Review of Potential Pseudomonas Weaponry, Relevant to the Pseudomonas–Aspergillus Interplay, for the Mycology Community
Abstract
1. Introduction
2. Why Pseudomonas aeruginosa?
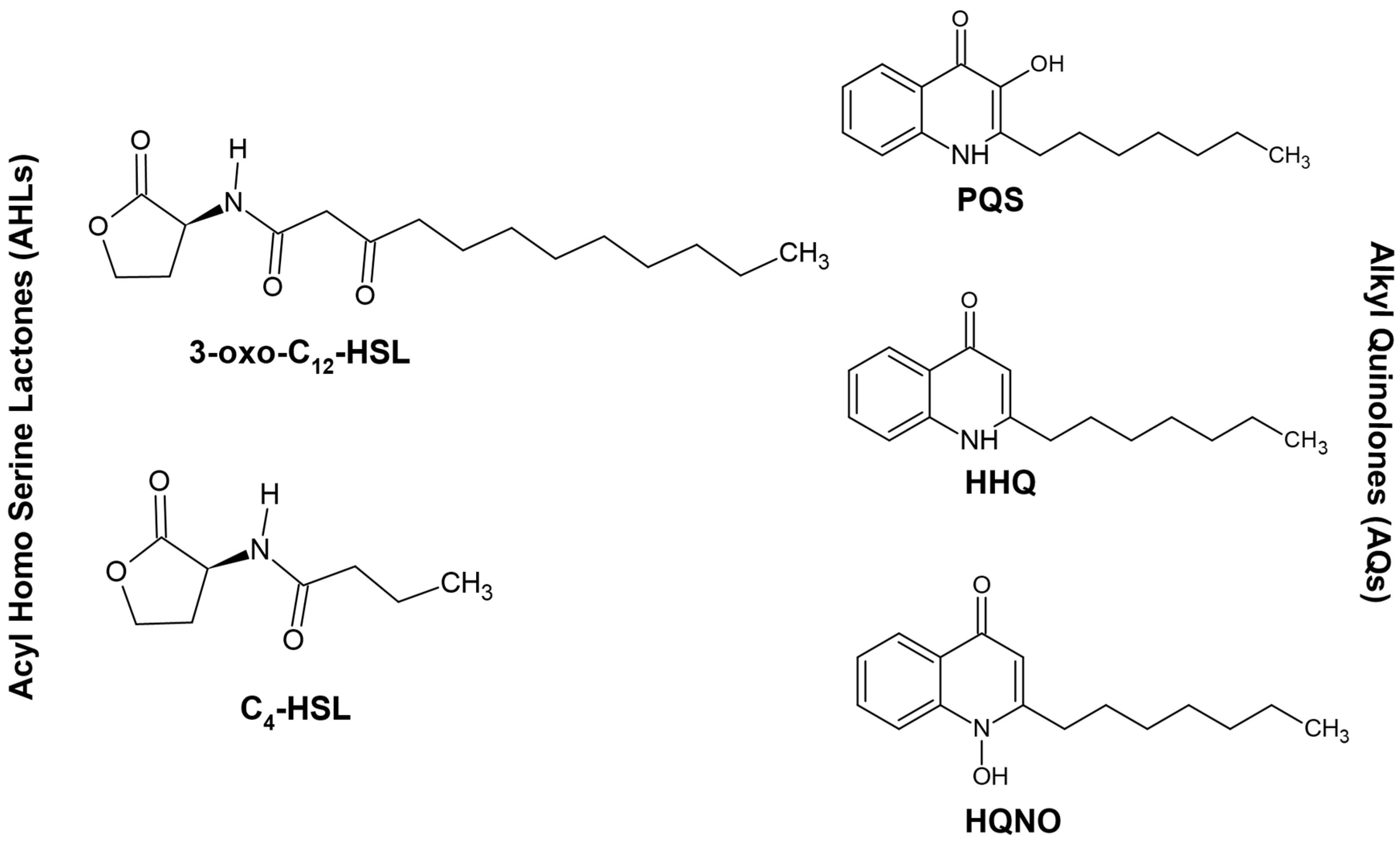
3. Quorum Sensing and P. aeruginosa
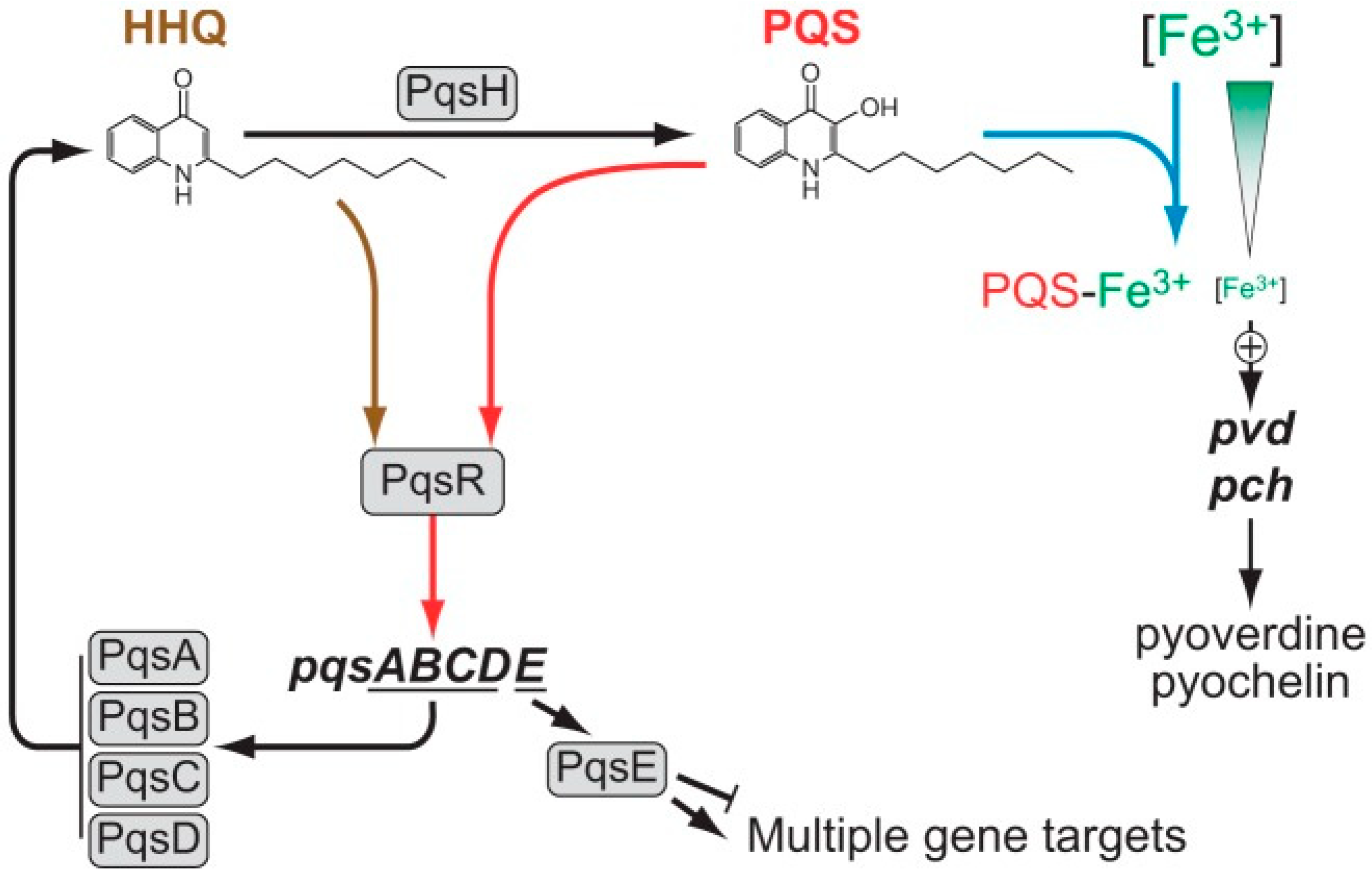
4. Interplay between Different Quorum Signaling Molecules and the Role of LasI/RhlI Systems in the Quorum-Sensing Circuitry
5. Involvement of Quorum-Sensing Molecules in P. aeruginosa Virulence and Antifungal Activity
6. Siderophores in P. aeruginosa and Their Anti-Aspergillus Effects
7. Elastase in P. aeruginosa and Antifungal Effects
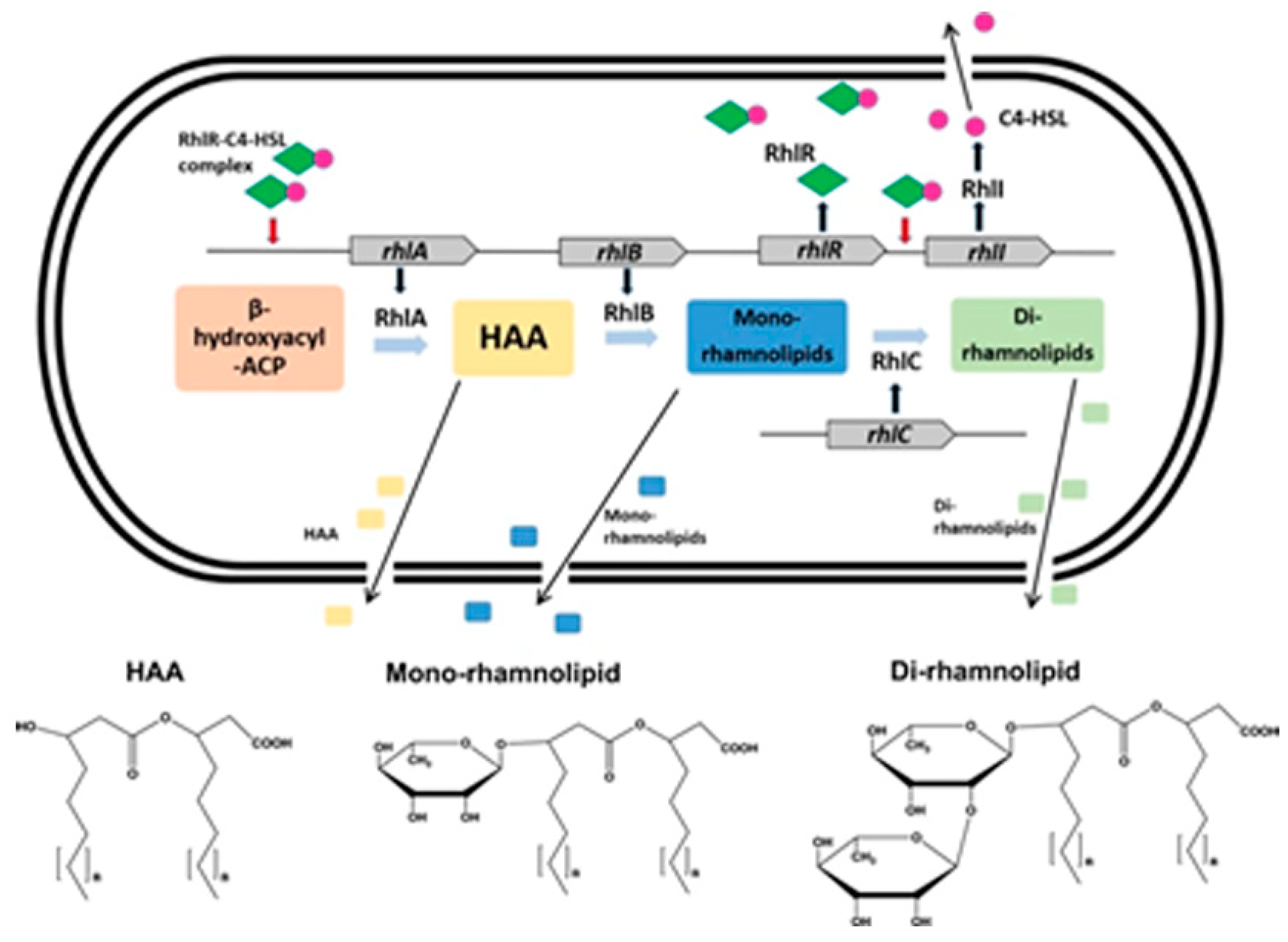
8. Rhamnolipids in P. aeruginosa and Their Antifungal Effects
9. Phenazines and Their Antifungal Role in P. aeruginosa
10. Role of Secretion Systems in P. aeruginosa Virulence and Quorum-Sensing Response
11. In Vivo Interactions between P. aeruginosa and A. fumigatus
12. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Williams, H.D.; Davies, J.C. Basic science for the chest physician: Pseudomonas aeruginosa and the cystic fibrosis airway. Thorax 2012, 67, 465–467. [Google Scholar] [CrossRef]
- Lipuma, J.J. The changing microbial epidemiology in cystic fibrosis. Clin. Microbiol. Rev. 2010, 23, 299–323. [Google Scholar] [CrossRef] [PubMed]
- Nagano, Y.; Millar, B.C.; Johnson, E.; Goldsmith, C.E.; Elborn, J.S.; Rendall, J.; Moore, J.E. Fungal infections in patients with cystic fibrosis. Rev. Med. Microbiol. 2007, 18, 11–16. [Google Scholar] [CrossRef]
- Sabino, R.; Ferreira, J.A.; Moss, R.B.; Valente, J.; Veríssimo, C.; Carolino, E.; Clemons, K.V.; Everson, C.; Banaei, N.; Penner, J.; et al. Molecular epidemiology of Aspergillus collected from cystic fibrosis patients. J. Cyst. Fibros. 2015, 14, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Mangan, A. Interactions between some aural Aspergillus species and bacteria. J. Gen. Microbiol. 1969, 58, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Blyth, W.; Forey, A. The influence of respiratory bacteria and their biochemical fractions on Aspergillus fumigatus. Sabouraudia 1971, 9, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.R.; Taylor, G.W.; Rutman, A.; Høiby, N.; Cole, P.J.; Wilson, R. Pseudomonas aeruginosa pyocyanin and 1-hydroxyphenazine inhibit fungal growth. J. Clin. Pathol. 1999, 52, 385–387. [Google Scholar] [CrossRef] [PubMed]
- Mowat, E.; Rajendran, R.; Williams, C.; McCulloch, E.; Jones, B.; Lang, S.; Ramage, G. Pseudomonas aeruginosa and their small diffusible extracellular molecules inhibit Aspergillus fumigatus biofilm formation. FEMS Microbiol. Lett. 2010, 313, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Moree, W.J.; Phelan, V.V.; Wu, C.H.; Bandeira, N.; Cornett, D.S.; Duggan, B.M.; Dorrestein, P.C. Interkingdom metabolic transformations captured by microbial imaging mass spectrometry. Proc. Natl. Acad. Sci. USA 2012, 109, 13811–13816. [Google Scholar] [CrossRef]
- Illakkiam, D.; Ponraj, P.; Shankar, M.; Muthusubramanian, S.; Rajendhran, J.; Gunasekaran, P. Identification and structure elucidation of a novel antifungal compound produced by Pseudomonas aeruginosa PGPR2 against Macrophomina phaseolina. Appl. Biochem. Biotechnol. 2013, 171, 2176–2185. [Google Scholar] [CrossRef]
- Manavathu, E.K.; Vager, D.L.; Vazquez, J.A. Development and antimicrobial susceptibility studies of in vitro monomicrobial and polymicrobial biofilm models with Aspergillus fumigatus and Pseudomonas aeruginosa. BMC Microbiol. 2014, 14, 53. [Google Scholar] [CrossRef] [PubMed]
- Briard, B.; Bomme, P.; Lechner, B.E.; Mislin, G.L.; Lair, V.; Prévost, M.C.; Latgé, J.P.; Haas, H.; Beauvais, A. Pseudomonas aeruginosa manipulates redox and iron homeostasis of its microbiota partner Aspergillus fumigatus via phenazines. Sci. Rep. 2015, 5, 8220. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.; Rajendran, R.; Kerr, S.; Lappin, D.F.; Mackay, W.G.; Williams, C.; Ramage, G. Aspergillus fumigatus enhances elastase production in Pseudomonas aeruginosa co-cultures. Med. Mycol. 2015, 53, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.A.; Penner, J.C.; Moss, R.B.; Haagensen, J.A.; Clemons, K.V.; Spormann, A.M.; Nazik, H.; Cohen, K.; Banaei, N.; Carolino, E.; et al. Inhibition of Aspergillus fumigatus and its biofilm by Pseudomonas aeruginosa is dependent on the source, phenotype and growth conditions of the bacterium. PLoS ONE 2015, 10, e0134692. [Google Scholar] [CrossRef]
- Shirazi, F.; Ferreira, J.A.; Stevens, D.A.; Clemons, K.V.; Kontoyiannis, D.P. Biofilm filtrates of Pseudomonas aeruginosa strains isolated from cystic fibrosis patients inhibit preformed Aspergillus fumigatus biofilms via apoptosis. PLoS ONE 2016, 11, e0150155. [Google Scholar] [CrossRef]
- Penner, J.C.; Ferreira, J.A.G.; Secor, P.R.; Sweere, J.M.; Birukova, M.K.; Joubert, L.M.; Haagensen, J.; Garcia, O.; Malkovskiy, A.V.; Kaber, G.; et al. Pf4 bacteriophage produced by Pseudomonas aeruginosa inhibits Aspergillus fumigatus metabolism via iron sequestration. Microbiology 2016, 162, 1583–1594. [Google Scholar] [CrossRef]
- Reen, F.J.; Phelan, J.P.; Woods, D.F.; Shanahan, R.; Cano, R.; Clarke, S.; McGlacken, G.P.; O’Gara, F. Harnessing bacterial signals for suppression of biofilm formation in the nosocomial fungal pathogen Aspergillus fumigatus. Front. Microbiol. 2016, 7, 2074. [Google Scholar] [CrossRef]
- Anand, R.; Clemons, K.V.; Stevens, D.A. Effect of anaerobiasis or hypoxia on Pseudomonas aeruginosa inhibition of Aspergillus fumigatus biofilm. Arch. Microbiol. 2017, 199, 881–890. [Google Scholar] [CrossRef]
- Briard, B.; Rasoldier, V.; Bomme, P.; ElAouad, N.; Guerreiro, C.; Chassagne, P.; Muszkieta, L.; Latgé, J.P.; Mulard, L.; Beauvais, A. Dirhamnolipids secreted from Pseudomonas aeruginosa modify antifungal susceptibility of Aspergillus fumigatus by inhibiting β1,3 glucan synthase activity. ISME J. 2017, 11, 1578–1591. [Google Scholar] [CrossRef]
- Sass, G.; Stevens, D.A. Aspergillus fumigatus (Af) hydroxamate siderophores protect formation of Af biofilms from the Pseudomonas aeruginosa (Pa) product pyoverdine. Open Forum Infect Dis. 2017, 4, S114. [Google Scholar] [CrossRef][Green Version]
- Nazik, H.; Moss, R.B.; Karna, V.; Clemons, K.V.; Banaei, N.; Cohen, K.; Choudhary, V.; Stevens, D.A. Are cystic fibrosis Aspergillus fumigatus isolates different? Intermicrobial interactions with Pseudomonas. Mycopathol. 2017, 182, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Reece, E.; Segurado, R.; Jackson, A.; McClean, S.; Renwick, J.; Greally, P. Co-colonisation with Aspergillus fumigatus and Pseudomonas aeruginosa is associated with poorer health in cystic fibrosis patients: An Irish registry analysis. BMC Pulm. Med. 2017, 17, 70. [Google Scholar] [CrossRef] [PubMed]
- Sass, G.; Nazik, H.; Penner, J.; Shah, H.; Ansari, S.R.; Clemons, K.V.; Groleau, M.C.; Dietl, A.M.; Visca, P.; Haas, H.; et al. Studies of Pseudomonas aeruginosa mutants indicate pyoverdine as the central factor in inhibition of Aspergillus fumigatus biofilm. J. Bacteriol. 2017, 200, e00345-17. [Google Scholar] [CrossRef] [PubMed]
- Anand, R.; Moss, R.B.; Sass, G.; Banaei, N.; Clemons, K.V.; Martinez, M.; Stevens, D.A. Small colony variants of Pseudomonas aeruginosa display heterogeneity in inhibiting Aspergillus fumigatus biofilm. Mycopathol. 2018, 183, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Sass, G.; Ansari, S.R.; Dietl, A.M.; Déziel, E.; Haas, H.; Stevens, D.A. Intermicrobial interaction: Aspergillus fumigatus siderophores protect against competition by Pseudomonas aeruginosa. PLoS ONE 2019, 14, e0216085. [Google Scholar] [CrossRef]
- Nazik, H.; Sass, G.; Ansari, S.R.; Ertekin, R.; Haas, H.; Déziel, E.; Stevens, D.A. Novel intermicrobial molecular interaction: Pseudomonas aeruginosa Quinolone Signal (PQS) modulates Aspergillus fumigatus response to iron. Microbiology 2020, 166, 44–55. [Google Scholar] [CrossRef]
- Branski, L.K.; Al-Mousawi, A.; Rivero, H.; Jeschke, M.G.; Sanford, A.P.; Herndon, D.N. Emerging infections in burns. Surg. Infect. (Larchmt) 2009, 10, 389–397. [Google Scholar] [CrossRef]
- De Bentzmann, S.; Plésiat, P. The Pseudomonas aeruginosa opportunistic pathogen and human infections. Environ. Microbiol. 2011, 13, 1655–1665. [Google Scholar] [CrossRef]
- Tielen, P.; Narten, M.; Rosin, N.; Biegler, I.; Haddad, I.; Hogardt, M.; Neubauer, R.; Schobert, M.; Wiehlmann, L.; Jahn, D. Genotypic and phenotypic characterization of Pseudomonas aeruginosa isolates from urinary tract infections. Int. J. Med. Microbiol. 2011, 301, 282–292. [Google Scholar] [CrossRef]
- Shoseyov, D.; Brownlee, K.G.; Conway, S.P.; Kerem, E. Aspergillus bronchitis in cystic fibrosis. Chest 2006, 130, 222–226. [Google Scholar] [CrossRef]
- Amin, R.; Dupuis, A.; Aaron, S.D.; Ratjen, F. The effect of chronic infection with Aspergillus fumigatus on lung function and hospitalization in patients with cystic fibrosis. Chest 2010, 137, 171–176. [Google Scholar] [CrossRef] [PubMed]
- de Boer, K.; Vandemheen, K.L.; Tullis, E.; Doucette, S.; Fergusson, D.; Freitag, A.; Paterson, N.; Jackson, M.; Lougheed, M.D.; Kumar, V.; et al. Exacerbation frequency and clinical outcomes in adult patients with cystic fibrosis. Thorax 2011, 66, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, K.A.; Ranganathan, S.; Park, J.; Skoric, B.; Adams, A.M.; Simpson, S.J.; Robins-Browne, R.M.; Franklin, P.J.; de Klerk, N.H.; Sly, P.D.; et al. Early respiratory infection is associated with reduced spirometry in children with cystic fibrosis. Am. J. Respir. Crit. Care Med. 2014, 190, 1111–1116. [Google Scholar] [CrossRef] [PubMed]
- Lamont, I.L.; Konings, A.F.; Reid, D.W. Iron acquisition by Pseudomonas aeruginosa in the lungs of patients with cystic fibrosis. BioMetals 2009, 22, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Haas, H. Iron—A Key Nexus in the Virulence of Aspergillus fumigatus. Front. Microbiol. 2012, 3, 28. [Google Scholar] [CrossRef]
- Cornelis, P.; Dingemans, J. Pseudomonas aeruginosa adapts its iron uptake strategies in function of the type of infections. Front. Cell Infect Microbiol. 2013, 3, 75. [Google Scholar] [CrossRef]
- Matthaiou, E.I.; Sass, G.; Stevens, D.A.; Hsu, J.L. Iron: An essential nutrient for Aspergillus fumigatus and a fulcrum for pathogenesis. Curr. Opin. Infect Dis. 2018, 31, 506–511. [Google Scholar] [CrossRef]
- Latifi, A.; Winson, M.K.; Foglino, M.; Bycroft, B.W.; Stewart, G.S.; Lazdunski, A.; Williams, P. Multiple homologues of LuxR and LuxI control expression of virulence determinants and secondary metabolites through quorum sensing in Pseudomonas aeruginosa PAO1. Mol. Microbiol. 1995, 17, 333–343. [Google Scholar] [CrossRef]
- Lee, D.G.; Urbach, J.M.; Wu, G.; Liberati, N.T.; Feinbaum, R.L.; Miyata, S.; Diggins, L.T.; He, J.; Saucier, M.; Déziel, E.; et al. Genomic analysis reveals that Pseudomonas aeruginosa virulence is combinatorial. Genome Biol. 2006, 7, R90. [Google Scholar] [CrossRef]
- Hauser, A.R. Pseudomonas aeruginosa: So many virulence factors, so little time. Crit. Care. Med. 2011, 39, 2193–2194. [Google Scholar] [CrossRef]
- Jimenez, P.N.; Koch, G.; Thompson, J.A.; Xavier, K.B.; Cool, R.H.; Quax, W.J. The Multiple Signaling Systems Regulating Virulence in Pseudomonas aeruginosa. Microbiol. Mol. Biol. Rev. 2012, 76, 46–65. [Google Scholar] [CrossRef] [PubMed]
- Peek, M.E.; Bhatnagar, A.; McCarty, N.A.; Zughaier, S.M. Pyoverdine, the Major Siderophore in Pseudomonas aeruginosa, Evades NGAL Recognition. Interdiscip Perspect Infect Dis. 2012, 2012, 843509. [Google Scholar] [CrossRef] [PubMed]
- Coggan, K.A.; Wolfgang, M.C. Global regulatory pathways and cross-talk control Pseudomonas aeruginosa environmental lifestyle and virulence phenotype. Curr. Issues Mol. Biol. 2012, 14, 47–70. [Google Scholar] [PubMed]
- Balasubramanian, D.; Schneper, L.; Kumari, H.; Mathee, K. A dynamic and intricate regulatory network determines Pseudomonas aeruginosa virulence. Nucleic Acids Res. 2013, 41, 1–20. [Google Scholar] [CrossRef]
- Davies, J.C.; Bilton, D. Bugs, biofilms, and resistance in cystic fibrosis. Respir. Care 2009, 54, 628–640. [Google Scholar] [CrossRef]
- Folkesson, A.; Jelsbak, L.; Yang, L.; Johansen, H.K.; Ciofu, O.; Høiby, N.; Molin, S. Adaptation of Pseudomonas aeruginosa to the cystic fibrosis airway: An evolutionary perspective. Nat. Rev. Microbiol. 2012, 10, 841–851. [Google Scholar] [CrossRef]
- Smyth, A.R.; Hurley, M.N. Targeting the Pseudomonas aeruginosa biofilm to combat infections in patients with cystic fibrosis. Drugs Future 2010, 35, 1007–1014. [Google Scholar] [CrossRef]
- Stover, C.K.; Pham, X.Q.; Erwin, A.L.; Mizoguchi, S.D.; Warrener, P.; Hickey, M.J.; Brinkman, F.S.; Hufnagle, W.O.; Kowalik, D.J.; Lagrou, M.; et al. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 2000, 406, 959–964. [Google Scholar] [CrossRef]
- Pesci, E.C.; Milbank, J.B.; Pearson, J.P.; McKnight, S.; Kende, A.S.; Greenberg, E.P.; Iglewski, B.H. Quinolone signaling in the cell-to-cell communication system of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 1999, 96, 11229–11234. [Google Scholar] [CrossRef]
- Wade, D.S.; Calfee, M.W.; Rocha, E.R.; Ling, E.A.; Engstrom, E.; Coleman, J.P.; Pesci, E.C. Regulation of Pseudomonas quinolone signal synthesis in Pseudomonas aeruginosa. J. Bacteriol. 2005, 187, 4372–4380. [Google Scholar] [CrossRef]
- Williams, P.; Cámara, M. Quorum sensing and environmental adaptation in Pseudomonas aeruginosa: A tale of regulatory networks and multifunctional signal molecules. Curr. Opin. Microbiol. 2009, 12, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Fuqua, W.C.; Winans, S.C.; Greenberg, E.P. Quorum sensing in bacteria: The LuxR-LuxI family of cell density-responsive transcriptional regulators. J. Bacteriol. 1994, 176, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Déziel, E.; Lépine, F.; Milot, S.; He, J.; Mindrinos, M.N.; Tompkins, R.G.; Rahme, L.G. Analysis of Pseudomonas aeruginosa 4-hydroxy-2-alkylquinolines (HAQs) reveals a role for 4-hydroxy-2-heptylquinoline in cell-to-cell communication. Proc. Natl. Acad. Sci. USA 2004, 101, 1339–1344. [Google Scholar] [CrossRef] [PubMed]
- Wilder, C.N.; Diggle, S.P.; Schuster, M. Cooperation and cheating in Pseudomonas aeruginosa: The roles of the las, rhl and pqs quorum-sensing systems. ISME J. 2011, 5, 1332–1343. [Google Scholar] [CrossRef] [PubMed]
- Pustelny, C.; Albers, A.; Büldt-Karentzopoulos, K.; Parschat, K.; Chhabra, S.R.; Cámara, M.; Williams, P.; Fetzner, S. Dioxygenase-mediated quenching of quinolone-dependent quorum sensing in Pseudomonas aeruginosa. Chem. Biol. 2009, 16, 1259–1267. [Google Scholar] [CrossRef]
- Tashiro, Y.; Yawata, Y.; Toyofuku, M.; Uchiyama, H.; Nomura, N. Interspecies interaction between Pseudomonas aeruginosa and other microorganisms. Microbes. Environ. 2013, 28, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Nouwens, A.S.; Beatson, S.A.; Whitchurch, C.B.; Walsh, B.J.; Schweizer, H.P.; Mattick, J.S.; Cordwell, S.J. Proteome analysis of extracellular proteins regulated by the las and rhl quorum sensing systems in Pseudomonas aeruginosa PAO1. Microbiology 2003, 149, 1311–1322. [Google Scholar] [CrossRef]
- Whiteley, M.; Greenberg, E.P. Promoter specificity elements in Pseudomonas aeruginosa quorum-sensing-controlled genes. J. Bacteriol. 2001, 183, 5529–5534. [Google Scholar] [CrossRef]
- Price-Whelan, A.; Dietrich, L.E.; Newman, D.K. Rethinking ‘secondary’ metabolism: Physiological roles for phenazine antibiotics [published correction appears in Nat. Chem. Biol. 2006, 2, 221]. Nat. Chem. Biol. 2006, 2, 71–78. [Google Scholar] [CrossRef]
- Xiao, G.; Déziel, E.; He, J.; Lépine, F.; Lesic, B.; Castonguay, M.H.; Milot, S.; Tampakaki, A.P.; Stachel, S.E.; Rahme, L.G. MvfR, a key Pseudomonas aeruginosa pathogenicity LTTR-class regulatory protein, has dual ligands. Mol. Microbiol. 2006, 62, 1689–1699. [Google Scholar] [CrossRef]
- Latifi, A.; Foglino, M.; Tanaka, K.; Williams, P.; Lazdunski, A. A hierarchical quorum-sensing cascade in Pseudomonas aeruginosa links the transcriptional activators LasR and RhIR (VsmR) to expression of the stationary-phase sigma factor RpoS. Mol. Microbiol. 1996, 21, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Schuster, M.; Urbanowski, M.L.; Greenberg, E.P. Promoter specificity in Pseudomonas aeruginosa quorum sensing revealed by DNA binding of purified LasR. Proc. Natl. Acad. Sci. USA 2004, 101, 15833–15839. [Google Scholar] [CrossRef] [PubMed]
- Schuster, M.; Greenberg, E.P. Early activation of quorum sensing in Pseudomonas aeruginosa reveals the architecture of a complex regulon. BMC Genomics 2007, 8, 287. [Google Scholar] [CrossRef] [PubMed]
- Whiteley, M.; Lee, K.M.; Greenberg, E.P. Identification of genes controlled by quorum sensing in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 1999, 96, 13904–13909. [Google Scholar] [CrossRef] [PubMed]
- Winzer, K.; Falconer, C.; Garber, N.C.; Diggle, S.P.; Camara, M.; Williams, P. The Pseudomonas aeruginosa lectins PA-IL and PA-IIL are controlled by quorum sensing and by RpoS. J. Bacteriol. 2000, 182, 6401–6411. [Google Scholar] [CrossRef]
- Heurlier, K.; Dénervaud, V.; Haenni, M.; Guy, L.; Krishnapillai, V.; Haas, D. Quorum-sensing-negative (lasR) mutants of Pseudomonas aeruginosa avoid cell lysis and death. J. Bacteriol. 2005, 187, 4875–4883. [Google Scholar] [CrossRef]
- Déziel, E.; Gopalan, S.; Tampakaki, A.P.; Lépine, F.; Padfield, K.E.; Saucier, M.; Xiao, G.; Rahme, L.G. The contribution of MvfR to Pseudomonas aeruginosa pathogenesis and quorum sensing circuitry regulation: Multiple quorum sensing-regulated genes are modulated without affecting lasRI, rhlRI or the production of N-acyl-l-homoserine lactones. Mol. Microbiol. 2005, 55, 998–1014. [Google Scholar] [CrossRef]
- Diggle, S.P.; Winzer, K.; Chhabra, S.R.; Worrall, K.E.; Cámara, M.; Williams, P. The Pseudomonas aeruginosa quinolone signal molecule overcomes the cell density-dependency of the quorum sensing hierarchy, regulates rhl-dependent genes at the onset of stationary phase and can be produced in the absence of LasR. Mol. Microbiol. 2003, 50, 29–43. [Google Scholar] [CrossRef]
- Dekimpe, V.; Déziel, E. Revisiting the quorum-sensing hierarchy in Pseudomonas aeruginosa: The transcriptional regulator RhlR regulates LasR-specific factors. Microbiology 2009, 155, 712–723. [Google Scholar] [CrossRef]
- Feltner, J.B.; Wolter, D.J.; Pope, C.E.; Groleau, M.C.; Smalley, N.E.; Greenberg, E.P.; Mayer-Hamblett, N.; Burns, J.; Déziel, E.; Hoffman, L.R.; et al. LasR Variant Cystic Fibrosis Isolates Reveal an Adaptable Quorum-Sensing Hierarchy in Pseudomonas aeruginosa. mBio 2016, 7, e01513–e01516. [Google Scholar] [CrossRef]
- Lee, J.; Wu, J.; Deng, Y.; Wang, J.; Wang, C.; Wang, J.; Chang, C.; Dong, Y.; Williams, P.; Zhang, L.H. A cell-cell communication signal integrates quorum sensing and stress response [published correction appears in Nat. Chem. Biol. 2013, 9, 406]. Nat. Chem. Biol. 2013, 9, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, P. Putting an end to the Pseudomonas aeruginosa IQS controversy. Microbiologyopen. 2020, 9, e962. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Cornelis, P.; Guillemyn, K.; Ballet, S.; Christophersen, C.; Hammerich, O. Structure revision of N-mercapto-4-formyl-carbostyril produced by Pseudomonas fluorescens G308 to 2-(2-hydroxyphenyl)thiazole-4-carbaldehyde [aeruginaldehyde]. Nat. Prod. Commun. 2014, 9, 789–794. [Google Scholar]
- Trottmann, F.; Franke, J.; Ishida, K.; García-Altares, M.; Hertweck, C. A pair of bacterial siderophores releases and traps an inter-cellular signal molecule: An unusual case of natural nitrone bioconjugation. Angew. Chem. Int. Ed. 2019, 58, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Ilangovan, A.; Fletcher, M.; Rampioni, G.; Pustelny, C.; Rumbaugh, K.; Heeb, S.; Cámara, M.; Truman, A.; Chhabra, S.R.; Emsley, J.; et al. Structural basis for native agonist and synthetic inhibitor recognition by the Pseudomonas aeruginosa quorum sensing regulator PqsR (MvfR). PLoS Pathog. 2013, 9, e1003508. [Google Scholar] [CrossRef]
- Gallagher, L.A.; McKnight, S.L.; Kuznetsova, M.S.; Pesci, E.C.; Manoil, C. Functions required for extracellular quinolone signaling by Pseudomonas aeruginosa. J. Bacteriol. 2002, 184, 6472–6480. [Google Scholar] [CrossRef]
- Choi, Y.; Park, H.Y.; Park, S.J.; Park, S.J.; Kim, S.K.; Ha, C.; Im, S.J.; Lee, J.H. Growth phase-differential quorum sensing regulation of anthranilate metabolism in Pseudomonas aeruginosa. [Published correction appears in Mol Cells. 2011, 32, 597]. Mol. Cells. 2011, 32, 57–65. [Google Scholar] [CrossRef]
- Coleman, J.P.; Hudson, L.L.; McKnight, S.L.; Farrow, J.M., 3rd; Calfee, M.W.; Lindsey, C.A.; Pesci, E.C. Pseudomonas aeruginosa PqsA is an anthranilate-coenzyme A ligase. J. Bacteriol. 2008, 190, 1247–1255. [Google Scholar] [CrossRef]
- Mukherjee, S.; Moustafa, D.A.; Stergioula, V.; Smith, C.D.; Goldberg, J.B.; Bassler, B.L. The PqsE and RhlR proteins are an autoinducer synthase-receptor pair that control virulence and biofilm development in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 2018, 115, E9411–E9418. [Google Scholar] [CrossRef]
- Drees, S.L.; Fetzner, S. PqsE of Pseudomonas aeruginosa Acts as Pathway-Specific Thioesterase in the Biosynthesis of Alkylquinolone Signaling Molecules. Chem. Biol. 2015, 22, 611–618. [Google Scholar] [CrossRef]
- Qazi, S.; Middleton, B.; Muharram, S.H.; Cockayne, A.; Hill, P.; O’Shea, P.; Chhabra, S.R.; Cámara, M.; Williams, P. N-acylhomoserine lactones antagonize virulence gene expression and quorum sensing in Staphylococcus aureus. Infect Immun. 2006, 74, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Shiner, E.K.; Terentyev, D.; Bryan, A.; Sennoune, S.; Martinez-Zaguilan, R.; Li, G.; Gyorke, S.; Williams, S.C.; Rumbaugh, K.P. Pseudomonas aeruginosa autoinducer modulates host cell responses through calcium signalling. Cell Microbiol. 2006, 8, 1601–1610. [Google Scholar] [CrossRef] [PubMed]
- Bredenbruch, F.; Geffers, R.; Nimtz, M.; Buer, J.; Häussler, S. The Pseudomonas aeruginosa quinolone signal (PQS) has an iron-chelating activity. Environ. Microbiol. 2006, 8, 1318–1329. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Cheng, J.; Wang, Y.; Shen, X. The Pseudomonas Quinolone Signal (PQS): Not Just for Quorum Sensing Anymore. Front. Cell Infect Microbiol. 2018, 8, 230. [Google Scholar] [CrossRef]
- Diggle, S.P.; Matthijs, S.; Wright, V.J.; Fletcher, M.P.; Chhabra, S.R.; Lamont, I.L.; Kong, X.; Hider, R.C.; Cornelis, P.; Cámara, M.; et al. The Pseudomonas aeruginosa 4-quinolone signal molecules HHQ and PQS play multifunctional roles in quorum sensing and iron entrapment. Chem. Biol. 2007, 14, 87–96. [Google Scholar] [CrossRef]
- Bala, A.; Kumar, L.; Chhibber, S.; Harjai, K. Augmentation of virulence related traits of pqs mutants by Pseudomonas quinolone signal through membrane vesicles. J. Basic Microbiol. 2015, 55, 566–578. [Google Scholar] [CrossRef]
- Popat, R.; Harrison, F.; da Silva, A.C.; Easton, S.A.; McNally, L.; Williams, P.; Diggle, S.P. Environmental modification via a quorum sensing molecule influences the social landscape of siderophore production. Proc. Biol. Sci. 2017, 284, 20170200. [Google Scholar] [CrossRef]
- Rampioni, G.; Pustelny, C.; Fletcher, M.P.; Wright, V.J.; Bruce, M.; Rumbaugh, K.P.; Heeb, S.; Cámara, M.; Williams, P. Transcriptomic analysis reveals a global alkyl-quinolone-independent regulatory role for PqsE in facilitating the environmental adaptation of Pseudomonas aeruginosa to plant and animal hosts. Environ. Microbiol. 2010, 12, 1659–1673. [Google Scholar] [CrossRef]
- Britigan, B.E.; Rasmussen, G.T.; Olakanmi, O.; Cox, C.D. Iron acquisition from Pseudomonas aeruginosa siderophores by human phagocytes: An additional mechanism of host defense through iron sequestration? Infect Immun. 2000, 68, 1271–1275. [Google Scholar] [CrossRef]
- Phelan, V.V.; Moree, W.J.; Aguilar, J.; Cornett, D.S.; Koumoutsi, A.; Noble, S.M.; Pogliano, K.; Guerrero, C.A.; Dorrestein, P.C. Impact of a Transposon Insertion in phzF2 on the Specialized Metabolite Production and Interkingdom Interactions of Pseudomonas aeruginosa. J. Bacteriol. 2014, 196, 1683–1693. [Google Scholar] [CrossRef]
- Cox, C.D.; Graham, R. Isolation of an iron-binding compound from Pseudomonas aeruginosa. J. Bacteriol. 1979, 137, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Adler, C.; Corbalán, N.S.; Seyedsayamdost, M.R.; Pomares, M.F.; de Cristóbal, R.E.; Clardy, J.; Kolter, R.; Vincent, P.A. Catecholate siderophores protect bacteria from pyochelin toxicity. PLoS ONE 2012, 7, e46754. [Google Scholar] [CrossRef] [PubMed]
- Briard, B.; Mislin, G.L.A.; Latgé, J.P.; Beauvais, A. Interactions between Aspergillus fumigatus and Pulmonary Bacteria: Current State of the Field, New Data, and Future Perspective. J. Fungi (Basel). 2019, 5, 48. [Google Scholar] [CrossRef] [PubMed]
- Sass, G.; Miller Conrad, L.C.; Nguyen, T.; Stevens, D.A. The Pseudomonas aeruginosa product pyochelin interferes with Trypanosoma cruzi infection and multiplication in vitro. Trans. R. Soc. Trop. Med. Hyg. 2020, in press. [Google Scholar] [CrossRef]
- Brandel, J.; Humbert, N.; Elhabiri, M.; Schalk, I.J.; Mislin, G.L.A.; Albrecht-Gary, A.M. Pyochelin, a siderophore of Pseudomonas aeruginosa: Physicochemical characterization of the iron(III), copper(II) and zinc(II) complexes. Dalton. Trans. 2012, 41, 2820–2834. [Google Scholar] [CrossRef]
- Margalit, A.; Carolan, J.C.; Shehan, D.; Kavanagh, K. The Aspergillus fumigatus secretome alters the proteome of Pseudomonas aeruginosa to stimulate bacterial growth: Implications for co-infection [published online ahead of print, 2020 May 23]. Mol. Cell Proteom. 2020, mcp.RA120.002059. [Google Scholar] [CrossRef]
- Labows, J.N.; McGinley, K.J.; Webster, G.F.; Leyden, J.J. Headspace analysis of volatile metabolites of Pseudomonas aeruginosa and related species by gas chromatography-mass spectrometry. J. Clin. Microbiol. 1980, 12, 521–526. [Google Scholar] [CrossRef]
- Zechman, J.M.; Labows, J.N., Jr. Volatiles of Pseudomonas aeruginosa and related species by automated headspace concentration--gas chromatography. Can. J. Microbiol. 1985, 31, 232–237. [Google Scholar] [CrossRef]
- Goeminne, P.C.; Vandendriessche, T.; Van Eldere, J.; Nicolai, B.M.; Hertog, M.L.; Dupont, L.J. Detection of Pseudomonas aeruginosa in sputum headspace through volatile organic compound analysis. Respir. Res. 2012, 13, 87. [Google Scholar] [CrossRef]
- Shestivska, V.; Spaněl, P.; Dryahina, K.; Sovová, K.; Smith, D.; Musílek, M.; Nemec, A. Variability in the concentrations of volatile metabolites emitted by genotypically different strains of Pseudomonas aeruginosa. J. Appl. Microbiol. 2012, 113, 701–713. [Google Scholar] [CrossRef]
- Bean, H.D.; Rees, C.A.; Hill, J.E. Comparative analysis of the volatile metabolomes of Pseudomonas aeruginosa clinical isolates. J Breath Res. 2016, 10, 047102. [Google Scholar] [CrossRef] [PubMed]
- Pabary, R.; Huang, J.; Kumar, S.; Alton, E.W.; Bush, A.; Hanna, G.B.; Davies, J.C. Does mass spectrometric breath analysis detect Pseudomonas aeruginosa in cystic fibrosis? Eur. Respir. J. 2016, 47, 994–997. [Google Scholar] [CrossRef] [PubMed]
- Nasir, M.; Bean, H.D.; Smolinska, A.; Rees, C.A.; Zemanick, E.T.; Hill, J.E. Volatile molecules from bronchoalveolar lavage fluid can ‘rule-in’ Pseudomonas aeruginosa and ‘rule-out’ Staphylococcus aureus infections in cystic fibrosis patients. Sci. Rep. 2018, 8, 826. [Google Scholar] [CrossRef] [PubMed]
- Kviatkovski, I.; Shushan, S.; Oron, Y.; Frumin, I.; Amir, D.; Secundo, L.; Livne, E.; Weissbrod, A.; Sobel, N.; Helman, Y. Smelling Pseudomonas aeruginosa infections using a whole-cell biosensor - An alternative for the gold-standard culturing assay. J. Biotechnol. 2018, 267, 45–49. [Google Scholar] [CrossRef]
- Suarez-Cuartin, G.; Giner, J.; Merino, J.L.; Rodrigo-Troyano, A.; Feliu, A.; Perea, L.; Sanchez-Reus, F.; Castillo, D.; Plaza, V.; Chalmers, J.D.; et al. Identification of Pseudomonas aeruginosa and airway bacterial colonization by an electronic nose in bronchiectasis. Respir. Med. 2018, 136, 111–117. [Google Scholar] [CrossRef]
- Heddergott, C.; Calvo, A.M.; Latgé, J.P. The volatome of Aspergillus fumigatus. Eukaryot Cell. 2014, 13, 1014–1025. [Google Scholar] [CrossRef]
- de Heer, K.; Vonk, S.I.; Kok, M.; Kolader, M.; Zwinderman, A.H.; van Oers, M.H.; Sterk, P.J.; Visser, C.E. eNose technology can detect and classify human pathogenic molds in vitro: A proof-of-concept study of Aspergillus fumigatus and Rhizopus oryzae. J. Breath Res. 2016, 10, 036008. [Google Scholar] [CrossRef]
- de Heer, K.; Kok, M.G.; Fens, N.; Weersink, E.J.; Zwinderman, A.H.; van der Schee, M.P.; Visser, C.E.; van Oers, M.H.; Sterk, P.J. Detection of airway colonization by Aspergillus fumigatus by use of Electronic Nose Technology in patients with cystic fibrosis. J. Clin. Microbiol. 2016, 54, 569–575. [Google Scholar] [CrossRef]
- Briard, B.; Heddergott, C.; Latgé, J. Volatile compounds emitted by Pseudomonas aeruginosa stimulate growth of the fungal pathogen Aspergillus fumigatus. mBio 2016, 7, e00219-16. [Google Scholar] [CrossRef]
- Wretlind, B.; Pavlovskis, O.R. Pseudomonas aeruginosa Elastase and Its Role in Pseudomonas Infections. Rev. Infect Dis. 1983, 5 Suppl 5, S998–S1004. [Google Scholar] [CrossRef]
- Michalska, M.; Wolf, P. Pseudomonas Exotoxin A: Optimized by evolution for effective killing. Front. Microbiol. 2015, 6, 963. [Google Scholar] [CrossRef] [PubMed]
- Petermann, S.R.; Doetkott, C.; Rust, L. Elastase Deficiency Phenotype of Pseudomonas aeruginosa Canine Otitis Externa Isolates. Clin. Diagn. Lab. Immunol. 2001, 8, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Morihara, K.; Homma, J.Y. Pseudomonas proteases. In Bacterial Enzymes and Virulence; Holder, I.A., Ed.; CRC Press: Boca Raton, FL, USA, 1985; pp. 41–80. [Google Scholar]
- Kamath, S.; Kapatral, V.; Chakrabarty, A.M. Cellular function of elastase in Pseudomonas aeruginosa: Role in the cleavage of nucleoside diphosphate kinase and in alginate synthesis. Mol. Microbiol. 1998, 30, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Wood, T.L.; Gong, T.; Zhu, L.; Miller, J.; Miller, D.S.; Yin, B.; Wood, T.K. Rhamnolipids from Pseudomonas aeruginosa disperse the biofilms of sulfate-reducing bacteria. NPJ. Biofilms Microbiomes. 2018, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Déziel, E.; Lépine, F.; Milot, S.; Villemur, R. rhlA is required for the production of a novel biosurfactant promoting swarming motility in Pseudomonas aeruginosa: 3-(3-hydroxyalkanoyloxy) alkanoic acids (HAAs), the precursors of rhamnolipids. Microbiology 2003, 149, 2005–2013. [Google Scholar] [CrossRef]
- Köhler, T.; Curty, L.K.; Barja, F.; van Delden, C.; Pechère, J.C. Swarming of Pseudomonas aeruginosa is dependent on cell-to-cell signaling and requires flagella and pili. J. Bacteriol. 2000, 182, 5990–5996. [Google Scholar] [CrossRef]
- Boles, B.R.; Thoendel, M.; Singh, P.K. Rhamnolipids mediate detachment of Pseudomonas aeruginosa from biofilms. Mol. Microbiol. 2005, 57, 1210–1223. [Google Scholar] [CrossRef]
- Caiazza, N.C.; Shanks, R.M.Q.; O’Toole, G.A. Rhamnolipids modulate swarming motility patterns of Pseudomonas aeruginosa. J. Bacteriol. 2005, 187, 7351–7361. [Google Scholar] [CrossRef]
- Davey, M.E.; Caiazza, N.C.; O’Toole, G.A. Rhamnolipid surfactant production affects biofilm architecture in Pseudomonas aeruginosa PAO1. J. Bacteriol. 2003, 185, 1027–1036. [Google Scholar] [CrossRef]
- Abdel-Mawgoud, A.M.; Lépine, F.; Déziel, E. Rhamnolipids: Diversity of structures, microbial origins and roles. Appl. Microbiol. Biotechnol. 2010, 86, 1323–1336. [Google Scholar] [CrossRef]
- Sotirova, A.; Spasova, D.; Galabova, D.; Karpenko, E.; Shulga, A. Rhamnolipid–biosurfactant permeabilizing effects on Gram-positive and Gram-negative bacterial strains. Curr. Microbiol. 2008, 56, 639–644. [Google Scholar] [CrossRef]
- Gibson, J.; Sood, A.; Hogan, D.A. Pseudomonas aeruginosa-Candida albicans interactions: Localization and fungal toxicity of a phenazine derivative. Appl. Environ. Microbiol. 2009, 75, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Haba, E.; Pinazo, A.; Jauregui, O.; Espuny, M.J.; Infante, M.R.; Manresa, A. Physicochemical characterization and antimicrobial properties of rhamnolipids produced by Pseudomonas aeruginosa 47T2 NCBIM 40044. Biotechnol. Bioeng. 2003, 81, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Deepika, K.V.; Sridhar, P.R.; Bramhachari, P.V. Characterization and antifungal properties of rhamnolipids produced by mangrove sediment bacterium Pseudomonas aeruginosa strain KVD-HM52. Biocatal. Agric. Biotechnol. 2015, 4, 608–615. [Google Scholar] [CrossRef]
- Lu, J.; Huang, X.; Li, K.; Li, S.; Zhang, M.; Wang, Y.; Jiang, H.; Xu, Y. LysR family transcriptional regulator PqsR as repressor of pyoluteorin biosynthesis and activator of phenazine-1-carboxylic acid biosynthesis in Pseudomonas sp. M18. J. Biotechnol. 2009, 143, 1–9. [Google Scholar] [CrossRef]
- Grahl, N.; Kern, S.E.; Newman, D.K.; Hogan, D.A. The Yin and Yang of Phenazine Physiology. In Microbial Phenazines; Chincholkar, S., Thomashow, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 43–69. [Google Scholar]
- Pierson, L.S. 3rd.; Pierson, E.A. Metabolism and function of phenazines in bacteria: Impacts on the behavior of bacteria in the environment and biotechnological processes. Appl. Microbiol. Biotechnol. 2010, 86, 1659–1670. [Google Scholar] [CrossRef]
- Recinos, D.A.; Sekedat, M.D.; Hernandez, A.; Cohen, T.S.; Sakhtah, H.; Prince, A.S.; Price-Whelan, A.; Dietrich, L.E. Redundant phenazine operons in Pseudomonas aeruginosa exhibit environment-dependent expression and differential roles in pathogenicity. Proc. Natl. Acad. Sci. USA 2012, 109, 19420–19425. [Google Scholar] [CrossRef]
- Recinos, D.A. The Roles and Regulation of the Redundant Phenazine Biosynthetic Operons in Pseudomonas aeruginosa PA14. Doctoral Thesis, Columbia University, USA, 2012. [CrossRef]
- Higgins, S.; Heeb, S.; Rampioni, G.; Fletcher, M.P.; Williams, P.; Cámara, M. Differential Regulation of the Phenazine Biosynthetic Operons by Quorum Sensing in Pseudomonas aeruginosa PAO1-N. Front. Cell. Infect Microbiol. 2018, 8, 252. [Google Scholar] [CrossRef]
- Cui, Q.; Lv, H.; Qi, Z.; Jiang, B.; Xiao, B.; Liu, L.; Ge, Y.; Hu, X. Cross-Regulation between the phz1 and phz2 Operons Maintain a Balanced Level of Phenazine Biosynthesis in Pseudomonas aeruginosa PAO1. PLoS ONE 2016, 11, e0144447. [Google Scholar] [CrossRef]
- Wilson, R.; Sykes, D.A.; Watson, D.; Rutman, A.; Taylor, G.W.; Cole, P.J. Measurement of Pseudomonas aeruginosa phenazine pigments in sputum and assessment of their contribution to sputum sol toxicity for respiratory epithelium. Infect Immun. 1988, 56, 2515–2517. [Google Scholar] [CrossRef]
- Hunter, R.C.; Klepac-Ceraj, V.; Lorenzi, M.M.; Grotzinger, H.; Martin, T.R.; Newman, D.K. Phenazine content in the cystic fibrosis respiratory tract negatively correlates with lung function and microbial complexity. Am. J. Respir. Cell Mol. Biol. 2012, 47, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.M.; Messenger, A.J. Occurrence, biochemistry and physiology of phenazine pigment production. Adv. Microb. Physiol. 1986, 27, 211–275. [Google Scholar] [CrossRef] [PubMed]
- Cáp, M.; Váchová, L.; Palková, Z. Reactive oxygen species in the signaling and adaptation of multicellular microbial communities. Oxid. Med. Cell Longev. 2012, 2012, 976753. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Kim, J.; Liew, M.; Yan, J.K.; Herrera, O.; Bok, J.W.; Kelleher, N.L.; Keller, N.P.; Wang, Y. Redox metabolites signal polymicrobial biofilm development via the NapA oxidative stress cascade in Aspergillus. Curr. Biol. 2015, 25, 29–37. [Google Scholar] [CrossRef]
- Zhao, J.; Yu, W. Interaction between Pseudomonas aeruginosa and Aspergillus fumigatus in cystic fibrosis. PeerJ. 2018, 6, e5931. [Google Scholar] [CrossRef]
- Chatterjee, P.; Sass, G.; Nazik, H.; Déziel, E.; Stevens, D.A. In high iron environment, pyocyanin is a major anti-Aspergillus molecule. In Proceedings of the 9th Advances Against Aspergillosis and Mucormycosis, Lugano, Switzerland, 27–29 February 2020. abstract 75. [Google Scholar]
- Filloux, A. Protein Secretion Systems in Pseudomonas aeruginosa: An Essay on Diversity, Evolution, and Function. Front. Microbiol. 2011, 2, 155. [Google Scholar] [CrossRef]
- Létoffé, S.; Redeker, V.; Wandersman, C. Isolation and characterization of an extracellular haem-binding protein from Pseudomonas aeruginosa that shares function and sequence similarities with the Serratia marcescens HasA haemophore. Mol. Microbiol. 1998, 28, 1223–1234. [Google Scholar] [CrossRef]
- Depluverez, S.; Devos, S.; Devreese, B. The Role of Bacterial Secretion Systems in the Virulence of Gram-Negative Airway Pathogens Associated with Cystic Fibrosis. Front Microbiol. 2016, 7, 1336. [Google Scholar] [CrossRef]
- Hauser, A.R.; Cobb, E.; Bodi, M.; Mariscal, D.; Valles, J.; Engel, J.N.; Rello, J. Type III protein secretion is associated with poor clinical outcomes in patients with ventilator-associated pneumonia caused by Pseudomonas aeruginosa. Crit. Care Med. 2002, 30, 521–528. [Google Scholar] [CrossRef]
- Hauser, A.R. The type III secretion system of Pseudomonas aeruginosa: Infection by injection. Nat. Rev. Microbiol. 2009, 7, 654–665. [Google Scholar] [CrossRef]
- Sato, H.; Frank, D.W. ExoU is a potent intracellular phospholipase. Mol. Microbiol. 2004, 53, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Juhas, M.; Crook, D.W.; Dimopoulou, I.D.; Lunter, G.; Harding, R.M.; Ferguson, D.J.; Hood, D.W. Novel type IV secretion system involved in propagation of genomic islands. J. Bacteriol. 2007, 189, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.; Gdynia, A.; Tielen, P.; Rosenau, F.; Jaeger, K.E. The autotransporter esterase EstA of Pseudomonas aeruginosa is required for rhamnolipid production, cell motility, and biofilm formation. J. Bacteriol. 2007, 189, 6695–6703. [Google Scholar] [CrossRef]
- Lesic, B.; Starkey, M.; He, J.; Hazan, R.; Rahme, L.G. Quorum sensing differentially regulates Pseudomonas aeruginosa type VI secretion locus I and homologous loci II and III, which are required for pathogenesis. Microbiology 2009, 155, 2845–2855. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhang, W.; Cheng, J.; Yang, X.; Zhu, K.; Wang, Y.; Wei, G.; Qian, P.Y.; Luo, Z.Q.; Shen, X. A Pseudomonas T6SS effector recruits PQS-containing outer membrane vesicles for iron acquisition. Nat. Commun. 2017, 8, 14888. [Google Scholar] [CrossRef]
- Gallique, M.; Bouteiller, M.; Merieau, A. The Type VI Secretion System: A Dynamic System for Bacterial Communication? Front. Microbiol. 2017, 8, 1454. [Google Scholar] [CrossRef]
- Basler, M.; Mekalanos, J.J. Type 6 secretion dynamics within and between bacterial cells. Science 2012, 337, 815. [Google Scholar] [CrossRef]
- Basler, M.; Ho, B.T.; Mekalanos, J.J. Tit-for-tat: Type VI secretion system counterattack during bacterial cell-cell interactions. Cell 2013, 152, 884–894. [Google Scholar] [CrossRef]
- Mougous, J.D.; Cuff, M.E.; Raunser, S.; Shen, A.; Zhou, M.; Gifford, C.A.; Goodman, A.L.; Joachimiak, G.; Ordoñez, C.L.; Lory, S.; et al. A virulence locus of Pseudomonas aeruginosa encodes a protein secretion apparatus. Science 2006, 312, 1526–1530. [Google Scholar] [CrossRef]
- Ngo, T.D.; Ple, S.; Thomas, A.; Barette, C.; Fortune, A.; Bouzidi, Y.; Fauvarque, M.O.; Pereira de Freitas, R.; Francisco Hilario, F.; Attree, I.; et al. Chimeric Protein-Protein Interface Inhibitors Allow Efficient Inhibition of Type III Secretion Machinery and Pseudomonas aeruginosa Virulence. ACS Infect Dis. 2019, 5, 1843–1854. [Google Scholar] [CrossRef]
- Trunk, K.; Peltier, J.; Liu, Y.C.; Dill, B.D.; Walker, L.; Gow, N.A.R.; Stark, M.J.R.; Quinn, J.; Strahl, H.; Trost, M.; et al. The type VI secretion system deploys antifungal effectors against microbial competitors. Nat. Microbiol. 2018, 3, 920–931. [Google Scholar] [CrossRef] [PubMed]
- Trunk, K.; Coulthurst, S.J.; Quinn, J. A New Front in Microbial Warfare-Delivery of Antifungal Effectors by the Type VI Secretion System. J. fungi. (Basel) 2019, 5, 50. [Google Scholar] [CrossRef] [PubMed]
- Hood, R.D.; Singh, P.; Hsu, F.; Güvener, T.; Carl, M.A.; Trinidad, R.R.; Silverman, J.M.; Ohlson, B.B.; Hicks, K.G.; Plemel, R.L.; et al. A type VI secretion system of Pseudomonas aeruginosa targets a toxin to bacteria. Cell Host Microbe. 2010, 7, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Lezan, E.; Schmidt, A.; Basler, M. Abundance of bacterial Type VI secretion system components measured by targeted proteomics. Nat. Commun. 2019, 10, 2584. [Google Scholar] [CrossRef]
- Wagner, S.; Sommer, R.; Hinsberger, S.; Lu, C.; Hartmann, R.W.; Empting, M.; Titz, A. Novel Strategies for the Treatment of Pseudomonas aeruginosa Infections. J. Med. Chem. 2016, 59, 5929–5969. [Google Scholar] [CrossRef]
- Defoirdt, T. Quorum-Sensing Systems as Targets for Antivirulence Therapy. Trends Microbiol. 2018, 26, 313–328. [Google Scholar] [CrossRef]
- Köhler, T.; Perron, G.G.; Buckling, A.; van Delden, C. Quorum sensing inhibition selects for virulence and cooperation in Pseudomonas aeruginosa. PLoS Pathog. 2010, 6, e1000883. [Google Scholar] [CrossRef]
- Keyser, P.; Elofsson, M.; Rosell, S.; Wolf-Watz, H. Virulence blockers as alternatives to antibiotics: Type III secretion inhibitors against Gram-negative bacteria. J. Intern. Med. 2008, 264, 17–29. [Google Scholar] [CrossRef]
- Mulani, M.S.; Kamble, E.E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging Strategies to Combat ESKAPE Pathogens in the Era of Antimicrobial Resistance: A Review. Front. Microbiol. 2019, 10, 539. [Google Scholar] [CrossRef]
- Vance, R.E.; Rietsch, A.; Mekalanos, J.J. Role of the type III secreted exoenzymes S, T, and Y in systemic spread of Pseudomonas aeruginosa PAO1 in vivo. Infect Immun. 2005, 73, 1706–1713. [Google Scholar] [CrossRef]
- Fleiszig, S.M.; Zaidi, T.S.; Fletcher, E.L.; Preston, M.J.; Pier, G.B. Pseudomonas aeruginosa invades corneal epithelial cells during experimental infection. Infect Immun. 1994, 62, 3485–3493. [Google Scholar] [CrossRef] [PubMed]
- Arnoldo, A.; Curak, J.; Kittanakom, S.; Chevelev, I.; Lee, V.T.; Sahebol-Amri, M.; Koscik, B.; Ljuma, L.; Roy, P.J.; Bedalov, A.; et al. Identification of small molecule inhibitors of Pseudomonas aeruginosa exoenzyme S using a yeast phenotypic screen. PLoS Genet. 2008, 4, e1000005. [Google Scholar] [CrossRef]
- Marsden, A.E.; King, J.M.; Spies, M.A.; Kim, O.K.; Yahr, T.L. Inhibition of Pseudomonas aeruginosa ExsA DNA-Binding Activity by N-Hydroxybenzimidazoles. Antimicrob Agents Chemother. 2016, 60, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Bowlin, N.O.; Williams, J.D.; Knoten, C.A.; Torhan, M.C.; Tashjian, T.F.; Li, B.; Aiello, D.; Mecsas, J.; Hauser, A.R.; Peet, N.P.; et al. Mutations in the Pseudomonas aeruginosa needle protein gene pscF confer resistance to phenoxyacetamide inhibitors of the type III secretion system. Antimicrob Agents Chemother. 2014, 58, 2211–2220. [Google Scholar] [CrossRef]
- Williams, J.D.; Torhan, M.C.; Neelagiri, V.R.; Brown, C.; Bowlin, N.O.; Di, M.; McCarthy, C.T.; Aiello, D.; Peet, N.P.; Bowlin, T.L.; et al. Synthesis and structure-activity relationships of novel phenoxyacetamide inhibitors of the Pseudomonas aeruginosa type III secretion system (T3SS). Bioorg. Med. Chem. 2015, 23, 1027–1043. [Google Scholar] [CrossRef]
- Sheremet, A.B.; Zigangirova, N.A.; Zayakin, E.S.; Luyksaar, S.I.; Kapotina, L.N.; Nesterenko, L.N.; Kobets, N.V.; Gintsburg, A.L. Small Molecule Inhibitor of Type Three Secretion System Belonging to a Class 2,4-disubstituted-4H-[1,3,4]-thiadiazine-5-ones Improves Survival and Decreases Bacterial Loads in an Airway Pseudomonas aeruginosa Infection in Mice. Biomed. Res. Int. 2018, 2018, 5810767. [Google Scholar] [CrossRef]
- Swietnicki, W.; Czarny, A.; Antkowiak, L.; Zaczynska, E.; Kolodziejczak, M.; Sycz, J.; Stachowicz, L.; Alicka, M.; Marycz, K. Identification of a potent inhibitor of type II secretion system from Pseudomonas aeruginosa. Biochem. Biophys. Res. Commun. 2019, 513, 688–693. [Google Scholar] [CrossRef]
- Massai, F.; Saleeb, M.; Doruk, T.; Elofsson, M.; Forsberg, Å. Development, Optimization, and Validation of a High Throughput Screening Assay for Identification of Tat and Type II Secretion Inhibitors of Pseudomonas aeruginosa. Front Cell Infect Microbiol. 2019, 9, 250. [Google Scholar] [CrossRef]
- Mitsuyama, J.; Kizawa, K.; Minami, S.; Watanabe, Y.; Yamaguchi, K. Evaluation of antimicrobial agents using an experimental pulmonary superinfection model with Aspergillus fumigatus and Pseudomonas aeruginosa in leukopenic mice. J. Infect Chemother. 2003, 9, 144–150. [Google Scholar] [CrossRef]
- Grassmé, H.; Becker, K.A.; Zhang, Y.; Gulbins, E. CFTR-dependent susceptibility of the cystic fibrosis-host to Pseudomonas aeruginosa. Int. J. Med. Microbiol. 2010, 300, 578–583. [Google Scholar] [CrossRef]
- Bragonzi, A. Murine models of acute and chronic lung infection with cystic fibrosis pathogens. Int. J. Med. Microbiol. 2010, 300, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Davidson, D.J.; Dorin, J.R.; McLachlan, G.; Ranaldi, V.; Lamb, D.; Doherty, C.; Govan, J.; Porteous, D.J. Lung disease in the cystic fibrosis mouse exposed to bacterial pathogens. Nat. Genet. 1995, 9, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Van Heeckeren, A.M.; Schluchter, M.D. Murine models of chronic Pseudomonas aeruginosa lung infection. Lab. Anim. 2002, 36, 291–312. [Google Scholar] [CrossRef] [PubMed]
- Sønderholm, M.; Kragh, K.N.; Koren, K.; Jakobsen, T.H.; Darch, S.E.; Alhede, M.; Jensen, P.Ø.; Whiteley, M.; Kühl, M.; Bjarnsholt, T. Pseudomonas aeruginosa Aggregate Formation in an Alginate Bead Model System Exhibits In Vivo-Like Characteristics. Appl. Environ. Microbiol. 2017, 83, e00113-17. [Google Scholar] [CrossRef] [PubMed]
- Cash, H.A.; Woods, D.E.; McCullough, B.; Johanson, W.G., Jr.; Bass, J.A. A rat model of chronic respiratory infection with Pseudomonas aeruginosa. Am. Rev. Respir. Dis. 1979, 119, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Heeckeren, A.; Walenga, R.; Konstan, M.W.; Bonfield, T.; Davis, P.B.; Ferkol, T. Excessive inflammatory response of cystic fibrosis mice to bronchopulmonary infection with Pseudomonas aeruginosa. J. Clin. Invest. 1997, 100, 2810–2815. [Google Scholar] [CrossRef]
- Ding, F.M.; Zhu, S.L.; Shen, C.; Ji, X.L.; Zhou, X. Regulatory T cell activity is partly inhibited in a mouse model of chronic Pseudomonas aeruginosa lung infection. Exp. Lung. Res. 2015, 41, 44–55. [Google Scholar] [CrossRef]
- Moser, C.; Jensen, P.O.; Kobayashi, O.; Hougen, H.P.; Song, Z.; Rygaard, J.; Kharazmi, A.; H. By, N. Improved outcome of chronic Pseudomonas aeruginosa lung infection is associated with induction of a Th1-dominated cytokine response. Clin. Exp. Immunol. 2002, 127, 206–213. [Google Scholar] [CrossRef]
- Bayes, H.K.; Ritchie, N.D.; Evans, T.J. Interleukin-17 Is Required for Control of Chronic Lung Infection Caused by Pseudomonas aeruginosa. Infect Immun. 2016, 84, 3507–3516. [Google Scholar] [CrossRef]
- Bayes, H.K.; Ritchie, N.; Irvine, S.; Evans, T.J. A murine model of early Pseudomonas aeruginosa lung disease with transition to chronic infection. Sci. Rep. 2016, 6, 35838. [Google Scholar] [CrossRef]
- Cai, S.; Li, Y.; Wang, K.; Cen, Y.; Lu, H.; Dong, B.; Chen, Y.; Kong, J. Pathogenic Effects of Biofilm on Pseudomonas aeruginosa Pulmonary Infection and Its Relationship to Cytokines. Med. Sci. Monit. 2016, 22, 4869–4874. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yu, H.; Hanes, M.; Chrisp, C.E.; Boucher, J.C.; Deretic, V. Microbial pathogenesis in cystic fibrosis: Pulmonary clearance of mucoid Pseudomonas aeruginosa and inflammation in a mouse model of repeated respiratory challenge. Infect Immun. 1998, 66, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Alcalá-Franco, B.; Montanari, S.; Cigana, C.; Bertoni, G.; Oliver, A.; Bragonzi, A. Antibiotic pressure compensates the biological cost associated with Pseudomonas aeruginosa hypermutable phenotypes in vitro and in a murine model of chronic airways infection. J. Antimicrob. Chemother. 2012, 67, 962–969. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, N.; Rasmussen, T.B.; Jensen, P.Ø.; Stub, C.; Hentzer, M.; Molin, S.; Ciofu, O.; Givskov, M.; Johansen, H.K.; Høiby, N. Novel mouse model of chronic Pseudomonas aeruginosa lung infection mimicking cystic fibrosis [published correction appears in Infect Immun. 2005 Aug;73(8):5290]. Infect Immun. 2005, 73, 2504–2514. [Google Scholar] [CrossRef]
- Pedersen, S.S.; Shand, G.H.; Hansen, B.L.; Hansen, G.N. Induction of experimental chronic Pseudomonas aeruginosa lung infection with P. aeruginosa entrapped in alginate microspheres. APMIS 1990, 98, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Facchini, M.; De Fino, I.; Riva, C.; Bragonzi, A. Long term chronic Pseudomonas aeruginosa airway infection in mice. J. Vis. Exp. 2014, 85, 51019. [Google Scholar] [CrossRef] [PubMed]
- Bayes, H.K.; Ritchie, N.D.; Ward, C.; Corris, P.A.; Brodlie, M.; Evans, T.J. IL-22 exacerbates weight loss in a murine model of chronic pulmonary Pseudomonas aeruginosa infection. J. Cyst. Fibros. 2016, 15, 759–768. [Google Scholar] [CrossRef]
- Hector, A.; Frey, N.; Hartl, D. Update on host-pathogen interactions in cystic fibrosis lung disease. Mol. Cell Pediatr. 2016, 3, 12. [Google Scholar] [CrossRef]
- Granchelli, A.M.; Adler, F.R.; Keogh, R.H.; Kartsonaki, C.; Cox, D.R.; Liou, T.G. Microbial Interactions in the Cystic Fibrosis Airway. J Clin Microbiol. 2018, 56, e00354-18. [Google Scholar] [CrossRef]
- Paugam, A.; Baixench, M.T.; Demazes-Dufeu, N.; Burgel, P.R.; Sauter, E.; Kanaan, R.; Dusser, D.; Dupouy-Camet, J.; Hubert, D. Characteristics and consequences of airway colonization by filamentous fungi in 201 adult patients with cystic fibrosis in France. Med. Mycol. 2010, 48 Suppl. 1, S32–S36. [Google Scholar] [CrossRef]
- Singh, A.; Ralhan, A.; Schwarz, C.; Hartl, D.; Hector, A. Fungal Pathogens in CF Airways: Leave or Treat? Mycopathologia 2018, 183, 119–137. [Google Scholar] [CrossRef] [PubMed]
- Harun, S.N.; Wainwright, C.E.; Grimwood, K.; Hennig, S. Australasian Cystic Fibrosis Bronchoalveolar Lavage (ACFBAL) study group. Aspergillus and progression of lung disease in children with cystic fibrosis. Thorax. 2019, 74, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Bargon, J.; Dauletbaev, N.; Köhler, B.; Wolf, M.; Posselt, H.G.; Wagner, T.O. Prophylactic antibiotic therapy is associated with an increased prevalence of Aspergillus colonization in adult cystic fibrosis patients. Respir. Med. 1999, 93, 835–838. [Google Scholar] [CrossRef]
- Baxter, C.G.; Rautemaa, R.; Jones, A.M.; Webb, A.K.; Bull, M.; Mahenthiralingam, E.; Denning, D.W. Intravenous antibiotics reduce the presence of Aspergillus in adult cystic fibrosis sputum. Thorax. 2013, 68, 652–657. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatterjee, P.; Sass, G.; Swietnicki, W.; Stevens, D.A. Review of Potential Pseudomonas Weaponry, Relevant to the Pseudomonas–Aspergillus Interplay, for the Mycology Community. J. Fungi 2020, 6, 81. https://doi.org/10.3390/jof6020081
Chatterjee P, Sass G, Swietnicki W, Stevens DA. Review of Potential Pseudomonas Weaponry, Relevant to the Pseudomonas–Aspergillus Interplay, for the Mycology Community. Journal of Fungi. 2020; 6(2):81. https://doi.org/10.3390/jof6020081
Chicago/Turabian StyleChatterjee, Paulami, Gabriele Sass, Wieslaw Swietnicki, and David A. Stevens. 2020. "Review of Potential Pseudomonas Weaponry, Relevant to the Pseudomonas–Aspergillus Interplay, for the Mycology Community" Journal of Fungi 6, no. 2: 81. https://doi.org/10.3390/jof6020081
APA StyleChatterjee, P., Sass, G., Swietnicki, W., & Stevens, D. A. (2020). Review of Potential Pseudomonas Weaponry, Relevant to the Pseudomonas–Aspergillus Interplay, for the Mycology Community. Journal of Fungi, 6(2), 81. https://doi.org/10.3390/jof6020081





