Applications of Invertebrate Animal Models to Dimorphic Fungal Infections
Abstract
1. Introduction
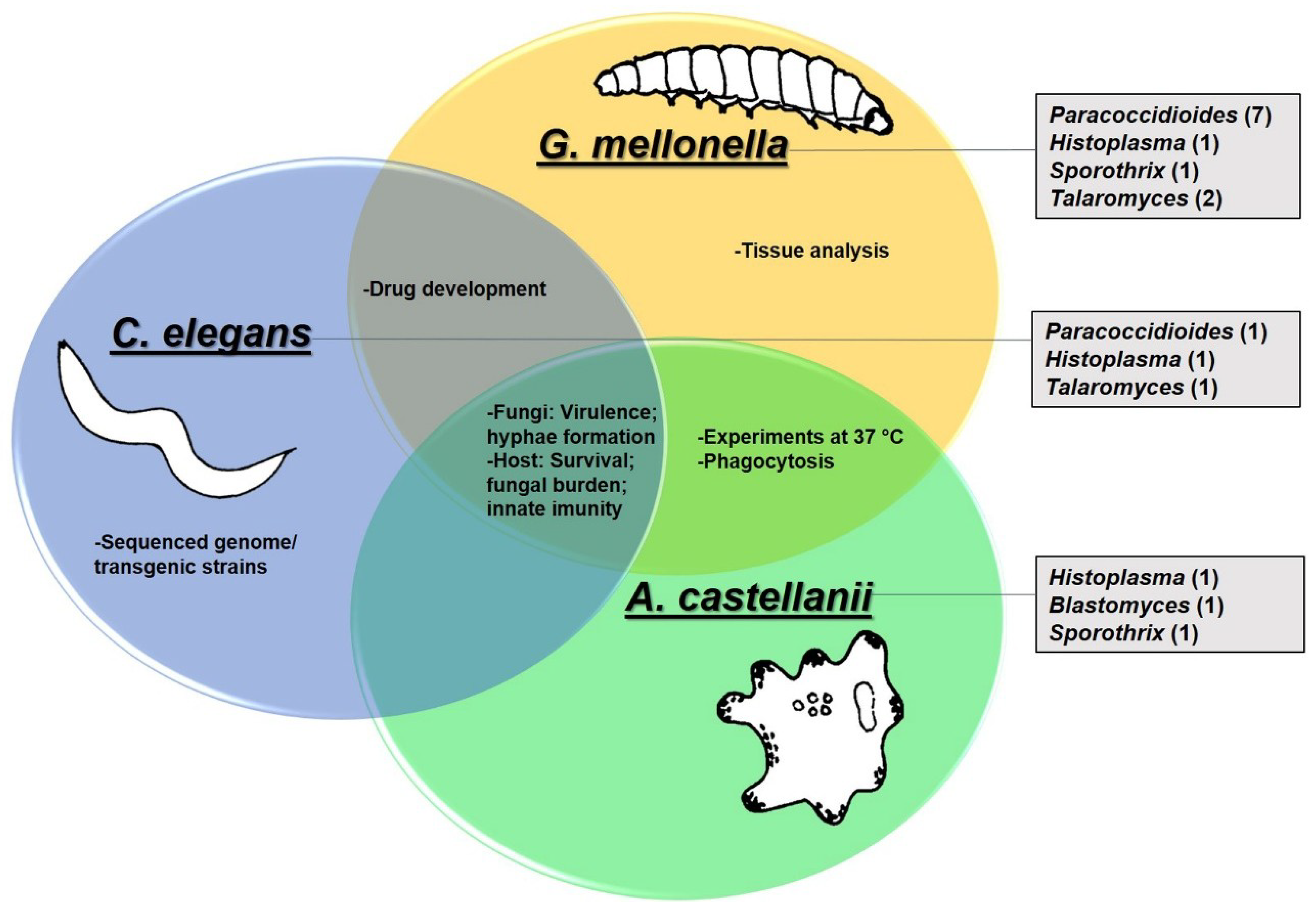
2. Galleria mellonella
3. Caenorhabditis elegans
4. Acanthamoeba castellanii
5. Use of G. mellonella, C. elegans and A. castellanii to Study Dimorphic Fungi
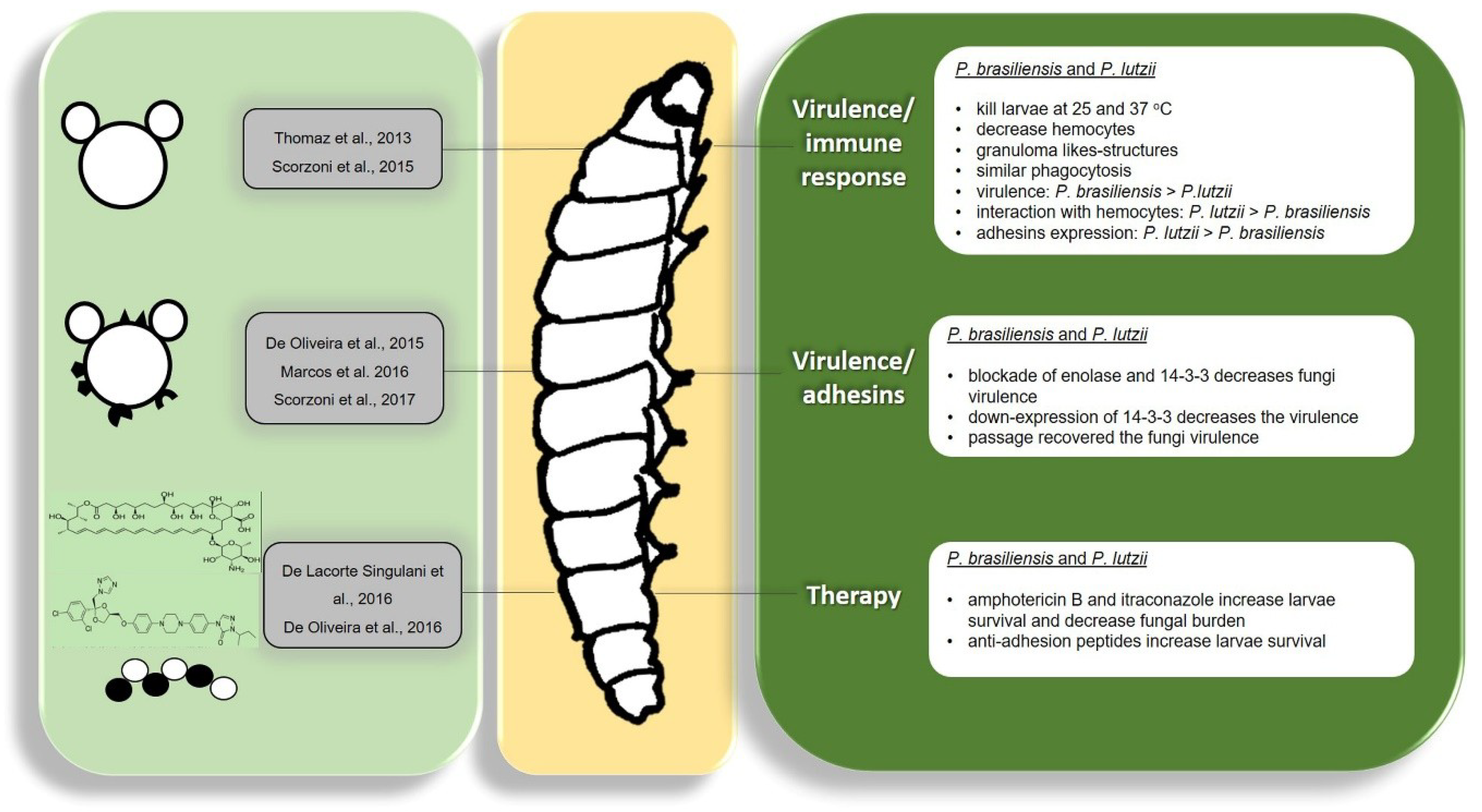
5.1. Paracoccidioides spp.
5.2. Histoplasma capsulatum
5.3. Blastomyces dermatitidis
5.4. Sporothrix spp.
5.5. Talaromyces marneffei (Penicillium marneffei)
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Festing, S.; Wilkinson, R. The ethics of animal research: Talking Point on the use of animals in scientific research. EMBO Rep. 2007, 8, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Doke, S.K.; Dhawale, S.C. Alternatives to animal testing: A review. Saudi Pharm. J. 2015, 23, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Russell, W.; Burch, R. The Principles of Humane Experimental Technique; Methuen: London, UK, 1959; ISBN 0 900767 78 2. [Google Scholar]
- Arora, T.; Mehta, A.K.; Joshi, V.; Mehta, K.D.; Rathor, N.; Mediratta, P.K.; Sharma, K.K. Substitute of Animals in Drug Research: An Approach Towards Fulfillment of 4R’s. Indian J. Pharm. Sci. 2011, 73, 1–6. [Google Scholar] [PubMed]
- Banks, R.E. The 4th R of research. Contemp Top. Lab. Anim Sci 1995, 34, 50–51. [Google Scholar] [PubMed]
- Hohl, T.M. Overview of vertebrate animal models of fungal infection. J. Immunol. Methods 2014, 410, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Segal, E.; Frenkel, M. Experimental in Vivo Models of Candidiasis. J. Fungi (Basel) 2018, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Goldani, L.Z.; Wirth, F. Animal Models and Antifungal Agents in Paracoccidioidomycosis: An Overview. Mycopathologia 2017, 182, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, J.; Connolly, P.; Schnizlein-Bick, C.; Durkin, M.; Kohler, S.; Smedema, M.; Brizendine, E.; Hector, R.; Wheat, J. Comparison of nikkomycin Z with amphotericin B and itraconazole for treatment of histoplasmosis in a murine model. Antimicrob. Agents Chemother. 2000, 44, 1624–1629. [Google Scholar] [CrossRef] [PubMed]
- Mylonakis, E.; Casadevall, A.; Ausubel, F.M. Exploiting amoeboid and non-vertebrate animal model systems to study the virulence of human pathogenic fungi. PLoS Pathog. 2007, 3, e101. [Google Scholar] [CrossRef] [PubMed]
- Glavis-Bloom, J.; Muhammed, M.; Mylonakis, E. Of model hosts and man: Using Caenorhabditis elegans, Drosophila melanogaster and Galleria mellonella as model hosts for infectious disease research. Adv. Exp. Med. Biol. 2012, 710, 11–17. [Google Scholar] [PubMed]
- Jorjao, A.L.; Oliveira, L.D.; Scorzoni, L.; Figueiredo-Godoi, L.M.A.; Prata, M.C.A.; Jorge, A.O.C.; Junqueira, J.C. From moths to caterpillars: Ideal conditions for Galleria mellonella rearing for in vivo microbiological studies. Virulence 2017, 9, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Okoli, I.; Coleman, J.J.; Tampakakis, E.; Tempakakis, E.; An, W.F.; Holson, E.; Wagner, F.; Conery, A.L.; Larkins-Ford, J.; Wu, G.; et al. Identification of antifungal compounds active against Candida albicans using an improved high-throughput Caenorhabditis elegans assay. PLoS ONE 2009, 4, e7025. [Google Scholar] [CrossRef] [PubMed]
- Sangalli-Leite, F.; Scorzoni, L.; Alves de Paula E Silva, A.C.; da Silva, J.F.; de Oliveira, H.C.; de Lacorte Singulani, J.; Gullo, F.P.; Moraes da Silva, R.; Regasini, L.O.; Siqueira da Silva, D.H.; et al. Synergistic effect of pedalitin and amphotericin B against Cryptococcus neoformans by in vitro and in vivo evaluation. Int. J. Antimicrob. Agents 2016, 48, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Desalermos, A.; Tan, X.; Rajamuthiah, R.; Arvanitis, M.; Wang, Y.; Li, D.; Kourkoumpetis, T.K.; Fuchs, B.B.; Mylonakis, E. A multi-host approach for the systematic analysis of virulence factors in Cryptococcus neoformans. J. Infect. Dis. 2015, 211, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Desalermos, A.; Fuchs, B.B.; Mylonakis, E. Selecting an invertebrate model host for the study of fungal pathogenesis. PLoS Pathog 2012, 8, e1002451. [Google Scholar] [CrossRef] [PubMed]
- Kwadha, C.A.; Ong’amo, G.O.; Ndegwa, P.N.; Raina, S.K.; Fombong, A.T. The Biology and Control of the Greater Wax Moth, Galleria mellonella. Insects 2017, 8, 61. [Google Scholar] [CrossRef] [PubMed]
- Götz, P.; Matha, V.; Vilcinskas, A. Effects of the entomopathogenic fungus Metarhizium anisopliae and its secondary metabolites on morphology and cytoskeleton of plasmatocytes isolated from the greater wax moth, Galleria mellonella. J. Insect Physiol. 1997, 43, 1149–1159. [Google Scholar] [PubMed]
- Cotter, G.; Doyle, S.; Kavanagh, K. Development of an insect model for the in vivo pathogenicity testing of yeasts. FEMS Immunol. Med. Microbiol. 2000, 27, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Krams, I.; Kecko, S.; Kangassalo, K.; Moore, F.R.; Jankevics, E.; Inashkina, I.; Krama, T.; Lietuvietis, V.; Meija, L.; Rantala, M.J. Effects of food quality on trade-offs among growth, immunity and survival in the greater wax moth Galleria mellonella. Insect Sci. 2015, 22, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Boman, H.G.; Hultmark, D. Cell-free immunity in insects. Annu. Rev. Microbiol. 1987, 41, 103–126. [Google Scholar] [CrossRef] [PubMed]
- Tojo, S.; Naganuma, F.; Arakawa1, K.; Yokoo1, S. Involvement of both granular cells and plasmatocytes in phagocytic reactions in the greater wax moth, Galleria mellonella. J. Insect Physiol. 2000, 46, 1129–1135. [Google Scholar] [CrossRef]
- Tsai, C.J.; Loh, J.M.; Proft, T. Galleria mellonella infection models for the study of bacterial diseases and for antimicrobial drug testing. Virulence 2016, 7, 214–229. [Google Scholar] [CrossRef] [PubMed]
- Thomaz, L.; García-Rodas, R.; Guimarães, A.J.; Taborda, C.P.; Zaragoza, O.; Nosanchuk, J.D. Galleria mellonella as a model host to study Paracoccidioides lutzii and Histoplasma capsulatum. Virulence 2013, 4, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Scorzoni, L.; Alves de Paula e Silva, A.C.; Singulani, J.d.L.; Leite, F.S.; de Oliveira, H.C.; Moraes da Silva, R.A.; Fusco-Almeida, A.M.; Soares Mendes-Giannini, M.J. Comparison of virulence between Paracoccidioides brasiliensis and Paracoccidioides lutzii using Galleria mellonella as a host model. Virulence 2015, 6, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Scorzoni, L.; de Paula E Silva, A.C.A.; de Oliveira, H.C.; Marcos, C.M.; Singulani, J.L.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J.S. Can passage in Galleria mellonella activate virulence factors of Paracoccidioides brasiliensis as in the murine model? Med. Mycol. 2018, 56, 374–377. [Google Scholar] [CrossRef] [PubMed]
- de Lacorte Singulani, J.; Scorzoni, L.; de Paula E Silva, A.C.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J. Evaluation of the efficacy of antifungal drugs against Paracoccidioides brasiliensis and Paracoccidioides lutzii in a Galleria mellonella model. Int. J. Antimicrob. Agents 2016, 48, 292–297. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, H.C.; Michaloski, J.S.; da Silva, J.F.; Scorzoni, L.; de Paula E Silva, A.C.; Marcos, C.M.; Assato, P.A.; Yamazaki, D.S.; Fusco-Almeida, A.M.; Giordano, R.J.; et al. Peptides Derived from a Phage Display Library Inhibit Adhesion and Protect the Host against Infection by Paracoccidioides brasiliensis and Paracoccidioides lutzii. Front. Pharmacol. 2016, 7, 509. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, H.C.; da Silva, J.e.F.; Scorzoni, L.; Marcos, C.M.; Rossi, S.A.; de Paula E Silva, A.C.; Assato, P.A.; da Silva, R.A.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J. Importance of adhesins in virulence of Paracoccidioides spp. Front. Microbiol. 2015, 6, 303. [Google Scholar] [CrossRef] [PubMed]
- Marcos, C.M.; da Silva, J.F.; de Oliveira, H.C.; Assato, P.A.; Singulani, J.L.; Lopez, A.M.; Tamayo, D.P.; Hernandez-Ruiz, O.; McEwen, J.G.; Mendes-Giannini, M.J.; et al. Decreased expression of 14-3-3 in Paracoccidioides brasiliensis confirms its involvement in fungal pathogenesis. Virulence 2016, 7, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Clavijo-Giraldo, D.M.; Matínez-Alvarez, J.A.; Lopes-Bezerra, L.M.; Ponce-Noyola, P.; Franco, B.; Almeida, R.S.; Mora-Montes, H.M. Analysis of Sporothrix schenckii sensu stricto and Sporothrix brasiliensis virulence in Galleria mellonella. J. Microbiol. Methods 2016, 122, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Suwunnakorn, S.; Cooper, J.C.R.; Kummasook, A.; Pongpom, M.; Vanittanakom, P.; Vanittanakom, N. Role of the rttA gene in morphogenesis, stress response, and virulence in the human pathogenic fungus Penicillium marneffei. Medical Mycology 2015, 53, 119–131. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, X.; Li, D.; Xi, L.; Mylonakis, E. Galleria mellonella Larvae as an Infection Model for Penicillium marneffei. Mycopathologia 2015, 180, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Vogel, H.; Altincicek, B.; Glockner, G.; Vilcinskas, A. A comprehensive transcriptome and immune-gene repertoire of the lepidopteran model host Galleria mellonella. BMC Genomics 2011, 12, 308. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, K.; Vilcinskas, A. Development and immunity-related microRNAs of the lepidopteran model host Galleria mellonella. BMC Genomics 2014, 15, 705. [Google Scholar] [CrossRef] [PubMed]
- Borman, A.M. Of mice and men and larvae: Galleria mellonella to model the early host-pathogen interactions after fungal infection. Virulence 2018, 9, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, G.; Kavanagh, K. Analysis of the early cellular and humoral responses of Galleria mellonella larvae to infection by Candida albicans. Virulence 2018, 9, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.; Kavanagh, K. Caspofungin primes the immune response of the larvae of Galleria mellonella and induces a non-specific antimicrobial response. J. Med. Microbiol. 2011, 60, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Schulenburg, H.; Félix, M.A. The Natural Biotic Environment of. Genetics 2017, 206, 55–86. [Google Scholar] [CrossRef] [PubMed]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [PubMed]
- Corsi, A.K.; Wightman, B.; Chalfie, M. A Transparent Window into Biology: A Primer on Caenorhabditis elegans. Genetics 2015, 200, 387–407. [Google Scholar] [CrossRef] [PubMed]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Feng, X.; Guang, S. Targeted genome engineering in. Cell. Biosci. 2016, 6, 60. [Google Scholar] [CrossRef] [PubMed]
- Lamitina, T. Functional genomic approaches in C. elegans. Methods Mol. Biol. 2006, 351, 127–138. [Google Scholar] [PubMed]
- Ewbank, J.J.; Zugasti, O.C. elegans: Model host and tool for antimicrobial drug discovery. Dis. Model. Mech. 2011, 4, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Arvanitis, M.; Glavis-Bloom, J.; Mylonakis, E. Invertebrate models of fungal infection. Biochim. Biophys. Acta. 2013, 1832, 1378–1383. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.G.; Blackwell, G.G.; Raabe, R.C.; Bettinger, J.C. An Assay for Measuring the Effects of Ethanol on the Locomotion Speed of Caenorhabditis elegans. J. Vis. Exp. 2015. [Google Scholar] [CrossRef] [PubMed]
- Breger, J.; Fuchs, B.B.; Aperis, G.; Moy, T.I.; Ausubel, F.M.; Mylonakis, E. Antifungal chemical compounds identified using a C. elegans pathogenicity assay. PLoS Pathog. 2007, 3, e18. [Google Scholar] [CrossRef] [PubMed]
- Mylonakis, E.; Ausubel, F.M.; Perfect, J.R.; Heitman, J.; Calderwood, S.B. Killing of Caenorhabditis elegans by Cryptococcus neoformans as a model of yeast pathogenesis. Proc. Natl. Acad. Sci. USA 2002, 99, 15675–15680. [Google Scholar] [CrossRef] [PubMed]
- Scorzoni, L.; de Lucas, M.P.; Mesa-Arango, A.C.; Fusco-Almeida, A.M.; Lozano, E.; Cuenca-Estrella, M.; Mendes-Giannini, M.J.; Zaragoza, O. Antifungal efficacy during Candida krusei infection in non-conventional models correlates with the yeast in vitro susceptibility profile. PLoS ONE 2013, 8, e60047. [Google Scholar] [CrossRef] [PubMed]
- Chisholm, A.D.; Hardin, J. Epidermal morphogenesis. WormBook 2005. [Google Scholar] [CrossRef]
- McGhee, J.D. The C. elegans intestine. WormBook 2007. [Google Scholar] [CrossRef] [PubMed]
- Irazoqui, J.E.; Urbach, J.M.; Ausubel, F.M. Evolution of host innate defence: Insights from Caenorhabditis elegans and primitive invertebrates. Nat. Rev. Immunol. 2010, 10, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Ermolaeva, M.A.; Schumacher, B. Insights from the worm: The C. elegans model for innate immunity. Semin. Immunol. 2014, 26, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Ewbank, J.J.; Pujol, N. Local and long-range activation of innate immunity by infection and damage in C. elegans. Curr. Opin. Immunol. 2016, 38, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Vizuete, A.; Veal, E.A. Caenorhabditis elegans as a model for understanding ROS function in physiology and disease. Redox. Biol. 2017, 11, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Pukkila-Worley, R.; Peleg, A.Y.; Tampakakis, E.; Mylonakis, E. Candida albicans hyphal formation and virulence assessed using a Caenorhabditis elegans infection model. Eukaryot. Cell 2009, 8, 1750–1758. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.C.R.; Fuchs, B.B.; Alves, V.S.; Jayamani, E.; Colombo, A.L.; Mylonakis, E. Pathogenesis of the Candida parapsilosis Complex in the Model Host Caenorhabditis elegans. Genes (Basel) 2018, 9, 401. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.H.; Ayyadevara, S.; McEwen, J.E.; Shmookler Reis, R.J. Histoplasma capsulatum and Caenorhabditis elegans: A simple nematode model for an innate immune response to fungal infection. Med. Mycol. 2009, 47, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Scorzoni, L.; de Lucas, M.P.; Singulani, J.L.; de Oliveira, H.C.; Assato, P.A.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J.S. Evaluation of Caenorhabditis elegans as a host model for Paracoccidioides brasiliensis and Paracoccidioides lutzii. Pathog. Dis. 2018, 76. [Google Scholar] [CrossRef] [PubMed]
- Boyce, K.J.; Andrianopoulos, A. Fungal dimorphism: The switch from hyphae to yeast is a specialized morphogenetic adaptation allowing colonization of a host. FEMS Microbiol Rev. 2015, 39, 797–811. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, G.M. Fungal Dimorphism and Virulence: Molecular Mechanisms for Temperature Adaptation, Immune Evasion, and In Vivo Survival. Mediators Inflamm. 2017, 2017, 8491383. [Google Scholar] [CrossRef] [PubMed]
- Marsh, E.K.; May, R.C. Caenorhabditis elegans, a model organism for investigating immunity. Appl. Environ. Microbiol. 2012, 78, 2075–2081. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, S.A.; Voorhies, M.; Gebhart, D.; Sil, A. Genome-Wide Reprogramming of Transcript Architecture by Temperature Specifies the Developmental States of the Human Pathogen Histoplasma. PLoS Genet. 2015. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Li, D.; Xi, L.; Mylonakis, E. Caenorhabditis elegans: A Simple Nematode Infection Model for Penicillium marneffei. PLoS ONE 2014, 9, e108764. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Munguía, B.; Omaña-Molina, M.; González-Lázaro, M.; González-Robles, A.; Bonilla, P.; Martínez-Palomo, A. Ultrastructural study of encystation and excystation in Acanthamoeba castellanii. J. Eukaryot. Microbiol. 2005, 52, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Robaei, D.; Carnt, N.; Minassian, D.C.; Dart, J.K. Therapeutic and optical keratoplasty in the management of Acanthamoeba keratitis: Risk factors, outcomes, and summary of the literature. Ophthalmology 2015, 122, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, R.; Khan, N.A. Biology and pathogenesis of Acanthamoeba. Parasit. Vectors 2012, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.; Lohan, A.J.; Liu, B.; Lagkouvardos, I.; Roy, S.; Zafar, N.; Bertelli, C.; Schilde, C.; Kianianmomeni, A.; Bürglin, T.R.; et al. Genome of Acanthamoeba castellanii highlights extensive lateral gene transfer and early evolution of tyrosine kinase signaling. Genome. Biol. 2013, 14, R11. [Google Scholar] [CrossRef] [PubMed]
- Greub, G.; Raoult, D. Microorganisms resistant to free-living amoebae. Clin. Microbiol. Rev. 2004, 17, 413–433. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, A.J.; Gomes, K.X.; Cortines, J.R.; Peralta, J.M.; Peralta, R.H. Acanthamoeba spp. as a universal host for pathogenic microorganisms: One bridge from environment to host virulence. Microbiol. Res. 2016, 193, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Steenbergen, J.N.; Nosanchuk, J.D.; Malliaris, S.D.; Casadevall, A. Interaction of Blastomyces dermatitidis, Sporothrix schenckii, and Histoplasma capsulatum with Acanthamoeba castellanii. Infect. Immun. 2004, 72, 3478–3488. [Google Scholar] [CrossRef] [PubMed]
- Martinez, R. New Trends in Paracoccidioidomycosis Epidemiology. J. Fungi (Basel) 2017, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, Z.F.; Silva, D.; Lazera, M.; Petri, V.; Oliveira, R.M.; Sabroza, P.C.; Wanke, B. Paracoccidioidomycosis mortality in Brazil (1980-1995). Cad. Saude. Publica. 2002, 18, 1441–1454. [Google Scholar] [CrossRef] [PubMed]
- Bocca, A.L.; Amaral, A.C.; Teixeira, M.M.; Sato, P.K.; Sato, P.; Shikanai-Yasuda, M.A.; Soares Felipe, M.S. Paracoccidioidomycosis: Eco-epidemiology, taxonomy and clinical and therapeutic issues. Future Microbiol. 2013, 8, 1177–1191. [Google Scholar] [CrossRef] [PubMed]
- Matute, D.R.; McEwen, J.G.; Puccia, R.; Montes, B.A.; San-Blas, G.; Bagagli, E.; Rauscher, J.T.; Restrepo, A.; Morais, F.; Niño-Vega, G.; et al. Cryptic speciation and recombination in the fungus Paracoccidioides brasiliensis as revealed by gene genealogies. Mol. Biol. Evol. 2006, 23, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Turissini, D.A.; Gomez, O.M.; Teixeira, M.M.; McEwen, J.G.; Matute, D.R. Species boundaries in the human pathogen Paracoccidioides. Fungal Genet. Biol. 2017, 106, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.M.; Theodoro, R.C.; de Carvalho, M.J.; Fernandes, L.; Paes, H.C.; Hahn, R.C.; Mendoza, L.; Bagagli, E.; San-Blas, G.; Felipe, M.S. Phylogenetic analysis reveals a high level of speciation in the Paracoccidioides genus. Mol. Phylogenet. Evol. 2009, 52, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Carrero, L.L.; Niño-Vega, G.; Teixeira, M.M.; Carvalho, M.J.; Soares, C.M.; Pereira, M.; Jesuino, R.S.; McEwen, J.G.; Mendoza, L.; Taylor, J.W.; et al. New Paracoccidioides brasiliensis isolate reveals unexpected genomic variability in this human pathogen. Fungal Genet. Biol. 2008, 45, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Mendes-Giannini, M.J.; Taylor, M.L.; Bouchara, J.B.; Burger, E.; Calich, V.L.; Escalante, E.D.; Hanna, S.A.; Lenzi, H.L.; Machado, M.P.; Miyaji, M.; et al. Pathogenesis II: Fungal responses to host responses: Interaction of host cells with fungi. Med. Mycol. 2000, 38, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Mendes-Giannini, M.J.; Monteiro da Silva, J.L.; de Fátima da Silva, J.; Donofrio, F.C.; Miranda, E.T.; Andreotti, P.F.; Soares, C.P. Interactions of Paracoccidioides brasiliensis with host cells: Recent advances. Mycopathologia 2008, 165, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Clemons, K.V.; Calich, V.L.; Burger, E.; Filler, S.G.; Grazziutti, M.; Murphy, J.; Roilides, E.; Campa, A.; Dias, M.R.; Edwards, J.E.; et al. Pathogenesis I: Interactions of host cells and fungi. Med Mycol. 2000, 38, 99–111. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Puccia, R.; Vallejo, M.C.; Matsuo, A.L.; Longo, L.V. The paracoccidioides cell wall: Past and present layers toward understanding interaction with the host. Front Microbiol. 2011, 2, 257. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, H.C.; Assato, P.A.; Marcos, C.M.; Scorzoni, L.; de Paula E Silva, A.C.; Da Silva, J.e.F.; Singulani, J.e.L.; Alarcon, K.M.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J. Paracoccidioides-host Interaction: An Overview on Recent Advances in the Paracoccidioidomycosis. Front Microbiol. 2015, 6, 1319. [Google Scholar] [CrossRef] [PubMed]
- Hanna, S.A.; Monteiro da Silva, J.L.; Giannini, M.J. Adherence and intracellular parasitism of Paracoccidioides brasiliensis in Vero cells. Microbes Infect. 2000, 2, 877–884. [Google Scholar] [CrossRef]
- Andreotti, P.F.; Monteiro da Silva, J.L.; Bailão, A.M.; Soares, C.M.; Benard, G.; Soares, C.P.; Mendes-Giannini, M.J. Isolation and partial characterization of a 30 kDa adhesin from Paracoccidioides brasiliensis. Microbes Infect. 2005, 7, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Marcos, C.M.; de Fátima da Silva, J.; de Oliveira, H.C.; Moraes da Silva, R.A.; Mendes-Giannini, M.J.; Fusco-Almeida, A.M. Surface-expressed enolase contributes to the adhesion of Paracoccidioides brasiliensis to host cells. FEMS Yeast Res. 2012, 12, 557–570. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.e.F.; de Oliveira, H.C.; Marcos, C.M.; da Silva, R.A.; da Costa, T.A.; Calich, V.L.; Almeida, A.M.; Mendes-Giannini, M.J. Paracoccidoides brasiliensis 30 kDa Adhesin: Identification as a 14-3-3 Protein, Cloning and Subcellular Localization in Infection Models. PLoS ONE 2013, 8, e62533. [Google Scholar] [CrossRef] [PubMed]
- da Silva Neto, B.R.; de Fátima da Silva, J.; Mendes-Giannini, M.J.; Lenzi, H.L.; de Almeida Soares, C.M.; Pereira, M. The malate synthase of Paracoccidioides brasiliensis is a linked surface protein that behaves as an anchorless adhesin. BMC Microbiol. 2009, 9, 272. [Google Scholar] [CrossRef] [PubMed]
- Bailão, A.M.; Schrank, A.; Borges, C.L.; Dutra, V.; Walquíria Inês Molinari-Madlum, E.E.; Soares Felipe, M.S.; Soares Mendes-Giannini, M.J.; Martins, W.S.; Pereira, M.; Maria de Almeida Soares, C. Differential gene expression by Paracoccidioides brasiliensis in host interaction conditions: Representational difference analysis identifies candidate genes associated with fungal pathogenesis. Microbes Infect. 2006, 8, 2686–2697. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, S.V.; Fonseca, F.L.; Rodrigues, M.L.; Mundodi, V.; Abi-Chacra, E.A.; Winters, M.S.; Alderete, J.F.; de Almeida Soares, C.M. Paracoccidioides brasiliensis enolase is a surface protein that binds plasminogen and mediates interaction of yeast forms with host cells. Infect. Immun. 2010, 78, 4040–4050. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, T.; da Silva, J.F.; Vicentin, J.; de Oliveira, H.C.; Assato, P.A.; Marcos, C.M.; de Paula E Silva, A.C.; da Silva, R.A.; Regasini, L.O.; Silva, D.H.; et al. Anti-apoptotic effects of decyl gallate on the induction of apoptosis in A549 pneumocytes by Paracoccidioides brasiliensis gp43. Med. Mycol. 2017, 55, 890–894. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.F.; Vicentim, J.; Oliveira, H.C.; Marcos, C.M.; Assato, P.A.; Andreotti, P.F.; Silva, J.L.; Soares, C.P.; Benard, G.; Almeida, A.M.; et al. Influence of the Paracoccidioides brasiliensis 14-3-3 and gp43 proteins on the induction of apoptosis in A549 epithelial cells. Mem. Inst. Oswaldo Cruz 2015, 110, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Assato, P.A.; da Silva, J.e.F.; de Oliveira, H.C.; Marcos, C.M.; Rossi, D.; Valentini, S.R.; Mendes-Giannini, M.J.; Zanelli, C.F.; Fusco-Almeida, A.M. Functional analysis of Paracoccidioides brasiliensis 14-3-3 adhesin expressed in Saccharomyces cerevisiae. BMC Microbiol. 2015, 15, 256. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.S.; Báo, S.N.; Andreotti, P.F.; de Faria, F.P.; Felipe, M.S.; dos Santos Feitosa, L.; Mendes-Giannini, M.J.; Soares, C.M. Glyceraldehyde-3-phosphate dehydrogenase of Paracoccidioides brasiliensis is a cell surface protein involved in fungal adhesion to extracellular matrix proteins and interaction with cells. Infect. Immun. 2006, 74, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Brummer, E.; Restrepo, A.; Hanson, L.H.; Stevens, D.A. Virulence of Paracoccidiodes brasiliensis: The influence of in vitro passage and storage. Mycopathologia 1990, 109, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Shikanai-Yasuda, M.A.; Mendes, R.P.; Colombo, A.L.; Queiroz-Telles, F.; Kono, A.S.G.; Paniago, A.M.M.; Nathan, A.; Valle, A.C.F.D.; Bagagli, E.; Benard, G.; et al. Brazilian guidelines for the clinical management of paracoccidioidomycosis. Rev. Soc. Bras. Med. Trop 2017, 50, 715–740. [Google Scholar] [CrossRef] [PubMed]
- de Macedo, P.M.; Almeida-Paes, R.; Freitas, D.F.; Varon, A.G.; Paixão, A.G.; Romão, A.R.; Coutinho, Z.F.; Pizzini, C.V.; Zancopé-Oliveira, R.M.; Francesconi do Valle, A.C. Acute juvenile Paracoccidioidomycosis: A 9-year cohort study in the endemic area of Rio de Janeiro, Brazil. PLoS Negl. Trop Dis. 2017, 11, e0005500. [Google Scholar] [CrossRef] [PubMed]
- Wheat, L.J.; Freifeld, A.G.; Kleiman, M.B.; Baddley, J.W.; McKinsey, D.S.; Loyd, J.E.; Kauffman, C.A.; America, I.D.S.o. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2007, 45, 807–825. [Google Scholar] [CrossRef] [PubMed]
- Garfoot, A.L.; Rappleye, C.A. Histoplasma capsulatum surmounts obstacles to intracellular pathogenesis. FEBS J. 2016, 283, 619–633. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.A.; Rappleye, C.A. Histoplasma mechanisms of pathogenesis–one portfolio doesn’t fit all. FEMS Microbiol. Lett. 2011, 324, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cordero, R.J.; Liedke, S.C.; de S Araújo, G.R.; Martinez, L.R.; Nimrichter, L.; Frases, S.; Peralta, J.M.; Casadevall, A.; Rodrigues, M.L.; Nosanchuk, J.D.; et al. Enhanced virulence of Histoplasma capsulatum through transfer and surface incorporation of glycans from Cryptococcus neoformans during co-infection. Sci. Rep. 2016, 6, 21765. [Google Scholar] [CrossRef] [PubMed]
- Retallack, D.M.; Heinecke, E.L.; Gibbons, R.; Deepe, G.S.; Woods, J.P. The URA5 gene is necessary for Histoplasma capsulatum growth during infection of mouse and human cells. Infect. Immun. 1999, 67, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Deepe, G.S.; Gibbons, R.S.; Smulian, A.G. Histoplasma capsulatum manifests preferential invasion of phagocytic subpopulations in murine lungs. J. Leukoc. Biol. 2008, 84, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Sugar, A.M.; Picard, M. Macrophage- and oxidant-mediated inhibition of the ability of live Blastomyces dermatitidis conidia to transform to the pathogenic yeast phase: Implications for the pathogenesis of dimorphic fungal infections. J. Infect. Dis. 1991, 163, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Eissenberg, L.G.; Poirier, S.; Goldman, W.E. Phenotypic variation and persistence of Histoplasma capsulatum yeasts in host cells. Infect. Immun. 1996, 64, 5310–5314. [Google Scholar] [PubMed]
- Kujoth, G.C.; Sullivan, T.D.; Merkhofer, R.; Lee, T.J.; Wang, H.; Brandhorst, T.; Wüthrich, M.; Klein, B.S. CRISPR/Cas9-Mediated Gene Disruption Reveals the Importance of Zinc Metabolism for Fitness of the Dimorphic Fungal Pathogen Blastomyces dermatitidis. MBio 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Brandhorst, T.T.; Wüthrich, M.; Warner, T.; Klein, B. Targeted gene disruption reveals an adhesin indispensable for pathogenicity of Blastomyces dermatitidis. J. Exp. Med. 1999, 189, 1207–1216. [Google Scholar] [CrossRef] [PubMed]
- Finkel-Jimenez, B.; Wüthrich, M.; Klein, B.S. BAD1, an essential virulence factor of Blastomyces dermatitidis, suppresses host TNF-alpha production through TGF-beta-dependent and -independent mechanisms. J. Immunol. 2002, 168, 5746–5755. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, A.; Bonifaz, A.; Gutierrez-Galhardo, M.C.; Mochizuki, T.; Li, S. Global epidemiology of sporotrichosis. Med. Mycol. 2015, 53, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Marimon, R.; Cano, J.; Gené, J.; Sutton, D.A.; Kawasaki, M.; Guarro, J. Sporothrix brasiliensis, S. globosa, and S. mexicana, Three New Sporothrix Species of Clinical Interest. J. Clin. Microbiol. 2007, 45, 3198–3206. [Google Scholar] [CrossRef] [PubMed]
- García Carnero, L.C.; Lozoya Pérez, N.E.; González Hernández, S.E.; Martínez Álvarez, J.A. Immunity and Treatment of Sporotrichosis. J. Fungi (Basel) 2018, 4, 100. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Bezerra, L.M.; Mora-Montes, H.M.; Zhang, Y.; Nino-Vega, G.; Rodrigues, A.M.; de Camargo, Z.P.; de Hoog, S. Sporotrichosis between 1898 and 2017: The evolution of knowledge on a changeable disease and on emerging etiological agents. Med. Mycol. 2018, 56, 126–143. [Google Scholar] [CrossRef] [PubMed]
- Barros, M.B.d.L.; de Almeida Paes, R.; Schubach, A.O. Sporothrix schenckii and Sporotrichosis. Clin. Microbiol. Rev. 2011, 24, 633–654. [Google Scholar] [CrossRef] [PubMed]
- Castro, R.A.; Kubitschek-Barreira, P.H.; Teixeira, P.A.; Sanches, G.F.; Teixeira, M.M.; Quintella, L.P.; Almeida, S.R.; Costa, R.O.; Camargo, Z.P.; Felipe, M.S.; et al. Differences in cell morphometry, cell wall topography and gp70 expression correlate with the virulence of Sporothrix brasiliensis clinical isolates. PLoS ONE 2013, 8, e75656. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, G.F.; dos Santos, P.O.; Rodrigues, A.M.; Sasaki, A.A.; Burger, E.; de Camargo, Z.P. Characterization of virulence profile, protein secretion and immunogenicity of different Sporothrix schenckii sensu stricto isolates compared with S. globosa and S. brasiliensis species. Virulence 2013, 4, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.M.; Bagagli, E.; de Camargo, Z.P.; Bosco, S.e.M. Sporothrix schenckii sensu stricto isolated from soil in an armadillo’s burrow. Mycopathologia 2014, 177, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, J.; Albuquerque, P.C.; Wolf, J.M.; Nascimento, R.; Pereira, M.D.; Nosanchuk, J.D.; Rodrigues, M.L. Analysis of multiple components involved in the interaction between Cryptococcus neoformans and Acanthamoeba castellanii. Fungal Biology 2017, 121, 602–614. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, J.D.; Falkow, S.; Tompkins, L.S.; Bermudez, L.E. Interaction of Mycobacterium avium with environmental amoebae enhances virulence. Infect. Immun. 1997, 65, 3759–3767. [Google Scholar] [PubMed]
- Steenbergen, J.N.; Shuman, H.A.; Casadevall, A. Cryptococcus neoformans interactions with amoebae suggest an explanation for its virulence and intracellular pathogenic strategy in macrophages. Proc. Natl. Acad. Sci. USA 2001, 98, 15245–15250. [Google Scholar] [CrossRef] [PubMed]
- Malliaris, S.D.; Steenbergen, J.N.; Casadevall, A. Cryptococcus neoformans var. gattii can exploit Acanthamoeba castellanii for growth. Med. Mycol. 2004, 42, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Vanittanakom, N.; Cooper, C.R.; Fisher, M.C.; Sirisanthana, T. Penicillium marneffei Infection and Recent Advances in the Epidemiology and Molecular Biology Aspects. Clin. Microbiol. Rev. 2006, 19, 95–110. [Google Scholar] [CrossRef] [PubMed]
- Supparatpinyo, K.; Khamwan, C.; Baosoung, V.; Sirisanthana, T.; Nelson, K.E. Disseminated Penicillium marneffei infection in Southeast Asia. Lancet 1994, 344, 110–113. [Google Scholar] [CrossRef]
- Yuen, K.; Wong, S.S.; Chau, P.; Tsang, D.N. Serodiagnosis of Penicillium marneffei infection. Lancet 1994, 344, 444–445. [Google Scholar] [CrossRef]
- Son, V.T.; Khue, P.M.; Strobel, M. Penicilliosis and AIDS in Haiphong, Vietnam: Evolution and predictive factors of death. Med. Mal. Infect. 2014, 44, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.Y.; Wong, K.F. Penicillium marneffei Infection in AIDS. Patholog. Res. Int. 2011, 2011, 764293. [Google Scholar] [PubMed]
- Chan, J.F.W.; Lau, S.K.P.; Yuen, K.-Y.; Woo, P.C.Y. Talaromyces (Penicillium) marneffei infection in non-HIV-infected patients. Emerg. Microbes. Infect. 2016, 5, e19. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Liao, H.; Zhang, J.; Zhong, X.; Tan, C.; Lu, D. Differences in clinical characteristics and prognosis of Penicilliosis among HIV-negative patients with or without underlying disease in Southern China: A retrospective study. BMC Infect. Dis. 2015, 15, 525. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Qiu, Y.; Lu, D.; Zhang, J.; Zhong, X.; Liu, G. A Retrospective Analysis of 7 Human Immunodeficiency Virus-Negative Infants Infected by Penicillium marneffei. Medicine 2015, 94, e1439. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singulani, J.L.; Scorzoni, L.; De Oliveira, H.C.; Marcos, C.M.; Assato, P.A.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J.S. Applications of Invertebrate Animal Models to Dimorphic Fungal Infections. J. Fungi 2018, 4, 118. https://doi.org/10.3390/jof4040118
Singulani JL, Scorzoni L, De Oliveira HC, Marcos CM, Assato PA, Fusco-Almeida AM, Mendes-Giannini MJS. Applications of Invertebrate Animal Models to Dimorphic Fungal Infections. Journal of Fungi. 2018; 4(4):118. https://doi.org/10.3390/jof4040118
Chicago/Turabian StyleSingulani, Junya L., Liliana Scorzoni, Haroldo C. De Oliveira, Caroline M. Marcos, Patricia A. Assato, Ana Marisa Fusco-Almeida, and Maria José S. Mendes-Giannini. 2018. "Applications of Invertebrate Animal Models to Dimorphic Fungal Infections" Journal of Fungi 4, no. 4: 118. https://doi.org/10.3390/jof4040118
APA StyleSingulani, J. L., Scorzoni, L., De Oliveira, H. C., Marcos, C. M., Assato, P. A., Fusco-Almeida, A. M., & Mendes-Giannini, M. J. S. (2018). Applications of Invertebrate Animal Models to Dimorphic Fungal Infections. Journal of Fungi, 4(4), 118. https://doi.org/10.3390/jof4040118






