The Dysbiosis and Inter-Kingdom Synergy Model in Oropharyngeal Candidiasis, a New Perspective in Pathogenesis
Abstract
1. Introduction
1.1. Oral Mucosal Homeostasis, Relationships between Resident Bacterial Microbiota and C. albicans in Health
1.2. Immunocompomised Hosts Have Altered Oral Bacterial Microbiomes but Not Significantly Altered Mycobiomes
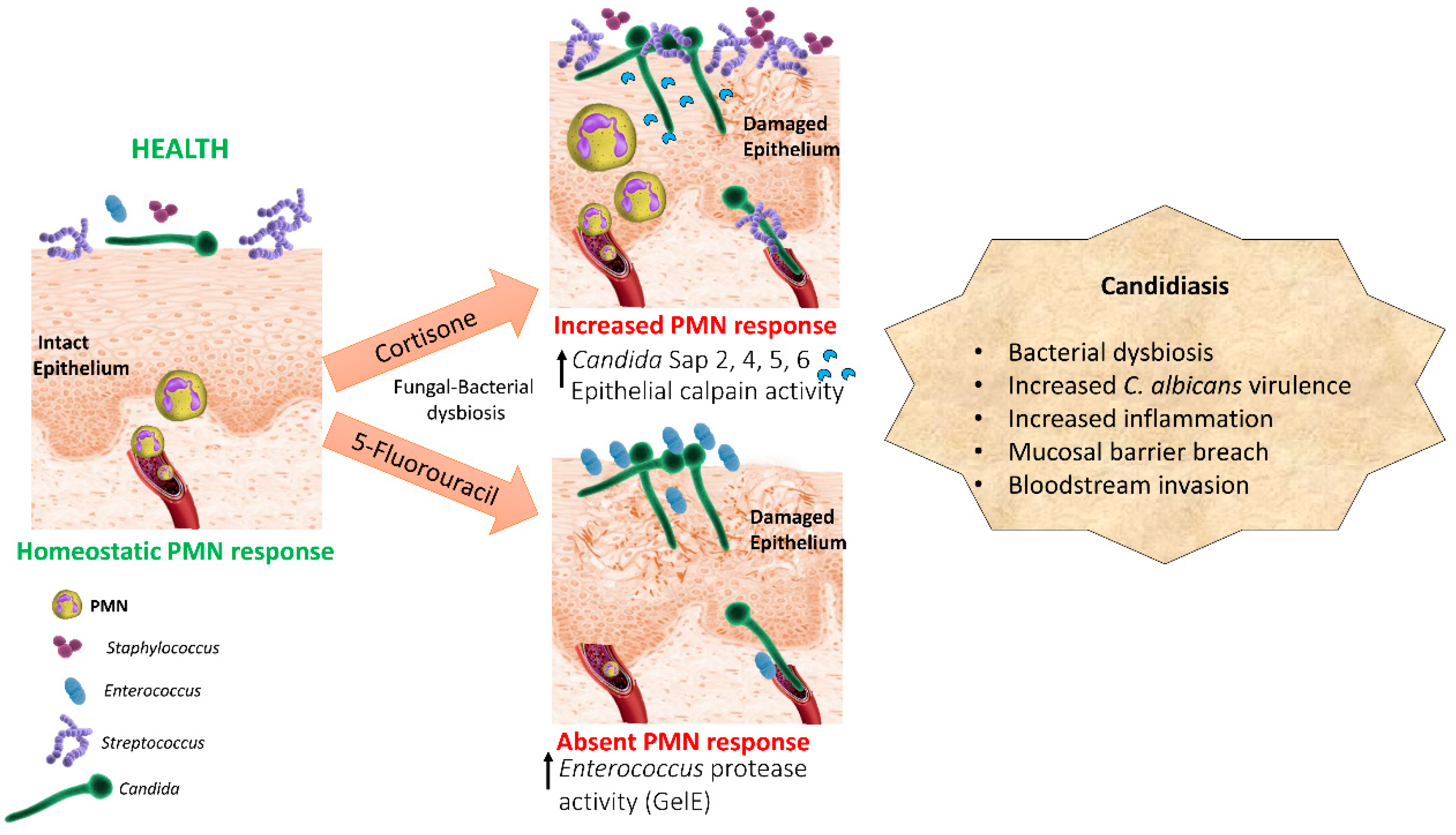
2. Oropharyngeal Candidiasis as a Result of a Dysbiotic Oral Microbiome
2.1. Synergism between Candida and Oral Opportunistic Bacteria in Mouse Models
2.2. A Mouse Chemotherapy Model Provides Proof of Concept for the Role of Dysbiotic Bacterial Communities in Candida Pathogenesis
2.3. Evidence for Bacterial Dybiosis in Oropharyngeal Candidiasis in Humans
3. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Nagy, K.N.; Sonkodi, I.; Szoke, I.; Nagy, E.; Newman, H.N. The microflora associated with human oral carcinomas. Oral Oncol. 1998, 3434, 304–308. [Google Scholar] [CrossRef]
- Brent, N.B. Thrush in the breastfeeding dyad: Results of a survey on diagnosis and treatment. Clin. Pediat. 2001, 4040, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Nicolatou-Galitis, O.; Dardoufas, K.; Markoulatos, P.; Sotiropoulou-Lontou, A.; Kyprianou, K.; Kolitsi, G.; Pissakas, G.; Skarleas, C.; Kouloulias, V.; Papanicolaou, V.; et al. Oral pseudomembranous candidiasis, herpes simplex virus-1 infection, and oral mucositis in head and neck cancer patients receiving radiotherapy and granulocyte-macrophage colony-stimulating factor (GM-CSF) mouthwash. J. Oral Pathol. Med. 2001, 3030, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Lucatorto, F.M.; Franker, C.; Hardy, W.D.; Chafey, S. Treatment of refractory oral candidiasis with fluconazole. A case report. Oral Surg. Oral Med. Oral Pathol. 1991, 7171, 42–44. [Google Scholar] [CrossRef]
- Redding, S.W.; Kirkpatrick, W.R.; Coco, B.J.; Sadkowski, L.; Fothergill, A.W.; Rinaldi, M.G.; Eng, T.Y.; Patterson, T.F. Candida glabrata oropharyngeal candidiasis in patients receiving radiation treatment for head and neck cancer. J. Clin. Microbiol. 2002, 4040, 1879–1881. [Google Scholar] [CrossRef]
- Jarvis, W.R. Epidemiology of nosocomial fungal infections, with emphasis on Candida species. Clin. Infect. Dis. 1995, 2020, 1526–1530. [Google Scholar] [CrossRef] [PubMed]
- Teoh, F.; Pavelka, N. How Chemotherapy Increases the Risk of Systemic Candidiasis in Cancer Patients: Current Paradigm and Future Directions. Pathogens 2016, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Meunier, F.; Gerard, M.; Richard, V.; Debusscher, L.; Bleiberg, H.; Malengrau, A. Hepatic candidosis in a patient with acute leukemia. Mycoses 1989, 3232, 421–426. [Google Scholar]
- Cole, G.T.; Halawa, A.A.; Anaissie, E.J. The role of the gastrointestinal tract in hematogenous candidiasis: From the laboratory to the bedside. Clin. Infect. Dis. 1996, 22, S73–S88. [Google Scholar] [CrossRef]
- Gow, N.A.; van de Veerdonk, F.L.; Brown, A.J.; Netea, M.G. Candida albicans morphogenesis and host defence: Discriminating invasion from colonization. Nat. Rev. Microbiol. 2011, 1010, 112–122. [Google Scholar] [CrossRef]
- Ranjan, A.; Dongari-Bagtzoglou, A. Tipping the Balance: C albicans Adaptation in Polymicrobial Environments. J. Fungi. (Basel) 2018, 18, 112. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, M.; Ranjan, A.; Thompson, A.; Diaz, P.I.; Sobue, T.; Maas, K.; Dongari-Bagtzoglou, A. Candida albicans induces mucosal bacterial dysbiosis that promotes invasive infection. PLoS Pathog. 2019, 22, e1007717. [Google Scholar] [CrossRef] [PubMed]
- Diaz, P.I.; Xie, Z.; Sobue, T.; Thompson, A.; Biyikoglu, B.; Ricker, A.; Ikonomou, L.; Dongari-Bagtzoglou, A. Synergistic interaction between Candida albicans and commensal oral streptococci in a novel in vitro mucosal model. Infect. Immun. 2012, 8080, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Falsetta, M.L.; Klein, M.I.; Colonne, P.M.; Scott-Anne, K.; Gregoire, S.; Pai, C.H.; Gonzalez-Begne, M.; Watson, G.; Krysan, D.J.; Bowen, W.H.; et al. Symbiotic relationship between Streptococcus mutans and Candida albicans synergizes virulence of plaque biofilms in vivo. Infect. Immun. 2014, 82, 1968–1981. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Sobue, T.; Thompson, A.; Xie, Z.; Poon, K.; Ricker, A.; Cervantes, J.; Diaz, P.I.; Dongari-Bagtzoglou, A. Streptococcal co-infection augments Candida pathogenicity by amplifying the mucosal inflammatory response. Cell. Microbiol. 2014, 16, 214–231. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Sobue, T.; Bertolini, M.; Thompson, A.; Dongari-Bagtzoglou, A. Streptococcus oralis and Candida albicans Synergistically Activate mu-Calpain to Degrade E-cadherin From Oral Epithelial Junctions. J. Infect. Dis. 2016, 214, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Sobue, T.; Bertolini, M.; Thompson, A.; Vickerman, M.; Nobile, C.J.; Dongari-Bagtzoglou, A. S. oralis activates the Efg1 filamentation pathway in C. albicans to promote cross-kingdom interactions and mucosal biofilms. Virulence 2017, 8, 1602–1617. [Google Scholar] [CrossRef]
- Kim, D.; Liu, Y.; Benhamou, R.I.; Sanchez, H.; Simon-Soro, A.; Li, Y.; Hwang, G.; Fridman, M.; Andes, D.R.; Koo, H. Bacterial-derived exopolysaccharides enhance antifungal drug tolerance in a cross-kingdom oral biofilm. ISME J. 2018, 12, 1427–1442. [Google Scholar] [CrossRef] [PubMed]
- Bandara, H.M.H.N.; Panduwawala, C.P.; Samaranayake, L.P. Biodiversity of the human oral mycobiome in health and disease. Oral Dis. 2019, 25, 363–371. [Google Scholar] [CrossRef]
- Ghannoum, M.A.; Jurevic, R.J.; Mukherjee, P.K.; Cui, F.; Sikaroodi, M.; Naqvi, A.; Gillevet, P.M. Characterization of the oral fungal microbiome (mycobiome) in healthy individuals. PLoS Pathog. 2010, 8, e1000713. [Google Scholar] [CrossRef]
- Dupuy, A.K.; David, M.S.; Li, L.; Heider, T.N.; Peterson, J.D.; Montano, E.A.; Dongari-Bagtzoglou, A.; Diaz, P.I.; Strausbaugh, L.D. Redefining the human oral mycobiome with improved practices in amplicon-based taxonomy: Discovery of Malassezia as a prominent commensal. PLoS ONE 2014, 9, e90899. [Google Scholar] [CrossRef] [PubMed]
- Diaz, P.I.; Hong, B.Y.; Dupuy, A.K.; Choquette, L.; Thompson, A.; Salner, A.L.; Schauer, P.K.; Hegde, U.; Burleson, J.A.; Strausbaugh, L.D.; et al. Integrated Analysis of Clinical and Microbiome Risk Factors Associated with the Development of Oral Candidiasis during Cancer Chemotherapy. J. Fungi. (Basel) 2019, 5, 49. [Google Scholar] [CrossRef] [PubMed]
- Russell, C.; Lay, K.M. Natural history of Candida species and yeasts in the oral cavities of infants. Arch. Oral Biol. 1973, 18, 957–962. [Google Scholar] [CrossRef]
- Kleinegger, C.L.; Lockhart, S.R.; Vargas, K.; Soll, D.R. Frequency, intensity, species, and strains of oral Candida vary as a function of host age. J. Clin. Microbiol. 1996, 34, 2246–2254. [Google Scholar] [PubMed]
- Kraneveld, E.A.; Buijs, M.J.; Bonder, M.J.; Visser, M.; Keijser, B.J.; Crielaard, W.; Zaura, E. The relation between oral Candida load and bacterial microbiome profiles in Dutch older adults. PLoS ONE 2012, 7, e42770. [Google Scholar] [CrossRef] [PubMed]
- Monif, G.R.; Carson, H.J. Female genital tract bacterial coisolates with Candida albicans in patients without clinical vaginitis. Infect. Dis. Obstet. Gynecol. 1998, 6, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Cools, P.; Jespers, V.; Hardy, L.; Crucitti, T.; Delany-Moretlwe, S.; Mwaura, M.; Ndayisaba, G.F.; van de Wijgert, J.H.; Vaneechoutte, M. A Multi-Country Cross-Sectional Study of Vaginal Carriage of Group B Streptococci (GBS) and Escherichia coli in Resource-Poor Settings: Prevalences and Risk Factors. PLoS ONE 2016, 11, e0148052. [Google Scholar] [CrossRef] [PubMed]
- Bayó, M.; Berlanga, M.; Agut, M. Vaginal microbiota in healthy pregnant women and prenatal screening of group B streptococci (GBS). Int. Microbiol. 2002, 5, 87–90. [Google Scholar] [CrossRef]
- Iliev, I.D.; Funari, V.A.; Taylor, K.D.; Nguyen, Q.; Reyes, C.N.; Strom, S.P.; Brown, J.; Becker, C.A.; Fleshner, P.R.; Dubinsky, M.; et al. Interactions between commensal fungi and the C-type lectin receptor Dectin-1 influence colitis. Science 2012, 336, 1314–1317. [Google Scholar] [CrossRef]
- Mason, K.L.; Erb Downward, J.R.; Mason, K.D.; Falkowski, N.R.; Eaton, K.A.; Kao, J.Y.; Young, V.B.; Huffnagle, G.B. Candida albicans and bacterial microbiota interactions in the cecum during recolonization following broad-spectrum antibiotic therapy. Infect. Immun. 2012, 80, 3371–3380. [Google Scholar] [CrossRef]
- Fan, D.; Coughlin, L.A.; Neubauer, M.M.; Kim, J.; Kim, M.S.; Zhan, X.; Simms-Waldrip, T.R.; Xie, Y.; Hooper, L.V.; Koh, A.Y. Activation of HIF-1α and LL-37 by commensal bacteria inhibits Candida albicans colonization. Nat. Med. 2015, 21, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Shankar, J.; Solis, N.V.; Mounaud, S.; Szpakowski, S.; Liu, H.; Losada, L.; Nierman, W.C.; Filler, S.G. Using Bayesian modelling to investigate factors governing antibiotic-induced Candida albicans colonization of the GI tract. Sci. Rep. 2015, 5, 8131. [Google Scholar] [CrossRef] [PubMed]
- Mason, K.L.; Erb Downward, J.R.; Falkowski, N.R.; Young, V.B.; Kao, J.Y.; Huffnagle, G.B. Interplay between the gastric bacterial microbiota and Candida albicans during postantibiotic recolonization and gastritis. Infect. Immun. 2012, 80, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.Y. Murine models of Candida gastrointestinal colonization and dissemination. Eukaryot. Cell. 2013, 12, 1416–1422. [Google Scholar] [CrossRef] [PubMed]
- Passalacqua, G.; Albano, M.; Canonica, G.W.; Bachert, C.; Van Cauwenberge, P.; Davies, R.J.; Durham, S.R.; Kontou-Fili, K.; Horak, F.; Malling, H.J. Inhaled and nasal corticosteroids: Safety aspects. Allergy 2000, 55, 16–33. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Majumdar, B.; Sarode, S.C.; Sarode, G.S.; Awan, K.H. Oropharyngeal Candidosis in HIV-Infected Patients-An Update. Front. Microbiol. 2018, 9, 980. [Google Scholar] [CrossRef] [PubMed]
- de Repentigny, L.; Goupil, M.; Jolicoeur, P. Oropharyngeal Candidiasis in HIV Infection: Analysis of Impaired Mucosal Immune Response to Candida albicans in Mice Expressing the HIV-1 Transgene. Pathogens 2015, 4, 406–421. [Google Scholar] [CrossRef] [PubMed]
- Diaz, P.I.; Hong, B.Y.; Frias-Lopez, J.; Dupuy, A.K.; Angeloni, M.; Abusleme, L.; Terzi, E.; Ioannidou, E.; Strausbaugh, L.D.; Dongari-Bagtzoglou, A. Transplantation-associated long-term immunosuppression promotes oral colonization by potentially opportunistic pathogens without impacting other members of the salivary bacteriome. Clin. Vaccine Immunol. 2013, 20, 920–930. [Google Scholar] [CrossRef]
- Holler, E.; Butzhammer, P.; Schmid, K.; Hundsrucker, C.; Koestler, J.; Peter, K.; Zhu, W.; Sporrer, D.; Hehlgans, T.; Kreutz, M.; et al. Metagenomic analysis of the stool microbiome in patients receiving allogeneic stem cell transplantation: Loss of diversity is associated with use of systemic antibiotics and more pronounced in gastrointestinal graft-versus-host disease. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2014, 20, 640–645. [Google Scholar] [CrossRef]
- Charlson, E.S.; Diamond, J.M.; Bittinger, K.; Fitzgerald, A.S.; Yadav, A.; Haas, A.R.; Bushman, F.D.; Collman, R.G. Lung-enriched organisms and aberrant bacterial and fungal respiratory microbiota after lung transplant. Am. J. Respir. Crit. Care Med. 2012, 186, 536–545. [Google Scholar] [CrossRef]
- Hong, B.Y.; Sobue, T.; Choquette, L.; Dupuy, A.K.; Thompson, A.; Burleson, J.A.; Salner, A.L.; Schauer, P.K.; Joshi, P.; Fox, E.; et al. Chemotherapy-induced oral mucositis is associated with detrimental bacterial dysbiosis. Microbiome 2019, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.B.; Mouridsen, H.T.; Bergmann, O.J.; Reibel, J.; Brünner, N.; Nauntofte, B. Oral mucosal lesions, microbial changes, and taste disturbances induced by adjuvant chemotherapy in breast cancer patients. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2008, 106, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Villafuerte, K.R.V.; Martinez, C.J.H.; Dantas, F.T.; Carrara, H.H.A.; Dos Reis, F.J.C.; Palioto, D.B. The impact of chemotherapeutic treatment on the oral microbiota of patients with cancer: A systematic review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 552–566. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Chandra, J.; Retuerto, M.; Tatsuoka, C.; Ghannoum, M.A.; McComsey, G.A. Dysbiosis in the oral bacterial and fungal microbiome of HIV-infected subjects is associated with clinical and immunologic variables of HIV infection. PLoS ONE 2018, 13, e0200285. [Google Scholar] [CrossRef]
- Bertolini, M.; Sobue, T.; Thompson, A.; Dongari-Bagtzoglou, A. Chemotherapy Induces Oral Mucositis in Mice Without Additional Noxious Stimuli. Transl. Oncol. 2017, 10, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, R.T.; Harmsen, A.G. The relative contribution of resident pulmonary alveolar macrophage and inflammatory polymorphonuclear neutrophils in host resistance to pulmonary infection by Candida albicans. Mycopathologia 1989, 108, 95–105. [Google Scholar] [PubMed]
- Leendertse, M.; Willems, R.J.; Giebelen, I.A.; Roelofs, J.J.; Bonten, M.J.; van der Poll, T. Neutrophils are essential for rapid clearance of Enterococcus faecium in mice. Infect. Immun. 2009, 77, 485–491. [Google Scholar] [CrossRef]
- Dutzan, N.; Abusleme, L.; Bridgeman, H.; Greenwell-Wild, T.; Zangerle-Murray, T.; Fife, M.E.; Bouladoux, N.; Linley, H.; Brenchley, L.; Wemyss, K.; et al. On-going Mechanical Damage from Mastication Drives Homeostatic Th17 Cell Responses at the Oral Barrier. Immunity 2017, 46, 133–147. [Google Scholar] [CrossRef]
- Sobue, T.; Diaz, P.; Xu, H.; Bertolini, M.; Dongari-Bagtzoglou, A. Experimental Models of C. albicans-Streptococcal Co-infection. Methods Mol. Biol. 2016, 1356, 137–152. [Google Scholar] [CrossRef]
- Witchley, J.N.; Penumetcha, P.; Abon, N.V.; Woolford, C.A.; Mitchell, A.P.; Noble, S.M. Candida albicans Morphogenesis Programs Control the Balance between Gut Commensalism and Invasive Infection. Cell Host Microbe 2019, 25, 432–443. [Google Scholar] [CrossRef]
- Kong, E.F.; Kucharíková, S.; Van Dijck, P.; Peters, B.M.; Shirtliff, M.E.; Jabra-Rizk, M.A. Clinical implications of oral candidiasis: Host tissue damage and disseminated bacterial disease. Infect Immun. 2015, 83, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Peters, B.M.; Noverr, M.C. Candida albicans-Staphylococcus aureus polymicrobial peritonitis modulates host innate immunity. Infect Immun. 2013, 81, 2178–2189. [Google Scholar] [CrossRef] [PubMed]
- Dongari-Bagtzoglou, A.; Kashleva, H.; Dwivedi, P.; Diaz, P.; Vasilakos, J. Characterization of mucosal Candida albicans biofilms. PLoS ONE 2009, 4, e7967. [Google Scholar] [CrossRef]
- Morse, D.J.; Smith, A.; Wilson, M.J.; Marsh, L.; White, L.; Posso, R.; Bradshaw, D.J.; Wei, X.; Lewis, M.A.O.; Williams, D.W. Molecular community profiling of the bacterial microbiota associated with denture-related stomatitis. Sci. Rep. 2019, 9, 10228. [Google Scholar] [CrossRef] [PubMed]
- Abusleme, L.; Diaz, P.I.; Freeman, A.F.; Greenwell-Wild, T.; Brenchley, L.; Desai, J.V.; Ng, W.I.; Holland, S.M.; Lionakis, M.S.; Segre, J.A.; et al. Human defects in STAT3 promote oral mucosal fungal and bacterial dysbiosis. JCI Insight. 2018, 3, 122061. [Google Scholar] [CrossRef] [PubMed]
- Sparo, M.; Delpech, G.; García Allende, N. Impact on Public Health of the Spread of High-Level Resistance to Gentamicin and Vancomycin in Enterococci. Front. Microbiol. 2018, 9, 3073. [Google Scholar] [CrossRef] [PubMed]
- Komiyama, E.Y.; Lepesqueur, L.S.; Yassuda, C.G.; Samaranayake, L.P.; Parahitiyawa, N.B.; Balducci, I.; Koga-Ito, C.Y. Enterococcus Species in the Oral Cavity: Prevalence, Virulence Factors and Antimicrobial Susceptibility. PLoS ONE 2016, 11, e0163001. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, L.S.; Soares Ferreira, S.M.; Souza, C.O.; Souto, R.; Colombo, A.P. Clinical and microbiological profiles of human immunodeficiency virus (HIV)-seropositive Brazilians undergoing highly active antiretroviral therapy and HIV-seronegative Brazilians with chronic periodontitis. J. Periodontol. 2007, 78, 87–96. [Google Scholar] [CrossRef]
- Gonçalves, L.S.; Souto, R.; Colombo, A.P. Detection of Helicobacter pylori, Enterococcus faecalis, and Pseudomonas aeruginosa in the subgingival biofilm of HIV-infected subjects undergoing HAART with chronic periodontitis. Eur. J. Clin. Microbiol. Infect. Dis. 2009, 28, 1335–1342. [Google Scholar] [CrossRef]
- Yang, C.H.; Chew, K.Y.; Solomkin, J.S.; Lin, P.Y.; Chiang, Y.C.; Kuo, Y.R. Surgical site infections among high-risk patients in clean-contaminated head and neck reconstructive surgery: Concordance with preoperative oral flora. Ann. Plast. Surg. 2013, 71, S55–S60. [Google Scholar] [CrossRef]
- Osakabe, L.; Utsumi, A.; Saito, B.; Okamatsu, Y.; Kinouchi, H.; Nakamaki, T.; Hironaka, S. Influence of Oral Anaerobic Bacteria on Hematopoietic Stem Cell Transplantation Patients: Oral Mucositis and General Condition. Transplant. Proc. 2017, 49, 2176–2182. [Google Scholar] [CrossRef] [PubMed]
- Lalla, R.V.; Latortue, M.C.; Hong, C.H.; Ariyawardana, A.; D’Amato-Palumbo, S.; Fischer, D.J.; Martof, A.; Nicolatou-Galitis, O.; Patton, L.L.; Elting, L.S.; et al. Fungal Infections Section, Oral Care Study Group, Multinational Association of Supportive Care in Cancer (MASCC)/International Society of Oral Oncology (ISOO). A systematic review of oral fungal infections in patients receiving cancer therapy. Support Care Cancer 2010, 18, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Olczak-Kowalczyk, D.; Daszkiewicz, M.; Krasuska-Sławińska Dembowska-Bagińska, B.; Gozdowski, D.; Daszkiewicz, P.; Fronc, B.; Semczuk, K. Bacteria and Candida yeasts in inflammations of the oral mucosa in children with secondary immunodeficiency. J. Oral. Pathol. Med. 2012, 41, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Carlson, E.; Johnson, G. Protection by Candida albicans of Staphylococcus aureus in the establishment of dual infection in mice. Infect. Immun. 1985, 50, 655–659. [Google Scholar] [PubMed]
- Lagkouvardos, I.; Joseph, D.; Kapfhammer, M.; Giritli, S.; Horn, M.; Haller, D.; Clavel, T. IMNGS: A comprehensive open resource of processed 16S rRNA microbial profiles for ecology and diversity studies. Sci. Rep. 2016, 6, 33721. [Google Scholar] [CrossRef] [PubMed]
- Niimi, M.; Firth, N.A.; Cannon, R.D. Antifungal drug resistance of oral fungi. Odontology 2010, 98, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Gligorov, J.; Bastit, L.; Gervais, H.; Henni, M.; Kahila, W.; Lepille, D.; Luporsi, E.; Sasso, G.; Varette, C.; Azria, D.; et al. Prevalence and treatment management of oropharyngeal candidiasis in cancer patients: Results of the French CANDIDOSCOPE study. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 532–539. [Google Scholar] [CrossRef]
- Elahi, S.; Pang, G.; Ashman, R.; Clancy, R. Enhanced clearance of Candida albicans from the oral cavities of mice following oral administration of Lactobacillus acidophilus. Clin. Exp. Immunol. 2005, 141, 29–36. [Google Scholar] [CrossRef]
- Matsubara, V.H.; Silva, E.G.; Paula, C.R.; Ishikawa, K.H.; Nakamae, A.E. Treatment with probiotics in experimental oral colonization by Candida albicans in murine model (DBA/2). Oral Dis. 2012, 18, 260–264. [Google Scholar] [CrossRef]
- Hatakka, K.; Ahola, A.J.; Yli-Knuuttila, H.; Richardson, M.; Poussa, T.; Meurman, J.H.; Korpela, R. Probiotics reduce the prevalence of oral candida in the elderly—a randomized controlled trial. J. Dent. Res. 2007, 86, 125–130. [Google Scholar] [CrossRef]
- Ishikawa, K.H.; Mayer, M.P.; Miyazima, T.Y.; Matsubara, V.H.; Silva, E.G.; Paula, C.R.; Campos, T.T.; Nakamae, A.E. A multispecies probiotic reduces oral Candida colonization in denture wearers. J Prosthodont. 2015, 24, 194–199. [Google Scholar] [CrossRef]
- Matsubara, V.H.; Ishikawa, K.H.; Ando-Suguimoto, E.S.; Bueno-Silva, B.; Nakamae, A.E.M.; Mayer, M.P.A. Probiotic Bacteria Alter Pattern-Recognition Receptor Expression and Cytokine Profile in a Human Macrophage Model Challenged with Candida albicans and Lipopolysaccharide. Front. Microbiol. 2017, 8, 2280. [Google Scholar] [CrossRef]
- Yeaman, M.R.; Filler, S.G.; Schmidt, C.S.; Ibrahim, A.S.; Edwards, J.E., Jr.; Hennessey, J.P., Jr. Applying Convergent Immunity to Innovative Vaccines Targeting Staphylococcus aureus. Front. Immunol. 2014, 5, 463. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertolini, M.; Dongari-Bagtzoglou, A. The Dysbiosis and Inter-Kingdom Synergy Model in Oropharyngeal Candidiasis, a New Perspective in Pathogenesis. J. Fungi 2019, 5, 87. https://doi.org/10.3390/jof5040087
Bertolini M, Dongari-Bagtzoglou A. The Dysbiosis and Inter-Kingdom Synergy Model in Oropharyngeal Candidiasis, a New Perspective in Pathogenesis. Journal of Fungi. 2019; 5(4):87. https://doi.org/10.3390/jof5040087
Chicago/Turabian StyleBertolini, Martinna, and Anna Dongari-Bagtzoglou. 2019. "The Dysbiosis and Inter-Kingdom Synergy Model in Oropharyngeal Candidiasis, a New Perspective in Pathogenesis" Journal of Fungi 5, no. 4: 87. https://doi.org/10.3390/jof5040087
APA StyleBertolini, M., & Dongari-Bagtzoglou, A. (2019). The Dysbiosis and Inter-Kingdom Synergy Model in Oropharyngeal Candidiasis, a New Perspective in Pathogenesis. Journal of Fungi, 5(4), 87. https://doi.org/10.3390/jof5040087



