Disease Entities in Mucormycosis
Abstract
1. Introduction
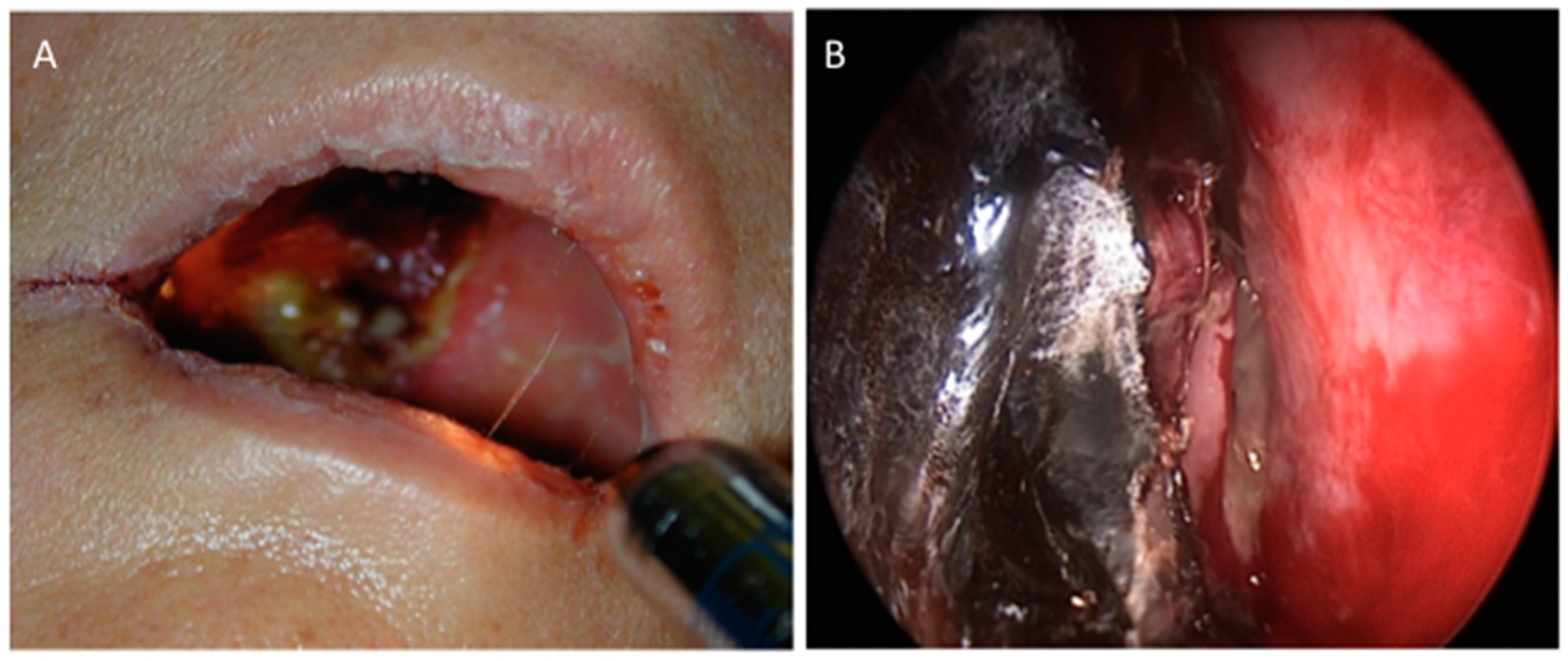
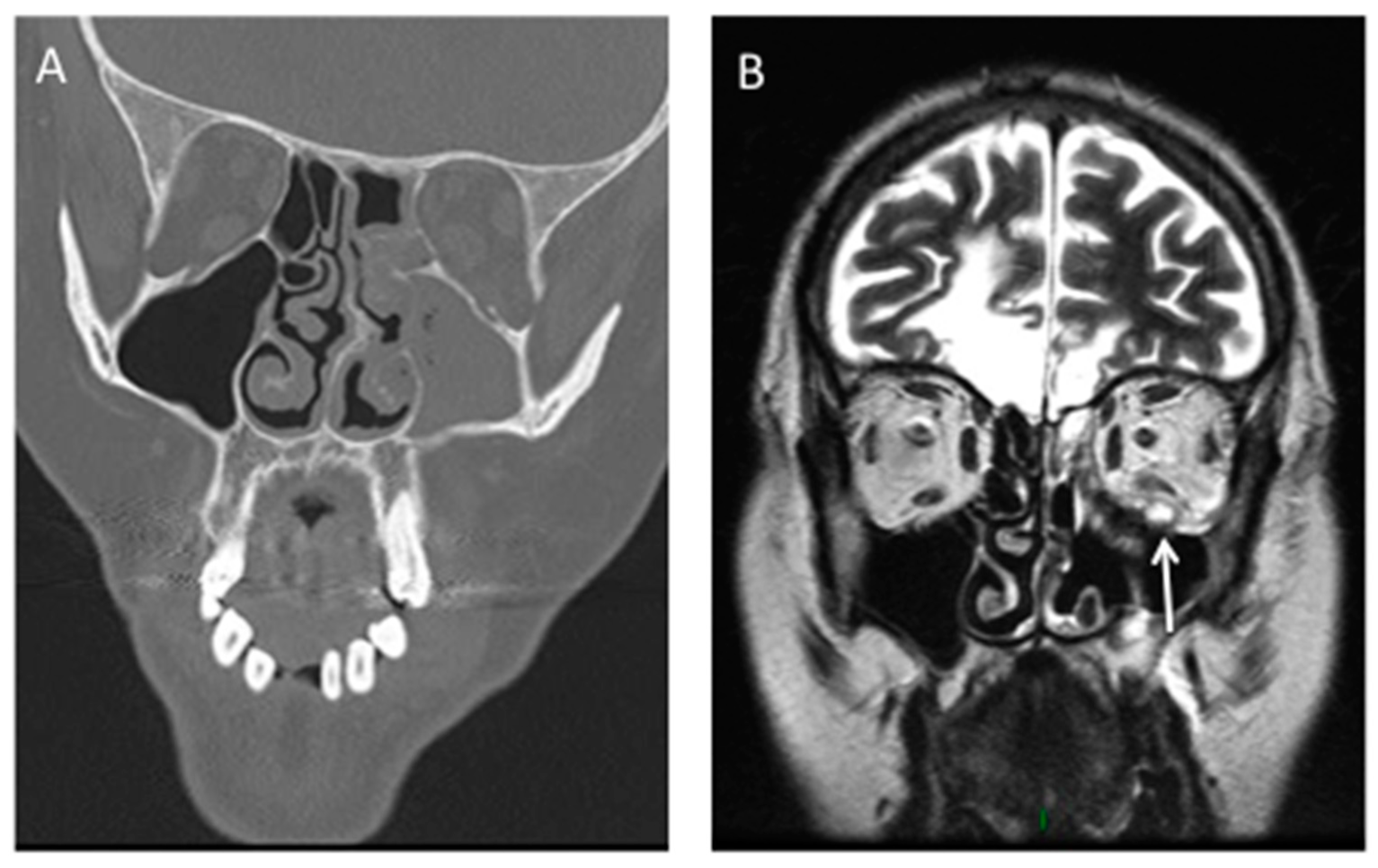
2. Rhino-Orbito-Cerebral Mucormycosis (ROCM)
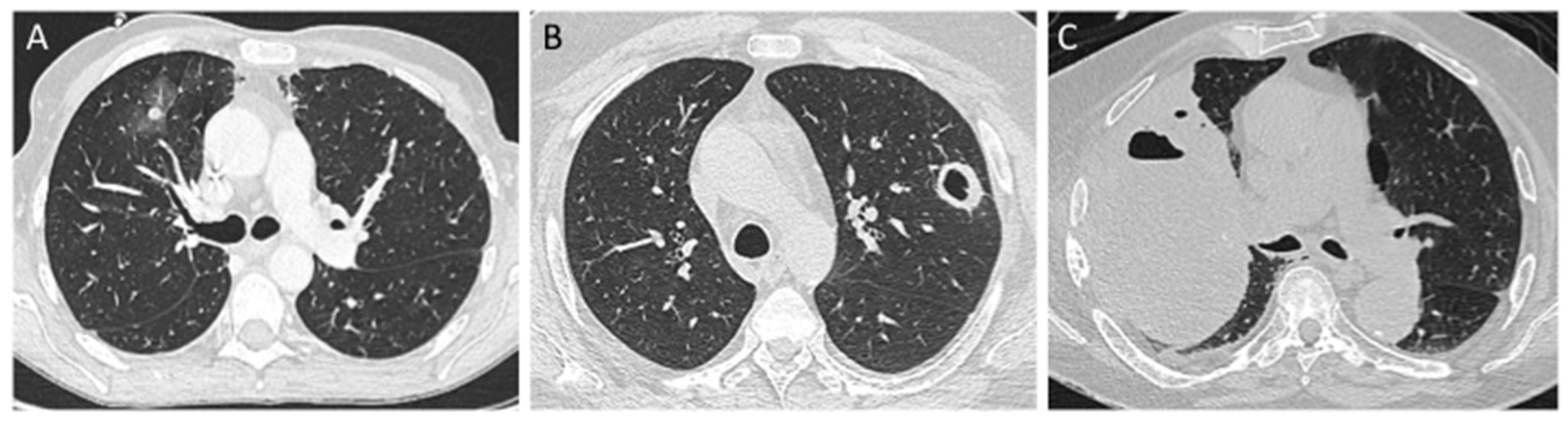
3. Pulmonary Mucormycosis (PM)
4. Cutaneous Mucormycosis
5. Gastro-Intestinal Mucormycosis
6. Disseminated Mucormycosis
7. Uncommon Presentations
8. Health-Care Associated Mucormycosis (HCM)
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bitar, D.; Van Cauteren, D.; Lanternier, F.; Dannaoui, E.; Che, D.; Dromer, F.; Desenclos, J.C.; Lortholary, O. Increasing incidence of zygomycosis (mucormycosis), France, 1997–2006. Emerg. Infect. Dis. 2009, 15, 1395–1401. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Spellberg, B.; Phan, Q.T.; Fu, Y.; Fu, Y.; Lee, A.S.; Edwards, J.E.; Filler, S.G.; Ibrahim, A.S. The endothelial cell receptor GRP78 is required for mucormycosis pathogenesis in diabetic mice. J. Clin. Invest. 2010, 120, 1914–1924. [Google Scholar] [CrossRef]
- Roden, M.M.; Zaoutis, T.E.; Buchanan, W.L.; Knudsen, T.A.; Sarkisova, T.A.; Schaufele, R.L.; Sein, M.; Sein, T.; Chiou, C.C.; Chu, J.H.; et al. Epidemiology and outcome of zygomycosis: A review of 929 reported cases. Clin. Infect. Dis. 2005, 41, 634–653. [Google Scholar] [CrossRef] [PubMed]
- Lanternier, F.; Dannaoui, E.; Morizot, G.; Elie, C.; Garcia-Hermoso, D.; Huerre, M.; Bitar, D.; Dromer, F.; Lortholary, O. French Mycosis Study Group A global analysis of mucormycosis in France: The RetroZygo Study (2005–2007). Clin. Infect. Dis. 2012, 54, S35–S43. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.; Keighley, C.; Wolfe, R.; Lee, W.L.; Slavin, M.A.; Kong, D.C.M.; Chen, S.C.A. The epidemiology and clinical manifestations of mucormycosis: A systematic review and meta-analysis of case reports. Clin. Microbiol. Infect. 2019, 25, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Skiada, A.; Pagano, L.; Groll, A.; Zimmerli, S.; Dupont, B.; Lagrou, K.; Lass-Florl, C.; Bouza, E.; Klimko, N.; Gaustad, P.; et al. Zygomycosis in Europe: Analysis of 230 cases accrued by the registry of the European Confederation of Medical Mycology (ECMM) Working Group on Zygomycosis between 2005 and 2007. Clin. Microbiol. Infect. 2011, 17, 1859–1867. [Google Scholar] [CrossRef] [PubMed]
- Pagano, L.; Valentini, C.G.; Posteraro, B.; Girmenia, C.; Ossi, C.; Pan, A.; Candoni, A.; Nosari, A.; Riva, M.; Cattaneo, C.; et al. Zygomycosis in Italy: A survey of FIMUA-ECMM (Federazione Italiana di Micopatologia Umana ed Animale and European Confederation of Medical Mycology). J. Chemother. Florence Italy 2009, 21, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Kontoyiannis, D.P.; Azie, N.; Franks, B.; Horn, D.L. Prospective antifungal therapy (PATH) alliance(®): Focus on mucormycosis. Mycoses 2014, 57, 240–246. [Google Scholar] [CrossRef]
- Vaughan, C.; Bartolo, A.; Vallabh, N.; Leong, S.C. A meta-analysis of survival factors in rhino-orbital-cerebral mucormycosis-has anything changed in the past 20 years? Clin. Otolaryngol. Off. J. ENT-UK Off. J. Neth. Soc. Oto-Rhino-Laryngol. Cervico-Facial Surg. 2018, 43, 1454–1464. [Google Scholar] [CrossRef]
- Ibrahim, A.S.; Spellberg, B.; Walsh, T.J.; Kontoyiannis, D.P. Pathogenesis of mucormycosis. Clin. Infect. Dis. 2012, 54, S16–S22. [Google Scholar] [CrossRef]
- Vironneau, P.; Kania, R.; Morizot, G.; Elie, C.; Garcia-Hermoso, D.; Herman, P.; Lortholary, O.; Lanternier, F. French Mycosis Study Group Local control of rhino-orbito-cerebral mucormycosis dramatically impacts survival. Clin. Microbiol. Infect. 2014, 20, O336–O339. [Google Scholar] [CrossRef] [PubMed]
- Son, J.H.; Lim, H.B.; Lee, S.H.; Yang, J.W.; Lee, S.B. Early Differential Diagnosis of Rhino-Orbito-Cerebral Mucormycosis and Bacterial Orbital Cellulitis: Based on Computed Tomography Findings. PLoS ONE 2016, 11, e0160897. [Google Scholar] [CrossRef]
- Tissot, F.; Agrawal, S.; Pagano, L.; Petrikkos, G.; Groll, A.H.; Skiada, A.; Lass-Flörl, C.; Calandra, T.; Viscoli, C.; Herbrecht, R. ECIL-6 guidelines for the treatment of invasive candidiasis, aspergillosis and mucormycosis in leukemia and hematopoietic stem cell transplant patients. Haematologica 2017, 102, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Yohai, R.A.; Bullock, J.D.; Aziz, A.A.; Markert, R.J. Survival factors in rhino-orbital-cerebral mucormycosis. Surv. Ophthalmol. 1994, 39, 3–22. [Google Scholar] [CrossRef]
- Danion, F.; Aguilar, C.; Catherinot, E.; Alanio, A.; DeWolf, S.; Lortholary, O.; Lanternier, F. Mucormycosis: New Developments into a Persistently Devastating Infection. Semin. Respir. Crit. Care Med. 2015, 36, 692–705. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.Y.; Aguado, J.M.; Bonatti, H.; Forrest, G.; Gupta, K.L.; Safdar, N.; John, G.T.; Pursell, K.J.; Muñoz, P.; Patel, R.; et al. Pulmonary zygomycosis in solid organ transplant recipients in the current era. Am. J. Transplant. 2009, 9, 2166–2171. [Google Scholar] [CrossRef] [PubMed]
- Park, B.J.; Pappas, P.G.; Wannemuehler, K.A.; Alexander, B.D.; Anaissie, E.J.; Andes, D.R.; Baddley, J.W.; Brown, J.M.; Brumble, L.M.; Freifeld, A.G.; et al. Invasive non-Aspergillus mold infections in transplant recipients, United States, 2001–2006. Emerg. Infect. Dis. 2011, 17, 1855–1864. [Google Scholar] [CrossRef] [PubMed]
- Pagano, L.; Offidani, M.; Fianchi, L.; Nosari, A.; Candoni, A.; Picardi, M.; Corvatta, L.; D’Antonio, D.; Girmenia, C.; Martino, P.; et al. Mucormycosis in hematologic patients. Haematologica 2004, 89, 207–214. [Google Scholar]
- Lee, F.Y.; Mossad, S.B.; Adal, K.A. Pulmonary mucormycosis: The last 30 years. Arch. Intern. Med. 1999, 159, 1301–1309. [Google Scholar] [CrossRef]
- Wahba, H.; Truong, M.T.; Lei, X.; Kontoyiannis, D.P.; Marom, E.M. Reversed halo sign in invasive pulmonary fungal infections. Clin. Infect. Dis. 2008, 46, 1733–1737. [Google Scholar] [CrossRef]
- Jung, J.; Kim, M.Y.; Lee, H.J.; Park, Y.S.; Lee, S.-O.; Choi, S.-H.; Kim, Y.S.; Woo, J.H.; Kim, S.-H. Comparison of computed tomographic findings in pulmonary mucormycosis and invasive pulmonary aspergillosis. Clin. Microbiol. Infect. 2015, 21, 684.e11–684.e18. [Google Scholar] [CrossRef] [PubMed]
- Legouge, C.; Caillot, D.; Chrétien, M.L.; Lafon, I.; Ferrant, E.; Audia, S.; Pagès, P.B.; Roques, M.; Estivalet, L.; Martin, L.; et al. The reversed halo sign: Pathognomonic pattern of pulmonary mucormycosis in leukemic patients with neutropenia? Clin. Infect. Dis. 2014, 58, 672–678. [Google Scholar] [CrossRef]
- Bourcier, J.; Heudes, P.-M.; Morio, F.; Gastinne, T.; Chevallier, P.; Rialland-Battisti, F.; Garandeau, C.; Danner-Boucher, I.; Le Pape, P.; Frampas, E.; et al. Prevalence of the reversed halo sign in neutropenic patients compared with non-neutropenic patients: Data from a single-centre study involving 27 patients with pulmonary mucormycosis (2003–2016). Mycoses 2017, 60, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Neblett Fanfair, R.; Benedict, K.; Bos, J.; Bennett, S.D.; Lo, Y.C.; Adebanjo, T.; Etienne, K.; Deak, E.; Derado, G.; Shieh, W.J.; et al. Necrotizing cutaneous mucormycosis after a tornado in Joplin, Missouri, in 2011. N. Engl. J. Med. 2012, 367, 2214–2225. [Google Scholar] [CrossRef]
- Warkentien, T.; Rodriguez, C.; Lloyd, B.; Wells, J.; Weintrob, A.; Dunne, J.R.; Ganesan, A.; Li, P.; Bradley, W.; Gaskins, L.J.; et al. Invasive mold infections following combat-related injuries. Clin. Infect. Dis. 2012, 55, 1441–1449. [Google Scholar] [CrossRef] [PubMed]
- Lelievre, L.; Garcia-Hermoso, D.; Abdoul, H.; Hivelin, M.; Chouaki, T.; Toubas, D.; Mamez, A.-C.; Lantieri, L.; Lortholary, O.; Lanternier, F.; et al. Posttraumatic mucormycosis: A nationwide study in France and review of the literature. Medicine (Baltimore) 2014, 93, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Skiada, A.; Petrikkos, G. Cutaneous zygomycosis. Clin. Microbiol. Infect. 2009, 15, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Petrikkos, G.; Skiada, A.; Lortholary, O.; Roilides, E.; Walsh, T.J.; Kontoyiannis, D.P. Epidemiology and clinical manifestations of mucormycosis. Clin. Infect. Dis. 2012, 54, S23–S34. [Google Scholar] [CrossRef]
- Agha, F.P.; Lee, H.H.; Boland, C.R.; Bradley, S.F. Mucormycoma of the colon: Early diagnosis and successful management. AJR Am. J. Roentgenol. 1985, 145, 739–741. [Google Scholar] [CrossRef]
- Dioverti, M.V.; Cawcutt, K.A.; Abidi, M.; Sohail, M.R.; Walker, R.C.; Osmon, D.R. Gastrointestinal mucormycosis in immunocompromised hosts. Mycoses 2015, 58, 714–718. [Google Scholar] [CrossRef]
- Rammaert, B.; Lanternier, F.; Zahar, J.-R.; Dannaoui, E.; Bougnoux, M.-E.; Lecuit, M.; Lortholary, O. Healthcare-associated mucormycosis. Clin. Infect. Dis. 2012, 54, S44–S54. [Google Scholar] [CrossRef] [PubMed]
- Taj-Aldeen, S.J.; Gamaletsou, M.N.; Rammaert, B.; Sipsas, N.V.; Zeller, V.; Roilides, E.; Kontoyiannis, D.P.; Henry, M.; Petraitis, V.; Moriyama, B.; et al. Bone and joint infections caused by mucormycetes: A challenging osteoarticular mycosis of the twenty-first century. Med. Mycol. 2017, 55, 691–704. [Google Scholar] [CrossRef]
- Kerezoudis, P.; Watts, C.R.; Bydon, M.; Dababneh, A.S.; Deyo, C.N.; Frye, J.M.; Kelley, P.C.; Kemp, A.M.; Palraj, B.V.; Pupillo, G.T. Diagnosis and Treatment of Isolated Cerebral Mucormycosis: Patient-Level Data Meta-Analysis and Mayo Clinic Experience. World Neurosurg. 2018, 123, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Duffy, J.; Harris, J.; Gade, L.; Sehulster, L.; Newhouse, E.; O’Connell, H.; Noble-Wang, J.; Rao, C.; Balajee, S.A.; Chiller, T. Mucormycosis outbreak associated with hospital linens. Pediatr. Infect. Dis. J. 2014, 33, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Cheng, V.C.C.; Chen, J.H.K.; Wong, S.C.Y.; Leung, S.S.M.; So, S.Y.C.; Lung, D.C.; Lee, W.-M.; Trendell-Smith, N.J.; Chan, W.-M.; Ng, D.; et al. Hospital Outbreak of Pulmonary and Cutaneous Zygomycosis due to Contaminated Linen Items From Substandard Laundry. Clin. Infect. Dis. 2016, 62, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Sundermann, A.J.; Clancy, C.J.; Pasculle, A.W.; Liu, G.; Cumbie, R.B.; Driscoll, E.; Ayres, A.; Donahue, L.; Pergam, S.A.; Abbo, L.; et al. How Clean Is the Linen at My Hospital? The Mucorales on Unclean Linen Discovery Study of Large United States Transplant and Cancer Centers. Clin. Infect. Dis. 2018, 68, 850–853. [Google Scholar] [CrossRef] [PubMed]
- Legrand, M.; Gits-Muselli, M.; Boutin, L.; Garcia-Hermoso, D.; Maurel, V.; Soussi, S.; Benyamina, M.; Ferry, A.; Chaussard, M.; Hamane, S.; et al. Detection of Circulating Mucorales DNA in Critically Ill Burn Patients: Preliminary Report of a Screening Strategy for Early Diagnosis and Treatment. Clin. Infect. Dis. 2016, 63, 1312–1317. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, A.; Chatterjee, S.S.; Das, A.; Panda, N.; Shivaprakash, M.R.; Kaur, A.; Varma, S.C.; Singhi, S.; Bhansali, A.; Sakhuja, V. Invasive zygomycosis in India: Experience in a tertiary care hospital. Postgrad. Med. J. 2009, 85, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Corzo-León, D.E.; Chora-Hernández, L.D.; Rodríguez-Zulueta, A.P.; Walsh, T.J. Diabetes mellitus as the major risk factor for mucormycosis in Mexico: Epidemiology, diagnosis, and outcomes of reported cases. Med. Mycol. 2018, 56, 29–43. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serris, A.; Danion, F.; Lanternier, F. Disease Entities in Mucormycosis. J. Fungi 2019, 5, 23. https://doi.org/10.3390/jof5010023
Serris A, Danion F, Lanternier F. Disease Entities in Mucormycosis. Journal of Fungi. 2019; 5(1):23. https://doi.org/10.3390/jof5010023
Chicago/Turabian StyleSerris, Alexandra, François Danion, and Fanny Lanternier. 2019. "Disease Entities in Mucormycosis" Journal of Fungi 5, no. 1: 23. https://doi.org/10.3390/jof5010023
APA StyleSerris, A., Danion, F., & Lanternier, F. (2019). Disease Entities in Mucormycosis. Journal of Fungi, 5(1), 23. https://doi.org/10.3390/jof5010023




