The Impact of Gene Dosage and Heterozygosity on the Diploid Pathobiont Candida albicans
Abstract
1. Introduction
2. The Heterozygous Diploid Genome of C. albicans
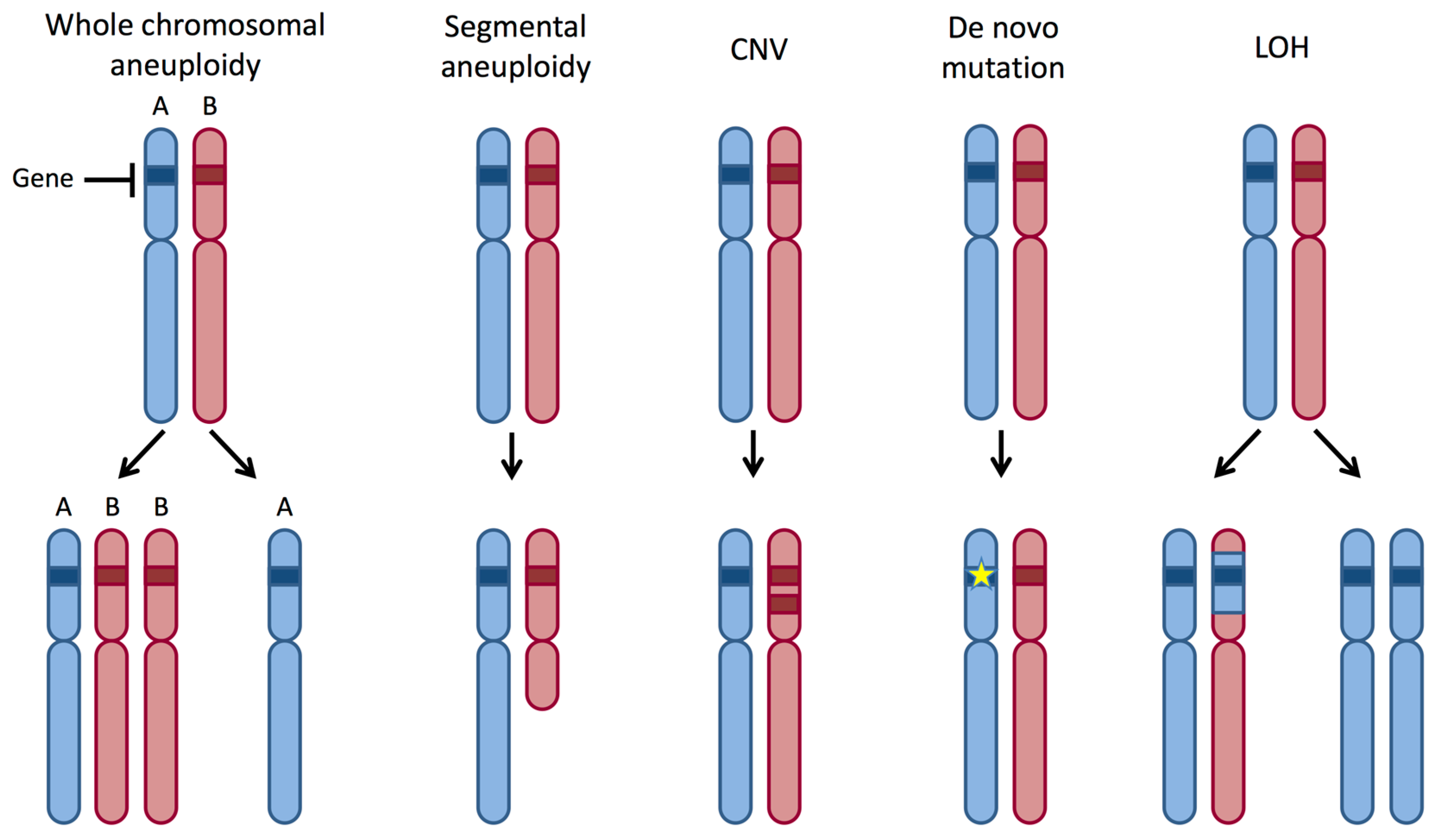
3. Mating, Ploidy Shifts and Aneuploidy
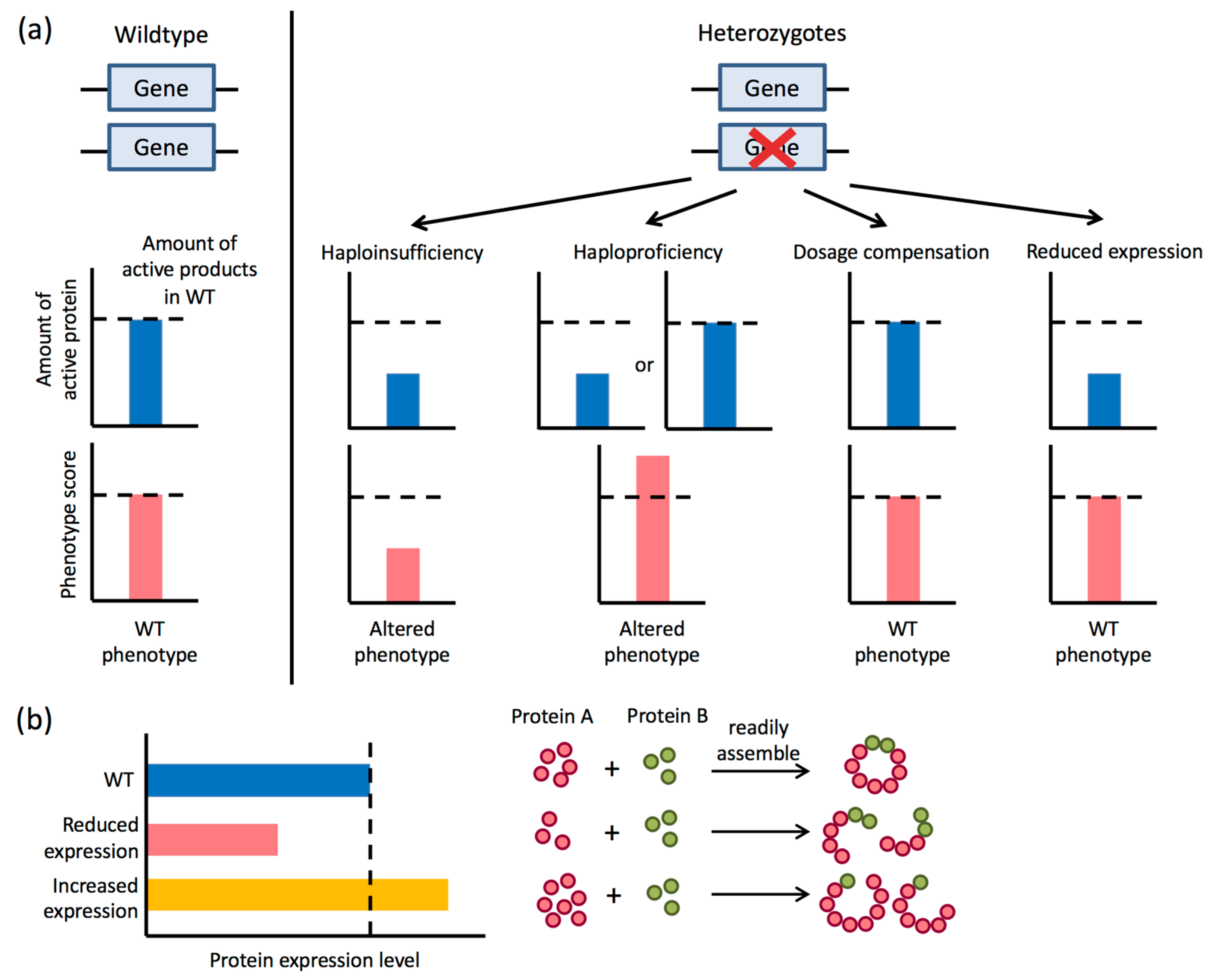
4. Hemizygosity and Haploinsufficiency in C. albicans
5. LOH Is Frequent in C. albicans
6. LOH Events Are Linked to Phenotypic Change and Host Adaptation
7. LOH and Drug Resistance in C. albicans
8. Aneuploidy and LOH during Strain Construction in C. albicans
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pavelka, N.; Rancati, G.; Zhu, J.; Bradford, W.D.; Saraf, A.; Florens, L.; Sanderson, B.W.; Hattem, G.L.; Li, R. Aneuploidy confers quantitative proteome changes and phenotypic variation in budding yeast. Nature 2010, 468, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Stingele, S.; Stoehr, G.; Peplowska, K.; Cox, J.; Mann, M.; Storchova, Z. Global analysis of genome, transcriptome and proteome reveals the response to aneuploidy in human cells. Mol. Syst. Biol. 2012, 8, 608. [Google Scholar] [CrossRef] [PubMed]
- Huettel, B.; Kreil, D.P.; Matzke, M.; Matzke, A.J. Effects of aneuploidy on genome structure, expression, and interphase organization in Arabidopsis thaliana. PLoS Genet. 2008, 4, e1000226. [Google Scholar] [CrossRef] [PubMed]
- Sheltzer, J.M.; Torres, E.M.; Dunham, M.J.; Amon, A. Transcriptional consequences of aneuploidy. Proc. Natl. Acad. Sci. USA 2012, 109, 12644–12649. [Google Scholar] [CrossRef] [PubMed]
- Mank, J.E. Sex chromosome dosage compensation: Definitely not for everyone. Trends Genet. 2013, 29, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Gu, W.; Hurles, M.E.; Lupski, J.R. Copy number variation in human health, disease, and evolution. Annu. Rev. Genomics Hum. Genet. 2009, 10, 451–481. [Google Scholar] [CrossRef]
- Sebat, J.; Lakshmi, B.; Malhotra, D.; Troge, J.; Lese-Martin, C.; Walsh, T.; Yamrom, B.; Yoon, S.; Krasnitz, A.; Kendall, J.; et al. Strong association of de novo copy number mutations with autism. Science 2007, 316, 445–449. [Google Scholar] [CrossRef]
- Santarosa, M.; Ashworth, A. Haploinsufficiency for tumour suppressor genes: When you don’t need to go all the way. Biochim. Biophys. Acta 2004, 1654, 105–122. [Google Scholar] [CrossRef]
- Dang, V.T.; Kassahn, K.S.; Marcos, A.E.; Ragan, M.A. Identification of human haploinsufficient genes and their genomic proximity to segmental duplications. Eur. J. Hum. Genet. 2008, 16, 1350–1357. [Google Scholar] [CrossRef]
- Delneri, D.; Hoyle, D.C.; Gkargkas, K.; Cross, E.J.; Rash, B.; Zeef, L.; Leong, H.S.; Davey, H.M.; Hayes, A.; Kell, D.B.; et al. Identification and characterization of high-flux-control genes of yeast through competition analyses in continuous cultures. Nat. Genet. 2008, 40, 113–117. [Google Scholar] [CrossRef]
- Ohnuki, S.; Ohya, Y. High-dimensional single-cell phenotyping reveals extensive haploinsufficiency. PLoS Biol. 2018, 16, e2005130. [Google Scholar] [CrossRef] [PubMed]
- Veitia, R.A.; Potier, M.C. Gene dosage imbalances: Action, reaction, and models. Trends Biochem. Sci. 2015, 40, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Li, G.W.; Burkhardt, D.; Gross, C.; Weissman, J.S. Quantifying absolute protein synthesis rates reveals principles underlying allocation of cellular resources. Cell 2014, 157, 624–635. [Google Scholar] [CrossRef] [PubMed]
- Papp, B.; Pal, C.; Hurst, L.D. Dosage sensitivity and the evolution of gene families in yeast. Nature 2003, 424, 194–197. [Google Scholar] [CrossRef] [PubMed]
- Morrill, S.A.; Amon, A. Why haploinsufficiency persists. Proc. Natl. Acad. Sci. USA 2019, 116, 11866–11871. [Google Scholar] [CrossRef]
- Moran, C.; Grussemeyer, C.A.; Spalding, J.R.; Benjamin, D.K., Jr.; Reed, S.D. Candida albicans and non-albicans bloodstream infections in adult and pediatric patients: Comparison of mortality and costs. Pediatr. Infect. Dis. J. 2009, 28, 433–435. [Google Scholar] [CrossRef] [PubMed]
- Olaiya, A.F.; Sogin, S.J. Ploidy determination of Canadida albicans. J. Bacteriol. 1979, 140, 1043–1049. [Google Scholar]
- Riggsby, W.S.; Torres-Bauza, L.J.; Wills, J.W.; Townes, T.M. DNA content, kinetic complexity, and the ploidy question in Candida albicans. Mol. Cell. Biol. 1982, 2, 853–862. [Google Scholar] [CrossRef]
- Butler, G.; Rasmussen, M.D.; Lin, M.F.; Santos, M.A.; Sakthikumar, S.; Munro, C.A.; Rheinbay, E.; Grabherr, M.; Forche, A.; Reedy, J.L.; et al. Evolution of pathogenicity and sexual reproduction in eight Candida genomes. Nature 2009, 459, 657–662. [Google Scholar] [CrossRef]
- Hirakawa, M.P.; Martinez, D.A.; Sakthikumar, S.; Anderson, M.Z.; Berlin, A.; Gujja, S.; Zeng, Q.; Zisson, E.; Wang, J.M.; Greenberg, J.M.; et al. Genetic and phenotypic intra-species variation in Candida albicans. Genome Res. 2015, 25, 413–425. [Google Scholar] [CrossRef]
- Ropars, J.; Maufrais, C.; Diogo, D.; Marcet-Houben, M.; Perin, A.; Sertour, N.; Mosca, K.; Permal, E.; Laval, G.; Bouchier, C.; et al. Gene flow contributes to diversification of the major fungal pathogen Candida albicans. Nat. Commun. 2018, 9, 2253. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.M.; Bennett, R.J.; Anderson, M.Z. The genome of the human pathogen Candida albicans is shaped by mutation and cryptic sexual recombination. MBio 2018, 9, e01205-18. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.; Federspiel, N.A.; Chibana, H.; Dungan, J.; Kalman, S.; Magee, B.B.; Newport, G.; Thorstenson, Y.R.; Agabian, N.; Magee, P.T.; et al. The diploid genome sequence of Candida albicans. Proc. Natl. Acad. Sci. USA 2004, 101, 7329–7334. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Raja, J.; Andaluz, E.; Magee, B.; Calderone, R.; Larriba, G. A single SNP, G929T (Gly310Val), determines the presence of a functional and a non-functional allele of HIS4 in Candida albicans SC5314: Detection of the non-functional allele in laboratory strains. Fungal Genet. Biol. 2008, 45, 527–541. [Google Scholar] [CrossRef] [PubMed]
- Muzzey, D.; Schwartz, K.; Weissman, J.S.; Sherlock, G. Assembly of a phased diploid Candida albicans genome facilitates allele-specific measurements and provides a simple model for repeat and indel structure. Genome Biol. 2013, 14, R97. [Google Scholar] [CrossRef] [PubMed]
- Staib, P.; Kretschmar, M.; Nichterlein, T.; Hof, H.; Morschhauser, J. Host versus in vitro signals and intrastrain allelic differences in the expression of a Candida albicans virulence gene. Mol. Microbiol. 2002, 44, 1351–1366. [Google Scholar] [CrossRef]
- Elmore, M.H.; Gibbons, J.G.; Rokas, A. Assessing the genome-wide effect of promoter region tandem repeat natural variation on gene expression. G3 (Bethesda) 2012, 2, 1643–1649. [Google Scholar] [CrossRef]
- Muzzey, D.; Sherlock, G.; Weissman, J.S. Extensive and coordinated control of allele-specific expression by both transcription and translation in Candida albicans. Genome Res. 2014, 24, 963–973. [Google Scholar] [CrossRef]
- Hoyer, L.L.; Cota, E. Candida albicans agglutinin-like sequence (Als) family vignettes: A review of Als protein structure and function. Front. Microbiol. 2016, 7, 280. [Google Scholar] [CrossRef]
- Oh, S.H.; Cheng, G.; Nuessen, J.A.; Jajko, R.; Yeater, K.M.; Zhao, X.; Pujol, C.; Soll, D.R.; Hoyer, L.L. Functional specificity of Candida albicans Als3p proteins and clade specificity of ALS3 alleles discriminated by the number of copies of the tandem repeat sequence in the central domain. Microbiology 2005, 151, 673–681. [Google Scholar] [CrossRef]
- Zhang, N.; Harrex, A.L.; Holland, B.R.; Fenton, L.E.; Cannon, R.D.; Schmid, J. Sixty alleles of the ALS7 open reading frame in Candida albicans: ALS7 is a hypermutable contingency locus. Genome Res. 2003, 13, 2005–2017. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Pujol, C.; Soll, D.R.; Hoyer, L.L. Allelic variation in the contiguous loci encoding Candida albicans ALS5, ALS1 and ALS9. Microbiology 2003, 149, 2947–2960. [Google Scholar] [CrossRef] [PubMed]
- Braun, B.R.; van Het Hoog, M.; d’Enfert, C.; Martchenko, M.; Dungan, J.; Kuo, A.; Inglis, D.O.; Uhl, M.A.; Hogues, H.; Berriman, M.; et al. A human-curated annotation of the Candida albicans genome. PLoS Genet. 2005, 1, e1. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, M.; Zhang, N.; Schmid, J. Biological roles of protein-coding tandem repeats in the yeast Candida albicans. J. Fungi (Basel) 2018, 4, 78. [Google Scholar] [CrossRef] [PubMed]
- Hull, C.M.; Raisner, R.M.; Johnson, A.D. Evidence for mating of the “asexual” yeast Candida albicans in a mammalian host. Science 2000, 289, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Magee, B.B.; Magee, P.T. Induction of mating in Candida albicans by construction of MTLa and MTLalpha strains. Science 2000, 289, 310–313. [Google Scholar] [CrossRef]
- Hull, C.M.; Johnson, A.D. Identification of a mating type-like locus in the asexual pathogenic yeast Candida albicans. Science 1999, 285, 1271–1275. [Google Scholar] [CrossRef]
- Lockhart, S.R.; Pujol, C.; Daniels, K.J.; Miller, M.G.; Johnson, A.D.; Pfaller, M.A.; Soll, D.R. In Candida albicans, white-opaque switchers are homozygous for mating type. Genetics 2002, 162, 737–745. [Google Scholar]
- Srikantha, T.; Daniels, K.J.; Pujol, C.; Sahni, N.; Yi, S.; Soll, D.R. Nonsex genes in the mating type locus of Candida albicans play roles in a/alpha biofilm formation, including impermeability and fluconazole resistance. PLoS Pathog. 2012, 8, e1002476. [Google Scholar] [CrossRef]
- Ibrahim, A.S.; Magee, B.B.; Sheppard, D.C.; Yang, M.; Kauffman, S.; Becker, J.; Edwards, J.E., Jr.; Magee, P.T. Effects of ploidy and mating type on virulence of Candida albicans. Infect. Immun. 2005, 73, 7366–7374. [Google Scholar] [CrossRef]
- Rustchenko, E. Chromosome instability in Candida albicans. FEMS Yeast Res. 2007, 7, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Slutsky, B.; Staebell, M.; Anderson, J.; Risen, L.; Pfaller, M.; Soll, D.R. “White-opaque transition”: A second high-frequency switching system in Candida albicans. J. Bacteriol. 1987, 169, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.G.; Johnson, A.D. White-opaque switching in Candida albicans is controlled by mating-type locus homeodomain proteins and allows efficient mating. Cell 2002, 110, 293–302. [Google Scholar] [CrossRef]
- Thomson, G.J.; Hernon, C.; Austriaco, N.; Shapiro, R.S.; Belenky, P.; Bennett, R.J. Metabolism-induced oxidative stress and DNA damage selectively trigger genome instability in polyploid fungal cells. EMBO J. 2019, 38, e101597. [Google Scholar] [CrossRef]
- Galitski, T.; Saldanha, A.J.; Styles, C.A.; Lander, E.S.; Fink, G.R. Ploidy regulation of gene expression. Science 1999, 285, 251–254. [Google Scholar] [CrossRef]
- Wu, C.Y.; Rolfe, P.A.; Gifford, D.K.; Fink, G.R. Control of transcription by cell size. PLoS Biol. 2010, 8, e1000523. [Google Scholar] [CrossRef]
- Bennett, R.J.; Johnson, A.D. Completion of a parasexual cycle in Candida albicans by induced chromosome loss in tetraploid strains. EMBO J. 2003, 22, 2505–2515. [Google Scholar] [CrossRef]
- Forche, A.; Alby, K.; Schaefer, D.; Johnson, A.D.; Berman, J.; Bennett, R.J. The parasexual cycle in Candida albicans provides an alternative pathway to meiosis for the formation of recombinant strains. PLoS Biol. 2008, 6, e110. [Google Scholar] [CrossRef]
- Hirakawa, M.P.; Chyou, D.E.; Huang, D.; Slan, A.R.; Bennett, R.J. Parasex generates phenotypic diversity de novo and impacts drug resistance and virulence in Candida albicans. Genetics 2017, 207, 1195–1211. [Google Scholar] [CrossRef]
- Hickman, M.A.; Paulson, C.; Dudley, A.M.; Berman, J. Parasexual ploidy reduction drives population heterogeneity through random and transient aneuploidy in Candida albicans. Genetics 2015, 200, 781–794. [Google Scholar] [CrossRef]
- Anderson, M.Z.; Thomson, G.J.; Hirakawa, M.P.; Bennett, R.J. A ‘parameiosis’ drives depolyploidization and homologous recombination in Candida albicans. Nat. Commun. 2019, 10, 4388. [Google Scholar] [CrossRef] [PubMed]
- Alby, K.; Bennett, R.J. Interspecies pheromone signaling promotes biofilm formation and same-sex mating in Candida albicans. Proc. Natl. Acad. Sci. USA 2011, 108, 2510–2515. [Google Scholar] [CrossRef] [PubMed]
- Alby, K.; Schaefer, D.; Bennett, R.J. Homothallic and heterothallic mating in the opportunistic pathogen Candida albicans. Nature 2009, 460, 890–893. [Google Scholar] [CrossRef] [PubMed]
- Guan, G.; Tao, L.; Yue, H.; Liang, W.; Gong, J.; Bing, J.; Zheng, Q.; Veri, A.O.; Fan, S.; Robbins, N.; et al. Environment-induced same-sex mating in the yeast Candida albicans through the Hsf1-Hsp90 pathway. PLoS Biol. 2019, 17, e2006966. [Google Scholar] [CrossRef]
- Legrand, M.; Lephart, P.; Forche, A.; Mueller, F.M.; Walsh, T.; Magee, P.T.; Magee, B.B. Homozygosity at the MTL locus in clinical strains of Candida albicans: Karyotypic rearrangements and tetraploid formation. Mol. Microbiol. 2004, 52, 1451–1462. [Google Scholar] [CrossRef]
- Tavanti, A.; Davidson, A.D.; Fordyce, M.J.; Gow, N.A.; Maiden, M.C.; Odds, F.C. Population structure and properties of Candida albicans, as determined by multilocus sequence typing. J. Clin. Microbiol. 2005, 43, 5601–5613. [Google Scholar] [CrossRef]
- Greenberg, J.R.; Price, N.P.; Oliver, R.P.; Sherman, F.; Rustchenko, E. Candida albicans SOU1 encodes a sorbose reductase required for L-sorbose utilization. Yeast 2005, 22, 957–969. [Google Scholar] [CrossRef]
- Janbon, G.; Sherman, F.; Rustchenko, E. Monosomy of a specific chromosome determines L-sorbose utilization: A novel regulatory mechanism in Candida albicans. Proc. Natl. Acad. Sci. USA 1998, 95, 5150–5155. [Google Scholar] [CrossRef]
- Janbon, G.; Sherman, F.; Rustchenko, E. Appearance and properties of L-sorbose-utilizing mutants of Candida albicans obtained on a selective plate. Genetics 1999, 153, 653–664. [Google Scholar]
- Kabir, M.A.; Ahmad, A.; Greenberg, J.R.; Wang, Y.K.; Rustchenko, E. Loss and gain of chromosome 5 controls growth of Candida albicans on sorbose due to dispersed redundant negative regulators. Proc. Natl. Acad. Sci. USA 2005, 102, 12147–12152. [Google Scholar] [CrossRef]
- Wu, W.; Pujol, C.; Lockhart, S.R.; Soll, D.R. Chromosome loss followed by duplication is the major mechanism of spontaneous mating-type locus homozygosis in Candida albicans. Genetics 2005, 169, 1311–1327. [Google Scholar] [CrossRef] [PubMed]
- Feri, A.; Loll-Krippleber, R.; Commere, P.H.; Maufrais, C.; Sertour, N.; Schwartz, K.; Sherlock, G.; Bougnoux, M.E.; d’Enfert, C.; Legrand, M. Analysis of repair mechanisms following an induced double-strand break uncovers recessive deleterious alleles in the Candida albicans diploid genome. MBio 2016, 7, e01109-16. [Google Scholar] [CrossRef] [PubMed]
- Marton, T.; Feri, A.; Commere, P.H.; Maufrais, C.; d’Enfert, C.; Legrand, M. Identification of recessive lethal alleles in the diploid genome of a Candida albicans laboratory strain unveils a potential role of repetitive sequences in buffering their deleterious impact. mSphere 2019, 4, e00709-18. [Google Scholar] [CrossRef] [PubMed]
- Andaluz, E.; Bellido, A.; Gomez-Raja, J.; Selmecki, A.; Bouchonville, K.; Calderone, R.; Berman, J.; Larriba, G. Rad52 function prevents chromosome loss and truncation in Candida albicans. Mol. Microbiol. 2011, 79, 1462–1482. [Google Scholar] [CrossRef]
- Hickman, M.A.; Zeng, G.; Forche, A.; Hirakawa, M.P.; Abbey, D.; Harrison, B.D.; Wang, Y.M.; Su, C.H.; Bennett, R.J.; Wang, Y.; et al. The ‘obligate diploid’ Candida albicans forms mating-competent haploids. Nature 2013, 494, 55–59. [Google Scholar] [CrossRef]
- Selmecki, A.; Forche, A.; Berman, J. Aneuploidy and isochromosome formation in drug-resistant Candida albicans. Science 2006, 313, 367–370. [Google Scholar] [CrossRef]
- Ene, I.V.; Farrer, R.A.; Hirakawa, M.P.; Agwamba, K.; Cuomo, C.A.; Bennett, R.J. Global analysis of mutations driving microevolution of a heterozygous diploid fungal pathogen. Proc. Natl. Acad. Sci. USA 2018, 115, E8688–E8697. [Google Scholar] [CrossRef]
- Forche, A.; Solis, N.V.; Swidergall, M.; Thomas, R.; Guyer, A.; Beach, A.; Cromie, G.A.; Le, G.T.; Lowell, E.; Pavelka, N.; et al. Selection of Candida albicans trisomy during oropharyngeal infection results in a commensal-like phenotype. PLoS Genet. 2019, 15, e1008137. [Google Scholar] [CrossRef]
- Ford, C.B.; Funt, J.M.; Abbey, D.; Issi, L.; Guiducci, C.; Martinez, D.A.; Delorey, T.; Li, B.Y.; White, T.C.; Cuomo, C.; et al. The evolution of drug resistance in clinical isolates of Candida albicans. eLife 2015, 4, e00662. [Google Scholar] [CrossRef]
- Selmecki, A.; Gerami-Nejad, M.; Paulson, C.; Forche, A.; Berman, J. An isochromosome confers drug resistance in vivo by amplification of two genes, ERG11 and TAC1. Mol. Microbiol. 2008, 68, 624–641. [Google Scholar] [CrossRef]
- Harrison, B.D.; Hashemi, J.; Bibi, M.; Pulver, R.; Bavli, D.; Nahmias, Y.; Wellington, M.; Sapiro, G.; Berman, J. A tetraploid intermediate precedes aneuploid formation in yeasts exposed to fluconazole. PLoS Biol. 2014, 12, e1001815. [Google Scholar] [CrossRef] [PubMed]
- Tucker, C.; Bhattacharya, S.; Wakabayashi, H.; Bellaousov, S.; Kravets, A.; Welle, S.L.; Myers, J.; Hayes, J.J.; Bulger, M.; Rustchenko, E. Transcriptional regulation on aneuploid chromosomes in diverse Candida albicans mutants. Sci. Rep. 2018, 8, 1630. [Google Scholar] [CrossRef] [PubMed]
- Arbour, M.; Epp, E.; Hogues, H.; Sellam, A.; Lacroix, C.; Rauceo, J.; Mitchell, A.; Whiteway, M.; Nantel, A. Widespread occurrence of chromosomal aneuploidy following the routine production of Candida albicans mutants. FEMS Yeast Res. 2009, 9, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Bouchonville, K.; Forche, A.; Tang, K.E.; Selmecki, A.; Berman, J. Aneuploid chromosomes are highly unstable during DNA transformation of Candida albicans. Eukaryot Cell 2009, 8, 1554–1566. [Google Scholar] [CrossRef]
- Bennett, R.J.; Forche, A.; Berman, J. Rapid mechanisms for generating genome diversity: Whole ploidy shifts, aneuploidy, and loss of heterozygosity. Cold Spring Harb. Perspect. Med. 2014, 4, a019604. [Google Scholar] [CrossRef]
- Morrow, C.A.; Fraser, J.A. Ploidy variation as an adaptive mechanism in human pathogenic fungi. Semin. Cell Dev. Biol. 2013, 24, 339–346. [Google Scholar] [CrossRef]
- Berman, J. Ploidy plasticity: A rapid and reversible strategy for adaptation to stress. FEMS Yeast Res. 2016, 16, fow020. [Google Scholar] [CrossRef]
- Uhl, M.A.; Biery, M.; Craig, N.; Johnson, A.D. Haploinsufficiency-based large-scale forward genetic analysis of filamentous growth in the diploid human fungal pathogen C.albicans. EMBO J. 2003, 22, 2668–2678. [Google Scholar] [CrossRef]
- Braun, B.R.; Johnson, A.D. TUP1, CPH1 and EFG1 make independent contributions to filamentation in Candida albicans. Genetics 2000, 155, 57–67. [Google Scholar]
- Lo, H.J.; Kohler, J.R.; DiDomenico, B.; Loebenberg, D.; Cacciapuoti, A.; Fink, G.R. Nonfilamentous C. albicans mutants are avirulent. Cell 1997, 90, 939–949. [Google Scholar] [CrossRef]
- Oh, J.; Fung, E.; Schlecht, U.; Davis, R.W.; Giaever, G.; St Onge, R.P.; Deutschbauer, A.; Nislow, C. Gene annotation and drug target discovery in Candida albicans with a tagged transposon mutant collection. PLoS Pathog. 2010, 6, e1001140. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Jiang, B.; Ketela, T.; Lemieux, S.; Veillette, K.; Martel, N.; Davison, J.; Sillaots, S.; Trosok, S.; Bachewich, C.; et al. Genome-wide fitness test and mechanism-of-action studies of inhibitory compounds in Candida albicans. PLoS Pathog. 2007, 3, e92. [Google Scholar] [CrossRef]
- Chaillot, J.; Cook, M.A.; Corbeil, J.; Sellam, A. Genome-wide screen for haploinsufficient cell size genes in the opportunistic yeast Candida albicans. G3 (Bethesda) 2017, 7, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Bharucha, N.; Chabrier-Rosello, Y.; Xu, T.; Johnson, C.; Sobczynski, S.; Song, Q.; Dobry, C.J.; Eckwahl, M.J.; Anderson, C.P.; Benjamin, A.J.; et al. A large-scale complex haploinsufficiency-based genetic interaction screen in Candida albicans: Analysis of the RAM network during morphogenesis. PLoS Genet. 2011, 7, e1002058. [Google Scholar] [CrossRef] [PubMed]
- Saputo, S.; Norman, K.L.; Murante, T.; Horton, B.N.; Diaz Jde, L.; DiDone, L.; Colquhoun, J.; Schroeder, J.W.; Simmons, L.A.; Kumar, A.; et al. Complex haploinsufficiency-based genetic analysis of the NDR/Lats kinase Cbk1 provides insight into its multiple functions in Candida albicans. Genetics 2016, 203, 1217–1233. [Google Scholar] [CrossRef] [PubMed]
- Glazier, V.E.; Murante, T.; Koselny, K.; Murante, D.; Esqueda, M.; Wall, G.A.; Wellington, M.; Hung, C.Y.; Kumar, A.; Krysan, D.J. Systematic complex haploinsufficiency-based genetic analysis of Candida albicans transcription factors: Tools and applications to virulence-associated phenotypes. G3 (Bethesda) 2018, 8, 1299–1314. [Google Scholar] [CrossRef]
- Glazier, V.E.; Murante, T.; Murante, D.; Koselny, K.; Liu, Y.; Kim, D.; Koo, H.; Krysan, D.J. Genetic analysis of the Candida albicans biofilm transcription factor network using simple and complex haploinsufficiency. PLoS Genet. 2017, 13, e1006948. [Google Scholar] [CrossRef]
- Glazier, V.E.; Krysan, D.J. Transcription factor network efficiency in the regulation of Candida albicans biofilms: It is a small world. Curr. Genet. 2018, 64, 883–888. [Google Scholar] [CrossRef]
- Nobile, C.J.; Fox, E.P.; Nett, J.E.; Sorrells, T.R.; Mitrovich, Q.M.; Hernday, A.D.; Tuch, B.B.; Andes, D.R.; Johnson, A.D. A recently evolved transcriptional network controls biofilm development in Candida albicans. Cell 2012, 148, 126–138. [Google Scholar] [CrossRef]
- Forche, A.; Abbey, D.; Pisithkul, T.; Weinzierl, M.A.; Ringstrom, T.; Bruck, D.; Petersen, K.; Berman, J. Stress alters rates and types of loss of heterozygosity in Candida albicans. MBio 2011, 2, e00129-11. [Google Scholar] [CrossRef]
- Ciudad, T.; Bellido, A.; Hermosa, B.; Andaluz, E.; Larriba, G. DLH1, the Candida albicans homologue of the meiosis-specific DMC1, is not involved in DNA repair but catalyses spontaneous interhomologue recombination and might promote non-crossover events. Cell. Microbiol. 2019, e13137. [Google Scholar] [CrossRef] [PubMed]
- Takagi, Y.; Akada, R.; Kumagai, H.; Yamamoto, K.; Tamaki, H. Loss of heterozygosity is induced in Candida albicans by ultraviolet irradiation. Appl. Microbiol. Biotechnol. 2008, 77, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- Forche, A.; Magee, P.T.; Selmecki, A.; Berman, J.; May, G. Evolution in Candida albicans populations during a single passage through a mouse host. Genetics 2009, 182, 799–811. [Google Scholar] [CrossRef] [PubMed]
- Forche, A.; Cromie, G.; Gerstein, A.C.; Solis, N.V.; Pisithkul, T.; Srifa, W.; Jeffery, E.; Abbey, D.; Filler, S.G.; Dudley, A.M.; et al. Rapid phenotypic and genotypic diversification after exposure to the oral host niche in Candida albicans. Genetics 2018, 209, 725–741. [Google Scholar] [CrossRef]
- Sitterle, E.; Maufrais, C.; Sertour, N.; Palayret, M.; d’Enfert, C.; Bougnoux, M.E. Within-host genomic diversity of Candida albicans in healthy carriers. Sci. Rep. 2019, 9, 2563. [Google Scholar] [CrossRef]
- Todd, R.T.; Wikoff, T.D.; Forche, A.; Selmecki, A. Genome plasticity in Candida albicans is driven by long repeat sequences. eLife 2019, 8, e45954. [Google Scholar] [CrossRef]
- Tao, L.; Du, H.; Guan, G.; Dai, Y.; Nobile, C.J.; Liang, W.; Cao, C.; Zhang, Q.; Zhong, J.; Huang, G. Discovery of a “white-gray-opaque” tristable phenotypic switching system in Candida albicans: Roles of non-genetic diversity in host adaptation. PLoS Biol. 2014, 12, e1001830. [Google Scholar] [CrossRef]
- Liang, S.H.; Anderson, M.Z.; Hirakawa, M.P.; Wang, J.M.; Frazer, C.; Alaalm, L.M.; Thomson, G.J.; Ene, I.V.; Bennett, R.J. Hemizygosity enables a mutational transition governing fungal virulence and commensalism. Cell Host Microbe 2019, 25, 418–431.e6. [Google Scholar] [CrossRef]
- Pande, K.; Chen, C.; Noble, S.M. Passage through the mammalian gut triggers a phenotypic switch that promotes Candida albicans commensalism. Nat. Genet. 2013, 45, 1088–1091. [Google Scholar] [CrossRef]
- Pierce, J.V.; Kumamoto, C.A. Variation in Candida albicans EFG1 expression enables host-dependent changes in colonizing fungal populations. MBio 2012, 3, e00117-12. [Google Scholar] [CrossRef]
- Pierce, J.V.; Dignard, D.; Whiteway, M.; Kumamoto, C.A. Normal adaptation of Candida albicans to the murine gastrointestinal tract requires Efg1p-dependent regulation of metabolic and host defense genes. Eukaryot Cell 2013, 12, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Stoldt, V.R.; Sonneborn, A.; Leuker, C.E.; Ernst, J.F. Efg1p, an essential regulator of morphogenesis of the human pathogen Candida albicans, is a member of a conserved class of bHLH proteins regulating morphogenetic processes in fungi. EMBO J. 1997, 16, 1982–1991. [Google Scholar] [CrossRef] [PubMed]
- Tso, G.H.W.; Reales-Calderon, J.A.; Tan, A.S.M.; Sem, X.; Le, G.T.T.; Tan, T.G.; Lai, G.C.; Srinivasan, K.G.; Yurieva, M.; Liao, W.; et al. Experimental evolution of a fungal pathogen into a gut symbiont. Science 2018, 362, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Witchley, J.N.; Penumetcha, P.; Abon, N.V.; Woolford, C.A.; Mitchell, A.P.; Noble, S.M. Candida albicans morphogenesis programs control the balance between gut commensalism and invasive infection. Cell Host Microbe 2019, 25, 432–443.e6. [Google Scholar] [CrossRef] [PubMed]
- Bohm, L.; Torsin, S.; Tint, S.H.; Eckstein, M.T.; Ludwig, T.; Perez, J.C. The yeast form of the fungus Candida albicans promotes persistence in the gut of gnotobiotic mice. PLoS Pathog. 2017, 13, e1006699. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Clark, S.T.; Surendra, A.; Copeland, J.K.; Wang, P.W.; Ammar, R.; Collins, C.; Tullis, D.E.; Nislow, C.; Hwang, D.M.; et al. Global analysis of the fungal microbiome in cystic fibrosis patients reveals loss of function of the transcriptional repressor Nrg1 as a mechanism of pathogen adaptation. PLoS Pathog. 2015, 11, e1005308. [Google Scholar] [CrossRef]
- Murad, A.M.; Leng, P.; Straffon, M.; Wishart, J.; Macaskill, S.; MacCallum, D.; Schnell, N.; Talibi, D.; Marechal, D.; Tekaia, F.; et al. NRG1 represses yeast-hypha morphogenesis and hypha-specific gene expression in Candida albicans. EMBO J. 2001, 20, 4742–4752. [Google Scholar] [CrossRef]
- Sasse, C.; Dunkel, N.; Schafer, T.; Schneider, S.; Dierolf, F.; Ohlsen, K.; Morschhauser, J. The stepwise acquisition of fluconazole resistance mutations causes a gradual loss of fitness in Candida albicans. Mol. Microbiol. 2012, 86, 539–556. [Google Scholar] [CrossRef]
- Coste, A.T.; Karababa, M.; Ischer, F.; Bille, J.; Sanglard, D. TAC1, transcriptional activator of CDR genes, is a new transcription factor involved in the regulation of Candida albicans ABC transporters CDR1 and CDR2. Eukaryot Cell 2004, 3, 1639–1652. [Google Scholar] [CrossRef]
- White, T.C. The presence of an R467K amino acid substitution and loss of allelic variation correlate with an azole-resistant lanosterol 14alpha demethylase in Candida albicans. Antimicrob. Agents Chemother. 1997, 41, 1488–1494. [Google Scholar] [CrossRef]
- Favre, B.; Didmon, M.; Ryder, N.S. Multiple amino acid substitutions in lanosterol 14alpha-demethylase contribute to azole resistance in Candida albicans. Microbiology 1999, 145 Pt 10, 2715–2725. [Google Scholar] [CrossRef]
- Sanglard, D.; Ischer, F.; Koymans, L.; Bille, J. Amino acid substitutions in the cytochrome P-450 lanosterol 14alpha-demethylase (CYP51A1) from azole-resistant Candida albicans clinical isolates contribute to resistance to azole antifungal agents. Antimicrob. Agents Chemother. 1998, 42, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Coste, A.; Selmecki, A.; Forche, A.; Diogo, D.; Bougnoux, M.E.; d’Enfert, C.; Berman, J.; Sanglard, D. Genotypic evolution of azole resistance mechanisms in sequential Candida albicans isolates. Eukaryot Cell 2007, 6, 1889–1904. [Google Scholar] [CrossRef] [PubMed]
- Coste, A.T.; Turner, V.; Ischer, F.; Morschhauser, J.; Forche, A.; Selmecki, A.; Berman, J.; Bille, J.; Sanglard, D. A mutation in Tac1p, a transcription factor regulating CDR1 and CDR2, is coupled with loss of heterozygosity at Chromosome 5 to mediate antifungal resistance in Candida albicans. Genetics 2006, 172, 2139–2156. [Google Scholar] [CrossRef] [PubMed]
- Morschhauser, J.; Barker, K.S.; Liu, T.T.; Bla, B.W.J.; Homayouni, R.; Rogers, P.D. The transcription factor Mrr1p controls expression of the MDR1 efflux pump and mediates multidrug resistance in Candida albicans. PLoS Pathog. 2007, 3, e164. [Google Scholar] [CrossRef] [PubMed]
- Dunkel, N.; Blass, J.; Rogers, P.D.; Morschhauser, J. Mutations in the multi-drug resistance regulator MRR1, followed by loss of heterozygosity, are the main cause of MDR1 overexpression in fluconazole-resistant Candida albicans strains. Mol. Microbiol. 2008, 69, 827–840. [Google Scholar] [CrossRef] [PubMed]
- Dunkel, N.; Liu, T.T.; Barker, K.S.; Homayouni, R.; Morschhauser, J.; Rogers, P.D. A gain-of-function mutation in the transcription factor Upc2p causes upregulation of ergosterol biosynthesis genes and increased fluconazole resistance in a clinical Candida albicans isolate. Eukaryot Cell 2008, 7, 1180–1190. [Google Scholar] [CrossRef] [PubMed]
- Asai, K.; Tsuchimori, N.; Okonogi, K.; Perfect, J.R.; Gotoh, O.; Yoshida, Y. Formation of azole-resistant Candida albicans by mutation of sterol 14-demethylase P450. Antimicrob. Agents Chemother. 1999, 43, 1163–1169. [Google Scholar] [CrossRef]
- Heilmann, C.J.; Schneider, S.; Barker, K.S.; Rogers, P.D.; Morschhauser, J. An A643T mutation in the transcription factor Upc2p causes constitutive ERG11 upregulation and increased fluconazole resistance in Candida albicans. Antimicrob. Agents Chemother. 2010, 54, 353–359. [Google Scholar] [CrossRef]
- Morschhauser, J. The development of fluconazole resistance in Candida albicans—An example of microevolution of a fungal pathogen. J. Microbiol. 2016, 54, 192–201. [Google Scholar] [CrossRef]
- Rustad, T.R.; Stevens, D.A.; Pfaller, M.A.; White, T.C. Homozygosity at the Candida albicans MTL locus associated with azole resistance. Microbiology 2002, 148, 1061–1072. [Google Scholar] [CrossRef] [PubMed]
- Pujol, C.; Messer, S.A.; Pfaller, M.; Soll, D.R. Drug resistance is not directly affected by mating type locus zygosity in Candida albicans. Antimicrob. Agents Chemother. 2003, 47, 1207–1212. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Manoharlal, R.; Gorantala, J.; Sharma, M.; Sanglard, D.; Prasad, R. PAP1 [poly(A) polymerase 1] homozygosity and hyperadenylation are major determinants of increased mRNA stability of CDR1 in azole-resistant clinical isolates of Candida albicans. Microbiology 2010, 156, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Popp, C.; Ramirez-Zavala, B.; Schwanfelder, S.; Kruger, I.; Morschhauser, J. Evolution of fluconazole-resistant Candida albicans strains by drug-induced mating competence and parasexual recombination. MBio 2019, 10, e02740-18. [Google Scholar] [CrossRef]
- Ahmad, A.; Kabir, M.A.; Kravets, A.; Andaluz, E.; Larriba, G.; Rustchenko, E. Chromosome instability and unusual features of some widely used strains of Candida albicans. Yeast 2008, 25, 433–448. [Google Scholar] [CrossRef]
- Selmecki, A.; Bergmann, S.; Berman, J. Comparative genome hybridization reveals widespread aneuploidy in Candida albicans laboratory strains. Mol. Microbiol. 2005, 55, 1553–1565. [Google Scholar] [CrossRef]
- Legrand, M.; Forche, A.; Selmecki, A.; Chan, C.; Kirkpatrick, D.T.; Berman, J. Haplotype mapping of a diploid non-meiotic organism using existing and induced aneuploidies. PLoS Genet. 2008, 4, e1. [Google Scholar] [CrossRef]
- Abbey, D.; Hickman, M.; Gresham, D.; Berman, J. High-resolution SNP/CGH microarrays reveal the accumulation of loss of heterozygosity in commonly used Candida albicans strains. G3 (Bethesda) 2011, 1, 523–530. [Google Scholar] [CrossRef]
- Sanglard, D.; Hube, B.; Monod, M.; Odds, F.C.; Gow, N.A. A triple deletion of the secreted aspartyl proteinase genes SAP4, SAP5, and SAP6 of Candida albicans causes attenuated virulence. Infect. Immun. 1997, 65, 3539–3546. [Google Scholar]
- Dunkel, N.; Morschhauser, J. Loss of heterozygosity at an unlinked genomic locus is responsible for the phenotype of a Candida albicans sap4Δ sap5Δ sap6Δ mutant. Eukaryot Cell 2011, 10, 54–62. [Google Scholar] [CrossRef]
- Ciudad, T.; Hickman, M.; Bellido, A.; Berman, J.; Larriba, G. Phenotypic consequences of a spontaneous loss of heterozygosity in a common laboratory strain of Candida albicans. Genetics 2016, 203, 1161–1176. [Google Scholar] [CrossRef] [PubMed]
- Coelho, M.C.; Pinto, R.M.; Murray, A.W. Heterozygous mutations cause genetic instability in a yeast model of cancer evolution. Nature 2019, 566, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Jakobson, C.M.; Jarosz, D.F. Molecular origins of complex heritability in natural genotype-to-phenotype relationships. Cell Syst. 2019, 8, 363–379.e3. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, S.-H.; Bennett, R.J. The Impact of Gene Dosage and Heterozygosity on the Diploid Pathobiont Candida albicans. J. Fungi 2020, 6, 10. https://doi.org/10.3390/jof6010010
Liang S-H, Bennett RJ. The Impact of Gene Dosage and Heterozygosity on the Diploid Pathobiont Candida albicans. Journal of Fungi. 2020; 6(1):10. https://doi.org/10.3390/jof6010010
Chicago/Turabian StyleLiang, Shen-Huan, and Richard J. Bennett. 2020. "The Impact of Gene Dosage and Heterozygosity on the Diploid Pathobiont Candida albicans" Journal of Fungi 6, no. 1: 10. https://doi.org/10.3390/jof6010010
APA StyleLiang, S.-H., & Bennett, R. J. (2020). The Impact of Gene Dosage and Heterozygosity on the Diploid Pathobiont Candida albicans. Journal of Fungi, 6(1), 10. https://doi.org/10.3390/jof6010010




