Siderophore-Based Molecular Imaging of Fungal and Bacterial Infections—Current Status and Future Perspectives
Abstract
1. Introduction
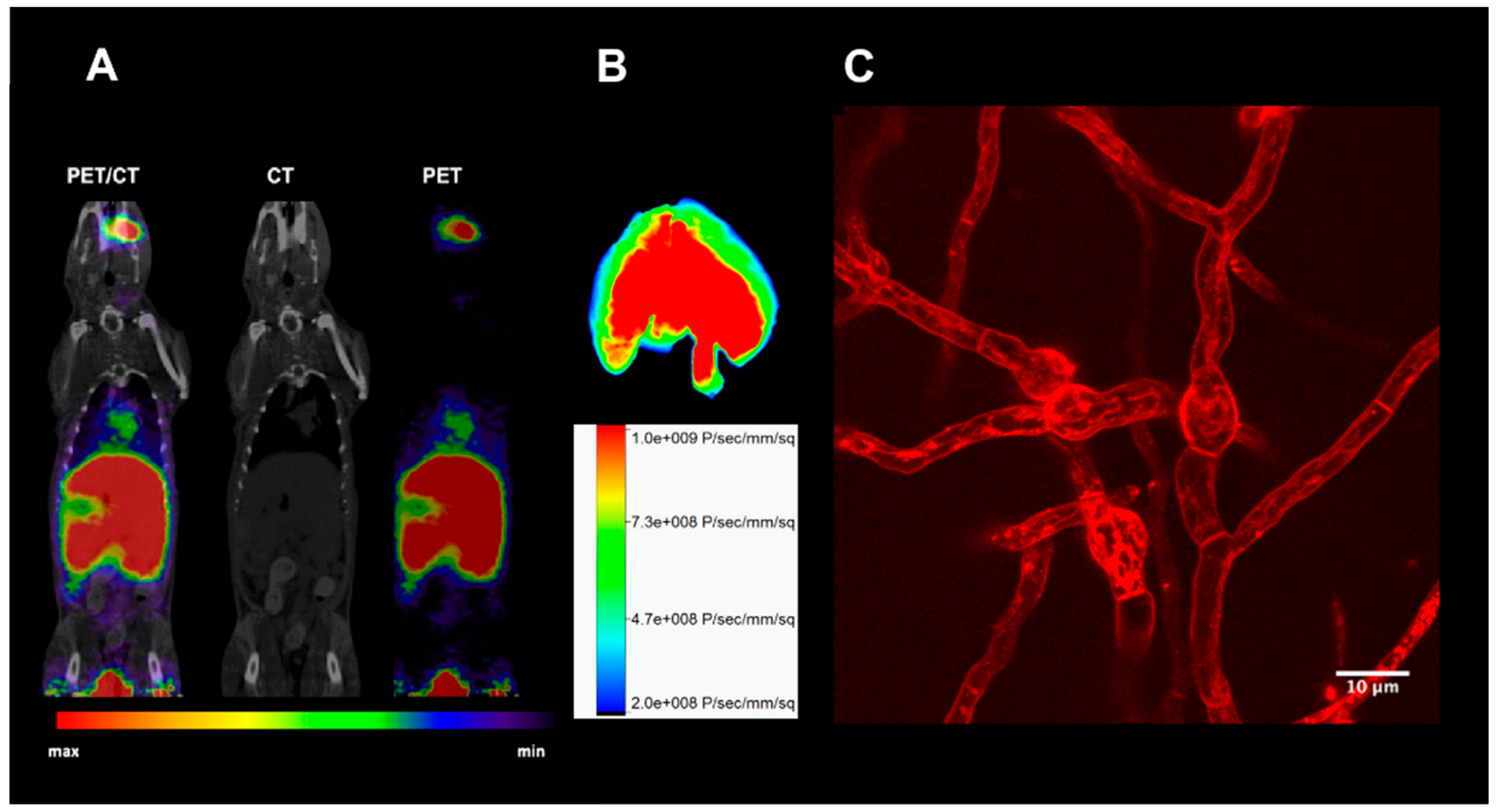
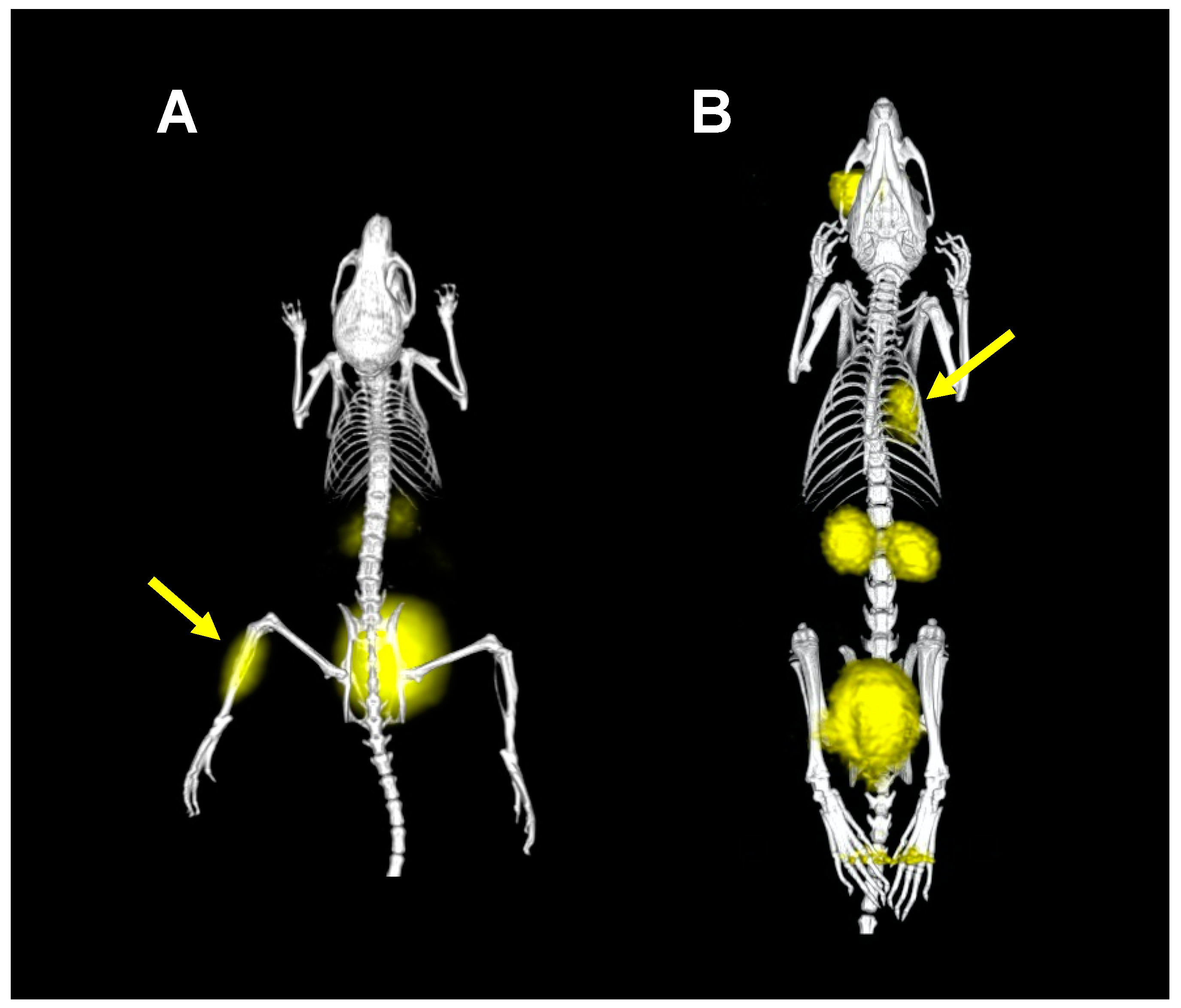
2. Proof of Principle: [68Ga]Ga-TAFC for Imaging Fungal Infections
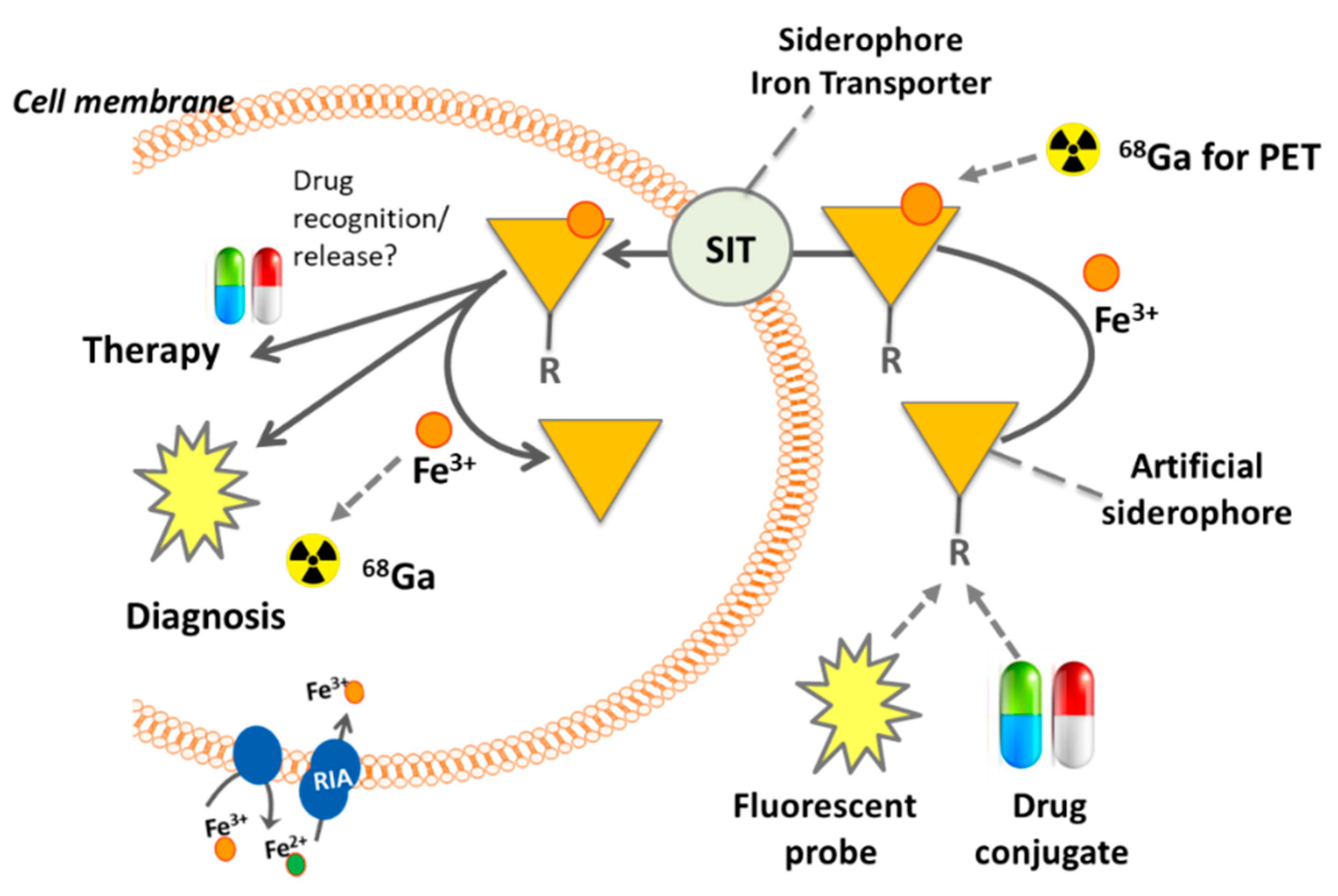
3. Siderophore Modifications
4. Other Siderophores for Molecular Imaging of Microbial Infections with PET
5. Siderophore Diagnostics from Urine
6. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CT | computed tomography |
| Cy | cyanine |
| DAFC | diacetylfusarinine C |
| DFO | desferrioxamine |
| DFO-B | desferrioxamine B |
| DFO-E | desferrioxamine E |
| FsC | fusarinine C |
| MirB | major facilitator iron regulated B (TAFC transporting SIT) |
| PET | positron emission tomography |
| PVD | pyoverdine |
| SIT | siderophore iron transporter |
| TAFC | triacetylfusarinine C |
References
- Cassat, J.E.; Skaar, E.P. Iron in infection and immunity. Cell Host Microbe 2013, 13, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Schrettl, M.; Bignell, E.; Kragl, C.; Joechl, C.; Rogers, T.; Arst, H.N.; Haynes, K.; Haas, H. Siderophore biosynthesis but not reductive iron assimilation is essential for Aspergillus fumigatus virulence. J. Exp. Med. 2004, 200, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, A.; Fedorova, N.D.; Crabtree, J.; Yu, Y.; Kim, S.; Chen, D.; Loss, O.; Cairns, T.; Goldman, G.; Armstrong-James, D.; et al. Sub-telomere directed gene expression during initiation of invasive aspergillosis. PLoS Pathog. 2008, 4, e1000154. [Google Scholar] [CrossRef] [PubMed]
- Schrettl, M.; Kim, H.S.; Eisendle, M.; Kragl, C.; Nierman, W.C.; Heinekamp, T.; Werner, E.R.; Jacobsen, I.; Illmer, P.; Yi, H.; et al. SreA-mediated iron regulation in Aspergillus fumigatus. Mol. Microbiol. 2008, 70, 27–43. [Google Scholar] [CrossRef]
- Hider, R.C.; Kong, X. Chemistry and biology of siderophores. Nat. Prod. Rep. 2010, 27, 637. [Google Scholar] [CrossRef]
- Haas, H.; Eisendle, M.; Turgeon, B.G. Siderophores in fungal physiology and virulence. Annu. Rev. Phytopathol. 2008, 46, 149–187. [Google Scholar] [CrossRef] [PubMed]
- Haas, H. Fungal siderophore metabolism with a focus on Aspergillus fumigatus. Nat. Prod. Rep. 2014, 31, 1266–1276. [Google Scholar] [CrossRef]
- Raymond-Bouchard, I.; Carroll, C.S.; Nesbitt, J.R.; Henry, K.A.; Pinto, L.J.; Moinzadeh, M.; Scott, J.K.; Moore, M.M. Structural requirements for the activity of the MirB ferrisiderophore transporter of Aspergillus fumigatus. Eukaryot. Cell 2012, 11, 1333–1344. [Google Scholar] [CrossRef]
- Kragl, C.; Schrettl, M.; Abt, B.; Sarg, B.; Lindner, H.H.; Haas, H. EstB-mediated hydrolysis of the siderophore triacetylfusarinine C optimizes iron uptake of Aspergillus fumigatus. Eukaryot. Cell 2007, 6, 1278–1285. [Google Scholar] [CrossRef]
- Gründlinger, M.; Gsaller, F.; Schrettl, M.; Lindner, H.; Haas, H. Aspergillus fumigatus SidJ mediates intracellular siderophore hydrolysis. Appl. Environ. Microbiol. 2013, 79, 7534–7536. [Google Scholar] [CrossRef]
- Ecker, F.; Haas, H.; Groll, M.; Huber, E.M. Iron scavenging in Aspergillus species: Structural and biochemical insights into fungal siderophore esterases. Angew. Chemie-Int. Ed. 2018, 57, 14624–14629. [Google Scholar] [CrossRef] [PubMed]
- Gsaller, F.; Eisendle, M.; Lechner, B.E.; Schrettl, M.; Lindner, H.; Müller, D.; Geley, S.; Haas, H. The interplay between vacuolar and siderophore-mediated iron storage in Aspergillus fumigatus. Metallomics 2012, 4, 1262–1270. [Google Scholar] [CrossRef] [PubMed]
- Sanguinetti, M.; Posteraro, B.; Beigelman-Aubry, C.; Lamoth, F.; Dunet, V.; Slavin, M.; Richardson, M.D. Diagnosis and treatment of invasive fungal infections: Looking ahead. J. Antimicrob. Chemother. 2019, 74, ii27–ii37. [Google Scholar] [CrossRef] [PubMed]
- Pelc, N.J.; Kinahan, P.E.; Pettigrew, R.I. Special Section Guest Editorial: Positron emission tomography: History, current status, and future prospects. J. Med. Imaging 2017, 4, 011001. [Google Scholar] [CrossRef] [PubMed]
- Fadeev, E.A.; Luo, M.; Groves, J.T. Synthesis, structure, and molecular dynamics of Gallium complexes of schizokinen and the amphiphilic siderophore acinetoferrin. J. Am. Chem. Soc. 2004, 126, 12065–12075. [Google Scholar] [CrossRef] [PubMed]
- Velikyan, I. Continued rapid growth in 68 Ga applications: Update 2013 to June 2014. J. Label. Compd. Radiopharm. 2015, 58, 99–121. [Google Scholar] [CrossRef] [PubMed]
- Petrik, M.; Haas, H.; Dobrozemsky, G.; Lass-Florl, C.; Helbok, A.; Blatzer, M.; Dietrich, H.; Decristoforo, C. 68Ga-Siderophores for PET imaging of invasive pulmonaryaspergillosis: Proof of principle. J. Nucl. Med. 2010, 51, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Petrik, M.; Haas, H.; Schrettl, M.; Helbok, A.; Blatzer, M.; Decristoforo, C. In vitro and in vivo evaluation of selected 68Ga-siderophores for infection imaging. Nucl. Med. Biol. 2012, 39, 361–369. [Google Scholar] [CrossRef]
- Petrik, M.; Franssen, G.M.; Haas, H.; Laverman, P.; Hörtnagl, C.; Schrettl, M.; Helbok, A.; Lass-Flörl, C.; Decristoforo, C. Preclinical evaluation of two 68Ga-siderophores as potential radiopharmaceuticals for Aspergillus fumigatus infection imaging. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 1175–1183. [Google Scholar] [CrossRef]
- Petrik, M.; Haas, H.; Laverman, P.; Schrettl, M.; Franssen, G.M.; Blatzer, M.; Decristoforo, C. 68Ga-Triacetylfusarinine C and 68Ga-Ferrioxamine E for Aspergillus infection imaging: Uptake specificity in various microorganisms. Mol. Imaging Biol. 2014, 16, 102–108. [Google Scholar] [CrossRef]
- Kaeopookum, P.; Summer, D.; Pfister, J.; Orasch, T.; Lechner, B.E.; Petrik, M.; Novy, Z.; Matuszczak, B.; Rangger, C.; Haas, H.; et al. Modifying the siderophore Triacetylfusarinine C for molecular imaging of fungal infection. Mol. Imaging Biol. 2019, 21, 1097–1106. [Google Scholar]
- Pfister, J.; Summer, D.; Petrik, M.; Khoylou, M.; Lichius, A.; Kaeopookum, P.; Kochinke, L.; Orasch, T.; Haas, H.; Decristoforo, C.; et al. Hybrid imaging of Aspergillus fumigatus pulmonary infection with fluorescent, 68Ga-labelled siderophores. Biomolecules 2020, 10, 168. [Google Scholar] [CrossRef]
- Skriba, A.; Pluhacek, T.; Palyzova, A.; Novy, Z.; Lemr, K.; Hajduch, M.; Petrik, M.; Havlicek, V. Early and non-invasive diagnosis of aspergillosis revealed by infection kinetics monitored in a rat model. Front. Microbiol. 2018, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Navalkissoor, S.; Gnanasegaran, G.; Baum, R. Theranostics and precision medicine special feature. Br. J. Radiol. 2018, 91, 20189004. [Google Scholar] [PubMed]
- Haas, H.; Petrik, M.; Decristoforo, C. An iron-mimicking, Trojan horse entering fungi—Has the time come for molecular imaging of fungal infections? PLoS Pathog. 2015, 11, 1–7. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ji, C.; Juárez-Hernández, R.E.; Miller, M.J. Exploiting bacterial iron acquisition: Siderophore conjugates. Future Med. Chem. 2012, 4, 297–313. [Google Scholar] [CrossRef] [PubMed]
- Gumienna-Kontecka, E.; Carver, P.L. Building a Trojan horse: Siderophore-drug conjugates for the treatment of infectious diseases. In Essential Metals in Medicine: Therapeutic Use and Toxicity of Metal Ions in the Clinic; Carver, P.L., Ed.; De Gruyter: Berlin, Germany; Boston, MA, USA, 2019; Volume 19, pp. 331–358. ISBN 9783110527872. [Google Scholar]
- Lu, Y.; Wang, H.; Wang, Z.; Cong, Y.; Zhang, P.; Liu, G.; Liu, C.; Chi, Z.; Chi, Z. Metabolic rewiring improves the production of the fungal active targeting molecule Fusarinine C. ACS Synth. Biol. 2019, 8, 1755–1765. [Google Scholar] [CrossRef]
- Shanzer, A.; Libman, J. Biomimetic siderophores: From structural probes to diagnosic tools. Met. Ions Biol. Syst. 1998, 35, 329. [Google Scholar]
- Besserglick, J.; Olshvang, E.; Szebesczyk, A.; Englander, J.; Levinson, D.; Hadar, Y.; Gumienna-Kontecka, E.; Shanzer, A. Ferrichrome has found its match: Biomimetic Analogues with diversified activity map discrete microbial targets. Chem.-Eur. J. 2017, 23, 13181–13191. [Google Scholar]
- Marvig, R.L.; Damkiær, S.; Khademi, S.M.H.; Markussen, T.M.; Molin, S.; Jelsbak, L. Within-Host Evolution of Pseudomonas aeruginosa reveals adaptation toward iron acquisition from hemoglobin. MBio 2014, 5, 1–8. [Google Scholar] [CrossRef]
- Minandri, F.; Imperi, F.; Frangipani, E.; Bonchi, C.; Visaggio, D.; Facchini, M.; Pasquali, P.; Bragonzi, A.; Visca, P. Role of iron uptake systems in Pseudomonas aeruginosa virulence and airway infection. Infect. Immun. 2016, 84, 2324–2335. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Wang, X.; Xu, H.; Xu, Y.; Ling, J.; Zhang, D.; Gao, S.; Liu, X. Roles of iron acquisition systems in virulence of extraintestinal pathogenic Escherichia coli: Salmochelin and aerobactin contribute more to virulence than heme in a chicken infection model. BMC Microbiol. 2012, 12, 143. [Google Scholar]
- Sheldon, J.R.; Heinrichs, D.E. Recent developments in understanding the iron acquisition strategies of gram positive pathogens. FEMS Microbiol. Rev. 2015, 39, 592–630. [Google Scholar] [PubMed]
- Cendrowski, S.; MacArthur, W.; Hanna, P. Bacillus anthracis requires siderophore biosynthesis for growth in macrophages and mouse virulence. Mol. Microbiol. 2004, 51, 407–417. [Google Scholar] [CrossRef]
- Caza, M.; Kronstad, J.W. Shared and distinct mechanisms of iron acquisition by bacterial and fungal pathogens of humans. Front. Cell. Infect. Microbiol. 2013, 3, 1–23. [Google Scholar] [CrossRef]
- Petrik, M.; Zhai, C.; Novy, Z.; Urbanek, L.; Haas, H.; Decristoforo, C. In Vitro and in vivo comparison of selected Ga-68 and Zr-89 labelled siderophores. Mol. Imaging Biol. 2016, 18, 344–352. [Google Scholar] [CrossRef]
- Petrik, M.; Umlaufova, E.; Raclavsky, V.; Palyzova, A.; Havlicek, V.; Haas, H.; Novy, Z.; Dolezal, D.; Hajduch, M.; Decristoforo, C. Imaging of Pseudomonas aeruginosa infection with Ga-68 labelled pyoverdine for positron emission tomography. Sci. Rep. 2018, 8, 15698. [Google Scholar] [CrossRef]
- Petrik, M.; Vlckova, A.; Novy, Z.; Urbanek, L.; Haas, H. Selected 68Ga-siderophores versus 68Ga-colloid and 68Ga-citrate: Biodistribution and small animal imaging in mice. Biomed. Pap. Med Fac. Univ. Palacky Olomouc Czechoslov. 2015, 159, 60–66. [Google Scholar]
- Sass, G.; Nazik, H.; Penner, J.; Shah, H.; Ansari, S.R.; Clemons, K.V.; Groleau, M.C.; Dietl, A.M.; Visca, P.; Haas, H.; et al. Studies of Pseudomonas aeruginosa mutants indicate pyoverdine as the central factor in inhibition of Aspergillus fumigatus biofilm. J. Bacteriol. 2017, 200, e00345-17. [Google Scholar] [CrossRef]
- Ioppolo, J.A.; Caldwell, D.; Beiraghi, O.; Llano, L.; Blacker, M.; Valliant, J.F.; Berti, P.J. 67 Ga-labeled deferoxamine derivatives for imaging bacterial infection: Preparation and screening of functionalized siderophore complexes. Nucl. Med. Biol. 2017, 52, 32–41. [Google Scholar] [CrossRef]
- Hoenigl, M.; Orasch, T.; Faserl, K.; Prattes, J.; Loeffler, J.; Springer, J.; Gsaller, F.; Reischies, F.; Duettmann, W.; Raggam, R.B.; et al. Triacetylfusarinine C: A urine biomarker for diagnosis of invasive aspergillosis. J. Infect. 2018, 78, 150–157. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrik, M.; Pfister, J.; Misslinger, M.; Decristoforo, C.; Haas, H. Siderophore-Based Molecular Imaging of Fungal and Bacterial Infections—Current Status and Future Perspectives. J. Fungi 2020, 6, 73. https://doi.org/10.3390/jof6020073
Petrik M, Pfister J, Misslinger M, Decristoforo C, Haas H. Siderophore-Based Molecular Imaging of Fungal and Bacterial Infections—Current Status and Future Perspectives. Journal of Fungi. 2020; 6(2):73. https://doi.org/10.3390/jof6020073
Chicago/Turabian StylePetrik, Milos, Joachim Pfister, Matthias Misslinger, Clemens Decristoforo, and Hubertus Haas. 2020. "Siderophore-Based Molecular Imaging of Fungal and Bacterial Infections—Current Status and Future Perspectives" Journal of Fungi 6, no. 2: 73. https://doi.org/10.3390/jof6020073
APA StylePetrik, M., Pfister, J., Misslinger, M., Decristoforo, C., & Haas, H. (2020). Siderophore-Based Molecular Imaging of Fungal and Bacterial Infections—Current Status and Future Perspectives. Journal of Fungi, 6(2), 73. https://doi.org/10.3390/jof6020073





