Abstract
Infections caused by Candida species have been increasing in the last decades and can result in local or systemic infections, with high morbidity and mortality. After Candida albicans, Candida glabrata is one of the most prevalent pathogenic fungi in humans. In addition to the high antifungal drugs resistance and inability to form hyphae or secret hydrolases, C. glabrata retain many virulence factors that contribute to its extreme aggressiveness and result in a low therapeutic response and serious recurrent candidiasis, particularly biofilm formation ability. For their extraordinary organization, especially regarding the complex structure of the matrix, biofilms are very resistant to antifungal treatments. Thus, new approaches to the treatment of C. glabrata’s biofilms are emerging. In this article, the knowledge available on C. glabrata’s resistance will be highlighted, with a special focus on biofilms, as well as new therapeutic alternatives to control them.
Keywords:
Candida species; Candida glabrata; biofilm; candidiasis; resistance; antifungal; infection 1. Introduction
1.1. Biology of Candida glabrata
Historically, Candida glabrata strains were originally classified in the genus Torulopsis due to its lack of filaments forms formation. However, in 1978 it was determined that the ability to form hyphae and/or pseudohyphae was not a reliable distinguishing factor of members of genus Candida species, and it was proposed that T. glabrata should be classified in the genus Candida, due to its human pathogenicity [1]. In fact, in contrast to other Candida species, C. glabrata is not polymorphic, growing only as blastoconidia and regarding the genetic aspects of Candida species, a critical distinguishing characteristic of this species is its haploid genome, in opposition to the diploid genome of Candida albicans and other Candida species. It should be highlighted that C. glabrata cells (1–4 µm) are noticeably smaller than C. albicans (4–6 µm), Candida tropicalis (4–8 µm), and other Candida species blastoconidia [2] (Figure 1). In Sabouraud dextrose agar culture medium, C. glabrata strains forms glistening, smooth, and cream-coloured colonies, which are relatively indistinguishable from those of other Candida species except for their relative size (Figure 1), which can be quite small [3]. On CHROMagarTM Candida (CHROMagar, Paris, France), a differential agar medium, it is possible to distinguish a number of different Candida species by colour; as a result of distinct biochemical reactions, C. glabrata colonies appear white, pink to purple (Figure 1), in contrast to C. albicans colonies which are blue-green. Concerning the biochemical reactions of Candida species, C. glabrata ferments and assimilates only glucose and trehalose, contrary to C. albicans, which ferments and/or assimilates a high number of sugars excluding sucrose [4,5]. Whereas C. albicans, Candida parapsilosis, and C. tropicalis are moderately closely related species of the CUG clade, which share a unique codon exchange from leucine to serine, C. glabrata is actually a “misnomer”, for it is really much more closely related to the Saccharomyces cerevisiae than to C. albicans [6,7]. As mentioned, oppositely to the other Candida species, but equally to its cousin S. cerevisiae, C. glabrata is strictly haploid and typically grows only in the yeast form [8].
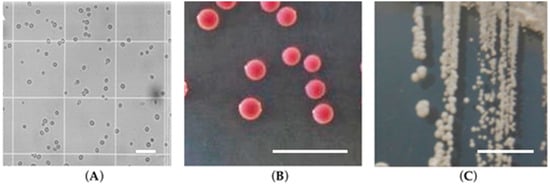
Figure 1.
Candida glabrata cells: (A) microscopy structure; (B) on CHROMagarTM Candida; (C) on Sabouraud dextrose agar (adapted from [4]). The scale corresponds to 50 μm with a magnification of 200×.
1.2. Epidemiology and Virulence Factors of Candida glabrata
For many years C. glabrata was considered a relatively non-pathogenic saprophyte of the normal flora of healthy individuals and certainly not readily associated with serious infections in humans. However, following the widespread and increased use of immunosuppressive therapy together with broad–spectrum antibiotic and antifungal therapies, the frequency of mucosal and systemic infections caused by C. glabrata has been growing significantly [9]. In fact, whilst mycological studies have been shown that C. albicans represents approximately 80% of the clinical isolates, in the last few decades, the number of candidiasis due to non-Candida albicans Candida (NCAC) species has meaningfully raised, namely in regard to C. glabrata strains [1,10,11]. Some studies suggest that fungemia has been associated with NCAC species [11,12,13]. The incidence of C. glabrata is higher in adults than in children, and is lower in neonates [14,15]. In the European Confederation of Medical Mycology survey, the frequency rates of candidiasis attributed to C. glabrata were around 14% [16] and 15% of all Candida-related systemic bloodstream infections [17,18]. This is extremely important since, compared to other Candida species infections, the mortality rate associated with C. glabrata is the highest [19].
Subsequently to the introduction of the highly active antiretroviral therapy, a reduction in the percentage of oropharyngeal infections, the colonisation by Candida species, and a decline in the frequency of fluconazole resistance in patients with HIV infection have been recorded [20]. However, Candida species are still the most frequent cause of systemic mycosis in our time [21,22]. During 1995–1996 and 1997–1998, a national programme of surveillance of bloodstream infections in the USA [23], and the SENTRY international programme of surveillance of bloodstream infections in the USA, Canada, and South America [24], showed the rising importance of NCAC, which accounted for between 44% and 48% of cases of fungaemia. Among NCAC, C. glabrata clearly stood out, with an increased prevalence observed through the study period in all three geographical regions, becoming the second most frequent species after C. albicans in the USA and Canada [23,24] and with a mortality rate associated with bloodstream infections of 49% in a retrospective series of 139 cases [25]. In the European SENTRY programme C. glabrata was the third most common NCAC, after C. parapsilosis [26]. In considering the SENTRY programme of USA from 1997–1999, NCAC were usually more susceptible to fluconazole, but continued surveillance is needed to confirm this tendency, as it is known that this may be not accurate in present days, mostly for C. glabrata and Candida krusei, which are known to be intrinsically resistant to fluconazole [23,24,26,27,28,29,30,31].
Until recently, few studies had evaluated independent risk factors associated with nosocomial C. glabrata acquisition and subsequent infection. Little is known about the hospital reservoirs of C. glabrata, but with C. albicans, probable sources include a complex interaction of environmental and human reservoirs [32]. Vasquez and colleagues [33] revealed that patients with a new acquisition of C. glabrata had extended and repeated hospitalizations prior to antifungal use compared to patients with no Candida species exposition. Candida glabrata has been also often isolated from patients with oral candidiasis, alone or coupled with C. albicans clinical isolates [4,34] and thus, has been related to recurrent systemic infections [35,36]. The propensity of C. glabrata for dissemination and the high mortality associated could be related to the virulence factors that this species exhibits, namely the elevated rates of resistance to the traditional antifungals.
The relatively nonpathogenic nature of C. glabrata in animal models [37,38] suggests that it has only few virulence attributes. However, the high mortality rate and the rapidity of the spread of disease would suggest the contrary [1]. In fact, in opposition to its inability to form hyphae and/or pseudohyphae and secret proteases, C. glabrata retain many virulence factors such as the capacity to secrete phospholipases, lipases, and haemolysins that contribute towards an extreme aggressiveness resulting in a low therapeutic response and serious recurrent candidiasis [10,39]. However, its most worrying virulence factor is its strong capability to form biofilms [10,40,41]. Biofilms are known as surface-associated communities of microorganisms embedded in an extracellular matrix [42,43], which confer significant antifungal therapy and host immune responses [4,10,41]. Candida glabrata clinical isolates have the ability to form a compact biofilm structure in different multilayers [40,41], with proteins, carbohydrates (e.g., β-1,3 glucans), and ergosterol in their matrixes [40,41,44]. The first step of C. glabrata biofilm development is adhesion and/or colonisation of yeast cells to an abiotic or/and biotic surface [4,10]. Adhesion is an extremely important step, not only in the biofilm formation, but also in the infections processes, and the extent of adhesion is dependent on C. glabrata cells’ characteristics, and host and/or abiotic surface properties, such as cell-surface hydrophobicity and cell wall composition [4,45]. The Candida glabrata cell wall is the site for physicochemical interactions between the microorganism and the surfaces, leading to its adherence. Despite the lack of studies concerning this issue, it is assumed that the cell surface of C. glabrata cells reportedly exhibit a degree of hydrophobicity comparable with C. albicans [46]. Interestingly, however, while the hydrophobicity of C. albicans was extremely sensitive to specific growth conditions, numerous isolates of C. glabrata were relatively insensitive to those same growth conditions [47]. Similar to C. albicans, C. glabrata adhesion phenomenon is mediated by epithelial adhesins (Epa) that have a comparable structure to the Als proteins [48]. The family of EPA genes are composed of 17–23 genes depending on the strain, however, EPA1, EPA6, and EPA7 are the most important adhesins [49]. Deletion of the EPA1 gene reduces C. glabrata adherence in vitro to host epithelial cells [50] and the adherence of this adhesin was inhibited in the presence of lactose [51]. In addition, usually C. glabrata strains are unable to express EPA6 in vitro, however, it is expressed during urinary infection, due to low levels of nicotinic acid [51]. Groot et al. [52] identified another family of adhesins involved in the first stage of C. glabrata biofilm development, namely Awp adhesins. Initially, four Awp adhesins (Awp1–4) were identified using liquid chromatography tandem mass spectrometry [52], and a subsequent study revealed the gene expression profile of the seven Awp adhesins (Awp1–7) [53]. Initial attachment of C. glabrata cells is followed by cell division, and this proliferation leads to the formation of a basal layer of anchoring microcolonies, with subsequent biofilm maturation [4,44]. Biofilm conditions and high cell density are adhesion inducers, activating EPA6, whereas EPA1 is triggered typically in the lag phase and the C. glabrata biofilm maturation is characterized by the production of the extracellular matrix [4,54]. A study using isolated mutant strains allowed the identification of four other genes involved in biofilm formation: silent information regulator (SIR4), telomere-binding (RIF1), EPA6, and serine-threonine protein kinase, YAK1.
2. General Mechanisms of Antifungal Drug Resistance
Clinical resistance is the result of a failure in the infection treatment [55]. Regarding susceptibility or resistance, Candida is defined as susceptible or resistant by the level of antifungal drug activity associated with a high likelihood of therapeutic success or therapeutic failure, correspondingly. There has been an epidemiological change from C. albicans to NCAC species, with C. glabrata and C. krusei emerging as important and potentially antifungal resistant causes of candidaemia [56]. It is important to mention that C. glabrata has been frequently reported as exhibiting variable karyotypes between isolates [57,58,59,60,61,62,63,64], and several studies with Candida species have demonstrated that these karyotypes are relatively stable, suggesting that the karyotype of virulent species is more stable than virulent ones [65]. The major karyotypic differences between C. glabrata strains are linked to a small number of chromosomal translocations. Along with variation in the subtelomeric EPA genes, the other genomic rearrangements are copy number variations in tandem gene repeats, encoding putative, or known cell wall proteins [58].
Bader et al. [66] analysed the derivates in C. glabrata strains’ genome, which were shown to be indistinguishable by multi locus sequence typing, but dissimilar phenotypic groups that were linked with specific karyotypic changes were also spotted. Chromosomal aberrations and functional adaptations can occur during infection and under antimicrobial therapy, but also under laboratory conditions deprived of extreme selective pressures, and can significantly affect phenotypic properties (e.g., the cell wall carbohydrate composition and quantitative changes in adhesion genes expression), being noticed slightly less than subtelomeric genes loss or differences in the number of macrosatellite repeats within adhesion genes. Another study also revealed that chromatin alterations could happen as essential strategies of survival, which would simplify a reprogramming of cellular energy metabolism in macrophage-internalized C. glabrata cells, and provide protection against DNA damage [67]. Thus, similar to all Candida species, C. glabrata have the competence to respond to environmental alterations, allowing them to adapt to the presence of antifungal agents, thereby providing protection against antimicrobial therapies [68].
Cell wall fluctuations, but mostly its immunoevasion and intracellular persistence, may be the crucial factors in the great ability of C. glabrata to persist in the course of multiple antifungal treatments and to develop multidrug resistance [69,70]. Consequently, different mechanisms of resistance vary among drugs, typically because of the mode of action of each class of antifungal. There are, presently, three main antifungal classes: azoles, polyenes and echinocandins. The azoles are known to have fungistatic activity, targeting the ergosterol biosynthetic pathway, by binding to the Cyp51 family of cytochrome P450—the 14-α sterol demethylases that are encoded by ERG11. They are responsible for the lack in the capacity to build and renew sterols in the cellular membranes, changing membrane fluidity and function of vital processes such as signalling, transport, exocytosis, and endocytosis [10]. Fluconazole, voriconazole, itraconazole, clotrimazole, and posaconazole are main examples. The polyenes (e.g., amphotericin B, nystatin) and echinocandins (e.g., micafungin, caspofungin, anidulafungin) are fungicidal drugs. The first ones bind to the ergosterol of the fungal cell membrane establishing transmembrane aggregates pores, which causes membrane depolarization which subsequently increases its permeability to monovalent protons and cations. This allows the passage of intracellular molecules to the external environment, initiating an osmotic imbalance, and finally cell death [71,72,73]. Echinocandins interfere with the fungal cell wall synthesis through a non-competitive inhibition of β-1,3-glucan synthesis [74], which results in the weakening of the cell wall, breakdown of cellular integrity and, finally, cell lysis [75].
2.1. Azole’s Resistance
During the past few decades there was a remarkable increase in mucosal infections caused by Candida species due to the increase of immunosuppressive diseases (e.g., cancer, AIDS), which was associated with an extraordinary emergence of resistance to azoles [76]. In the early 1990s, fluconazole became the first choice drug in the treatment and prophylaxis of oro-oesophageal candidiasis, and resistance was subsequently described in up to 41% of the patients in the following years [77,78,79]. Also, an increase was observed in the cases of fungaemia caused by NCAC species, specially C. glabrata and C. krusei [19,80,81,82]. As recognized, C. glabrata grows only as a yeast form in vivo and its adhesion is relatively weak [83,84,85]. Thus, it is believed that the increase of C. glabrata infections is due to that same inherent low susceptibility to azoles [86] and that the acquired resistance is a result of rare mutations that are selected by drug pressure [87]. Studies appear to conclude that the azole resistance develops gradually as a consequence of successive adjustments due to the continuous pressure exerted by the drug [87,88,89]. The acquired resistance of C. glabrata to azole drugs is linked to several mechanisms, but the most common is the induction of efflux pumps, encoded by the ABC-transporter genes (CDR1 and CDR2, SNQ2) or to MDR belonging to the major facilitator superfamily (MFS) that lead to decreased drug concentration [90]. In C. glabrata, the transcription factor Pdr1 is involved in resistance to azoles through upregulation of CDR1, CDR2, and SNQ2 [91]. The mitochondrial dysfunction associated with the development of the “petite mutants”, which have mitochondrial DNA deficiency and upregulate the ABC transporter genes, highly amplifying resistance to azoles, leading to a drastic improvement of fitness in C. glabrata. These mutants upregulate ABC transporter genes, displaying enlarged resistance to azole drugs [92]. A number of ABC transporters, including Cdr1, Pdh1 (also known as CDR2), Yor1, and Snq2, contribute to xenobiotic drug efflux. The transcription factor Pdr1 is the main regulator of ABC transporter gene expression and the key component of Pleiotropic Drug Resistance (PDR) [93,94]. In C. glabrata, Pdr1 forms a heterodimer with Stb5 in S. cerevisiae and transcriptional analysis pointed out a shared region among the homologues of these two genes, PDR1 and STB5, and many of the genes upregulated by overexpression of PDR1 were upregulated by the deletion of STB5. Accordingly, the PDR1 overexpression and STB5 deletion are correlated [95,96]. It was found that the overexpression of STB5 in C. glabrata represses azole resistance, while its deletion produces a minor intensification in resistance. Expression analysis assays recognized that STB5 shares many transcriptional targets with PDR1 but, unlike the second, it is a negative regulator of pleiotropic drug resistance (including the ABC transporter genes CDR1, PDH1, and YOR1) [91,96]. A study by Farahyar et al. [97] demonstrated that CDR1 and CDR2 genes are expectedly upregulated in azole-resistant isolates (≥2-fold) and that, fatty acid activator 1 (FAA1) gene presented a ≥2-fold expression in resistant isolates, when compared to the susceptible isolates and the reference strain. The work also revealed that not only the ABC transporter genes, but also small hydrophobic compounds and lipid metabolism may have a huge responsibility in azole drug resistance of C. glabrata [97]. A study of Ferrari and colleagues [91] involving transcription profiling with microarrays showed that more than 385 genes are differentially regulated by a selected number of the gain-of-function mutations (GOFs) expressed in the same genetic background, with a minimal overlap in co-regulated genes. CDR1 and PUP1 (for PDR1 upregulated and encoding a mitochondrial protein) were generally upregulated by all tested GOFs. While both genes mediated azole resistance, their deletions resulted in a decline in virulence and a decrease in tissue load. Their individual overexpression was shown to partially restore phenotypes obtained in clinical isolates [91]. Kaur and colleagues [98] made a screening of a library of 9216 random insertion mutants, and identified a set of 27 genes, which upon mutation, conferred alterations in fluconazole susceptibility in C. glabrata. These genes included ABC transporters (PDR5 and PDR16), genes involved in retrograde signalling from mitochondria to nucleus (RTG2) and genes involved in diverse cellular functions (activation of RNA polymerase II transcription, calcium homeostasis, ribosomal biogenesis, mitochondrial function, nuclear ubiquitin ligase function, and cell wall biosynthesis). Similarly, using a mutant defective in calcium uptake, the same authors noticed that the strains with a flaw in a putative plasma membrane calcium channel were modestly more susceptible to fluconazole, but revealed a significant loss of viability upon prolonged fluconazole exposure. This result suggests that calcium signalling is necessary for the survival of azole stress in C. glabrata and that, in the absence of Ca2+ signalling, fluconazole has a fungicidal rather than a fungistatic effect on C. glabrata [99]. Another azole-related resistance mechanism is the decreased affinity, or even incapacity, of these drugs to bind. The high ability to upregulate ERG11, CDR1, and PDR1 expression is, normally, followed by azole exposure [100]. All the genes linked to the biosynthesis of ergosterol are likely to be upregulated in the case of azole pressure, nonetheless, ERG 1, 3, 6, 7, 9, and especially ERG11, are the most studied ERG genes. ERG11, which converts lanosterol into 4, 4-dimethylcolesta-8,14,24-trienol, is markedly more mentioned as a central point regarding the increase of ergosterol bioproduction, in response to the azole attack on the C. glabrata cell membrane [87,101]. Potential mechanisms for the azole resistance include a small affinity of its lanosterol 14α-demethylase. The resistance mechanisms in ERG11 occur through the acquisition of point mutations in the gene encoding for the Erg11. Quite a few mutations in ERG11 have been described, but only a few are directly connected to azole resistance [102,103]. The overexpression or upregulation of the target enzyme of the azoles is also an important resistant mechanism linked to azole drugs [55]. Recently, a study in three different hospitals in Poland was developed in order to determine the mechanisms of resistance to azoles in C. glabrata clinical isolates in this country [104]. The authors used a Sensititre Yeast One test and discovered that, from the 81 studied strains, 18 were resistant to fluconazole, and 15 were cross-resistant to all other azoles tested. RT-qPCR studies showed that 13 of 15 azole-resistant strains presented upregulation of the CDR1 gene encoding the efflux pump, but no upregulation of the expression of CDR2. Also, no upregulation of the ERG11 gene was observed. This study confirms that the gene profile of the resistant isolates of C. glabrata azoles is variable between countries and strains, although certain genes are commonly up or downregulated [104]. Miyazaki et al. [99] studied the effects of calcineurin, a serine-threonine-specific protein phosphatase [105]. This protein emerged as a new target of antifungal therapy founded on studies in several pathogenic fungi, probably because azole antifungals and calcineurin inhibitors have mild synergistic effects against C. glabrata wild-type strains [98,106,107]. The results of this group have shown that the C. glabrata calcineurin mutant presented augmented susceptibility to azoles and cell wall-damaging agents and had lower virulence. Though the mutant lacking Crz1 presented a cell wall-associated phenotype intermediate to that of the calcineurin mutant and was modestly reduced in virulence, it did not improve azole susceptibility, thereby suggesting that calcineurin regulates both Crz1-dependent and independent pathways depending on the type of stress [99]. Chen et al. [108] disclosed that AP1 (which encodes a transcription factor related to stress responses) plays a critical role in reaction to various stresses in C. glabrata and decreases the stress through transcriptional activation of its target genes, including FLR1. The deletion of this gene only caused an amplified sensitivity to fluconazole. Candida glabrata clinical isolates are known to have the aldo-keto-reductase superfamily upregulated in the resistant isolates. RT-qPCR analysis revealed a AKR mRNA expression twice of that seen in the sensitive isolates, associated with increased fluconazole and itraconazole resistance, thus suggesting that upregulation of the AKR gene might give a new insight into the mechanism of azole resistance [49,109]. Although isolation of such C. glabrata mutants from patients has been rarely reported, Ferrari et al. [92] have successfully characterized two sequential and related C. glabrata isolates recovered from the same patient undergoing azole therapy: BPY40 (azole susceptible) and BPY41 (azole resistant). BPY41 had a mitochondrial dysfunction with upregulation of the ABC-transporter genes of C. glabrata. Testing the virulence of the “petite mutants” in mice with systemic and vaginal murine infection models, the authors showed that, even with in vitro growth deficiency, BPY41 was more virulent than BPY40. The authors also found an increase in the oxido-reductive metabolism and in the stress response in BPY41, which was consistent with mitochondrial dysfunction, and that certain genes involved in cell wall adaptation were upregulated in BPY41 compared to BPY40 [92]. Finally, Taff et al. [110] identified two glucan transferases and an exo-glucanase that deliver glucan from the cell to the extracellular matrix, playing a biofilm-specific role, by mediating the distribution and organization of mature biofilm matrix.
2.2. Polyene’s Resistance
The first cases of resistance to amphotericin B arose in parallel with the increase in the number of invasive infections caused by several genus of fungi, many of them with primary or intrinsic resistance to amphotericin B and usually associated with a high mortality [111,112,113]. Although C. glabrata is frequently considered to be susceptible to amphotericin B, it has a clear tendency to have higher minimum inhibitory concentration (MIC) values to polyenes than C. albicans [76,114]. Polyene resistance is still poorly understood and not well documented, particularly in C. glabrata, but the molecular mechanisms primarily include the replacement of some or all of the polyene-binding sterols, and the reorientation or the camouflaging of studying a clinical isolate of C. glabrata. Vandeputte and colleagues [115] revealed lower ergosterol content in its membrane in comparison to the wild type, and also found a nonsense mutation in the ERG6 gene that leads to a decrease in ergosterol content. Discrepancies of the cell wall were also observed, which were associated with developed susceptibility to cell wall-perturbing agents, with a high rate of cell mortality [115]. In another clinical isolate of C. glabrata recovered from a patient treated with amphotericin B and with a poor susceptibility to polyenes, a deficiency of ergosterol and an accumulation of late sterol intermediates was detected, emphasizing a defect in the final steps of the ergosterol pathway. Sequencing exposed a unique missense mutation in ERG6 (substitution of a cysteine by a phenylalanine in the corresponding protein). RT-qPCR demonstrated an overexpression of the genes that encode enzymes involved in late steps of the ergosterol pathway. The complementation of this strain with a wild-type copy of the ERG6 gene regenerated the susceptibility to polyenes and the standard morphology [116].
2.3. Echinocandin’s Resistance
Echinocandins are the first-line agents in the treatment of candidaemia [74]. Three mechanisms can induce the reduced echinocandin susceptibility [55]: acquired FKS mutations [117] which confer low β-1,3-d-glucan synthase sensitivity, higher MIC values, and clinical failure [118]; adaptive stress responses, which result in high cell wall chitin content with a paradoxical growth in vitro [119]; and finally, intrinsic FKS mutations, also resulting in elevated MIC levels (but in a lower level of reduced β-1,3-d-glucan synthase sensitivity when compared with the acquired FKS mutations) [118,120,121]. The GAS gene family is also a regulator in the production of β-1,3 glucan in this species [52]. GAS1, GAS2, and GAS5 are glycosylphosphatidylinositol (GPI)-anchored cell surface proteins [122], which are involved in the production of β-1,3 glucan in C. glabrata [123]. A study performed from patients with C. glabrata bloodstream infections showed that the resistance to echinocandins increased from 4.9% to 12.3% between 2001 and 2010. In addition, among the 78 fluconazole resistant isolates, 14.1% were resistant to one or more echinocandin and almost 8% of the isolates had a FKS mutation (FKS1/FKS2 mutations), which appeared due to a prior echinocandin therapy. Additionally, nearly all revealed intermediate or resistant MIC values to one echinocandin [124]. Thompson III et al. [125] performed sequentiation of hot spots studies in 2008, known to confer echinocandin resistance, and the fallouts revealed an F659V substitution within the FKS2 region of the glucan synthase complex [125]. Curiously, micafungin MIC levels of C. glabrata FKS hot spot mutant isolates were observed to be less elevated than those obtained for the other echinocandins, showing that the efficacy of micafungin could be differentially dependent on specific FKS gene mutations [126]. Shields et al. [117] analysed several echinocandin MIC levels and found that the average MICs values of caspofungin and anidulafungin were higher for patients who failed therapy. Several Candida species isolates observed in vitro reflect a curious high-dose paradox which is being linked to a complex network of pathways, causing slightly elevated MIC levels, in which cells appear to regain susceptibility at high levels of a drug [127,128]. This has the potential to contribute to clinical resistance [55] and it is extremely important to differentiate these low-level drug tolerance and adaptive mechanisms from the Fks1-mediated mechanisms that have been observed in clinical isolates and can result in treatment failure [55,129].
3. Resistance Mechanisms Related to Biofilms
Fungi in general, and Candida species in particular, are not motile microorganisms. The biofilm structure, thus, reflects the sequence of cell division events that occur during a biofilm development and results in an exceptionally resistant profile of the biofilm cells to one or several antifungal drugs. The infections are complicated by the presence of robust inducible gene networks encoding different proteins that confer tolerance or resistances to many available antifungal drugs [130]. These resistances can be classified as either microbiological or clinical [55]. The first is defined by the presence of a developed or mutational resistance mechanism to a specific drug. It depends directly on the microorganism and it is distributed into two groups: primary or innate, in which fungi are resistant prior to drug exposure, and secondary or acquired, when it appears in response to a drug exposure.
Several general mechanisms of biofilm drug resistance are thought to confer resistance to multiple classes of antifungals in Candida species biofilms: the up-regulation of efflux pumps, cell wall composition, increased cell density and quorum sensing effect, presence of an extracellular matrix, changes in metabolism, the presence of persister cells, and cellular signalling and stress responses [101,130,131,132,133].
3.1. Up-Regulation of Efflux Pumps and Cell Wall Composition
Two main classes of efflux pumps contribute to antifungal drug resistance: the ATP binding cassette (ABC) transporter superfamily containing CDR1 and CDR2, and the MFS superfamily containing MDR1 [130,134,135]. While under treatment with antifungals, biofilm cells up-regulate these transporters within six hours of surface contact both in vitro and in vivo, even in the absence of drug [136,137,138,139]. In 2013, our group [41] described that the usual ABC transporters were upregulated in biofilms of three C. glabrata strains. Similarly, another ABC transporter, PDR1, was evaluated and was found to be overexpressed. These alterations were linked to modifications in the structure of C. glabrata biofilms by creating cell clusters, which could be a possible mechanism of biofilm tolerance to fluconazole. The surface adherence alone seems enough to intensify the expression of the genes encoding the efflux pumps [140], which are also up-regulated in mature biofilms, demonstrating that they continue to mediate drug resistance throughout biofilm development [141,142,143].
3.2. Increased Cell Density and Quorum Sensing
It is well recognized that the inoculum size can affect susceptibility results [130,144,145,146,147]. Thus, using the microtiter method to test Candida species for drug susceptibility, an optimal inoculum size range was defined as a clinical standard. Indeed, if this cell concentration is augmented in the drug resistance assays, resistance specifically to fluconazole, ketoconazole, amphotericin B, and caspofungin is increased up to twenty-fold [147]. On the contrary, if biofilms are dissociated and analysed at a lower density in the same assays, they show drug susceptibilities at the level of planktonic cells evaluated at the same cellular density.
In the biofilm environment, Candida species cells have the ability to communicate with each other via quorum sensing (via numerous signalling molecules), which is directly dependent on the cell density [148].
3.3. Extracellular Matrix
In Candida species biofilms, carbohydrates, proteins, and nucleic acids form the extracellular matrix that surround the cells in the biofilms [149,150,151,152,153,154]. It is thought that biofilms prevent, to a greater or lesser extent, the penetration of antifungal drugs through their structure, by the establishment of a diffusion barrier, which acts as an ion-exchange resin, binding charged antibiotic molecules, contributing to biofilm drug resistance, including in the case of C. glabrata [44,150,155]. The most important components are the β-glucans, which are polymers of the fungal cell wall and are a substantial constituent of the biofilm Candida species matrix. When induced, the disruption of β-1,3-glucans or a β-1,3-glucanase treatment have been shown to increase susceptibility of biofilms to fluconazole and the addition of exogenous β-1,3 glucans has been demonstrated to result in the rise of resistance to fluconazole in planktonic cells [155]. Additionally, it is possible that biofilms can also sequester amphotericin B, as it has been shown that β-1,3-glucans can bind specifically to this drug [44,156]. It is known that planktonic cells generally rely on irreversible genetic changes to maintain a resistant phenotype, while biofilms are able to persist due to their physical presence and the density of the population, which affords an almost inducible resistant phenotype notwithstanding of distinct genetic alterations [130,157]. The application of DNA microarray and proteomic technologies can facilitate a more detailed analysis of the biofilm lifestyle [158,159] Specific biofilm formation genes are being brought up regarding different roles in biofilm resistance: peroxisomal catalase (CTA1), the biosynthesis and degradation of tyrosine genes (ARO), the muscle creatine kinase (MSK), the heat shock protein 90 (HSP 90), the sphingolipid biosynthesis (SKN 1 and KRE1), SIR, RIF, and, finally, the extracellular matrix (ECM) regulators: zinc regulated genes (ZAP1), g-carbonic anhydrase (GCAL1), alcohol dehydrogenase (ADH5), and also cell surface hydrophobicity (CSH1) [130,159].
3.4. Metabolism and Stress Response
Alterations in temperature or specific nutrients, restricted nutrient availability, ionic stress, and variations in osmolality and oxidative stress are all acknowledged as antifungal resistance mechanisms of biofilms. A study showed that the resistance to chlorhexidine, fluconazole, amphotericin B, and nystatin increased as biofilms mature over time, corresponding to a growth in metabolic activity over biofilm maturation. However, in these experiments, cell number was not controlled, thus making it unclear if this is a true demonstration of metabolic activity [159]. Baillie and Douglas [160] have shown that, at lower growth rates, planktonic cells are more resistant to amphotericin B, and biofilms are equally resistant over a range of growth rates, thereby suggesting that growth rate plays only a minor role in Candida species biofilm drug resistance. In another study [161], the same authors demonstrated that neither glucose nor iron limitation disturbs Candida species biofilm resistance to amphotericin B. However, iron limitation increased the susceptibility of dispersed daughter cells from biofilms to amphotericin B, detected by a number of cells which induce responses by signalling pathways [162]. Seneviratne et al. [163] showed that there is a positive regulatory protein response associated with the stress response in biofilms of stressed C. glabrata, as displayed by the heat shock and other stress proteins (Hsp12, Trx1, and Pep4).
3.5. Persister Cells
Inside the biofilm cell population, persister cells form a unique group that is formed randomly, phenotypically dormant, highly tolerant to antifungal drugs [164], and which is a key mechanism of resistance in chronic infections [130]. Yeast persister cells were first discovered as a small population in C. albicans biofilms [165,166]. These cells were extremely drug resistant in a manner that was independent of drug efflux pumps and the composition of the cell membrane. Al-Dhaheri and Douglas [166] reported persister cells in biofilms treated with amphotericin B from isolates of Candida species. Candida species persister cells are exclusively recovered from biofilms and not from planktonic populations, notwithstanding their growth phase, and involve attachment to a substrate to initiate the dormant phenotype. These cells are believed to be a phenotypic variant of the wild type strain, for they are the result of a biofilm with new subpopulations [133]. Bojsen and colleagues [167] performed a study in order to evaluate whether resistance mechanisms on amphotericin B were shared between biofilm and planktonic populations. A multiplexed barcode sequencing screening of a combined group of gene-deletion mutants cultivated as biofilm and planktonic cells associated with an assay for resistance to the ergosterol-targeting fungicide amphotericin B was executed. The results revealed that the biofilm and planktonic population had substantial overlap in amphotericin B-persistent mutants. Also, the authors were able to demonstrate that the mutants defective in sterol metabolism, ribosome biosynthesis, and in the TORC1 (ubiquitin binding activity, role in cellular response to starvation, regulation of cell growth, etc.) and in the Ras pathways (protein signal transduction) displayed an amplified persistence when treated with amphotericin B. The ras1, ras2, and tor1 mutants had a high-persister phenotype compared to wild-type biofilm and planktonic cells exposed to the TORC1 pathway inhibitor rapamycin, and, on the other hand, the inhibition of TORC1 with rapamycin similarly improved the proportion of persisters in C. glabrata. With these results, the authors demonstrated that a decreased TORC1-mediated induction of ribosome biosynthesis via Ras can originate the development of amphotericin B-persister cells in planktonic populations, but also in biofilms [167].
4. Cross and/or Multidrug Resistance
Antifungal drug resistance is particularly more serious when it develops not only against the administered drug, but also to other non-related chemical compounds, and C. glabrata has emerged as a major health threat since it also rapidly acquires resistance to multiple drug classes. The use of echinocandins and development of cross-resistance is creating apprehension, for the signs of multidrug resistance amongst azoles and echinocandins have already been described [168,169,170]. Studies with clinical isolates obtained from patients in several epidemiological studies show that not only has multiple antifungal resistance been described in isolated events, but also that cross-resistance among more than one class of antifungal drugs is growing [171]. As a result, 11.1%–58.3% of C. glabrata isolates resistant to echinocandins had cross resistance against fluconazole or other azoles [168,169,172], and cross resistance of C. glabrata of echinocandins and amphotericin B have also been reported [173]. Estimates regarding the percentage of non-susceptible or resistant C. glabrata clinical isolates against four antifungal drugs used in clinical practice, across several geographic regions are: fluconazole, 3.4%–70%; amphotericin B, 2.5%–60%; caspofungin, 1.3%–16.2%, and 5-flucytosine, 0.8%–35%, which are clearly concerning numbers [171,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193]. Additionally, the latest cross-resistance between amphotericin B and azoles or caspofungin in Candida species are increasingly worrying [103,194,195,196,197]. Not in all cases, but in most of them, this multidrug resistance phenomenon depends on the activity of ABC transporters and MFS [198], which are known to be regulated by the Pdr1 transcription factor, which is recognized to be the major regulator of multidrug resistance in C. glabrata [94]. As previously explained, C. glabrata expresses three ABC transporters (CDR1, CDR2, and SNQ2). Studies revealed that the deletion of CDR1 in an azole-resistant strain leads to the intracellular accumulation of fluconazole and hypersusceptibility to other azoles. The additional CDR2 deletion worsens this phenotype [91,199]. Finally, the deletion of SNQ2 leads to an amplified susceptibility to several azoles, but also to 4-nitroquinoline-N-oxide, in an azole-resistant strain [200]. Recently, Healey and colleagues [201] showed a mutator phenotype caused by a mismatch repair defect which is prevalent in C. glabrata clinical isolates. These strains possess alterations in mismatch repair gene MSH2 which leads them to display an advanced predisposition to develop antifungal treatment in vitro and in mouse models of colonization. Also, the authors found that 55% of all C. glabrata that are recovered from patients have these genetic characteristics, which is clinically very concerning. The genetic mechanism involved in this process supports the acquisition of resistance to multiple antifungals, partially explaining the higher rates of triazole and multidrug resistance associated with C. glabrata [201]. Beforehand, Nishikawa and colleagues [202,203,204] identified an activator-targeted KIX domain in the human MED15 Mediator subunit that is structurally conserved in Gal11/Med15 Mediator subunits in fungi. This Gal11/Med15 KIX domain is involved in Pdr1 orthologues and in the clinically important C. glabrata multidrug resistance pathogenenis [205]. In a recent work, Nishikawa et al. [204] implemented a sequential biochemical and in vivo high-throughput screens to identify small-molecule inhibitors of the interaction of the C. glabrata Pdr1 activation domain with the C. glabrata Gal11A KIX domain, which is linked to the C. glabrata multidrug resistance. Results showed that iKIX1 inhibits Pdr1-dependent gene activation and re-sensitizes drug-resistant C. glabrata to azole antifungals in vitro and in animal models for disseminated and urinary tract C. glabrata infection [204]. The sirtuins Sir2 and Hst1 control the expression of several genes including adhesins required for host colonization and niacin transporters needed for growth in C. glabrata. With the knowledge that these sirtuins can be inactivated during infection, Orta-Zavalza et al. [206] proved that their inhibition could change the response of C. glabrata to other stressful conditions. The results showed that a deletion of HST1 reduced the susceptibility of C. glabrata to fluconazole and hydrogen peroxide. Pdr1 and CDR1 mediated the fluconazole resistance phenotype of the Δ hst1 cells, while the transcriptional activator Msn4 and the catalase Cta1 were required to provide oxidative stress resistance. Also, the authors showed that the transcription factor Sum1 interacts with Hst1 and participates in the regulation of these genes. The findings state that Hst1 acts as a regulator of stress resistance associated-genes [206].
Candida species seems to tolerate stress induced by weak acids, which appears to be a key factor in their persistence and virulence in antifungal drugs. MFS transporters are integrated into two families: the DHA1 (drug: H+ antiporter family 1), with 12 transmembrane domains, and the DHA2 (drug: H+ antiporter family 2), with 14 transmembrane domains. Other transporters, Qdr2 and Tpo3, have also been studied [198]. The first was acknowledged to be a cause of resistance to imidazoles (e.g., clotrimazole, miconazole, tioconazole, and ketoconazole) and was proven to play an active part in the efflux of these drugs. Its expression was found to be stimulated in clotrimazole-stressed cells, under Pdr1 control [207]. The second was demonstrated to be linked to the resistance to both imidazoles and triazoles (e.g., fluconazole), and to the polyamine spermine, found in high concentrations in the urogenital tract. The authors also found that TPO3 was upregulated in C. glabrata cells exposed to spermine, in a Pdr1-dependent manner [198]. Likewise, Tpo3 appears to be linked to the efflux of azoles and spermine, and the control of the intracellular concentration of this polyamine appears to be important for azole resistance [208]. Another MFS H+ antiporter, Aqr1, from C. glabrata, was also identified by Costa et al. [209]. This MFS antiporter is a determinant of resistance to acetic acid, flucytosine, and clotrimazole (frequently found in the vaginal mucosa, probably contributing to the persistence in this niche). It is known that these antifungals act synergistically with acetic acid against this pathogen. Aqr1 (located in plasma membrane and in the membrane vesicles) was suggested to play a role in intermediating the extrusion of chemical compounds, dropping the intracellular accumulation of 3H-flucytosine and, in a minor degree, of 3H-clotrimazole, which is reliable with a direct role in antifungal drug efflux. When an AQR1 deletion was performed, no effect could be noticed on the intracellular accumulation of 14C-acetic acid, thus suggesting that its role in acetic acid resistance may be indirect, possibly through the transport of a so far undisclosed physiological substrate. The pre-exposure to flucytosine or clotrimazole was found to make C. glabrata cells more tolerant to acetic acid stress. Therefore, Costa et al. [209] showed that Aqr1 is an antifungal drug resistance determinant and it may play an essential part in C. glabrata persistent colonization and multidrug resistance.
Hull et al. [210] identified a clinical isolate of C. glabrata (CG156) that displayed flocculent growth and cross-resistance to fluconazole, voriconazole, and amphotericin B. In this work, CG156 was found to be a low-efflux isolate and when grown on sterol-supplemented, its cultures reached higher cell densities, with shorter lag phases, and showed variations in cellular sterol composition that did not affect its azole-resistant phenotype. When this isolate was grown in the presence of ergosterol, it showed increased sensitivity to the polyene and when grown with cholesterol it became more resistant. The results therefore indicate that some clinical isolates might persist as slow-growing agents of chronic infections, possibly since they can survive without sterol auxotrophy; possess mutated Erg11; lack cellular ergosterol (high-level resistance to polyenes); and can opportunistically exploit a wide spectrum of host/environmental sterols for growth. The authors also indicate that the altered cellular sterol composition of CG156 may affect intracellular signalling and trafficking pathways, as the efflux machinery [199,211,212] and any transport proteins that are proposed to mediate azole import via facilitated diffusion [213].
Other cases of induced cross-resistance to azole drugs in C. glabrata were related to resistance against both azoles and amphotericin B [198,199,212] and related to prochloraz (an agricultural antifungal). The original mechanism responsible for this phenomenon was found to be the upregulation of multidrug transporters [214]. Also in C. glabrata, Flr1 has been proven to be involved in the resistance of benomyl (a pesticide used in agriculture), but no connection was found between this transporter and antifungal resistance [108].
Vermitsky and colleagues [94] showed that treatment with terbinafine (allylamines antifungal class), which targets the enzyme squalene epoxidase in the ergosterol biosynthetic pathway, presented upregulation on ERG11 as previously reported, however, unlike the azoles, this drug had minimal effect on CDR1 and PDH1. Several studies with histatins have been performed, since these salivary cationic proteins had been found to have effectiveness against diverse fungi [215,216,217,218,219]. Unfortunately, further studies disclosed that they have expressively less activity against C. glabrata, with current speculation suggesting that C. glabrata might escape histatin 5 activity by applying fermentative pathways, as theoretically glucose can either be fermented or assimilated. Moreover, C. glabrata is a Crabtree-positive fungus [220], thus its respiration could be negatively affected by certain levels of glucose [221]. The same results that confirmed an apparently fundamental and extensive resistance of C. glabrata to histatin 5 showed that it is not related to the resistance mechanisms of azoles.
5. Alternative Therapies for the Treatment of Infections Related to Candida glabrata
Considering the increasing number of Candida species with drug resistance, namely C. glabrata, the identification of efficient alternative therapies to the current antifungal agents is crucial. Many approaches are currently being pursued, including the development of novel antifungal agents, the exploitation of the antimicrobial properties of plant derivatives and honey, and the development of photodynamic therapy. An overview of the most recent advances in these approaches is provided in the following paragraphs.
5.1. Plant Essential Oils and Extracts
Despite the efforts to discover novel and more efficient chemical antifungal molecules, these are often associated with a variety of adverse side effects. This prompted the search for safer alternatives, among which are plants. Medicinal plants have been used since ancient times for therapeutic purposes. For example, superficial candidiasis is traditionally treated with a topical application of calendula and commiphora [222]. Currently, these plants are being investigated to determine the active principles responsible for their therapeutic effects and to understand how to apply these principles for antifungal treatment [223,224,225]. Several plant secondary metabolites such as tannins, terpenoids, alkaloids, flavonoids, and glycosides have been found to have antimicrobial properties in vitro [226,227]. Plant antifungal properties are less explored than antibacterial properties, but they are thought to derive from the same secondary metabolites. To explore the antifungal potential of plants, both essential oils and plant extracts have been used. Both are promising antifungal agents because of their relative safety, wide acceptance (a consequence of the traditional use), and are renewable in nature [228,229,230].
Plant essential oils are odorous volatile natural complex compounds. They are found only in 10% of the plant kingdom [231] and are generally in low quantities (rarely exceeding 1% of the plant mass) [232]. They are typically recovered from plants by distillation methods, are hydrophobic [233,234], and are composed of variable mixtures of many compounds (e.g., terpenoids such as alcohols, esters, aldehydes, ketones, ethers, phenols and epoxides, and low molecular weight aliphatic hydrocarbons [235,236]).
Likewise, plant extracts are of complex composition. These can be extracted from different plant organs, giving rise to a variable composition (in components and quantities) and therefore therapeutic effect. For example, Mirtus communis L. (myrtle) leaf and flower extracts are rich in tannins while the stem is rich in flavonoids [237]. Furthermore, the composition of the extracts is also variable according to the solvent used for extract preparation [238].
Several plant essential oils and extracts have been investigated for possible antifungal properties. These include those obtained from Origanum vulgare (oregano), Cinnamomum zeylanicum (cinnamon), Lippia graveolens (Mexican oregano), Thymus vulgaris (thyme), Salvia officinalis (sage), Rosmarinus officinalis (rosemary), Ocimum basilicum (basil), Zingiber officinale (ginger), Eucalyptus globulus (blue gum), Juglans regia (walnut), Pterospartum tridentatum (common poppy), Rubus ulmifolius (blackberry), and Glycyrrhiza glabra L. (licorice). Antimicrobial activity against C. glabrata isolates was observed for Origanum vulgare, Lippia graveolens, and Cinnamomum zeylanicum essential oils, with the first two being more active against fluconazole-susceptible C. glabrata, and the latter showing the best antifungal activity against the fluconazole-resistant C. glabrata isolates [239]. Extracts from Juglans regia, Eucalyptus globulus, Pterospartum tridentatum, and Rubus ulmifolius were also effective against several Candida species, especially C. glabrata, followed by C. albicans, C. parapsilosis, and C. tropicalis. The effects of Juglans regia and Eucalyptus globulus were assessed in more detail revealing a fungistatic and not fungicidal activity [240]. Glycyrrhiza glabra extracts have shown promising inhibitory results against several Candida strains, especially C. tropicalis and C. glabrata. These extracts have also demonstrated anti-biofilm activity against the two Candida species, although a double concentration of extract was generally required to obtain an antifungal effect in biofilm similar to that of planktonic cells [241].
In summary, plant essential oils and extracts are promising alternatives to current chemical antifungal agents. However, it is important to note that the establishment of standards for the therapeutic use of these plant derivatives to treat Candida species infections will require overcoming the variation in composition between samples, resulting from a multitude of factors that include, but are not restricted to, genotype, cultivation area, time of harvesting, and processing methods [242].
5.2. Honey
Honey is a natural sweet substance produced by honeybees from the nectar of flowers or from secretions of living parts of plants, which are transformed in the upper aero-digestive tract of the bee. Consequently, the chemical composition of honey varies depending on the botanical source. Honey is then stored in the honeycomb where it ripens and matures [243].
The first human use of honey traces back to 8000 years ago. Since then, honey has played an important role in traditional medicine, and is now also finding its place in modern medicine. In fact, studies have reported the bactericidal, bacteriostatic, antiviral, antioxidant, anti-inflammatory, and anti-tumoral properties of honey [244,245,246,247]. However, although a number of studies have investigated the antimicrobial properties of honey against bacteria, few have focused on its antifungal properties [248,249,250,251,252,253]. Nevertheless, the few reports currently available show promising results. Irish et al. [250] evaluated three floral honeys (Jarrah honey, Medihoney Antibacterial Honey Barrier, Comvita Would Care 18+) and one artificial honey simulating honey’s typical high sugar levels against C. albicans, C. glabrata, and C. dubliniensis. They found that C. dubliniensis was the most susceptible species to the activity of honey, while C. glabrata was the least susceptible. They also reported greater antifungal activities of the floral honeys compared to the artificial honey. This observation was also reported in the study of Estevinho et al. [243], where a synthetic honey solution was tested to determine the antifungal activity that was attributable to sugars, only to find that the activity was reduced compared to natural honey. It is therefore suggested that the component(s) of honey responsible for the antifungal properties are not sugar based.
Additional in vitro and in vivo evaluations are necessary to fully assess the antifungal potential of honey. For in vivo applications, honey may be limited to topical treatments, not being a viable option to treat candidaemia. However, honey may be used prophylactically to prevent more serious infections. A few studies have already demonstrated this possibility. For example, prevention of exit site infection by coating catheters with honey was found to be at least as effective as povidone iodine [254] or mupirocin [255].
5.3. Photodynamic Therapy (PDT)
Many studies have investigated the use of photodynamic therapy (PDT) to fight fungi infections [256]. PDT uses a photosensitive substance activated by a light source of a specific wavelength. The activation of photosensitizers added to cells and microorganisms, by an appropriate wavelength of light in the presence of oxygen, promote a phototoxic response of the cells, usually via oxidative damage [257]. The PDT sensitization depends on the parameters related to the concentration, time of incubation, and type of photosensitizer, as well as the physiological state of the microorganisms, time of exposure, and energy density of the laser [258,259]. Although photodynamic approaches are well established experimentally for the treatment of certain cutaneous infections, there is limited information about their mechanism of action for specific pathogens as well as the risks to healthy tissues [260].
The action of different photosensitizers, mainly phenothiazine dyes, porphyrins, and phthalocyanines, has been investigated. Many studies have demonstrated the efficacy of phenothiazine dyes such as methylene blue, new methylene blue, and toluidine blue in PDT for the reduction of fungi [256,258,259,261]. Malachite green, a cationic dye of the triarylmethane family (e.g., crystal violet) is another option for a photosensitizer. These dies have been shown to effectively reduce the number of Candida species cells. Dovigo et al. [262] observed that the fungicidal effect of PDT was strain-dependent. Significant decreases in biofilm viability were observed for three strains of C. albicans and for two strains of C. glabrata. Moreover, single-species biofilms were less susceptible to PDT than their planktonic counterparts.
As a consequence of the use of non-specific oxidizing agents, organisms resistant to conventional antifungal agents may be successfully killed by PDT and the development of resistance to this therapy seems unlikely, making this a very promising therapy [257]. In fact, the data to date suggests that photodynamic treatment approaches hold great promise for combating certain fungal pathogens.
5.4. Antifungal Agents with New Targets and New Sources
As a consequence of the increasing resistance demonstrated by Candida species to the currently available antifungal drugs, efforts are being made to develop novel and more effective antifungals. Different molecules are being discovered, synthesized, and evaluated for their capacity to control Candida species growth. For example, Vartak et al. [263] isolated a new polyene macrolide antibiotic from the fermentation broth of Streptomyces species. After purification, they evaluated the antimicrobial activity against several fungi (Aspergillus fumigatus, C. albicans, C. krusei, C. glabrata, Cryptococcus neoformans, Trichophyton species, and fluconazole-resistant C. krusei and C. glabrata strains) and Gram-positive and Gram-negative bacteria. They found the polyene macrolide to be specifically active against fungi, being unable to inhibit bacterial growth.
Glucosides modified in their saccharide units have been synthesized and their activity against Candida species evaluated by de Souza et al. [264]. One of the modified glucosides showed promising results, with fungistatic (threefold higher than fluconazole) and fungicidal activity against C. glabrata. The authors consider this compound to be a novel structural pattern in the development of new antifungal drugs. Different aldehydes, hydrazones, and hydrazines have also been assessed against several Candida species, among which 4-pyridin-2-ylbenzaldehyde and tert-butyl-(2Z)-2-(3,4,5-trihydroxybenzylidine)hydrazine carboxylate have shown the most promising results [265]. The activity of these compounds is thought to be on the fungal membrane. A series of fatty N-acyldiamines, prepared from fatty methyl esters and 1,2-ethylenediamine, 1,3-propanediamine, or 1,4-butanediamine, have also demonstrated moderate to good antifungal activity against C. albicans, C. tropicalis, C. glabrata, and C. parapsilosis [266].
Abrão et al. [267] evaluated the anti-candidal activity of new Mannich base-type eugenol derivatives and found that 4-allyl-2-methoxy-6-(morpholin-4-ylmethyl) phenyl benzoate and 4-{5-allyl-2[(4-chlorobenzoyl)oxy]-3-methoxybenzyl}morpholin-4-ium chloride were the most effective, particularly against C. glabrata, C. albicans, and C. krusei, with IC50 values below those of fluconazole. Raman et al. [268] evaluated the activity of amphiphilic, helical β-peptide structural mimetics of natural antimicrobial α-peptides against clinically isolated and drug resistant Candida strains. Candida tropicalis was the most susceptible to the activity of the β-peptide, while C. glabrata was the least susceptible. Interestingly, they report that β-peptides are mostly ineffective at disrupting Candida species biofilms, but they can prevent the formation of C. albicans, C. glabrata, C. parapsilosis and C. tropicalis biofilms. β-peptides thus seem to be a promising class of molecules to use as therapeutics.
Silva-Dias et al. [269] evaluated the antifungal properties of cerium, a lanthanide member, against planktonic and sessile Candida cells. The activity of cerium appears to result from severe cellular metabolic activity impairment and membrane damage. This compound was shown to effectively prevent biofilm formation both in vitro and in vivo in segments of polyurethane catheters in mouse models, and also to almost eradicate established biofilms when used at high concentrations. Cerium is therefore suggested as a possible antifungal agent to prevent the formation of biofilm-associated infections in clinical settings, for example, by catheter coating.
Additionally, antibacterial agents are being evaluated for their antifungal activity. For example, chloramphenicol, a bacteriostatic antibiotic, was evaluated against 30 representative yeast strains [270]. The antifungal activity of chloramphenicol was comparable to other known antifungal compounds (e.g., caspofungin acetate, ketoconazole, and metronidazole). However, it had no activity against most C. albicans tested, as well as C. famata, C. glabrata, C. haemolonei and Cryptococcus neoformans.
The combination of common antifungal agents with other antifungal molecules is also being assessed. For example, Ning et al. [271] has shown that the combination of epigallocatechin gallate (EGCG) with miconazole, fluconazole, or amphotericin B has a synergistic effect against planktonic and biofilm cells of Candida species (Fractional inhibitory concentrations (FIC) ≤ 50). They suggest that combined treatment of antifungal agents and EGCG may lower the dosages of antimycotics necessary to treat infections, thus preventing possible adverse effects and the emergence of resistant strains.
Another approach under consideration is the design of drugs with extended persistence and controlled release. For this purpose, nanotechnology creates new possibilities. For example, Perera et al. [272] evaluated the encapsulation of citric acid into a Mg-Al-layered double hydroxide (LDH). Citric acid has antifungal properties and its encapsulation would allow its slow release in topical skin formulations. The nanoparticles obtained were introduced into a body cream formulation, which demonstrated a prolonged slow release up to 8 h in aqueous medium under different pH values (3–5). The same body cream demonstrated an improved antifungal activity against C. albicans and C. glabrata, but not C. tropicalis. Also, Silva et al. [273] presented results with silver nanoparticles having a significant effect on reducing C. glabrata biofilm.
Defence mutualisms between social insects and microorganisms have been also largely studied, since the symbiotic nature of endophytic microorganisms favours metabolic interactions with their host and their environment, increasing the production of bioactive compounds [274]. Nirma et al. [275] reported the discovery of a Pseudallescheria boydii strain isolated from Nasutitermes species The microbial symbiont produces two metabolites with antifungal activity: tyroscherin and N-methyltyroscherin, shown to be effective antifungal agents with favourable selectivity indices for C. albicans and C. parapsilosis. Later, the same authors [276] discovered ilicicolinic acids A, C, and D and ilicicolinal isolated from a fungus (Neonectria discophora SNB-CN63) that was isolated from a termite (Nasutitermes corniger) nest in French Guiana, which showed in vitro against the same Candida species. Also in French Guiana, other authors [274] isolated fungi and bacteria from plants. Three active fungal extracts were fractionated, resulting in the isolation of eight compounds, which exhibited antifungal and cytotoxic potential against C. albicans ATCC10213.
6. Conclusions
Fungal infections have been increasing significantly in recent years, contributing to high morbidity and mortality, especially in immunocompromised individuals. The quick rise in the incidence of single, cross, and multidrug antifungal resistance within C. glabrata strains make it crucial to further increase the data on the virulence and resistance mechanisms associated with this species. Also, the capacity to form biofilms is one of the most important features in Candida species pathogenicity, creating a dangerous prospect of ineffective therapies against these infections. Candida glabrata biofilms are extremely refractory to antimicrobial therapy and more difficult to manage due to the natural properties mode of growth. They are able to withstand much higher concentrations of antifungal drugs compared with the planktonic cells, making C. glabrata biofilm infections extremely challenging to treat.
The research for an improved comprehension on the mechanisms of drug resistance, but also the search for alternatives to antifungal therapies, is becoming more important over time. Figure 2 summarizes the mechanisms of antifungal resistance and the present alternative therapies related to C. glabrata biofilms.
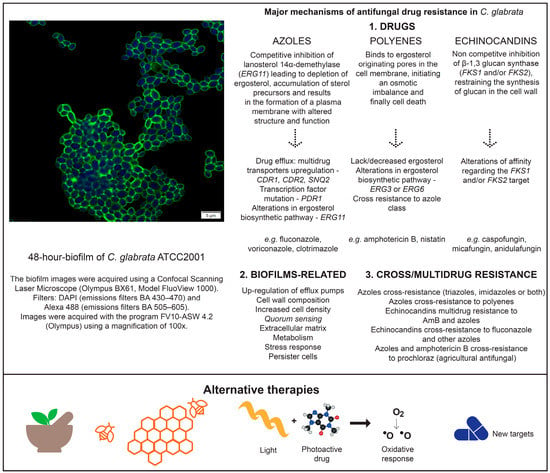
Figure 2.
Mechanisms of antifungal resistance and alternatives therapies associated to C. glabrata biofilms.
Acknowledgments
This work was supported by the Programa Operacional, Fatores de competitividade—COMPETE and by national funds through FCT—Fundação para a Ciência e a Tecnologia on the scope of the projects FCT PTDC/SAU-MIC/119069/2010, RECI/EBB-EBI/0179/2012, and PEst-OE/EQB/LA0023/2013, Célia F. Rodrigues’ SFRH/BD/93078/2013 PhD grant, Maria Elisa Rodrigues’ Grant SFRH/BD/93078/2013. The authors thank the Project “BioHealth—Biotechnology and Bioengineering approaches to improve Programa Operacional Regional do Norte (ON.2—O Novo Norte), QREN, FEDER.
Author Contributions
All authors contributed to the script.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fidel, P.; Vazquez, J.; Sobel, J. Candida glabrata: Review of epidemiology, pathogenesis, and clinical disease with comparison to C. albicans. Clin. Microbiol. Rev. 1999, 12, 80–96. [Google Scholar]
- Introduction and historical perspectives. In Candida and Candidiasis; Calderone, R. (Ed.) ASM Press: Washington, DC, USA, 2002; pp. 15–25.
- Larone, H. Medically Important Fungi: A Guide to Identification, 4th ed.; ASM Press: Washington, DC, USA, 2002. [Google Scholar]
- Silva, S.; Negri, M.; Henriques, M.; Oliveira, R.; Williams, D.W.; Azeredo, J. Candida glabrata, Candida parapsilosis and Candida tropicalis: Biology, epidemiology, pathogenicity and antifungal resistance. FEMS Microbiol. Rev. 2012, 36, 288–305. [Google Scholar] [CrossRef] [PubMed]
- Odds, F. Candida and Candidiasis, 2nd ed.; Baillierir Tindall.: London, UK, 1988. [Google Scholar]
- Dujon, B.; Sherman, D.; Fischer, G.; Durrens, P.; Casaregola, S.; Lafontaine, I.; de Montigny, J.; Marck, C.; Neuvéglise, C.; Talla, E.; et al. Genome evolution in yeasts. Nature 2004, 430, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Brunke, S.; Hube, B. Two unlike cousins: Candida albicans and C. glabrata infection strategies. Cell. Microbiol. 2013, 15, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Domergue, R.; Zupancic, M.L.; Cormack, B.P. A yeast by any other name: Candida glabrata and its interaction with the host. Curr. Opin. Microbiol. 2005, 8, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Hajjeh, R.A.R.A.; Sofair, A.N.A.N.; Harrison, L.H.L.H.; Lyon, G.M.M.; Arthington-Skaggs, B.A.B.A.; Mirza, S.A.S.A.; Phelan, M.; Morgan, J.; Lee-Yang, W.; Ciblak, M.A.M.A.; et al. Incidence of bloodstream infections due to Candida species and in vitro susceptibilities of isolates collected from 1998 to 2000 in a population-based active surveillance program. J. Clin. Microbiol. 2004, 42, 1519–1527. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.F.; Silva, S.; Henriques, M. Candida glabrata: A review of its features and resistance. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 673–688. [Google Scholar] [CrossRef] [PubMed]
- Colombo, A.L.; Guimaraes, T.; Silva, L.R.B.F.; Monfardini, L.P.; de Almeida Monfardini, L.P.; Cunha, A.K.B.; Rady, P.; Alves, T.; Rosas, R.C. Prospective observational study of candidemia in Sao Paulo, Brazil: Incidence rate, epidemiology, and predictors of mortality. Infect. Control Hosp. Epidemiol. 2007, 28, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Righi, E.; Costa, A.; Fasce, R.; Molinari, M.P.; Rosso, R.; Pallavicini, F.B.; Viscoli, C.; Pfaller, M.; Diekema, D.; et al. Epidemiological trends in nosocomial candidemia in intensive care. BMC Infect. Dis. 2006, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Hasan, F.; Xess, I.; Wang, X.; Jain, N.; Fries, B.C. Biofilm formation in clinical Candida isolates and its association with virulence. Microbes Infect. 2009, 11, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Krcmery, V.; Barnes, A.J. Non-albicans Candida spp. Causing fungaemia: pathogenicity and antifungal resistance. J. Hosp. Infect. 2002, 50, 243–260. [Google Scholar] [CrossRef] [PubMed]
- Krcmery, V. Torulopsis glabrata an emerging yeast pathogen in cancer patients. Int. J. Antimicrob. Agents 1999, 11, 1–6. [Google Scholar] [CrossRef]
- Tortorano, A.M.; Kibbler, C.; Peman, J.; Bernhardt, H.; Klingspor, L.; Grillot, R. Candidaemia in Europe: epidemiology and resistance. Int. J. Antimicrob. Agents 2006, 27, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Sardi, J.; Scorzoni, L.; Bernardi, T.; Fusco-Almeida, A.; Mendes Giannini, M. Candida species: Current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J. Med. Microbiol. 2013, 62, 10–24. [Google Scholar] [PubMed]
- Lim, C.S.-Y.; Rosli, R.; Seow, H.F.; Chong, P.P. Candida and invasive candidiasis: back to basics. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Abi-Said, D.; Anaissie, E.; Uzun, O.; Pinzcowski, H.; Vartivarian, S. The epidemiology of hematogeneous candidiasis caused by different Candida species. Clin. Infect. Dis. 1997, 24, 1122–1128. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.; Lozano-Chiu, M.; Rex, J. Declining rates of oropharyngeal candidiasis and carriage of Candida albicans associated with trends toward reduced rates of carriage of fluconazole-resistant C. albicans in human immunodeficiency virus-infected patients. Clin. Infect. Dis. 1998, 27, 1291–1294. [Google Scholar] [CrossRef] [PubMed]
- Beck-Sague, C.; Jarvis, W. National nosocomial infectious surveillance system. Secular trends in the epidemiology of nosocomial fungal infections in the United States, 1980–1990. J. Infect. Dis. 1993, 167, 1247–1251. [Google Scholar] [CrossRef] [PubMed]
- Rees, J.; Pinner, R.; Hajjeh, R.; Brandt, M.; Reingold, A. The epidemiological features of invasive mycotic infections in the San Francisco Bay area, 1992–1993: results of population-based laboratory active surveillance. Clin. Infect. Dis. 1998, 7, 1138–1147. [Google Scholar] [CrossRef]
- Pfaller, M.; Jones, R.; Messer, S.; Edmond, M.; Wenzel, R. National surveillance of nosocomial blood stream infection due to species of Candida other than Candida albicans: frequency of occurrence and antifungal susceptibility in the SCOPE Program. Diagn. Microbiol. Infect. Dis. 1998, 30, 121–129. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Jones, R.N.; Doern, G.V.; Sader, H.S.; Messer, S.A.; Houston, A.; Coffman, S.; Hollis, R.J. Bloodstream infections due to Candida species: SENTRY antimicrobial surveillance program in North America and Latin America, 1997–1998. Antimicrob. Agents Chemother. 2000, 44, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Gumbo, T.; Isada, C.; Hall, G.; Karafa, M.; Gordon, S. Candida glabrata fungemia. Clinical features of 139 patients. Medicine 1999, 78, 220–227. [Google Scholar] [CrossRef]
- Pfaller, M.; Jones, R.; Doern, G.; Fluit, A.; Verhoef, J.; Sader, H.; Messer, S.; Houston, A.; Coffman, S.; Hollis, R. International surveillance of blood stream infections due to Candida species in the European SENTRY Program: species distribution and antifungal susceptibility including the investigational triazole and echinocandin agents. SENTRY Participant Group (Euro). Diagn. Microbiol. Infect. Dis. 1999, 35, 19–25. [Google Scholar] [CrossRef]
- Rodriguez-Tudela, J.; Cuenca-Estrella, M. Estudio multicéntrico sobre funguemias por levaduras en España (abril-junio de 1997). Grupo de trabajo para el estudio de las funguemias. Rev. Clin. Esp. 1999, 199, 356–361. [Google Scholar] [PubMed]
- Pfaller, M.; Diekema, D.; Jones, R.; Sader, H.; Fluit, A.; Hollis, R.; Messer, S.; Group, T.S.P. International surveillance of bloodstream infections due to Candida species: Frequency of occurrence and in vitro susceptibilities to fluconazole, ravuconazole, and voriconazole of isolates collected from 1997 through 1999 in the SENTRY Antimicrobial Surveillance Program. J. Clin. Microbiol. 2001, 39, 3254–3259. [Google Scholar] [CrossRef] [PubMed]
- Wingard, J. Importance of Candida species other than C. albicans as pathogens in oncology patients. Clin. Infect. Dis. 1995, 20, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Rex, J.; Pfaller, M.; Barry, A.; Nelson, P.; Webb, C. Antifungal susceptibility testing of isolates from a randomized, multicenter trial of fluconazole versus amphotericin B as treatment of nonneutropenic patients with candidemia. Antimicrob. Agents Chemother. 1995, 39, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Abbas, J.; Bodey, G.P.; Hanna, H.A.; Mardani, M.; Girgawy, E.; Abi-Said, D.; Whimbey, E.; Hachem, R.; Raad, I. Candida krusei fungemia. An escalating serious infection in immunocompromised patients. Arch. Intern. Med. 2000, 160, 2659–2664. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, J.A.; Sanchez, V.; Dmuchowski, C.; Dembry, L.M.; Sobel, J.D.; Zervos, M.J. Nosocomial acquisition of Candida albicans: an epidemiologic study. J. Infect. Dis. 1993, 168, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, J.A.; Dembry, L.M.; Sanchez, V.; Vazquez, M.A.; Sobel, J.D.; Dmuchowski, C.; Zervos, M.J. Nosocomial Candida glabrata colonization: An epidemiologic study. J. Clin. Microbiol. 1998, 36, 421–426. [Google Scholar] [PubMed]
- Martins, M.; Henriques, M.; Ribeiro, A.P.; Fernandes, R.; Gonçalves, V.; Seabra, Á.; Azeredo, J.; Oliveira, R. Oral Candida carriage of patients attending a dental clinic in Braga, Portugal. Rev. Iberoam. Micol. 2010, 27, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Masiá Canuto, M.; Gutiérrez Rodero, F.; Ortiz de la Tabla Ducasse, V.; Hernández Aguado, I.; Martín González, C.; Sánchez Sevillano, A.; Martín Hidalgo, A. Determinants for the development of oropharyngeal colonization or infection by fluconazole-resistant candida strains in HIV-Infected Patients. Eur. J. Clin. Microbiol. Infect. Dis. 2000, 19, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Redding, S.W.; Kirkpatrick, W.R.; Coco, B.J.; Sadkowski, L.; Fothergill, A.W.; Rinaldi, M.G.; Eng, T.Y.; Patterson, T.F. Candida glabrata oropharyngeal candidiasis in patients receiving radiation treatment for head and neck cancer. J. Clin. Microbiol. 2002, 40, 1879–1881. [Google Scholar] [CrossRef] [PubMed]
- De Bernardis, F.; Chiani, P.; Ciccozzi, M.; Pellegrini, G.; Ceddia, T.; D’Offizzi, G.; Quinti, I.; Sullivan, P.A.; Cassone, A. Elevated aspartic proteinase secretion and experimental pathogenicity of Candida albicans isolates from oral cavities of subjects infected with human immunodeficiency virus. Infect. Immun. 1996, 64, 466–471. [Google Scholar] [PubMed]
- Fidel, P.L.; Cutright, J.L.; Tait, L.; Sobel, J.D. A murine model of Candida glabrata vaginitis. J. Infect. Dis. 1996, 173, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Negri, M.; Silva, S.; Henriques, M.; Oliveira, R. Insights into Candida tropicalis nosocomial infections and virulence factors. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 1399–1412. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Henriques, M.; Martins, A.; Oliveira, R.; Williams, D.; Azeredo, J. Biofilms of non-Candida albicans Candida species: Quantification, structure and matrix composition. Med. Mycol. 2009, 47, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, E.; Silva, S.; Rodrigues, C.F.; Alves, C.T.; Azeredo, J.; Henriques, M. Effects of fluconazole on Candida glabrata biofilms and its relationship with ABC transporter gene expression. Biofouling 2014, 30, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Lewandowski, Z.; Caldwell, D.E.; Korber, D.R.; Lappin-Scott, H.M. Microbial Biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.; Costerton, J. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.F.; Silva, S.; Azeredo, J.; Henriques, M. Candida glabrata’s recurrent infections: biofilm formation during Amphotericin B treatment. Lett. Appl. Microbiol. 2016, 63, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Klotz, S.A.; Drutz, D.J.; Zajic, J.E. Factors Governing Adherence of Candida Species to Plastic Surfaces. Infect. Immun. 1985, 50, 97–101. [Google Scholar] [PubMed]
- Hazen, K.C.; Plotkin, B.J.; Klimas, D.M. Influence of growth conditions on cell surface hydrophobicity of Candida albicans and Candida glabrata. Infect. Immun. 1986, 54, 269–271. [Google Scholar] [PubMed]
- Kikutani, H.; Makino, S. The murine autoimmune diabetes model: NOD and related strains. Adv. Immunol. 1992, 51, 285–322. [Google Scholar] [PubMed]
- De Las Peñas, A.; Pan, S.J.; Castaño, I.; Alder, J.; Cregg, R.; Cormack, B.P. Virulence-related surface glycoproteins in the yeast pathogen Candida glabrata are encoded in subtelomeric clusters and subject to RAP1- and SIR-dependent transcriptional silencing. Genes Dev. 2003, 17, 2245–2258. [Google Scholar] [CrossRef] [PubMed]
- Castano, I.; Pan, S.; Zupancic, M.; Hennequin, C.; Dujon, B.; Cormack, B. Telomere length control and transcriptional regulation of subtelomeric adhesins in Candida glabrata. Mol. Microbiol. 2005, 55, 1246–1258. [Google Scholar] [CrossRef] [PubMed]
- Sundstrom, P. Adhesion in Candida spp. Cell. Microbiol. 2002, 4, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Filler, S.G. Candida–host cell receptor–ligand interactions. Curr. Opin. Microbiol. 2006, 9, 333–339. [Google Scholar] [CrossRef] [PubMed]
- de Groot, P.W.J.; Kraneveld, E.A.; Yin, Q.Y.; Dekker, H.L.; Gross, U.; Crielaard, W.; de Koster, C.G.; Bader, O.; Klis, F.M.; Weig, M. The cell wall of the human pathogen Candida glabrata: differential incorporation of novel adhesin-like wall proteins. Eukaryot. Cell 2008, 7, 1951–1964. [Google Scholar] [CrossRef] [PubMed]
- Kraneveld, E.A.; de Soet, J.J.; Deng, D.M.; Dekker, H.L.; de Koster, C.G.; Klis, F.M.; Crielaard, W.; de Groot, P.W.J. Identification and differential gene expression of adhesin-like wall proteins in Candida glabrata biofilms. Mycopathologia 2011, 172, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Negri, M.; Henriques, M.; Oliveira, R.; Williams, D.W.; Azeredo, J. Adherence and biofilm formation of non-Candida albicans Candida species. Trends Microbiol. 2011, 19, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Alcazar-Fuoli, L.; Mellado, E. Current status of antifungal resistance and its impact on clinical practice. Br. J. Haematol. 2014, 166. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, A.A.; Farley, M.M.; Harrison, L.H.; Stein, B.; Hollick, R.; Lockhart, S.R.; Magill, S.S.; Derado, G.; Park, B.J.; Chiller, T.M. Changes in incidence and antifungal drug resistance in candidemia: Results from population-based laboratory surveillance in Atlanta and Baltimore, 2008–2011. Clin. Infect. Dis. 2012, 55, 1352–1361. [Google Scholar] [CrossRef] [PubMed]
- Shahi, P.; Moye-Rowley, W.S. Coordinate control of lipid composition and drug transport activities is required for normal multidrug resistance in fungi. Biochim. Biophys. Acta 2009, 1794, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Muller, H.; Thierry, A.; Coppée, J.-Y.; Gouyette, C.; Hennequin, C.; Sismeiro, O.; Talla, E.; Dujon, B.; Fairhead, C. Genomic polymorphism in the population of Candida glabrata: gene copy-number variation and chromosomal translocations. Fungal Genet. Biol. 2009, 46, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Thierry, A.; Bouchier, C.; Dujon, B.; Richard, G.-F. Megasatellites: a peculiar class of giant minisatellites in genes involved in cell adhesion and pathogenicity in Candida glabrata. Nucleic Acids Res. 2008, 36, 5970–5982. [Google Scholar] [CrossRef] [PubMed]
- Barchiesi, F.; Falconi Di Francesco, L.; Arzeni, D.; Caselli, F.; Gallo, D.; Scalise, G. Electrophoretic Karyotyping and Triazole Susceptibility of Candida glabrata Clinical Isolates. Eur. J. Clin. Microbiol. Infect. Dis. 1999, 18, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, C.S.; Merz, W.G. Electrophoretic karyotypes of Torulopsis glabrata. J. Clin. Microbiol. 1989, 27, 2165–2168. [Google Scholar] [PubMed]
- Klempp-Selb, B.; Rimek, D.; Kappe, R. Karyotyping of Candida albicans and Candida glabrata from patients with Candida sepsis. Mycoses 2000, 43, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Chen, Y.C.; Lo, H.J.; Chen, K.W.; Li, S.Y. Assessment of Candida glabrata strain relatedness by pulsed-field gel electrophoresis and multilocus sequence typing. J. Clin. Microbiol. 2007, 45, 2452–2459. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Chae, M.J.; Song, J.W.; Jung, S.-I.; Cho, D.; Kee, S.J.; Kim, S.H.; Shin, M.G.; Suh, S.P.; Ryang, D.W. Changes in karyotype and azole susceptibility of sequential bloodstream isolates from patients with Candida glabrata candidemia. J. Clin. Microbiol. 2007, 45, 2385–2391. [Google Scholar] [CrossRef] [PubMed]
- Magee, B.B.; Sanchez, M.D.; Saunders, D.; Harris, D.; Berriman, M.; Magee, P.T. Extensive chromosome rearrangements distinguish the karyotype of the hypovirulent species Candida dubliniensis from the virulent Candida albicans. Fungal Genet. Biol. 2008, 45, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Bader, O.; Schwarz, A.; Kraneveld, E.A.; Tangwattanachuleeporn, M.; Schmidt, P.; Jacobsen, M.D.; Gross, U.; De Groot, P.W.J.; Weig, M. Gross karyotypic and phenotypic alterations among different progenies of the Candida glabrata CBS138/ATCC2001 reference strain. PLoS ONE 2012, 7, e52218. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.N.; Balusu, S.; Gorityala, N.; Dandu, L.; Kaur, R. Functional genomic analysis of Candida glabrata-macrophage interaction: role of chromatin remodeling in virulence. PLoS Pathog. 2012, 8, e1002863. [Google Scholar] [CrossRef] [PubMed]
- Jandric, Z.; Schuller, C. Stress response in Candida glabrata: pieces of a fragmented picture. Futur. Mirobiology 2011, 6, 1475–1484. [Google Scholar] [CrossRef] [PubMed]
- Riera, M.; Mogensen, E.; d’Enfert, C.; Janbon, G. New regulators of biofilm development in Candida glabrata. Res. Microbiol. 2012, 163, 297–307. [Google Scholar] [CrossRef]
- Iraqui, I.; Garcia-Sanchez, S.; Aubert, S.; Dromer, F.; Ghigo, J.M.; d’Enfert, C.; Janbon, G. The YAK1P kinase controls expression of adhesins and biofilm formation in Candida glabrata in a SIR4P-dependent pathway. Mol. Microbiol. 2005, 55, 1259–1271. [Google Scholar] [CrossRef] [PubMed]
- Lemke, A.; Kiderlen, A.F.; Kayser, O. Amphotericin B. Appl. Microbiol. Biotechnol. 2005, 68, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Baginski, M.; Czub, J. Amphotericin B and its new derivatives-mode of action. Curr. Drug Metab. 2009, 10, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Botero, M.C.; Puentes-Herrera, M.; Cortés, J.A. Formas lipídicas de anfotericina. Rev. Chil. Infectol. 2014, 31, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Perlin, D.S. Resistance to echinocandin- class antifungal drugs. Drug Resist. Updat. 2007, 10, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Debono, M.; Gordee, R.S. Antibiotics that inhibit fungal cell wall development. Annu. Rev. Microbiol. 1994, 48, 471–497. [Google Scholar] [CrossRef] [PubMed]
- Canuto, M.M.; Rodero, F.G. Antifungal drug resistance to azoles and polyenes. Lancet Infect. Dis. 2002, 2, 550–563. [Google Scholar] [CrossRef]
- Maenza, J.; Merz, W.; Romagnoli, M.; Keruly, J.; Moore, R.; Gallant, J. Infection due to fluconazole- resistant Candida in patients with AIDS: Prevalence and microbiology. Clin. Infect. Dis. 1997, 24, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Laguna, F.; Rodríguez-Tudela, J.L.; Martínez-Súarez, J.V.; Polo, R.; Valencia, E.; Díaz-Guerra, T.M.; Dronda, F.; Pulido, F.; Martínez-Suárez, J. Patterns of fluconazole susceptibility in isolates from human immunodeficiency virus-infected patients with oropharyngeal candidiasis due to Candida albicans. Clin. Infect. Dis. 1997, 24, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Revankar, S.G.; Kirkpatrick, W.R.; McAtee, R.K.; Dib, O.P.; Fothergill, A.W.; Redding, S.W.; Rinaldi, M.G.; Patterson, T.F. Detection and significance of fluconazole resistance in oropharyngeal Candidiasis in human immunodeficiency virus-infected patients. J. Infect. Dis. 1996, 174, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Wingard, J.; Merz, W.; Rinaldi, M.; Jonhson, T.; Karp, J.; Saral, R. Increase in Candida krusei infection among patients with bone marrow transplantation and neutropenia treated prophylactically with fluconazole. N. Engl. J. Med. 1991, 325, 1274–1277. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, F.; Wall, P.; Cohen, J. Trends in antifungal use and antifungal isolates in a university hospital 1990–1992. J. Hosp. Infect. 1995, 31, 149–152. [Google Scholar] [CrossRef]
- Nguyen, M.L.H.; Peacock, J.E.; Morris, A.J.; Tanner, D.C.; Nguyen, M.L.H.; Snydman, D.R.; Wagener, M.M.; Rinaldi, M.G.; Yu, V.L. The changing face of candidemia: emergence of non- Candida albicans species and antifungal resistance. Am. J. Med. 1996, 100, 617–623. [Google Scholar] [CrossRef]
- Van Bambeke, F.; Balzi, E.; Tulkens, P.M. Antibiotic Efflux Pumps. Biochem. Pharmacol. 2000, 60, 457–470. [Google Scholar] [CrossRef]
- Wilson, D.; Thewes, S.; Zakikhany, K.; Fradin, C.; Albrecht, A.; Almeida, R.; Brunke, S.; Grosse, K.; Martin, R.; Mayer, F.; et al. Identifying infection-associated genes of Candida albicans in the postgenomic era. FEMS Yeast Res. 2009, 9, 688–700. [Google Scholar] [CrossRef] [PubMed]
- Brunke, S.; Seider, K.; Almeida, R.; Heyken, A.; Fleck, C.; Brock, M.; Barz, D.; Rupp, S.; Hube, B. Candida glabrata tryptophan-based pigment production via the Ehrlich pathway. Mol. Microbiol. 2010, 76, 25–47. [Google Scholar] [CrossRef] [PubMed]
- Tscherner, M.; Schwarzmüller, T.; Kuchler, K. Pathogenesis and Antifungal Drug Resistance of the Human Fungal Pathogen Candida glabrata. Pharmaceuticals 2011, 4, 169–186. [Google Scholar] [CrossRef]
- Henry, K.; Nickels, J.; Edlind, T. Upregulation of ERG genes in Candida species by azoles and other sterol biosynthesis inhibitors. Antimicrob. Agents Chemother. 2000, 44, 2693–2700. [Google Scholar] [CrossRef] [PubMed]
- Stead, D.A.; Walker, J.; Holcombe, L.; Gibbs, S.R.S.; Yin, Z.; Selway, L.; Butler, G.; Brown, A.J.P.; Haynes, K. Impact of the transcriptional regulator, ACE2, on the Candida glabrata secretome. Proteomics 2010, 10, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Calcagno, A.; Bignell, E.; Warn, P.; Jones, M.; Denning, D.; Muhlschlegel, F.; Rogers, T.; Haynes, K. C. glabrata STE12 is required for wild-type levels of virulence and nitrogen starvation induced filamentation. Mol. Microbiol. 2003, 50, 1309–1318. [Google Scholar] [CrossRef] [PubMed]
- Kanafani, Z.A.; Perfect, J.R. Resistance to antifungal agents: mechanisms and clinical impact. Clin. Infect. Dis. 2008, 46, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.; Sanguinetti, M.; Torelli, R.; Posteraro, B.; Sanglard, D. Contribution of CgPDR1-regulated genes in enhanced virulence of azole-resistant Candida glabrata. PLoS ONE 2011, 6, e17589. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.; Sanguinetti, M.; De Bernardis, F.; Torelli, R.; Posteraro, B.; Vandeputte, P.; Sanglard, D. Loss of mitochondrial functions associated with azole resistance in Candida glabrata results in enhanced virulence in mice. Antimicrob. Agents Chemother. 2011, 55, 1852–1860. [Google Scholar] [CrossRef] [PubMed]
- Noble, J.A.; Tsai, H.F.; Suffis, S.D.; Su, Q.; Myers, T.G.; Bennett, J.E. STB5 is a negative regulator of azole resistance in Candida glabrata. Antimicrob. Agents Chemother. 2013, 57, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Vermitsky, J.; Edlind, T.D. Azole Resistance in Candida glabrata: Coordinate upregulation of multidrug transporters and evidence for a PDR1-like transcription factor. Antimicrob. Agents Chemother. 2004, 48, 3773–3781. [Google Scholar] [CrossRef] [PubMed]
- Vale-Silva, L.; Ischer, F.; Leibundgut-Landmann, S.; Sanglard, D. Gain-of-function mutations in PDR1, a regulator of antifungal drug resistance in Candida glabrata, control adherence to host cells. Infect. Immun. 2013, 81, 1709–1720. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Schmidt, J.A.; Moye-Rowley, W.S. Regulation of the CGPDR1 transcription factor from the pathogen Candida glabrata. Eukaryot. Cell 2011, 10, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Farahyar, S.; Zaini, F.; Kordbacheh, P.; Rezaie, S.; Falahati, M.; Safara, M. Expression of efflux pumps and fatty acid activator one genes in azole resistant Candida glabrata isolated from immunocompromised patients. Acta Med. Iran. 2015, 54, 458–464. [Google Scholar]
- Kaur, R.; Castan, I.; Cormack, B.P. Functional genomic analysis of fluconazole susceptibility in the pathogenic yeast Candida glabrata: Roles of calcium signaling and mitochondria. Antimicrob. Agents Chemother. 2004, 48, 1600–1613. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Yamauchi, S.; Inamine, T.; Nagayoshi, Y.; Saijo, T.; Izumikawa, K.; Seki, M.; Kakeya, H.; Yamamoto, Y.; Yanagihara, K.; et al. Roles of calcineurin and Crz1 in antifungal susceptibility and virulence of Candida glabrata. Antimicrob. Agents Chemother. 2010, 54, 1639–1643. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Ma, B.; Cormack, B.P. A family of glycosylphosphatidylinositol-linked aspartyl proteases is required for virulence of Candida glabrata. Proc. Natl. Acad. Sci. USA. 2007, 104, 7628–7633. [Google Scholar] [CrossRef] [PubMed]
- Akins, R.A. An update on antifungal targets and mechanisms of resistance in Candida albicans. Med. Mycol. 2005, 43, 285–318. [Google Scholar] [CrossRef] [PubMed]
- Marichal, P.; Koymans, L.; Willemsens, S.; Bellens, D.; Verhasselt, P.; Luyten, W.; Borgers, M.; Ramaekers, F.C.; Odds, F.C.; Bossche, H.V. Contribution of mutations in the cytochrome P450 14-α-demethylase (Erg11p, Cyp51p) to azole resistance in Candida albicans. Microbiology 1999, 145, 2701–2713. [Google Scholar] [CrossRef]
- Morio, F.; Pagniez, F.; Lacroix, C.; Miegeville, M.; Le Pape, P. Amino acid substitutions in the Candida albicans sterol D5,6-desaturase (Erg3p) confer azole resistance: characterization of two novel mutants with impaired virulence. J. Antimicrob. Chemother. 2012, 67, 2131–2138. [Google Scholar] [CrossRef] [PubMed]
- Szweda, P.; Gucwa, K.; Romanowska, E.; Dzierzanowska-Fangrat, K.; Naumiuk, L.; Brillowska-Dabrowska, A.; Wojciechowska-Koszko, I.; Milewski, S. Mechanisms of azole resistance among clinical isolates of Candida glabrata in Poland. J. Med. Microbiol. 2015, 64, 610–619. [Google Scholar] [CrossRef]
- Aramburu, J.; Rao, A.; Klee, C.B. Calcineurin: from structure to function. Curr. Top. Cell. Regul. 2000, 36, 237–295. [Google Scholar] [PubMed]
- Onyewu, C.; Blankenship, J.R.; Del Poeta, M.; Heitman, J. Ergosterol biosynthesis inhibitors become fungicidal when combined with calcineurin inhibitors against Candida albicans, Candida glabrata, and Candida krusei. Antimicrob. Agents Chemother. 2003, 47, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.C.; Goldstein, A.L.; Blankenship, J.R.; Del Poeta, M.; Davis, D.; Cardenas, M.E.; Perfect, J.R.; McCusker, J.H.; Heitman, J. Calcineurin is essential for survival during membrane stress in Candida albicans. EMBO J. 2002, 21, 546–559. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.H.; Miyazaki, T.; Tsai, H.F.; Bennett, J.E. The bZip transcription factor CGAP1P is involved in multidrug resistance and required for activation of multidrug transporter gene CgFLR1 in Candida glabrata. Gene 2007, 386, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Farahyar, S.; Zaini, F.; Kordbacheh, P.; Rezaie, S.; Safara, M.; Raoofian, R.; Heidari, M. Overexpression of Aldo-Keto-reductase in Azole-resistant clinical isolates of Candida glabrata determined by cDNA-AFLP. Daru J. Pharm. Sci. 2013, 21, 1. [Google Scholar] [CrossRef] [PubMed]
- Taff, H.T.; Nett, J.E.; Zarnowski, R.; Ross, K.M.; Sanchez, H.; Cain, M.T.; Hamaker, J.; Mitchell, A.P.; Andes, D.R. A Candida biofilm-induced pathway for matrix glucan delivery: implications for drug resistance. PLoS Pathog. 2012, 8, e1002848. [Google Scholar] [CrossRef] [PubMed]
- Berenguer, J.; Rodriguez-Tudela, J.; Richard, C.; Alvarez, M.; Sanz, M.; Gaztelurrutia, L.; Ayats, J.; Martinez-Suarez, J. Deep infections caused by Scedosporium prolificans. A report on 16 cases in Spain and a review of the literature. Scedosporium prolificans Spanish Study Group. Medicine 1997, 76, 256–265. [Google Scholar] [CrossRef]
- Boutati, E.; Anaissie, E. Fusarium, a significant emerging pathogen in patients with hematologic malignancy: ten years’ experience at a cancer center and implications for management. Blood 1997, 90, 999–1008. [Google Scholar] [PubMed]
- Tritz, D.; Woods, G. Fatal disseminated infection with Aspergillus terreus in immunocompromised hosts. Clin. Infect. Dis. 1993, 16, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Rex, J.H.J.; Walsh, T.J.; Sobel, J.D.J.; Filler, S.G.; Pappas, P.G.; Dismukes, W.E.; Edwards, J.E. Practice guidelines for the treatment of candidiasis. Clin. Infect. Dis. 2000, 30, 662–678. [Google Scholar] [CrossRef] [PubMed]
- Vandeputte, P.; Tronchin, G.; Larcher, G.; Ernoult, E.; Bergès, T.; Chabasse, D.; Bouchara, J.-P. A nonsense mutation in the ERG6 gene leads to reduced susceptibility to polyenes in a clinical isolate of Candida glabrata. Antimicrob. Agents Chemother. 2008, 52, 3701–3709. [Google Scholar] [CrossRef]
- Vandeputte, P.; Tronchin, G.; Bergès, T.; Hennequin, C.; Chabasse, D.; Bouchara, J.P. Reduced susceptibility to polyenes associated with a missense mutation in the ERG6 gene in a clinical isolate of Candida glabrata with pseudohyphal growth. Antimicrob. Agents Chemother. 2007, 51, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Shields, R.K.; Nguyen, M.H.; Press, E.G.; Kwa, A.L.; Cheng, S.; Du, C.; Clancy, C.J. The presence of an FKS mutation rather than MIC is an independent risk factor for failure of echinocandin therapy among patients with invasive candidiasis due to Candida glabrata. Antimicrob. Agents Chemother. 2012, 56, 4862–4869. [Google Scholar] [CrossRef] [PubMed]
- Beyda, N.D.; Lewis, R.E.; Garey, K.W. Echinocandin resistance in Candida species: mechanisms of reduced susceptibility and therapeutic approaches. Ann. Pharmacother. 2012, 46, 1086–1096. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.A.; Gow, N.A.; Munro, C.A. Fungal echinocandin resistance. Fungal Genet. Biol. 2010, 47, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Barchiesi, F.; Barchiesi, F.; Spreghini, E.; Tomas- setti, S.; Della Vittoria, A.; Arzeni, D.; Manso, E.; Scalise, G. Effects of caspofungin against Candida guilliermondii and Candida parapsilosis. Antimicrob. Agents Chemother. 2006, 2719–2727. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Effron, G.; Katiyar, S.K.; Park, S.; Edlind, T.D.; Perlin, D.S. A naturally occurring proline-to-alanine amino acid change in Fks1p in Candida parapsilosis, Candida orthopsilosis, and Candida metapsilosis accounts for reduced echinocandin susceptibility. Antimicrob. Agents Chemother. 2008, 52, 2305–2312. [Google Scholar] [CrossRef] [PubMed]
- Vai, M.; Orlandi, I.; Cavadini, P.; Alberghina, L.; Popolo, L. Candida albicans homologue of GGP1/GAS1 gene is functional in Saccharomyces cerevisiae and contains the determinants for glycosylphosphatidylinositol attachment. Yeast 1996, 12, 361–368. [Google Scholar] [CrossRef]
- Garcia-Effron, G.; Lee, S.; Park, S.; Cleary, J.D.; Perlin, D.S. Effect of Candida glabrata FKS1 and FKS2 mutations on echinocandin sensitivity and kinetics of 1,3-β-d-glucan synthase: implication for the existing susceptibility breakpoint. Antimicrob. Agents Chemother. 2009, 53, 3690–3699. [Google Scholar] [CrossRef] [PubMed]
- Alexander, B.D.; Johnson, M.D.; Pfeiffer, C.D.; Jimenez-Ortigosa, C.; Catania, J.; Booker, R.; Castanheira, M.; Messer, S.A.; Perlin, D.S.; Pfaller, M.A. Increasing echinocandin resistance in Candida glabrata: clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin. Infect. Dis. 2013, 56, 1724–1732. [Google Scholar] [CrossRef] [PubMed]
- Thompson, G.R.; Wiederhold, N.P.; Vallor, A.C.; Villareal, N.C.; Lewis, J.S.; Patterson, T.F. Development of caspofungin resistance following prolonged therapy for invasive candidiasis secondary to Candida glabrata infection. Antimicrob. Agents Chemother. 2008, 52, 3783–3785. [Google Scholar] [CrossRef] [PubMed]
- Arendrup, M.; Perlin, D.; Jensen, R.; Howard, S.; Goodwin, J.; Hopec, W. Differential in vivo activities of anidulafungin, caspofungin, and micafungin against Candida glabrata isolates with and without FSK resistance mutations. Antimicrob. Agents Chemother. 2012, 2435–2442. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.A.; White, T.C.; Perlin, D.S.; Selitrennikoff, C.P. Studies of the paradoxical effect of caspofungin at high drug concentrations. Diagn. Microbiol. Infect. Dis. 2005, 51, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Chamilos, G.; Lewis, R.E.; Albert, N.; Kontoyiannis, D.P. Paradoxical effect of echinocandins across candida species In vitro: Evidence for echinocandin-specific and candida species-related differences. Antimicrob. Agents Chemother. 2007, 51, 2257–2259. [Google Scholar] [CrossRef] [PubMed]
- Balashov, S.V.; Park, S.; Perlin, D.S. Assessing resistance to the echinocandin antifungal drug caspofungin in Candida albicans by profiling mutations in FKS1. Antimicrob. Agents Chemother. 2006, 50, 2058–2063. [Google Scholar] [CrossRef] [PubMed]
- Ramage, G.; Rajendran, R.; Sherry, L.; Williams, C. Fungal biofilm resistance. Int. J. Microbiol. 2012, 2012, 528521. [Google Scholar] [CrossRef] [PubMed]
- Mathé, L.; Van Dijck, P. Recent insights into Candida albicans biofilm resistance mechanisms. Curr. Genet. 2013, 59, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Taff, H.T.; Mitchell, K.F.; Edward, J.A.; Andes, D.R. Mechanisms of Candida biofilm drug resistance. Futur. Microbiol. 2013, 8, 1325–1337. [Google Scholar] [CrossRef] [PubMed]
- Fox, E.; Singh-babak, S.; Hartooni, N.; Nobile, C. Biofilms and antifungal resistance. In Antifungals from Genomics to Resistance and the Development of Novel Agents; Coste, A., Vandeputte, P., Eds.; Caister Academic Press: Poole, UK, 2015; pp. 71–90. [Google Scholar]
- Anderson, J.B. Evolution of antifungal-drug resistance: Mechanisms and pathogen fitness. Nat. Rev. Microbiol. 2005, 3, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Jabra-Rizk, M.A.; Falkler, W.A.; Meiller, T.F. Fungal biofilms and drug resistance. Emerg. Infect. Dis. 2004, 10, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Nett, J.; Lepak, A.; Marchillo, K.; Andes, D. Time course global gene expression analysis of an in vivo Candida biofilm. J. Infect. Dis. 2009, 200, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Ramage, G.; Vandewalle, K.; Wickes, B.; López-Ribot, J. Characteristics of biofilm formation by Candida albicans. Rev. Iberoam. Micol. 2001, 18, 163–170. [Google Scholar] [PubMed]
- Mukherjee, P.K.; Chandra, J.; Kuhn, D.M.; Ghannoum, M.A. Mechanism of fluconazole resistance in Candida albicans biofilms: phase-specific role of efflux pumps and membrane sterols. Infect. Immun. 2003, 71, 4333–4340. [Google Scholar] [CrossRef] [PubMed]
- Andes, D.; Nett, J.; Oschel, P.; Albrecht, R.; Marchillo, K.; Pitula, A. Development and characterization of an in vivo central venous catheter Candida albicans biofilm model. Infect. Immun. 2004, 72, 6023–6031. [Google Scholar] [CrossRef] [PubMed]
- Mateus, C.; Crow, S.A.; Ahearn, D.G. Adherence of Candida albicans to silicone induces immediate enhanced tolerance to fluconazole. Antimicrob. Agents Chemother. 2004, 48, 3358–3366. [Google Scholar] [CrossRef] [PubMed]
- Nobile, C.J.; Fox, E.P.; Nett, J.E.; Sorrells, T.R.; Mitrovich, Q.M.; Hernday, A.D.; Tuch, B.B.; Andes, D.R.; Johnson, A.D. A recently evolved transcriptional network controls biofilm development in Candida albicans. Cell 2012, 148, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Ramage, G.; Bachmann, S.; Patterson, T.F.; Wickes, B.L.; López-Ribot, J.L. Investigation of multidrug efflux pumps in relation to fluconazole resistance in Candida albicans biofilms. J. Antimicrob. Chemother. 2002, 49, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Yeater, K.; Chandra, J.; Cheng, G.; Mukherjee, P.; Zhao, X.; Rodriguez- Zas, S.; Kwast, K.; Ghannoum, M.; Hoyer, L. Temporal analysis of Candida albicans gene expression during biofilm development. Microbiology 2007, 2373–2385. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.H.; Yu, C.Y. Influence of incubation time, inoculum size, and glucose concentrations on spectrophotometric endpoint determinations for amphotericin B, fluconazole, and itraconazole. J. Clin. Microbiol. 1999, 37, 141–145. [Google Scholar] [PubMed]
- European Committee on Antimicrobial Susceptibility Testing—Antifungal agents, Breakpoint tables for interpretation of MICs, Version 8.0, valid from 2015-11-16. Available online: http://www.eucast.org/clinical_breakpoints/ (accessed on 1 December 2016).
- National Committee for Clinical Laboratory Standards. 1997. Available online: http://clsi.org/ (accessed on 1 December 2016).
- Perumal, P.; Mekala, S.; Chaffin, W.L. Role for cell density in antifungal drug resistance in Candida albicans biofilms. Antimicrob. Agents Chemother. 2007, 51, 2454–2463. [Google Scholar] [CrossRef] [PubMed]
- Hornby, J.; Jensen, E.; Lisec, A.; Tasto, J.; Jahnke, B.; Shoemaker, R.; Dussault, P.; Nickerson, K. Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl. Env. Microbiol. 2001, 67, 2982–2992. [Google Scholar] [CrossRef] [PubMed]
- Al-fattani, M.A.; Douglas, L.J. Penetration of candida biofilms by antifungal agents. Antimicrob. Agents Chemother. 2004, 48, 3291–3297. [Google Scholar] [CrossRef] [PubMed]
- Al-Fattani, M.A.; Douglas, L.J. Biofilm matrix of Candida albicans and Candida tropicalis: Chemical composition and role in drug resistance. J. Med. Microbiol. 2006, 55, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Lal, P.; Sharma, D.; Pruthi, P.; Pruthi, V. Exopolysaccharide analysis of biofilm-forming Candida albicans. J. Appl. Microbiol. 2010, 109, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.; Uppuluri, P.; Thomas, D.P.; Cleary, I.A.; Henriques, M.; Lopez-Ribot, J.L.; Oliveira, R. Presence of extracellular DNA in the Candida albicans biofilm matrix and its contribution to biofilms. Mycopathologia 2010, 169, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.; Henriques, M.; Lopez-Ribot, J.L.; Oliveira, R. Addition of DNase improves the in vitro activity of antifungal drugs against Candida albicans biofilms. Mycoses 2012, 55, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Kojic, E.M.; Darouiche, R.O. Candida infections of medical devices. Clin. Microbiol. Rev. 2004, 17, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, K.F.; Taff, H.T.; Cuevas, M.A.; Reinicke, E.L.; Sanchez, H.; Andes, D.R. Role of matrix β-1,3-glucan in antifungal resistance of non-albicans Candida biofilms. Antimicrob. Agents Chemother. 2013, 57, 1918–1920. [Google Scholar] [CrossRef] [PubMed]
- Vediyappan, G.; Rossignol, T.; D’Enfert, C. Interaction of Candida albicans biofilms with antifungals: Transcriptional response and binding of antifungals to β-glucans. Antimicrob. Agents Chemother. 2010. [Google Scholar] [CrossRef] [PubMed]
- Bandara, H.M.H.N.; Lam, O.L.T.; Watt, R.M.; Jin, L.J.; Samaranayake, L.P. Bacterial lipopolysaccharides variably modulate in vitro biofilm formation of Candida species. J. Med. Microbiol. 2010, 59, 1225–1234. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, S.C.; Smith, M.C.A.; Muston, P.; Holland, S.L.; Avery, S.V. Heterogeneous expression of the virulence-related adhesin EPA1 between individual cells and strains of the pathogen Candida glabrata. Eukaryot. Cell 2012, 11, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Chandra, J.; Kuhn, D.; Mukherjee, P.; Hoyer, L.; McCormick, T.; Ghannoum, M. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J. Bacteriol. 2001, 183, 5385–5394. [Google Scholar] [CrossRef] [PubMed]
- Baillie, G.S.; Douglas, L.J. Effect of growth rate on resistance of Candida albicans biofilms to antifungal agents. Antimicrob. Agents Chemother. 1998, 42, 1900–1905. [Google Scholar] [PubMed]
- Baillie, G.S.; Douglas, L.J. Iron-limited biofilms of Candida albicans and their susceptibility to amphotericin B. Antimicrob. Agents Chemother. 1998, 42, 2146–2149. [Google Scholar] [PubMed]
- Cannon, R.; Lamping, E.; Holmes, A. Candida albicans drug resistance—Another way to cope with stress. Microbiol 2007, 153, 3211–3217. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, C.J.; Wang, Y.; Jin, L.; Abiko, Y.; Samaranayake, L.P. Proteomics of drug resistance in Candida glabrata biofilms. Proteomics 2010, 10, 1444–1454. [Google Scholar] [CrossRef] [PubMed]
- LaFleur, M.D.; Kumamoto, C.A.; Lewis, K. Candida albicans biofilms produce antifungal-tolerant persister cells. Antimicrob. Agents Chemother. 2006, 50, 3839–3846. [Google Scholar] [CrossRef] [PubMed]
- Khot, P.D.; Suci, P.A.; Miller, R.L.; Nelson, R.D.; Tyler, B.J. A small subpopulation of blastospores in Candida albicans biofilms exhibit resistance to amphotericin B associated with differential regulation of ergosterol and β-1,6-glucan pathway genes. Antimicrob. Agents Chemother. 2006, 50, 3708–3716. [Google Scholar] [CrossRef] [PubMed]
- Al-Dhaheri, R.S.; Douglas, L.J. Absence of amphotericin B-tolerant persister cells in biofilms of some Candida species. Antimicrob. Agents Chemother. 2008, 52, 1884–1887. [Google Scholar] [CrossRef] [PubMed]
- Bojsen, R.; Regenberg, B.; Gresham, D.; Folkesson, A. A common mechanism involving the TORC1 pathway can lead to amphotericin B-persistence in biofilm and planktonic Saccharomyces cerevisiae populations. Sci. Rep. 2016, 6, 21874. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Farmakiotis, D.; Yang, D.; McCue, D.A.; Kantarjian, H.M.; Kontoyiannis, D.P.; Mathisen, M.S. The ever-evolving landscape of candidaemia in patients with acute leukaemia: non-susceptibility to caspofungin and multidrug resistance are associated with increased mortality. J. Antimicrob. Chemother. 2015. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Castanheira, M.; Lockhart, S.R.; Ahlquist, A.M.; Messer, S.A.; Jones, R.N. Frequency of decreased susceptibility and resistance to echinocandins among fluconazole-resistant bloodstream isolates of Candida glabrata. J. Clin. Microbiol. 2012, 50, 1199–1203. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, A.A.; Harrison, L.H.; Farley, M.M.; Hollick, R.; Stein, B.; Chiller, T.M.; Lockhart, S.R.; Park, B.J. Declining incidence of candidemia and the shifting epidemiology of Candida resistance in two US metropolitan areas, 2008-2013: Results from population-based surveillance. PLoS ONE 2015, 10, e0120452. [Google Scholar] [CrossRef] [PubMed]
- Razzaghi-Abyaneh, M.; Sadeghi, G.; Zeinali, E.; Alirezaee, M.; Shams-Ghahfarokhi, M.; Amani, A.; Mirahmadi, R.; Tolouei, R. Species distribution and antifungal susceptibility of Candida spp. isolated from superficial Candidiasis in outpatients in Iran. J. Mycol. Med. 2014, 24, e43–e50. [Google Scholar] [CrossRef] [PubMed]
- Sanguinetti, M.; Posteraro, B.; Lass-Florl, C. Antifungal drug resistance among Candida species: Mechanisms and clinical impact. Mycoses 2015, 2, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Farmakiotis, D.; Tarrand, J.J.; Kontoyiannis, D.P. Drug-resistant Candida glabrata infection in cancer patients. Emerg. Infect. Dis. 2014, 20, 1833–1840. [Google Scholar] [CrossRef] [PubMed]
- Mohamadi, J.; Motaghi, M.; Panahi, J.; Havasian, M.R.; Delpisheh, A.; Azizian, M.; Pakzad, I. Anti-fungal resistance in candida isolated from oral and diaper rash Candidiasis in neonates. Bioinformation 2014, 10, 667–670. [Google Scholar] [CrossRef] [PubMed]
- Nieto, M.C.; Tellería, O.; Cisterna, R. Sentinel surveillance of invasive candidiasis in Spain: Epidemiology and antifungal susceptibility. Diagn. Microbiol. Infect. Dis. 2015, 81, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Rhomberg, P.R.; Messer, S.A.; Jones, R.N.; Castanheira, M. Isavuconazole, micafungin, and 8 comparator antifungal agents’ susceptibility profiles for common and uncommon opportunistic fungi collected in 2013: temporal analysis of antifungal drug resistance using CLSI species-specific clinical breakpoints and proposed epidemiological cutoff values. Diagn. Microbiol. Infect. Dis. 2015, 82, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Schmalreck, A.F.; Willinger, B.; Haase, G.; Blum, G.; Lass-Flörl, C.; Fegeler, W.; Becker, K. Antifungal Susceptibility Testing-AFST Study Group Species and susceptibility distribution of 1062 clinical yeast isolates to azoles, echinocandins, flucytosine and amphotericin B from a multi-centre study. Mycoses 2012, 55, e124–e137. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.Y.; Yang, Y.P.; Zhang, Y.; Li, W.; Wang, J.D.; Huang, W.M.; Fan, Y.M. Molecular identification and antifungal susceptibility of 186 Candida isolates from vulvovaginal Candidiasis in Southern China. J. Med. Microbiol. 2015, 64, 390–393. [Google Scholar] [CrossRef] [PubMed]
- Zarei Mahmoudabadi, A.; Rezaei-Matehkolaei, A.; Ghanavati, F. The Susceptibility Patterns of Candida Species Isolated From Urine Samples to Posaconazole and Caspofungin. Jundishapur J. Microbiol. 2015, 8. [Google Scholar] [CrossRef] [PubMed]
- Posteraro, B.; Spanu, T.; Fiori, B.; De Maio, F.; De Carolis, E.; Giaquinto, A.; Prete, V.; De Angelis, G.; Torelli, R.; D’Inzeo, T.; et al. Antifungal susceptibility profiles of bloodstream yeast isolates by sensititre yeastone over nine years at a large Italian teaching hospital. Antimicrob. Agents Chemother. 2015, 59, 3944–3955. [Google Scholar] [CrossRef] [PubMed]
- Czaika, V.; Nenoff, P.; Glöckner, A.; Becker, K.; Fegeler, W.; Schmalreck, A.F. Detection of azole susceptibility patterns in clinical yeast strains isolated from 1998 to 2008. New Microbiol. 2014, 37, 465–494. [Google Scholar] [PubMed]
- Pfaller, M.A.; Messer, S.A.; Boyken, L.; Tendolkar, S.; Hollis, R.J.; Diekema, D.J. Geographic variation in the susceptibilities of invasive isolates of Candida glabrata to seven systemically active antifungal agents: A global assessment from the ARTEMIS Antifungal Surveillance Program conducted in 2001 and 2002. J. Clin. Microbiol. 2004, 42, 3142–3146. [Google Scholar] [CrossRef] [PubMed]
- Gopinathan, S.; Janagond, A.B.; Agatha, D.; P R, T. Detection of FUR1 Gene in 5-flucytosine resistant candida isolates in vaginal candidiasis patients. J. Clin. Diagn. Res. 2013, 7, 2452–2455. [Google Scholar] [CrossRef] [PubMed]
- Haddadi, P.; Zareifar, S.; Badiee, P.; Alborzi, A.; Mokhtari, M.; Zomorodian, K.; Pakshir, K.; Jafarian, H. Yeast colonization and drug susceptibility pattern in the pediatric patients with neutropenia. Jundishapur J. Microbiol. 2014, 7, e11858. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.H.; Melake, N.A.; Somily, A.M.; Zakaria, A.S.; Baddour, M.M.; Mahmoud, A.Z. The effect of antifungal combination on transcripts of a subset of drug-resistance genes in clinical isolates of Candida species induced biofilms. Saudi Pharm. J. SPJ Off. Publ. Saudi Pharm. Soc. 2015, 23, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Silva, L.; Ferreira-Paim, K.; Mora, D.J.; Da Silva, P.R.; Andrade, A.A.; Araujo, N.E.; Pedrosa, A.L.; Silva-Vergara, M.L. Susceptibility profile of clinical and environmental isolates of Cryptococcus neoformans and Cryptococcus gattii in Uberaba, Minas Gerais, Brazil. Med. Mycol. 2013, 51, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Oxman, D.A.; Chow, J.K.; Frendl, G.; Hadley, S.; Hershkovitz, S.; Ireland, P.; McDermott, L.A.; Tsai, K.; Marty, F.M.; Kontoyiannis, D.P.; Golan, Y. Candidaemia associated with decreased in vitro fluconazole susceptibility: is Candida speciation predictive of the susceptibility pattern? J. Antimicrob. Chemother. 2010, 65, 1460–1465. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Messer, S.A.; Jones, R.N.; Castanheira, M. Antifungal susceptibilities of Candida, Cryptococcus neoformans and Aspergillus fumigatus from the Asia and Western Pacific region: Data from the SENTRY antifungal surveillance program (2010–2012). J. Antibiot. 2015, 68, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Chang, T.Y.; Liu, J.W.; Chen, F.J.; Chien, C.C.; Tang, Y.F.; Lu, C.H. Correlation of anti-fungal susceptibility with clinical outcomes in patients with cryptococcal meningitis. BMC Infect. Dis. 2012, 12, 361. [Google Scholar] [CrossRef] [PubMed]
- Matos, C.S.; de Souza Andrade, A.; Oliveira, N.S.; Barros, T.F. Microbiological characteristics of clinical isolates of Cryptococcus spp. in Bahia, Brazil: Molecular types and antifungal susceptibilities. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 1647–1652. [Google Scholar] [CrossRef] [PubMed]
- Asadzadeh, M.; Al-Sweih, N.A.; Ahmad, S.; Khan, Z.U. Antifungal susceptibility of clinical Candida parapsilosis isolates in Kuwait. Mycoses 2008, 51, 318–323. [Google Scholar] [CrossRef]
- Hegazi, M.; Abdelkader, A.; Zaki, M.; El-Deek, B. Characteristics and risk factors of candidemia in pediatric intensive care unit of a tertiary care children’s hospital in Egypt. J. Infect. Dev. Ctries. 2014, 8, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Zaoutis, T.E.; Foraker, E.; McGowan, K.L.; Mortensen, J.; Campos, J.; Walsh, T.J.; Klein, J.D. Antifungal susceptibility of Candida spp. isolated from pediatric patients: A survey of 4 children’s hospitals. Diagn. Microbiol. Infect. Dis. 2005, 52, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Krogh-Madsen, M.; Arendrup, M.C.; Heslet, L.; Knudsen, J.D. Amphotericin B and Ca- spofungin resistance in Candida glabrata isolates recovered from a critically ill patient. Clin. Infect. Dis. 2006, 42, 938–944. [Google Scholar] [CrossRef] [PubMed]
- Martel, C.M.; Parker, J.E.; Bader, O.; Weig, M.; Gross, U.; Warrilow, A.G.S.; Rolley, N.; Kelly, D.E.; Kelly, S.L. Identification and characterization of four azole-resistant Erg3 mutants of Candida albicans. Antimicrob. Agents Chemother. 2010, 54, 4527–4533. [Google Scholar] [CrossRef] [PubMed]
- Eddouzi, J.; Parker, J.E.; Vale-Silva, L.A.; Coste, A.; Ischer, F.; Kelly, S.; Manai, M.; Sanglard, D. Molecular mechanisms of drug resistance in clinical Candida species isolated from Tunisian hospitals. Antimicrob. Agents Chemother. 2013, 3182–3193. [Google Scholar] [CrossRef] [PubMed]
- Forastiero, A.; Mesa-Arango, A.C.; Alastruey-Izquierdo, A.; Alcazar-Fuoli, L.; Bernal-Martinez, L.; Pelaez, T.; Lopez, J.F.; Grimalt, J.O.; Gomez- Lopez, A.; Cuesta, I.; Zaragoza, O.; Mellado, E. Candida tropicalis antifungal cross-resistance is related to different azole target (Erg11p) modifications. Antimicrob. Agents Chemother. 2013, 57, 4769–4781. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Pais, P.; Teixeira, M.C. Multidrug resistance in pathogenic yeasts: Emphasis on the role of ABC and MFS multidrug transporters. In The Battle Against Microbial Pathogens: Basic Science, Technological Advances and Educational Programs; Formatex: Badajoz, Spain, 2015; pp. 947–954. [Google Scholar]
- Sanglard, D.; Ischer, F.; Bille, J. Role of ATP-binding-cassette transporter genes in high-frequency acquisition of resistance to azole antifungals in Candida glabrata. Antimicrob. Agents Chemother. 2001, 45, 1174–1183. [Google Scholar] [CrossRef] [PubMed]
- Torelli, R.; Posteraro, B.; Ferrari, S.; La Sorda, M.; Fadda, G.; Sanglard, D.; Sanguinetti, M. The ATP-binding cassette transporter-encoding gene CgSNQ2 is contributing to the CgPDR1-dependent azole resistance of Candida glabrata. Mol. Microbiol. 2008, 68, 186–201. [Google Scholar] [CrossRef] [PubMed]
- Healey, K.R.; Zhao, Y.; Perez, W.B.; Lockhart, S.R.; Sobel, J.D.; Farmakiotis, D.; Kontoyiannis, D.P.; Sanglard, D.; Taj-Aldeen, S.J.; Alexander, B.D.; et al. Prevalent mutator genotype identified in fungal pathogen Candida glabrata promotes multi-drug resistance. Nat. Commun. 2016, 7, 11128. [Google Scholar] [CrossRef] [PubMed]
- Thakur, J.K.; Arthanari, H.; Yang, F.; Pan, S.J.; Fan, X.; Breger, J.; Frueh, D.P.; Gulshan, K.; Li, D.K.; Mylonakis, E.; et al. A nuclear receptor-like pathway regulating multidrug resistance in fungi. Nature 2008, 452, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Vought, B.W.; Satterlee, J.S.; Walker, A.K.; Sun, Z.-Y.J.; Watts, J.L.; DeBeaumont, R.; Mako Saito, R.; Hyberts, S.G.; Yang, S.; Macol, C.; et al. An ARC/Mediator subunit required for SREBP control of cholesterol and lipid homeostasis. Nature 2006, 442, 700–704. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, J.L.; Boeszoermenyi, A.; Vale-Silva, L.A.; Torelli, R.; Posteraro, B.; Sohn, Y.J.; Ji, F.; Gelev, V.; Sanglard, D.; Sanguinetti, M.; et al. Inhibiting fungal multidrug resistance by disrupting an activator-Mediator interaction. Nature 2016, 530, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Moye-Rowley, W.S. Multidrug resistance in fungi: Regulation of transporter-encoding gene expression. Front. Physiol. 2014, 5 APR, 143. [Google Scholar] [CrossRef] [PubMed]
- Orta-Zavalza, E.; Guerrero-Serrano, G.; Gutiérrez-Escobedo, G.; Cañas-Villamar, I.; Juárez-Cepeda, J.; Castaño, I.; De Las Peñas, A. Local silencing controls the oxidative stress response and the multidrug resistance in Candida glabrata. Mol. Microbiol. 2013, 88, 1135–1148. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Pires, C.; Cabrito, T.R.; Renaudin, A.; Ohno, M.; Chibana, H.; Sá-Correia, I.; Teixeira, M.C. Candida glabrata drug:H+ antiporter CgQdr2 confers imidazole drug resistance, being activated by transcription factor CgPdr1. Antimicrob. Agents Chemother. 2013, 57, 3159–3167. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Nunes, J.; Henriques, A.; Mira, N.P.; Nakayama, H.; Chibana, H.; Teixeira, M.C. Candida glabrata drug:H+ antiporter CgTpo3 (ORF CAGL0I10384g): role in azole drug resistance and polyamine homeostasis. J. Antimicrob. Chemother. 2014, 69, 1767–1776. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Henriques, A.; Pires, C.; Nunes, J.; Ohno, M.; Chibana, H.; Sá-Correia, I.; Teixeira, M.C. The dual role of Candida glabrata drug:H+ antiporter CgAqr1 (ORF CAGL0J09944g) in antifungal drug and acetic acid resistance. Front. Microbiol. 2013, 4, 170. [Google Scholar] [CrossRef] [PubMed]
- Hull, C.M.; Parker, J.E.; Bader, O.; Weig, M.; Gross, U.; Warrilow, A.G.S.; Kelly, D.E.; Kelly, S.L. Facultative sterol uptake in an ergosterol-deficient clinical isolate of Candida glabrata harboring a missense mutation in ERG11 and exhibiting cross-resistance to azoles and amphotericin B. Antimicrob. Agents Chemother. 2012, 56, 4223–4232. [Google Scholar] [CrossRef] [PubMed]
- Brun, S.; Bergès, T.; Poupard, P.; Vauzelle-Moreau, C.; Renier, G.; Chabasse, D.; Bouchara, J.-P. Mechanisms of azole resistance in petite mutants of Candida glabrata. Antimicrob. Agents Chemother. 2004, 48, 1788–1796. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.; Ischer, F.; Calabrese, D.; Posteraro, B.; Sanguinetti, M.; Fadda, G.; Rohde, B.; Bauser, C.; Bader, O.; Sanglard, D. Gain of function mutations in CgPDR1 of Candida glabrata not only mediate antifungal resistance but also enhance virulence. PLoS Pathog. 2009, 5, e1000268. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, B.E.; Oltean, H.N.; Oliver, B.G.; Hoot, S.J.; Leyde, S.E.; Hedstrom, L.; White, T.C.; BE, M. Azole drugs are imported by facilitated diffusion in Candida albicans and other pathogenic fungi. PLoS Pathog. 2010, 6, e1001126. [Google Scholar] [CrossRef] [PubMed]
- Miranda, I.M.; Silva, R.M.; Estevinho, M.; Pina-vaz, C. Environmental azole fungicide, prochloraz, can induce cross-resistance to medical triazoles in Candida glabrata. FEMS Yeast Res. 2014, 1–5. [Google Scholar]
- Helmerhorst, E.; Venuleo, C.; Sanglard, D.; Oppenheim, F. Roles of cellular respiration, CgCDR1, and CgCDR2 in Candida glabrata resistance to histatin. Antimicrob. Agents Chemother. 2006, 1100–1103. [Google Scholar] [CrossRef] [PubMed]
- Edgerton, M.; Koshlukova, S. Salivary histatin 5 and its similarities to the other antimicrobial proteins in human saliva. Adv. Dent. Res. 2000, 14, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Helmerhorst, E.; Hancock, R.; Devine, D.; Oppenheim, F. The antifungal mechanisms of antimicrobial proteins. In Mammalian Antimicrobial Proteins; Hancock, R., Devine, D., Eds.; Cambridge University Press: Cambridge, UK, 2004; pp. 245–277. [Google Scholar]
- Oppenheim, F. Salivary histidine-rich proteins. In Human Saliva: Clinical Chemistry and Microbiology; Tenovuo, J., Ed.; CRC Press: Boca Raton, FL, USA, 1989; pp. 151–160. [Google Scholar]
- Oppenheim, F.; Xu, T.; McMillian, F.; Levitz, S.; Diamond, R.; Offner, G.; Troxler, R. Histatins, a novel family of histidinerich proteins in human parotid secretion. Isolation, characterization, primary structure, and fungistatic effects on Candida albicans. J. Biol. Chem. 1988, 263, 7472–7477. [Google Scholar] [PubMed]
- Tsai, H.; Bobek, L. Human salivary histatins: promising antifungal therapeutic agents. Crit. Rev. Oral Biol. Med. 1998, 9, 480–497. [Google Scholar] [CrossRef] [PubMed]
- van Urk, H.; Voll, W.; Scheffers, W.; van Dijken, J. Transient-state analysis of metabolic fluxes in Crabtree-positive and Crabtreenegative yeasts. Appl. Environ. Microbiol. 1990, 56, 281–287. [Google Scholar] [PubMed]
- Dalirsani, Z.; Adibpour, M.; Aghazadeh, M.; Amirchaghmaghi, M.; Falaki, F.; Mozafari, P.M.; Hamzei, F.M. In vitro comparison of inhibitory activity of 10 plant extracts against Candida albicans. Aust. J. Basic Appl. Sci. 2011, 5, 930–935. [Google Scholar]
- Junio, H.A.; Sy-Cordero, A.A.; Ettefagh, K.A.; Burns, J.T.; Micko, K.T.; Graf, T.N.; Richter, S.J.; Cannon, R.E.; Oberlies, N.H.; Cech, N.B. Synergy Directed Fractionation of Botanical Medicines: A Case Study with Goldenseal (Hydrastis canadensis). J. Nat. Prod. 2011, 74, 1621–1629. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.; Fernandes Júnior, A. Biological properties of medicinal plants: A review of their antimicrobial activity. J. Venom. Anim. Toxins Incl. Trop. Dis. 2010, 16, 402–413. [Google Scholar] [CrossRef]
- Martins, N.; Barros, L.; Santos-Buelga, C.; Henriques, M.; Silva, S.; Ferreira, I.C.F.R. Evaluation of bioactive properties and phenolic compounds in different extracts prepared from Salvia officinalis L. Food Chem. 2015, 170, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Dahanukar, S.A.; Kulkarni, R.A.; Rege, N.N. Pharmacology of medicinal plants and natural products. Indian J. Pharmacol. 2000, 32, S81–S118. [Google Scholar]
- Cowan, M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [PubMed]
- Sawamura, M. Aroma and functional properties of Japanese yuzu (Citrus junos Tanaka) essential oil. Aroma Res. 2000, 1, 14–19. [Google Scholar]
- Ormancey, X.; Sisalli, S.; Coutiere, P. Formulation of essential oils in functional parfumery. Parfum. Cosmet. Actual. 2001, 157, 30–40. [Google Scholar]
- Sharanappa, R.; Vidyasagar, G. Anti-Candida activity of medicinal plants. A review. Int. J. Pharm. Pharm. Sci. 2013, 5, 9–16. [Google Scholar]
- Djilani, A.; Dicko, A. The therapeutic benefits of essential oils. Nutrition, well-being and health. InTech 2012, 155–178. [Google Scholar]
- The Chemistry of Aromatherapeutic Oils, 3rd ed.; Bowles, J.E. (Ed.) Griffin Press: Salisbury, Australia, 2003.
- Gupta, V.; Mittal, P.; Bansal, P.; Khokra, S.L.; Kaushik, D. Pharmacological Potential of Matricaria recutita-A Review. Int. J. Pharm. Sci. Drug Res. 2010, 2, 12–16. [Google Scholar]
- Martín, Á.; Varona, S.; Navarrete, A.; Cocero, M.J. Encapsulation and co-precipitation processes with supercritical fluids: applications with essential oils. Open Chem. Eng. J. 2010, 4, 31–41. [Google Scholar] [CrossRef]
- Dorman, H.; Deans, S. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Aleksic, V.; Knezevic, P. Antimicrobial and antioxidative activity of extracts and essential oils of Myrtus communis L. Microbiol. Res. 2014, 169, 240–254. [Google Scholar] [CrossRef] [PubMed]
- Aidi Wannes, W.; Mhamdi, B.; Sriti, J.; Ben Jemia, M.; Ouchikh, O.; Hamdaoui, G.; Kchouk, M.E.; Marzouk, B.; Wannes, A. Antioxidant activities of the essential oils and methanol extracts from myrtle (Myrtus communis var. Italica L.) leaf, stem and flower. Food Chem. Toxicol. 2010, 48, 1362–1370. [Google Scholar] [CrossRef] [PubMed]
- Lapornik, B.; Prošek, M.; Wondra, A. Comparison of extracts prepared from plant by-products using different solvents and extraction time. J. Food Eng. 2005, 71, 214–222. [Google Scholar] [CrossRef]
- Pozzatti, P.; Scheid, L.; Spader, T.; Atayde, M.; Santurio, J.; Alves, S. In vitro activity of essential oils extracted from plants used as spices against fluconazole-resistant and fluconazole-susceptible Candida spp. Canadian. Can. J. Microbiol. 2008, 54, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Martins, N.; Ferreira, I.C.F.R.; Barros, L.; Carvalho, A.M.; Henriques, M.; Silva, S. Plants used in folk medicine: The potential of their hydromethanolic extracts against Candida species. Ind. Crops Prod. 2015, 66, 62–67. [Google Scholar] [CrossRef]
- Martins, N.; Ferreira, I.C.F.R.; Henriques, M.; Silva, S. In vitro anti-Candida activity of Glycyrrhiza glabra L. Ind. Crops Prod. 2016, 83, 81–85. [Google Scholar] [CrossRef]
- Prabhakar, K.; Kumar, L.S.; Rajendran, S.; Chandrasekaran, M.; Bhaskar, K.; Sajit Khan, A.K. Antifungal Activity of Plant Extracts against Candida Species from Oral Lesions. Indian J. Pharm. Sci. 2008, 70, 801–803. [Google Scholar] [CrossRef] [PubMed]
- Estevinho, L.; Afonso, S.; Feás, X. Antifungal effect of lavender honey against Candida albicans, Candida krusei and Cryptococcus neoformans. J. Food Sci. Technol. 2011, 48, 640–643. [Google Scholar] [CrossRef] [PubMed]
- Estevinho, L.; Pereira, A.P.; Moreira, L.; Dias, L.G.; Pereira, E. Antioxidant and antimicrobial effects of phenolic compounds extracts of Northeast Portugal honey. Food Chem. Toxicol. 2008, 46, 3774–3779. [Google Scholar] [CrossRef] [PubMed]
- Bardy, J.; Nicholas, S.; Kathleen, M.; Alexander, M. A systematic review of honey uses and its potential value within oncology care. J. Clin. Nurs. 2008, 17, 2604–2623. [Google Scholar] [CrossRef] [PubMed]
- Lusby, E.; Coombes, A.; Wilkinson, J. Bactericidal Activity of Different Honeys against Pathogenic Bacteria. Arch. Med. Res. 2005, 36, 464–467. [Google Scholar] [CrossRef] [PubMed]
- Molan, P. Why honey is effective as a medicine. Bee World 2001, 82, 22–40. [Google Scholar] [CrossRef]
- Eteraf-Oskouei, T.; Najafi, M. Traditional and Modern Uses of Natural Honey in Human Diseases: A Review. Iran. J. Basic Med. Sci. 2013, 16, 731–742. [Google Scholar] [PubMed]
- Theunissen, F.; Grobler, S.; Gedalia, I. The antifungal action of three South African honeys on Candida albicans. Apidologie 2001, 32, 371–379. [Google Scholar] [CrossRef]
- Irish, J.; Carter, D.; Shokohi, T.; Blair, S. Honey has an antifungal effect against Candida species. Med. Mycol. 2006, 44, 289–291. [Google Scholar] [CrossRef] [PubMed]
- Küçük, M.; Kolaylı, S.; Karaoğlu, Ş.; Ulusoy, E.; Baltacı, C.; Candan, F. Biological activities and chemical composition of three honeys of different types from Anatolia. Food Chem. 2007, 100, 526–534. [Google Scholar] [CrossRef]
- Boukraa, L.; Benbarek, H.; Moussa, A. Synergistic action of starch and honey against Candida albicans in correlation with diastase number. Brazilian J. Microbiol. 2008, 39, 40–43. [Google Scholar] [CrossRef]
- Koc, A.N.; Silici, S.; Ercal, B.D.; Kasap, F.; Hörmet-Öz, H.T.; Mavus-Buldu, H. Antifungal Activity of Turkish Honey against Candida spp. and Trichosporon spp: An in vitro evaluation. Med. Mycol. 2009, 47, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Quadri, M.; Huraib, S. Manuka honey for central vein catheter exit site care. Semin. Dial. 1999, 12, 396–399. [Google Scholar] [CrossRef]
- Johnson, D.W.; van Eps, C.; Mudge, D.W.; Wiggins, K.J.; Armstrong, K.; Hawley, C.M.; Campbell, S.B.; Isbel, N.M.; Nimmo, G.R.; Gibbs, H. Randomized, controlled trial of topical exit-site application of honey (medihoney) versus mupirocin for the prevention of catheter-associated infections in hemodialysis patients. J. Am. Soc. Nephrol. 2005, 16, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Freire, F.; Ferraresi, C.; Jorge, A.O.C.; Hamblin, M.R. Photodynamic therapy of oral Candida infection in a mouse model. J. Photochem. Photobiol. B Biol. 2016, 159, 161–168. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Mima, E.G.; Pavarina, A.C.; Dovigo, L.N.; Vergani, C.E.; de Souza Costa, C.A.; Kurachi, C.; Bagnato, V.S. Susceptibility of Candida albicans to photodynamic therapy in a murine model of oral candidosis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010, 109, 392–401. [Google Scholar] [CrossRef] [PubMed]
- de Souza, S.C.; Junqueira, J.C.; Balducci, I.; Koga-Ito, C.Y.; Munin, E.; Jorge, A.O.C. Photosensitization of different Candida species by low power laser light. J. Photochem. Photobiol. B Biol. 2006, 83, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.C.; Junqueira, J.C.; Rossoni, R.D.; Pereira, C.A.; Munin, E.; Jorge, A.O.C. Comparison of the photodynamic fungicidal efficacy of methylene blue, toluidine blue, malachite green and low-power laser irradiation alone against Candida albicans. Lasers Med. Sci. 2010, 25, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Martinez, L.R.; Nimrichter, L.; Nosanchuk, J.D.; Baltazar, L.M.; Ray, A.; Santos, D.A.; Cisalpino, P.S.; Friedman, A.J. Antimicrobial photodynamic therapy: an effective alternative approach to control fungal infections. Front. Microbiol. 2015, 6, 202. [Google Scholar]
- Giroldo, L.M.; Felipe, M.P.; de Oliveira, M.A.; Munin, E.; Alves, L.P.; Costa, M.S. Photodynamic antimicrobial chemotherapy (PACT) with methylene blue increases membrane permeability in Candida albicans. Lasers Med. Sci. 2009, 24, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Dovigo, L.N.; Pavarina, A.C.; de Oliveira Mima, E.G.; Giampaolo, E.T.; Vergani, C.E.; Bagnato, V.S. Fungicidal effect of photodynamic therapy against fluconazole-resistant Candida albicans and Candida glabrata. Mycoses 2011, 54, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Vartak, A.; Mutalik, V.; Parab, R.R.; Shanbhag, P.; Bhave, S.; Mishra, P.D.; Mahajan, G.B. Isolation of a new broad spectrum antifungal polyene from Streptomyces sp. MTCC 5680. Lett. Appl. Microbiol. 2014, 58, 591–596. [Google Scholar] [CrossRef] [PubMed]
- de Souza, T.B.; de Oliveira Brito, K.M.; Silva, N.C.; Rocha, R.P.; de Sousa, G.F.; Duarte, L.P.; Coelho, L.F.L.; Dias, A.L.T.; Veloso, M.P.; Carvalho, D.T.; et al. New eugenol glucoside-based derivative shows fungistatic and fungicidal activity against opportunistic Candida glabrata. Chem. Biol. Drug Des. 2015, 87, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Casanova, B.B.; Muniz, M.N.; Oliveira, T.; Oliveira, L.F.; Machado, M.M.; Fuentefria, A.M.; Gosmann, G.; Gnoatto, S.C.B. Synthesis and biological evaluation of some hydrazone derivatives as anti-inflammatory agents. Lett. Drug Des. Discov. 2012, 9, 310–315. [Google Scholar]
- Ferreira, B.D.S.; de Almeida, A.M.; Nascimento, T.C.; de Castro, P.P.; Silva, V.L.; Diniz, C.G.; Le Hyaric, M. Synthesis and biological evaluation of a new series of N-acyldiamines as potential antibacterial and antifungal agents. Bioorg. Med. Chem. Lett. 2014, 24, 4626–4629. [Google Scholar] [CrossRef] [PubMed]
- Abrão, P.H.O.; Pizi, R.B.; de Souza, T.B.; Silva, N.C.; Fregnan, A.M.; Silva, F.N.; Coelho, L.F.L.; Malaquias, L.C.C.; Dias, A.L.T.; Dias, D.F.; Veloso, M.P.; Carvalho, D.T. Synthesis and biological evaluation of new eugenol mannich bases as promising antifungal agents. Chem. Biol. Drug Des. 2015, 86, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Raman, N.; Lee, M.-R.; Lynn, D.; Palecek, S. Antifungal Activity of 14-Helical β-peptides against planktonic cells and biofilms of Candida species. Pharmaceuticals 2015, 8, 483–503. [Google Scholar] [CrossRef] [PubMed]
- Silva-Dias, A.; Miranda, I.M.; Branco, J.; Cobrado, L.; Monteiro-Soares, M.; Pina-Vaz, C.; Rodrigues, A.G. In vitro antifungal activity and in vivo antibiofilm activity of cerium nitrate against Candida species. J. Antimicrob. Chemother. 2014, 70, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Joseph, M.R.P.; Al-Hakami, A.M.; Assiry, M.M.; Jamil, A.S.; Assiry, A. M.; Shaker, M.A.; Hamid, M.E. In vitro anti-yeast activity of chloramphenicol: A preliminary report. J. Med. Mycol. 2015, 25, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.; Ling, J.; Wu, C.D. Synergistic effects of tea catechin epigallocatechin gallate and antimycotics against oral Candida species. Arch. Oral Biol. 2015, 60, 1565–1570. [Google Scholar] [CrossRef] [PubMed]
- Perera, J.; Weerasekera, M.; Kottegoda, N. Slow release anti-fungal skin formulations based on citric acid intercalated layered double hydroxides nanohybrids. Chem. Cent. J. 2015, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Pires, P.; Monteiro, D.R.; Negri, M.; Gorup, L.F.; Camargo, E.R.; Barbosa, D.B.; Oliveira, R.; Williams, D.W.; Henriques, M.; Azeredo, J. The effect of silver nanoparticles and nystatin on mixed biofilms of Candida glabrata and Candida albicans on acrylic. Med. Mycol. 2013, 51, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Casella, T.M.; Eparvier, V.; Mandavid, H.; Bendelac, A.; Odonne, G.; Dayan, L.; Duplais, C.; Espindola, L.S.; Stien, D. Antimicrobial and cytotoxic secondary metabolites from tropical leaf endophytes: Isolation of antibacterial agent pyrrocidine C from Lewia infectoria SNB-GTC2402. Phytochemistry 2013, 96, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Nirma, C.; Eparvier, V.; Stien, D. Antifungal Agents from Pseudallescheria boydii SNB-CN73 Isolated from a Nasutitermes sp. Termite. J. Nat. Prod. 2013, 76, 988–991. [Google Scholar] [CrossRef] [PubMed]
- Nirma, C.; Eparvier, V.; Stien, D. Antibacterial ilicicolinic acids c and d and ilicicolinal from neonectria discophora SNB-CN63 isolated from a termite nest. J. Nat. Prod. 2015, 78, 159–162. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).