14 | Stress response | [39] |
| HAP42 | Orf19.1481 | 0.49 | 0.54 | ||
| HAP5 | Orf19.1973 | 0.15 | 0.33 | Stress response, Metabolism | [39,85] |
| HCM1 | Orf19.4853 | 18.4 | 0.27 | Stress response, Filamentation | [39,86] |
| HFL1 | Orf19.3063 | <0.05 | 2.11 | DNA replication | [87] |
| INO2 | Orf19.7539 | <0.04 | 0.28 | Transcription | [88] |
| INO4 | Orf19.837.1 | 0.33 | 0.81 | Transcription | [88] |
| ISW2 | Orf19.7401 | 3.40 | 2.89 | Stress response | [89] |
| KAR4 | Orf19.3736 | 0.51 | 1.23 | Mating | [90,91] |
| LYS143 | Orf19.4776 | 7.25 | 0.88 | Biofilm formation | [43] |
| LYS144 | Orf19.5380 | 1.29 | 0.42 | Biofilm formation | [43] |
| MAC1 | Orf19.7068 | 0.05 | 0.63 | Copper ion homeostasis, Filamentation | [92] |
| MIG1 | Orf19.4318 | <0.03 | 1.37 | Metabolism | [93,94,95] |
| MIG2 | Orf19.5326 | 1.62 | 0.63 | Metabolism | [93] |
| MSN4 | Orf19.4752 | 1.88 | 0.21 | Biofilm formation | [43] |
| NDT80 | Orf19.2119 | 0.10 | 1.87 | Drug resistance, Biofilm formation | [48,96] |
| NTO1 | Orf19.5910 | 2.80 | 0.66 | Stress response | [89] |
| OPI1 | Orf19.1543 | 4.03 | 0.42 | Filamentation, Metabolism | [97,98] |
| PTH2 | Orf19.4231 | 2.96 | 0.24 | ||
| RAP1 | Orf19.1773 | 16.0 | 0.64 | Telomere recombination, Filamentation | [99,100,101] |
| RBF1 | Orf19.5558 | N/A OP | <0.03 | Filamentation | [102] |
| RCA1 | Orf19.6102 | 0.11 | 0.53 | CO2 sensing, Drug resistance | [103,104] |
| REP1 | Orf19.7521 | 0.68 | 2.43 | Drug resistance | [105] |
| RFG1 | Orf19.2823 | 1.05 | 2.12 | Filamentation, Stress response | [106,107,108] |
| RFX1 | Orf19.3865 | 1.78 | 1.67 | Stress response | [109] |
| RFX2 | Orf19.4590 | 1.46 | 0.58 | Stress response | [109] |
| RME1 | Orf19.4438 | 2.15 | 0.57 | Drug resistance | [110] |
| RPN4 | Orf19.1069 | 18.2 | 0.67 | Intracellular proteolysis | [111,112] |
| RTG1 | Orf19.4722 | 0.47 | 0.35 | Metabolism | [113] |
| RTG3 | Orf19.2315 | 0.36 | 0.46 | Metabolism | [113] |
| SEF2 | Orf19.1926 | 1.09 | 0.31 | ||
| SFL1 | Orf19.454 | 0.88 | 1.95 | Flocculation, Filamentation | [114,115] |
| SKN7 | Orf19.971 | 1.13 | 0.65 | Stress response | [116] |
| SKO1 | Orf19.1032 | 0.56 | 0.42 | Stress response, Filamentation | [117,118,119] |
| STP2 | Orf19.4961 | 9.18 | 0.15 | Metabolism | [120] |
| STP4 | Orf19.909 | 3.25 | 0.47 | Metabolism | [120] |
| SWI4 | Orf19.4545 | 0.22 | 1.02 | Cell cycle | [121] |
| TYE7 | Orf19.4941 | 2.04 | 0.97 | Metabolism | [82] |
| UGA33 | Orf19.7317 | 0.99 | 0.60 | Adherence | [43] |
| UME6 | Orf19.1822 | 0.62 | 2.02 | Filamentation, CO2 sensing | [122,123,124,125] |
| UME7 | Orf19.2745 | 0.50 | 1.44 | Adherence | [43] |
| UPC2 | Orf19.391 | 0.94 | 3.13 | Drug resistance, Metabolism | [126,127,128,129] |
| WAR1 | Orf19.1035 | 0.31 | 0.94 | Stress response | [130] |
| XBP1 | Orf19.5210 | 0.16 | 0.85 | Stress response | [39] |
| ZCF16 | Orf19.2808 | 1.47 | 1.19 | Biofilm formation | [131] |
| ZCF17 | Orf19.3305 | 1.31 | 2.22 | Adherence | [43] |
| ZCF2 | Orf19.431 | 0.59 | 0.36 | Stress response | [132,133] |
| ZCF20 | Orf19.4145 | 0.66 | 0.46 | Iron homeostasis | [134] |
| ZCF21 | Orf19.4166 | 0.25 | 0.02 | ||
| ZCF22 | Orf19.4251 | 1.77 | 0.93 | ||
| ZCF24 | Orf19.4524 | 0.96 | 0.31 | Stress response | [39] |
| ZCF25 | Orf19.4568 | 8.48 | 0.34 | ||
| ZCF27 | Orf19.4649 | 0.53 | 1.46 | Filamentation | [43] |
| ZCF30 | Orf19.5251 | 1.08 | 0.60 | ||
| ZCF31 | Orf19.5924 | 0.42 | 3.24 | Stress response | [83] |
| ZCF34 | Orf19.6182 | 0.35 | 0.22 | Stress response | [83] |
| ZCF7 | Orf19.1685 | 4.73 | 0.37 | ||
| ZCF8 | Orf19.1718 | 0.48 | 0.46 | Adherence | [76] |
| ZFU2 | Orf19.6781 | 0.52 | 2.26 | ||
| ZFU3 | Orf19.6888 | 0.20 | 0.06 | Biofilm formation | [48,131] |
| ZMS1 | Orf19.5026 | 0.36 | 0.84 | ||
| Orf19.1150 | 1.22 | 0.78 | |||
| Orf19.1274 | 0.70 | 1.20 | |||
| Orf19.1577 | 0.89 | 0.68 | |||
| Orf19.1757 | 1.04 | 0.61 | |||
| Orf19.217 | 0.60 | 0.57 | |||
| Orf19.2476 | 1.91 | 2.49 | |||
| Orf19.2612 | 2.38 | 1.40 | |||
| Orf19.2961 | 7.02 | 2.05 | |||
| Orf19.3928 | 5.71 | 0.23 | |||
| Orf19.7098 | 7.77 | 1.10 | |||
| Gene Name | Orf19# | Protein Function | Known Effect on White-Opaque Switch in Mutant Strain | Reference | |
|---|---|---|---|---|---|
| Wh --> Op 1 | Op --> Wh 1 | ||||
| YNG2 | Orf19.878 | Histone Acetyltransferases (Writers) | 23.8 | 0.01 | [5] |
| SPT10 | Orf19.2361 | no effect | no effect | [135] | |
| HPA2 | Orf19.6323 | no effect | no effect | [135] | |
| RTT109 | Orf19.7491 | 0.10 | 6.98 | [157] | |
| NAT4 | Orf19.4664 | 0.12 | 3.42 | [135] | |
| SAS2 | Orf19.2087 | no effect | no effect | [135] | |
| HAT1 | Orf19.779 | 7.60 | 0.13 | [140] | |
| ELP3 | Orf19.7387 | no effect | no effect | [135] | |
| SET1 | Orf 9.6009 | Histone Methyl Transferases (Writers) | 1.73 | 0.98 3 | [135] |
| SET2 | Orf19.175 | no effect | no effect | [135] | |
| DOT1 | Orf19.740 | no effect | no effect | [135] | |
| HDA1 | Orf19.2606 | Histone Deacetylases (Erasers) | 2.73 | 1.06 3 | [29,31] * |
| HDA2 | Orf19.6952 | 3.33 | no data | [158] | |
| HDA3 | Orf19.7344 | 3.67 | no data | [158] | |
| RPD3 | Orf19.2834 | 33.3 | 49.7 | [137] | |
| RPD31 | Orf19.6801 | 2.85 | 1.23 3 | [136] | |
| HST1 | Orf19.4761 | 1.29 3 | 0.37 | [135] | |
| HST2 | Orf19.2580 | 0.04 | 1.86 3 | [135] | |
| HST34 | Orf19.1934 | 6.00 | no effect | [24] | |
| HOS1 | Orf19.4411 | no effect | no effect | [135] | |
| HOS2 | Orf19.5377 | 0.13 | 2.29 | [135] | |
| HOS3 | Orf19.2772 | no effect | no effect | [135] | |
| SET3 | Orf19.7221 | 0.16 | 2.71 | [135] | |
| PHO132 | Orf19.4444 | Phosphatases (Erasers) | 0.93 3 | 5.01 | [135] |
| ORF19.4736 | Orf19.4736 | no effect | no effect | [135] | |
| SWR1 | Orf19.1871 | Chromatin Remodelers | 16.0 | 0.01 | [5] |
| CAC2 | Orf19.6670 | Histone Chaperones | 3.75 | 2.53 | [139] |
| HIR1 | Orf19.2099 | no effect | no effect | [139] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qasim, M.N.; Valle Arevalo, A.; Nobile, C.J.; Hernday, A.D. The Roles of Chromatin Accessibility in Regulating the Candida albicans White-Opaque Phenotypic Switch. J. Fungi 2021, 7, 37. https://doi.org/10.3390/jof7010037
Qasim MN, Valle Arevalo A, Nobile CJ, Hernday AD. The Roles of Chromatin Accessibility in Regulating the Candida albicans White-Opaque Phenotypic Switch. Journal of Fungi. 2021; 7(1):37. https://doi.org/10.3390/jof7010037
Chicago/Turabian StyleQasim, Mohammad N., Ashley Valle Arevalo, Clarissa J. Nobile, and Aaron D. Hernday. 2021. "The Roles of Chromatin Accessibility in Regulating the Candida albicans White-Opaque Phenotypic Switch" Journal of Fungi 7, no. 1: 37. https://doi.org/10.3390/jof7010037
APA StyleQasim, M. N., Valle Arevalo, A., Nobile, C. J., & Hernday, A. D. (2021). The Roles of Chromatin Accessibility in Regulating the Candida albicans White-Opaque Phenotypic Switch. Journal of Fungi, 7(1), 37. https://doi.org/10.3390/jof7010037







