The CWI Pathway: Regulation of the Transcriptional Adaptive Response to Cell Wall Stress in Yeast
Abstract
:1. Cell Wall Organization and Structure in S. cerevisiae
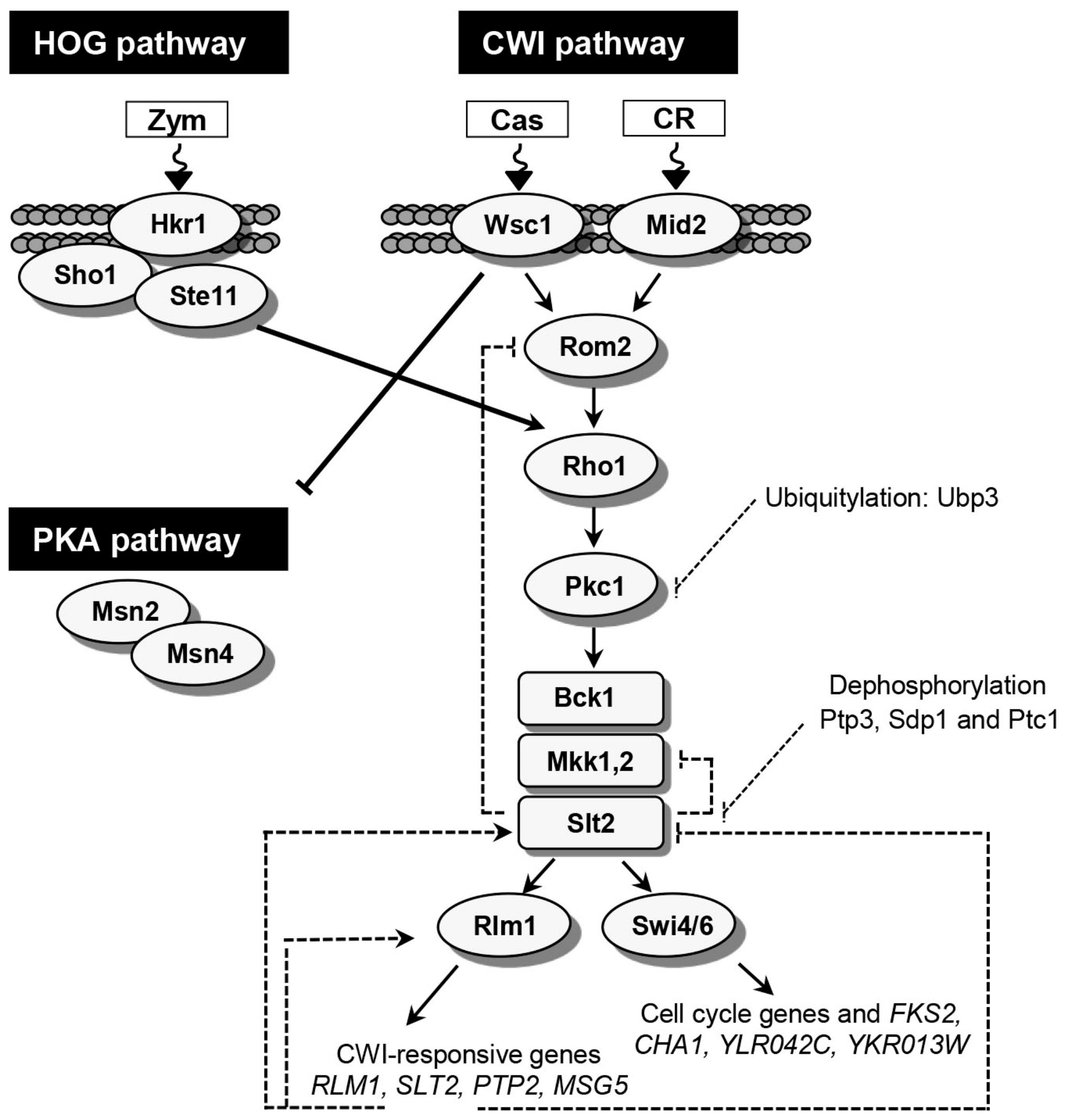
2. Yeast Adaptive Response to Cell Wall Stress: The CWI Pathway
3. The Transcriptional Program to Cell Wall Stress: Regulatory Mechanisms
4. Modulation of the CWI Pathway through the Transcriptional Response
5. Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Levin, D.E. Regulation of cell wall biogenesis in Saccharomyces cerevisiae: The cell wall integrity signaling pathway. Genetics 2011, 189, 1145–1175. [Google Scholar] [CrossRef] [PubMed]
- Orlean, P. Architecture and biosynthesis of the Saccharomyces cerevisiae cell wall. Genetics 2012, 192, 775–818. [Google Scholar] [CrossRef] [PubMed]
- Kapteyn, J.C.; Montijn, R.C.; Vink, E.; de la Cruz, J.; Llobell, A.; Douwes, J.E.; Shimoi, H.; Lipke, P.N.; Klis, F.M. Retention of Saccharomyces cerevisiae cell wall proteins through a phosphodiester-linked β-1,3-/β-1,6-glucan heteropolymer. Glycobiology 1996, 6, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Kollar, R.; Reinhold, B.B.; Petrakova, E.; Yeh, H.J.; Ashwell, G.; Drgonova, J.; Kapteyn, J.C.; Klis, F.M.; Cabib, E. Architecture of the yeast cell wall. β(1→6)-glucan interconnects mannoprotein, β(1→3)-glucan, and chitin. J. Biol. Chem. 1997, 272, 17762–17775. [Google Scholar] [CrossRef] [PubMed]
- Kollar, R.; Petrakova, E.; Ashwell, G.; Robbins, P.W.; Cabib, E. Architecture of the yeast cell wall. The linkage between chitin and β(1→3)-glucan. J. Biol. Chem. 1995, 270, 1170–1178. [Google Scholar] [CrossRef] [PubMed]
- Cabib, E.; Blanco, N.; Grau, C.; Rodríguez-Peña, J.M.; Arroyo, J. Crh1p and Crh2p are required for the cross-linking of chitin to β(1–6)glucan in the Saccharomyces cerevisiae cell wall. Mol. Microbiol. 2007, 63, 921–935. [Google Scholar] [CrossRef] [PubMed]
- Dranginis, A.M.; Rauceo, J.M.; Coronado, J.E.; Lipke, P.N. A biochemical guide to yeast adhesins: Glycoproteins for social and antisocial occasions. Microbiol. Mol. Biol. Rev. 2007, 71, 282–294. [Google Scholar] [CrossRef] [PubMed]
- Mouyna, I.; Fontaine, T.; Vai, M.; Monod, M.; Fonzi, W.A.; Diaquin, M.; Popolo, L.; Hartland, R.P.; Latgé, J.P. Glycosylphosphatidylinositol-anchored glucanosyltransferases play an active role in the biosynthesis of the fungal cell wall. J. Biol. Chem. 2000, 275, 14882–14889. [Google Scholar] [CrossRef] [PubMed]
- De Groot, P.W.; Ram, A.F.; Klis, F.M. Features and functions of covalently linked proteins in fungal cell walls. Fungal Genet. Biol. 2005, 42, 657–675. [Google Scholar] [CrossRef] [PubMed]
- Kapteyn, J.C.; Van Egmond, P.; Sievi, E.; Van Den, E.H.; Makarow, M.; Klis, F.M. The contribution of the O-glycosylated protein Pir2p/Hsp150 to the construction of the yeast cell wall in wild-type cells and β-1,6-glucan-deficient mutants. Mol. Microbiol. 1999, 31, 1835–1844. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, M.A.J.; Kinoshita, T.; Hart, G.W. Glycosylphosphatidylinositol anchors. In Essentials of Glycobiology; Varki, A., Cummings, R.D., Esko, J.D., Freeze, H.H., Stanley, P., Bertozzi, C.R., Hart, G.W., Etzler, M.E., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2009. [Google Scholar]
- Gow, N.A.R.; Latge, J.P.; Munro, C.A. The fungal cell wall: Structure, biosynthesis, and function. Microbiol. Spectr. 2017, 5, 1–25. [Google Scholar]
- Synytsya, A.; Novak, M. Structural diversity of fungal glucans. Carbohydr. Polym. 2013, 92, 792–809. [Google Scholar] [CrossRef] [PubMed]
- Free, S.J. Fungal cell wall organization and biosynthesis. Adv. Genet. 2013, 81, 33–82. [Google Scholar] [PubMed]
- Lesage, G.; Bussey, H. Cell wall assembly in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2006, 70, 317–343. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Herrera, J.; Elorza, M.V.; Valentin, E.; Sentandreu, R. Molecular organization of the cell wall of Candida albicans and its relation to pathogenicity. FEMS Yeast Res. 2006, 6, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Aimanianda, V.; Clavaud, C.; Simenel, C.; Fontaine, T.; Delepierre, M.; Latgé, J.P. Cell wall β-(1,6)-glucan of Saccharomyces cerevisiae: Structural characterization and in situ synthesis. J. Biol. Chem. 2009, 284, 13401–13412. [Google Scholar] [CrossRef] [PubMed]
- Latgé, J.P. The cell wall: A carbohydrate armour for the fungal cell. Mol. Microbiol. 2007, 66, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Tada, R.; Latgé, J.P.; Aimanianda, V. Undressing the fungal cell wall/cell membrane—The antifungal drug targets. Curr. Pharm. Des. 2013, 19, 3738–3747. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, M.B.; Douglas, C.M. Lipopeptide inhibitors of fungal glucan synthase. J. Med. Vet. Mycol. 1997, 35, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Bal, A.M. The echinocandins: Three useful choices or three too many? Int. J. Antimicrob. Agents 2010, 35, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.A.; Gow, N.A.; Munro, C.A. Fungal echinocandin resistance. Fungal Genet. Biol. 2010, 47, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Fortwendel, J.R.; Juvvadi, P.R.; Perfect, B.Z.; Rogg, L.E.; Perfect, J.R.; Steinbach, W.J. Transcriptional regulation of chitin synthases by calcineurin controls paradoxical growth of Aspergillus fumigatus in response to caspofungin. Antimicrob. Agents Chemother. 2010, 54, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Meyer, V.; Damveld, R.A.; Arentshorst, M.; Stahl, U.; van den Hondel, C.A.; Ram, A.F. Survival in the presence of antifungals: Genome-wide expression profiling of Aspergillus niger in response to sublethal concentrations of caspofungin and fenpropimorph. J. Biol. Chem. 2007, 282, 32935–32948. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.A.; Lee, K.K.; Munro, C.A.; Gow, N.A. Caspofungin treatment of Aspergillus fumigatus results in ChsG-dependent upregulation of chitin synthesis and the formation of chitin-rich microcolonies. Antimicrob. Agents Chemother. 2015, 59, 5932–5941. [Google Scholar] [CrossRef] [PubMed]
- Schiavone, M.; Vax, A.; Formosa, C.; Martin-Yken, H.; Dague, E.; Francois, J.M. A combined chemical and enzymatic method to determine quantitatively the polysaccharide components in the cell wall of yeasts. FEMS Yeast Res. 2014, 14, 933–947. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Uscanga, B.; Francois, J.M. A study of the yeast cell wall composition and structure in response to growth conditions and mode of cultivation. Lett. Appl. Microbiol. 2003, 37, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Klis, F.M.; Boorsma, A.; De Groot, P.W. Cell wall construction in Saccharomyces cerevisiae. Yeast 2006, 23, 185–202. [Google Scholar] [CrossRef] [PubMed]
- Popolo, L.; Gualtieri, T.; Ragni, E. The yeast cell-wall salvage pathway. Med. Mycol. 2001, 39 (Suppl. 1), 111–121. [Google Scholar] [CrossRef] [PubMed]
- García, R.; Bermejo, C.; Grau, C.; Pérez, R.; Rodríguez-Peña, J.M.; Francois, J.; Nombela, C.; Arroyo, J. The global transcriptional response to transient cell wall damage in Saccharomyces cerevisiae and its regulation by the cell integrity signaling pathway. J. Biol. Chem. 2004, 279, 15183–15195. [Google Scholar] [CrossRef] [PubMed]
- Ketela, T.; Green, R.; Bussey, H. Saccharomyces cerevisiae Mid2p is a potential cell wall stress sensor and upstream activator of the Pkc1-Mpk1 cell integrity pathway. J. Bacteriol. 1999, 181, 3330–3340. [Google Scholar] [PubMed]
- Rodríguez-Peña, J.M.; Díez-Muñiz, S.; Bermejo, C.; Nombela, C.; Arroyo, J. Activation of the yeast cell wall integrity MAPK pathway by zymolyase depends on protease and glucanase activities and requires the mucin-like protein Hkr1 but not Msb2. FEBS Lett. 2013, 587, 3675–3680. [Google Scholar] [CrossRef] [PubMed]
- Charoenbhakdi, S.; Dokpikul, T.; Burphan, T.; Techo, T.; Auesukaree, C. Vacuolar H+-ATPase protects Saccharomyces cerevisiae cells against ethanol-induced oxidative and cell wall stresses. Appl. Environ. Microbiol. 2016, 82, 3121–3130. [Google Scholar] [CrossRef] [PubMed]
- Smits, G.J.; Kapteyn, J.C.; Van Den, E.H.; Klis, F.M. Cell wall dynamics in yeast. Curr. Opin. Microbiol. 1999, 2, 348–352. [Google Scholar] [CrossRef]
- Dallies, N.; Francois, J.; Paquet, V. A new method for quantitative determination of polysaccharides in the yeast cell wall. Application to the cell wall defective mutants of Saccharomyces cerevisiae. Yeast 1998, 14, 1297–1306. [Google Scholar] [CrossRef]
- Gentzsch, M.; Tanner, W. The PMT gene family: Protein O-glycosylation in Saccharomyces cerevisiae is vital. EMBO J. 1996, 15, 5752–5759. [Google Scholar] [PubMed]
- Popolo, L.; Gilardelli, D.; Bonfante, P.; Vai, M. Increase in chitin as an essential response to defects in assembly of cell wall polymers in the ggp1Δ mutant of Saccharomyces cerevisiae. J. Bacteriol. 1997, 179, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, J.; Hutzler, J.; Bermejo, C.; Ragni, E.; García-Cantalejo, J.; Botias, P.; Piberger, H.; Schott, A.; Sanz, A.B.; Strahl, S. Functional and genomic analyses of blocked protein O-mannosylation in baker’s yeast. Mol. Microbiol. 2011, 79, 1529–1546. [Google Scholar] [CrossRef] [PubMed]
- De Nobel, H.; Ruiz, C.; Martin, H.; Morris, W.; Brul, S.; Molina, M.; Klis, F.M. Cell wall perturbation in yeast results in dual phosphorylation of the Slt2/Mpk1 MAP kinase and in an Slt2-mediated increase in FKS2-LacZ expression, glucanase resistance and thermotolerance. Microbiology 2000, 146, 2121–2132. [Google Scholar] [CrossRef] [PubMed]
- Jung, U.S.; Levin, D.E. Genome-wide analysis of gene expression regulated by the yeast cell wall integrity signalling pathway. Mol. Microbiol. 1999, 34, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Kapteyn, J.C.; Ram, A.F.; Groos, E.M.; Kollar, R.; Montijn, R.C.; Van Den, E.H.; Llobell, A.; Cabib, E.; Klis, F.M. Altered extent of cross-linking of β 1,6-glucosylated mannoproteins to chitin in Saccharomyces cerevisiae mutants with reduced cell wall β 1,3-glucan content. J. Bacteriol. 1997, 179, 6279–6284. [Google Scholar] [CrossRef] [PubMed]
- Delley, P.A.; Hall, M.N. Cell wall stress depolarizes cell growth via hyperactivation of Rho1. J. Cell. Biol. 1999, 147, 163–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapteyn, J.C.; Van Den Ende, H.; Klis, F.M. The contribution of cell wall proteins to the organization of the yeast cell wall. Biochim. Biophys. Acta 1999, 1426, 373–383. [Google Scholar] [CrossRef]
- Ene, I.V.; Walker, L.A.; Schiavone, M.; Lee, K.K.; Martin-Yken, H.; Dague, E.; Gow, N.A.; Munro, C.A.; Brown, A.J. Cell wall remodeling enzymes modulate fungal cell wall elasticity and osmotic stress resistance. mBio 2015, 6, e00986. [Google Scholar] [CrossRef] [PubMed]
- Lagorce, A.; Hauser, N.C.; Labourdette, D.; Rodríguez, C.; Martin-Yken, H.; Arroyo, J.; Hoheisel, J.D.; Francois, J. Genome-wide analysis of the response to cell wall mutations in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 2003, 278, 20345–20357. [Google Scholar] [CrossRef] [PubMed]
- Dichtl, K.; Samantaray, S.; Wagener, J. Cell wall integrity signalling in human pathogenic fungi. Cell. Microbiol. 2016, 18, 1228–1238. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Takaesu, G.; Hagiwara, M.; Irie, K.; Matsumoto, K. Characterization of a serum response factor-like protein in Saccharomyces cerevisiae, Rlm1, which has transcriptional activity regulated by the Mpk1 (Slt2) mitogen-activated protein kinase pathway. Mol. Cell. Biol. 1997, 17, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Baetz, K.; Moffat, J.; Haynes, J.; Chang, M.; Andrews, B. Transcriptional coregulation by the cell integrity mitogen-activated protein kinase Slt2 and the cell cycle regulator Swi4. Mol. Cell. Biol. 2001, 21, 6515–6528. [Google Scholar] [CrossRef] [PubMed]
- Boorsma, A.; De Nobel, H.; ter Riet, B.; Bargmann, B.; Brul, S.; Hellingwerf, K.J.; Klis, F.M. Characterization of the transcriptional response to cell wall stress in Saccharomyces cerevisiae. Yeast 2004, 21, 413–427. [Google Scholar] [CrossRef] [PubMed]
- García, R.; Rodríguez-Peña, J.M.; Bermejo, C.; Nombela, C.; Arroyo, J. The high osmotic response and cell wall integrity pathways cooperate to regulate transcriptional responses to zymolyase-induced cell wall stress in Saccharomyces cerevisiae. J. Biol. Chem. 2009, 284, 10901–10911. [Google Scholar] [CrossRef] [PubMed]
- Reinoso-Martin, C.; Schuller, C.; Schuetzer-Muehlbauer, M.; Kuchler, K. The yeast protein kinase c cell integrity pathway mediates tolerance to the antifungal drug caspofungin through activation of Slt2p mitogen-activated protein kinase signaling. Eukaryot. Cell 2003, 2, 1200–1210. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.K.; Rogers, P.D.; Baerson, S.R.; Jacob, M.R.; Barker, K.S.; Cleary, J.D.; Walker, L.A.; Nagle, D.G.; Clark, A.M. Genome-wide expression profiling of the response to polyene, pyrimidine, azole, and echinocandin antifungal agents in Saccharomyces cerevisiae. J. Biol. Chem. 2003, 278, 34998–35015. [Google Scholar] [CrossRef] [PubMed]
- Bermejo, C.; García, R.; Straede, A.; Rodríguez-Peña, J.M.; Nombela, C.; Heinisch, J.J.; Arroyo, J. Characterization of sensor-specific stress response by transcriptional profiling of wsc1 and mid2 deletion strains and chimeric sensors in Saccharomyces cerevisiae. OMICS J. Integr. Biol. 2010, 14, 679–688. [Google Scholar] [CrossRef] [PubMed]
- García, R.; Bravo, E.; Díez-Muñiz, S.; Nombela, C.; Rodríguez-Peña, J.M.; Arroyo, J. A novel connection between the cell wall integrity and the PKA pathways regulates cell wall stress response in yeast. Sci. Rep. 2017, 7, 5703. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, J.; Bermejo, C.; García, R.; Rodríguez-Peña, J.M. Genomics in the detection of damage in microbial systems: Cell wall stress in yeast. Clin. Microbiol. Infect. 2009, 15, 44–46. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.B.; Gasch, A.P. Stress-activated genomic expression changes serve a preparative role for impending stress in yeast. Mol. Biol. Cell 2008, 19, 4580–4587. [Google Scholar] [CrossRef] [PubMed]
- Westfall, P.J.; Patterson, J.C.; Chen, R.E.; Thorner, J. Stress resistance and signal fidelity independent of nuclear MAPK function. Proc. Natl. Acad. Sci. USA 2008, 105, 12212–12217. [Google Scholar] [CrossRef] [PubMed]
- Lagorce, A.; Berre-Anton, V.; Aguilar-Uscanga, B.; Martin-Yken, H.; Dagkessamanskaia, A.; Francois, J. Involvement of GFA1, which encodes glutamine-fructose-6-phosphate amidotransferase, in the activation of the chitin synthesis pathway in response to cell-wall defects in Saccharomyces cerevisiae. Eur. J. Biochem. 2002, 269, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.A.; Mol, P.C.; Bowers, B.; Silverman, S.J.; Valdivieso, M.H.; Durán, A.; Cabib, E. The function of chitin synthases 2 and 3 in the Saccharomyces cerevisiae cell cycle. J. Cell. Biol. 1991, 114, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, J.; Farkas, V.; Sanz, A.B.; Cabib, E. Strengthening the fungal cell wall through chitin-glucan cross-links: Effects on morphogenesis and cell integrity. Cell. Microbiol. 2016, 18, 1239–1250. [Google Scholar] [CrossRef] [PubMed]
- Cabib, E.; Arroyo, J. How carbohydrates sculpt cells: Chemical control of morphogenesis in the yeast cell wall. Nat. Rev. Microbiol. 2013, 11, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Ragni, E.; Sipiczki, M.; Strahl, S. Characterization of Ccw12p, a major key player in cell wall stability of Saccharomyces cerevisiae. Yeast 2007, 24, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Sanz, A.B.; García, R.; Rodríguez-Peña, J.M.; Díez-Muñiz, S.; Nombela, C.; Peterson, C.L.; Arroyo, J. Chromatin remodeling by the SWI/SNF complex is essential for transcription mediated by the yeast cell wall integrity MAPK pathway. Mol. Biol. Cell 2012, 23, 2805–2817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, U.S.; Sobering, A.K.; Romeo, M.J.; Levin, D.E. Regulation of the yeast Rlm1 transcription factor by the Mpk1 cell wall integrity MAP kinase. Mol. Microbiol. 2002, 46, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Sanz, A.B.; García, R.; Rodríguez-Peña, J.M.; Nombela, C.; Arroyo, J. Cooperation between SAGA and SWI/SNF complexes is required for efficient transcriptional responses regulated by the yeast MAPK Slt2. Nucleic Acids Res. 2016, 44, 7159–7172. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Levin, D.E. Mpk1 MAPK association with the Paf1 complex blocks Sen1-mediated premature transcription termination. Cell 2011, 144, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Levin, D.E. Transcriptional reporters for genes activated by cell wall stress through a non-catalytic mechanism involving Mpk1 and SBF. Yeast 2010, 27, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Truman, A.W.; Levin, D.E. Yeast Mpk1 mitogen-activated protein kinase activates transcription through Swi4/Swi6 by a noncatalytic mechanism that requires upstream signal. Mol. Cell. Biol. 2008, 28, 2579–2589. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Posas, F. Response to hyperosmotic stress. Genetics 2012, 192, 289–318. [Google Scholar] [CrossRef] [PubMed]
- Bermejo, C.; Rodríguez, E.; García, R.; Rodríguez-Peña, J.M.; de la Concepcion, M.L.R.; Rivas, C.; Arias, P.; Nombela, C.; Posas, F.; Arroyo, J. The sequential activation of the yeast HOG and SLT2 pathways is required for cell survival to cell wall stress. Mol. Biol. Cell. 2008, 19, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.D.; Verveer, P.J.; Bastiaens, P.I. Growth factor-induced MAPK network topology shapes Erk response determining PC-12 cell fate. Nat. Cell Biol. 2007, 9, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Mattison, C.P.; Spencer, S.S.; Kresge, K.A.; Lee, J.; Ota, I.M. Differential regulation of the cell wall integrity mitogen-activated protein kinase pathway in budding yeast by the protein tyrosine phosphatases Ptp2 and Ptp3. Mol. Cell. Biol. 1999, 19, 7651–7660. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Walker, L.; Novick, P.; Ferro-Novick, S. Ptc1p regulates cortical er inheritance via Slt2p. EMBO J. 2006, 25, 4413–4422. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Ruiz, A.; Serrano, R.; Arino, J.; Casamayor, A. Transcriptional profiling of the protein phosphatase 2c family in yeast provides insights into the unique functional roles of Ptc1. J. Biol. Chem. 2006, 281, 35057–35069. [Google Scholar] [CrossRef] [PubMed]
- Flandez, M.; Cosano, I.C.; Nombela, C.; Martin, H.; Molina, M. Reciprocal regulation between Slt2 MAPK and isoforms of Msg5 dual-specificity protein phosphatase modulates the yeast cell integrity pathway. J. Biol. Chem. 2004, 279, 11027–11034. [Google Scholar] [CrossRef] [PubMed]
- Collister, M.; Didmon, M.P.; MacIsaac, F.; Stark, M.J.; MacDonald, N.Q.; Keyse, S.M. YIL113W encodes a functional dual-specificity protein phosphatase which specifically interacts with and inactivates the Slt2/Mpk1p MAP kinase in S. cerevisiae. FEBS Lett. 2002, 527, 186–192. [Google Scholar] [CrossRef]
- Hahn, J.S.; Thiele, D.J. Regulation of the Saccharomyces cerevisiae Slt2 kinase pathway by the stress-inducible Sdp1 dual specificity phosphatase. J. Biol. Chem. 2002, 277, 21278–21284. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Shen, X.; Yan, G.; Ma, D.; Bai, X.; Li, S.; Jiang, Y. A MAP kinase dependent feedback mechanism controls Rho1 GTPase and actin distribution in yeast. PLoS ONE 2009, 4, e6089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jimenez-Sanchez, M.; Cid, V.J.; Molina, M. Retrophosphorylation of Mkk1 and Mkk2 MAPKKS by the Slt2 MAPK in the yeast cell integrity pathway. J. Biol. Chem. 2007, 282, 31174–31185. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, M.; Ayalew, M.; Ruff, J.A. Down-regulation of Pkc1-mediated signaling by the deubiquitinating enzyme Ubp3. J. Biol. Chem. 2008, 283, 1954–1961. [Google Scholar] [CrossRef] [PubMed]
- Becskei, A.; Serrano, L. Engineering stability in gene networks by autoregulation. Nature 2000, 405, 590–593. [Google Scholar] [CrossRef] [PubMed]
- Singh, V. Recent advancements in synthetic biology: Current status and challenges. Gene 2014, 535, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.J.; Nelson, B.; Marton, M.J.; Stoughton, R.; Meyer, M.R.; Bennett, H.A.; He, Y.D.; Dai, H.; Walker, W.L.; Hughes, T.R.; et al. Signaling and circuitry of multiple MAPK pathways revealed by a matrix of global gene expression profiles. Science 2000, 287, 873–880. [Google Scholar] [CrossRef] [PubMed]
- García, R.; Sanz, A.B.; Rodríguez-Peña, J.M.; Nombela, C.; Arroyo, J. Rlm1 mediates positive autoregulatory transcriptional feedback that is essential for Slt2-dependent gene expression. J. Cell Sci. 2016, 129, 1649–1660. [Google Scholar] [CrossRef] [PubMed]
- Wiederhold, N.P. The antifungal arsenal: Alternative drugs and future targets. Int. J. Antimicrob. Agents 2017, in press. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Miller, M.J. Polyoxins and nikkomycins: Progress in synthetic and biological studies. Curr. Pharm. Des. 1999, 5, 73–99. [Google Scholar] [PubMed]
- Cowen, L.E.; Steinbach, W.J. Stress, drugs, and evolution: The role of cellular signaling in fungal drug resistance. Eukaryot. Cell 2008, 7, 747–764. [Google Scholar] [CrossRef] [PubMed]
- Verwer, P.E.; van Duijn, M.L.; Tavakol, M.; Bakker-Woudenberg, I.A.; van de Sande, W.W. Reshuffling of Aspergillus fumigatus cell wall components chitin and β-glucan under the influence of caspofungin or nikkomycin z alone or in combination. Antimicrob. Agents Chemother. 2012, 56, 1595–1598. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.A.; Gow, N.A.; Munro, C.A. Elevated chitin content reduces the susceptibility of Candida species to caspofungin. Antimicrob. Agents Chemother. 2013, 57, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.A.; Munro, C.A.; de Bruijn, I.; Lenardon, M.D.; McKinnon, A.; Gow, N.A. Stimulation of chitin synthesis rescues Candida albicans from echinocandins. PLoS Pathog. 2008, 4, e1000040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Peña, J.M.; Pérez-Diaz, R.M.; Alvarez, S.; Bermejo, C.; García, R.; Santiago, C.; Nombela, C.; Arroyo, J. The ‘yeast cell wall chip’—A tool to analyse the regulation of cell wall biogenesis in Saccharomyces cerevisiae. Microbiology 2005, 151, 2241–2249. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Peña, J.M.; Díez-Muñiz, S.; Nombela, C.; Arroyo, J. A yeast strain biosensor to detect cell wall-perturbing agents. J. Biotechnol. 2008, 133, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Kitichantaropas, Y.; Boonchird, C.; Sugiyama, M.; Kaneko, Y.; Harashima, S.; Auesukaree, C. Cellular mechanisms contributing to multiple stress tolerance in Saccharomyces cerevisiae strains with potential use in high-temperature ethanol fermentation. AMB Express 2016, 6, 107. [Google Scholar] [CrossRef] [PubMed]
- Bashir, K.M.I.; Choi, J.S. Clinical and Physiological Perspectives of β-Glucans: The Past, Present, and Future. Int. J. Mol. Sci. 2017, 18, 1906. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanz, A.B.; García, R.; Rodríguez-Peña, J.M.; Arroyo, J. The CWI Pathway: Regulation of the Transcriptional Adaptive Response to Cell Wall Stress in Yeast. J. Fungi 2018, 4, 1. https://doi.org/10.3390/jof4010001
Sanz AB, García R, Rodríguez-Peña JM, Arroyo J. The CWI Pathway: Regulation of the Transcriptional Adaptive Response to Cell Wall Stress in Yeast. Journal of Fungi. 2018; 4(1):1. https://doi.org/10.3390/jof4010001
Chicago/Turabian StyleSanz, Ana Belén, Raúl García, José M. Rodríguez-Peña, and Javier Arroyo. 2018. "The CWI Pathway: Regulation of the Transcriptional Adaptive Response to Cell Wall Stress in Yeast" Journal of Fungi 4, no. 1: 1. https://doi.org/10.3390/jof4010001
APA StyleSanz, A. B., García, R., Rodríguez-Peña, J. M., & Arroyo, J. (2018). The CWI Pathway: Regulation of the Transcriptional Adaptive Response to Cell Wall Stress in Yeast. Journal of Fungi, 4(1), 1. https://doi.org/10.3390/jof4010001





