Immune Response of Galleria mellonella against Human Fungal Pathogens
Abstract
1. Introduction
2. Advantages and Disadvantages of the G. mellonella Model
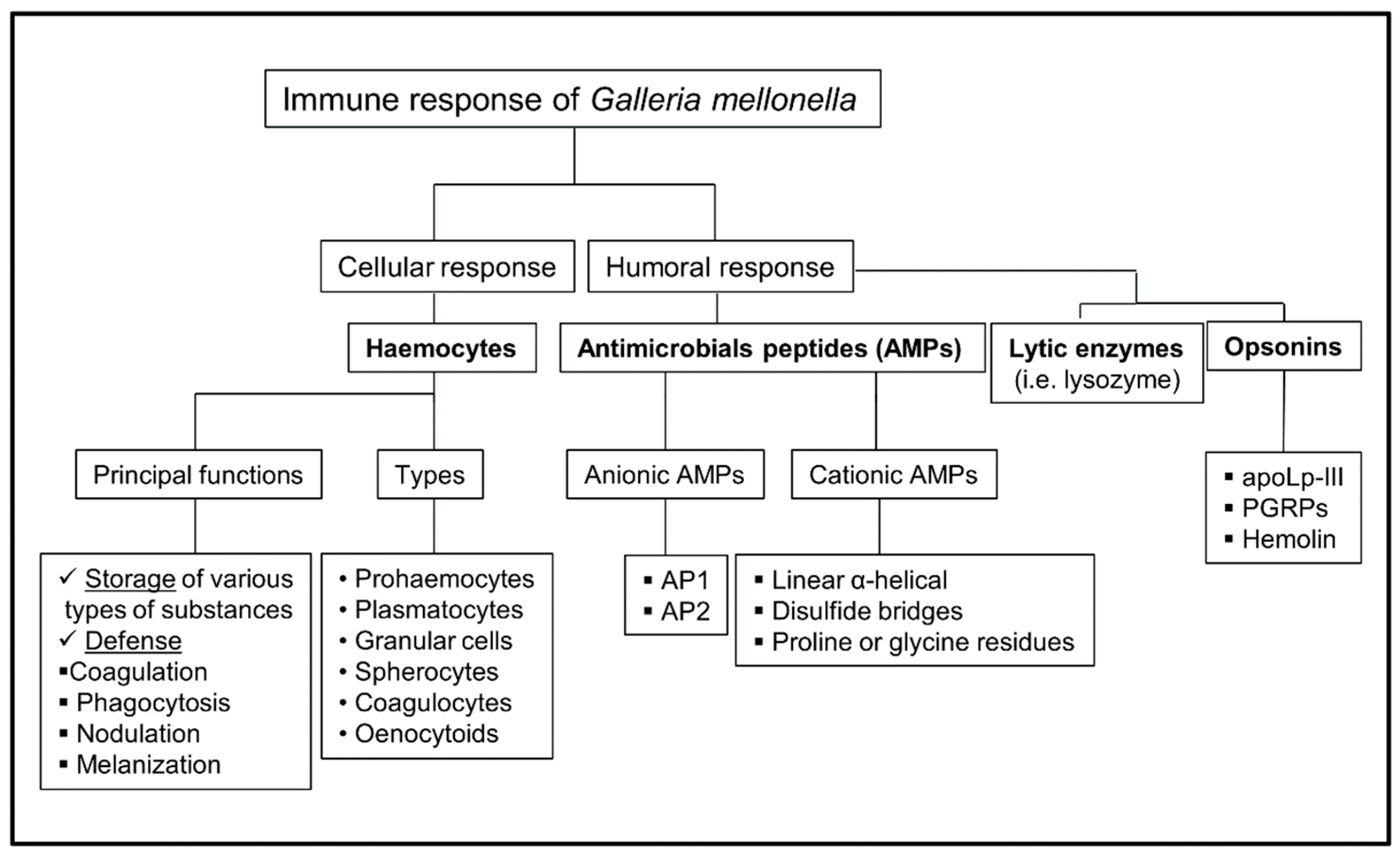
3. Immune Response of G. mellonella
4. Cellular Immune Response
5. Humoral Immune Response
5.1. Antimicrobials Peptides (AMPs)
5.2. Lytic Enzymes (Lysozymes)
5.3. Melanization
5.4. Opsonins
6. Galleria mellonella as a Model to Study Fungal Pathogens
7. Response of G. mellonella to Different Fungi
7.1. Candida spp.
7.2. Aspergillus fumigatus
7.3. Cryptococcus neoformans
7.4. Other Fungal Pathogens
8. Future Perspectives
Author Contributions
Funding
Acknowledgements
Conflicts of Interest
References
- Trevijano-Contador, N.; Zaragoza, O. Expanding the use of alternative models to investigate novel aspects of immunity to microbial pathogens. Virulence 2014, 5, 454–456. [Google Scholar] [CrossRef] [PubMed]
- Vilmos, P.; Kurucz, E. Insect immunity: Evolutionary roots of the mammalian innate immune system. Immunol. Lett. 1998, 62, 59–66. [Google Scholar] [CrossRef]
- Junqueira, J.C. Galleria mellonella as a model host for human pathogens: Recent studies and new perspectives. Virulence 2012, 3, 474–476. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, B.B.; O’Brien, E.; Khoury, J.B.; Mylonakis, E. Methods for using Galleria mellonella as a model host to study fungal pathogenesis. Virulence 2010, 1, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.J.; Loh, J.M.; Proft, T. Galleria mellonella infection models for the study of bacterial diseases and for antimicrobial drug testing. Virulence 2016, 7, 214–229. [Google Scholar] [CrossRef] [PubMed]
- Trevijano-Contador, N.; Herrero-Fernandez, I.; Garcia-Barbazan, I.; Scorzoni, L.; Rueda, C.; Rossi, S.A.; Garcia-Rodas, R.; Zaragoza, O. Cryptococcus neoformans induces antimicrobial responses and behaves as a facultative intracellular pathogen in the non mammalian model Galleria mellonella. Virulence 2015, 6, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Reeves, E.P.; Messina, C.G.; Doyle, S.; Kavanagh, K. Correlation between gliotoxin production and virulence of Aspergillus fumigatus in Galleria mellonella. Mycopathologia 2004, 158, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.; Thomas, D.Y.; Whiteway, M.; Kavanagh, K. Correlation between virulence of Candida albicans mutants in mice and Galleria mellonella larvae. FEMS Immunol. Med. Microbiol. 2002, 34, 153–157. [Google Scholar] [CrossRef]
- Cotter, G.; Doyle, S.; Kavanagh, K. Development of an insect model for the in vivo pathogenicity testing of yeasts. FEMS Immunol. Med. Microbiol. 2000, 27, 163–169. [Google Scholar] [CrossRef]
- Dunphy, G.B.; Oberholzer, U.; Whiteway, M.; Zakarian, R.J.; Boomer, I. Virulence of Candida albicans mutants toward larval Galleria mellonella (insecta, lepidoptera, galleridae). Can. J. Microbiol. 2003, 49, 514–524. [Google Scholar] [CrossRef]
- Mylonakis, E.; Moreno, R.; El Khoury, J.B.; Idnurm, A.; Heitman, J.; Calderwood, S.B.; Ausubel, F.M.; Diener, A. Galleria mellonella as a model system to study Cryptococcus neoformans pathogenesis. Infect. Immunity 2005, 73, 3842–3850. [Google Scholar] [CrossRef]
- Aperis, G.; Fuchs, B.B.; Anderson, C.A.; Warner, J.E.; Calderwood, S.B.; Mylonakis, E. Galleria mellonella as a model host to study infection by the francisella tularensis live vaccine strain. Microbes Infect. 2007, 9, 729–734. [Google Scholar] [CrossRef]
- Lange, A.; Beier, S.; Huson, D.H.; Parusel, R.; Iglauer, F.; Frick, J.S. Genome sequence of Galleria mellonella (greater wax moth). Genome Announc. 2018, 6, e01220-17. [Google Scholar] [CrossRef]
- Kelly, J.; Kavanagh, K. Caspofungin primes the immune response of the larvae of Galleria mellonella and induces a non-specific antimicrobial response. J. Med. Microbiol. 2011, 60, 189–196. [Google Scholar] [CrossRef]
- Maguire, R.; Duggan, O.; Kavanagh, K. Evaluation of Galleria mellonella larvae as an in vivo model for assessing the relative toxicity of food preservative agents. Cell Biol. Toxicol. 2016, 32, 209–216. [Google Scholar] [CrossRef]
- Kavanagh, K.; Reeves, E.P. Exploiting the potential of insects for in vivo pathogenicity testing of microbial pathogens. FEMS Microbiol. Rev. 2004, 28, 101–112. [Google Scholar] [CrossRef]
- James, R.R.; Xu, J. Mechanisms by which pesticides affect insect immunity. J. Invertebr. Pathol. 2012, 109, 175–182. [Google Scholar] [CrossRef]
- Boman, H.G.; Hultmark, D. Cell-free immunity in insects. Annu. Rev. Microbiol. 1987, 41, 103–126. [Google Scholar] [CrossRef]
- Tojo, S.; Naganuma, F.; Arakawa, K.; Yokoo, S. Involvement of both granular cells and plasmatocytes in phagocytic reactions in the greater wax moth, Galleria mellonella. J. Insect. Physiol. 2000, 46, 1129–1135. [Google Scholar] [CrossRef]
- Browne, N.; Heelan, M.; Kavanagh, K. An analysis of the structural and functional similarities of insect hemocytes and mammalian phagocytes. Virulence 2013, 4, 597–603. [Google Scholar] [CrossRef]
- Altincicek, B.; Stotzel, S.; Wygrecka, M.; Preissner, K.T.; Vilcinskas, A. Host-derived extracellular nucleic acids enhance innate immune responses, induce coagulation, and prolong survival upon infection in insects. J. Immunol. 2008, 181, 2705–2712. [Google Scholar] [CrossRef]
- Gillespie, J.P.; Kanost, M.R.; Trenczek, T. Biological mediators of insect immunity. Annu. Rev. Entomol. 1997, 42, 611–643. [Google Scholar] [CrossRef]
- Schmit, A.R.; Ratcliffe, N.A. The encapsulation of foreign tissue implants in Galleria mellonella larvae. J. Insect. Physiol. 1977, 23, 175–184. [Google Scholar] [CrossRef]
- Bergin, D.; Reeves, E.P.; Renwick, J.; Wientjes, F.B.; Kavanagh, K. Superoxide production in Galleria mellonella hemocytes: Identification of proteins homologous to the nadph oxidase complex of human neutrophils. Infect. Immunity 2005, 73, 4161–4170. [Google Scholar] [CrossRef]
- Renwick, J.; Reeves, E.P.; Wientjes, F.B.; Kavanagh, K. Translocation of proteins homologous to human neutrophil p47phox and p67phox to the cell membrane in activated hemocytes of Galleria mellonella. Dev. Comp. Immunol. 2007, 31, 347–359. [Google Scholar] [CrossRef]
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef]
- Brown, S.E.; Howard, A.; Kasprzak, A.B.; Gordon, K.H.; East, P.D. The discovery and analysis of a diverged family of novel antifungal moricin-like peptides in the wax moth Galleria mellonella. Insect Biochem. Mol. Biol. 2008, 38, 201–212. [Google Scholar] [CrossRef]
- Kim, C.H.; Lee, J.H.; Kim, I.; Seo, S.J.; Son, S.M.; Lee, K.Y.; Lee, I.H. Purification and cdna cloning of a cecropin-like peptide from the great wax moth, Galleria mellonella. Mol. Cells 2004, 17, 262–266. [Google Scholar]
- Brown, S.E.; Howard, A.; Kasprzak, A.B.; Gordon, K.H.; East, P.D. A peptidomics study reveals the impressive antimicrobial peptide arsenal of the wax moth Galleria mellonella. Insect Biochem. Mol. Biol. 2009, 39, 792–800. [Google Scholar] [CrossRef]
- Langen, G.; Imani, J.; Altincicek, B.; Kieseritzky, G.; Kogel, K.H.; Vilcinskas, A. Transgenic expression of gallerimycin, a novel antifungal insect defensin from the greater wax moth Galleria mellonella, confers resistance to pathogenic fungi in tobacco. Biol. Chem. 2006, 387, 549–557. [Google Scholar] [CrossRef]
- Cytrynska, M.; Mak, P.; Zdybicka-Barabas, A.; Suder, P.; Jakubowicz, T. Purification and characterization of eight peptides from Galleria mellonella immune hemolymph. Peptides 2007, 28, 533–546. [Google Scholar] [CrossRef]
- Kawaoka, S.; Katsuma, S.; Daimon, T.; Isono, R.; Omuro, N.; Mita, K.; Shimada, T. Functional analysis of four gloverin-like genes in the silkworm, Bombyx mori. Arch. Insect Biochem. Physiol. 2008, 67, 87–96. [Google Scholar] [CrossRef]
- Bulet, P.; Hetru, C.; Dimarcq, J.L.; Hoffmann, D. Antimicrobial peptides in insects; structure and function. Dev. Comp. Immunol. 1999, 23, 329–344. [Google Scholar] [CrossRef]
- Sowa-Jasilek, A.; Zdybicka-Barabas, A.; Staczek, S.; Wydrych, J.; Mak, P.; Jakubowicz, T.; Cytrynska, M. Studies on the role of insect hemolymph polypeptides: Galleria mellonella anionic peptide 2 and lysozyme. Peptides 2014, 53, 194–201. [Google Scholar] [CrossRef]
- Lockey, T.D.; Ourth, D.D. Purification and characterization of lysozyme from hemolymph of heliothis virescens larvae. Biochem. Biophys. Res. Commun. 1996, 220, 502–508. [Google Scholar] [CrossRef]
- Mohrig, W.; Messner, B. Lysozyme as antibacterial agent in honey and bees venom. Acta Biol. Med. Ger. 1968, 21, 85–95. [Google Scholar]
- Vogel, H.; Altincicek, B.; Glockner, G.; Vilcinskas, A. A comprehensive transcriptome and immune-gene repertoire of the lepidopteran model host Galleria mellonella. BMC Genom. 2011, 12, 308. [Google Scholar] [CrossRef]
- Yu, K.H.; Kim, K.N.; Lee, J.H.; Lee, H.S.; Kim, S.H.; Cho, K.Y.; Nam, M.H.; Lee, I.H. Comparative study on characteristics of lysozymes from the hemolymph of three lepidopteran larvae, Galleria mellonella, Bombyx mori, Agrius convolvuli. Dev. Comp. Immunol. 2002, 26, 707–713. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, Y.Y.; Chang, J.Y.; Park, M.S.; Kho, H.S. The effects of peroxidase on the enzymatic and candidacidal activities of lysozyme. Arch. Oral Biol. 2010, 55, 607–612. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Nakaoka, C.; Hiratani, T.; Abe, S.; Uchida, K.; Yamaguchi, H. Synergy of lysozyme and lanoconazole on the morphology of Candida albicans. J. Electron Microsc. 2001, 50, 41–49. [Google Scholar] [CrossRef]
- Wu, T.; Samaranayake, L.P.; Leung, W.K.; Sullivan, P.A. Inhibition of growth and secreted aspartyl proteinase production in Candida albicans by lysozyme. J. Med. Microbiol. 1999, 48, 721–730. [Google Scholar] [CrossRef]
- Ratcliffe, N.A.; Rowley, A.F.; Fitzgerald, S.W.; Rhodes, C.P. Invertebrate immunity: Basic concepts and recent advances. Int. Rev. Cytol. 1985, 97, 183–350. [Google Scholar]
- Götz, P. Encapsulation in arthropods. Immunity Invertebr. 1986, 153–170. [Google Scholar]
- Masaaki Ashida, H.Y. Limited proteolysis of prophenoloxidase during activation by microbial products in insect plasma and effect of phenoloxidase on electrophoretic mobilities of plasma proteins. J. Insect Biochem. 1988, 18, 11–19. [Google Scholar] [CrossRef]
- Soderhall, K. Prophenoloxidase-activating cascade as a recognition and defense system in arthropods. Immunity Invertebr. 1986, 208–223. [Google Scholar]
- Soderhall, K.S.; Smith, V.J. The prophenoloxidase activating system. The biochemistry of its activation and role in arthropod cellular immunity, with special reference to crustacenad. Immunity Arthropods 1986, 251–285. [Google Scholar]
- Yu, X.Q.; Kanost, M.R. Immulectin-2, a lipopolysaccharide-specific lectin from an insect, manduca sexta, is induced in response to gram-negative bacteria. J. Biol. Chem. 2000, 275, 37373–37381. [Google Scholar] [CrossRef]
- Yu, X.Q.; Kanost, M.R. Binding of hemolin to bacterial lipopolysaccharide and lipoteichoic acid. An immunoglobulin superfamily member from insects as a pattern-recognition receptor. Eur. J. Biochem. 2002, 269, 1827–1834. [Google Scholar] [CrossRef]
- Yoshida, H.; Kinoshita, K.; Ashida, M. Purification of a peptidoglycan recognition protein from hemolymph of the silkworm, Bombyx mori. J. Biol. Chem. 1996, 271, 13854–13860. [Google Scholar] [CrossRef]
- Wang, X.; Rocheleau, T.A.; Fuchs, J.F.; Christensen, B.M. Beta 1, 3-glucan recognition protein from the mosquito, armigeres subalbatus, is involved in the recognition of distinct types of bacteria in innate immune responses. Cell. Microbiol. 2006, 8, 1581–1590. [Google Scholar] [CrossRef]
- Blacklock, B.J.; Ryan, R.O. Hemolymph lipid transport. Insect Biochem. Mol. Biol. 1994, 24, 855–873. [Google Scholar] [CrossRef]
- Sun, D.; Ziegler, R.; Milligan, C.E.; Fahrbach, S.; Schwartz, L.M. Apolipophorin iii is dramatically up-regulated during the programmed death of insect skeletal muscle and neurons. J. Neurobiol. 1995, 26, 119–129. [Google Scholar] [CrossRef]
- Ladendorff, N.E.; Kanost, M.R. Isolation and characterization of bacteria-induced protein p4 from hemolymph of manduca sexta. Arch. Insect Biochem. Physiol. 1990, 15, 33–41. [Google Scholar] [CrossRef]
- Terenius, O. Hemolin-a lepidopteran anti-viral defense factor? Dev. Comp. Immunol. 2008, 32, 311–316. [Google Scholar] [CrossRef]
- Hoffmann, J.A. The immune response of drosophila. Nature 2003, 426, 33–38. [Google Scholar] [CrossRef]
- Alcazar-Fuoli, L.; Buitrago, M.; Gomez-Lopez, A.; Mellado, E. An alternative host model of a mixed fungal infection by azole susceptible and resistant aspergillus spp strains. Virulence 2015, 6, 376–384. [Google Scholar] [CrossRef]
- Gomez-Lopez, A.; Forastiero, A.; Cendejas-Bueno, E.; Gregson, L.; Mellado, E.; Howard, S.J.; Livermore, J.L.; Hope, W.W.; Cuenca-Estrella, M. An invertebrate model to evaluate virulence in Aspergillus fumigatus: The role of azole resistance. Med. Mycol. 2014, 52, 311–319. [Google Scholar] [CrossRef]
- Thomaz, L.; Garcia-Rodas, R.; Guimaraes, A.J.; Taborda, C.P.; Zaragoza, O.; Nosanchuk, J.D. Galleria mellonella as a model host to study Paracoccidioides lutzii and histoplasma capsulatum. Virulence 2013, 4, 139–146. [Google Scholar] [CrossRef]
- Desalermos, A.; Tan, X.; Rajamuthiah, R.; Arvanitis, M.; Wang, Y.; Li, D.; Kourkoumpetis, T.K.; Fuchs, B.B.; Mylonakis, E. A multi-host approach for the systematic analysis of virulence factors in Cryptococcus neoformans. J. Infect. Dis. 2015, 211, 298–305. [Google Scholar] [CrossRef]
- Slater, J.L.; Gregson, L.; Denning, D.W.; Warn, P.A. Pathogenicity of Aspergillus fumigatus mutants assessed in Galleria mellonella matches that in mice. Med. Mycol. 2011, 49 (Suppl. 1), S107–S113. [Google Scholar] [CrossRef]
- Halldorsdottir, A.M.; Carayannopoulos, M.O.; Scrivner, M.; Gronowski, A.M. Method evaluation for total beta-human chorionic gonadotropin using urine and the advia centaur. Clin. Chem. 2003, 49, 1421–1422. [Google Scholar] [CrossRef]
- Fidel, P.L., Jr. Candida-host interactions in hiv disease: Implications for oropharyngeal candidiasis. Adv. Dent. Res. 2011, 23, 45–49. [Google Scholar] [CrossRef]
- Benjamin, D.K., Jr.; Garges, H.; Steinbach, W.J. Candida bloodstream infection in neonates. Semin. Perinatol. 2003, 27, 375–383. [Google Scholar] [CrossRef]
- Li, D.D.; Deng, L.; Hu, G.H.; Zhao, L.X.; Hu, D.D.; Jiang, Y.Y.; Wang, Y. Using Galleria mellonella—Candida albicans infection model to evaluate antifungal agents. Biol. Pharm. Bull. 2013, 36, 1482–1487. [Google Scholar] [CrossRef]
- Sherry, L.; Rajendran, R.; Lappin, D.F.; Borghi, E.; Perdoni, F.; Falleni, M.; Tosi, D.; Smith, K.; Williams, C.; Jones, B.; et al. Biofilms formed by Candida albicans bloodstream isolates display phenotypic and transcriptional heterogeneity that are associated with resistance and pathogenicity. BMC Microbiol. 2014, 14, 182. [Google Scholar] [CrossRef]
- Mensa, J.; Pitart, C.; Marco, F. Treatment of critically ill patients with candidemia. Int. J. Antimicrob. Agents 2008, 32 (Suppl. 2), S93–S97. [Google Scholar] [CrossRef]
- Wisplinghoff, H.; Bischoff, T.; Tallent, S.M.; Seifert, H.; Wenzel, R.P.; Edmond, M.B. Nosocomial bloodstream infections in us hospitals: Analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 2004, 39, 309–317. [Google Scholar] [CrossRef]
- Santos, R.B.; Scorzoni, L.; Namba, A.M.; Rossoni, R.D.; Jorge, A.O.C.; Junqueira, J.C. Lactobacillus species increase the survival of Galleria mellonella infected with Candida albicans and non-albicans Candida clinical isolates. Med. Mycol. 2018. [Google Scholar] [CrossRef]
- Silva, L.N.; Campos-Silva, R.; Ramos, L.S.; Trentin, D.S.; Macedo, A.J.; Branquinha, M.H.; Santos, A.L.S. Virulence of Candida haemulonii complex in Galleria mellonella and efficacy of classical antifungal drugs: A comparative study with other clinically relevant non-albicans Candida species. FEMS Yeast Res. 2018, 18, foy082. [Google Scholar] [CrossRef]
- Moralez, A.T.; Perini, H.F.; Furlaneto-Maia, L.; Almeida, R.S.; Panagio, L.A.; Furlaneto, M.C. Phenotypic switching of Candida tropicalis is associated with cell damage in epithelial cells and virulence in Galleria mellonella model. Virulence 2016, 7, 379–386. [Google Scholar] [CrossRef]
- Mesa-Arango, A.C.; Forastiero, A.; Bernal-Martinez, L.; Cuenca-Estrella, M.; Mellado, E.; Zaragoza, O. The non-mammalian host Galleria mellonella can be used to study the virulence of the fungal pathogen Candida tropicalis and the efficacy of antifungal drugs during infection by this pathogenic yeast. Med. Mycol. 2012, 51, 461–472. [Google Scholar] [CrossRef]
- Ames, L.; Duxbury, S.; Pawlowska, B.; Ho, H.L.; Haynes, K.; Bates, S. Galleria mellonella as a host model to study candida glabrata virulence and antifungal efficacy. Virulence 2017, 8, 1909–1917. [Google Scholar] [CrossRef]
- Scorzoni, L.; de Lucas, M.P.; Mesa-Arango, A.C.; Fusco-Almeida, A.M.; Lozano, E.; Cuenca-Estrella, M.; Mendes-Giannini, M.J.; Zaragoza, O. Antifungal efficacy during Candida krusei infection in non-conventional models correlates with the yeast in vitro susceptibility profile. PLoS ONE 2013, 8, e60047. [Google Scholar] [CrossRef]
- Fuchs, B.B.; Li, Y.; Li, D.; Johnston, T.; Hendricks, G.; Li, G.; Rajamuthiah, R.; Mylonakis, E. Micafungin elicits an immunomodulatory effect in Galleria mellonella and mice. Mycopathologia 2016, 181, 17–25. [Google Scholar] [CrossRef]
- Banville, N.; Fallon, J.; McLoughlin, K.; Kavanagh, K. Disruption of haemocyte function by exposure to cytochalasin b or nocodazole increases the susceptibility of Galleria mellonella larvae to infection. Microbes Infect. 2011, 13, 1191–1198. [Google Scholar] [CrossRef]
- Sheehan, G.; Kavanagh, K. Analysis of the early cellular and humoral responses of Galleria mellonella larvae to infection by Candida albicans. Virulence 2018, 9, 163–172. [Google Scholar] [CrossRef]
- Kim, C.H.; Shin, Y.P.; Noh, M.Y.; Jo, Y.H.; Han, Y.S.; Seong, Y.S.; Lee, I.H. An insect multiligand recognition protein functions as an opsonin for the phagocytosis of microorganisms. J. Biol. Chem. 2010, 285, 25243–25250. [Google Scholar] [CrossRef]
- Zdybicka-Barabas, A.; Staczek, S.; Mak, P.; Piersiak, T.; Skrzypiec, K.; Cytrynska, M. The effect of Galleria mellonella apolipophorin iii on yeasts and filamentous fungi. J. Insect. Physiol. 2012, 58, 164–177. [Google Scholar] [CrossRef]
- Weers, P.M.; Ryan, R.O. Apolipophorin iii: A lipid-triggered molecular switch. Insect Biochem. Mol. Biol. 2003, 33, 1249–1260. [Google Scholar] [CrossRef]
- Staczek, S.; Zdybicka-Barabas, A.; Mak, P.; Sowa-Jasilek, A.; Kedracka-Krok, S.; Jankowska, U.; Suder, P.; Wydrych, J.; Grygorczuk, K.; Jakubowicz, T.; et al. Studies on localization and protein ligands of Galleria mellonella apolipophorin iii during immune response against different pathogens. J. Insect. Physiol. 2018, 105, 18–27. [Google Scholar] [CrossRef]
- Camilli, G.; Tabouret, G.; Quintin, J. The complexity of fungal beta-glucan in health and disease: Effects on the mononuclear phagocyte system. Front. Immunol. 2018, 9, 673. [Google Scholar] [CrossRef]
- Mowlds, P.; Coates, C.; Renwick, J.; Kavanagh, K. Dose-dependent cellular and humoral responses in Galleria mellonella larvae following beta-glucan inoculation. Microbes Infect. 2010, 12, 146–153. [Google Scholar] [CrossRef]
- Bergin, D.; Murphy, L.; Keenan, J.; Clynes, M.; Kavanagh, K. Pre-exposure to yeast protects larvae of Galleria mellonella from a subsequent lethal infection by Candida albicans and is mediated by the increased expression of antimicrobial peptides. Microbes Infect. 2006, 8, 2105–2112. [Google Scholar] [CrossRef]
- Sowa-Jasilek, A.; Zdybicka-Barabas, A.; Staczek, S.; Wydrych, J.; Skrzypiec, K.; Mak, P.; Derylo, K.; Tchorzewski, M.; Cytrynska, M. Galleria mellonella lysozyme induces apoptotic changes in Candida albicans cells. Microbiol. Res. 2016, 193, 121–131. [Google Scholar] [CrossRef]
- Sheehan, G.; Clarke, G.; Kavanagh, K. Characterisation of the cellular and proteomic response of Galleria mellonella larvae to the development of invasive aspergillosis. BMC Microbiol. 2018, 18, 63. [Google Scholar] [CrossRef]
- Fallon, J.P.; Reeves, E.P.; Kavanagh, K. The Aspergillus fumigatus toxin fumagillin suppresses the immune response of Galleria mellonella larvae by inhibiting the action of haemocytes. Microbiology 2011, 157, 1481–1488. [Google Scholar] [CrossRef]
- Fallon, J.P.; Troy, N.; Kavanagh, K. Pre-exposure of Galleria mellonella larvae to different doses of Aspergillus fumigatus conidia causes differential activation of cellular and humoral immune responses. Virulence 2011, 2, 413–421. [Google Scholar] [CrossRef]
- Jackson, J.C.; Higgins, L.A.; Lin, X. Conidiation color mutants of Aspergillus fumigatus are highly pathogenic to the heterologous insect host Galleria mellonella. PLoS ONE 2009, 4, e4224. [Google Scholar] [CrossRef]
- Lazera, M.S.; Salmito Cavalcanti, M.A.; Londero, A.T.; Trilles, L.; Nishikawa, M.M.; Wanke, B. Possible primary ecological niche of Cryptococcus neoformans. Med. Mycol. 2000, 38, 379–383. [Google Scholar] [CrossRef]
- Casadevall, A.; Perfect, J. Cryptococcus Neoformans; ASM: Washington, DC, USA, 1998; p. 541. [Google Scholar]
- Venn-Watson, S.; Daniels, R.; Smith, C. Thirty year retrospective evaluation of pneumonia in a bottlenose dolphin Tursiops truncatus population. Dis. Aquat. Organ. 2012, 99, 237–242. [Google Scholar] [CrossRef]
- Warpeha, K.M.; Park, Y.D.; Williamson, P.R. Susceptibility of intact germinating arabidopsis thaliana to human fungal pathogens Cryptococcus neoformans and C. gattii. Appl. Environ. Microbiol. 2013, 79, 2979–2988. [Google Scholar] [CrossRef]
- Steenbergen, J.N.; Shuman, H.A.; Casadevall, A. Cryptococcus neoformans interactions with amoebae suggest an explanation for its virulence and intracellular pathogenic strategy in macrophages. Proc. Natl. Acad. Sci. USA 2001, 98, 15245–15250. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.M.; Huang, M.; Botts, M.R.; Hull, C.M.; Huttenlocher, A. A zebrafish model of cryptococcal infection reveals roles for macrophages, endothelial cells, and neutrophils in the establishment and control of sustained fungemia. Infect. Immunity 2016, 84, 3047–3062. [Google Scholar] [CrossRef] [PubMed]
- Zaragoza, O.; Rodrigues, M.L.; De Jesus, M.; Frases, S.; Dadachova, E.; Casadevall, A. The capsule of the fungal pathogen Cryptococcus neoformans. Adv. Appl. Microbiol. 2009, 68, 133–216. [Google Scholar] [PubMed]
- Heitman, J.; Kozel, T.R.; Kwon-Chung, K.J.; Perferct, J.R.; Casadevall, A. Cryptococcus. From Human Pathogen to Model Yeast; ASM Press: Washington, DC, USA, 2011. [Google Scholar]
- Smith, L.M.; Dixon, E.F.; May, R.C. The fungal pathogen Cryptococcus neoformans manipulates macrophage phagosome maturation. Cell. Microbiol. 2015, 17, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Tucker, S.C.; Casadevall, A. Replication of Cryptococcus neoformans in macrophages is accompanied by phagosomal permeabilization and accumulation of vesicles containing polysaccharide in the cytoplasm. Proc. Natl. Acad. Sci. USA 2002, 99, 3165–3170. [Google Scholar] [CrossRef] [PubMed]
- Doering, T.L. How sweet it is! Cell wall biogenesis and polysaccharide capsule formation in Cryptococcus neoformans. Annu. Rev. Microbiol. 2009, 63, 223–247. [Google Scholar] [CrossRef]
- O’Meara, T.R.; Alspaugh, J.A. The Cryptococcus neoformans capsule: A sword and a shield. Clin. Microbiol. Rev. 2012, 25, 387–408. [Google Scholar] [CrossRef]
- Feldmesser, M.; Kress, Y.; Casadevall, A. Dynamic changes in the morphology of Cryptococcus neoformans during murine pulmonary infection. Microbiology 2001, 147, 2355–2365. [Google Scholar] [CrossRef]
- Okagaki, L.H.; Strain, A.K.; Nielsen, J.N.; Charlier, C.; Baltes, N.J.; Chretien, F.; Heitman, J.; Dromer, F.; Nielsen, K. Cryptococcal cell morphology affects host cell interactions and pathogenicity. PLoS Pathog. 2010, 6, e1000953. [Google Scholar] [CrossRef]
- Zaragoza, O.; Garcia-Rodas, R.; Nosanchuk, J.D.; Cuenca-Estrella, M.; Rodriguez-Tudela, J.L.; Casadevall, A. Fungal cell gigantism during mammalian infection. PLoS Pathog. 2010, 6, e1000945. [Google Scholar] [CrossRef]
- Zaragoza, O.; Nielsen, K. Titan cells in Cryptococcus neoformans: Cells with a giant impact. Curr. Opin. Microbiol. 2013, 16, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Trevijano-Contador, N.; de Oliveira, H.C.; Garcia-Rodas, R.; Rossi, S.A.; Llorente, I.; Zaballos, A.; Janbon, G.; Arino, J.; Zaragoza, O. Cryptococcus neoformans can form titan-like cells in vitro in response to multiple signals. PLoS Pathog. 2018, 14, e1007007. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rodas, R.; Casadevall, A.; Rodriguez-Tudela, J.L.; Cuenca-Estrella, M.; Zaragoza, O. Cryptococcus neoformans capsular enlargement and cellular gigantism during Galleria mellonella infection. PLoS ONE 2011, 6, e24485. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Velasco, G.Y.; Prados-Rosales, R.C.; Ortiz-Urquiza, A.; Quesada-Moraga, E.; Di Pietro, A. Galleria mellonella as model host for the trans-kingdom pathogen fusarium oxysporum. Fungal Genet. Biol. 2011, 48, 1124–1129. [Google Scholar] [CrossRef]
- Coleman, J.J.; Muhammed, M.; Kasperkovitz, P.V.; Vyas, J.M.; Mylonakis, E. Fusarium pathogenesis investigated using Galleria mellonella as a heterologous host. Fungal Biol. 2011, 115, 1279–1289. [Google Scholar] [CrossRef]
- Munoz-Gomez, A.; Corredor, M.; Benitez-Paez, A.; Pelaez, C. Development of quantitative proteomics using itraq based on the immunological response of Galleria mellonella larvae challenged with fusarium oxysporum microconidia. PLoS ONE 2014, 9, e112179. [Google Scholar] [CrossRef]
- Kashino, S.S.; Singer-Vermes, L.M.; Calich, V.L.; Burger, E. Alterations in the pathogenicity of one Paracoccidioides brasiliensis isolate do not correlative with its in vitro growth. Mycopathologia 1990, 111, 173–180. [Google Scholar] [CrossRef]
- de Oliveira, H.C.; da Silva Jde, F.; Scorzoni, L.; Marcos, C.M.; Rossi, S.A.; de Paula, E.S.A.C.; Assato, P.A.; da Silva, R.A.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J. Importance of adhesins in virulence of paracoccidioides spp. Front Microbiol. 2015, 6, 303. [Google Scholar] [CrossRef]
- Scorzoni, L.; de Paula e Silva, A.C.; Singulani Jde, L.; Leite, F.S.; de Oliveira, H.C.; da Silva, R.A.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J. Comparison of virulence between Paracoccidioides brasiliensis and Paracoccidioides lutzii using Galleria mellonella as a host model. Virulence 2015, 6, 766–776. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trevijano-Contador, N.; Zaragoza, O. Immune Response of Galleria mellonella against Human Fungal Pathogens. J. Fungi 2019, 5, 3. https://doi.org/10.3390/jof5010003
Trevijano-Contador N, Zaragoza O. Immune Response of Galleria mellonella against Human Fungal Pathogens. Journal of Fungi. 2019; 5(1):3. https://doi.org/10.3390/jof5010003
Chicago/Turabian StyleTrevijano-Contador, Nuria, and Oscar Zaragoza. 2019. "Immune Response of Galleria mellonella against Human Fungal Pathogens" Journal of Fungi 5, no. 1: 3. https://doi.org/10.3390/jof5010003
APA StyleTrevijano-Contador, N., & Zaragoza, O. (2019). Immune Response of Galleria mellonella against Human Fungal Pathogens. Journal of Fungi, 5(1), 3. https://doi.org/10.3390/jof5010003




