Using Expanded Natural Killer Cells as Therapy for Invasive Aspergillosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Expansion of Natural Killer Cells
2.2. Preparation of Aspergillus fumigatus
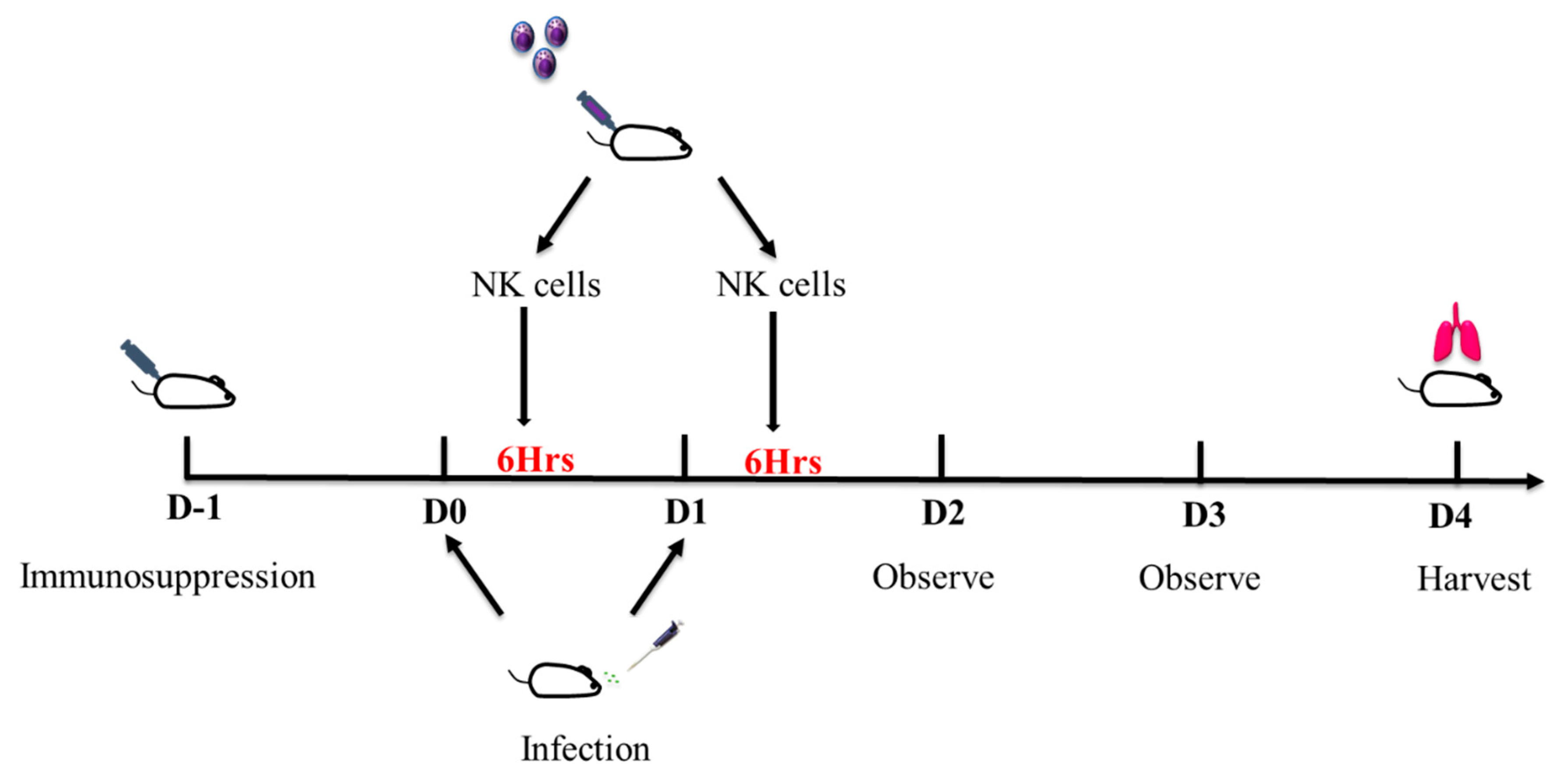
2.3. Pulmonary Aspergillosis Mice Model
2.4. Immunosuppression
2.5. Fungal Load Quantification
2.6. Histology
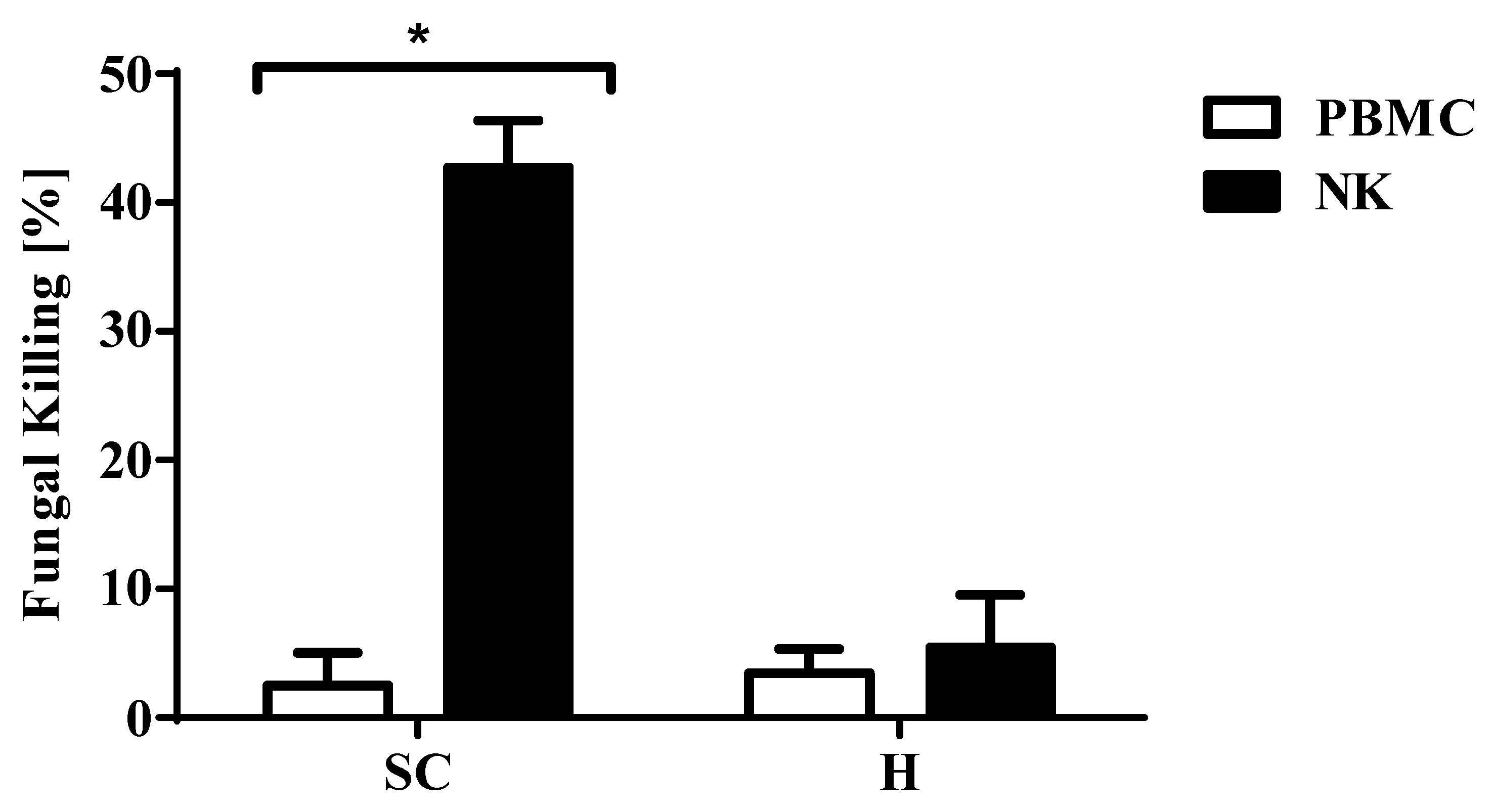
2.7. Fungal Killing Assay
2.8. Dectin-1 Inhibition Assay
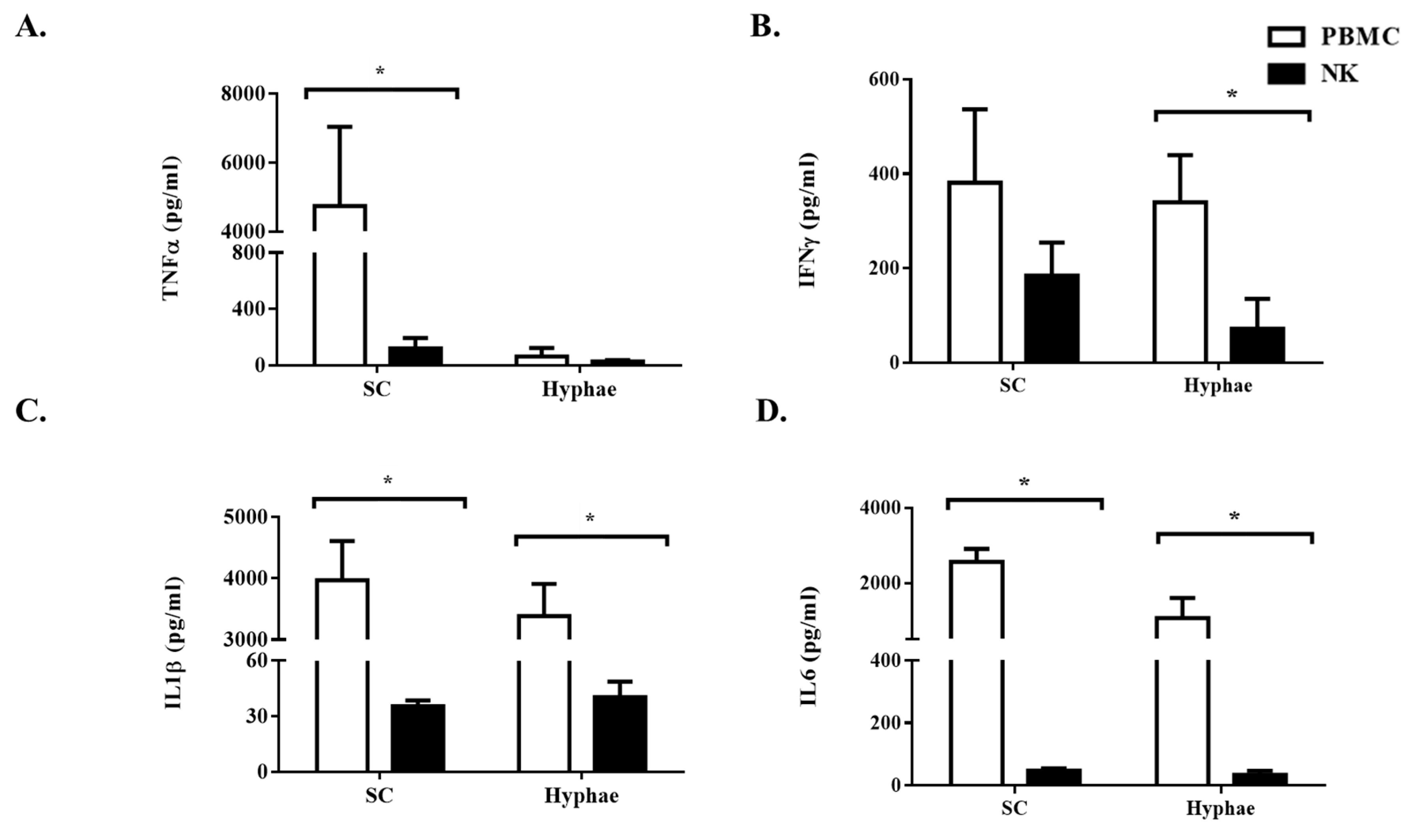
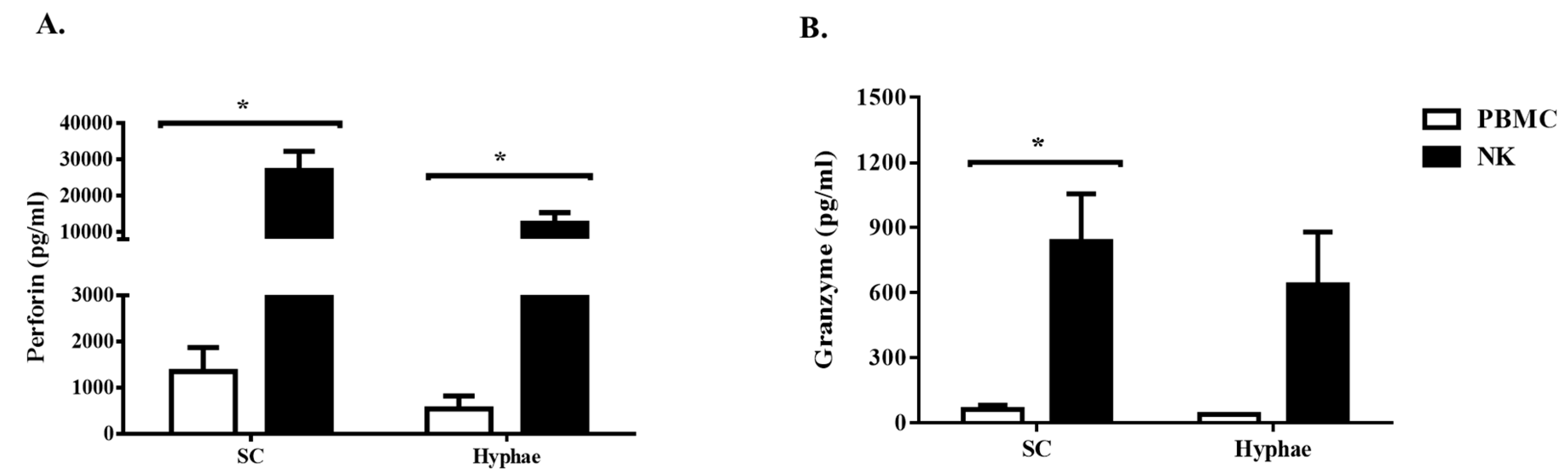
2.9. Cytokine and Perforin Quantification
2.10. Statistics
3. Results
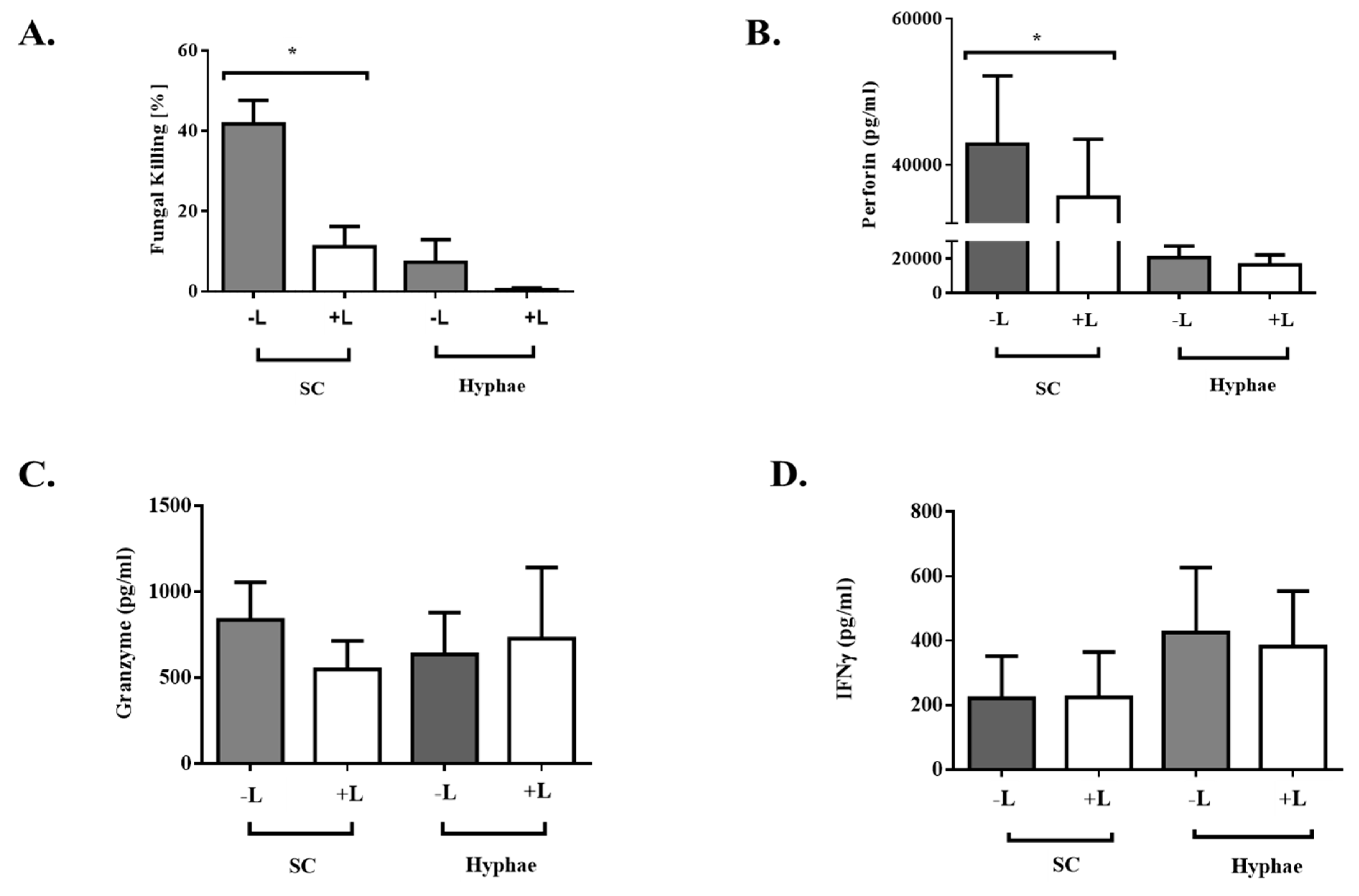
3.1. Expanded Natural Killer Cells had Enhanced Fungicidal Activity Against Aspergillus conidia
3.2. The Antifungal Activity of Expanded NK Cells Is Not Attributable to Enhanced Proinflammatory Cytokine Response
3.3. Expanded NK Cells Release High Level of Perforin
3.4. Effect of Dectin-1 Inhibition on Expanded NK Cells
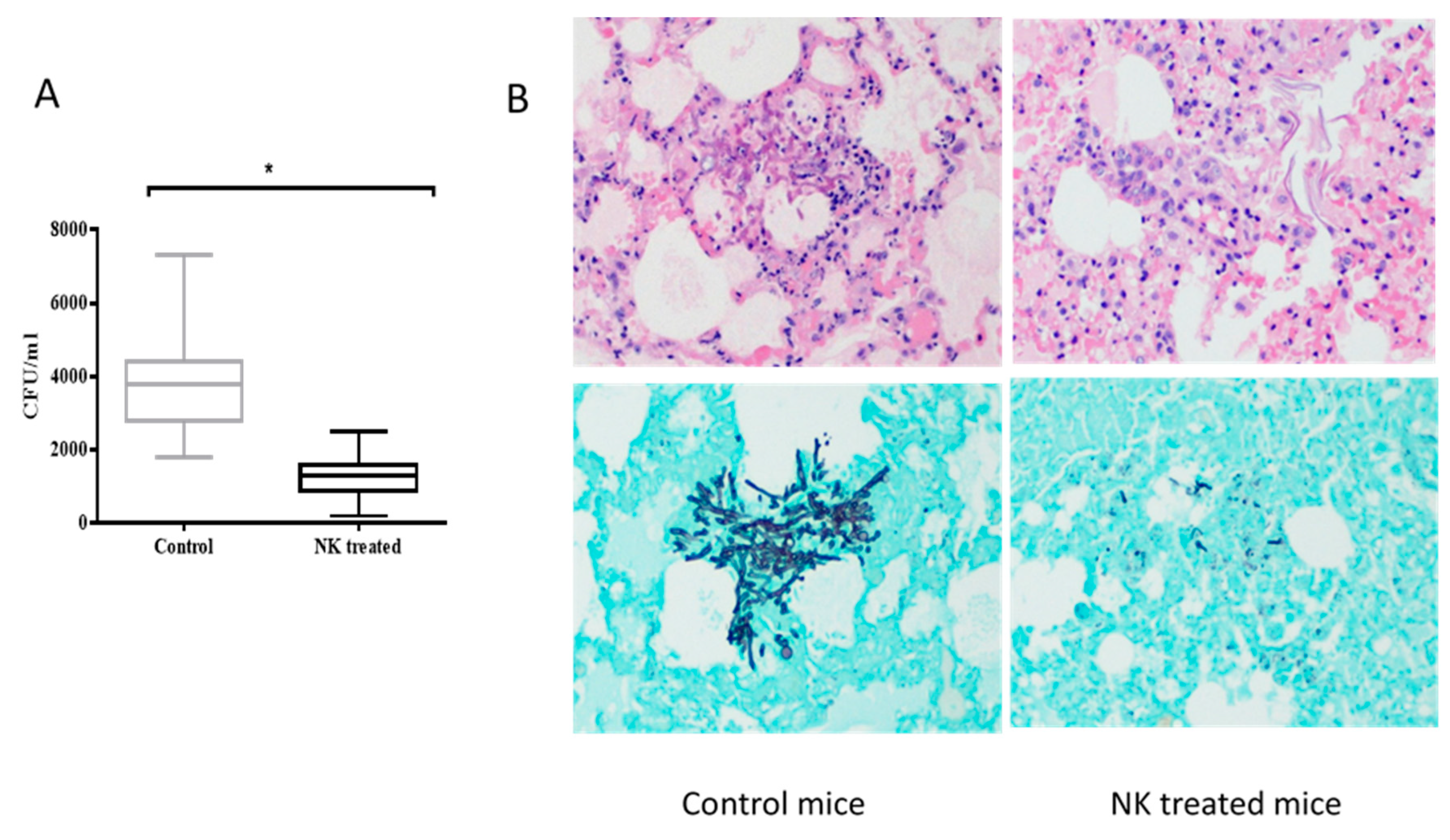
3.5. In vivo Antifungal Activity of Expanded NK Cells Against Invasive Pulmonary Aspergillosis
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Steinbach, W.J.; Marr, K.A.; Anaissie, E.J.; Azie, N.; Quan, S.-P.; Meier-Kriesche, H.-U.; Apewokin, S.; Horn, D.L. Clinical epidemiology of 960 patients with invasive aspergillosis from the PATH Alliance registry. J. Infect. 2012, 65, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Lass-Flörl, C.; Roilides, E.; Löffler, J.; Wilflingseder, D.; Romani, L. Minireview: Host defence in invasive aspergillosis. Mycoses 2013, 56, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, S.; Win, M.S.; Chai, L.Y.A. Optimizing Outcomes in Immunocompromised Hosts: Understanding the Role of Immunotherapy in Invasive Fungal Diseases. Front. Microbiol. 2015, 6, 1322. [Google Scholar] [CrossRef] [PubMed]
- Bouzani, M.; Ok, M.; McCormick, A.; Ebel, F.; Kurzai, O.; Morton, C.O.; Einsele, H.; Loeffler, J. Human NK Cells Display Important Antifungal Activity againstAspergillus fumigatus, Which Is Directly Mediated by IFN-γ Release. J. Immunol. 2011, 187, 1369–1376. [Google Scholar] [CrossRef]
- Schmidt, S.; Zimmermann, S.-Y.; Tramsen, L.; Koehl, U.; Lehrnbecher, T. Natural Killer Cells and Antifungal Host Response. Clin. Vaccine Immunol. 2013, 20, 452–458. [Google Scholar] [CrossRef][Green Version]
- Beitzen-Heineke, A.; Bouzani, M.; Schmitt, A.-L.; Kurzai, O.; Hünniger, K.; Einsele, H.; Loeffler, J. Human Invariant Natural Killer T cells possess immune-modulating functions duringAspergillusinfection. Med Mycol. 2015, 54, 169–176. [Google Scholar] [CrossRef]
- Ziegler, S.; Weiss, E.; Schmitt, A.-L.; Schlegel, J.; Burgert, A.; Terpitz, U.; Sauer, M.; Moretta, L.; Sivori, S.; Leonhardt, I.; et al. CD56 Is a Pathogen Recognition Receptor on Human Natural Killer Cells. Sci. Rep. 2017, 7, 6138. [Google Scholar] [CrossRef]
- Bouzani, M.; Ok, M.; Kurzai, O.; Einsele, H.; Loeffler, J. Human Natural Killer Cells Are Able to Kill Aspergillus Fumigatus but Not Via the Perforin - Granzyme Pathway. Blood 2009, 114, 1640. [Google Scholar] [CrossRef]
- Schmidt, S.; Tramsen, L.; Hanisch, M.; Latgé, J.-P.; Huenecke, S.; Koehl, U.; Lehrnbecher, T. Human Natural Killer Cells Exhibit Direct Activity Against Aspergillus fumigatus Hyphae, But Not Against Resting Conidia. J. Infect. Dis. 2011, 203, 430–435. [Google Scholar] [CrossRef]
- Lehrnbecher, T.; Kalkum, M.; Champer, J.; Tramsen, L.; Schmidt, S.; Klingebiel, T. Immunotherapy in invasive fungal infection--focus on invasive aspergillosis. Curr. Pharm. Des. 2013, 19, 3689–3712. [Google Scholar] [CrossRef]
- Becker, P.S.A.; Suck, G.; Nowakowska, P.; Ullrich, E.; Seifried, E.; Bader, P.; Tonn, T.; Seidl, C. Selection and expansion of natural killer cells for NK cell-based immunotherapy. Cancer Immunol. Immunother. 2016, 65, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.; Campana, D. Expansion and Activation of Natural Killer Cells for Cancer Immunotherapy. Ann. Lab. Med. 2009, 29, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, T.; Chang, Y.-H.; Campana, D. Expanded and Activated Natural Killer Cells for Immunotherapy of Hepatocellular Carcinoma. Cancer Immunol. Res. 2016, 4, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Chai, L.Y.A.; Kullberg, B.J.; Vonk, A.G.; Warris, A.; Cambi, A.; Latgeé, J.-P.; Joosten, L.A.B.; Van Der Meer, J.W.M.; Netea, M. Modulation of Toll-Like Receptor 2 (TLR2) and TLR4 Responses by Aspergillus fumigatus. Infect. Immun. 2009, 77, 2184–2192. [Google Scholar] [CrossRef] [PubMed]
- Marx, J.; Vudathala, D.; Murphy, L.; Rankin, S.; Hankenson, F.C. Antibiotic Administration in the Drinking Water of Mice. J. Am. Assoc. Lab. Anim. Sci. 2014, 53, 301–306. [Google Scholar]
- Mehrad, B.; Strieter, R.M.; Standiford, T.J. Role of TNF-α in pulmonary host defense in murine invasive aspergillosis. J. Immunol. 1999, 162, 1633–1640. [Google Scholar]
- Osińska, I.; Popko, K.; Demkow, U. Perforin: An important player in immune response. Central Eur. J. Immunol. 2014, 1, 109–115. [Google Scholar] [CrossRef]
- Djeu, J.Y.; Jiang, K.; Wei, S. A view to a kill: Signals triggering cytotoxicity. Clin. Cancer Res. 2002, 8, 636. [Google Scholar]
- Klingemann, H.-G.; Martinson, J. Ex vivo expansion of natural killer cells for clinical applications. Cytotherapy 2004, 6, 15–22. [Google Scholar] [CrossRef]
- Fujisaki, H.; Kakuda, H.; Shimasaki, N.; Imai, C.; Ma, J.; Lockey, T.; Eldridge, P.; Leung, W.H.; Campana, D. Expansion of Highly Cytotoxic Human Natural Killer Cells for Cancer Cell Therapy. Cancer Res. 2009, 69, 4010–4017. [Google Scholar] [CrossRef]
- Li, S.S.; Kyei, S.K.; Timm-McCann, M.; Ogbomo, H.; Jones, G.J.; Shi, M.; Xiang, R.F.; Oykhman, P.; Huston, S.M.; Islam, A.; et al. The NK Receptor NKp30 Mediates Direct Fungal Recognition and Killing and Is Diminished in NK Cells from HIV-Infected Patients. Cell Host Microbe 2013, 14, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Garg, T.K.; Szmania, S.M.; Khan, J.A.; Hoering, A.; Malbrough, P.A.; Moreno-Bost, A.; Greenway, A.D.; Lingo, J.D.; Li, X.; Yaccoby, S.; et al. Highly activated and expanded natural killer cells for multiple myeloma immunotherapy. Haematologica 2012, 97, 1348–1356. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Hughes, M.A.; Burdick, M.; Strieter, R.M.; Mehrad, B. Early NK Cell-Derived IFN-γ Is Essential to Host Defense in Neutropenic Invasive Aspergillosis. J. Immunol. 2009, 182, 4306–4312. [Google Scholar] [CrossRef] [PubMed]
- Ogbomo, H.; Mody, C.H. Granule-Dependent Natural Killer Cell Cytotoxicity to Fungal Pathogens. Front. Immunol. 2017, 7, 692. [Google Scholar] [CrossRef] [PubMed]
- Sutton, P.; Waring, P.; Muüllbacher, A. Exacerbation of invasive aspergillosis by the immunosuppressive fungal metabolite, gliotoxin. Immunol. Cell Biol. 1996, 74, 318–322. [Google Scholar] [CrossRef]
- Schneider, A.; Blatzer, M.; Posch, W.; Schubert, R.; Lass-Flörl, C.; Schmidt, S.; Lehrnbecher, T. Aspergillus fumigatus responds to natural killer (NK) cells with upregulation of stress related genes and inhibits the immunoregulatory function of NK cells. Oncotarget 2016, 7, 71062–71071. [Google Scholar] [CrossRef] [PubMed]
- Herbrecht, R.; Denning, D.W.; Patterson, T.F.; Bennett, J.E.; Greene, R.E.; Oestmann, J.-W.; Kern, W.V.; Marr, K.A.; Ribaud, P.; Lortholary, O.; et al. Voriconazole versus Amphotericin B for Primary Therapy of Invasive Aspergillosis. New Engl. J. Med. 2002, 347, 408–415. [Google Scholar] [CrossRef]
- Brown, G.D. Dectin-1: A signalling non-TLR pattern-recognition receptor. Nat. Rev. Immunol. 2005, 6, 33–43. [Google Scholar] [CrossRef]
- Taylor, P.R.; Brown, G.D.; Reid, D.M.; Willment, J.A.; Martinez-Pomares, L.; Gordon, S.; Wong, S.Y.C. The β-Glucan Receptor, Dectin-1, Is Predominantly Expressed on the Surface of Cells of the Monocyte/Macrophage and Neutrophil Lineages. J. Immunol. 2002, 169, 3876–3882. [Google Scholar] [CrossRef]
- Chiba, S.; Ikushima, H.; Ueki, H.; Yanai, H.; Kimura, Y.; Hangai, S.; Nishio, J.; Negishi, H.; Tamura, T.; Saijo, S.; et al. Recognition of tumor cells by Dectin-1 orchestrates innate immune cells for anti-tumor responses. eLife 2014, 3, e04177. [Google Scholar] [CrossRef]
- Hohl, T.M.; Feldmesser, M.; Perlin, D.S.; Pamer, E.G. Caspofungin modulates inflammatory responses to Aspergillus fumigatus through stage-specific effects on fungal β-glucan exposure. J. Infect. Dis. 2008, 198, 176–185. [Google Scholar] [CrossRef]
- Dubey, L.K.; Moeller, J.B.; Schlosser, A.; Sorensen, G.L.; Holmskov, U. Chitin enhances serum IgE in Aspergillus fumigatus induced allergy in mice. Immunobiol. 2015, 220, 714–721. [Google Scholar] [CrossRef]
- Chai, L.Y.; Vonk, A.G.; Kullberg, B.J.; Verweij, P.E.; Verschueren, I.; Van Der Meer, J.W.; Joosten, L.A.; Latgé, J.-P.; Netea, M.G. Aspergillus fumigatus cell wall components differentially modulate host TLR2 and TLR4 responses. Microbes Infect. 2011, 13, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.L.; Aimanianda, V.; Wang, X.; Gresnigt, M.S.; Ammerdorffer, A.; Jacobs, C.W.; Gazendam, R.P.; Joosten, L.A.B.; Netea, M.G.; Latgeé, J.-P.; et al. Aspergillus Cell Wall Chitin Induces Anti- and Proinflammatory Cytokines in Human PBMCs via the Fc-γ Receptor/Syk/PI3K Pathway. mBio 2016, 7, e01823-15. [Google Scholar] [CrossRef]
- Agrawal, S.; Gupta, S.; Agrawal, A. Human Dendritic Cells Activated via Dectin-1 Are Efficient at Priming Th17, Cytotoxic CD8 T and B Cell Responses. PLOS ONE 2010, 5, e13418. [Google Scholar] [CrossRef] [PubMed]
- Pagano, L.; Akova, M.; Dimopoulos, G.; Herbrecht, R.; Drgona, L.; Blijlevens, N. Risk assessment and prognostic factors for mould-related diseases in immunocompromised patients. J. Antimicrob. Chemother. 2010, 66, i5–i14. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.; Shook, D.R.; Shimasaki, N.; Chang, Y.-H.; Fujisaki, H.; Campana, D. Cytotoxicity of Activated Natural Killer Cells against Pediatric Solid Tumors. Clin. Cancer Res. 2010, 16, 3901–3909. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soe, W.M.; Lim, J.H.J.; Williams, D.L.; Goh, J.G.; Tan, Z.; Sam, Q.H.; Chotirmall, S.H.; Ali, N.A.B.M.; Lee, S.C.; Seet, J.E.; et al. Using Expanded Natural Killer Cells as Therapy for Invasive Aspergillosis. J. Fungi 2020, 6, 231. https://doi.org/10.3390/jof6040231
Soe WM, Lim JHJ, Williams DL, Goh JG, Tan Z, Sam QH, Chotirmall SH, Ali NABM, Lee SC, Seet JE, et al. Using Expanded Natural Killer Cells as Therapy for Invasive Aspergillosis. Journal of Fungi. 2020; 6(4):231. https://doi.org/10.3390/jof6040231
Chicago/Turabian StyleSoe, Win Mar, Joan Hui Juan Lim, David L. Williams, Jessamine Geraldine Goh, Zhaohong Tan, Qi Hui Sam, Sanjay H. Chotirmall, Nur A’tikah Binte Mohamed Ali, Soo Chin Lee, Ju Ee Seet, and et al. 2020. "Using Expanded Natural Killer Cells as Therapy for Invasive Aspergillosis" Journal of Fungi 6, no. 4: 231. https://doi.org/10.3390/jof6040231
APA StyleSoe, W. M., Lim, J. H. J., Williams, D. L., Goh, J. G., Tan, Z., Sam, Q. H., Chotirmall, S. H., Ali, N. A. B. M., Lee, S. C., Seet, J. E., Ravikumar, S., & Chai, L. Y. A. (2020). Using Expanded Natural Killer Cells as Therapy for Invasive Aspergillosis. Journal of Fungi, 6(4), 231. https://doi.org/10.3390/jof6040231




