Candida albicans Morphology-Dependent Host FGF-2 Response as a Potential Therapeutic Target
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Cell Culture
2.3. Fungal Strains and Growth Conditions
2.4. HUVECs Challenge with C. albicans
2.5. HUVECs Challenge with C. albicans Spent Medium
2.6. HUVECs Challenge with C. albicans Nonviable (Heat-Killed and PFA-Treated) Strains
2.7. HUVECs Challenge with Candidalysin Peptide
2.8. Evaluation of FGF-2 Monotherapy and Combination Therapy with Fluconazole in a Murine Model of Systemic Candidiasis
2.9. Statistics
3. Results
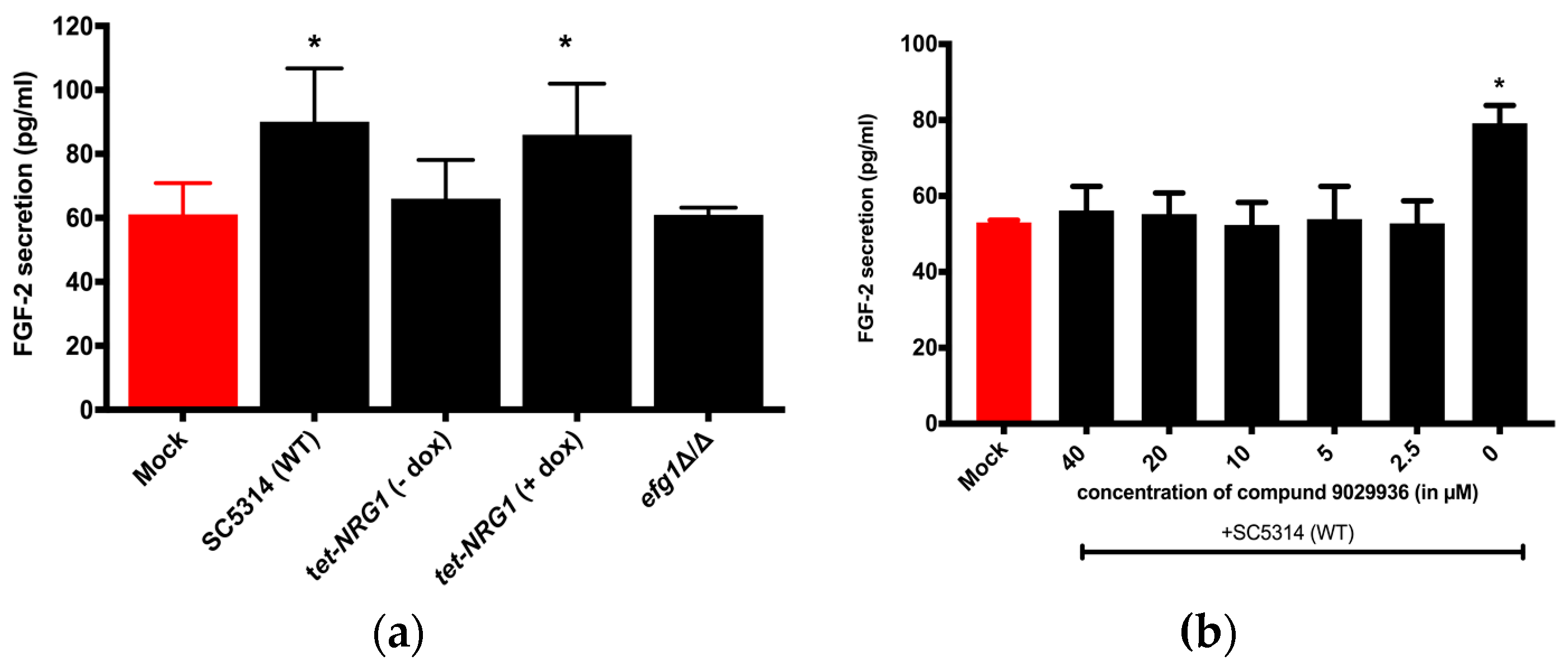
3.1. FGF-2 Protein Secretion from Hosts is Dependent on the Morphology of the C. albicans
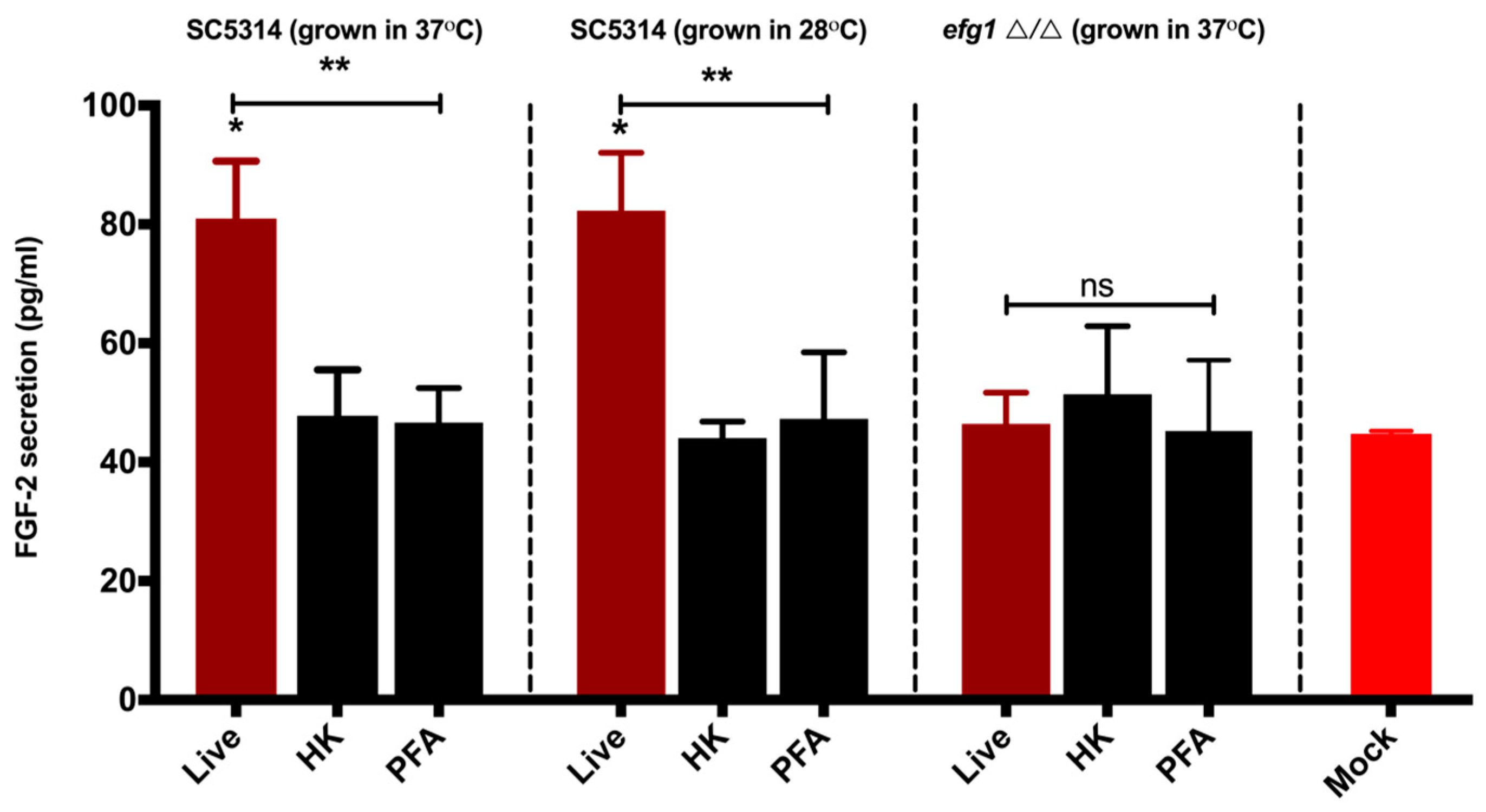
3.2. Viable C. albicans Hyphae are Required for the Induction of the FGF-2 Response
3.3. Candidalysin Induces Host FGF-2 Protein Secretion
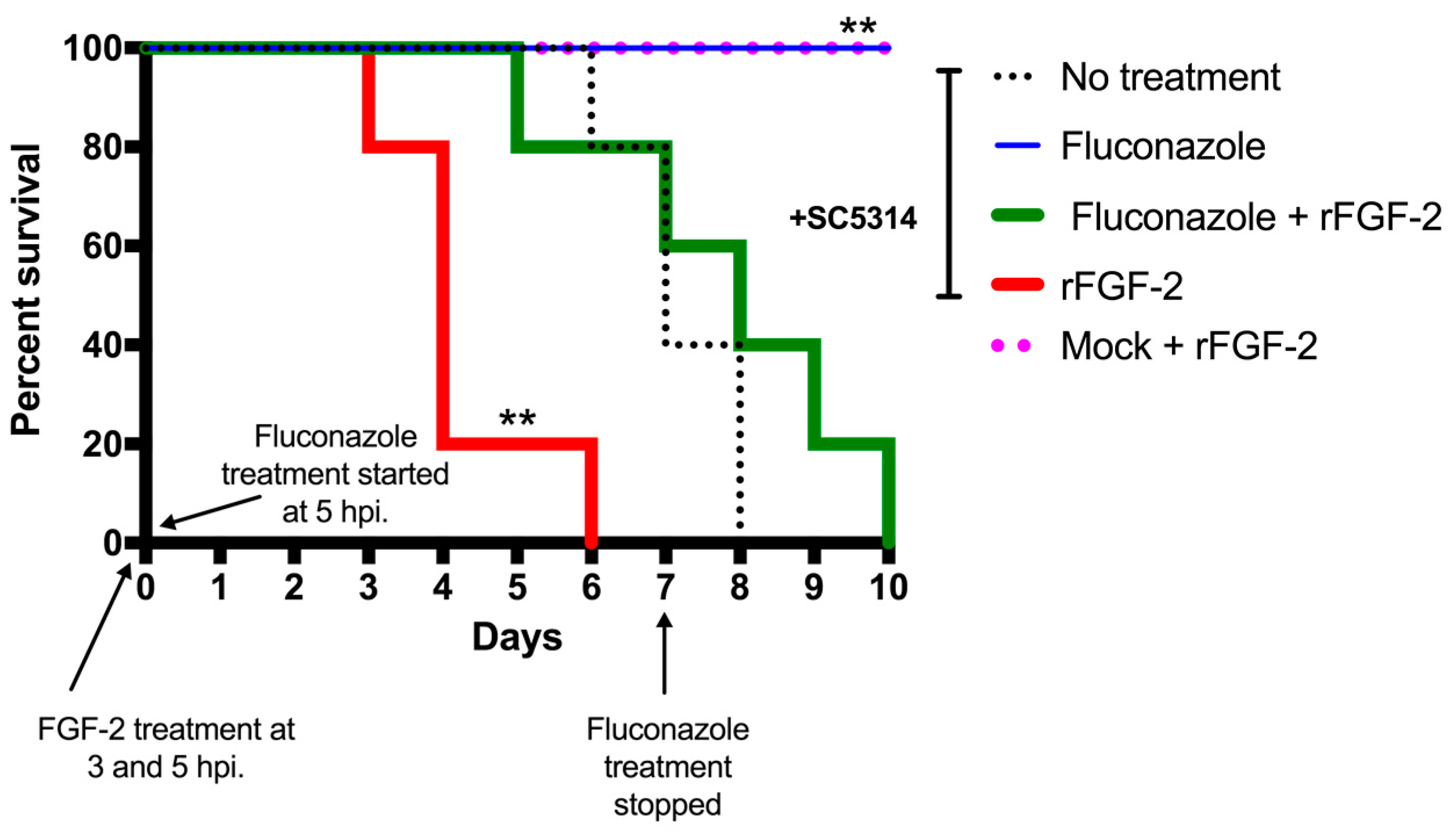
3.4. FGF-2 Enhances Mortality Rate in a Murine Model of Systemic Candidiasis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brown, G.D.; Denning, D.W.; Gow, N.A.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden killers: Human fungal infections. Sci. Transl. Med. 2012, 4, 165rv113. [Google Scholar] [CrossRef] [PubMed]
- Carpino, N.; Naseem, S.; Frank, D.M.; Konopka, J.B. Modulating host signaling pathways to promote resistance to infection by Candida albicans. Front. Cell Infect. Microbiol. 2017, 7, 481. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of invasive candidiasis: A persistent public health problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef] [PubMed]
- Kullberg, B.J.; Arendrup, M.C. Invasive Candidiasis. N. Engl. J. Med. 2015, 373, 1445–1456. [Google Scholar] [CrossRef] [PubMed]
- Nett, J.E.; Andes, D.R. Antifungal Agents: Spectrum of activity, pharmacology, and clinical indications. Infect. Dis. Clin. N. Am. 2016, 30, 51–83. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, R.S.; Robbins, N.; Cowen, L.E. Regulatory circuitry governing fungal development, drug resistance, and disease. Microbiol. Mol. Biol. Rev. 2011, 75, 213–267. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, R.S.; Uppuluri, P.; Zaas, A.K.; Collins, C.; Senn, H.; Perfect, J.R.; Heitman, J.; Cowen, L.E. Hsp90 orchestrates temperature-dependent Candida albicans morphogenesis via Ras1-PKA signaling. Curr. Biol. 2009, 19, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Sanguinetti, M.; Posteraro, B.; Lass-Florl, C. Antifungal drug resistance among Candida species: Mechanisms and clinical impact. Mycoses 2015, 58, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J.; Klagsbrun, M. Angiogenic factors. Science 1987, 235, 442. [Google Scholar] [CrossRef] [PubMed]
- Nishida, N.; Yano, H.; Nishida, T.; Kamura, T.; Kojiro, M. Angiogenesis in cancer. Vasc. Health Risk Manag. 2006, 2, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Rifkin, D.B. Autocrine activities of basic fibroblast growth factor: Regulation of endothelial cell movement, plasminogen activator synthesis, and DNA synthesis. J. Cell Biol. 1988, 107, 1199–1205. [Google Scholar] [CrossRef] [PubMed]
- Seghezzi, G.; Patel, S.; Ren, C.J.; Gualandris, A.; Pintucci, G.; Robbins, E.S.; Shapiro, R.L.; Galloway, A.C.; Rifkin, D.B.; Mignatti, P. Fibroblast Growth Factor-2 (FGF-2) induces Vascular Endothelial Growth Factor (VEGF) expression in the endothelial cells of forming capillaries: An autocrine mechanism contributing to angiogenesis. J. Cell Biol. 1998, 141, 1659. [Google Scholar] [CrossRef] [PubMed]
- Pepper, M.S.; Ferrara, N.; Orci, L.; Montesano, R. Potent synergism between vascular endothelial growth factor and basic fibroblast growth factor in the induction of angiogenesis in vitro. Biochem. Biophys. Res. Commun. 1992, 189, 824–831. [Google Scholar] [CrossRef]
- Akl, M.R.; Nagpal, P.; Ayoub, N.M.; Tai, B.; Prabhu, S.A.; Capac, C.M.; Gliksman, M.; Goy, A.; Suh, K.S. Molecular and clinical significance of fibroblast growth factor 2 (FGF2 /bFGF) in malignancies of solid and hematological cancers for personalized therapies. Oncotarget 2016, 7, 44735–44762. [Google Scholar] [CrossRef] [PubMed]
- Metzner, T.; Bedeir, A.; Held, G.; Peter-Vörösmarty, B.; Ghassemi, S.; Heinzle, C.; Spiegl-Kreinecker, S.; Marian, B.; Holzmann, K.; Grasl-Kraupp, B.; et al. Fibroblast Growth Factor receptors as therapeutic targets in human melanoma: Synergism with BRAF inhibition. J. Investig. Dermatol. 2011, 131, 2087–2095. [Google Scholar] [CrossRef] [PubMed]
- Brady, N.; Chuntova, P.; Bade, L.K.; Schwertfeger, K.L. The FGF/FGFR axis as a therapeutic target in breast cancer. Expert Rev. Endocrinol. Metab. 2013, 8, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ami, R.; Albert, N.D.; Lewis, R.E.; Kontoyiannis, D.P. Proangiogenic growth factors potentiate in situ angiogenesis and enhance antifungal drug activity in murine invasive aspergillosis. J. Infect. Dis. 2013, 207, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Ashman, R.B.; Papadimitriou, J.M. Endothelial cell proliferation associated with lesions of murine systemic candidiasis. Infect. Immun. 1994, 62, 5151–5153. [Google Scholar] [PubMed]
- Lee, S.C.; Li, A.; Calo, S.; Inoue, M.; Tonthat, N.K.; Bain, J.M.; Louw, J.; Shinohara, M.L.; Erwig, L.P.; Schumacher, M.A.; et al. Calcineurin orchestrates dimorphic transitions, antifungal drug responses and host-pathogen interactions of the pathogenic mucoralean fungus Mucor circinelloides. Mol. Microbiol. 2015, 97, 844–865. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Pittman, K.J.; Barker, J.R.; Salinas, R.E.; Stanaway, I.B.; Williams, G.D.; Carroll, R.J.; Balmat, T.; Ingham, A.; Gopalakrishnan, A.M.; et al. An atlas of genetic variation linking pathogen-induced cellular traits to human disease. Cell Host Microbe 2018, 24, 308–323.e6. [Google Scholar] [CrossRef] [PubMed]
- Barker, K.S.; Park, H.; Phan, Q.T.; Xu, L.; Homayouni, R.; Rogers, P.D.; Filler, S.G. Transcriptome profile of the vascular endothelial cell response to Candida albicans. J. Infect. Dis. 2008, 198, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Uppuluri, P.; Pierce, C.G.; Thomas, D.P.; Bubeck, S.S.; Saville, S.P.; Lopez-Ribot, J.L. The transcriptional regulator Nrg1p controls Candida albicans biofilm formation and dispersion. Eukaryot. Cell 2010, 9, 1531–1537. [Google Scholar] [CrossRef] [PubMed]
- Romo, J.A.; Pierce, C.G.; Chaturvedi, A.K.; Lazzell, A.L.; McHardy, S.F.; Saville, S.P.; Lopez-Ribot, J.L. Development of anti-virulence approaches for candidiasis via a novel series of small-molecule inhibitors of candida albicans filamentation. MBio 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Gillum, A.M.; Tsay, E.Y.; Kirsch, D.R. Isolation of the Candida albicans gene for orotidine-5’-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol. Gen. Genet. 1984, 198, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Ramage, G.; VandeWalle, K.; Lopez-Ribot, J.L.; Wickes, B.L. The filamentation pathway controlled by the Efg1 regulator protein is required for normal biofilm formation and development in Candida albicans. FEMS Microbiol. Lett. 2002, 214, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Saville, S.P.; Lazzell, A.L.; Monteagudo, C.; Lopez-Ribot, J.L. Engineered control of cell morphology in vivo reveals distinct roles for yeast and filamentous forms of Candida albicans during infection. Eukaryot. Cell 2003, 2, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Noble, S.M.; Johnson, A.D. Strains and strategies for large-scale gene deletion studies of the diploid human fungal pathogen Candida albicans. Eukaryot. Cell 2005, 4, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Cleary, I.A.; Reinhard, S.M.; Miller, C.L.; Murdoch, C.; Thornhill, M.H.; Lazzell, A.L.; Monteagudo, C.; Thomas, D.P.; Saville, S.P. Candida albicans adhesin Als3p is dispensable for virulence in the mouse model of disseminated candidiasis. Microbiology 2011, 157, 1806–1815. [Google Scholar] [CrossRef] [PubMed]
- Homann, O.R.; Dea, J.; Noble, S.M.; Johnson, A.D. A phenotypic profile of the Candida albicans regulatory network. PLoS Genet. 2009, 5, e1000783. [Google Scholar] [CrossRef] [PubMed]
- Moyes, D.L.; Wilson, D.; Richardson, J.P.; Mogavero, S.; Tang, S.X.; Wernecke, J.; Höfs, S.; Gratacap, R.L.; Robbins, J.; Runglall, M.; et al. Candidalysin is a fungal peptide toxin critical for mucosal infection. Nature 2016, 532, 64. [Google Scholar] [CrossRef] [PubMed]
- Lermann, U.; Morschhauser, J. Secreted aspartic proteases are not required for invasion of reconstituted human epithelia by Candida albicans. Microbiology 2008, 154, 3281–3295. [Google Scholar] [CrossRef] [PubMed]
- Quintin, J.; Voigt, J.; van der Voort, R.; Jacobsen, I.D.; Verschueren, I.; Hube, B.; Giamarellos-Bourboulis, E.J.; van der Meer, J.W.; Joosten, L.A.; Kurzai, O.; et al. Differential role of NK cells against Candida albicans infection in immunocompetent or immunocompromised mice. Eur J. Immunol. 2014, 44, 2405–2414. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Filler, S.G. Candida albicans Als3, a multifunctional adhesin and invasin. Eukaryot. Cell 2011, 10, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Nobile, C.J.; Andes, D.R.; Nett, J.E.; Smith, F.J., Jr.; Yue, F.; Phan, Q.-T.; Edwards, J.E., Jr.; Filler, S.G.; Mitchell, A.P. Critical role of Bcr1-dependent adhesins in C. albicans biofilm formation in vitro and in vivo. PLoS Pathog. 2006, 2, e63. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.P.; Mogavero, S.; Moyes, D.L.; Blagojevic, M.; Krüger, T.; Verma, A.H.; Coleman, B.M.; De La Cruz Diaz, J.; Schulz, D.; Ponde, N.O.; et al. Processing of Candida albicans Ece1p is critical for candidalysin maturation and fungal virulence. mBio 2018, 9, e02178-17. [Google Scholar] [CrossRef] [PubMed]
- Grubb, S.E.; Murdoch, C.; Sudbery, P.E.; Saville, S.P.; Lopez-Ribot, J.L.; Thornhill, M.H. Candida albicans-endothelial cell interactions: A key step in the pathogenesis of systemic candidiasis. Infect. Immun. 2008, 76, 4370–4377. [Google Scholar] [CrossRef] [PubMed]
- Desai, J.V. Candida albicans Hyphae: From Growth Initiation to Invasion. J. Fungi (Basel) 2018, 4. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, I.D.; Wilson, D.; Wächtler, B.; Brunke, S.; Naglik, J.R.; Hube, B. Candida albicans dimorphism as a therapeutic target. Expert Rev. Anti Infect. Ther. 2012, 10, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Gow, N.A.; van de Veerdonk, F.L.; Brown, A.J.; Netea, M.G. Candida albicans morphogenesis and host defence: Discriminating invasion from colonization. Nat. Rev. Microbiol. 2011, 10, 112–122. [Google Scholar] [CrossRef] [PubMed]
- da Silva Dantas, A.; Lee, K.K.; Raziunaite, I.; Schaefer, K.; Wagener, J.; Yadav, B.; Gow, N.A. Cell biology of Candida albicans-host interactions. Curr. Opin. Microbiol. 2016, 34, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Gong, Y.; Liu, L.; Zhou, Y.; Fang, X.; Zhang, C.; Li, Y.; Li, J. The use of human umbilical vein endothelial cells (HUVECs) as an in vitro model to assess the toxicity of nanoparticles to endothelium: A review. J. Appl. Toxicol. 2017, 37, 1359–1369. [Google Scholar] [CrossRef] [PubMed]
- Antoine, M.; Wirz, W.; Tag, C.G.; Mavituna, M.; Emans, N.; Korff, T.; Stoldt, V.; Gressner, A.M.; Kiefer, P. Expression pattern of fibroblast growth factors (FGFs), their receptors and antagonists in primary endothelial cells and vascular smooth muscle cells. Growth Factors 2005, 23, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Müller, V.; Viemann, D.; Schmidt, M.; Endres, N.; Ludwig, S.; Leverkus, M.; Roth, J.; Goebeler, M. Candida albicans triggers activation of distinct signaling pathways to establish a proinflammatory gene expression program in primary human endothelial cells. J. Immunol. 2007, 179, 8435. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.; Mayer, F.L.; Miramon, P.; Citiulo, F.; Slesiona, S.; Jacobsen, I.D.; Hube, B. Distinct roles of Candida albicans-specific genes in host-pathogen interactions. Eukaryot. Cell 2014, 13, 977–989. [Google Scholar] [CrossRef] [PubMed]
- Orozco, A.S.; Zhou, X.; Filler, S.G. Mechanisms of the proinflammatory response of endothelial cells to Candida albicans infection. Infect. Immun. 2000, 68, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Phan, Q.T.; Belanger, P.H.; Filler, S.G. Role of hyphal formation in interactions of Candida albicans with endothelial cells. Infect. Immun. 2000, 68, 3485–3490. [Google Scholar] [CrossRef] [PubMed]
- Gow, N.A.; Netea, M.G.; Munro, C.A.; Ferwerda, G.; Bates, S.; Mora-Montes, H.M.; Walker, L.; Jansen, T.; Jacobs, L.; Tsoni, V.; et al. Immune recognition of Candida albicans beta-glucan by dectin-1. J. Infect. Dis. 2007, 196, 1565–1571. [Google Scholar] [CrossRef] [PubMed]
- Rowen, J.L.; Tate, J.M. Heat-Killed Candida albicans elicit more interleukin 8 (IL-8) from human monocytes than live yeast. Pediatr. Res. 1998, 43, 156. [Google Scholar] [CrossRef]
- van de Veerdonk, F.L.; Marijnissen, R.J.; Kullberg, B.J.; Koenen, H.J.P.M.; Cheng, S.-C.; Joosten, I.; van den Berg, W.B.; Williams, D.L.; van der Meer, J.W.M.; Joosten, L.A.B.; et al. The macrophage mannose receptor induces IL-17 in Response to Candida albicans. Cell Host Microbe 2009, 5, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Saraswat, D.; Tati, S.; Edgerton, M. Novel aggregation properties of Candida albicans secreted aspartyl proteinase Sap6 mediate virulence in oral Candidiasis. Infect. Immun. 2015, 83, 2614–2626. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.P.; Willems, H.M.E.; Moyes, D.L.; Shoaie, S.; Barker, K.S.; Tan, S.L.; Palmer, G.E.; Hube, B.; Naglik, J.R.; Peters, B.M. Candidalysin drives epithelial signaling, neutrophil recruitment, and immunopathology at the vaginal mucosa. Infect. Immun. 2017. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.; Naglik, J.R.; Hube, B. The missing link between Candida albicans hyphal morphogenesis and host cell damage. PLoS Pathog. 2016, 12, e1005867. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ami, R.; Lewis, R.E.; Leventakos, K.; Kontoyiannis, D.P. Aspergillus fumigatus inhibits angiogenesis through the production of gliotoxin and other secondary metabolites. Blood 2009, 114, 5393–5399. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T. Angiogenin: An antimicrobial ribonuclease. Nat. Immunol. 2003, 4, 213. [Google Scholar] [CrossRef] [PubMed]
- Datta, M.; Via, L.E.; Kamoun, W.S.; Liu, C.; Chen, W.; Seano, G.; Weiner, D.M.; Schimel, D.; England, K.; Martin, J.D.; et al. Anti-vascular endothelial growth factor treatment normalizes tuberculosis granuloma vasculature and improves small molecule delivery. Proc. Natl. Acad. Sci. USA 2015, 112, 1827–1832. [Google Scholar] [CrossRef] [PubMed]
- Oehlers, S.H.; Cronan, M.R.; Scott, N.R.; Thomas, M.I.; Okuda, K.S.; Walton, E.M.; Beerman, R.W.; Crosier, P.S.; Tobin, D.M. Interception of host angiogenic signalling limits mycobacterial growth. Nature 2015, 517, 612–615. [Google Scholar] [CrossRef] [PubMed]
- Polena, H.; Boudou, F.; Tilleul, S.; Dubois-Colas, N.; Lecointe, C.; Rakotosamimanana, N.; Pelizzola, M.; Andriamandimby, S.F.; Raharimanga, V.; Charles, P.; et al. Mycobacterium tuberculosis exploits the formation of new blood vessels for its dissemination. Sci. Rep. 2016, 6, 33162. [Google Scholar] [CrossRef] [PubMed]
- Van De Veerdonk, F.L.; Netea, M.G.; Joosten, L.A.; Van Der Meer, J.W.M.; Kullberg, B.J. Novel strategies for the prevention and treatment of Candida infections: The potential of immunotherapy. FEMS Microbiol. Rev. 2010, 34, 1063–1075. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vellanki, S.; Huh, E.Y.; Saville, S.P.; Lee, S.C. Candida albicans Morphology-Dependent Host FGF-2 Response as a Potential Therapeutic Target. J. Fungi 2019, 5, 22. https://doi.org/10.3390/jof5010022
Vellanki S, Huh EY, Saville SP, Lee SC. Candida albicans Morphology-Dependent Host FGF-2 Response as a Potential Therapeutic Target. Journal of Fungi. 2019; 5(1):22. https://doi.org/10.3390/jof5010022
Chicago/Turabian StyleVellanki, Sandeep, Eun Young Huh, Stephen P. Saville, and Soo Chan Lee. 2019. "Candida albicans Morphology-Dependent Host FGF-2 Response as a Potential Therapeutic Target" Journal of Fungi 5, no. 1: 22. https://doi.org/10.3390/jof5010022
APA StyleVellanki, S., Huh, E. Y., Saville, S. P., & Lee, S. C. (2019). Candida albicans Morphology-Dependent Host FGF-2 Response as a Potential Therapeutic Target. Journal of Fungi, 5(1), 22. https://doi.org/10.3390/jof5010022




