Identification and Biocontrol of Fusarium oxysporum Affecting Lucky Bamboo (Dracaena sanderiana Hort. ex. Mast.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Bioagent Bacteria, and Plants Used in the Study
2.2. Isolation of Pathogenic Fungi
2.3. Pathogenicity and Virulence Tests
2.4. Identification of Pathogenic Fungal Isolates
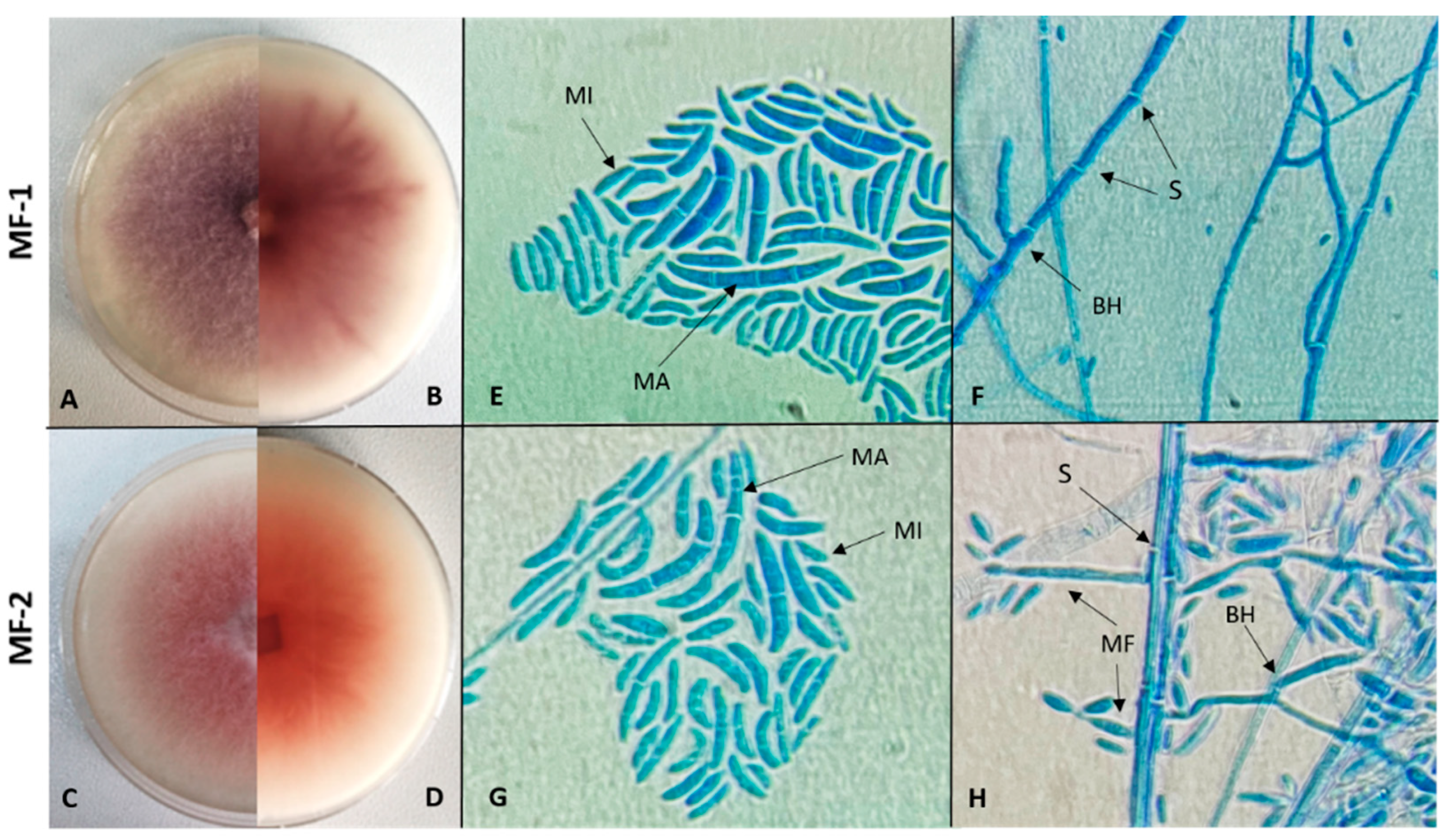
2.4.1. Macroscopic and Microscopic Identification of Pathogenic Fungal Isolates
2.4.2. Molecular Identification of Pathogenic Fungal Isolates
2.5. Purification of Chitinase Enzyme from Bacteria
2.6. Determining Chitinase Enzyme Activity
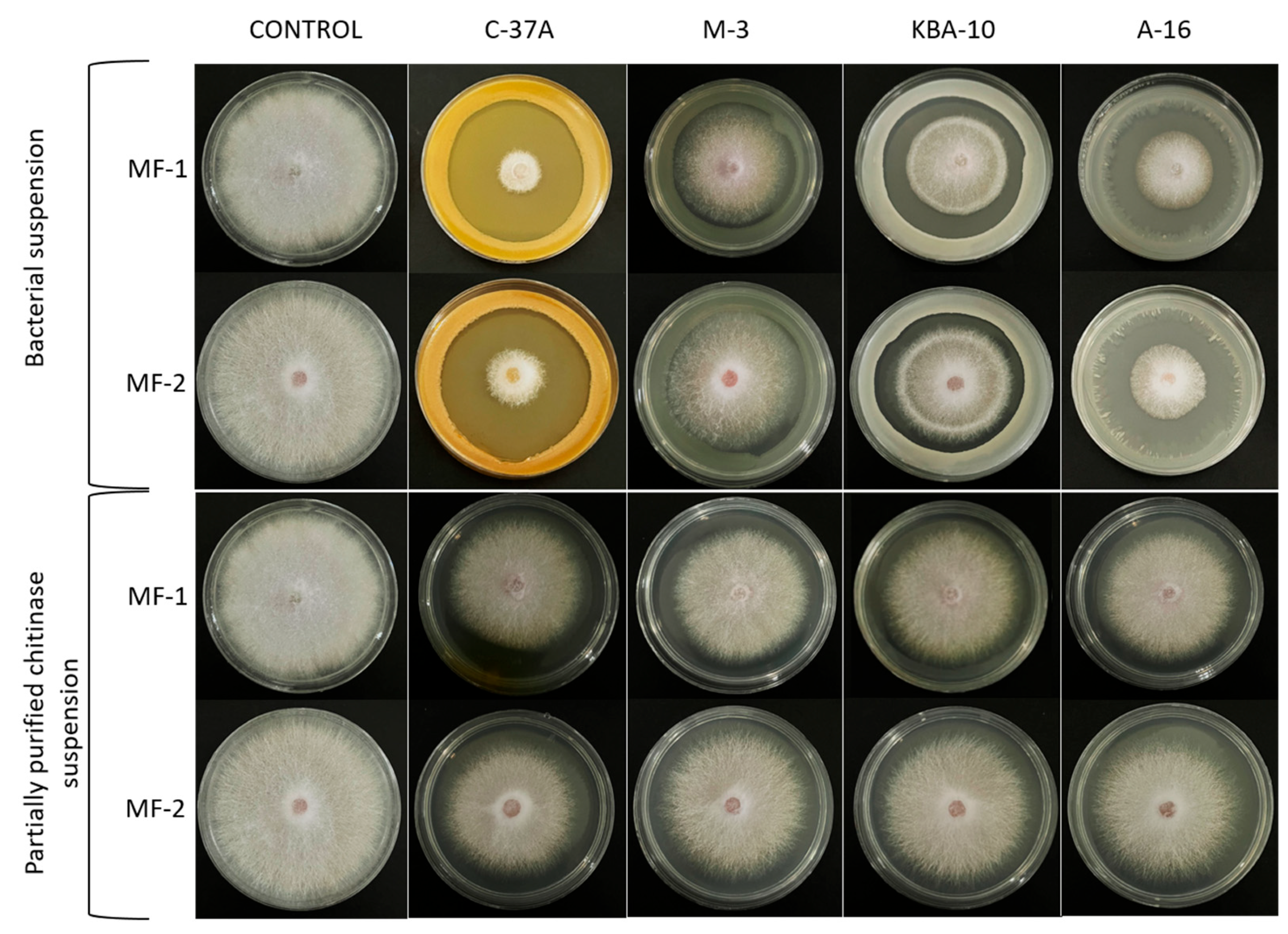
2.7. Determination of the In Vitro Antifungal Potential of Bacterial Isolates and Their Partially Purified Chitinase Enzymes
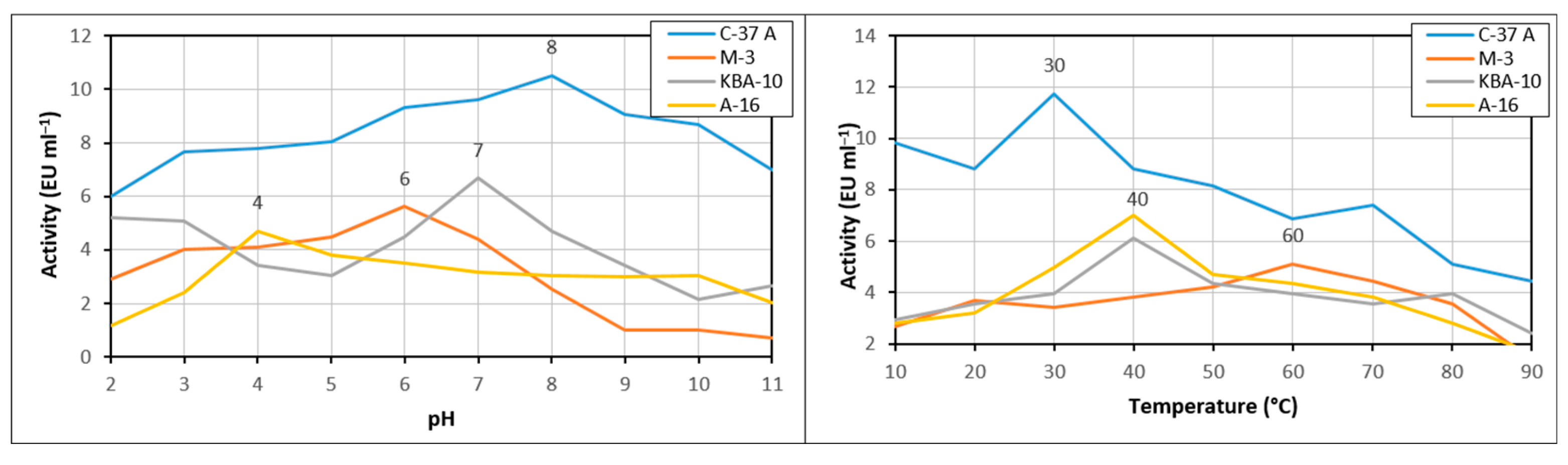
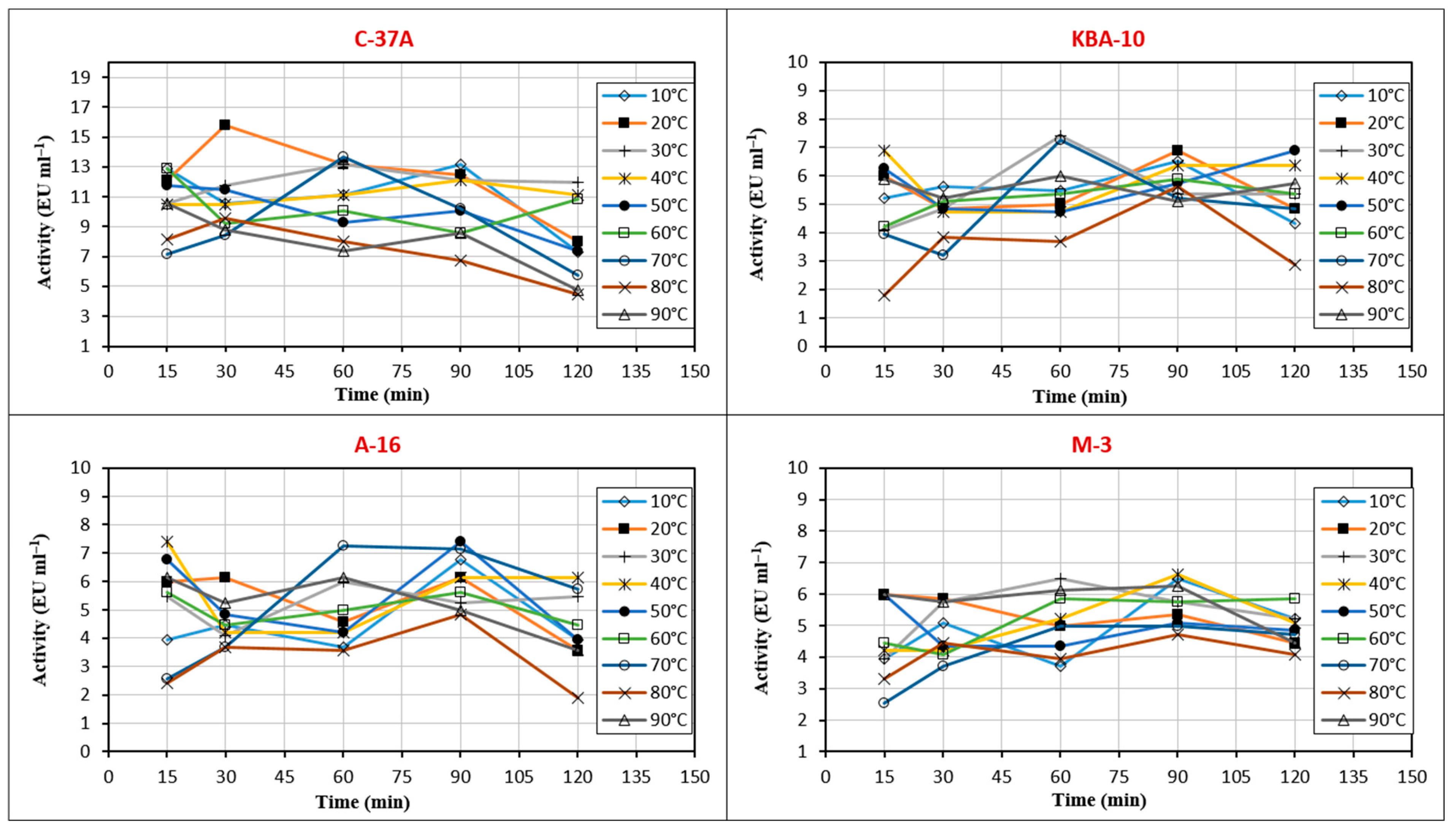
2.8. Characterization of Optimum and Stable Temperature and pH Conditions of the Chitinase Enzyme
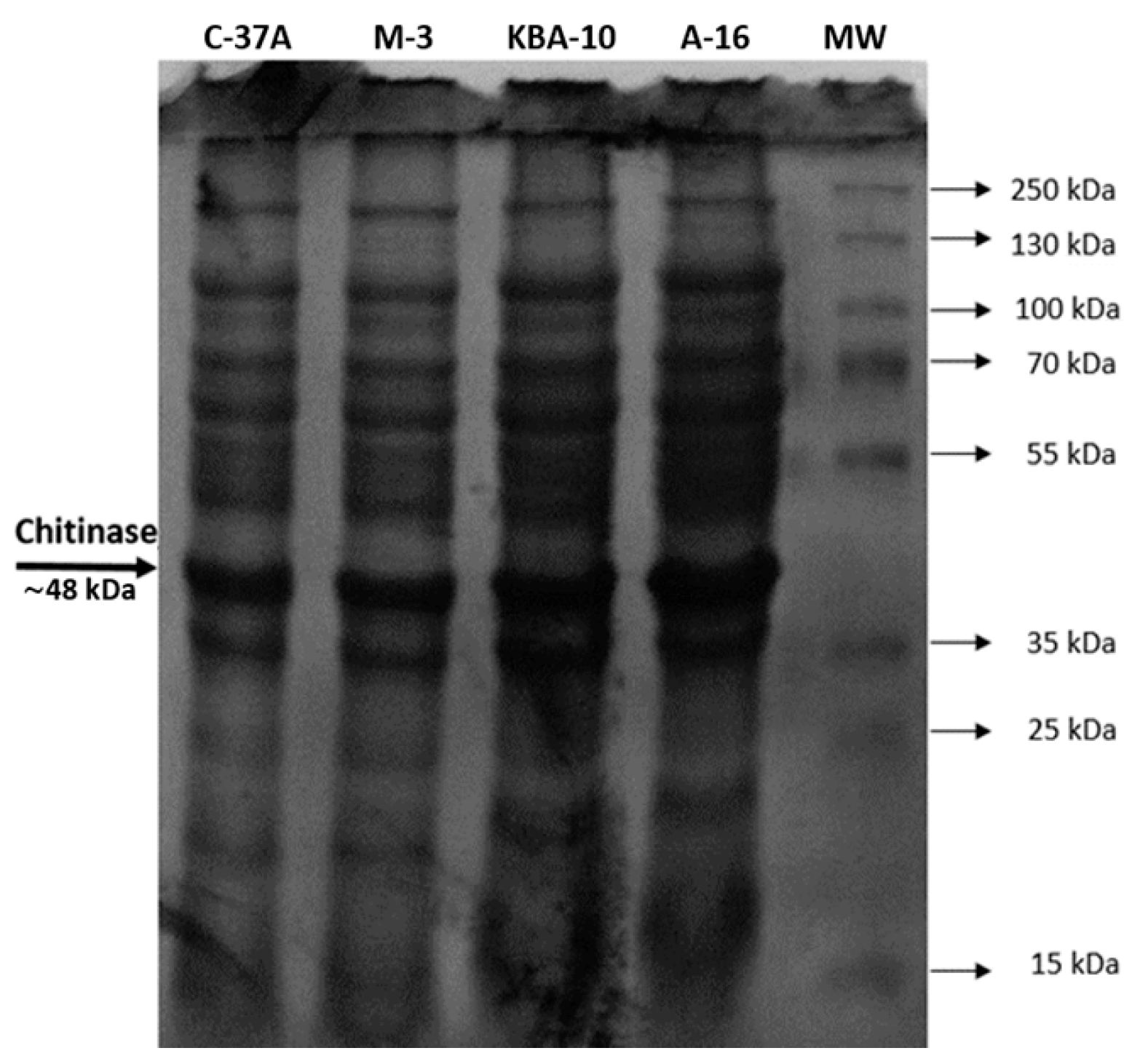
2.9. SDS-PAGE Electrophoresis
2.10. Protein Determination
2.11. Statistical Analysis
3. Results
3.1. Isolation and Identification of Fusarium Isolates
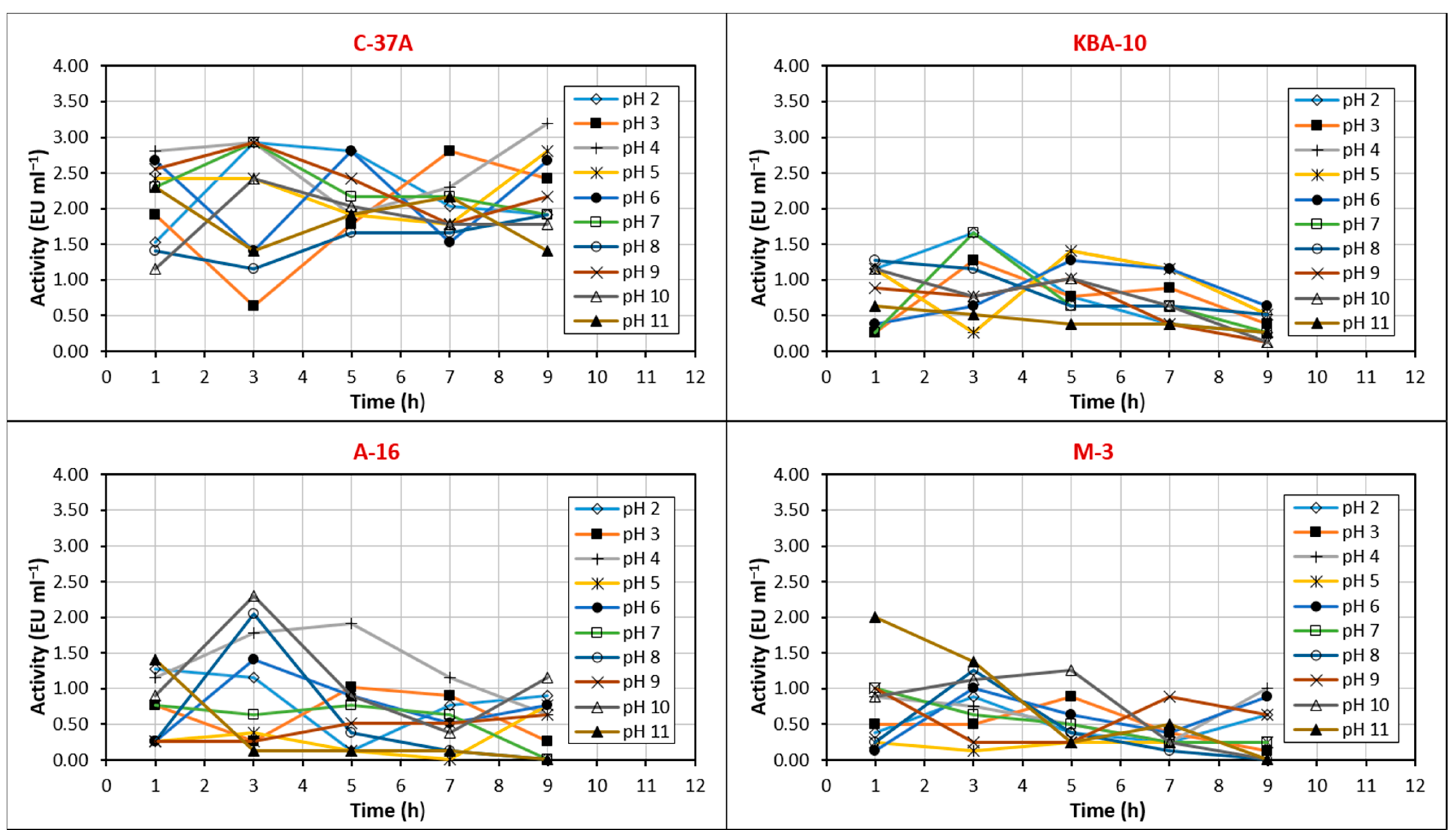
3.2. Partial Purification and Activity Profile of Chitinase Enzyme
3.3. Optimum pH and Temperature Values of the Chitinase Enzyme
3.4. Stable Temperature and pH Values of the Chitinase Enzyme
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hugh, T.W.; Tan, X. Plant Magic: Auspicious and Inauspicious Plants from Around the World; Marshall Cavendish Editions: Singapore, 2008; p. 62. [Google Scholar]
- Grewal, H.S.; Kaur, R.; Arora, J.S. Effect of growth regulators on shoot and root formation in Dracaena. Indian J. Hortic. 1999, 56, 375–381. [Google Scholar]
- Damen, T.H.; Van der Burg, W.J.; Wiland-Szymańska, J.; Sosef, M.S.M. Taxonomic novelties in African Dracaena (Dracaenaceae). Blumea-Biodivers. Evol. Biogeogr. Plants 2018, 63, 31–53. [Google Scholar] [CrossRef]
- Lahuf, A.A. Alternaria alternata causes leaf blight of rosy periwinkle (Catharanthus roseus) in Iraq. Australas. Plant Dis. Notes 2019, 14, 4. [Google Scholar] [CrossRef]
- Abdel-Rahman, T.F.; El-Morsy, S.A.; Halawa, A.E.A. Occurrence of stem and leaf spots on lucky bamboo (Dracaena sanderiana hort. ex. mast.) plants in vase and its cure with safe means. J. Plant Prot. Pathol. 2020, 11, 705–713. [Google Scholar] [CrossRef]
- Hilal, A.; El-Argawy, E.; Korany, A.E.; Fekry, T. Chemical and Biological Control of Dracaena marginata Leaf Spots in Northern Egypt. Int. J. Agric. Biol. 2016, 18, 1201–1212. [Google Scholar] [CrossRef]
- Abdelhameed, R.M.; Darwesh, O.M.; El-Shahat, M. Synthesis of arylidene hydrazinylpyrido [2, 3-d] pyrimidin-4-ones as potent anti-microbial agents. Heliyon 2020, 6, e04956. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Zhang, Y.; Wang, X.; Qian, Y.; Wu, W.; Li, J.; Zeng, Q. Occurrence of Anthracnose Caused by Colletotrichum gloeosporioides on Lucky Bamboo in China. Preprints 2023, 2023091217. [Google Scholar] [CrossRef]
- Abedi-Tizaki, M.; Zafari, D.; Sadeghi, J. First report of Fusarium solani causing stem rot of Dracaena in Iran. J. Plant Prot. Res. 2016, 56, 100–103. [Google Scholar] [CrossRef]
- Elshahawy, I.E.; Darwesh, O.M. Preventive and curative effect of difenoconazole+ azoxytrobin and thiophanate-methyl against lucky bamboo anthracnose disease caused by Colletotrichum dracaenophilum. Heliyon 2023, 9, e14444. [Google Scholar] [CrossRef]
- Abdel-Rahman, T.F.; Abdel-Megeed, A.; Salem, M.Z. Characterization and control of Rhizoctonia solani affecting lucky bamboo (Dracaena sanderiana hort. ex. Mast.) using some bioagents. Sci. Rep. 2023, 13, 6691. [Google Scholar] [CrossRef]
- Lecomte, C.; Alabouvette, C.; Edel-Hermann, V.; Robert, F.; Steinberg, C. Biological control of ornamental plant diseases caused by Fusarium oxysporum: A review. Biol. Control 2016, 101, 17–30. [Google Scholar] [CrossRef]
- Dikbas, N.; Dadasoglu, F.; Kotan, R.; Cakir, A. Influence of summer savory essential oil (Satureja hortensis) on decay of strawberry and grape. J. Essent. Oil Bear. Plants 2011, 14, 151–160. [Google Scholar] [CrossRef]
- Mohammadi, P.; Kotan, R. Biberde bakteriyel leke hastalığının etmeni Xanthomonas axonopodis pv. vesicatoria’nın kontrolünde kullanılabilecek ve bitki gelişimi üzerine de etkili olan bakteriyel biyopestisitin geliştirilmesi. In Proceedings of the Türkiye V. Bitki Koruma Kongresi, Antalya, Türkiye, 3–5 February 2014. [Google Scholar]
- Şenol Kotan, M.; Dikbaş, N.; Kotan, R. Solid carrier bacterial formulations against Fusarium root and stem rot disease in cucumber. J. Plant Pathol. 2024, 106, 153–163. [Google Scholar] [CrossRef]
- Senol, M.; Nadaroglu, H.; Dikbas, N.; Kotan, R. Purification of Chitinase enzymes from Bacillus subtilis bacteria TV-125, investigation of kinetic properties and antifungal activity against Fusarium culmorum. Ann. Clin. Microbiol. Antimicrob. 2014, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Anand, G.; Yadav, D.; Bar, M. Microbial Chitinases: Potential Applications in Agriculture. Microbial Enzymes: Production, Purif. Ind. Appl. 2025, 1, 421–436. [Google Scholar] [CrossRef]
- Tozlu, E.; Demirci, E. Incidence and characterization of sunflower stem rot disease caused by Sclerotinia sclerotiorum and S. minor in Pasinler Plain of Erzurum, and reaction of some sunflower cultivars to the pathogens. Plant Prot. Bull. 2008, 48, 19–33. [Google Scholar]
- Aung, S.L.L.; Liu, H.F.; Pei, D.F.; Lu, B.B.; Oo, M.M.; Deng, J.X. Morphology and molecular characterization of a fungus from the Alternaria alternata species complex causing black spots on Pyrus sinkiangensis (Koerle pear). Mycobiology 2020, 48, 233–239. [Google Scholar] [CrossRef]
- Leck, A. Preparation of lactophenol cotton blue slide mounts. Community Eye Health 1999, 12, 24. [Google Scholar]
- Kim, K.W.; Park, E.W. Optical clearing of apple tissues for in vivo imaging of the pathogenic behavior of the fungus Botryosphaeria dothidea on host surfaces. Appl. Microsc. 2025, 55, 4. [Google Scholar] [CrossRef]
- Uysal, A.; Kurt, Ş.; Guarnaccia, V. Distribution and characterization of Colletotrichum species associated with Citrus anthracnose in eastern Mediterranean region of Turkey. Eur. J. Plant Pathol. 2022, 163, 125–141. [Google Scholar] [CrossRef]
- Vigneshwaran, K.; Rajamohan, K.; Balabaskar, P.; Udhyakumar, R.; Sivasakthivelan, P. Molecular, Morphological identification, and Virulence profiling of Fusarium oxysporum f. sp. lycopersici (Sacc.) (WC Snyder & HN Hansen) associated with Root-knot Nematode inciting Fusarium wilt of tomato. Physiol. Mol. Plant Pathol. 2025, 138, 102741. [Google Scholar] [CrossRef]
- Dikbaş, N.; Uçar, S.; Tozlu, E.; Kotan, M.Ş.; Kotan, R. Antifungal activity of partially purified bacterial chitinase against Alternaria alternata. Erwerbs-Obstbau 2023, 65, 761–766. [Google Scholar] [CrossRef]
- Mohammadi, P.; Tozlu, E.; Kotan, R.; Kotan, M.Ş. Potential of Some Bacteria for Biological Control of Postharvest Citrus Green Mould Caused by Penicillium digitatum. Plant Prot. Sci. 2017, 53, 134–143. [Google Scholar] [CrossRef]
- Kotan, R.; Sahin, F.; Demirci, E.; Eken, C. Biological control of the potato dry rot caused by Fusarium species using PGPR strains. Biol. Control 2009, 50, 194–198. [Google Scholar] [CrossRef]
- Saribuga, E.; Dikbaş, N.; Nadaroğlu, H.; Senol, M. Purification. characterization of phytase enzyme from Lactobacillus acidophilus bacteria and determination of it’s some kinetic properties. J. Pure Appl. Microbiol. 2014, 8, 2373–2378. [Google Scholar] [CrossRef]
- Dikbaş, N.; Alım, Ş.; Uçar, S.; Şenol Kotan, M. Biochemical properties of phytase immobilized and its effect on growth parameters of tomato. J. Plant Nutr. Soil Sci. 2024, 187, 533–544. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Demir, Y.; Beydemir, Ş. Purification, refolding, and characterization of recombinant human paraoxonase-1. Turk. J. Chem. 2015, 39, 764–776. [Google Scholar] [CrossRef]
- Bradford, M.M.A. Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–251. [Google Scholar] [CrossRef]
- Karagöz, F.P.; Demir, Y.; Kotan, M.Ş.; Dursun, A.; Beydemir, Ş.; Dikbaş, N. Purification of the phytase enzyme from Lactobacillus plantarum: The effect on pansy growth and macro–micro element content. Biotechnol. Appl. Biochem. 2021, 68, 1067–1075. [Google Scholar] [CrossRef]
- Bald, J.G.; Suzukı, T.; Doyle, A. Pathogenicity of Fusarium oxysporum to Easter lily, Narcissus and Gladiolus. Ann. Appl. Biol. 1971, 67, 331–342. [Google Scholar] [CrossRef]
- Gullino, M.L.; Katan, J.; Garibaldi, A. Fusarium Wilts of Greenhouse Vegetable and Ornamental Crops; American Phytopathological Society (APS Press): St. Paul, MN, USA, 2017; pp. 5–9. [Google Scholar] [CrossRef]
- Booth, C. Fusarium. Laboratory Guide to the Identification of the Major Species; Commonwealth Mycological Institute: London, UK, 1977; 58p. [Google Scholar]
- Teixeira, L.M.; Coelho, L.; Tebaldi, N.D. Characterization of Fusarium oxysporum isolates and resistance of passion fruit genotypes to fusariosis. Rev. Bras. De Frutic. 2017, 39, e-415. [Google Scholar] [CrossRef]
- Gargouri Jbir, T.; Zitnick-Anderson, K.; Pasche, J.S.; Kalil, A.K. Characterization of Fusarium oxysporum f. sp. pisi Associated with Root Rot of Field Pea in North Dakota and the Effects of Temperature on Aggressiveness. Plant Dis. 2024, 108, 365–374. [Google Scholar] [CrossRef]
- Stoeva, D.; Gencheva, D.; Yordanova, R.; Beev, G. Molecular Marker-Based Identification and Genetic Diversity Evaluation of Fusarium spp.—A Review. Acta Microbiol. Bulg. 2023, 39, 376–385. [Google Scholar] [CrossRef]
- Doan, C.T.; Tran, T.N.; Wang, S.L. Production of thermophilic chitinase by Paenibacillus sp. TKU052 by bioprocessing of chitinous fishery wastes and its application in N-acetyl-D-glucosamine production. Polymers 2021, 13, 3048. [Google Scholar] [CrossRef]
- Aktas, C.; Ruzgar, D.; Gurkok, S.; Gormez, A. Purification and characterization of Stenotrophomonas maltophilia chitinase with antifungal and insecticidal properties. Prep. Biochem. Biotechnol. 2023, 53, 797–806. [Google Scholar] [CrossRef]
- Hamid, R.; Khan, M.A.; Ahmad, M.; Ahmad, M.M.; Abdin, M.Z.; Musarrat, J.; Javed, S. Chitinases: An update. J. Pharm. Bioallied Sci. 2013, 5, 21–29. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Nagpure, A.; Gupta, R.K. Bacterial chitinases: Properties and potential. Critical reviews in biotechnology. Crit. Rev. Biotechnol. 2007, 27, 21–28. [Google Scholar] [CrossRef]
- Dikbaş, N.; Uçar, S.; Tozlu, G.; Öznülüer Özer, T.; Kotan, R. Bacterial chitinase biochemical properties, immobilization on zinc oxide (ZnO) nanoparticle and its effect on Sitophilus zeamais as a potential insecticide. World J. Microbiol. Biotechnol. 2021, 37, 173. [Google Scholar] [CrossRef]
- Chaiharn, M.; Pikomol, A.; Lumyoung, S. Purification and characterization of an extracellular chitinase from Bacillus thuringiensis R 176 using solid state fermentation with shrimp shells waste. Chiang Mai J. Sci. 2019, 46, 495–509. [Google Scholar]
- Malik, M.S.; Rehman, A.; Khan, I.U.; Khan, T.A.; Jamil, M.; Rha, E.S.; Anees, M. Thermo-neutrophilic cellulases and chitinases characterized from a novel putative antifungal biocontrol agent: Bacillus subtilis TD11. PLoS ONE 2023, 18, e0281102. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xie, J.; Wang, S.; Feng, H.; Wang, G. Thermostability Improvement of the Chitinase from Bacillus circulans for Efficient Chitin Oligosaccharide Production via Computational Design. Biomolecules 2025, 15, 330. [Google Scholar] [CrossRef] [PubMed]
- Cheba, B.A.; Zaghloul, T.I.; EL-Mahdy, A.R.; EL-Massry, M.H. Effect of pH and temperature on Bacillus sp. R2 chitinase activity and stability. Procedia Technol. 2016, 22, 471–477. [Google Scholar] [CrossRef]
- Du, J.; Duan, S.; Miao, J.; Zhai, M.; Cao, Y. Purification and characterization of chitinase from Paenibacillus sp. Biotechnol. Appl. Biochem. 2021, 68, 30–40. [Google Scholar] [CrossRef]
- Akeed, Y.; Atrash, F.; Naffaa, W. Partial purification and characterization of chitinase produced by Bacillus licheniformis B307. Heliyon 2020, 6, e03858. [Google Scholar] [CrossRef]
- Almeida, L.S.; Inoue, S.K.G.; Marques, J.M.; da Silva, J.K.R. A review of antifungal activity of bacterial strains and their secondary metabolites against Fusarium species. Rev. Environ. Sci. Bio/Technol. 2025, 1–38. [Google Scholar] [CrossRef]
- Yan, L.I.U.; Jing, T.A.O.; Yujun, Y.A.N.; Bin, L.I.; Hui, L.I.; Chun, L.I. Biocontrol efficiency of Bacillus subtilis SL-13 and characterization of an antifungal chitinase. Chin. J. Chem. Eng. 2011, 19, 128–134. [Google Scholar] [CrossRef]
- Pleban, S.; Chernin, L.; Chet, I. Chitinolytic activity of an endophytic strain of Bacillus cereus. Lett. Appl. Microbiol. 1997, 25, 284–288. [Google Scholar] [CrossRef]
- Shali, A.; Ghasemi, S.; Ahmadian, G.; Ranjbar, G.; Dehestani, A.; Khalesi, N.; Vahed, M. Bacillus pumilus SG2 chitinases induced and regulated by chitin, show inhibitory activity against Fusarium graminearum and Bipolaris sorokiniana. Phytoparasitica 2010, 38, 141–147. [Google Scholar] [CrossRef]

| Application | Virulence Score (0–4) | Disease Severity (%) |
|---|---|---|
| MF-1 | 3.8 ± 0.4 | 90 ± 12.2 |
| MF-2 | 1.6 ± 0.5 | 40 ± 10.0 |
| Control | 0 ± 0 | 0 ± 0 |
| Bacteria | Purification Step | Volume (mL) | Activity (EU/mL) | Protein (mg/mL) | Total Activity (EU/mL) | Total Protein (mg) | Specific Activity (EU/mg) | Recovery % | Purification Fold |
|---|---|---|---|---|---|---|---|---|---|
| Crude Extract | 40 | 10.83 | 4.53 | 433.20 | 181.40 | 2.39 | 100 | 1 | |
| C-37A | Ammonium Sulfate Precipitation (40–60%) | 40 | 9.44 | 3.87 | 377.60 | 154.8 | 2.43 | 87.16 | 1.01 |
| Crude Extract | 40 | 2.55 | 1.87 | 102 | 74.80 | 1.36 | 100 | 1 | |
| M-3 | Ammonium Sulfate Precipitation (60–80%) | 40 | 0.50 | 0.41 | 20 | 16.40 | 1.22 | 19.61 | 0.90 |
| Crude Extract | 40 | 5.10 | 2.85 | 204 | 114 | 1.78 | 100 | 1 | |
| KBA-10 | Ammonium Sulfate Precipitation (0–20%) | 40 | 0.51 | 0.35 | 20.40 | 14 | 1.46 | 10 | 0.82 |
| Crude Extract | 40 | 2.81 | 1.06 | 44.96 | 42.40 | 1.06 | 100 | 1 | |
| A-16 | Ammonium Sulfate Precipitation (60–80%) | 40 | 1.02 | 0.39 | 40.8 | 15.39 | 2.65 | 90.74 | 2.5 |
| Bacteria | MF-1 | MF-2 | |||
|---|---|---|---|---|---|
| FDGD (mm) | PIR (%) | FDGD (mm) | PIR (%) | ||
| Bacterial isolates | C-37A | 14.2 ± 0.2 a | 83.3 ± 0.5 a | 18.0 ± 0.7 a | 75.5 ± 1.4 a |
| M-3 | 40.5 ± 1.1 d | 29.3 ± 2.2 d | 40.2 ± 0.2 d | 30.3 ± 0.5 d | |
| KBA-10 | 32.2 ± 0.6 c | 46.4 ± 1.3 c | 36.3 ± 1.0 c | 38.1 ± 2.1 c | |
| A-16 | 21.5 ± 0.4 b | 68.3 ± 0.8 b | 25.3 ± 1.3 b | 60.5 ±2.6 b | |
| Control | 54.8 ± 0.2 e | 0.0 ± 0.5 e | 55.0 ± 0.0 e | 0.0 ± 0.0 e | |
| F-Values * | 1390.8 | 1384.1 | 640.2 | 638.0 | |
| Partial purification enzyme solution | C-37A | 41.2 ± 2.0 a | 28.0 ± 4.2 a | 43.7 ± 1.0 a | 23.1 ± 2.1 a |
| M-3 | 42.8 ± 0.2 ab | 24.6 ± 0.5 ab | 44.3 ± 1.3 a | 21.7 ± 2.6 a | |
| KBA-10 | 42.8 ± 0.5 ab | 24.6 ± 1.0 ab | 44.7 ± 0.5 a | 21.1 ± 0.9 a | |
| A-16 | 44.0 ± 0.4 b | 22.2 ± 0.8 b | 44.0 ± 0.8 a | 22.5 ± 1.7 a | |
| Control | 54.8 ± 0.2 c | 0.0 ± 0.5 c | 55.0 ± 0.0 b | 0.0 ± 0.0 b | |
| F-Values * | 64.4 | 64.6 | 67.5 | 67.8 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Şenol Kotan, M. Identification and Biocontrol of Fusarium oxysporum Affecting Lucky Bamboo (Dracaena sanderiana Hort. ex. Mast.). J. Fungi 2025, 11, 655. https://doi.org/10.3390/jof11090655
Şenol Kotan M. Identification and Biocontrol of Fusarium oxysporum Affecting Lucky Bamboo (Dracaena sanderiana Hort. ex. Mast.). Journal of Fungi. 2025; 11(9):655. https://doi.org/10.3390/jof11090655
Chicago/Turabian StyleŞenol Kotan, Merve. 2025. "Identification and Biocontrol of Fusarium oxysporum Affecting Lucky Bamboo (Dracaena sanderiana Hort. ex. Mast.)" Journal of Fungi 11, no. 9: 655. https://doi.org/10.3390/jof11090655
APA StyleŞenol Kotan, M. (2025). Identification and Biocontrol of Fusarium oxysporum Affecting Lucky Bamboo (Dracaena sanderiana Hort. ex. Mast.). Journal of Fungi, 11(9), 655. https://doi.org/10.3390/jof11090655






