Clinical Relevance and Characteristics of Aspergillus calidoustus and Other Aspergillus Species of Section Usti
Abstract
1. Introduction
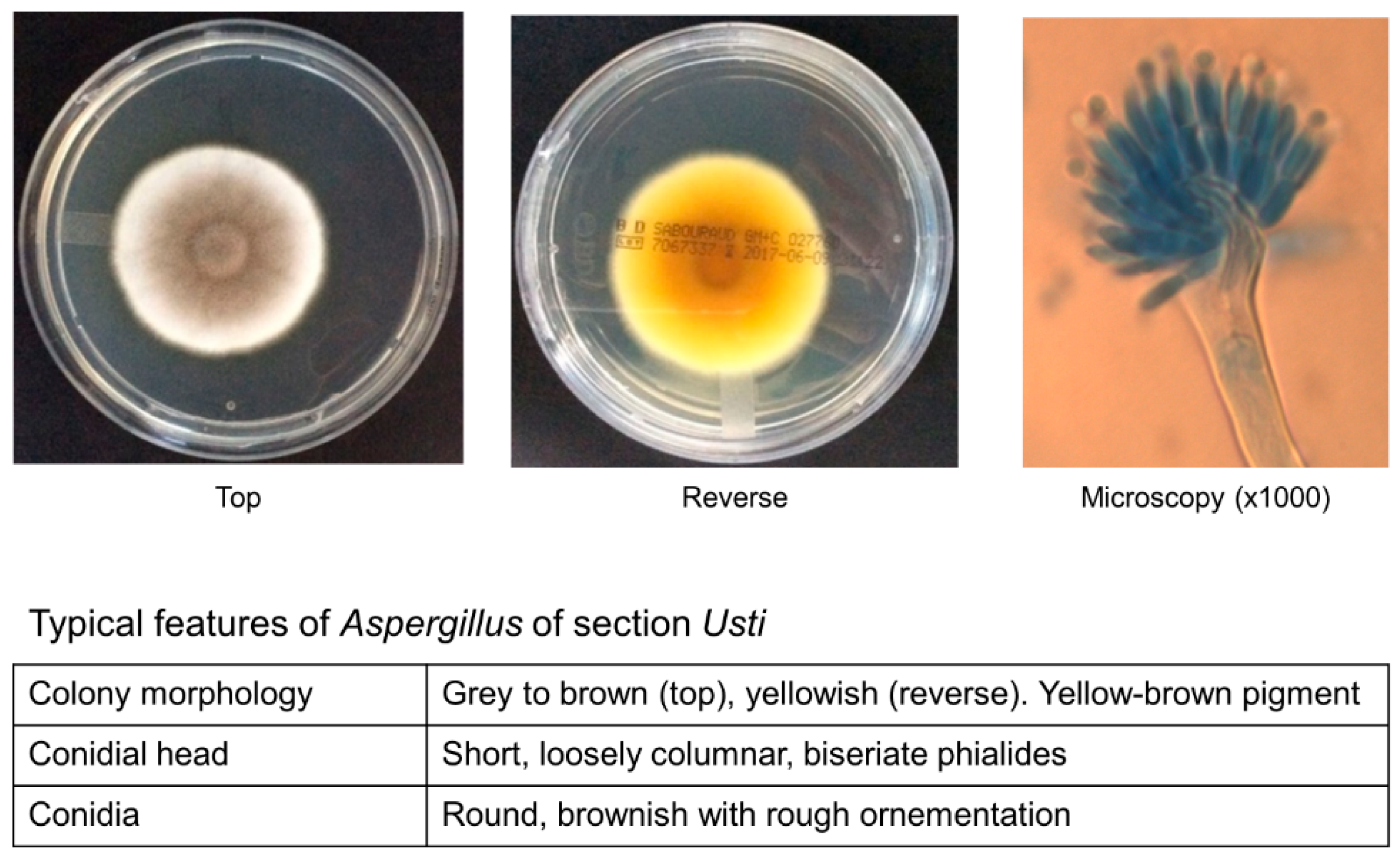
2. Taxonomy and Microbiology
3. Epidemiology and Clinical Characteristics
4. Treatment
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kontoyiannis, D.P.; Marr, K.A.; Park, B.J.; Alexander, B.D.; Anaissie, E.J.; Walsh, T.J.; Ito, J.; Andes, D.R.; Baddley, J.W.; Brown, J.M.; et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: Overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin. Infect. Dis. 2010, 50, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Pagano, L.; Caira, M.; Candoni, A.; Offidani, M.; Martino, B.; Specchia, G.; Pastore, D.; Stanzani, M.; Cattaneo, C.; Fanci, R.; et al. Invasive aspergillosis in patients with acute myeloid leukemia: A SEIFEM-2008 registry study. Haematologica 2010, 95, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G.; Alexander, B.D.; Andes, D.R.; Hadley, S.; Kauffman, C.A.; Freifeld, A.; Anaissie, E.J.; Brumble, L.M.; Herwaldt, L.; Ito, J.; et al. Invasive fungal infections among organ transplant recipients: Results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin. Infect. Dis. 2010, 50, 1101–1111. [Google Scholar] [CrossRef]
- Steinbach, W.J.; Marr, K.A.; Anaissie, E.J.; Azie, N.; Quan, S.P.; Meier-Kriesche, H.U.; Apewokin, S.; Horn, D.L. Clinical epidemiology of 960 patients with invasive aspergillosis from the PATH Alliance registry. J. Infect. 2012, 65, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Lamoth, F.; Chung, S.J.; Damonti, L.; Alexander, B.D. Changing Epidemiology of Invasive Mold Infections in Patients Receiving Azole Prophylaxis. Clin. Infect. Dis. 2017, 64, 1619–1621. [Google Scholar] [CrossRef]
- Samson, R.A.; Varga, J.; Meijer, M.; Frisvad, J.C. New taxa in Aspergillus section Usti. Stud. Mycol. 2011, 69, 81–97. [Google Scholar] [CrossRef]
- Hageskal, G.; Kristensen, R.; Fristad, R.F.; Skaar, I. Emerging pathogen Aspergillus calidoustus colonizes water distribution systems. Med. Mycol. 2011, 49, 588–593. [Google Scholar]
- Novakova, A.; Hubka, V.; Saiz-Jimenez, C.; Kolarik, M. Aspergillus baeticus sp. nov. and Aspergillus thesauricus sp. nov., two species in section Usti from Spanish caves. Int J. Syst. Evol. Microbiol. 2012, 62, 2778–2785. [Google Scholar] [CrossRef]
- Polizzi, V.; Adams, A.; Malysheva, S.V.; De Saeger, S.; Van Peteghem, C.; Moretti, A.; Picco, A.M.; De Kimpe, N. Identification of volatile markers for indoor fungal growth and chemotaxonomic classification of Aspergillus species. Fungal Biol. 2012, 116, 941–953. [Google Scholar] [CrossRef]
- Carrizosa, J.; Levison, M.E.; Lawrence, T.; Kaye, D. Cure of Aspergillus ustus endocarditis on a prosthetic valve. Arch. Intern. Med. 1974, 133, 486–490. [Google Scholar] [CrossRef]
- Glampedakis, E.; Cassaing, S.; Fekkar, A.; Dannaoui, E.; Bougnoux, M.E.; Bretagne, S.; Neofytos, D.; Schreiber, P.W.; Hennequin, C.; Morio, F.; et al. Invasive aspergillosis due to Aspergillus section Usti: A multicenter retrospective study. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Seroy, J.; Antiporta, P.; Grim, S.A.; Proia, L.A.; Singh, K.; Clark, N.M. Aspergillus calidoustus case series and review of the literature. Transpl. Infect. Dis. 2017, 19. [Google Scholar] [CrossRef] [PubMed]
- Jurjevic, Z.; Peterson, S.W. Aspergillus asper sp. nov. and Aspergillus collinsii sp. nov., from Aspergillus section Usti. Int. J. Syst. Evol. Microbiol. 2016, 66, 2566–2572. [Google Scholar] [CrossRef] [PubMed]
- Varga, J.; Houbraken, J.; Van Der Lee, H.A.; Verweij, P.E.; Samson, R.A. Aspergillus calidoustus sp. nov., causative agent of human infections previously assigned to Aspergillus ustus. Eukaryot. Cell 2008, 7, 630–638. [Google Scholar] [CrossRef]
- Ait-Ammar, N.; Levesque, E.; Murat, J.B.; Imbert, S.; Foulet, F.; Dannaoui, E.; Botterel, F. Aspergillus pseudodeflectus: A new human pathogen in liver transplant patients. BMC Infect. Dis. 2018, 18, 648. [Google Scholar] [CrossRef]
- Fakih, M.G.; Barden, G.E.; Oakes, C.A.; Berenson, C.S. First reported case of Aspergillus granulosus infection in a cardiac transplant patient. J. Clin. Microbiol. 1995, 33, 471–473. [Google Scholar] [CrossRef]
- Sutton, D.A.; Wickes, B.L.; Romanelli, A.M.; Rinaldi, M.G.; Thompson, E.H.; Fothergill, A.W.; Dishop, M.K.; Elidemir, O.; Mallory, G.B.; Moonnamakal, S.P.; et al. Cerebral aspergillosis caused by Aspergillus granulosus. J. Clin. Microbiol. 2009, 47, 3386–3390. [Google Scholar] [CrossRef]
- Donnelly, J.P.; Chen, S.C.; Kauffman, C.A.; Steinbach, W.J.; Baddley, J.W.; Verweij, P.E.; Clancy, C.J.; Wingard, J.R.; Lockhart, S.R.; Groll, A.H.; et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2019, 46. [Google Scholar] [CrossRef]
- McMullen, A.R.; Wallace, M.A.; Pincus, D.H.; Wilkey, K.; Burnham, C.A. Evaluation of the Vitek MS Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry System for Identification of Clinically Relevant Filamentous Fungi. J. Clin. Microbiol. 2016, 54, 2068–2073. [Google Scholar] [CrossRef]
- Houbraken, J.; Due, M.; Varga, J.; Meijer, M.; Frisvad, J.C.; Samson, R.A. Polyphasic taxonomy of Aspergillus section Usti. Stud. Mycol. 2007, 59, 107–128. [Google Scholar] [CrossRef]
- Alastruey-Izquierdo, A.; Cuesta, I.; Houbraken, J.; Cuenca-Estrella, M.; Monzon, A.; Rodriguez-Tudela, J.L. In vitro activity of nine antifungal agents against clinical isolates of Aspergillus calidoustus. Med. Mycol. 2010, 48, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Ghannoum, M.; Long, L.; Larkin, E.L.; Isham, N.; Sherif, R.; Borroto-Esoda, K.; Barat, S.; Angulo, D. Evaluation of the Antifungal Activity of the Novel Oral Glucan Synthase Inhibitor SCY-078, Singly and in Combination, for the Treatment of Invasive Aspergillosis. Antimicrob. Agents Chemother. 2018, 62, e00244-18. [Google Scholar] [CrossRef]
- Wiederhold, N.P.; Locke, J.B.; Daruwala, P.; Bartizal, K. Rezafungin (CD101) demonstrates potent in vitro activity against Aspergillus, including azole-resistant Aspergillus fumigatus isolates and cryptic species. J. Antimicrob. Chemother. 2018, 73, 3063–3067. [Google Scholar] [CrossRef] [PubMed]
- Glampedakis, E.; Coste, A.T.; Aruanno, M.; Bachmann, D.; Delarze, E.; Erard, V.; Lamoth, F. Efficacy of Antifungal Monotherapies and Combinations against Aspergillus calidoustus. Antimicrob. Agents Chemother. 2018, 62, e01137-18. [Google Scholar] [CrossRef]
- Balajee, S.A.; Kano, R.; Baddley, J.W.; Moser, S.A.; Marr, K.A.; Alexander, B.D.; Andes, D.; Kontoyiannis, D.P.; Perrone, G.; Peterson, S.; et al. Molecular identification of Aspergillus species collected for the Transplant-Associated Infection Surveillance Network. J. Clin. Microbiol. 2009, 47, 3138–3141. [Google Scholar] [CrossRef]
- Egli, A.; Fuller, J.; Humar, A.; Lien, D.; Weinkauf, J.; Nador, R.; Kapasi, A.; Kumar, D. Emergence of Aspergillus calidoustus infection in the era of posttransplantation azole prophylaxis. Transplantation 2012, 94, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Panackal, A.A.; Imhof, A.; Hanley, E.W.; Marr, K.A. Aspergillus ustus infections among transplant recipients. Emerg. Infect. Dis. 2006, 12, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Ullmann, A.J.; Aguado, J.M.; Arikan-Akdagli, S.; Denning, D.W.; Groll, A.H.; Lagrou, K.; Lass-Florl, C.; Lewis, R.E.; Munoz, P.; Verweij, P.E.; et al. Diagnosis and management of Aspergillus diseases: Executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin. Microbiol. Infect. 2018, 24 (Suppl. 1), e1–e38. [Google Scholar] [CrossRef]
- Mendoza, M.A.; Anderson, A.; Morris, M.I.; Lekakis, L.; Simkins, J.; Prado, C.E.; Martinez, O.V.; Komanduri, K.V.; Camargo, J.F. Successful Treatment of Invasive Fungal Infection Due to Highly Resistant Aspergillus calidoustus in an Allogeneic Hematopoietic Cell Transplant Recipient. Mycopathologia 2020, 185, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Hosseini-Yeganeh, M.; McLachlan, A.J. Tissue distribution of terbinafine in rats. J. Pharm. Sci. 2001, 90, 1817–1828. [Google Scholar] [CrossRef] [PubMed]
- Seidel, D.; Meissner, A.; Lackner, M.; Piepenbrock, E.; Salmanton-Garcia, J.; Stecher, M.; Mellinghoff, S.; Hamprecht, A.; Duran Graeff, L.; Kohler, P.; et al. Prognostic factors in 264 adults with invasive Scedosporium spp. and Lomentospora prolificans infection reported in the literature and FungiScope((R)). Crit. Rev. Microbiol. 2019, 45, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Lamoth, F.; Kontoyiannis, D.P. Therapeutic Challenges of Non-Aspergillus Invasive Mold Infections in Immunosuppressed Patients. Antimicrob. Agents Chemother. 2019, 63, e01244-19. [Google Scholar] [CrossRef]
- Rivero-Menendez, O.; Cuenca-Estrella, M.; Alastruey-Izquierdo, A. In vitro activity of olorofim (F901318) against clinical isolates of cryptic species of Aspergillus by EUCAST and CLSI methodologies. J. Antimicrob. Chemother. 2019, 74, 1586–1590. [Google Scholar] [CrossRef] [PubMed]
- Rivero-Menendez, O.; Cuenca-Estrella, M.; Alastruey-Izquierdo, A. In vitro activity of APX001A against rare moulds using EUCAST and CLSI methodologies. J. Antimicrob. Chemother. 2019, 74, 1295–1299. [Google Scholar] [CrossRef] [PubMed]
| Isolated in Clinical Specimens | Environmental Samples Only | |
|---|---|---|
| Proven/probable IA 1 cases A. calidoustus2 A. pseudodeflectus3 A. granulosus4 A. ustus5 | A. amylovorus A. asper A. baeticus A. californicus A. carlsbadensis A. cavernicola A. collinsii A. deflectus A. egyptiacus A. elongatus | A. turkensis A. germanicus A. heterothallicus A. kassunensis A. lucknowensis A. monodii A. pseudoustus A. subsessilis A. thessauricus |
| Colonization only A. insuetus A. keveii A. puniceus | ||
| Antifungal Drug Classes | Evidences | Comments |
|---|---|---|
| Amphotericin B | Relatively good in vitro activity (MIC 0.25–2 µg/mL) [11,21] Effective in a Galleria model [24] | Recommended as first-line on the basis of optimal in vitro activity (use lipid-based formulation) |
| Mold-active azoles | Relatively low in vitro activity (MIC 2 – 16 µg/mL): isavuconazole > voriconazole > posaconazole [11,21] Voriconazole effective in a Galleria model of infection [24] Caveat: breakthrough infections frequently reported (mainly under posaconazole, but also voriconazole) | Pre-clinical and clinical data suggest possible use in selected situations (e.g., less severe cases or second-line/maintenance treatment, absence of previous mold-active azole prophylaxis) Avoid posaconazole |
| Echinocandins | Fungistatic effect: micafungin/anidulafungin > caspofungin [21] | May be used in combination with either amphotericin B or triazoles despite no evidence of synergism Few experience as monotherapy, use only if no other alternatives (preferably micafungin or anidulafungin) |
| Terbinafine | Relatively good in vitro activity (MIC 0.25–1 µg/mL) [21,24] Effective in a Galleria model of infection [24] In vitro and in vivo (Galleria) synergism with voriconazole, posaconazole and isavuconazole [24] In vitro antagonism with amphotericin B [24] Accumulation in skin (no sustained levels in blood) [30] | May be combined with voriconazole (or isavuconazole) in selected situations (see above, possible interest in patients with skin lesions or alternative to amphotericin B in case of intolerance) Use as monotherapy not recommended |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glampedakis, E.; Erard, V.; Lamoth, F. Clinical Relevance and Characteristics of Aspergillus calidoustus and Other Aspergillus Species of Section Usti. J. Fungi 2020, 6, 84. https://doi.org/10.3390/jof6020084
Glampedakis E, Erard V, Lamoth F. Clinical Relevance and Characteristics of Aspergillus calidoustus and Other Aspergillus Species of Section Usti. Journal of Fungi. 2020; 6(2):84. https://doi.org/10.3390/jof6020084
Chicago/Turabian StyleGlampedakis, Emmanouil, Véronique Erard, and Frederic Lamoth. 2020. "Clinical Relevance and Characteristics of Aspergillus calidoustus and Other Aspergillus Species of Section Usti" Journal of Fungi 6, no. 2: 84. https://doi.org/10.3390/jof6020084
APA StyleGlampedakis, E., Erard, V., & Lamoth, F. (2020). Clinical Relevance and Characteristics of Aspergillus calidoustus and Other Aspergillus Species of Section Usti. Journal of Fungi, 6(2), 84. https://doi.org/10.3390/jof6020084





