Abstract
The ability to form biofilms is a common feature of microorganisms, such as bacteria or fungi. These consortiums can colonize a variety of surfaces, such as host tissues, dentures, and catheters, resulting in infections highly resistant to drugs, when compared with their planktonic counterparts. This refractory effect is particularly critical in polymicrobial biofilms involving both fungi and bacteria. This review emphasizes Candida spp.-bacteria biofilms, the epidemiology of this community, the challenges in the eradication of such biofilms, and the most relevant treatments.
1. An Overview of Single and Polymicrobial Biofilms Involving Candida spp. and Bacterial Species
Microorganisms can naturally accumulate on a wide variety of surfaces where they form sessile communities, as mono or polymicrobial biofilms. Household/industrial, biomaterials and/or biological surfaces are some of the substrates that can be colonized by microorganisms [1]. Indeed, the ability to form biofilms is an important virulence factor for pathogenic microorganisms. It is defined as complex and dynamic microbial 3D structures, consisting of attached cells encased in a self-synthesized matrix of extracellular polymeric substances (EPSs) [2]. Sessile cells are protected from the surrounding environment by the extracellular matrix that covers the cell and is therefore a key factor of drug resistance [3,4]. Biofilm-associated infections are more difficult to treat and control since sessile cells are 10- to 1000-fold more resistant than their planktonic counterparts [5]. This mode of growth confers some advantages to its members, including exchange of substrate, resistance to antimicrobial drugs, immune system, mechanical and environmental stresses, adhesion ability, nutritional sources, and cellular communication [6]. Accordingly, cellular structures enable microbial communication by quorum sensing and their adaptation to several stressful conditions and presents a propensity to cause diseases. Due to their “adaptative” resistance, once established, bacterial and fungal biofilm-associated infections are very hard to treat and eradicate.
A typical microbial biofilm formation involves several steps namely the attachment to biotic or abiotic surfaces (formation of micro-colonies/development of young biofilm), maturation (differentiation of structured mature biofilm), and detachment (dispersal of mature biofilm) [7]. Thus, biofilms can serve as a reservoir for pathogenic cells and their release can cause septicemia and evolve into invasive systemic infections of organs and tissues. External factors contribute and influence the biofilm’s characteristics. Among them, the surface where the biofilm is growing, the nutrients available, and the inhibitors present in the surrounding environment or the presence of antagonistic microorganisms are the most relevant [8].
Depending on the situation, bacterial biofilms can be either beneficial or problematic [9]. Still, bacterial biofilms are usually pathogenic and responsible for several diseases, such as nosocomial infections [10]. Among the bacteria responsible for this kind of infection, the majority are bacteria belonging to the known ESKAPE group, namely Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Psedomonas aeruginosa, and Enterobacter spp. [11]. Infections derived from these bacteria are nowadays known as being extremely difficult to handle, mostly due to their biofilm formation ability. Additionally, these microorganisms can be found in different contexts, including environmental, industrial, and clinical, which aggravates the possibility of infection. The progress of medical science and the widespread use of medical devices and artificial organs have given rise to the emergence of bacterial biofilm infections [7,12]. Actually, it has been reported that the majority of devices with medical applications may result in biofilm infections, with 65% of all bacterial infections being related to bacterial biofilms [13,14].
Due to the heterogeneity of microorganisms present in the human flora, biofilms that can be generated are mostly polymicrobial, involving either species of the same genus or species from different kingdoms (cross-kingdom microorganisms, such as bacteria and fungi) [15]. Polymicrobial biofilms are now recognized as having higher complicated management [16]. In fact, the diversity, complexity, and the different pathogens associated with polymicrobial biofilms can significantly contribute to severe clinical implications [16]. The interspecies interactions exhibited by polymicrobial biofilms are relevant to the colonization, host response, drug resistance, and disease progression [17,18]. Therefore, all contributions on several aspects behind biofilm formation (e.g., mechanisms of adhesion and signaling involved in multispecies interaction) and antimicrobial resistance are crucial for development of new strategies in the treatment and prevention of polymicrobial biofilms’ infections. The impairment of microbial adhesion and biofilm formation are crucial steps and targets of therapeutic strategies aimed at the inhibition and development of polymicrobial diseases. Correspondingly, the co-aggregation occurring during the polymicrobial biofilm formation is well studied for common pathogens. Candida spp., namely Candida albicans, was shown to easily form biofilms in combination with other microorganisms, namely bacteria and other yeasts. As an example, C. albicans was shown to co-habit with strains, such as Staphylococcus aureus, Streptococcus mutans, and Fusobacterium spp. [19], but more examples of polymicrobial biofilms will be given in the next sections.
Approximately 80% of microbial infections are associated with biofilms, exhibiting high mortality rates [20]. Both yeast and bacteria are able to adhere to biotic or abiotic surfaces, developing into those highly organized communities, which is their preferred mode of growth. Therefore, biofilms formed by yeasts and/or bacteria, and consequently their associated infections, have become increasingly important. Moreover, bacteria and fungi of the Candida genus are often found in multispecies biofilms in vivo [21], with fungal–bacterial interactions research on the rise. Multi-species biofilms can display different behaviors, namely mutually beneficial (co-aggregation), competitive, and antagonistic interactions. An example of a beneficial interaction between species that live in the same biofilm is the interaction of bacteria and C. albicans in the case of oral biofilms. On the other hand, a competitive and antagonistic interaction in multispecies biofilms is observed in the case of C. albicans and Pseudomonas aeruginosa [21].
This review highlights important aspects related to Candida spp./bacterial mixed biofilms, namely their epidemiology, microbial resistance, and recent advances in the management of this kind of consortia. Moreover, the challenges found in the development of effective therapies against polymicrobial biofilms are also addressed herein.
2. Epidemiology of Candida spp./Bacteria Single and Mixed Biofilms
Biofilms’ infections caused by a single microbial species or by a mixture of bacterial and fungal species have increased significantly, contributing to high levels of morbidity and mortality. Indeed, the presence of both eukaryotic and prokaryotic pathogens makes the infections difficult to diagnose as well as to treat, requiring complex multi-drug treatment strategies [17]. Antimicrobials directed towards one species in a mixed-species biofilm often facilitate non-targeted organisms to thrive and continue the infection [22]. In this sense, mixed biofilms represent an understudied and clinically relevant health problem, with the potential to serve as an infectious reservoir for a variety of microorganisms, including bacteria and fungi [17].
2.1. Epidemiology of Candida spp. Single Biofilms
Candida spp. are major human fungal pathogens, which cause both mucosal and deep tissue infections. The ability of these yeasts to form biofilms on medical devices has a profound effect on its capacity to cause human disease [23]. Infection occurs in 60% of these cases, with Candida spp. being responsible for up to 20% of these [24] and mortality rates as high as 30% [25,26,27]. Candida albicans can form biofilms on almost any medical device [27], including vascular and urinary catheters, joint prostheses, cardiac valves, artificial vascular bypass devices, pacemakers, ventricular assist devices, and central nervous system shunts [27,28]. Among them, catheter-related infections are the major cause of morbidity and mortality among hospitalized patients, and microbial biofilms are associated with 90% of these infections. Candida spp. catheter-associated biofilms can lead to bloodstream infections, with an approximate incidence of one episode per 100 hospital admissions [27,29], as well as to urinary tract infections [27,30]. Up to 70% to 80% of Candida spp. bloodstream infections are associated with central venous catheters [27,30]. Also, Candida spp. endocarditis was previously considered a rare disease, but the incidence is increasing, partly because of the increased use of prosthetic intravascular devices. Likewise, biofilm formation on biotic surfaces has been reported, including both oral and vaginal tissues [31,32].
Among Candida spp., C. albicans is effectively the most predominant cause of invasive fungal infections and is a serious challenge for public health. However, while C. albicans is the fungal species most often isolated, the incidence of non-Candida albicans Candida species (NCACs) has recently increased [27,33,34]. In fact, more than half of the cases of Candida spp. infections in European countries were caused by C. albicans, followed by 14% for C. glabrata, 14% for Candida parapsilosis, 7% for Candida tropicalis, and 2% for Candida krusei [27,35]. A predominance of NCACs was also observed in the north of America. In Brazil, C. albicans accounted for 40.9% of cases, followed by C. tropicalis (20.9 %), C. parapsilosis (20.5%), and C. glabrata (4.9%) [27,36,37]. The change in epidemiology observed in past years could be associated with severe immunosuppression or illness, prematurity, exposure to broad-spectrum antibiotics, and older patients [27,38]. Among NCACs, C. parapsilosis has emerged as a significant pathogen with clinical manifestations, such as endophthalmitis, endocarditis, septic arthritis, peritonitis, and fungaemia, usually associated with invasive procedures or prosthetic devices [27,33,37,39]. A study isolated 100 strains of C. parapsilosis from a haemodialysis unit; 53% corresponded to C. parapsilosis and 47% were found to be Candida orthopsilosis [27]. Furthermore, candidaemia due to C. tropicalis has been associated with cancer in patients with leukemia or neutropenia [27,40], and Candida dubliniensis was frequently found in combination with other species, especially C. albicans. Also, it was detected a high prevalence of C. dubliniensis in the oral cavities of HIV-infected and AIDS patients [27,41,42]. Other species have been isolated and 400 out of 1356 isolates were identified as C. parapsilosis sensu lato (29.5%). This species was also isolated in Spain as the second most frequent from blood after C. albicans [27]. Of these 400 isolates, 364 were C. parapsilosis sensu stricto (90.7%), C. orthopsilosis (8.2%), and Candida metapsilosis (1.1%). The incidence of Candida guilliermondii and Candida rugosa is also increasing [37,43]. Candida rugosa (1.1%) has been described in the oral cavity of diabetic patients [27,44] and Candida lusitaniae is responsible for 1% to 2% of all candidaemias [27,44].
2.2. Epidemiology of Bacterial Single Biofilms
It is estimated that approximately 65% of all bacterial infections are associated with bacterial biofilms (device and non-device related) [45]. Single or mixed bacterial biofilms are correlated with a broad range of infections, from indwelling medical devices to chronic tissue infections (e.g., chronic wounds, cystic fibrosis (CF)) [46,47,48].
Although the number of anaerobic species involved in infections is lower, compared with the aerobic, and the infections are mainly formed by aerobes and anaerobes pathogens [49], the correct knowledge of the usual sites of colonization by anaerobes is helpful for the identification of the microorganisms involved and to estimate paths of invasion [50]. Indeed, the most identified are Porphyromonas spp., Prevotella spp., Fusobacterium spp., Pepto-streptococcus spp., and Actinomyces spp., infecting brain, spinal cord, neck, lungs, oral cavity, and upper airway. The Bacteroides fragilis group and Clostridium spp. are also isolated from patients with abdominal, gastrointestinal, or genital tract infections. Propionibacterium acnes has been linked to acne and endophthalmitis after cataract surgery, and Finegoldia magna can be frequently isolated from gynecological materials and specimens from skin and soft tissue infections [50].
One of the most common biofilm infections is periodontitis. In this infection, Porphyromonas gengivalis, Pseudomonas aerobicus, and Fusobacterium nucleatum are among the causative agents, which can also be the cause of biofilms on the mucosal surfaces in the oral cavity [51]. Other relevant infection is the colonization of teeth surfaces (tartar), which can lead to the invasion of mucosal cells, changing the flow of calcium in the epithelial cells, and the release of several toxins [52]. Eventually, osteomyelitis infections can have bacterial (e.g., S. aureus) or fungal origin (e.g., C. glabrata) [53,54], and the infections occur through the bloodstream, trauma, or foregoing infections [53].
Considering medical device infections (e.g., contact lenses, mechanical heart valves, peritoneal dialysis catheters, prosthetic joints, central venous catheters, pacemakers, urinary catheters, voice prostheses), the microorganisms can be introduced during implantation of a prosthesis or derived from a transient bacteremia. Actually, the implant tissue interface is linked to a local immunological depression of the host, which allows even the less virulent members of commensal flora to colonize the biomaterials [55]. After the insertion, the microorganisms adhere to biomaterials and grow to form a biofilm [56]. Around 40% of ventricular-assisted devices, 10% of ventricular shunts, 4% of pacemakers and defibrillators, 4% of mechanical heart valves, 2% of breast implants, and 2% of joint prostheses are affected by biofilm infections [57]. Staphylococcus aureus and Staphylococcus epidermidis are respectively at the first and second positions in staphylococcal species, followed by some emerging new pathogens, such as Staphylococcus hominis, Staphylococcus haemolyticus, Staphylococcus capitis, and Staphylococcus warneri [58,59,60]. Staphylococcus aureus frequently colonizes the human naris, and it is a major etiological agent of nosocomial infections [61]. Staphylococcus epidermidis (saprophytic of the human skin) has been alarmingly involved in outbreaks of community-acquired skin infections, progressively emerging as a main opportunistic species with the rising use of medical devices [62,63]. Moreover, P. aeruginosa, Enterococcus faecalis [64], and bacteria from the Enterobacteriaceae family are frequently detected on infected orthopedic implants [65]. All these microorganisms are producers or strong producers of biofilms, with distinctive matrices and implant locations [65,66,67]. For example, Enterobacteriaceae and Enterococcus spp. are recurrently identified after pelvis surgery [58], and Propionibacterium spp. (mainly Propionibacterium acnes) are related to infected shoulder implants [68]. Around 75% of the infections originate in these leading pathogenic species while 25% consist of over 50 species [58], an element that has relevant implications for anti-infective strategies.
Infections associated to cardiovascular implantable electronic devices are relatively infrequent, but carry a considerable risk of mortality and morbidity, demanding, in several occasions, the complete extraction of the device [69,70]. At the time of surgical implantation, there can be tissue damage, resulting in the accumulation of platelets and fibrin at the suture and on the devices. Pathogens have enhanced the aptitude to colonize these locations [15]. The microbial agents related to endocarditis differ on the time in which the infection becomes symptomatic: within 60 days from cardiac surgery, bacteria are mainly from nosocomial origin (intraoperative contamination); if the infection appears after 12 months, pathogens are typically entangled to native valve endocarditis: Viridans streptococci, S. aureus (leading cause of infections associated to cardiovascular implantable electronic devices [71]), Haemophilus aphrophilus, Actinobacillus actinomycetemcomitans, Cardiobacterium hominis, Eikenella spp., and Kingella spp. To sum up, infections occurring between 2 and 12 months are a microbial combination of the other two periods [72]. Nevertheless, there are also indications of an absence of a species shift between early and late infections [73] and a rise in species of the central nervous system, as dominant pathogens (after S. aureus) [71]. Gram-negative Bacillus spp., Enterococcus spp., and Candida spp. can also be related to endocarditis, and the origin of these can also be related to dental work [10].
Among the several microorganisms that attach to contact lenses, E. coli, P. aeruginosa, S. aureus, and S. epidermidis, but also species of Candida spp., Serratia spp., and Proteus spp., are among the most frequent. The adhesion varies on the water content, bacterial strain, substrate nature, electrolyte concentration, and the polymer composition. The lens storage boxes have been confirmed as a source of contamination [15]. The location and extent of biofilm formation on central venous catheters depends on the duration of catheterization, depending on factors, such as the nature of the fluid administered. In fact, Gram-positive bacteria (e.g., S. epidermidis and S. aureus) do not grow well in intravenous fluids, on contrary to Gram-negative aquatic bacteria (e.g., P. aeruginosa, Enterobacter spp., Klebsiella spp.) [10,74]. Ultimately, regarding urinary catheters, these are made of silicon or latex devices, and can have a closed or an open system for urine (more prone to contamination than the first) [75]. Urinary catheters are commonly contaminated by biofilms of S. epidermidis, E. coli, E. faecalis, P. aeruginosa, Proteus mirabilis, Klebsiella pneumoniae, and other Gram-negative bacteria [76].
2.3. Epidemiology of Candida spp. and Bacteria Mixed Biofilms
Humans are colonized by diverse populations of bacteria and fungi when in a healthy state and in the settings of disease, and the interactions between these microbial populations can be beneficial or detrimental to the host [77]. Candida spp. are the most common commensal fungus that coexist with hundreds of species of bacteria in the human body. Multiple Candida spp., such as C. albicans, C. tropicalis, C. glabrata, and C. krusei, have all been recovered either in combination or with other bacterial species [78,79]. Alarmingly, Candida spp.-associated polymicrobial infections have often resulted in high mortality and morbidity in both adults and children because of their increased dissemination behavior and the current lack of diagnostic sensitivity, especially in a biofilm mode of growth [79,80,81]. Some studies have explored the Candida spp.–bacterial interactions in opportunistic biofilm infections, such as those on the skin, and into systemic disease, in the lungs, in the oral cavity, in the gastrointestinal tract, and vulvovaginal. Mixed biofilms of C. albicans and S. epidermidis, Enterococcus spp. and S. aureus have been found in systemic infections [17,27,82]. In particular, S. aureus seems to have a certain tendency to interact with C. albicans, as suggested by the high frequency with which S. aureus is isolated from the blood of patients with candidemia. Considerably, staphylococcal species and Candida spp. have also been found to be associated in bloodstream infections of a neonatal population [79,81], and in infective endocarditis [79,83,84]. Candida albicans and S. aureus invasion were revealed to be clearly facilitated by ALS3 (a C. albicans adhesin). Candida albicans hyphae (highly immunogenic feature) attracts phagocytic cells, which rapidly surround S. aureus, before migrating to cervical lymph nodes, and leading to systemic disease, morbidity, and mortality, which suggests synergy of the infection between these two entities [85]. Importantly, a novel strategy showed that the adhesin Als3p binds to multiple staphylococcal adhesins. The work also revealed that this is necessary for C. albicans to transport S. aureus into the tissue and cause a disseminated infection in an oral co-colonization model. These tactics accelerate the invasion of S. aureus through mucosal barriers, leading to systemic infection in co-colonized patients [86]. Furthermore, variances in adhesion forces between S. aureus and different regions of C. albicans hyphae (“tip”, “middle”, “head”) were quantitatively confirmed, signposting that the head region is different from the remainder of the hyphae. Significantly, properties of the hyphal head region were shown to be comparable to those of budding yeast cells [87]. Notably, the interaction between these two pathogens may be lethal to the host, by causing both candidemia and bacteremia [79,80,88].
In the lungs, Candida spp. has been reported to interact with Burkholderia cenocepacia in patients with CF [79], and with Mycobacterium tuberculosis in patients with tuberculosis [79,89]. Curiously, antagonistic interactions between Candida spp. and bacterial species have also been observed in the lungs, with P. aeruginosa killing yeast hyphae and biofilms of C. albicans [27,79,90].
In the oral environment, Candida spp. have been found to co-exist with multiple bacterial species, including S. aureus, S. mutans (the main bacteria found on human caries), Streptococcus gordonii, E. coli, Klebsiella spp., and Pseudomonas spp. The formation of these polymicrobial biofilms has a direct correlation with the use of dentures, with biofilms forming on the surface of these dentures or on the oral mucosa itself [22,78,79,91,92]. Some bacterial species, such as S. gordonii, are able to enhance the development of hyphae and the formation of biofilm by C. albicans when in the presence of human saliva, thereby contributing to the establishment of a polymicrobial biofilm that is hard to treat [79].
Further, in the gastrointestinal tract, C. albicans often encounters E. faecalis [79,93,94]. These two pathogens seem to have antagonistic interactions when in polymicrobial biofilms, much like P. aeruginosa and C. albicans in the lung environment. On contrary, E. coli and C. albicans seem to work together to form biofilms in human tissues and body fluids [79,95]. The presence of Candida spp. in polymicrobial biofilms in the gastrointestinal tract has been shown as particularly problematic, as the associated infections have mortality rates quite higher than those of solely bacterial polymicrobial biofilms (75% compared to 30%) [79,96,97,98,99]. Finally, in the vulvovaginal environment, antagonistic effects have been reported between Lactobacillus spp. (Lactobacillus rhamnosus, Lactobacillus acidophilus, Lactobacillus plantarum, and Lactobacillus reuteri) and Candida spp., by inhibition of both hyphal and biofilm formation by the latter [79,100].
3. Candida/Bacteria Mixed Biofilms: Characterization and the Problematic of the Biofilms’ Drug Resistance
3.1. Mixed Candida spp./Bacteria Biofilms: Features, Pathogenicity, and Virulence
Candida spp. are the most common infectious fungal species in humans. Apart from their role as the main etiology for various types of candidiasis, Candida spp. are also related to polymicrobial infections. In these infections, several trans-kingdom polymicrobial interactions are formed, either synergistic or antagonistic, which may support the virulence and pathogenicity of both Candida spp. and bacteria while distinctively impacting the pathogen–host immune response (Figure 1) [79].
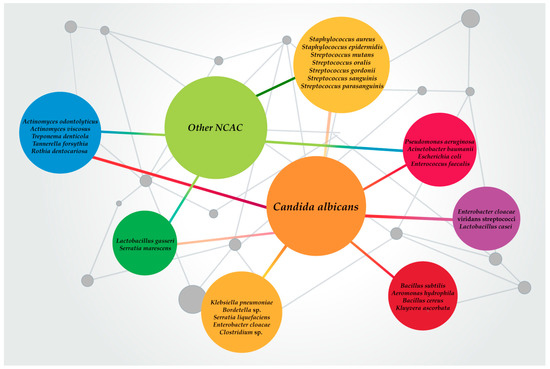
Figure 1.
Most relevant Candida spp.–bacteria mixed biofilms reported and studied in the last decades.
Understanding which species—fungi and/or bacteria—controls virulence, and the associated mechanisms, especially in biofilms, offers potential for novel therapies and underpinnings for further research problems.
It is established that different environmental conditions induce different interactions of bacteria with Candida spp., particularly, C. albicans [101]. Aerobic and anaerobic liquid co-cultures of C. albicans and several bacteria (Aeromonas hydrophila, Bacillus cereus, Bacillus subtilis, Clostridium spp., Enterobacter spp., K. pneumoniae, Kluyvera ascorbate, and Serratia marcescens) were used by Benadé and colleagues to study yeast–bacteria mixed cultures. Candida albicans growth was inhibited in the presence of bacterial growth, probably due to the presence of extracellular hydrolytic enzymes (e.g., chitinases and mannan-degrading), under aerobic conditions. Yet, this inhibition was not noticed under anaerobic conditions (no enzymes nor other compounds, such as prodigiosin from cultures of S. marcescens, were produced) and the growth in co-cultures was comparable to what is detected in pure cultures. A lower quantity of chitin was manufactured under anaerobic conditions, when compared to aerobic settings. Finally, the reduced production of the bacterial enzymes, prodigiosin, and mannan present in the yeast cell wall was linked to anaerobic growth and survival of C. albicans in the presence of bacteria [101]. Culture conditions also have influence in biofilms. Trypticase soy broth (TSB) and brain heart infusion (BHI) had higher biofilm formations and metabolic activity, and longer incubation periods with a fed-batch system and fetal bovine serum (FBS) revealed upper growth conditions in clinically isolated NCACs and S. epidermidis on silicone. This fact is relevant when designing or studying the mixed biofilms under in vitro conditions, probably being responsible for a higher or lower biomass production and, consequently, modifying the drug response [102]. As biofilms formed in silicone, some dental products can damage the oral microbiome homeostasis, inducing the mixed-species biofilm formation, allowing higher adhesion to dental prothesis. This is the case of denture adhesives (Ultra Corega Cream and Corega Strip), which increased the adhesion of C. albicans but not of L. casei. In fact, C. albicans biofilm formation by (single- and mixed-species) was higher on the strip adhesive. The authors did not observe any relations of synergism or antagonism between the two microorganisms [103].
Likewise, natural components of bacteria and fungi can condition mixed biofilm structure and functioning. For example, mannans located on the outer surface of C. albicans mediate Streptococcus mutans exoenzyme GtfB (β-glucosyltransferase) binding, so as to control in vivo cross-kingdom biofilm development, namely improving glucan-matrix production and regulating bacterial–fungal association. Recently, the GtfB binding properties to C. albicans was tested in strains defective in O-mannan (pmt4ΔΔ) or N-mannan outer chain (och1ΔΔ), and it was noticed that the binding was compromised (>3-fold reduction vs. parental strain) [64]. Moreover, the quantity of GtfB on the fungal surface was expressively cut, and the ability of C. albicans mutant strains to develop mixed-species biofilms with S. mutans was impaired (independent of hyphae or established fungal-biofilm regulators—EFG1, BCR1) [64]. As other authors have shown [104,105], the biofilm matrix stability was lower on the mutants, causing a high rate of biomass loss, which was also confirmed by in vivo assays [64,104,105]. The commensal protection of S. aureus against antimicrobials by C. albicans biofilm matrix has been reported. When grown together, the fungus offers a bacterial increased tolerance to antimicrobial drugs, which is secured by β-1,3-glucans secreted by the fungal cell. These polysaccharides can block the drugs’ penetration, and provide protection [104,105] through a coating of the bacteria. Notably, inhibiting β-1,3-glucans production, caspofungin indirectly sensitized the bacteria to antimicrobials [106]. In an in vitro model of the mixed-species biofilm C. albicans–S. epidermidis on polyvinyl chloride (PVC) material, the bacteria attached to the spores, pseudohyphae, and hyphae of C. albicans, originating a complex and dense network display. The biofilm was organized by viable and dead pathogens, and the surface of the mixed biofilms was rough, with living pathogens mostly in protrusive quotas and dead pathogens in concave aggregates [107]. Though the surface of PVC material was described as having slightly different biofilms, the formation is a dynamic process: Rapid growing in 24 h of co-culture and maximal thickness peaked at 48 h (matured at 48–72 h). Furthermore, there were noteworthy variances (p < 0.05) in the ratio of viable cells between the interior, middle, and outer layers [107]. Similarly, Bertolini et al. [108] evidenced that Candida spp.–streptococcal (Streptococcus oralis strain 34) mucosal biofilms exhibit distinctive structural and virulence features varying on growth conditions and hyphal morphotypes. Streptococcus oralis can stimulate fungal invasion and tissue damage, moisture, nutrient availability, and hyphal morphotype. Actually, the presence of commensal bacteria was shown to influence the architecture and virulence characteristics of mucosal fungal biofilms. A pioneer study in the University Hospital of Tlemcen CHU in Algeria studied the formation of mixed biofilm formation between C. albicans and several bacteria in peripheral venous catheters. The authors found that C. albicans have the potential to form mixed biofilms with Enterobacter cloacae, Bordetella spp., and Serratia liquefaciens, which were isolated from the same catheter as the yeasts. Depending on the microorganisms of the biofilms, a level of competition among bacteria and C. albicans was noticed that was directly associated to the composition of the medium and its pH [109].
Antagonism or synergism between Candida spp./bacteria mixed biofilms has also been discussed. Essentially, there is an antagonistic interaction of S. aureus toward C. glabrata during in vitro biofilm formation, induced by the presence of cell-free bacterial supernatant (CFBS). CFBS originated a strong decay in yeast viability and the formation of numerous lipid droplets, reactive oxygen species accumulation, as well as nuclear alterations, and DNA fragmentation signposting the initiation of an apoptotic mechanism [110]. Likewise, Martins et al. [111] explained, for the first time, the duality in C. albicans–C. rugosa biofilms, and suggested that C. albicans or Candida spp. can co-exist in biofilms exhibiting an apparent antagonism. Quite the reverse, earlier studies displayed synergistic interaction and increased mortality in animal models infected by dual species biofilms of S. aureus and C. albicans [112,113,114]. Zago et al. [115] clarified the dynamics of biofilm formation and the interface between C. albicans and methicillin-susceptible (MSSA) and -resistant S. aureus (MRSA). The results showed that C. albicans, MSSA, and MRSA can, in fact, co-exist in biofilms in an apparent synergism, with S. aureus cells preferentially coupled to C. albicans hyphal forms. Nevertheless, more studies are required, involving these and other Candida spp. and bacterial species.
Regarding protein receptors, Sap9 has been related to interactions amid fungal cells, and with interkingdom communication in the formation of polymicrobial biofilm communities. Compared with the parent strain, the sap9Δ mutant of C. albicans SC5314 produces smoother biofilms, with less blastopores and more hyphae. These features were stressed under flow (shear) conditions and in the presence of S. gordonii. Regarding dual-species biofilms (C. albicans sap9Δ and S. oralis, Streptococcus sanguinis, Streptococcus parasanguinis, S. mutans, or E. faecalis), all contained a higher number of entangled hyphae and bacteria bound to the substratum than the C. albicans wild type. Furthermore, the mutant hyphae amplified the cell surface hydrophobicity, had higher levels of the binding cell wall Als3 (~25%), and lower interaction with Eap1, which connects Sap9 in fungal cell–cell recognition [116]. The utility of intercellular adhesion A (icaA), fibrinogen binding protein (fbe), and accumulation-associated protein (aap) genes in the formation of S. epidermidis-C. albicans mixed-species biofilms was also explored. The thickness of S. epidermidis and C. albicans biofilms were inferior than that in the mixed biofilms. Plus, the growth speed in the mixed biofilms was greater than that in C. albicans, and in S. epidermidis at 48 h. Overall, mixed-species biofilms indicated a more complex structure and are thicker than single species biofilms of S. epidermidis or C. albicans, which can be correlated to higher expressions of the S. epidermidis icaA, fbe, and aap genes [117].
Prostaglandin E2 (PGE2) from C. albicans was proven to stimulates the growth of S. aureus–C. albicans in mixed biofilms, as reported by Krause et al. [118]. In fact, C. albicans PGE2 was determined as a central molecule stimulating growth and mixed S. aureus/C. albicans biofilm formation, though C. albicans derived farnesol, but not tyrosol, may also provide a similar stimulus but to a smaller degree [118].
Beforehand, it was described that microorganisms of a community, such as biofilms, secrete signaling chemical molecules to coordinate their cooperative behavior, in a phenomenon called quorum sensing. Kong et al. [119] confirmed that in the presence of farnesol, in biofilms (exogenously supplemented or secreted by C. albicans), S. aureus had a significantly enhanced tolerance to antimicrobials due to a broad stress response system, which can lead to upregulation of drug efflux pumps, and high resistance patterns. This work evidenced that, in mixed biofilms, C. albicans can improve the pathogenicity of S. aureus, with key therapeutic repercussions. Also, de Carvalho Dias et al. [120] stated that some soluble factors from single- and mixed-species biofilm of C. albicans and MSSA promote cell death and the inflammatory response. The soluble factors from mixed biofilms were the most toxic to the keratinocytes (NOK-si and HaCaT) cells. Single and mixed biofilms stimulated interleukin 6 (IL-6), nitrous oxide (NO), and tumor necrosis factor-alpha (TNF-α) production by J744A.1 macrophages [120].
3.2. Mixed Candida spp./Bacteria Biofilms vs. Oral Biofilms Features, Pathogenicity, and Virulence
As in oral infections, the coexistence of Candida spp. and bacteria in numerous other diseases is a critical issue, which questions and, in several circumstances, jeopardizes the effectiveness of the chosen therapeutics.
This is the case of patients with CF or other respiratory disorders (ORDs). Haiko et al. [121] collected and analyzed sputum samples from 130 patients with CF and 186 patients with ORD. Respectively, nearly 70% and 44% of the sputum samples of the CF patients and patients with ORD had pathogenic bacteria, particularly P. aeruginosa and S. aureus (CF patients). No difference was noted in the coexistence of pathogenic bacteria and Candida spp., yet P. aeruginosa and S. aureus coexisted with Candida spp. more frequently in CF patients than in patients with ORD. Curiously, adult CF patients were demonstrated to have a greater rate of coexistence of any pathogenic bacteria and Candida spp. than the children with CF and the adult patients with ORD [121]. Different pathogens have comparable medical settings and virulence approaches in order to origin infections. Formerly, Uppuluri et al. [122] proved that active and passive immunization with Hyr1 (rHyr1p-N) protected mice against lethal candidemia. The same authors revealed that C. albicans Hyr1 protein can be an immunotherapeutic target for Acinetobacter spp. infection. Hyr1p shares its homology with cell surface proteins of the multidrug-resistant (MDR) A. baumannii, such as membrane protein A (OmpA), which binds to C. albicans Hyr1, leading to a mixed-species biofilm. Its blocking or deletion notably reduced A. baumannii binding to C. albicans hyphae, diminishing mixed biofilms’ in vitro formation and improving the survival of diabetic or neutropenic mice infected with A. baumannii bacteremia or pneumonia.
Charles and colleagues [123] revealed that the in vivo decrease in anaerobic bacteria helps C. glabrata overgrowth (with a decrease IL-1β expression). Notably, at the same time, β-glucan treatment reestablishes the gut microbiota, mitigates colitis (increasing IL-10 production via PPARγ sensing) and, thus, C. glabrata elimination. During colitis development, a proliferation of E. coli and E. faecalis populations and a decline in Lactobacillus johnsonii and Bacteroides thetaiotaomicron was noted. The reduction in L. johnsonii was stressed by C. glabrata overgrowth [123]. Interactions between the gut-associated Bacteroides fragilis NCTC 9343, Bacteroides vulgatus ATCC 8482, and C. albicans were also explored [124]. Mostly, the yeast growth was not affected by the presence of the bacteria, but the Bacteroides spp. growth was expressively higher in the presence of C. albicans. The cell-free supernatant of 24-h-old C. albicans CAB 392 monocultures was able to increase the number of Bacteroides and the chloramphenicol sensitivity. Remarkably, the supplementation of Bacteroides monocultures with dead C. albicans CAB 392 cells (with outer cell wall mannan layers) also led to amplified bacterial concentrations. In fact, B. vulgatus ATCC 8482 used the mannan. The authors concluded that C. albicans can stimulate Bacteroides growth via aerobic respiration and/or antioxidant production [124]. Actually, the importance of mannans in single and mixed biofilms has previously been demonstrated [64,104].
Interactions between the bacteriome and mycobiome stress microbial dysbiosis in familial Crohn’s disease (CD) of northern France and Belgium have been discussed [125]. Using Ion Torrent sequencing, Hoarau and colleagues showed positive interkingdom correlations between C. tropicalis, S. marcescens, and E. coli, which were associated to CD dysbiosis. The amount of anti-Saccharomyces cerevisiae antibodies (ASCAa; a known CD biomarker) was related with the abundance of C. tropicalis. Biofilms of this species included blastopores while double- and triple-species biofilms involved hyphae. Serratia marcescens used fimbriae to co-aggregate or attach with C. tropicalis–E. coli while E. coli was apposed with C. tropicalis [125].
Bone infections (such as chronic osteomyelitis) caused by microbial biofilms are a noteworthy public health burden, with a relevant assorted morbidity and mortality. Authors have studied the pathogenesis of several species linked to this disease, performing in vitro and ex vivo assays, with several osteomyelitis pathogens in single and mixed biofilms [126]. Staphylococcus aureus, P. aeruginosa, C. albicans, and S. mutans were grown in hydroxyapatite, rat jawbone, or polystyrene wells, and in diverse media. All species produced mature biofilms within 7 days on all substrate surfaces regardless of the media. In fact, the results also showed that biofilms noticeably damaged the bone, which confirmed that osteomyelitis biofilms have the skill to directly resorb bone [126].
Diabetic foot ulcers (DFUs) are another major clinical problem aggravated by persistent bacterial infection. The understanding of macrophage–microbe interactions can lead to progress in targeted therapies for DFU healing. Macrophage gene expression and protein secretion have been shown to be disturbed by both microbial species as well as the human monocyte donor [127]. Indeed, Staphylococcus simulans and C. albicans instigate upregulation of genes associated with a pro-inflammatory (M1) phenotype. Pseudomonas aeruginosa triggers a rise in secretion of the pro-inflammatory cytokine and M1 marker tumor necrosis factor-alpha (TNFa) [127]. Similarly, the prevalence and impact of MDR microorganisms in microbial infected DFUs in north Egypt was recently studied [128]. Microbial profiles of diabetic foot patients with purulent wounds displayed a predominance of monomicrobial infections over polymicrobial infections (77.3% vs. 22.7%). A total of 24 bacterial isolates and 4 yeast isolates were identified. Strains of C. albicans, A. baumanni, S. aureus, and K. pneumonia were acknowledged, with a resistance on more than six of empirical antibiotics [128].
4. Management of Candida spp./Bacterial Biofilms: Is this the Impossible Mission?
Choosing the most suitable therapy to eradicate single or mixed Candida spp./bacterial biofilms has become one of the most actual challenging clinical goals. In the last years, several attempts have been made to select natural or synthetic new compounds with improved antimicrobial activity, or combining both, in order to increase this effect. Table 1 summarizes the most relevant ones and the following sections provide more detail of each one.

Table 1.
Effective new treatments to fight Candida spp./bacteria mixed biofilms.
4.1. Oral Disease Management
Endodontic biofilms are polymicrobial communities (bacteria–fungi) surrounded by a polymeric matrix of polysaccharides, resistant to usual intracanal irrigants, antimicrobial drugs, and to the host immunity. In order to prevent and treat main oral biofilm-associated infections, the in vitro effectiveness of a Cu/CaOH2-based endodontic paste, against S. aureus, P. aeruginosa, and C. albicans, was evaluated. The paste expressively cut both the microbial replication time and cell growth. Biofilms experienced a fall in the number of cells and levels of released pyoverdine [129]. Quaternary ammonium amphiphiles (e.g., benzalkonium chloride, BAC), used as a preservative in topical formulations for ocular, skin, or nasal purposes, are a class of compounds with a wide range of commercial and industrial uses. BAC was shown to have a wide antimicrobial activity and minor enveloped viruses. Nonetheless, there are some safety concerns about its irritant and cytotoxic effect on epithelial cells, which demands caution in its applications. Perinelli et al. [130] synthesized BAC analogues (such as derivatives of leucine esters: C10, C12, and C14). Although the cytotoxic effect was dependent on the length of the hydrophobic chain, in general, the compounds showed a promising antimicrobial activity (against S. aureus and Enterococcus spp., E. coli, P. aeruginosa, and C. albicans), as MIC values for C14-derivatives were equivalent to those of BAC [130]. Regarding therapeutic responses applying light, Pourhajibagher et al. [131] reported that an acrylic resin containing Undaria pinnatifida, using photo-activation with LED, has antimicrobial properties against planktonic and mixed biofilms forms of cariogenic microorganisms (S. mutans, S. sanguinis, and Lactobacillus acidophilus) and C. albicans, even at the lowest concentration. Correspondingly, photodynamic inactivation (PDI) on single- and multi-species biofilms of C. albicans and S. sanguinis showed reductions of 1.07 (single) and 0.39 log10 (mixed), demonstrating that PDI is a possible way to control these clinically important microorganisms [137]. In another work, Diogo et al. used photodynamic therapy (aPDT) with the Zn(II)chlorin e6 methyl ester (Zn(II)e6Me) activated by red light against monospecies and mixed-species biofilms of E. faecalis and C. albicans. The results proved that once activated with light for 60 or 90 s, Zn(II)e6Me damaged the normal microbial cell ultrastructure and removed approximately 60% of the biofilm’s biomass. Hence, these results show that aPDT might be an effective strategy for the eradication of endodontic biofilms in infected root canal systems. Further studies are, yet, needed [138].
In what concerns mouthwashes, Ardizzoni et al. [132] evaluated the antimicrobial activity of alcohol-free commercial mouthwashes with chlorhexidine digluconate (CHX), fluoride, essential oils, cetylpyridinium chloride (CC), and triclosan. Candida albicans and a cluster of viridans streptococci (frequently present in the oral cavity) were isolated from pharyngeal swabs and tested. The results showed that mouthwashes containing CHX and CC were the most successful in impairing biofilms and increasing the host response to C. albicans. Additionally, they were effective in damaging biofilm formation by viridans streptococci that cooperate with the cariogenic S. mutans, and ineffective against viridans streptococci that are natural competitors of S. mutans. On the contrary, in a mixed biofilm, the mouthwashes eradicated S. salivarius but failed to impair C. albicans’ biofilm forming ability [132]. Likewise, Tan et al. [133] showed that the combination activity of curcumin and 2-aminobenzimidazole against single- and mixed-species biofilms of C. albicans and S. aureus was curiously most potent on mixed biofilms. The antimicrobial activity of 0.2% polyhexamethilene biguanide (PHMB) to 2.5% NaOCl and 0.2% CHX in root canal models infected with E. faecalis, C. albicans, and S. epidermidis was also evaluated. PHMB reduced cell counts of all species. Both NaOCl and PHMB were efficient in eliminating E. faecalis and S. epidermidis from the mature dentin biofilm, but CHX was not satisfactory in this matter [135]. Comparably, S. epidermidis MFP5-5 and S. xylosus MFP28-3, C. albicans MFP8, C. parapsilosis MFP16-2, and Candida famata MFP29-1 were isolated from silicone facial prostheses by Ariani et al. [168]. In order to verify their antimicrobial activity, several agents used to clean facial prostheses were used: Antibacterial soap, essential oil-containing mouth rinse, ethanol 27%, chlorhexidine mouth rinse, and buttermilk. The results showed that antibacterial soap and buttermilk had the lowest activity. On the other side, CHX exhibited the highest reduction in colony forming units (CFUs) in 24-h, 2-week, and regrown mixed-species biofilms [168].
Streptococcus mutans is involved in tooth decay by the development of biofilm adhesion and caries, and the presence of C. albicans may exacerbate the demineralization process. The antimicrobial and anti-adhesion properties of micellar solutions of surfactants (cetylpyridinium chloride and cetyltrimethylammonium bromide and sufactin) and terpinen-4-ol (TP) (a natural plant product) were studied. All surfactants stimulated the antimicrobial activity of TP against S. mutans, proposing a specificity for membrane interactions that may be facilitated by surfactants [134]. Kim et al. [136] signposted that the association of topical antifungal fluconazole and povidone iodine (PI) can entirely suppress C. albicans oral carriage and mixed-biofilm formation without increasing the bacterial in vivo killing activity. PI increased the fluconazole efficacy, by disturbing the bacterial exopolysaccharide (EPS) matrix, through inhibition of α-glucan synthesis (which binds and sequesters fluconazole [172]) by S. mutans exoenzyme linked to the fungal surface. This study indicates that EPS inhibitors might be good anti-biofilmers to boost the killing efficacy.
Finally, the colonization of acidogenic bacteria and fungi on denture materials is linked with DS and dental caries. An innovative mixed-species acidogenic biofilm model was recently developed to measure antimicrobial properties against single- and mixed-species biofilm of C. albicans, Lactobacillus casei, and S. mutans. The novel fluoride-releasing copolymer was constituted by methyl methacrylate (MMA) and 2-hydroxyethyl methacrylate (HEMA) with polymethyl methacrylate (PMMA). The intermicrobial interactions in mixed-species acidogenic biofilms were sensitive to fluoride (in mixed-species biofilms, cell densities were reduced around 10-fold, when compared with non-fluoride material), dropping the formation of polymicrobial biofilms [139].
4.2. Innovative Treatments of Other Diseases
Hospital-acquired infections and multidrug-resistant bacteria are a substantial hazard to any healthcare system. The in vitro antimicrobial features of flexible Corning® light-diffusing fiber (LDF) on ESKAPE and other relevant pathogens (S. epidermidis, Streptococcus pyogenes, C. albicans, and E. coli) were measured. The authors found that the LDF delivery of 405 nm violet-blue light had a significant antimicrobial activity towards a wide range of pathogens under diverse experimental conditions [140]. Regarding in vivo assays, the drug had efficacy in invasive candidiasis, aspergillosis, and pneumocystis. High bioavailability, positive drug interaction, and tolerability profile was also observed.
During the present year, a new antibacterial and antifungal nanosystem composed of magnetic nanoparticles (MNPs) and a PBP10 peptide attached to the surface was synthesized [141]. MNPs were revealed to improve the antimicrobial activity of the phosphoinositide-binding domain of gelsolin, and control its mode of action against S. aureus MRSA Xen 30, P. aeruginosa Xen 5, and Candida spp., in both planktonic and biofilm forms. This effect reinforces the possibility of new treatment methods of infections [141].
Bacterial molecules and current drugs have also been under investigation for their antimicrobial activity. Otsuka et al. [143] demonstrated that the ZorO (type I toxin-antitoxin system), localized in the inner membrane, disturbs the plasma membrane integrity and potential when expressed in E. coli, also triggering the production of cytotoxic hydroxyl radicals. Exogenously added Ala-Leu-Leu-Arg-Leu peptide (ALLRL, required for ZorO toxicity) to S. aureus, Bacillus subtilis, and C. albicans, revealed to induce cell membrane damage and growth defect, with no effects on E. coli, revealing it as an attractive antimicrobial to Gram-positive bacteria and C. albicans. Although E. faecalis and C. albicans are common residents of the microbiome, both microorganisms are recorded by the Centers for Disease Control and Prevention, as serious global public health threats with amplified antimicrobial resistance directly related to these microorganisms have been recorded. EntV is a bacteriocin encoded by the EntV (ef1097) locus, documented for decreasing C. albicans virulence and biofilm formation by obstructing hyphal morphogenesis. Brown et al. indicated that the antifungal activity of the E. faecalis peptide EntV obliges protease cleavage only by gelatinase (GelE) and disulfide bond formation by DsbA. It was concluded that EntV, or an analogous compound, should be explored as a therapeutic alternative, alone or in combination with current drugs, against Gram-positive bacteria and C. albicans [144]. A diabetic class of drugs, thiazolidinediones (TZDs), was found to be successful quorum sensing quenchers, inhibiting biofilm formation. Prior findings confirmed this high antibiofilm effect of the TZD derivative thiazolidinedione-8 (S-8), in solution or incorporated into a sustained-release membrane (SRM-S-8) [173,174]. Analyzing the effect of SRM-S-8 on mixed C. albicans–S. mutans biofilm development, under flow conditions, indicated that the constant release of S-8 promotes enhanced penetration of the drug to deeper layers of dental polymicrobial biofilms, thus increasing the antimicrobial activity against the pathogens [145]. Rogiers et al. [151] concluded that anidulafungin (an antifungal drug) rises the antibacterial activity of tigecycline, thus acting synergistically, in polymicrobial biofilms of C. albicans–S. aureus on intraperitoneally implanted foreign bodies. Also, the abundance of S. aureus poly-β-(1,6)-N-acetylglucosamine was also cut with anidulafungin.
Acinetobacter baumannii is well adjusted to hospital environments. Its chronic infections endure predominantly due to its ability to form biofilms resistant to conventional drugs and to fragile host immune systems. A report disclosed the antibiofilm and antivirulence ability of the three most active flavonoids, fisetin, phloretin, and curcumin, against A. baumannii [146]. The antibiofilm activity was dose dependent, and curcumin had the highest activity, when compared with gallium nitrate (a biofilm inhibitor), inhibiting pellicle formation and the surface motility of A. baumannii. This compound also exposed antibiofilm activity against C. albicans and mixed cultures of C. albicans–A. baumannii. After a Caenorhabditis elegans infection model was treated with curcumin treatment, A. baumannii virulence was lowered, without cytotoxicity [146]. Later, the same authors, were able to indicate that voriconazole inhibits cross-kingdom interactions between C. albicans and A. viscosus through the ergosterol pathway [147]. They reported a higher biomass and virulence of mixed-species biofilms, when compared with the A. viscosus biofilm alone, and indicate voriconazole as a candidate strategy to combat root caries pathogens [147]. Eugenol showed concentration-dependent antibiofilm activity in single- and mixed-biofilms of drug-resistant strains of C. albicans and S. mutans, through multiple modes of action [148]. Importantly, in this work, the C. albicans strains used were resistant to fluconazole, itraconazole, ketoconazole, and amphotericin B, except C. albicans CAJ-01 and C. albicans MTCC3017, which were sensitive to fluconazole. Streptococcus mutans MTCC497 was resistant to ampicillin, azithromycin, ceftriaxone, and vancomycin [148]. In de Alteriis et al.’s [149] study, gH625-GCGKKKK (a derivative of the membranotropic peptide gH625) strongly inhibited the formation of mixed biofilms of clinical isolates of C. tropicalis–S. marcescens and C. tropicalis–S. aureus and reduced the biofilm architecture, interfering with cell adhesion and polymeric matrix, as well as eradicating the long-term polymicrobial biofilms on the silicone surface.
Elshinawy et al. [150] evaluated chitosan (Ch-NPs), silver nanoparticles (Ag-NPs), and ozonated olive oil (O3-oil), both single or combined against endodontic pathogens, such as E. faecalis, S. mutans, and C. albicans. Ch-NPs had lower MIC and MBC values, with a higher antimicrobial activity than O3-oil against E. faecalis, S. mutans, and C. albicans. Synergism was found between O3-oil and Ch-NPs (FIC index ≤0.5), diminishing mature viable biofilms on a premolar ex vivo model (6-log reductions), with a complete anti-fibroblast adherent effect. An innovative two-layer nitric oxide-generating system (NOx) exposed 2- and 10-log fold CFU reductions [152]. NOx was effective against S. aureus, P. aeruginosa, A. baumannii, E. coli, and Candida spp. single- and mixed-species biofilms, including multidrug-resistant strains. This work suggests NOx as a possible new group of antimicrobial drugs with strong, broad-spectrum activity, and, importantly, with no signs of resistance development. The good efficacy of electrospun membranes of poly(lactic acid) (carrier matrix) and carvacrol (essential oil with antimicrobial activity) against S. aureus and C. albicans in single and mixed cultures was demonstrated by Scaffaro and colleagues [153]. A significant decrease of CFUs, biomass, and metabolic activity of 24- and 48-h biofilms was proven. This system might be used as a new tool for skin and wound bacterial–fungal infections [153].
Tyrosol does not reduce hydrolytic enzymes and acid production by Candida spp. and S. mutans but significantly reduces the metabolic activity of single biofilms of Candida spp. [154]. The use of this compound as a substitute antimicrobial for topical therapies still requires more studies. Similarly, carboxymethyl chitosan (CM-chitosan) might be a possible antibiofilm agent to be applied on voice prostheses. Tan and colleagues [158] performed several studies using this compound. They determined the influence of carboxymethyl chitosan on the mixed biofilm formation of C. albicans, C. tropicalis, L. gasseri, S. salivarius, R. dentocariosa, and S. epidermidis, on silicone over a long-term period. Results showed that on surfaces preserved by carboxymethyl chitosan, the biofilm was less dense and there were less coats of cells and profuse cellular debris, plus damaged and morphologically altered yeast cells. Then, they showed that it inhibits mixed fungal and damages the cells of bacterial biofilms on silicone. CM–chitosan also repressed the adhesion of fungi and bacteria (>90%) and stopped biofilm formation (~46%–70%), when it was added after biofilm initiation. However, although CM–chitosan inhibited Candida spp. yeast-to-hyphal transition, it was not able to inhibit the metabolic activity of biofilms [159]. In other reports, the same authors revealed it is possible to inhibit the activity of Lactobacilli supernatant (cell free) against fungal–bacterial multispecies biofilms on silicone. In fact, the Lactobacilli supernatant inhibited several features: Adhesion of mixed biofilms (efficiency >90%/90 min) and the metabolic activity of the biofilms (72.23% and 58.36%), also damaging the cells. The Candida spp. yeast-to-hyphal transition was equally reduced. The results showed that the Lactobacilli supernatant can possibly be an antibiofilm agent (single and mixed) for prostheses [161]. The same authors also described an inhibitory effect of probiotic L. gasseri and L. rhamnosus supernatants on single and mixed NCACs biofilm (C. tropicalis, C. krusei, and C. parapsilosis) [175]. Finally, another work of Tan et al. exhibited that CM–chitosan is effective as a sole agent, inhibiting both monomicrobial and polymicrobial biofilms (of C. tropicalis and S. epidermidis) in microplates and also on the silicone surface in short- and long-term periods [160]. First, CM–chitosan constrained planktonic growth and adhesion. Then, biofilm formation was also repressed (90 min or 12 h after biofilm initiation), demonstrating that this compound can possibly be used as an antibiofilm agent to limit monomicrobial and polymicrobial biofilm.
Natural and quorum sensing compounds, such as marine compounds, essential oils (EOs), and extracts, have also been analyzed. Several marine bacterial exopolymers mediated green synthesis of noble metal nanoparticles (EP NPs) (derived from Eolian Islands, Italy, in the Mediterranean Sea) and have antimicrobial properties [142]. No activity was indicated for EP-gold NPs, except against E. coli, whereas EP-silver NPs exhibited a broad-spectrum of activity towards S. aureus, E. coli, P. aeruginosa, and C. albicans [142]. Budzyńska et al. [163] suggested C. albicans–S. aureus dual-species biofilm as an efficient target for the combination of EOs (geranium, citronella, and clove oils) and fluconazole or mupirocin. EOs of citrus species can prevent the formation of polymicrobial biofilms (P. aeruginosa and several pathogenic fungi). Pompia and grapefruit EOs constrained fungal growth (MIC: 50–250 mg/L), but no effect on P. aeruginosa growth was observed. Both citrus EOs inhibited the formation of bacterial and fungal single-species biofilms (minimum inhibitory concentration, MIC: 50 mg/L) and potentiated the activity of common antimicrobials. Finally, citrus EOs disturbed quorum sensing in P. aeruginosa and caused fast permeabilization of C. albicans membrane, demonstrating a possible application in the control of polymicrobial fungi–bacterial infections [163]. Combining drugs and essential oils lead to a limitation in dual-species biofilm formation and the elimination of the preformed mixed biofilm to a higher degree [162]. Soliman et at. [164] assessed the anti-Candida spp. activities of some medicinal plants. The ethanol extracts of Avicennia marina (Qurm), Ziziphus spina-Christi (Sidr), Portulaca oleracea (Baq’lah), Fagonia indica (Shoka’a), Lawsania inermis (Henna), Salvadora persica (Souwak), and Asphodelus tenuifolius (Kufer) were tested against C. albicans (yeasts and hypha), including cytotoxicity. The results proposed that L. inermis and P. oleracea extracts and/or their chemicals may be suited as antimicrobials against C. albicans, S. aureus, P. aeruginosa, E. coli, A. baumannii, and Klebsiella pneumoniae, with no associated toxicity [164]. Furthermore, it was detected that farnesol reduces the formation of single and mixed biofilms (total biomass 37%–90%; metabolic activity: 64%–96%) and cell viability (1.3–4.2 and 0.67–5.32 log10, respectively, for single- and mixed-species biofilms) [157,176]. Abdel-Rhman et al. [156] demonstrated that mixed-species biofilms, such as P. aeruginosa–C. albicans, can produce a protected environment that consents the tyrosol and farnesol (C. albicans quorum sensing compounds) might affect Egyptian clinical isolates of P. aeruginosa. Tyrosol proved to have antibacterial activity, also impeding the production of the virulence factors, hemolysin and protease, in P. aeruginosa, proposing that it powerfully affecta P. aeruginosa in mixed microbial biofilms. On the other side, farnesol faintly inhibited hemolysin production in this pathogen. Similarly, tyrosol showed inhibitory effects against single- and mixed-species biofilms formed by important oral pathogens. In fact, Arias et al. [155] evaluated single and mixed biofilms of C. albicans ATCC 10231, C. glabrata ATCC 90030, and S. mutans ATCC 25175 formed on acrylic resin (AR) and hydroxyapatite (HA) surfaces, in the presence of tyrosol, during 48 h. The molecule had an antibiofilm effect in single and mixed cultures (mostly at the highest concentration, 200 mmol/L), demonstrating that it can be valuable in the development of topical therapies dedicated to inhibiting biofilm-associated oral diseases.
Novel synthetic molecules are under exploration. The activity spectrum of the most active N1- and 2N-substituted 5-aryl-2-aminoimidazoles against single- and mixed-biofilms of Gram-positive and Gram-negative bacteria and C. albicans was assessed by Peeters et al. [165]. Molecules with substituents at both the N1 and 2N positions had high activity against mixed Gram-positive and yeast biofilms (monospecies and mixed), excluding biofilms formed by Gram-negative bacteria. The authors concluded that -aryl-2-aminoimidazoles can be used as anti-infective coatings on orthopedic implants, since, in general, the viability of bone cells was not disturbed, even inducing calcium deposition. Qu et al. [167] showed that guanylated polymethacrylates kill mixed fungal/bacterial biofilms, particularly C. albicans–S. aureus, denoting a possible use in antimicrobial lock therapy. The molecules displayed an increased efficacy, eradicating C. albicans–S. aureus mixed biofilms. Additionally, applying multiple combinations of current antimicrobial drugs, the performance was very good (99.98% of S. aureus and 82.2% of C. albicans were eliminated). When added to planktonic assays, the extracellular biofilm matrix, namely, β-1,3 glucans, offered protection to the cells, increasing the MIC of the polymethacrylates by 2- to 4-fold. The authors suggested that this mechanism might be lessened by chemical optimization of the polymer structure [167]. The antimicrobial activity of novel cellulose carbamates has also been assessed [169]. The compounds demonstrated both bactericide and fungicide in vitro activity. Particularly, ω-aminoethylcellulose carbamate showed high activity against C. albicans (IC50: 0.02 mg/mL) and S. aureus and IC50: 0.05 mg/mL). Besides, the antimicrobial activity and cytotoxicity was superior when p-amino-benzylamine was added, and a mixed cellulose carbamate had high biocompatibility, forming films on cotton and PES, with a strong activity against S. aureus and K. pneumoniae [169].
Finally, lock solutions have equally been under discussion for the prevention of biofilms. The current guidelines involve catheter removal, but the reinsertion can be defiant or risky. Lown et al. reported that a lock solution with micafungin, ethanol, and doxycycline inhibited C. albicans and mixed C. albicans–S. aureus biofilms. Beforehand, it was also confirmed the great in vitro activity of the same drugs as single agents for the prevention and treatment of C. albicans biofilms [177,178,179,180]. In this recent work, it was reported that a solution with 2% (v/v) ethanol, 0.01565 μg/mL micafungin, and 800 μg/mL doxycycline reduced metabolic activity (98%), with no fungal regrowth, applied once to prevent fungal biofilm formation. The solution also restrained the regrowth of C. albicans from mature polymicrobial biofilms, although this was not profusely bactericidal. Furthermore, when using 5% ethanol with low concentrations of micafungin and doxycycline, synergistic activity was found to prevent C. albicans single-biofilm formation [166].
5. Conclusions
Biofilm-associated infections require the multidisciplinary collaboration of experts of different areas of knowledge, including clinical microbiology, internal medicine, pharmacology, and basic science. To prevent microbial contamination, adhesion is a key step to avoid biofilm-associated infections. For that, prophylactic measures, such as good hygienic practices, are crucial. Although several progresses have been made to control and eradicate biofilm-related infections, new and innovative anti-biofilm approaches are still needed in order to ensure the effective management of biofilm infections.
Author Contributions
Conceptualization C.F.R., M.E.R. and F.G.; methodology C.F.R., M.E.R. and F.G.; investigation C.F.R., M.E.R. and F.G.; original draft preparation, C.F.R.; writing—review and editing, C.F.R., M.E.R. and F.G. All authors have read and agreed to the published version of the manuscript.
Funding
C.F.R. would like to thank for the UID/EQU/00511/2019 Project—Laboratory of Process Engineering, Environment, Biotechnology and Energy—LEPABE financed by national funds through FCT/MCTES (PIDDAC). M.E.R. and F.G. would like to thank for the Portuguese Foundation for Science and Technology (FCT) under the scope of the strategic funding of UID/BIO/04469/2019 unit and BioTecNorte operation (NORTE-01-0145-FEDER-000004) funded by the European Regional Development Fund under the scope of Norte2020-Programa Operacional Regional do Norte.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef]
- Serra, D.O.; Klauck, G.; Hengge, R. Vertical stratification of matrix production is essential for physical integrity and architecture of macrocolony biofilms of Escherichia coli. Environ. Microbiol. 2015, 17, 5073–5088. [Google Scholar] [CrossRef]
- Rodrigues, C.; Silva, S.; Henriques, M. Candida glabrata: A review of its features and resistance. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 673–688. [Google Scholar] [CrossRef]
- Rodrigues, M.E.; Silva, S.; Azeredo, J.; Henriques, M. Novel strategies to fight Candida species infection. Crit. Rev. Microbiol. 2014, 42, 594–606. [Google Scholar]
- Davies, D. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2003, 2, 114–122. [Google Scholar] [CrossRef]
- dos Santos, A.L.S.; Galdino, A.C.M.; de Mello, T.P.; de Ramos, L.S.; Branquinha, M.H.; Bolognese, A.M.; Columbano Neto, J.; Roudbary, M. What are the advantages of living in a community? A microbial biofilm perspective! Mem. Inst. Oswaldo Cruz 2018, 113, e180212. [Google Scholar] [CrossRef]
- Wu, H.; Moser, C.; Wang, H.Z.; Høiby, N.; Song, Z.J. Strategies for combating bacterial biofilm infections. Int. J. Oral Sci. 2015, 7, 1–7. [Google Scholar] [CrossRef]
- Cavalheiro, M.; Teixeira, M.C. Candida Biofilms: Threats, Challenges, and Promising Strategies. Front. Med. 2018, 5, 28. [Google Scholar] [CrossRef]
- Armbruster, C.R.; Parsek, M.R. New insight into the early stages of biofilm formation. Proc. Natl. Acad. Sci. USA 2018, 115, 4317–4319. [Google Scholar] [CrossRef]
- Bjarnsholt, T. The role of bacterial biofilms in chronic infections. Supplementum. APMIS 2013, 136. [Google Scholar] [CrossRef]
- Mulani, M.S.; Kamble, E.E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging strategies to combat ESKAPE pathogens in the era of antimicrobial resistance: A review. Front. Microbiol. 2019, 10, 539. [Google Scholar] [CrossRef]
- Percival, S.L.; Suleman, L.; Vuotto, C.; Donelli, G. Healthcare-Associated infections, medical devices and biofilms: Risk, tolerance and control. J. Med. Microbiol. 2015, 64, 323–334. [Google Scholar] [CrossRef]
- Lewis, K. Riddle of Biofilm Resistance. Antimicrob. Agents Chemother. 2001, 45, 999–1007. [Google Scholar] [CrossRef]
- Hancock, R.E.W. Rethinking the Antibiotic Discovery Paradigm. EBioMedicine 2015, 2, 629–630. [Google Scholar] [CrossRef][Green Version]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef]
- Ribeiro, S.M.; Felício, M.R.; Boas, E.V.; Gonçalves, S.; Costa, F.F.; Samy, R.P.; Santos, N.C.; Franco, O.L. New frontiers for anti-biofilm drug development. Pharmacol. Ther. 2016, 160, 133–144. [Google Scholar] [CrossRef]
- Harriott, M.M.; Noverr, M.C. Importance of Candida-bacterial polymicrobial biofilms in disease. Trends Microbiol. 2011, 19, 557–563. [Google Scholar] [CrossRef]
- Mahajan, A.; Singh, B.; Kashyap, D.; Kumar, A.; Mahajan, P. Interspecies communication and periodontal disease. Sci. World J. 2013, 2013, 765434. [Google Scholar] [CrossRef]
- Pathirana, R.U.; McCall, A.D.; Norris, H.L.; Edgerton, M. Filamentous non-albicans Candida species adhere to Candida albicans and benefit from dual biofilm growth. Front. Microbiol. 2019, 10, 1188. [Google Scholar] [CrossRef]
- Srinivasan, A.; Torres, N.S.; Leung, K.P.; Lopez-Ribot, J.L.; Ramasubramanian, A.K. nBioChip, a Lab-on-a-Chip Platform of Mono- and Polymicrobial Biofilms for High-Throughput Downstream Applications. mSphere 2017, 2, e00247-17. [Google Scholar] [CrossRef]
- Shirtliff, M.E.; Peters, B.M.; Jabra-Rizk, M.A. Cross-kingdom interactions: Candida albicans and bacteria. FEMS Microbiol. Lett. 2009, 299, 1–8. [Google Scholar] [CrossRef]
- Thein, Z.M.; Seneviratne, C.J.; Samaranayake, Y.H.; Samaranayake, L.P. Community lifestyle of Candida in mixed biofilms: A mini review. Mycoses 2009, 52, 467–475. [Google Scholar] [CrossRef]
- Hawser, S.P.; Douglas, L.J. Biofilm formation by Candida species on the surface of catheter materials in vitro. Infect. Immun. 1994, 62, 915–921. [Google Scholar]
- Kojic, E.M.; Darouiche, R.O. Candida infections of medical devices. Clin. Microbiol. Rev. 2004, 17, 255–267. [Google Scholar] [CrossRef]
- Viudes, A.; Pemán, J.; Cantón, E.; Úbeda, P.; López-Ribot, J.L.; Gobernado, M. Candidemia at a tertiary-care hospital: Epidemiology, treatment, clinical outcome and risk factors for death. Eur. J. Clin. Microbiol. Infect. Dis. 2002, 21, 767–774. [Google Scholar] [CrossRef]
- Finkel, J.S.; Mitchell, A.P. Genetic control of Candida albicans biofilm development. Nat. Rev. Microbiol. 2011, 9, 109. [Google Scholar] [CrossRef]
- Sardi, J.C.O.; Scorzoni, L.; Bernardi, T.; Fusco-Almeida, A.M.; Mendes Giannini, M.J.S.; Bernardi, T.; Scorzoni, L.; Fusco-Almeida, A.M.; Sardi, J.C.O.; Scorzoni, L.; et al. Candida species: Current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J. Med. Microbiol. 2013, 62, 10–24. [Google Scholar] [CrossRef]
- Byers, M.; Chapman, S.; Feldman, S.; Parent, A. Fluconazole pharmacokinetics in the cerebrospinal fluid of a child with Candida tropicalis meningitis. Pediatr. Infect. Dis. J. 1992, 11, 895–896. [Google Scholar]
- Didone, L.; Oga, D.; Krysan, D.J. A novel assay of biofilm antifungal activity reveals that amphotericin B and caspofungin lyse Candida albicans cells in biofilms. Yeast 2011, 28, 561–568. [Google Scholar] [CrossRef]
- Nett, J.; Lincoln, L.; Marchillo, K.; Massey, R.; Holoyda, K.; Hoff, B.; VanHandel, M.; Andes, D. Putative role of beta-1,3 glucans in Candida albicans biofilm resistance. Antimicrob. Agents Chemother. 2007, 51, 510–520. [Google Scholar] [CrossRef]
- Ozkan, S.; Kaynak, F.; Kalkanci, A.; Abbasoglu, U.; Kustimur, S. Slime production and proteinase activity of Candida species isolated from blood samples and the comparison of these activities with minimum inhibitory concentration values of antifungal agents. Mem. Inst. Oswaldo Cruz 2005, 100, 319–324. [Google Scholar] [CrossRef]
- Dongari-Bagtzoglou, A.; Dwivedi, P.; Ioannidou, E.; Shaqman, M.; Hull, D.; Burleson, J. Oral Candida infection and colonization in solid organ transplant recipients. Oral Microbiol. Immunol. 2009, 24, 249–254. [Google Scholar] [CrossRef]
- Almirante, B.; Rodríguez, D.; Park, B.J.; Cuenca-Estrella, M.; Planes, A.M.; Almela, M.; Mensa, J.; Sanchez, F.; Ayats, J.; Gimenez, M.; et al. Epidemiology and Predictors of Mortality in Cases of Candida Bloodstream Infection: Results from Population-Based Surveillance, Barcelona, Spain, from 2002 to 2003 Epidemiology and Predictors of Mortality in Cases of Candida Bloodstream Infection: Re. J. Clin. Microbiol. 2005, 43, 1829–1835. [Google Scholar] [CrossRef]
- Lai, C.C.; Wang, C.Y.; Liu, W.L.; Huang, Y.T.; Hsueh, P.R. Time to positivity of blood cultures of different Candida species causing fungaemia. J. Med. Microbiol. 2012, 61, 701–704. [Google Scholar] [CrossRef]
- Tortorano, A.M.; Kibbler, C.; Peman, J.; Bernhardt, H.; Klingspor, L.; Grillot, R. Candidaemia in Europe: Epidemiology and resistance. Int. J. Antimicrob. Agents 2006, 27, 359–366. [Google Scholar] [CrossRef]
- Colombo, A.L.; Nucci, M.; Park, B.J.; Nouér, S.A.; Arthington-Skaggs, B.; da Matta, D.A.; Warnock, D.; Morgan, J.; Brazilian Network Candidemia Study. Epidemiology of candidemia in Brazil: A nationwide sentinel surveillance of candidemia in eleven medical centers. J. Clin. Microbiol. 2006, 44, 2816–2823. [Google Scholar] [CrossRef]
- Nucci, M.; Queiroz-Telles, F.; Tobón, A.M.; Restrepo, A.; Colombo, A.L. Epidemiology of Opportunistic Fungal Infections in Latin America. Clin. Infect. Dis. 2010, 51, 561–570. [Google Scholar] [CrossRef]
- Horn, D.L.; Neofytos, D.; Anaissie, E.J.; Fishman, J.A.; Steinbach, W.J.; Olyaei, A.J.; Marr, K.A.; Pfaller, M.A.; Chang, C.; Webster, K.M. Epidemiology and Outcomes of Candidemia in 2019 Patients: Data from the Prospective Antifungal Therapy Alliance Registry. Clin. Infect. Dis. 2009, 48, 1695–1703. [Google Scholar] [CrossRef]
- Cantón, E.; Pemán, J.; Quindós, G.; Eraso, E.; Miranda-Zapico, I.; Álvarez, M.; Merino, P.; Campos-Herrero, I.; Marco, F.; De La Pedrosa, E.G.G.; et al. Prospective multicenter study of the epidemiology, molecular identification, and antifungal susceptibility of Candida parapsilosis, Candida orthopsilosis, and Candida metapsilosis isolated from patients with candidemia. Antimicrob. Agents Chemother. 2011, 55, 5590–5596. [Google Scholar] [CrossRef][Green Version]
- Colombo, A.L.; Guimarães, T.; Silva, L.R.B.F.; de Monfardini, L.P.A.; Cunha, A.K.B.; Rady, P.; Alves, T.; Rosas, R.C. Prospective Observational Study of Candidemia in São Paulo, Brazil: Incidence Rate, Epidemiology, and Predictors of Mortality. Infect. Control Hosp. Epidemiol. 2007, 28, 570–576. [Google Scholar] [CrossRef]
- Tintelnot, K.; Haase, G.; Seibold, M.; Bergmann, F.; Staemmler, M.; Franz, T.; Naumann, D. Evaluation of phenotypic markers for selection and identification of Candida dubliniensis. J. Clin. Microbiol. 2000, 38, 1599–1608. [Google Scholar]
- Lasker, B.A.; Elie, C.M.; Lott, T.J.; Espinel-Ingroff, A.; Gallagher, L.; Kuykendall, R.J.; Kellum, M.E.; Pruitt, W.R.; Warnock, D.W.; Rimland, D.; et al. Molecular epidemiology of Candida albicans strains isolated from the oropharynx of HIV-positive patients at successive clinic visits. Med. Mycol. 2001, 39, 341–352. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Messer, S.A.; Hollis, R.J.; Boyken, L.; Tendolkar, S.; Kroeger, J.; Diekema, D.J. Variation in susceptibility of bloodstream isolates of Candida glabrata to fluconazole according to patient age and geographic location in the United States in 2001 to 2007. J. Clin. Microbiol. 2009, 47, 3185–3190. [Google Scholar] [CrossRef]
- Pires-Gonçalves, R.H.; Miranda, E.T.; Baeza, L.C.; Matsumoto, M.T.; Zaia, J.E.; Mendes-Giannini, M.J.S. Genetic relatedness of commensal strains of Candida albicans carried in the oral cavity of patients’ dental prosthesis users in Brazil. Mycopathologia 2007, 164, 255–263. [Google Scholar] [CrossRef]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef]
- Ciofu, O.; Rojo-Molinero, E.; Macià, M.D.; Oliver, A. Antibiotic treatment of biofilm infections. APMIS 2017, 125, 304–319. [Google Scholar] [CrossRef]
- Rodrigues, C.F.; Rodrigues, M.; Henriques, M. Candida sp. Infections in Patients with Diabetes Mellitus. J. Clin. Med. 2019, 8, 76. [Google Scholar] [CrossRef]
- Vickery, K.; Hu, H.; Jacombs, A.S.; Bradshaw, D.A.; Deva, A.K. A review of bacterial biofilms and their role in device-associated infection. Healthc. Infect. 2013, 18, 61–66. [Google Scholar] [CrossRef]
- Vuotto, C.; Donelli, G. Anaerobes in biofilm-based healthcare-associated infections. Adv. Exp. Med. Biol. 2015, 830, 97–112. [Google Scholar]
- Japanese Society of Chemotherapy Committee on guidelines for treatment of anaerobic infections; Japanese Association for Anaerobic Infections Research. Chapter 1-1. Anaerobic infections (General): Epidemiology of anaerobic infections. J. Infect. Chemother. 2011, 17, 4–12. [Google Scholar] [CrossRef][Green Version]
- Watanabe, T.; Maruyama, F.; Nozawa, T.; Aoki, A.; Okano, S.; Shibata, Y.; Oshima, K.; Kurokawa, K.; Hattori, M.; Nakagawa, I.; et al. Complete genome sequence of the bacterium Porphyromonas gingivalis TDC60, which causes periodontal disease. J. Bacteriol. 2011, 193, 4259–4260. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Overman, P.R. Biofilm: A new view of plaque. J. Contemp. Dent. Pract. 2000, 1, 13–20. [Google Scholar] [CrossRef]
- Chihara, S.; Segreti, J. Osteomyelitis. Dis. Mon. 2010, 56, 6–31. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Fuchs, B.; Wang, Y.; Chen, W.; Yuen, G.; Chen, R.; Jayamani, E.; Anastassopoulou, C.; Pukkila-Worley, R.; Coleman, J.; et al. The role of Candida albicans SPT20 in filamentation, biofilm formation and pathogenesis. PLoS ONE 2014, 9, e94468. [Google Scholar] [CrossRef] [PubMed]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. A review of the clinical implications of anti-infective biomaterials andinfection-resistant surfaces. Biomaterials 2013, 34, 8018–8029. [Google Scholar] [CrossRef] [PubMed]
- Arciola, C.R.; Campoccia, D.; Speziale, P.; Montanaro, L.; Costerton, J.W. Biofilm formation in Staphylococcus implant infections. A review of molecular mechanisms and implications for biofilm-resistant materials. Biomaterials 2012, 33, 5967–5982. [Google Scholar] [CrossRef]
- Treatment of Infections Associated with Surgical Implants. PubMed-NCBI. Available online: https://www.ncbi.nlm.nih.gov/pubmed/15070792 (accessed on 8 November 2019).
- Arciola, C.R.; An, Y.H.; Campoccia, D.; Donati, M.E.; Montanaro, L. Etiology of implant orthopedic infections: A survey on 1027 clinical isolates. Int. J. Artif. Organs 2005, 28, 1091–1100. [Google Scholar] [CrossRef]
- Von Eiff, C.; Arciola, C.R.; Montanaro, L.; Becker, K.; Campoccia, D. Emerging staphylococcus species as new pathogens in implant infections. Int. J. Artif. Organs 2006, 29, 360–367. [Google Scholar] [CrossRef]
- Campoccia, D.; Montanaro, L.; Visai, L.; Corazzari, T.; Poggio, C.; Pegreffi, F.; Maso, A.; Pirini, V.; Ravaioli, S.; Cangini, I.; et al. Characterization of 26 Staphylococcus warneri isolates from orthopedic infections. Int. J. Artif. Organs 2010, 33, 575–581. [Google Scholar] [CrossRef]
- Campoccia, D.; Baldassarri, L.; Pirini, V.; Ravaioli, S.; Montanaro, L.; Arciola, C.R. Molecular epidemiology of Staphylococcus aureus from implant orthopaedic infections: Ribotypes, agr polymorphism, leukocidal toxins and antibiotic resistance. Biomaterials 2008, 29, 4108–4116. [Google Scholar] [CrossRef]
- Arciola, C.R.; Campoccia, D.; Gamberini, S.; Donati, M.E.; Pirini, V.; Visai, L.; Speziale, P.; Montanaro, L. Antibiotic resistance in exopolysaccharide-forming Staphylococcus epidermidis clinical isolates from orthopaedic implant infections. Biomaterials 2005, 26, 6530–6535. [Google Scholar] [CrossRef] [PubMed]
- Campoccia, D.; Montanaro, L.; Von Eiff, C.; Pirini, V.; Ravaioli, S.; Becker, K.; Arciola, C.R. Cluster analysis of ribotyping profiles of Staphylococcus epidermidis isolates recovered from foreign body-associated orthopedic infections. J. Biomed. Mater. Res. Part A 2009, 88, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Hwang, G.; Liu, Y.; Kim, D.; Li, Y.; Krysan, D.J.; Koo, H. Candida albicans mannans mediate Streptococcus mutans exoenzyme GtfB binding to modulate cross-kingdom biofilm development in vivo. PLoS Pathog. 2017, 13, e1006407. [Google Scholar] [CrossRef] [PubMed]
- Arciola, C.R.; Baldassarri, L.; Campoccia, D.; Creti, R.; Pirini, V.; Huebner, J.; Montanaro, L. Strong biofilm production, antibiotic multi-resistance and high gelE expression in epidemic clones of Enterococcus faecalis from orthopaedic implant infections. Biomaterials 2008, 29, 580–586. [Google Scholar] [CrossRef]
- Montanaro, L.; Poggi, A.; Visai, L.; Ravaioli, S.; Campoccia, D.; Speziale, P.; Arciola, C.R. Extracellular DNA in biofilms. Int. J. Artif. Organs 2011, 34, 824–831. [Google Scholar] [CrossRef]
- Frank, K.L.; Patel, R. Poly-N-acetylglucosamine is not a major component of the extracellular matrix in biofilms formed by icaADBC-positive Staphylococcus lugdunensis isolates. Infect. Immun. 2007, 75, 4728–4742. [Google Scholar] [CrossRef]
- Fernandez Sampedro, M.; Piper, K.E.; McDowell, A.; Patrick, S.; Mandrekar, J.N.; Rouse, M.S.; Steckelberg, J.M.; Patel, R. Species of Propionibacterium and Propionibacterium acnes phylotypes associated with orthopedic implants. Diagn. Microbiol. Infect. Dis. 2009, 64, 138–145. [Google Scholar] [CrossRef]
- Mond, H.G.; Proclemer, A. The 11th world survey of cardiac pacing and implantable cardioverter-defibrillators: Calendar year 2009—A world society of Arrhythmia’s project. PACE Pacing Clin. Electrophysiol. 2011, 34, 1013–1027. [Google Scholar] [CrossRef]
- Arnold, C.J.; Chu, V.H. Cardiovascular Implantable Electronic Device Infections. Infect. Dis. Clin. N. Am. 2018, 32, 811–825. [Google Scholar] [CrossRef]
- Brabham, W.W.; Wray, D.W.; Gold, M.R. Microbiologic characteristics and antimicrobial susceptibility of pacemaker/ICD infections: A moving target! J. Cardiovasc. Electrophysiol. 2012, 23, 382–383. [Google Scholar] [CrossRef]
- Karchmer, A.W.; Longworth, D.L. Infections of intracardiac devices. Infect. Dis. Clin. N. Am. 2002, 16, 477–505. [Google Scholar] [CrossRef]
- Jan, E.; Camou, F.; Texier-Maugein, J.; Whinnett, Z.; Caubet, O.; Ploux, S.; Pellegrin, J.-L.; Ritter, P.; Metayer, P.L.; Roudaut, R.; et al. Microbiologic Characteristics and In Vitro Susceptibility to Antimicrobials in a Large Population of Patients with Cardiovascular Implantable Electronic Device Infection. J. Cardiovasc. Electrophysiol. 2012, 23, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Maki, D.G. The prevention and management of device-related infection in infusion therapy. J. Med. 1980, 11, 239–253. [Google Scholar] [PubMed]
- Stickler, D.J. Bacterial biofilms and the encrustation of urethral catheters. Biofouling 1996, 9, 293–305. [Google Scholar] [CrossRef]
- Kirmusaoğlu, S.; Yurdugül, S.; Metin, A.; Vehid, S. The effect of urinary catheters on microbial biofilms and catheter associated urinary tract infections. Urol. J. 2017, 14, 3028–3034. [Google Scholar]
- Morales, D.K.; Hogan, D.A. Candida albicans interactions with bacteria in the context of human health and disease. PLoS Pathog. 2010, 6, e1000886. [Google Scholar] [CrossRef]
- Baena-Monroy, T.; Moreno-Maldonado, V.; Franco-Martínez, F.; Aldape-Barrios, B.; Quindós, G.; Sánchez-Vargas, L.O. Candida albicans, Staphylococcus aureus and Streptococcus mutans colonization in patients wearing dental prosthesis. Med. Oral Patol. Oral Cir. Bucal 2005, 10, E27–E39. [Google Scholar]
- Lebeaux, D.; Ghigo, J.M.; Beloin, C. Biofilm-Related Infections: Bridging the Gap between Clinical Management and Fundamental Aspects of Recalcitrance toward Antibiotics. Microb. Mol. Biol. Rev. 2014, 78, 3–9. [Google Scholar] [CrossRef]
- Klotz, S.A.; Chasin, B.S.; Powell, B.; Gaur, N.K.; Lipke, P.N. Polymicrobial bloodstream infections involving Candida species: Analysis of patients and review of the literature. Diagn. Microbiol. Infect. Dis. 2007, 59, 401–406. [Google Scholar] [CrossRef]
- Pammi, M.; Zhong, D.; Johnson, Y.; Revell, P.; Versalovic, J. Polymicrobial bloodstream infections in the neonatal intensive care unit are associated with increased mortality: A case-control study. BMC Infect. Dis. 2014, 14, 390. [Google Scholar] [CrossRef]
- Klotz, S.A.; Gaur, N.K.; De Armond, R.; Sheppard, D.; Khardori, N.; Edwards, J.E.; Lipke, P.N.; El-Azizi, M. Candida albicans Als proteins mediate aggregation with bacteria and yeasts. Med. Mycol. 2007, 45, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Marks, D.J.B.; Hyams, C.; Koo, C.Y.; Pavlou, M.; Robbins, J.; Koo, C.S.; Rodger, G.; Huggett, J.F.; Yap, J.; Macrae, M.B.; et al. Clinical features, microbiology and surgical outcomes of infective endocarditis: A 13-year study from a UK tertiary cardiothoracic referral centre. QJM 2015, 108, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Thuny, F.; Grisoli, D.; Collart, F.; Habib, G.; Raoult, D. Management of infective endocarditis: Challenges and perspectives. Lancet 2012, 379, 965–975. [Google Scholar] [CrossRef]
- Allison, D.L.; Scheres, N.; Willems, H.M.E.; Bode, C.S.; Krom, B.P.; Shirtliff, M.E. The host immune system facilitates disseminated Staphylococcus aureus disease due to phagocytic attraction to Candida albicans during coinfection: A case of bait and switch. Infect. Immun. 2019, 87, e00137-19. [Google Scholar] [CrossRef]
- Schlecht, L.M.; Peters, B.M.; Krom, B.P.; Freiberg, J.A.; Hänsch, G.M.; Filler, S.G.; Jabra-Rizk, M.A.; Shirtliff, M.E. Systemic Staphylococcus aureus infection mediated by Candida albicans hyphal invasion of mucosal tissue. Microbiology 2015, 161, 168–181. [Google Scholar] [CrossRef]
- Ovchinnikova, E.S.; Krom, B.P.; Busscher, H.J.; Van Der Mei, H.C. Evaluation of adhesion forces of Staphylococcus aureus along the length of Candida albicans hyphae. BMC Microbiol. 2012, 12, 281. [Google Scholar] [CrossRef]
- Sousa, C.; Botelho, C.; Rodrigues, D.; Azeredo, J.; Oliveira, R. Infective endocarditis in intravenous drug abusers: An update. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 2905–2910. [Google Scholar] [CrossRef]
- Ochieng, W.; Wanzala, P.; Bii, C.; Oishi, J.; Ichimura, H.; Lihana, R.; Mpoke, S.; Mwaniki, D.; Okoth, F.A. Tuberculosis and oral Candida species surveillance in HIV infected individuals in Northern Kenya, and the implications on tuberculin skin test screening for DOPT-P. E. Afr. Med. J. 2005, 82, 609. [Google Scholar] [CrossRef]
- Morales, D.K.; Jacobs, N.J.; Rajamani, S.; Krishnamurthy, M.; Cubillos-Ruiz, J.R.; Hogan, D.A. Antifungal mechanisms by which a novel Pseudomonas aeruginosa phenazine toxin kills Candida albicans in biofilms. Mol. Microbiol. 2010, 78, 1379–1392. [Google Scholar] [CrossRef]
- Douglas, L.J. Medical importance of biofilms in Candida infections. Rev. Iberoam. Micol. 2002, 19, 139–143. [Google Scholar]
- Metwalli, K.H.; Khan, S.A.; Krom, B.P.; Jabra-Rizk, M.A. Streptococcus mutans, Candida albicans, and the Human Mouth: A Sticky Situation. PLoS Pathog. 2013, 9, e1003616. [Google Scholar] [CrossRef] [PubMed]
- Stiefel, U.; Donskey, C.J. The Role of the Intestinal Tract as a Source for Transmission of Nosocomial Pathogens. Curr. Infect. Dis. Rep. 2004, 6, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Stuart, C.H.; Schwartz, S.A.; Beeson, T.J.; Owatz, C.B. Enterococcus faecalis: Its Role in Root Canal Treatment Failure and Current Concepts in Retreatment. J. Endod. 2006, 32, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Hermann, C.; Munzel, U.; Rüchel, R. Bacterial flora accompanying Candida yeasts in clinical specimens. Mycoses 1999, 42, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, E.J.C. Intra-Abdominal Anaerobic Infections: Bacteriology and Therapeutic Potential of Newer Antimicrobial Carbapenem, Fluoroquinolone, and Desfluoroquinolone Therapeutic Agents. Clin. Infect. Dis. 2002, 35, S106–S111. [Google Scholar] [CrossRef] [PubMed]
- Edey, M.; Hawley, C.M.; McDonald, S.P.; Brown, F.G.; Rosman, J.B.; Wiggins, K.J.; Bannister, K.M.; Johnson, D.W. Enterococcal peritonitis in Australian peritoneal dialysis patients: Predictors, treatment and outcomes in 116 cases. Nephrol. Dial. Transplant. 2010, 25, 1272–1278. [Google Scholar] [CrossRef] [PubMed]
- Barraclough, K.; Hawley, C.M.; McDonald, S.P.; Brown, F.G.; Rosman, J.B.; Wiggins, K.J.; Bannister, K.M.; Johnson, D.W. Polymicrobial Peritonitis in Peritoneal Dialysis Patients in Australia: Predictors, Treatment, and Outcomes. Am. J. Kidney Dis. 2010, 55, 121–131. [Google Scholar] [CrossRef]
- Dupont, H.; Paugam-Burtz, C.; Muller-Serieys, C.; Fierobe, L.; Chosidow, D.; Marmuse, J.P.; Mantz, J.; Desmonts, J.M.; Solomkin, J.S. Predictive factors of mortality due to polymicrobial peritonitis with Candida isolation in peritoneal fluid in critically III patients. Arch. Surg. 2002, 137, 1341–1346. [Google Scholar] [CrossRef]
- Orsi, C.F.; Sabia, C.; Ardizzoni, A.; Colombari, B.; Neglia, R.G.; Peppoloni, S.; Morace, G.; Blasi, E. Inhibitory effects of different lactobacilli on Candida albicans hyphal formation and biofilm development. J. Biol. Regul. Homeost. Agents 2014, 28, 743–752. [Google Scholar]
- Benadé, E.; Stone, W.; Mouton, M.; Postma, F.; Wilsenach, J.; Botha, A. Binary Interactions of Antagonistic Bacteria with Candida albicans Under Aerobic and Anaerobic Conditions. Microb. Ecol. 2016, 71, 645–659. [Google Scholar] [CrossRef]
- Tan, Y.; Leonhard, M.; Schneider-Stickler, B. Evaluation of culture conditions for mixed biofilm formation with clinically isolated non-albicans Candida species and Staphylococcus epidermidis on silicone. Microb. Pathog. 2017, 112, 215–220. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Junior, N.M.; Mendoza Marin, D.O.; Leite, A.R.P.; Pero, A.C.; Klein, M.I.; Compagnoni, M.A. Influence of the use of complete denture adhesives on microbial adhesion and biofilm formation by single- and mixed-species. PLoS ONE 2018, 13, e0203951. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.F.; Boas, D.; Haynes, K.; Henriques, M. The MNN2 Gene Knockout Modulates the Antifungal Resistance of Biofilms of Candida glabrata. Biomolecules 2018, 8, 130. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.F.; Henriques, M. Portrait of Matrix Gene Expression in Candida glabrata Biofilms with Stress Induced by Different Drugs. Genes 2018, 9, 205. [Google Scholar] [CrossRef] [PubMed]
- Kong, E.F.; Tsui, C.; Kucharkov, S.; Andes, D.; Van Dijck, P.; Jabra-Rizk, M.A. Commensal protection of Staphylococcus aureus against antimicrobials by Candida albicans biofilm matrix. MBio 2016, 7, e01365-16. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, X.; Huang, Y.; Zhao, G.; Lei, Y.; Ye, L.; Huang, Q.; Duan, W. Study on the Structure of Candida albicans–Staphylococcus epidermidis Mixed Species Biofilm on Polyvinyl Chloride Biomaterial. Cell Biochem. Biophys. 2015, 73, 461–468. [Google Scholar] [CrossRef]
- Bertolini, M.M.; Xu, H.; Sobue, T.; Nobile, C.J.; Del Bel Cury, A.A.; Dongari-Bagtzoglou, A. Candida-streptococcal mucosal biofilms display distinct structural and virulence characteristics depending on growth conditions and hyphal morphotypes. Mol. Oral Microbiol. 2015, 30, 307–322. [Google Scholar] [CrossRef]
- Seghir, A.; Boucherit-Otmani, Z.; Boucherit, K.; Sari-Belkharroubi, L.; Anselme-Bertrand, I. Évaluation du potentiel de formation de biofilms mixtes entre Candida albicans et quelques espèces bactériennes isolées de cathéters vasculaires périphériques au CHU de Tlemcen. Première étude en Algérie. J. Mycol. Med. 2015, 25, 123–129. [Google Scholar] [CrossRef]
- Camarillo-Márquez, O.; Córdova-Alcántara, I.M.; Hernández-Rodríguez, C.H.; García-Pérez, B.E.; Martínez-Rivera, M.A.; Rodríguez-Tovar, A.V. Antagonistic Interaction of Staphylococcus aureus Toward Candida glabrata During in vitro Biofilm Formation Is Caused by an Apoptotic Mechanism. Front. Microbiol. 2018, 9, 2031. [Google Scholar] [CrossRef]
- Martins, C.H.G.; Pires, R.H.; Cunha, A.O.; Pereira, C.A.M.; de Lacorte Singulani, J.; Abrão, F.; de Moraes, T.; Mendes-Giannini, M.J.S. Candida/Candida biofilms. First description of dual-species Candida albicans/C. rugosa biofilm. Fungal Biol. 2016, 120, 530–537. [Google Scholar] [CrossRef]
- Staib, F.; Grosse, G.; Mishra, S.K. Staphylococcus aureus and Candida albicans infection (animal experiments). Zentralbl. Bakteriol. Orig. A 1976, 234, 450–461. [Google Scholar] [PubMed]
- Carlson, E. Synergistic effect of Candida albicans and Staphylococcus aureus on mouse mortality. Infect. Immun. 1982, 38, 921–924. [Google Scholar] [PubMed]
- Peters, B.M.; Noverr, M.C. Candida albicans-Staphylococcus aureus Polymicrobial Peritonitis Modulates Host Innate Immunity. Infect. Immun. 2013, 81, 2178–2189. [Google Scholar] [CrossRef] [PubMed]
- Zago, C.E.; Silva, S.; Sanitá, P.V.; Barbugli, P.A.; Dias, C.M.I.; Lordello, V.B.; Vergani, C.E. Dynamics of biofilm formation and the Interaction between Candida albicans and methicillin-susceptible (MSSA) and-resistant Staphylococcus aureus (MRSA). PLoS ONE 2015, 10, e0123206. [Google Scholar] [CrossRef] [PubMed]
- Dutton, L.C.; Jenkinson, H.F.; Lamont, R.J.; Nobbs, A.H. Role of Candida albicans secreted aspartyl protease Sap9 in interkingdom biofilm formation. Pathog. Dis. 2016, 74, ftw005. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, Y.; Huang, Y.; Zhou, Y.; Zhao, G.; Ye, L.; Lei, Y.; Tang, Q. Function of intercellular adhesion a, fibrinogen binding protein, and accumulation-associated protein genes in formation of Staphylococcus epidermidis-Candida albicans mixed species biofilms. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2015, 29, 63–68. [Google Scholar] [PubMed]
- Krause, J.; Geginat, G.; Tammer, I. Prostaglandin E2 from Candida albicans Stimulates the Growth of Staphylococcus aureus in Mixed Biofilms. PLoS ONE 2015, 10, e0135404. [Google Scholar] [CrossRef]
- Kong, E.F.; Tsui, C.; Kucharíková, S.; Van Dijck, P.; Jabra-Rizk, M.A. Modulation of Staphylococcus aureus Response to Antimicrobials by the Candida albicans Quorum Sensing Molecule Farnesol. Antimicrob. Agents Chemother. 2017, 61, e01573-17. [Google Scholar] [CrossRef]
- de Carvalho Dias, K.; Barbugli, P.A.; de Patto, F.; Lordello, V.B.; de Aquino Penteado, L.; Medeiros, A.I.; Vergani, C.E. Soluble factors from biofilm of Candida albicans and Staphylococcus aureus promote cell death and inflammatory response. BMC Microbiol. 2017, 17, 146. [Google Scholar] [CrossRef]
- Haiko, J.; Saeedi, B.; Bagger, G.; Karpati, F.; Özenci, V. Coexistence of Candida species and bacteria in patients with cystic fibrosis. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1071–1077. [Google Scholar] [CrossRef]
- Uppuluri, P.; Lin, L.; Alqarihi, A.; Luo, G.; Youssef, E.G.; Alkhazraji, S.; Yount, N.Y.; Ibrahim, B.A.; Bolaris, M.A.; Edwards, J.E.; et al. The Hyr1 protein from the fungus Candida albicans is a cross kingdom immunotherapeutic target for Acinetobacter bacterial infection. PLoS Pathog. 2018, 14, e1007056. [Google Scholar] [CrossRef] [PubMed]
- Charlet, R.; Bortolus, C.; Barbet, M.; Sendid, B.; Jawhara, S. A decrease in anaerobic bacteria promotes Candida glabrata overgrowth while β-glucan treatment restores the gut microbiota and attenuates colitis. Gut Pathog. 2018, 10, 50. [Google Scholar] [CrossRef] [PubMed]
- Valentine, M.; Benadé, E.; Mouton, M.; Khan, W.; Botha, A. Binary interactions between the yeast Candida albicans and two gut-associated Bacteroides species. Microb. Pathog. 2019, 135, 103619. [Google Scholar] [CrossRef] [PubMed]
- Hoarau, G.; Mukherjee, P.K.; Gower-Rousseau, C.; Hager, C.; Chandra, J.; Retuerto, M.A.; Neut, C.; Vermeire, S.; Clemente, J.; Colombel, J.F.; et al. Bacteriome and Mycobiome Interactions Underscore Microbial Dysbiosis in Familial Crohn’s Disease. MBio 2016, 7, e01250-16. [Google Scholar] [CrossRef] [PubMed]
- Junka, A.; Szymczyk, P.; Ziółkowski, G.; Karuga-Kuzniewska, E.; Smutnicka, D.; Bil-Lula, I.; Bartoszewicz, M.; Mahabady, S.; Sedghizadeh, P.P. Bad to the Bone: On In Vitro and Ex Vivo Microbial Biofilm Ability to Directly Destroy Colonized Bone Surfaces without Participation of Host Immunity or Osteoclastogenesis. PLoS ONE 2017, 12, e0169565. [Google Scholar] [CrossRef] [PubMed]
- Deusenbery, C.B.; Kalan, L.; Meisel, J.S.; Gardner, S.E.; Grice, E.; Spiller, K.L. Human macrophage response to microbial supernatants from diabetic foot ulcers. Wound Repair Regen. 2019. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.A.; Tamer, T.M.; Rageh, A.A.; Abou-Zeid, A.M.; Abd El-Zaher, E.H.F.; Kenawy, E.-R. Insight into multidrug-resistant microorganisms from microbial infected diabetic foot ulcers. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 1261–1270. [Google Scholar] [CrossRef] [PubMed]
- Meto, A.; Colombari, B.; Sala, A.; Pericolini, E.; Meto, A.; Peppoloni, S.; Blasi, E. Antimicrobial and antibiofilm efficacy of a copper/calcium hydroxide-based endodontic paste against Staphylococcus aureus, Pseudomonas aeruginosa and Candida albicans. Dent. Mater. J. 2019, 38, 591–603. [Google Scholar] [CrossRef]
- Perinelli, D.R.; Petrelli, D.; Vitali, L.A.; Bonacucina, G.; Cespi, M.; Vllasaliu, D.; Giorgioni, G.; Palmieri, G.F. Quaternary Ammonium Leucine-Based Surfactants: The Effect of a Benzyl Group on Physicochemical Properties and Antimicrobial Activity. Pharmaceutics 2019, 11, 287. [Google Scholar] [CrossRef]
- Pourhajibagher, M.; Ghorbanzadeh, R.; Bahador, A. Antimicrobial properties of acrylic resins doped with Undaria pinnatifida exposed to light-emitting diode: In silico and in vitro assessments on multispecies biofilm-producing microbiota. Photodiagnosis Photodyn. Ther. 2019, 27, 210–215. [Google Scholar] [CrossRef]
- Ardizzoni, A.; Pericolini, E.; Paulone, S.; Orsi, C.F.; Castagnoli, A.; Oliva, I.; Strozzi, E.; Blasi, E. In vitro effects of commercial mouthwashes on several virulence traits of Candida albicans, viridans streptococci and Enterococcus faecalis colonizing the oral cavity. PLoS ONE 2018, 13, e0207262. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Leonhard, M.; Moser, D.; Ma, S.; Schneider-Stickler, B. Antibiofilm efficacy of curcumin in combination with 2-aminobenzimidazole against single- and mixed-species biofilms of Candida albicans and Staphylococcus aureus. Colloids Surfaces B Biointerfaces 2019, 174, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Bucci, A.R.; Marcelino, L.; Mendes, R.K.; Etchegaray, A. The antimicrobial and antiadhesion activities of micellar solutions of surfactin, {CTAB} and {CPCl} with terpinen-4-ol: Applications to control oral pathogens. World J. Microbiol. Biotechnol. 2018, 34, 86. [Google Scholar] [CrossRef] [PubMed]
- Medvedec Mikić, I.; Cigić, L.; Kero, D.; Kalibović Govorko, D.; Prpić Mehičić, G.; Tambić Andrašević, A.; Simeon, P. Antimicrobial effectiveness of polyhexamethylene biguanide on Enterococcus faecalis, Staphylococcus epidermidis and Candida albicans. Med. Glas. 2018, 15, 132–138. [Google Scholar]
- Kim, D.; Liu, Y.; Benhamou, R.I.; Sanchez, H.; Simón-Soro, Á.; Li, Y.; Hwang, G.; Fridman, M.; Andes, D.R.; Koo, H. Bacterial-derived exopolysaccharides enhance antifungal drug tolerance in a cross-kingdom oral biofilm. ISME J. 2018, 12, 1427–1442. [Google Scholar] [CrossRef] [PubMed]
- do Rosário Palma, A.L.; de Paula-Ramos, L.; Domingues, N.; Back-Brito, G.N.; de Oliveira, L.D.; Pereira, C.A.; Jorge, A.O.C. Biofilms of Candida albicans and Streptococcus sanguinis and their susceptibility to antimicrobial effects of photodynamic inactivation. Photodiagn. Photodyn. Ther. 2018, 24, 95–101. [Google Scholar] [CrossRef]
- Diogo, P.; Fernandes, C.; Caramelo, F.; Mota, M.; Miranda, I.M.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Uliana, M.P.; de Oliveira, K.T.; Santos, J.M.; et al. Antimicrobial Photodynamic Therapy against Endodontic Enterococcus faecalis and Candida albicans Mono and Mixed Biofilms in the Presence of Photosensitizers: A Comparative Study with Classical Endodontic Irrigants. Front. Microbiol. 2017, 8, 498. [Google Scholar] [CrossRef]
- Yassin, S.A.; German, M.J.; Rolland, S.L.; Rickard, A.H.; Jakubovics, N.S. Inhibition of multispecies biofilms by a fluoride-releasing dental prosthesis copolymer. J. Dent. 2016, 48, 62–70. [Google Scholar] [CrossRef]
- Shehatou, C.; Logunov, S.L.; Dunman, P.M.; Haidaris, C.G.; Klubben, W.S. Characterizing the Antimicrobial Properties of 405 nm Light and the Corning Light-Diffusing Fiber Delivery System. Lasers Surg. Med. 2019, 51, lsm.23132. [Google Scholar] [CrossRef]
- Bucki, R.; Niemirowicz-Laskowska, K.; Deptuła, P.; Wilczewska, A.Z.; Misiak, P.; Durnaś, B.; Fiedoruk, K.; Piktel, E.; Mystkowska, J.; Janmey, P.A. Susceptibility of microbial cells to the modified PIP2-binding sequence of gelsolin anchored on the surface of magnetic nanoparticles. J. Nanobiotechnol. 2019, 17, 81. [Google Scholar] [CrossRef]
- Scala, A.; Piperno, A.; Hada, A.; Astilean, S.; Vulpoi, A.; Ginestra, G.; Marino, A.; Nostro, A.; Zammuto, V.; Gugliandolo, C. Marine Bacterial Exopolymers-Mediated Green Synthesis of Noble Metal Nanoparticles with Antimicrobial Properties. Polymers 2019, 11, 1157. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, Y.; Ishikawa, T.; Takahashi, C.; Masuda, M. A Short Peptide Derived from the ZorO Toxin Functions as an Effective Antimicrobial. Toxins 2019, 11, 392. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.O.; Graham, C.E.; Cruz, M.R.; Singh, K.V.; Murray, B.E.; Lorenz, M.C.; Garsin, D.A. Antifungal Activity of the Enterococcus faecalis Peptide EntV Requires Protease Cleavage and Disulfide Bond Formation. MBio 2019, 10, e01334-19. [Google Scholar] [CrossRef] [PubMed]
- Feldman, M.; Shenderovich, J.; Lavy, E.; Friedman, M.; Steinberg, D. A Sustained-Release Membrane of Thiazolidinedione-8: Effect on Formation of a Candida/Bacteria Mixed Biofilm on Hydroxyapatite in a Continuous Flow Model. BioMed Res. Int. 2017, 2017, 3510124. [Google Scholar] [CrossRef] [PubMed]
- Raorane, C.J.; Lee, J.-H.; Kim, Y.-G.; Rajasekharan, S.K.; García-Contreras, R.; Lee, J. Antibiofilm and Antivirulence Efficacies of Flavonoids and Curcumin Against Acinetobacter baumannii. Front. Microbiol. 2019, 10, 990. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Zou, L.; Wu, J.; Liu, H.; Luo, T.; Zhou, X.; Li, W.; Ren, B. Voriconazole inhibits cross-kingdom interactions between Candida albicans and Actinomyces viscosus through the ergosterol pathway. Int. J. Antimicrob. Agents 2019, 53, 805–813. [Google Scholar] [CrossRef]
- Jafri, H.; Khan, M.S.A.; Ahmad, I. In vitro efficacy of eugenol in inhibiting single and mixed-biofilms of drug-resistant strains of Candida albicans and Streptococcus mutans. Phytomedicine 2019, 54, 206–213. [Google Scholar] [CrossRef]
- de Alteriis, E.; Lombardi, L.; Falanga, A.; Napolano, M.; Galdiero, S.; Siciliano, A.; Carotenuto, R.; Guida, M.; Galdiero, E. Polymicrobial antibiofilm activity of the membranotropic peptide gH625 and its analogue. Microb. Pathog. 2018, 125, 189–195. [Google Scholar] [CrossRef]
- Elshinawy, M.I.; Al-Madboly, L.A.; Ghoneim, W.M.; El-Deeb, N.M. Synergistic Effect of Newly Introduced Root Canal Medicaments-Ozonated Olive Oil and Chitosan Nanoparticles, Against Persistent Endodontic Pathogens. Front. Microbiol. 2018, 9, 1371. [Google Scholar] [CrossRef]
- Rogiers, O.; Holtappels, M.; Siala, W.; Lamkanfi, M.; Van Bambeke, F.; Lagrou, K.; Van Dijck, P.; Kucharíková, S. Anidulafungin increases the antibacterial activity of tigecycline in polymicrobial Candida albicans/Staphylococcus aureus biofilms on intraperitoneally implanted foreign bodies. J. Antimicrob. Chemother. 2018, 73, 2806–2814. [Google Scholar] [CrossRef]
- Waite, R.D.; Stewart, J.E.; Stephen, A.S.; Allaker, R.P. Activity of a nitric oxide-generating wound treatment system against wound pathogen biofilms. Int. J. Antimicrob. Agents 2018, 52, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Scaffaro, R.; Lopresti, F.; D’Arrigo, M.; Marino, A.; Nostro, A. Efficacy of poly(lactic acid)/carvacrol electrospun membranes against Staphylococcus aureus and Candida albicans in single and mixed cultures. Appl. Microbiol. Biotechnol. 2018, 102, 4171–4181. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, D.R.; Arias, L.S.; Fernandes, R.A.; Straioto, F.G.; Barros Barbosa, D.; Pessan, J.P.; Delbem, A.C.B. Role of tyrosol on Candida albicans, Candida glabrata and Streptococcus mutans biofilms developed on different surfaces. Am. J. Dent. 2017, 30, 35–39. [Google Scholar] [PubMed]
- Arias, L.S.; Delbem, A.C.B.; Fernandes, R.A.; Barbosa, D.B.; Monteiro, D.R. Activity of tyrosol against single and mixed-species oral biofilms. J. Appl. Microbiol. 2016, 120, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
- Hassan Abdel-Rhman, S.; Mostafa El-Mahdy, A.; El-Mowafy, M. Effect of Tyrosol and Farnesol on Virulence and Antibiotic Resistance of Clinical Isolates of Pseudomonas aeruginosa. BioMed Res. Int. 2015, 2015, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.A.; Monteiro, D.R.; Arias, L.S.; Fernandes, G.L.; Delbem, A.C.B.; Barbosa, D.B. Biofilm formation by Candida albicans and Streptococcus mutans in the presence of farnesol: A quantitative evaluation. Biofouling 2016, 32, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Leonhard, M.; Moser, D.; Ma, S.; Schneider-Stickler, B. Long-term antibiofilm activity of carboxymethyl chitosan on mixed biofilm on silicone. Laryngoscope 2016, 126, E404–E408. [Google Scholar] [CrossRef]
- Tan, Y.; Leonhard, M.; Moser, D.; Ma, S.; Schneider-Stickler, B. Inhibition of mixed fungal and bacterial biofilms on silicone by carboxymethyl chitosan. Colloids Surfaces B Biointerfaces 2016, 148, 193–199. [Google Scholar] [CrossRef]
- Tan, Y.; Leonhard, M.; Ma, S.; Moser, D.; Schneider-Stickler, B. Efficacy of carboxymethyl chitosan against Candida tropicalis and Staphylococcus epidermidis monomicrobial and polymicrobial biofilms. Int. J. Biol. Macromol. 2018, 110, 150–156. [Google Scholar] [CrossRef]
- Tan, Y.; Leonhard, M.; Moser, D.; Schneider-Stickler, B. Inhibition activity of Lactobacilli supernatant against fungal-bacterial multispecies biofilms on silicone. Microb. Pathog. 2017, 113, 197–201. [Google Scholar] [CrossRef]
- Budzyńska, A.; Różalska, S.; Sadowska, B.; Różalska, B. Candida albicans/Staphylococcus aureus Dual-Species Biofilm as a Target for the Combination of Essential Oils and Fluconazole or Mupirocin. Mycopathologia 2017, 182, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Pekmezovic, M.; Aleksic, I.; Barac, A.; Arsic-Arsenijevic, V.; Vasiljevic, B.; Nikodinovic-Runic, J.; Senerovic, L. Prevention of polymicrobial biofilms composed of Pseudomonas aeruginosa and pathogenic fungi by essential oils from selected Citrus species. Pathog. Dis. 2016, 74, ftw102. [Google Scholar] [CrossRef] [PubMed]
- Soliman, S.S.M.; Semreen, M.H.; El-Keblawy, A.A.; Abdullah, A.; Uppuluri, P.; Ibrahim, A.S. Assessment of herbal drugs for promising anti-Candida activity. BMC Complement. Altern. Med. 2017, 17, 257. [Google Scholar] [CrossRef] [PubMed]
- Peeters, E.; Hooyberghs, G.; Robijns, S.; Waldrant, K.; De Weerdt, A.; Delattin, N.; Liebens, V.; Kucharíková, S.; Tournu, H.; Verstraeten, N.; et al. Modulation of the Substitution Pattern of 5-Aryl-2-Aminoimidazoles Allows Fine-Tuning of Their Antibiofilm Activity Spectrum and Toxicity. Antimicrob. Agents Chemother. 2016, 60, 6483–6497. [Google Scholar] [CrossRef]
- Lown, L.; Peters, B.M.; Walraven, C.J.; Noverr, M.C.; Lee, S.A. An Optimized Lock Solution Containing Micafungin, Ethanol and Doxycycline Inhibits Candida albicans and Mixed C. albicans—Staphylococcus aureus Biofilms. PLoS ONE 2016, 11, e0159225. [Google Scholar] [CrossRef]
- Qu, Y.; Locock, K.; Verma-Gaur, J.; Hay, I.D.; Meagher, L.; Traven, A. Searching for new strategies against polymicrobial biofilm infections: Guanylated polymethacrylates kill mixed fungal/bacterial biofilms. J. Antimicrob. Chemother. 2016, 71, 413–421. [Google Scholar] [CrossRef]
- Ariani, N.; Visser, A.; Teulings, M.R.I.M.; Dijk, M.; Rahardjo, T.B.W.; Vissink, A.; van der Mei, H.C. Efficacy of cleansing agents in killing microorganisms in mixed species biofilms present on silicone facial prostheses—An in vitro study. Clin. Oral Investig. 2015, 19, 2285–2293. [Google Scholar] [CrossRef]
- Ganske, K.; Wiegand, C.; Hipler, U.-C.; Heinze, T. Synthesis of Novel Cellulose Carbamates Possessing Terminal Amino Groups and Their Bioactivity. Macromol. Biosci. 2016, 16, 451–461. [Google Scholar] [CrossRef]
- Zielińska, S.; Wójciak-Kosior, M.; Dziągwa-Becker, M.; Gleńsk, M.; Sowa, I.; Fijałkowski, K.; Rurańska-Smutnicka, D.; Matkowski, A.; Junka, A. The Activity of Isoquinoline Alkaloids and Extracts from Chelidonium majus against Pathogenic Bacteria and Candida sp. Toxins 2019, 11, 406. [Google Scholar] [CrossRef]
- Olejar, K.J.; Ricci, A.; Swift, S.; Zujovic, Z.; Gordon, K.C.; Fedrizzi, B.; Versari, A.; Kilmartin, P.A. Characterization of an Antioxidant and Antimicrobial Extract from Cool Climate, White Grape Marc. Antioxidants 2019, 8, 232. [Google Scholar] [CrossRef]
- Rodrigues, C.; Silva, S.; Azeredo, J.; Henriques, M. Detection and Quantification of Fluconazole Within Candida glabrata Biofilms. Mycopathologia 2015, 179, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Kagan, S.; Jabbour, A.; Sionov, E.; Alquntar, A.A.; Steinberg, D.; Srebnik, M.; Nir-Paz, R.; Weiss, A.; Polacheck, I. Anti-Candida albicans biofilm effect of novel heterocyclic compounds. J. Antimicrob. Chemother. 2014, 69, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Feldman, M.; Al-Quntar, A.; Polacheck, I.; Friedman, M.; Steinberg, D. Therapeutic Potential of Thiazolidinedione-8 as an Antibiofilm Agent against Candida albicans. PLoS ONE 2014, 9, e93225. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Leonhard, M.; Moser, D.; Ma, S.; Schneider-Stickler, B. Inhibitory effect of probiotic lactobacilli supernatants on single and mixed non- albicans Candida species biofilm. Arch. Oral Biol. 2018, 85, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Rocha, G.R.; Salamanca, E.J.F.; de Barros, A.L.; Lobo, C.I.V.; Klein, M.I. Effect of tt-farnesol and myricetin on in vitro biofilm formed by Streptococcus mutans and Candida albicans. BMC Complementary Altern. Med. 2018, 18, 61. [Google Scholar] [CrossRef]
- Černáková, L.; Jordao, L.; Bujdáková, H. Impact of Farnesol and Corsodyl ® on Candida albicans Forming Dual Biofilm With Streptococcus mutans. Oral Dis. 2018, 24, 6–9. [Google Scholar]
- Cateau, E.; Rodier, M.-H.; Imbert, C. In vitro efficacies of caspofungin or micafungin catheter lock solutions on Candida albicans biofilm growth. J. Antimicrob. Chemother. 2008, 62, 153–155. [Google Scholar] [CrossRef]
- Visek, J.; Ryskova, L.; Safranek, R.; Lasticova, M.; Blaha, V. In vitro comparison of efficacy of catheter locks in the treatment of catheter related blood stream infection. Clin. Nutr. ESPEN 2019, 30, 107–112. [Google Scholar] [CrossRef]
- Chandra, J.; Long, L.; Isham, N.; Mukherjee, P.K.; DiSciullo, G.; Appelt, K.; Ghannoum, M.A. In Vitro and In Vivo Activity of a Novel Catheter Lock Solution against Bacterial and Fungal Biofilms. Antimicrob. Agents Chemother. 2018, 62, e00722-18. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).