Oral Candidiasis: A Disease of Opportunity
Abstract
1. Introduction
1.1. Candida and Candidiasis Etymology and Historical Perspective
1.2. Candida albicans: An Opportunistic Pathogen
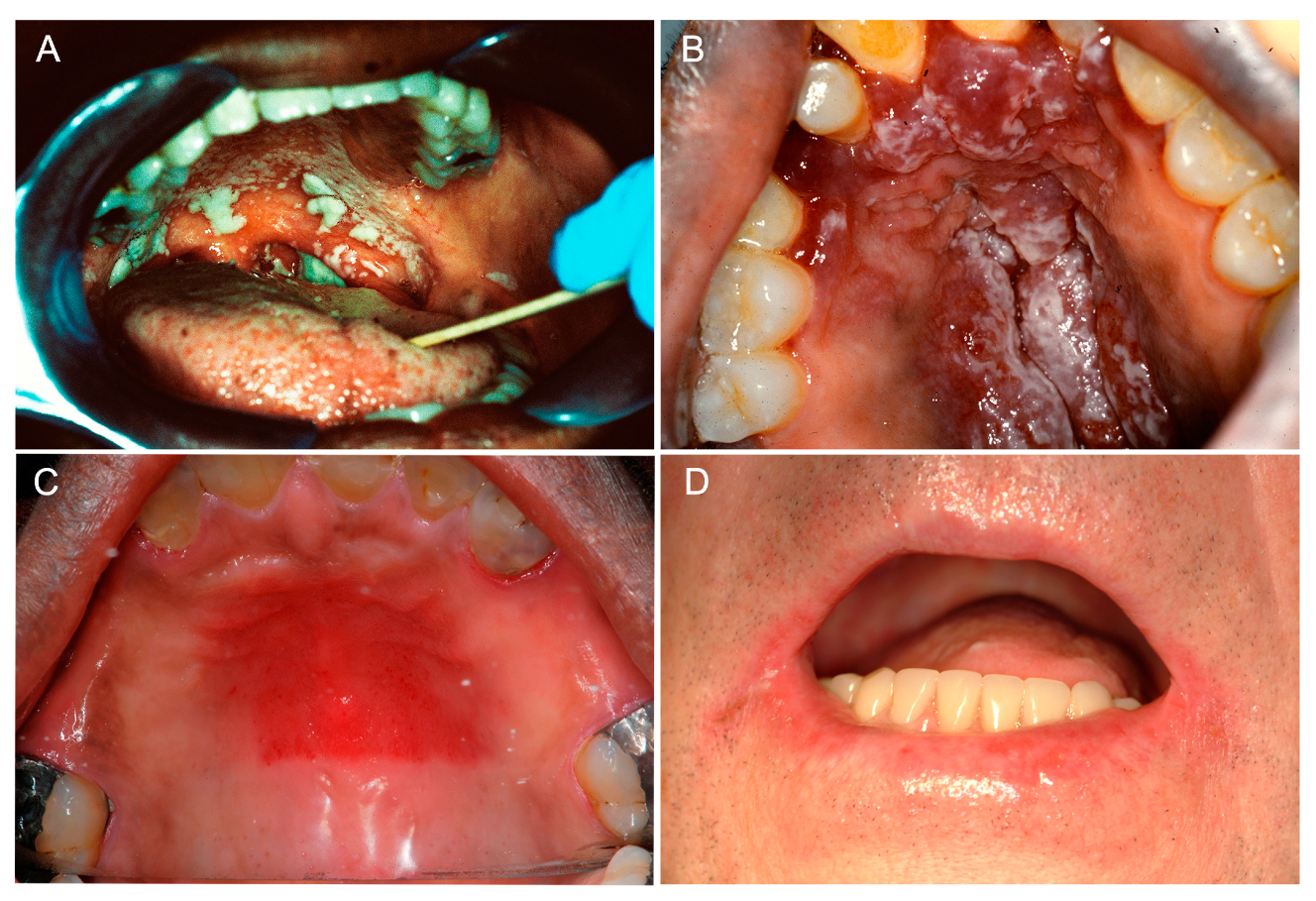
2. Clinical Manifestations of Oral Candidiasis
2.1. Acute Manifestations of Oral Candidiasis
2.1.1. Acute Pseudomembranous Candidiasis
2.1.2. Acute Erythematous Candidiasis
2.2. Chronic Manifestations of Oral Candidiasis
2.2.1. Chronic Erythematous Atrophic Candidiasis
2.2.2. Angular Cheilitis
2.2.3. Cheilocandidiasis
2.2.4. Chronic Hyperplastic Candidiasis
2.2.5. Median Rhomboid Glossitis
2.3. Chronic Mucocutaneous Candidiasis Syndromes
3. Predisposing Factors to Oral Candidiasis
3.1. Local Factors
3.1.1. Salivary Hypofunction
3.1.2. Denture Wearing
3.1.3. Topical Corticosteroid Therapy
3.1.4. Smoking
3.2. Systemic Factors
3.2.1. Age-Related Immunosenescence
3.2.2. Broad-Spectrum Antibiotics
3.2.3. HIV Infection and AIDS
3.2.4. Systemic Immunocompromise
3.2.5. Nutritional Deficiencies
4. Host Immune Response
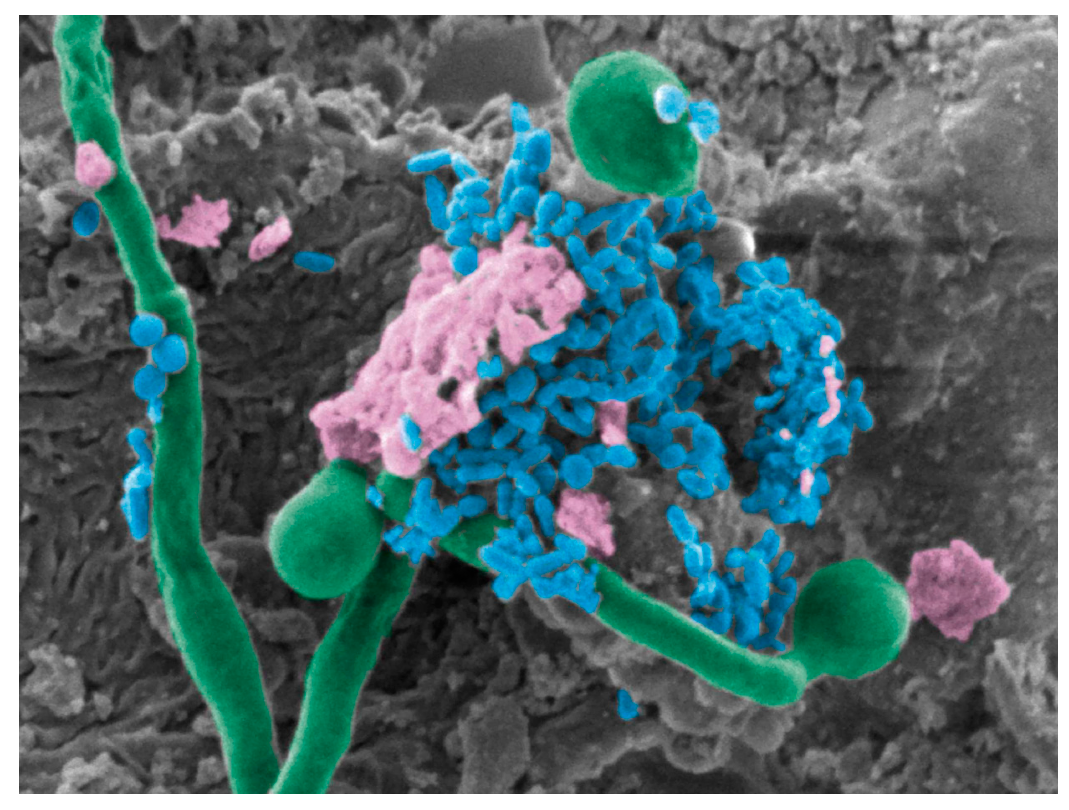
5. Candida albicans–Bacterial Interactions in the Oral Cavity
6. Animal Models
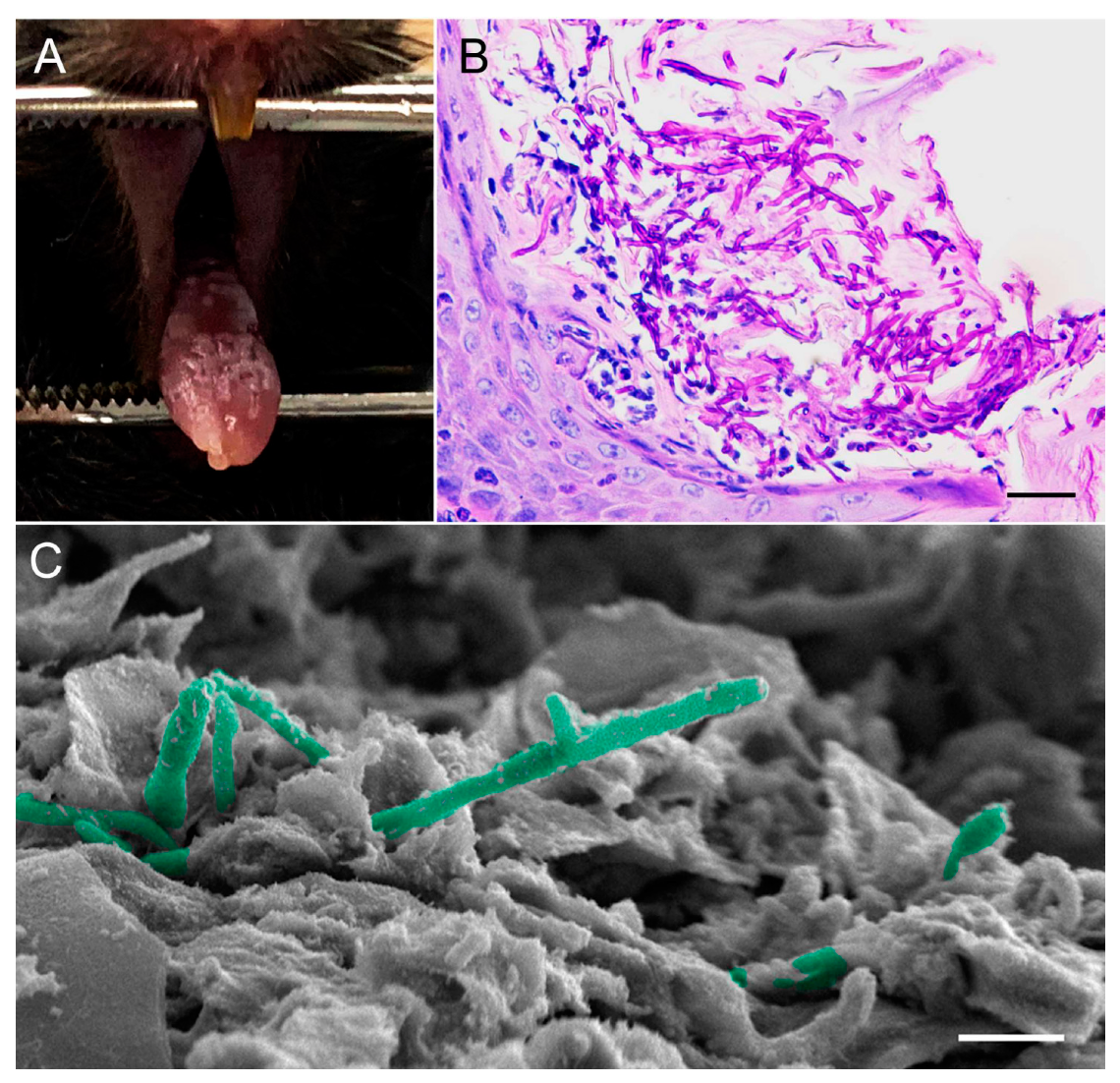
6.1. Mouse Model of Oropharyngeal Candidiasis
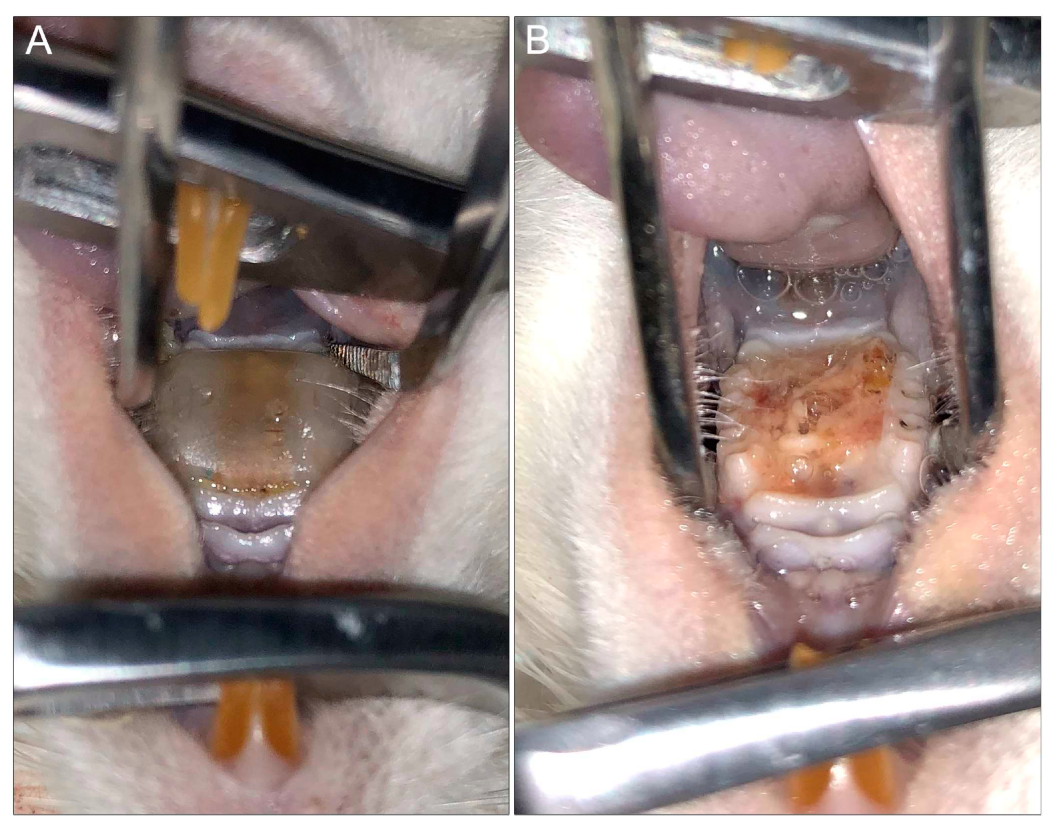
6.2. Rat Model of Denture Stomatitis
7. New Approaches in Antifungal Drug Discovery Anti-Virulence Drugs
8. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Millsop, J.W.; Faze, L.N. Oral candidiasis. Clin. Dermatol. 2016, 34, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Verma, R.; Murari, A.; Agrawal, A. Oral candidiasis: An overview. J. Oral Maxillofac. Pathol. 2014, 18, S81–S85. [Google Scholar] [PubMed]
- Hellstein, J.W.; Marek, C.L. Candidiasis: Red and white manifestations in the oral cavity. Head Neck Pathol. 2019, 13, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Hippocrates, C.-B. Epidemics; Williams and Wilkins: Baltimore, MD, USA, 1939; Volume 3. [Google Scholar]
- Knoke, M.; Bernhardt, H. The first description of an oesophageal candidosis by Bernhard von Langenbeck in 1839. Mycoses 2006, 49, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Calderone, R.A. Introduction and historical perspectives. In Candida and Candidiasis; Calderon, R.A., Ed.; ASM Press: Washington, DC, USA, 2002; pp. 3–13. [Google Scholar]
- Lynch, D.P. Oral candidiasis. History, classification, and clinical presentation. Oral Surg. Oral Med. Oral Pathol. 1994, 78, 189–193. [Google Scholar] [CrossRef]
- Barnett, J.A. A history of research on yeasts 8: Taxonomy. Yeast 2004, 21, 1141–1932. [Google Scholar] [CrossRef]
- Samaranayake, L.P. Oral mycoses in HIV infection. Oral Surg. Oral Med. Oral Pathol. 1992, 73, 171–180. [Google Scholar] [CrossRef]
- Fidel, P.L.J. Candida-host interactions in HIV disease implications for oropharyngeal candidiasis. Adv. Dent. Res. 2011, 23, 45–49. [Google Scholar] [CrossRef]
- Fidel, P.L., Jr. Candida-host interactions in HIV disease: Relationships in oropharyngeal candidiasis. Adv. Dent. Res. 2006, 19, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Majumdar, B.; Sarode, S.C.; Sarode, G.S.; Awan, K.H. Oropharyngeal candidosis in HIV-infected patients-An update. Front. Microbiol. 2018, 9, 980. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y. Looking into Candida albicans infection, host response, and antifungal strategies. Virulence 2015, 6, 307–308. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.A.O.; Williams, D.W. Diagnosis and management of oral candidosis. Br. Dent. J. 2017, 223, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.; Lewis, M. Pathogenesis and treatment of oral candidosis. J. Oral Microbiol. 2011, 3. [Google Scholar] [CrossRef] [PubMed]
- Naglik, J.R.; Moyes, D.L.; Waächtler, B.; Hube, B. Candida albicans interactions with epithelial cells and mucosal immunity. Microbes Infect. 2011, 13, 963–976. [Google Scholar] [CrossRef] [PubMed]
- Southern, P.; Horbul, J.; Maher, D.; Davis, D.A.C. albicans colonization of human mucosal surfaces. PLoS ONE 2008, 3, e2067. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.W.; Jordan, R.P.; Wei, X.-Q.; Alves, C.T.; Wise, M.P.; Wilson, M.J.; Lewis, M.A. Interactions of Candida albicans with host epithelial surfaces. J. Oral Microbiol. 2013, 5. [Google Scholar] [CrossRef]
- Fidel, P.J. Immunity to Candida. Oral Dis. 2002, 8, 69–75. [Google Scholar] [CrossRef]
- Mason, K.L.; Erb Downward, J.R.; Mason, K.D.; Falkowski, N.R.; Eaton, K.A.; Kao, J.Y.; Young, V.B.; Huffnagle, G.B. Candida albicans and bacterial microbiota interactions in the cecum during recolonization following broad-spectrum antibiotic therapy. Infect. Immun. 2012, 80, 3371–3380. [Google Scholar] [CrossRef]
- Liu, M.-B.; Xu, S.R.; He, Y.; Deng, G.-H.; Sheng, H.-F.; Huang, X.-M.; Ouyang, C.-Y.; Zhou, H.-W. Diverse vaginal microbiomes in reproductive-age women with vulvovaginal candidiasis. PLoS ONE 2013, 8, e79812. [Google Scholar] [CrossRef]
- Patil, S.; Rao, R.S.; Majumdar, B.; Anil, S. Clinical appearance of oral Candida infection and therapeutic strategies. Front. Microbiol. 2015, 6, 1391. [Google Scholar] [CrossRef]
- Jabra-Rizk, M.A.; Kong, E.; Tsui, C.; Nguyen, M.; Clancy, C.; Fidel, P.; Noverr, M. Candida albicans pathogenesis: Fitting within the host-microbe damage response framework. Infect. Immun. 2016, 84, 2724–2739. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.; Diekema, D. Epidemiology of invasive candidiasis: A persistent public health problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, S.; Mitchell, A. Mucosal biofilms of Candida albicans. Curr. Opin. Microbiol. 2011, 14, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Ellis, M. Invasive fungal infections: Evolving challenges for diagnosis and therapeutics. Mol. Immunol. 2002, 38, 947–957. [Google Scholar] [CrossRef]
- Höfs, S.; Mogavero, S.; Hube, B. Interaction of Candida albicans with host cells: Virulence factors, host defense, escape strategies and the microbiota. J. Microbiol. 2016, 53, 149–169. [Google Scholar] [CrossRef]
- Mayer, F.L.; Wilson, D.; Hube, B. Candida albicans pathogenecity mechanisms. Virulence 2013, 4, 119–128. [Google Scholar] [CrossRef]
- Hoyer, L.L. The ALS gene family of Candida albicans. Trends Microbiol. 2001, 9, 176–180. [Google Scholar] [CrossRef]
- Murciano, C.; Moyes, D.L.; Runglall, M.; Tobouti, P.; Islam, A.; Hoyer, L.L. Evaluation of the role of Candida albicans agglutinin-like sequence (Als) proteins in human oral epithelial cell interactions. PLoS ONE 2012, 7, e33362. [Google Scholar] [CrossRef]
- Phan, Q.T.; Myers, C.L.; Fu, F.; Sheppard, D.C.; Yeaman, M.R.; Welch, W.H.; Ibrahim, A.S.; Edwards, J.E., Jr.; Filler, S.G. Als3 Is a Candida albicans invasin that binds to cadherins and induces endocytosis by host cells. PLoS Biol. 2007, 5, e64. [Google Scholar] [CrossRef]
- de Groot, P.W.; Bader, O.; de Boer, A.D.; Weig, M.; Chauhan, N. Adhesins in human fungal pathogens: Glue with plenty of stick. Eukaryot. Cell 2013, 12, 470–481. [Google Scholar] [CrossRef]
- Silverman, R.J.; Nobbs, A.H.; Vickerman, M.M.; Barbour, M.E.; Jenkinson, H.F. Interaction of Candida albicans cell wall Als3 protein with Streptococcus gordonii AspB adhesin promotes development of mixed-species communities. Infect. Immun. 2010, 78, 4644–4652. [Google Scholar] [CrossRef] [PubMed]
- Peters, B.; Ovchinnikova, E.; Schlecht, L.; Hoyer, L.; Busscher, H.; van der Mei, H.; Krom, B.; Jabra-Rizk, M.; Shirtliff, M. Staphylococcus aureus adherence to Candida albicans hyphae is mediated by the hyphal adhesin Als3p. Microbiology 2012, 158, 2975–2986. [Google Scholar] [CrossRef] [PubMed]
- Swidergall, M.; Filler, S.G. Oropharyngeal candidiasis: Fungal invasion and epithelial cell responses. PLoS Pathog. 2017, 13, e1006056. [Google Scholar] [CrossRef] [PubMed]
- Sundstrom, P. Adhesion in Candida spp. Cell. Microbiol. 2002, 4, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Desai, J.V. Candida albicans hyphae: From growth initiation to invasion. J. Fungi 2018, 4, 10. [Google Scholar] [CrossRef]
- Wall, G.; Montelongo-Jauregui, D.; Vidal Bonifacio, B.; Lopez-Ribot, J.L.; Uppuluri, P. Candida albicans biofilm growth and dispersal: Contributions to pathogenesis. Curr. Opin. Microbiol. 2019, 11, 1–6. [Google Scholar] [CrossRef]
- Calderone, R.E. Candida and Candidiasis, 2nd ed.; ASM Press: Washington, DC, USA, 2012. [Google Scholar]
- Mathe, L.; Van Dijck, P. Recent insights into Candida albicans biofilm resistance. Curr. Genet. 2013, 59, 251–264. [Google Scholar] [CrossRef]
- Nett, J.; Andes, D. Candida albicans biofilm development, modeling a host-pathogen interaction. Curr. Opin. Microbiol. 2006, 9, 340–345. [Google Scholar] [CrossRef]
- Tsui, C.; Kong, E.; Jabra-Rizk, M. Pathogenesis of Candida albicans Biofilm. Pathog. Dis. 2016, 74, ftw018. [Google Scholar] [CrossRef]
- Phan, Q.T.; Belanger, P.H.; Filler, S.G. Role of hyphal formation in interactions of Candida albicans with endothelial cells. Infect. Immun. 2000, 68, 3485–3490. [Google Scholar] [CrossRef]
- Kadosh, D. Regulatory mechanisms controlling morphology and pathogenesis in Candida albicans. Curr. Opin. Microbiol. 2019, 52, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Lo, H.L.; Kohler, J.R.; DiDomenico, D.; Loebenberg, D.; Cacciapuoti, A.; Fink, G.R. Nonfilamentous C. albicans mutants are avirulent. Cell 1997, 90, 939–949. [Google Scholar] [CrossRef]
- Schaller, M.; Borelli, C.; Korting, H.C.; Hube, B. Hydrolytic enzymes as virulence factors of Candida albicans. Mycoses 2005, 48, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Naglik, J.R.; Challacombe, S.J.; Hube, B. Candida albicans secreted aspartyl proteinases in virulence and pathogenesis. Microbiol. Mol. Biol. Rev. 2003, 67, 400–428. [Google Scholar] [CrossRef]
- Naglik, J.R.; Gaffen, S.L.; Hube, B. Candidalysin: Discovery and function in Candida albicans infections. Curr. Opin. Microbiol. 2019, 52, 100–109. [Google Scholar] [CrossRef]
- Swidergall, M.; Khalaji, M.; Solis, N.V.; Moyes, D.L.; Drummond, R.A.; Hube, B.; Lionakis, M.S.; Murdoch, C.; Filler, S.G.; Naglik, J.R. Candidalysin is required for neutrophil recruitment and virulence during systemic Candida albicans infection. J. Infect. Dis. 2019, 220, 1477–1488. [Google Scholar] [CrossRef]
- Fox, E.; Bui, C.; Nett, J.; Hartooni, N.; Mui, M.; Andes, D.; Nobile, C.; Johnson, A. An expanded regulatory network temporally controls Candida albicans biofilm formation. Mol. Microbiol. 2015, 96, 1226–1239. [Google Scholar] [CrossRef]
- Nett, J.; Andes, D. Fungal Biofilms: In vivo models for discovery of anti-biofilm drugs. Microbiol. Spectr. 2015, 3. [Google Scholar] [CrossRef]
- Finkel, J.; Mitchell, A. Genetic control of Candida albicans biofilm development. Nat. Rev. Microbiol. 2011, 9, 109–118. [Google Scholar] [CrossRef]
- Tournu, H.; Van Dijck, P. Candida biofilms and the host: Models and new concepts for eradication. Int. J. Microbiol. 2012, 845352. [Google Scholar] [CrossRef]
- Costerton, J.W.; Montanaro, L.; Arciola, C.R. Biofilm in implant infections: Its production and regulation. Int. J. Artif. Org. 2005, 28, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K. Riddle of biofilm resistance. Antimicrob. Agents Chemother. 2001, 45, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Ghannoum, M.; Roilides, E.; Katragkou, A.; Petraitis, V.; Walsh, T. The role of echinocandins in Candida biofilm–related vascular catheter infections: In vitro and in vivo model systems. Clin. Infect. Dis. 2015, 61, S618–S621. [Google Scholar] [CrossRef] [PubMed]
- Hirota, K.; Yumoto, H.; Sapaar, B.; Matsuo, T.; Ichikawa, T.; Miyake, Y. Pathogenic factors in Candida biofilm-related infectious diseases. J. Appl. Microbiol. 2017, 122, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Al-Fattani, M.; Douglas, L. Biofilm matrix of Candida albicans and Candida tropicalis: Chemical composition and role in drug resistance. J. Med. Microbiol. 2006, 55, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Nett, J.; Lincoln, L.; Marchillo, K.; Massey, R.; Holoyda, K.; Hoff, B.; VanHandel, M.; Andes, D. Putative role of beta-1,3 glucans in Candida albicans biofilm resistance. Antimicrob. Agents Chemother. 2007, 51, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Nett, J.E.; Sanchez, H.; Andes, D.R. Interface of Candida albicans biofilm matrix-associated drug resistance and cell wall integrity regulation. Eukaryot. Cell 2011, 10, 1660–1669. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, K.F.; Zarnowskia, R.; Sancheza, H.; Edwarda, J.A.; Reinickea, E.L.; Netta, J.E.; Mitchell, A.P.; Andes, D.R. Community participation in biofilm matrix assembly and function. Proc. Nat. Acad. Sci. USA 2015, 112, 4092–4097. [Google Scholar] [CrossRef] [PubMed]
- Jabra-Rizk, M. Pathogenesis of polymicrobial biofilms. Open Mycol. J. 2011, 5, 39–43. [Google Scholar] [CrossRef]
- Nett, J.E.; Marchillo, K.; Spiegel, C.A.; Andes, D.R. Development and validation of an in vivo Candida albicans biofilm denture model. Infect. Immun. 2010, 78, 3650–3659. [Google Scholar] [CrossRef]
- Taff, H.; Mitchell, K.; Edward, J.; Andes, D. Mechanisms of Candida biofilm drug resistance. Future Microbiol. 2013, 8, 1325–1337. [Google Scholar] [CrossRef] [PubMed]
- Harriott, M.M.; Noverr, M.C. Importance of Candida-bacterial polymicrobial biofilms in disease. Trends Microbiol. 2011, 19, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Sultan, A.S.; Kong, E.F.; Rizk, A.M.; Jabra-Rizk, M.A. The oral microbiome: A lesson in co-existence. PLoS Pathog. 2018, 14, e1006719. [Google Scholar] [CrossRef] [PubMed]
- Scully, C.; Giovanni, L. Oral Candidosis. European Association of Oral Medicine. Available online: http://www.eaom.eu/pdf/content/oral_candidosis.pdf (accessed on 5 November 2019).
- Garcia-Cuesta, C.; Sarrion Pérez, M.G.; Bagán, J.V. Current treatment of oral candidiasis: A literature review. J. Clin. Exp. Dent. 2014, 6, e576–e582. [Google Scholar] [CrossRef]
- Peters, E.S., Jr.; Eisenberg, E. Oral candidiasis: The clinical diagnostic spectrum. J. Conn. State Dent. Assoc. 1990, 66, 34–37. [Google Scholar]
- Samaranayake, L.P.; Keung Leung, W.; Jin, L. Oral mucosal fungal infections. Periodontology 2000 2009, 49, 39–59. [Google Scholar] [CrossRef]
- Webb, B.C.; Thomas, C.J.; Willcox, M.D.P.; Harty, D.W.S.; Knox, K.W. Candida-associated denture stomatitis. Aetiology and management: A review. Part 3. Treatment of oral candidosis. Aust. Dent. J. 1998, 43, 244–249. [Google Scholar] [CrossRef]
- Ramage, G.; Tomsett, K.; Wickes, B.L.; Lopez-Ribot, J.L.; Redding, S.W. Denture stomatitis: A role for Candida biofilms. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2004, 98, 53–59. [Google Scholar] [CrossRef]
- Budtz-JöRgensen, E. The significance of Candida albicans in denture stomatitis. Eur. J. Oral Sci. 1974, 82, 151–190. [Google Scholar] [CrossRef]
- Gendreau, L.; Loewy, Z.G. Epidemiology and etiology of denture stomatitis. J. Prosthodont. 2011, 20, 251–260. [Google Scholar] [CrossRef]
- Baran, I.; Nalçaci, R. Self-reported denture hygiene habits and oral tissue conditions of complete denture wearers. Arch. Gerontol. Geriatr. 2009, 49, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Sultan, A.S.; Rizk, A.M.; Vila, T.; Ji, Y.; Masri, R.; Jabra-Rizk, M.A. Digital design of a universal rat intraoral device for therapeutic evaluation of topical formulation against Candida-associated denture stomatitis. Infect. Immun. 2019, in press. [Google Scholar] [CrossRef] [PubMed]
- Neville, B.; Damm, D.D.; Allen, C.; Chi, A.C. Fungal and Protozoal Diseases. In Oral and Maxillofacial Pathology, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 191–217. [Google Scholar]
- Samaranayake, L.; Parahitiyawa, N. Oral Medicine & Pathology. A Guide to Diagnosis and Management. In Infections of the Oral Mucosa; Tilakaratne, W., Ed.; Jaypee: New Delhi, India, 2014. [Google Scholar]
- Bakri, M.M.; Hussaini, M.H.; Holmes, R.A.; Cannon, D.R.; Rich, M.A. Revisiting the association between candidal infection and carcinoma, particularly oral squamous cell carcinoma. J. Oral Microbiol. 2010, 2. [Google Scholar] [CrossRef]
- Manfredi, M.P.L.; Giovati, L.; Alnuaimi, A.; McCullough, M.J. Oral and Maxillofacial Fungal Infections. In Contemporary Oral Medicine; Farah, C., Balasubramaniam, R., McCullough, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Humbert, L.; Cornu, M.; Proust-Lemoine, E.; Bayry, J.; Wermeau, J.L.; Vantyghem, M.C.; Sendid, B. Chronic mucocutaneous candidiasis in autoimmune polyendocrine syndrome Type 1. Front. Immunol. 2018, 9, 2570. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Amador, V.; Silverman, S., Jr.; Mayer, P.; Tyler, M.; Quivey, J. Candidal colonization and oral candidiasis in patients undergoing oral and pharyngeal radiation therapy. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1997, 84, 149–153. [Google Scholar] [CrossRef]
- Redding, S.W.; Zellars, R.C.; Kirkpatrick, W.R.; McAtee, R.K.; Caceres, M.A.; Fothergill, A.G.; Lopez-Ribot, J.L.; Bailey, C.W.; Rinald, i.M.G.; Paterson, T.F. Epidemiology of oropharyngeal Candida colonization and infection in patients receiving radiation for head and neck cancer. J. Clin. Microbiol. 1999, 37, 3896–3900. [Google Scholar] [CrossRef] [PubMed]
- Salvatori, O.; Puri, S.; Tati, S.; Edgerton, M. Innate immunity and saliva in Candida albicans-mediated oral diseases. J. Dent. Res. 2016, 95, 365–371. [Google Scholar] [CrossRef]
- Vila, T.; Rizk, A.M.; Sultan, A.S.; Jabra-Rizk, M.A. The power of saliva: Antimicrobial and beyond. PLoS Pathog. 2019, in press. [Google Scholar] [CrossRef]
- Sroussi, H.Y.; Epstein, J.B.; Bensadoun, R.J.; Saunders, D.P.; Lalla, R.V.; Migliorati, C.A.; Heaivilin, N.; Zumsteg, Z.S. Common oral complications of head and neck cancer radiation therapy: Mucositis, infections, saliva change, fibrosis, sensory dysfunctions, dental caries, periodontal disease, and osteoradionecrosis. Cancer Med. 2017, 6, 2918–2931. [Google Scholar] [CrossRef]
- Khan, S.A.; Fidel, P., Jr.; Al Thunayyan, A.; Meiller, T.; Jabra-Rizk, M.A. Impaired histatin-5 level and salivary antimicrobial activity against C. albicans in HIV-infected individuals. J. AIDS Clin. Res. 2013, 4, 1000193. [Google Scholar] [CrossRef] [PubMed]
- Iinuma, T.; Arai, Y.; Abe, Y.; Takayama, M.; Fukumoto, M.; Fukui, Y.; Iwase, T.; Takebayashi, T.; Hirose, N.; Gionhaku, N.; et al. Denture wearing during sleep doubles the risk of pneumonia in the very elderly. J. Dent. Res. 2015, 94, 28S–36S. [Google Scholar] [CrossRef] [PubMed]
- Newton, A.V. Denture sore mouth. A possible etiology. Br. Dent. J. 1962, 112, 357–360. [Google Scholar]
- Perić, M.; Živković, R.; Milić Lemić, A.; Radunović, M.; Miličić, B.; Arsić Arsenijević, V. The severity of denture stomatitis as related to risk factors and different Candida spp. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 126, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Scully, C.; Giovanni, L. Denture Related Stomatitis. European Association of Oral Medicine. Available online: http://www.eaom.eu/pdf/content/denture_related_stomatitis.pdf (accessed on 15 November 2019).
- Tejani, S.; Sultan, A.; Stojanov, I.; Woo, S.B. Candidal carriage predicts candidiasis during topical immunosuppressive therapy: A preliminary retrospective cohort study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 122, 448–454. [Google Scholar] [CrossRef]
- Mun, M.; Yap, T.; Alnuaimi, A.D.; Adams, G.G.; McCullough, M.J. Oral candidal carriage in asymptomatic patients. Aust. Dent. J. 2016, 61, 190–195. [Google Scholar] [CrossRef]
- Sultan, A.S.; Jessri, M.; Farah, C.S. Electronic nicotine delivery systems: Oral health implications and oral cancer risk. J. Oral Pathol. Med. 2018. [Google Scholar] [CrossRef]
- Alanazi, H.; Semlali, A.; Chmielewski, W.; Rouabhia, M. E-cigarettes increase Candida albicans growth and modulate its interaction with gingival epithelial cells. Int. J. Environ. Res. Public Health 2019, 16, 294. [Google Scholar] [CrossRef]
- Rad, M.; Kakoie, S.; Niliye Brojeni, F.; Pourdamghan, N. Effect of long-term smoking on whole-mouth salivary flow rate and oral health. J. Dent. Res. Dent. Clin. Dent. Prospects 2010, 4, 110–114. [Google Scholar]
- Krogh, P.; Hald, B.; Holmstrup, P. Possible mycological etiology of oral mucosal cancer: Catalytic potential of infecting Candida albicans and other yeasts in production of N-nitrosobenzylmethylamine. Carcinogenesis 1987, 8, 1543–1548. [Google Scholar] [CrossRef]
- Gasparoto, T.H.; de Oliveira, C.E.; Vieira, N.A.; Porto, V.C.; Gasparoto, C.T.; Campanelli, A.P.; Lara, V.S. The pattern recognition receptors expressed on neutrophils and the associated cytokine profile from different aged patients with Candida-related denture stomatitis. Exp. Gerontol. 2012, 47, 741–748. [Google Scholar] [CrossRef]
- Johnson, D.; Yeh, C.K.; Dodds, M.W. Effect of donor age on the concentartions of histatins in human parotid and submandibular/sublingual saliva. Arch. Oral Biol. 2000, 45, 731–740. [Google Scholar] [CrossRef]
- Farah, C.S.; Lynch, N.; McCullough, M.J. Oral fungal infections: An update for the general practitioner. Aust. Dent. J. 2010, 5, 48–54. [Google Scholar] [CrossRef]
- Vidya, K.M.; Rao, U.K.; Nittayananta, W.; Liu, H.; Owotade, F.J. Oral mycoses and other opportunistic infections in HIV: Therapy and emerging problems—A workshop report. Oral Dis. 2016, 22, 158–165. [Google Scholar] [CrossRef]
- de Repentigny, L.; Lewandowski, D.; Jolicoeur, P. Immunopathogenesis of oropharyngeal candidiasis in human immunodeficiency virus infection. Clin. Microbiol. Rev. 2004, 17, 729–759. [Google Scholar] [CrossRef]
- Novak, N.; Haberstok, J.; Bieber, T.; Allam, J.P. The immune privilege of the oral mucosa. Trends Mol. Med. 2008, 14, 191–198. [Google Scholar] [CrossRef]
- Gow, N.A.; Netea, M.G.; Munro, C.A.; Ferwerda, G.; Bates, B.; Mora-Montes, H.M.; Walker, l.; Jansen, T.; Jacobs, L.; Tsoni, V.; et al. Immune recognition of Candida albicans β-glucan by dectin-1. J. Infect. Dis. 2007, 196, 1565–1571. [Google Scholar] [CrossRef]
- Gow, N.A.; Hube, B. Importance of the Candida albicans cell wall during commensalism and infection. Curr. Opin. Microbiol. 2012, 15, 406–412. [Google Scholar] [CrossRef]
- Gow, N.A.; van de Veerdonk, F.L.; Brown, A.J.; Netea, M.G. Candida albicans morphogenesis and host defence: Discriminating invasion from colonization. Nat. Rev. Microbiol. 2012, 10, 112–122. [Google Scholar] [CrossRef]
- Li, M.; Chen, Q.; Tang, R.; Shen, Y.; Liu, W. The expression of beta-defensin-2,3 and LL-37 induced by Candida albicans phospholipomannan in human keratinocytes. J. Dermatol. Sci. 2011, 61, 72–75. [Google Scholar] [CrossRef]
- Naglik, J.R.; König, A.; Hube, B.; Gaffen, S.L. Candida albicans-epithelial interactions and induction of mucosal innate immunity. Curr. Opin. Microbiol. 2017, 40, 104–112. [Google Scholar] [CrossRef]
- Moyes, D.L.; Naglik, J.R. Mucosal immunity and Candida albicans infection. Clin. Dev. Immunol. 2011, 2011, 346307. [Google Scholar] [CrossRef]
- Netea, M.G.; Maródi, L. Innate immune mechanisms for recognition and uptake of Candida species. Trends Immunol. 2010, 31, 346–353. [Google Scholar] [CrossRef]
- Schaller, M.; Mailhammer, R.; Grassl, G.; Sander, C.A.; Hube, B.; Korting, H.C. Infection of human oral epithelia with Candida species induces cytokine expression correlated to the degree of virulence. J. Investig. Dermatol. 2002, 118, 652–657. [Google Scholar] [CrossRef]
- Ye, P.; Rodriguez, F.H.; Kanaly, S.; Stocking, K.L.; Schurr, J.; Schwarzenberger, P.; Oliver, P.; Huang, W.; Zhang, P.; Zhang, J. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J. Exp. Med. 2001, 194, 519–527. [Google Scholar] [CrossRef]
- Van der Meer, J.W.; van de Veerdonk, F.L.; Joosten, L.A.; Kullberg, B.J.; Netea, M.G. Severe Candida spp. infections: New insights into natural immunity. Int. J. Antimicrob. Agents 2010, 36, S58–S62. [Google Scholar] [CrossRef]
- Hebecker, B.; Naglik, J.R.; Hube, B.; Jacobsen, I.D. Pathogenicity mechanisms and host response during oral Candida albicans infections. Expert. Rev. Anti-Infect. Ther. 2014, 12, 867–879. [Google Scholar] [CrossRef]
- Meir, J.; Hartmann, E.; Eckstein, M.T.; Guiducci, E.; Kirchner, F.; Rosenwald, A.; Leibund Gut-Landmann, S.; Pérez, J.C. Identification of Candida albicans regulatory genes governing mucosal infection. Cell. Microbiol. 2018, 20, e12841. [Google Scholar] [CrossRef]
- Amerongen, A.; Veerman, E. Saliva: The defender of the oral cavity. Oral Dis. 2002, 8, 12–22. [Google Scholar] [CrossRef]
- Gorr, S. Antimicrobial peptides of the oral cavity. Periodontology 2000 2009, 51, 152–180. [Google Scholar] [CrossRef]
- Auvynet, C.; Rosenstein, Y. Multifunctional host defense peptides: Antimicrobial peptides, the small yet big players in innate and adaptive immunity. FEBS J. 2009, 276, 6497–6508. [Google Scholar] [CrossRef]
- Peters, B.M.; Shirtliff, M.E.; Jabra-Rizk, M.A. Antimicrobial peptides: Primeval molecules or future drugs? PLoS Pathog. 2010, 6, e1001067. [Google Scholar] [CrossRef]
- Khurshid, Z.; Naseem, M.; Yahya, F.; Mali, M.; Sannam, K.R.; Sahibzada, H.A.; Zafar, M.S.; Faraz, M.S.; Khan, E. Significance and diagnostic role of antimicrobial cathelicidins (LL-37) peptides in oral health. Biomolecules 2017, 7, 80. [Google Scholar] [CrossRef]
- Edgerton, M.; Koshlukova, S.; Lo, T.; Chrzan, B.; Straubinger, R.; Raj, P. Candidacidal activity of salivary histatins. J. Biol. Chem. 1998, 272, 20438–20447. [Google Scholar] [CrossRef]
- Oppenheim, F.G.; Xu, T.; McMillian, F.M.; Levitz, S.M.; Diamond, R.D.; Offner, G.D.; Troxler, R.F. Histatins, a novel family of histidine-rich proteins in human parotid secretion. J. Biol. Chem. 1988, 263, 7472–7477. [Google Scholar]
- Li, X.S.; Reddy, M.S.; Baev, D.; Edgerton, M. Candida albicans Ssa1/2p is the cell envelope binding protein for human salivary histatin 5. J. Biol. Chem. 2003, 278, 28553–28561. [Google Scholar] [CrossRef]
- Gyurko, C.; Lendenmann, U.; Helmerhorst, E.J.; Troxler, R.F.; Oppenheim, F.G. Killing of Candida albicans by histatin 5: Cellular uptake and energy requirement. Antonie Van Leeuwenhoek 2001, 79, 297–309. [Google Scholar] [CrossRef]
- Mochon, A.B.; Liu, H. The antimicrobial peptide histatin-5 causes a spatially restricted disruption on the Candida albicans surface allowing rapid entry of the peptide into the cytoplasm. PLoS Pathog. 2008, 4, e1000190. [Google Scholar] [CrossRef]
- Helmerhorst, E.J.; Troxler, R.F.; Oppenheim, F.G. The human salivary peptide histatin 5 exerts its antifungal activity through the formation of reactive oxygen species. Proc. Nat. Acad. Sci. USA 2001, 98, 14637–14642. [Google Scholar] [CrossRef]
- Vivino, F.B.; Bunya, V.Y.; Massaro-Giordano, G.; Johr, C.R.; Giattino, S.L.; Schorpion, A.; Shafer, B.; Peck, A.; Sivils, K.; Rasmussen, A.; et al. Sjogren’s syndrome: An update on disease pathogenesis, clinical manifestations and treatment. Clin. Immunol. 2019, 22. [Google Scholar] [CrossRef]
- Fox, P.C. Saliva and salivary gland alterations in HIV infection. J. Am. Dent. Assoc. 1991, 122, 46–48. [Google Scholar] [CrossRef]
- Gaffen, S.; Hernandez-Santos, N.; Peterson, A. IL-17 Signaling in host defense against Candida albicans. Immunol. Res. 2011, 50, 181–187. [Google Scholar] [CrossRef]
- Klein, R.S.; Harris, C.A.; Small, C.B.; Moll, B.; Lesser, M.; Friedland, G.H. Oral candidiasis in high-risk patients as the initial manifestation of the acquired immunodeficiency syndrome. N. Engl. J. Med. 1984, 311, 354–358. [Google Scholar] [CrossRef]
- Sallusto, F.; Lanzavecchia, A. The instructive role of dendritic cells on T-cell responses. Arthritis Res. 2002, 4, 127–132. [Google Scholar] [CrossRef]
- Conti, H.; Shen, F.; Nayyar, N.; Stocum, E.; Sun, J.N.; Lindemann, M.J.; Ho, A.W.; Hai, J.H.; Yu, J.J.; Jung, J.W.; et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J. Exp. Med. 2009, 206, 299–311. [Google Scholar] [CrossRef]
- Pirofski, L.-A.; Casadevall, A. Rethinking T cell immunity in oropharyngeal candidiasis. J. Exp. Med. 2009, 206, 269–273. [Google Scholar] [CrossRef]
- Conti, H.R.; Bruno, V.M.; Childs, E.E.; Daugherty, S.; Hunter, J.P.; Mengesha, B.G.; Saevig, D.L.; Hendricks, M.; Coleman, B.M.; Brane, L.; et al. IL-17 receptor signaling in oral epithelial cells is critical for protection against oropharyngeal candidiasis. Cell Host Microbe 2016, 20, 606–617. [Google Scholar] [CrossRef]
- Khader, S.A.; Gaffen, S.L.; Kolls, J.K. Th17 cells at the crossroads of innate and adaptive immunity against infectious diseases at the mucosa. Mucosal Immunol. 2009, 2, 403–411. [Google Scholar] [CrossRef]
- Onishi, R.M.; Gaffen, S.L. Interleukin-17 and its target genes: Mechanisms of interleukin-17 function in disease. Immunology 2010, 129, 311–321. [Google Scholar] [CrossRef]
- Liang, S.C.; Tan, X.Y.; Luxenberg, D.P.; Karim, R.; Dunussi-Joannopoulos, K.; Collins, M.; Fouser, L.A. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 2006, 203, 2271–2279. [Google Scholar] [CrossRef]
- Cua, D.J.; Tato, C.M. Innate IL-17-producing cells: The sentinels of the immune system. Nat. Rev. Immunol. 2010, 10, 479–489. [Google Scholar] [CrossRef]
- Gladiator, A.; Wangler, N.; Trautwein-Weidner, K.; Leibund-Gut-Landmann, S. Cutting edge: IL-17–secreting innate lymphoid cells are essential for host defense against fungal infection. J. Immunol. 2013, 190, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.H.; Richardson, J.P.; Zhou, C.; Coleman, B.M.; Moyes, D.L.; Ho, J.; Huppler, A.R.; Ramani, K.; McGeachy, M.J.; Mufazalov, I.A.; et al. Oral epithelial cells orchestrate innate type 17 responses to Candida albicans through the virulence factor candidalysin. Sci. Immunol. 2017, 2, eaam8834. [Google Scholar] [CrossRef] [PubMed]
- Puel, A.; Cypowyj, S.; Bustamante, J.; Wright, J.F.; Liu, L.; Lim, H.K.; Migaud, M.; Israel, L.; Chrabieh, M.; Audry, M. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science 2011, 332, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Conti, H.R.; Gaffen, S.L. Host responses to Candida albicans: Th17 cells and mucosal candidiasis. Microbes Infect. 2010, 12, 518–527. [Google Scholar] [CrossRef]
- Wade, W.G. The oral microbiome in health and disease. Pharmacol. Res. 2013, 69, 137–143. [Google Scholar] [CrossRef]
- Avila, M.; Ojcius, D.M.; Yilmaz, O. The oral microbiota: Living with a permanent guest. DNA Cell Biol. 2009, 28, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, H.F.; Lamont, R.J. Oral microbial communities in sickness and in health. Trends Microbiol. 2005, 13, 589–595. [Google Scholar] [CrossRef]
- Duran-Pinedo, A.E.; Frias-Lopez, J. Beyond microbial community composition: Functional activities of the oral microbiome in health and disease. Microbes Infect. 2015, 17, 505–516. [Google Scholar] [CrossRef]
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.; Yu, W.H.; Lakshmanan, A.; Wade, W.G. The human oral microbiome. J. Bacteriol. 2010, 192, 5002–5017. [Google Scholar] [CrossRef]
- Zaura, E.; Nicu, E.A.; Krom, B.P.; Keijser, B.J. Acquiring and maintaining a normal oral microbiome: Current perspective. Front. Cell. Infect. Microbiol. 2014, 4, 85. [Google Scholar] [CrossRef]
- Kolenbrander, P.E.; Anserson, R.X.; Blehert, D.S.; Egland, P.G.; Foster, J.S.; Palmer, R.J., Jr. Communication among oral bacteria. Microbiol. Mol. Biol. Rev. 2002, 66, 486–505. [Google Scholar] [CrossRef] [PubMed]
- Rickard, A.H.; Gilbert, P.; High, N.J.; Kolenbrander, P.E.; Handley, P.S. Bacterial coaggregation: An integral process in the development of multi-species biofilms. Trends Microbiol. 2003, 11, 94–100. [Google Scholar] [CrossRef]
- Kleinberg, T. Acids formed aerobically and anaerobically by pure and mixed cultures of oral bacteria from oxidizable sugar, organic acid and amino acid substrates. J. Dent. Res. 1999, 78, 211. [Google Scholar]
- O’Donnell, L.E.; Millhouse, E.; Sherry, L.; Kean, R.; Malcolm, J.; Nile, C.J.; Ramage, G. Polymicrobial Candida biofilms: Friends and foe in the oral cavity. FEMS Yeast Res. 2015, 15, fov077. [Google Scholar] [CrossRef]
- Jenkinson, H.F.; Barbour, M.E.; Jagger, D.C.; Miles, M.; Bamford, C.M.; Nobbs, A.H.; Dutton, L.C.; Silverman, R.J.; McNally, L.; Vickerman, M.M.; et al. Candida albicans—Bacteria interactions in biofilms and disease. Univ. Bristol Dent. Sch. 2008, 1–6. [Google Scholar]
- Van Dijck, P.; Jabra-Rizk, M.A. Fungal–Bacterial Interactions: In Health and Disease. In Candida Albicans: Cellular and Molecular Biology; Prasad, R., Ed.; Springer: Cham, Germany, 2017; pp. 115–143. [Google Scholar] [CrossRef]
- Nobbs, A.H.; Jenkinson, H.F. Interkingdom networking within the oral microbiome. Microbes Infect. 2015, 17, 484–492. [Google Scholar] [CrossRef]
- Jakubovics, N.S.; Yassin, S.A.; Rickard, A.H. Community interactions of oral streptococci. Adv. Appl. Microbiol. 2014, 87, 43–110. [Google Scholar]
- Montelongo-Jauregui, D.; Lopez-Ribot, J. Candida interactions with the oral bacterial microbiota. J. Fungi 2018, 4, 122. [Google Scholar] [CrossRef]
- Montelongo-Jauregui, D.; Saville, S.P.; Lopez-Ribot, J.L. Contributions of Candida albicans dimorphism, adhesive interactions, and extracellular matrix to the formation of dual-species biofilms with Streptococcus gordonii. mBio 2019, 10. [Google Scholar] [CrossRef]
- Diaz, P.I.; Xie, Z.; Sobue, T.; Thompson, A.; Biyikoglu, B.; Ricker, A.; Ikonomou, L.; Dongari-Bagtzoglou, A. Synergistic Interaction between Candida albicans and Commensal Oral Streptococci in a Novel In Vitro Mucosal Model. Infect. Immun. 2012, 80, 620–632. [Google Scholar] [CrossRef]
- Ellepola, K.; Liu, Y.; Cao, T.; Koo, H.; Seneviratne, C. Bacterial GtfB augments Candida albicans accumulation in cross-kingdom biofilms. J. Dent. Res. 2017, 96, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Dongari-Bagtzoglou, A. Shaping the oral mycobiota: Interactions of opportunistic fungi with oral bacteria and the host. Curr. Opin. Microbiol. 2015, 27, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Bamford, C.V.; d’Mello, A.; Nobbs, A.H.; Dutton, L.C.; Vickerman, M.M.; Jenkinson, H.F. Streptococcus gordonii modulates Candida albicans biofilm formation through intergeneric communication. Infect. Immun. 2009, 77, 3696–3704. [Google Scholar] [CrossRef] [PubMed]
- Metwalli, K.H.; Khan, S.A.; Krom, B.P.; Jabra-Rizk, M.A. Streptococcus mutans, Candida albicans and the human mouth: A sticky situation. PLoS Pathog. 2013, 9, e1003616. [Google Scholar] [CrossRef]
- Sztajer, H.; Szafranski, S.P.; Tomasch, J.; Reck, M.; Nimtz, M.; Rohde, M. Cross-feeding and interkingdom communication in dual-species biofilms of Streptococcus mutans and Candida albicans. ISME J. 2014, 8, 2256. [Google Scholar] [CrossRef]
- Falsetta, M.L.; Klein, M.I.; Colone, P.M.; Scott-Anne, K.; Gregoire, S.; Pai, C.-H.; Gonzalez, M.; Watson, G.; Krysan, D.J.; Bowen, W.H.; et al. Symbiotic relationship between Streptococcus mutans and Candida albicans synergizes the virulence of plaque-biofilms in vivo. Infect. Immun. 2014, 82, 1968–1981. [Google Scholar] [CrossRef]
- Krom, B.P.; Kidwai, S.; Ten Cate, J.M. Candida and other fungal species: Forgotten players of healthy oral microbiota. J. Dent. Res. 2014, 93, 445–451. [Google Scholar] [CrossRef]
- Bertolini, M.; Ranjan, A.; Thompson, A.; Diaz, P.I.; Sobue, T.; Maas, K.; Dongari-Bagtzoglou, A. Candida albicans induces mucosal bacterial dysbiosis that promotes invasive infection. PLoS Pathog. 2019, 15, e1007717. [Google Scholar] [CrossRef]
- Isalm, B.; Khan, S.N.; Khan, A.U. Dental caries: From infection to prevention. Med. Sci. Monit. 2007, 13, RA196–RA203. [Google Scholar]
- Zero, D.T.; Fontana, M.; Martinez-Mier, E.A.; Ferrera-Zandona, A.; Ando, M.; Gonzalez-Cabezas, C.; Bayne, S. The biology, prevention, diagnosis and treatment of dental caries. J. Am. Dent. Assoc. 2009, 140, 25S–34S. [Google Scholar] [CrossRef]
- Rouabhia, M.; Chmielewski, W. Diseases anssociated with oral polymicrobial biofilms. Open Mycol. J. 2012, 6, 27–32. [Google Scholar] [CrossRef]
- Bowen, W.; Koo, H. Biology of Streptococcus mutans-derived glucosyltransferases: Role in extracellular matrix formation of cariogenic biofilms. Caries Res. 2011, 45, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Valm, A.M. The structure of dental plaque microbial communities in the transition from health to dental caries and periodontal disease. J. Mol. Biol. 2019, 431, 2956–2969. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.I.; Hwang, G.; Santos, P.H.; Campanella, O.H.; Koo, H. Streptococcus mutans-derived extracellular matrix in cariogenic oral biofilms. Front. Cell. Infect. Microbiol. 2015, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, D.; Vicente, V.; Fraiz, F.; Lavoranti, O.; Svidzinski, T.; Pinheiro, R. Analysis of the in vitro adherence of Streprococcus mutans and Candida albicans. Braz. J. Microbiol. 2007, 38, 624–663. [Google Scholar] [CrossRef]
- Jarosz, L.M.; Deng, D.M.; van der Mei, H.; Crielaard, W.; Krom, B. Streptococcus mutans competemce-stimulating peptide inhibits Candida albicans hypha formation. Eukaryot. Cell 2009, 8, 1658–1664. [Google Scholar] [CrossRef]
- Xiao, J.; Huang, X.; Alkhers, N.; Alzamil, H.; Alzoubi, S.; Wu, T.T. Candida albicans and early childhood caries: A systematic review and meta-analysis. Caries Res. 2018, 52, 102–112. [Google Scholar] [CrossRef]
- Pereira, D.; Seneviratne, C.; Koga-Ito, C.; Samaranayake, L. Is the oral fungal pathogen Candida albicans a cariogen? Oral Dis. 2018, 24, 518–526. [Google Scholar] [CrossRef]
- Raja, M.; Hannan, A.; Ali, K. Association of oral candidal carriage with dental caries in children. Caries Res. 2010, 44, 272–276. [Google Scholar] [CrossRef]
- de Carvalho, F.G.; Silva, D.S.; Hebling, J.; Spolidorio, L.C.; Spolidorio, D.M.P. Presence of mutans streptococci and Candida spp. in dental plaque/dentine of carious teeth and early childhood caries. Arch Oral Biol. 2006, 51, 1024–1028. [Google Scholar] [CrossRef]
- Koo, H.; Bowen, W.H. Candida albicans and Streptococcus mutans: A potential synergistic alliance to cause virulent tooth decay in children. Future Microbiol. 2014, 9, 1295–1297. [Google Scholar] [CrossRef] [PubMed]
- Klinke, T.; Guggenheim, B.; Klimm, W.; Thurnheer, T. Dental caries in rats associated with Candida albicans. Caries Res. 2011, 45, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Lobo, C.I.V.; Rinaldi, T.B.; Christiano, C.M.S.; De Sales Leite, L.; Barbugli, P.A.; Klein, M.I. Dual-species biofilms of Streptococcus mutans and Candida albicans exhibit more biomass and are mutually beneficial compared with single-species biofilms. J. Oral Microbiol. 2019, 11, 1581520. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Scoffield, J.; Wu, R.; Deivanayagam, C.; Zou, J.; Wu, H. Antigen I/II mediates interactions between Streptococcus mutans and Candida albicans. Mol. Oral Microbiol. 2018, 33, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Hwang, G.; Marsh, G.; Gao, L.; Waugh, R.; Koo, H. Binding force dynamics of Streptococcus mutans–glucosyltransferase B to Candida albicans. J. Dent. Res. 2015, 94, 1310–1317. [Google Scholar] [CrossRef] [PubMed]
- Hwang, G.; Liu, Y.; Kim, D.; Li, Y.; Krysan, D.J.; Koo, H. Candida albicans mannans mediate Streptococcus mutans exoenzyme GtfB binding to modulate cross-kingdom biofilm development in vivo. PLoS Pathog. 2017, 13, e1006407. [Google Scholar] [CrossRef]
- Cavalcanti, I.M.; Nobbs, A.H.; Ricomini-Filho, A.P.; Jenkinson, H.F.; Del Bel Cury, A.A. Interkingdom cooperation between Candida albicans, Streptococcus oralis and Actinomyces oris modulates early biofilm development on denture material. Pathog. Dis. 2016, 74, ftw002. [Google Scholar] [CrossRef]
- Xu, H.; Sobue, T.; Thompson, A.; Xie, Z.; Poon, K.; Ricker, A.; Cervantes, J.; Diaz, P.I.; Dongari-Bagtzoglou, A. Streptococcal co-infection augments Candida pathogenicity by amplifying the mucosal inflammatory response. Cell. Microbiol. 2014, 16, 214–231. [Google Scholar] [CrossRef]
- Xu, H.; Sobue, T.; Bertolini, M.; Thompson, A.; Dongari-Bagtzoglou, A. Streptococcus oralis and Candida albicans synergistically activate mu-calpain to degrade E-cadherin from oral epithelial junctions. J. Infect. Dis. 2016, 214, 925–934. [Google Scholar] [CrossRef]
- Xu, H.; Sobue, T.; Bertolini, M.; Thompson, A.; Vickerman, M.; Nobile, C.J.; Dongari-Bagtzoglou, A.S. oralis ativates the Efg1 filamentation pathway in C. albicans to promote cross-kingdom interactions and mucosal biofilms. Virulence 2017, 8, 1602–1617. [Google Scholar] [CrossRef]
- Bamford, C.V.; Nobbs, A.H.; Barbour, M.E.; Lamont, R.J.; Jenkinson, H.F. Functional regions of Candida albicans hyphal cell wall protein Als3 that determine interaction with the oral bacterium Streptococcus gordonii. Microbiology 2015, 161, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Nobb, A.H.; Vickerman, M.M.; Jenkinson, H.F. Heterologous expression of Candida albicans cell wall-associated adhesin in Saccharomyces cerevisiae reveals differential specificities in adherence and biofilm formation and in binding to Streptococcus gordonii. Eukaryot. Cell 2010, 9, 1622–1634. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.R.; McNab, R.; Jenkinson, H.F. Candida albicans binding to the oral bacterium Streptococcus gordonii involves multiple adhesin-receptor interactions. Infect. Immun. 1996, 64, 4680–4685. [Google Scholar] [CrossRef] [PubMed]
- Montelongo-Jauregui, D.; Srinivasan, A.; Ramasubramanian, A.K.; Lopez-Ribot, J.L. An In vitro model for Candida albicans-Streptococcus gordonii biofilms on titanium surfaces. J. Fungi 2018, 4, 66. [Google Scholar] [CrossRef] [PubMed]
- Chinnici, J.; Yerke, L.; Tsou, C.; Busarajan, S.; Mancuso, R.; Sadhak, N.D.; Kim, J.; Maddi, A. Candida albicans cell wall integrity transcription factors regulate polymicrobial biofilm formation with Streptococcus gordonii. PeerJ 2019, 7, e7870. [Google Scholar] [CrossRef] [PubMed]
- Pihlstrom, B.L.; Michalowicz, B.S.; Johnson, N.W. Periodontal diseases. Lancet 2005, 366, 1809–1820. [Google Scholar] [CrossRef]
- Khan, S.A.; Kong, E.F.; Meiller, T.F.; Jabra-Rizk, M.A. Periodontal diseases: Bug induced, host promoted. PLoS Pathog. 2015, 11, e1004952. [Google Scholar] [CrossRef]
- Haffajee, A.D.; Socransky, S.S. Introduction to microbial aspects of periodontal biofilm communities, development and treatment. Periodontology 2000 2006, 42, 7–12. [Google Scholar] [CrossRef]
- Jabra-Rizk, M.A.; Ferreira, S.M.S.; Sabet, M.; Falkler, W.A.; Merz, W.G.; Meiller, T.F. Recovery of Candida dubliniensis and other yeast from human immunodeficiency virus-associated periodontal lesions. J. Clin. Microbiol. 2001, 39, 4520–4522. [Google Scholar] [CrossRef][Green Version]
- Thein, Z.M.; Samaranayake, Y.H.; Samaranayake, L.P. Effect of oral bacteria on growth and survival of Candida albicans biofilms. Arch. Oral Biol. 2006, 51, 672–680. [Google Scholar] [CrossRef]
- Urzua, B.; Hermosilla, G.; Gamonal, J.; Morales-Bozo, I.; Canals, M.; Barahona, S.; Cóccola, C.; Cifuentes, V. Yeast diversity in the oral microbiota of subjects with periodontitis: Candida albicans and Candida dubliniensis colonize the periodontal pockets. Med. Mycol. 2008, 46, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Sztukowska, M.N.; Dutton, L.C.; Delaney, C.; Ramsdale, M.; Ramage, G.; Jenkinson, H.F.; Nobbs, A.H.; Lamont, R.J. Community Development between Porphyromonas gingivalis and Candida albicans mediated by InlJ and Als3. mBio 2018, 9, e00202-18. [Google Scholar] [CrossRef] [PubMed]
- Bartnicka, D.; Karkowska-Kuleta, J.; Zawrotniak, M.; Satala, D.; Michalik, K.; Zielinska, G.; Bochenska, O.; Kozik, A.; Ciaston, I.; Koziel, J. Adhesive protein-mediated cross-talk between Candida albicans and Porphyromonas gingivalis in dual species biofilm protects the anaerobic bacterium in unfavorable oxic environment. Sci. Rep. 2019, 9, 4376. [Google Scholar] [CrossRef] [PubMed]
- Grimaudo, N.J.; Nesbitt, W.; Clark, W. Coaggregation of Candida albicans with oral Actinomyces species. Oral Microbiol. Immunol. 1996, 11, 59–61. [Google Scholar] [CrossRef]
- Jabra-Rizk, M.A.; Falkler, W.A., Jr.; Merz, W.G.; Kelley, J.I.; Baqui, A.A.M.A.; Meiller, T.F. Coaggregation of Candida dubliniensis with Fusobacterium nucleatum. J. Clin. Microbiol. 1999, 37, 1464–1468. [Google Scholar] [CrossRef]
- Wu, T.; Cen, L.; Kaplan, C.; Zhou, X.; Lux, R.; Shi, W.; He, X. Cellular Components Mediating Coadherence of Candida albicans and Fusobacterium nucleatum. J. Dent. Res. Dent. Clin. Dent. Prospects 2015, 94, 1432–1438. [Google Scholar] [CrossRef]
- Bor, B.; Cen, L.; Agnello, M.; Shi, W.; He, X. Morphological and physiological changes induced by contact-dependent interaction between Candida albicans and Fusobacterium nucleatum. Sci. Rep. 2016, 6, 27956. [Google Scholar] [CrossRef]
- Peters, B.; Jabra-Rizk, M.; Scheper, M.; Leid, J.; Costerton, J.; Shirtliff, M. Microbial interactions and differential protein expression in Staphylococcus aureus and Candida albicans dual-species biofilms. FEMS Immun. Med. Microbiol. 2010, 59, 493–503. [Google Scholar] [CrossRef]
- Vila, T.; Kong, E.F.; Ibrahim, A.; Piepenbrink, K.; Shetty, A.C.; McCracken, C.; Bruno, V.; Jabra-Rizk, M.A. Candida albicans quorum sensing molecule farnesol modulates staphyloxanthin production and activates the thiol-based oxidative-stress response in Staphylococcus aureus. Virulence 2019, 10, 625–642. [Google Scholar] [CrossRef]
- Kong, E.; Johnson, J.; Jabra-Rizk, M. Community-associated methicillin-resistant Staphylococcus aureus: An enemy amidst us. PLoS Pathog. 2016, 12, e1005837. [Google Scholar] [CrossRef]
- Kong, E.; Tsui, C.; Kucharíková, S.; Andes, D.; Van Dijck, P.; Jabra-Rizk, M.A. Commensal protection of Staphylococcus aureus against antimicrobials by Candida albicans biofilm matrix. mBio 2016, 7, e01365-16. [Google Scholar] [CrossRef] [PubMed]
- Kong, E.F.; Tsui, C.; Kucharíková, S.; Van Dijck, P.; Jabra-Rizk, M.A. Modulation of Staphylococcus aureus response to antimicrobials by the Candida albicans quorum sensing molecule farnesol. Antimicrob. Agents Chemother. 2017, 61, e01573-17. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.J.; Jackson, M.S.; Bagg, J. The ecology of Staphylococcus species in the oral cavity. J. Med. Microbiol. 2001, 50, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Baena-Monroy, T.; Moreno-Maldonado, V.; Franco-Martinez, F.; Aldape-Barrios, B.; Quindos, G.; Sanchez, V.; Luis, O. Candida albicas, Staphylococcus aureus and Streptococcus mutans colonization in patients wearing dental prosthesis. Med. Oral Patol. Oral Cir. Bucal 2005, 10, E27–E39. [Google Scholar] [PubMed]
- Tawara, Y.; Honma, K.; Naito, Y. Methicillin-resistant Staphylococcus aureus and Candida albicans on denture surfaces. Bull. Tokyo Dent. Coll. 1996, 37, 119–128. [Google Scholar]
- Cuesta, A.I.; Jewtuchowicz, V.; Brusca, M.I.; Nastri, M.L.; Rosa, A.C. Prevalence of Staphylococcus spp. and Candida spp in the oral cavity and periodontal pockets of periodontal disease patients. Acta Odontol. Latinoam. 2010, 23, 20–26. [Google Scholar] [PubMed]
- Schlecht, L.; Peters, B.; Krom, B.; Hänsch, G.; Filler, S.; Jabra-Rizk, M.; Shirtliff, M. Systemic Staphylococcus aureus infection mediated by Candida albicans hyphal invasion of mucosal tissue. Microbiology 2015, 161, 168–181. [Google Scholar] [CrossRef]
- Kong, E.; Kucharíková, S.; Van Dijck, P.; Peters, B.; Shirtliff, M.; Jabra-Rizk, M. Clinical implications of oral candidiasis: Host tissue damage and disseminated bacterial disease. Infect. Immun. 2015, 83, 604–613. [Google Scholar] [CrossRef]
- Szabo, E.K.; MacCallum, D.M. The contribution of mouse models to our understanding of systemic candidiasis. FEMS Microbiol. Lett. 2011, 320, 1–8. [Google Scholar] [CrossRef]
- Kong, E.; Tsui, C.; Boyce, H.; Ibrahim, A.; Hoag, S.; Karlsson, A.; Meiller, T.; Jabra-Rizk, M. Development and in vivo evaluation of a novel histatin-5 bioadhesive hydrogel formulation against oral candidiasis. Antimicrob. Agents Chemother. 2015, 60, 881–889. [Google Scholar] [CrossRef]
- Segal, E.; Frenkel, M. Experimental in vivo models of candidiasis. J. Fungi 2018, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Naglik, J.R.; Fidel, P.L.; Odds, F.C. Animal models of mucosal Candida infection. FEMS Microbiol. Lett. 2008, 283, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Conti, H.R.; Huppler, A.R.; Whibley, N.; Gaffen, S.L. Animal models for candidiasis. Curr. Protoc. Immunol. 2014, 105. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Pereira, C.A.; Junqueira, J.C.; Jorge, A.O.C. Recent mouse and rat methods for the study of experimental oral candidiasis. Virulence 2013, 4, 391–399. [Google Scholar] [CrossRef]
- Solis, N.V.; Filler, S.G. Mouse model of oropharyngeal candidiasis. Nat. Prot. 2012, 7, 637–642. [Google Scholar] [CrossRef]
- Mosci, P.; Pericolini, E.; Gabrielli, E.; Kenno, S.; Perito, S.; Bistoni, F.; d’Enfert, C.; Vecchiarelli, A. A novel bioluminescence mouse model for monitoring oropharyngeal candidiasis in mice. Virulence 2013, 4, 250–254. [Google Scholar] [CrossRef]
- de Repentigny, L.; Aumont, F.; Ripeau, J.S.; Fiorillo, M.; Kay, D.G.; Hanna, Z.; Jolicoeur, P. Mucosal candidiasis in transgenic mice expressing human immunodeficiency virus Type 1. J. Infect. Dis. 2002, 185, 1103–1114. [Google Scholar] [CrossRef]
- de Repentigny, L.; Goupil, M.; Jolicoeur, P. Oropharyngeal candidiasis in HIV infection: Analysis of impaired mucosal immune response to Candida albicans in mice expressing the HIV-1 transgene. Pathogens 2015, 4, 406–421. [Google Scholar] [CrossRef]
- Budtz-Jorgensen, E. Denture stomatitis. IV. An experimental model in monkeys. Acta Odontol. Scand. 1971, 29, 513–526. [Google Scholar] [CrossRef]
- Johnson, C.C.; Yu, A.; Lee, H.; Fidel, P.L.J.; Noverr, M.C. Development of a contemporary animal model of Candida albicans-associated denture stomatitisusing a novel intraoral denture system. Infect. Immun. 2012, 80, 1736–1743. [Google Scholar] [CrossRef]
- Tobouti, P.L.; Casaroto, A.R.; de Almeida, R.S.; de Paula Ramos, S.; Dionísio, T.J.; Porto, V.C.; Santos, C.F.; Lara, V.S. Expression of secreted aspartyl proteinases in an experimental model of Candida albicans-associated denture stomatitis. J. Prosthodont. 2016, 25, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Hotta, J.; Cral, W.G.; Sakima, V.T.; Lara, V.S.; Urban, V.M.; Neppelenbroek, K.H. Intraoral device for optimal antifungal delivery in a rat model. Curr. Drug Deliv. 2017, 14, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Yano, J.; Yu, A.; Fidel, P.L.J.; Noverr, M.C. Candida glabrata has no enhancing role in the pathogenesis of Candida-associated denture stomatitis in a rat model. mSphere 2019, 4, e00191-19. [Google Scholar] [CrossRef] [PubMed]
- Tobudic, S.; Kratzer, C.; Lassnigg, A.; Presterl, E. Antifungal susceptibility of Candida albicans in biofilms. Mycoses 2012, 55, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Nobile, C.J.; Johnson, A.D. Candida albicans biofilms and human disease. Annu. Rev. Microbiol. 2015, 69, 71–92. [Google Scholar] [CrossRef]
- Spampinato, C.; Leonardi, D. Candida infections, causes, targets, and resistance mechanisms: Traditional and alternative antifungal agents. Biomed Res. Int. 2013, 2013, 204–237. [Google Scholar] [CrossRef]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.; Benjamin, D.K.J.; Calandra, T.F.; Edwards, J.E.J.; Filler, S.G.; Fisher, J.F.; Kullberg, B.J.; Ostrosky-Zeichner, L.; et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 503–535. [Google Scholar] [CrossRef]
- Pierce, C.G.; Lopez-Ribot, J.L. Candidiasis drug discovery and development: New approaches targeting virulence for discovering and identifying new drugs. Expert Opin. Drug Discov. 2013, 8, 1117–1126. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Perlin, D.S. Echinocandin resistance: An emerging clinical problem? Curr. Opin. Infect. Dis. 2014, 27, 484–492. [Google Scholar] [CrossRef]
- Pristov, K.E.; Ghannoum, M.A. Resistance of Candida to azoles and echinocandins worldwide. Clin. Microbiol. Infect. 2019, 25, 792–798. [Google Scholar] [CrossRef]
- Vila, T.; Romo, J.A.; Pierce, C.G.; McHardy, S.F.; Saville, S.P.; Lopez-Ribot, J.L. Targeting Candida albicans filamentation for antifungal drug development. Virulence 2017, 8, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, D.M.; George, T.; Chandra, J.; Mukherjee, P.K.; Ghannoum, M.A. Antifungal susceptibility of Candida biofilms: Unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrob. Agents Chemother. 2002, 46, 1773–1780. [Google Scholar] [CrossRef]
- Hornby, J.M.; Jensen, E.C.; Lisec, A.D.; Tasto, J.J.; Jahnke, B.; Shoemaker, R.; Dussault, P.; Nickerson, K.W. Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl. Environ. Microbiol. 2001, 67, 2982–2992. [Google Scholar] [CrossRef] [PubMed]
- Shareck, J.; Belhumeur, P. Modulation of morphogenesis in Candida albicans by various small molecules. Eukaryot. Cell 2011, 10, 1004–1012. [Google Scholar] [CrossRef] [PubMed]
- Pierce, C.G.; Chaturvedi, A.K.; Lazzell, A.L.; Powell, A.T.; Saville, S.P.; McHardy, S.F.; Lopez-Ribot, J.L. A novel small molecule inhibitor of Candida albicans biofilm formation, filamentation and virulence with low potential for the development of resistance. NPJ Biofilms Microbiomes 2015, 1, 15012. [Google Scholar] [CrossRef]
- Romo, J.A.; Pierce, C.G.; Chaturvedi, A.K.; Lazzell, A.L.; McHardy, S.F.; Saville, S.P.; Lopez-Ribot, J.L. Development of anti-virulence approaches for candidiasis via a novel series of small-molecule inhibitors of Candida albicans filamentation. mBio 2017, 8, e01991-17. [Google Scholar] [CrossRef]
- Romo, J.A.; Zhang, H.; Cai, H.; Kadosh, D.; Koehler, J.R.; Saville, S.P.; Wang, Y.; Lopez-Ribot, J.L. Global transcriptomic analysis of the Candida albicans response to treatment with a novel inhibitor of filamentation. mSphere 2019, 4, e00620-19. [Google Scholar] [CrossRef]
- Chandra, J.; Ghannoum, M.A. CD101, a novel echinocandin, possesses potent antibiofilm activity against early and mature Candida albicans biofilms. Antimicrob. Agents Chemother. 2018, 62, e01750-17. [Google Scholar] [CrossRef]
- Lepak, A.J.; Zhao, M.; Van Scoy, B.; Ambrose, P.G.; Andes, D.R. Pharmacodynamics of a long-acting echinocandin, CD101, in a neutropenic invasive-candidiasis murine model using an extended-interval dosing design. Antimicrob. Agents Chemother. 2018, 62, e02154-17. [Google Scholar] [CrossRef]
- Zhao, M.; Lepak, A.J.; Van Scoy, B.; Bader, J.C.; Marchillo, K.; Vanhecker, J.; Ambrose, P.G.; Andes, D.R. In vivo pharmacokinetics and pharmacodynamics of APX001 against Candida spp. in a neutropenic disseminated candidiasis mouse model. Antimicrob. Agents Chemother. 2018, 62, e02542-17. [Google Scholar] [CrossRef]
| Adherence to Oral Epithelial Surface |
|
| Biofilm Formation |
|
| Evasion of Host Defenses |
|
| Invasion and Destruction of Host Tissue |
|
| Local Factors |
|
|
|
| Systemic Factors |
|
|
|
|
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vila, T.; Sultan, A.S.; Montelongo-Jauregui, D.; Jabra-Rizk, M.A. Oral Candidiasis: A Disease of Opportunity. J. Fungi 2020, 6, 15. https://doi.org/10.3390/jof6010015
Vila T, Sultan AS, Montelongo-Jauregui D, Jabra-Rizk MA. Oral Candidiasis: A Disease of Opportunity. Journal of Fungi. 2020; 6(1):15. https://doi.org/10.3390/jof6010015
Chicago/Turabian StyleVila, Taissa, Ahmed S. Sultan, Daniel Montelongo-Jauregui, and Mary Ann Jabra-Rizk. 2020. "Oral Candidiasis: A Disease of Opportunity" Journal of Fungi 6, no. 1: 15. https://doi.org/10.3390/jof6010015
APA StyleVila, T., Sultan, A. S., Montelongo-Jauregui, D., & Jabra-Rizk, M. A. (2020). Oral Candidiasis: A Disease of Opportunity. Journal of Fungi, 6(1), 15. https://doi.org/10.3390/jof6010015





