The Evolving Threat of Fusarium Wilt TR4 to Small-Scale Mixed Cultivar Banana Production in the Red River Basin of Northern Vietnam
Abstract
1. Introduction
2. Materials and Methods
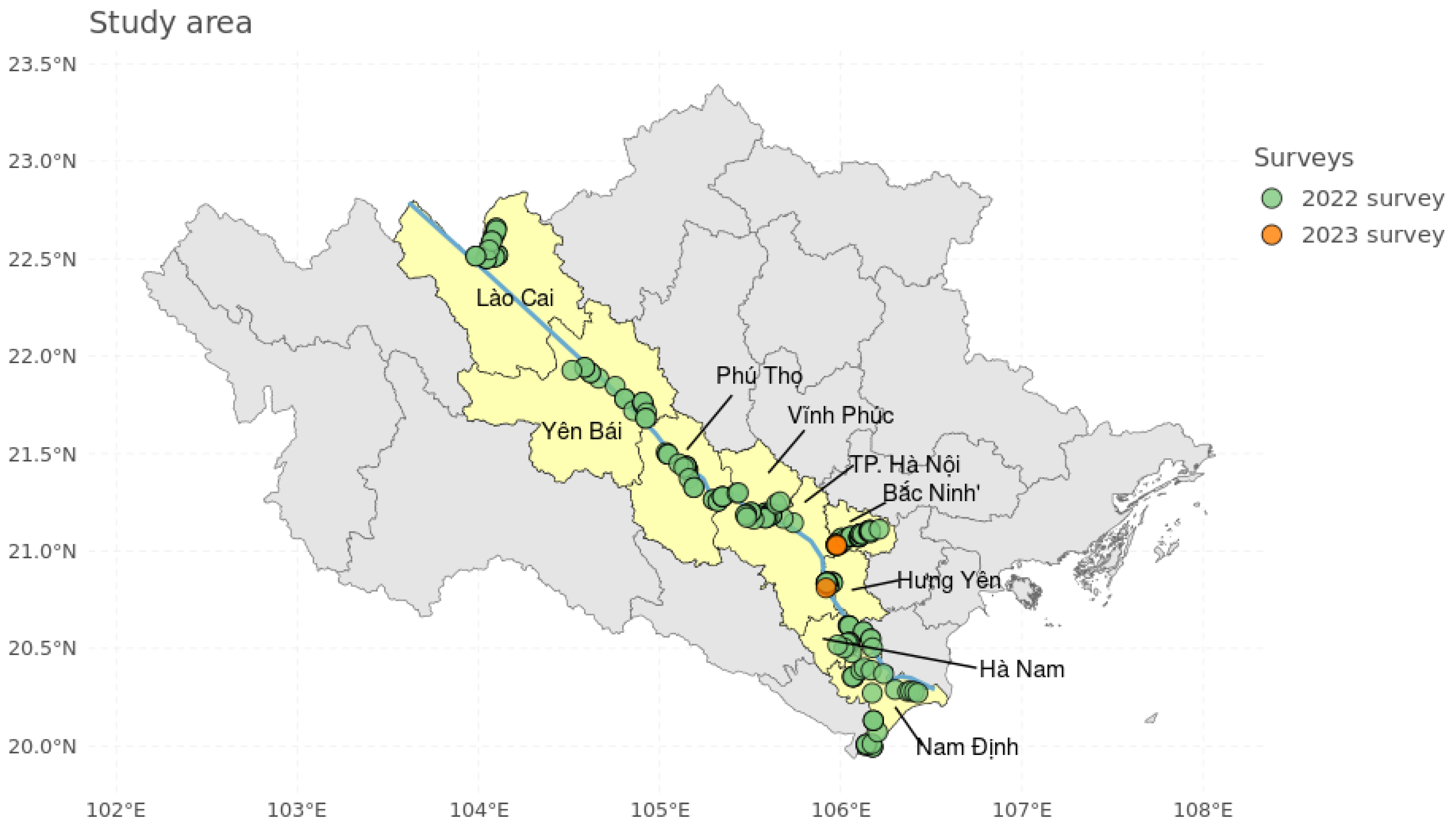
2.1. Field Surveys
2.2. Plant Sample Analysis
2.3. Screenhouse-Based Screening Trial
3. Data Analysis
4. Results
4.1. Field Survey
4.2. Screening Trial
5. Discussion
6. Conclusions
7. Future Perspectives and Challenges
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buddenhagen, I.W. Understanding strain diversity in Fusarium oxysporum f.sp. cubense and history of introduction of ‘tropical race 4’ to better manage banana production. Acta Hortic. 2009, 828, 193–204. [Google Scholar] [CrossRef]
- Ploetz, R.C. Management of Fusarium wilt of banana: A review with special reference to 105 tropical race 4. Crop Prot. 2015, 73, 7–15. [Google Scholar] [CrossRef]
- Siamak, S.B.; Zheng, S. Banana Fusarium wilt (Fusarium oxysporum f. sp. cubense) control and resistance, in the context of developing wilt-resistant bananas within sustainable production systems. Hortic. Plant J. 2018, 4, 208–218. [Google Scholar] [CrossRef]
- Bai, T.T.; Xu, S.; Rupp, F.; Fan, H.; Yin, K.; Guo, Z.; Zhang, L.; Yang, B.; Huang, Y.; Li, Y.; et al. Temporal variations of Fusarium oxysporum f. sp. cubense tropical race 4 population in a heavily infected banana field in Southwest China. Acta Agric. Scand. 2019, 69, 641–648. [Google Scholar] [CrossRef]
- Viljoen, A.; Ma, L.-J.; Molina, A.B. Fusarium wilt (Panama disease) and monoculture in banana production: Resurgence of a century-old disease. In Emerging Plant Diseases and Global Food Security; Ristaino, J.B., Records, A., Eds.; American Phytopathological Society: St. Paul, MN, USA, 2020; pp. 159–184. [Google Scholar]
- Dita, M.A.; Barquero, M.; Heck, D.; Mizubuti, E.S.; Staver, C.P. Fusarium wilt of banana: Current knowledge and epidemiology on research needs toward sustainable disease management. Front. Plant Sci. 2018, 9, 1468. [Google Scholar] [CrossRef]
- Zheng, S.-J.; García-Bastidas, F.A.; Li, X.; Zeng, L.; Bai, T.; Xu, S.; Yin, K.; Li, H.; Fu, G.; Yu, Y.; et al. New geographical insights of the latest expansion of Fusarium oxysporum f. sp. cubense tropical race 4 into the greater Mekong subregion. Front. Plant Sci. 2018, 9, 457. [Google Scholar] [CrossRef]
- García-Bastidas, F.; Ordóñez, N.; Konkol, J.; Al-Qasim, M.; Naser, Z.; Abdelwali, M.; Salem, N.; Waalwijk, C.; Ploetz, R.C.; Kema, G.H.J. First report of Fusarium oxysporum f. sp. cubense 86 tropical race 4 associated with Panama disease of banana outside Southeast Asia. Plant Dis. 2014, 98, 694. [Google Scholar] [CrossRef]
- O’Neill, W.T.; Henderson, J.; Pattemore, J.A.; O’Dwyer, C.; Perry, S.; Beasley, D.R.; Tan, Y.P.; Smyth, A.L.; Goosem, C.H.; Thomson, K.M.; et al. Detection of Fusarium oxysporum f. sp. cubense tropical race 4 strain in northern Queensland. Australas. Plant Dis. Notes 2016, 11, 33. [Google Scholar] [CrossRef]
- Dita, M.A.; Teixeira, L.A.J.; O’Neill, W.; Pattison, A.B.; Weinert, M.P.; Li, C.Y.; Zheng, S.J.; Staver, C.; Thangavelu, R.; Viljoen, A. Current state of Fusarium wilt of banana in the subtropics. Acta Hortic. 2020, 1272, 45–56. [Google Scholar] [CrossRef]
- Maymon, M.; Shpatz, U.; Harel, Y.M.; Levy, E.; Elkind, G.; Teverovski, E.; Gofman, R.; Haberman, A.; Zemorski, R.; Ezra, N.; et al. First report of Fusarium oxysporum f. sp. cubense tropical race 4 causing fusarium wilt of Cavendish bananas in Israel. Plant Dis. 2018, 102, 2655–2656. [Google Scholar] [CrossRef]
- Maymon, M.; Sela, N.; Shpatz, U.; Galpaz, N.; Freeman, S. The origin and current situation of Fusarium oxysporum f. sp. cubense tropical race 4 in Israel and the Middle East. Sci. Rep. 2020, 10, 1590. [Google Scholar] [CrossRef] [PubMed]
- Aguayo, J.; Cerf-Wendling, I.; Folscher, A.B.; Fourrier-Jeandel, C.; Ioos, R.; Mathews, M.C.; Mostert, D.; Renault, C.; Wilson, V.; Viljoen, A. First report of Fusarium oxysporum f. sp. cubense tropical race 4 (TR4) causing banana wilt in the Island of Mayotte. Plant Dis. 2021, 105, 5–13. [Google Scholar] [CrossRef]
- Viljoen, A.; Mostert, D.; Chiconela, T.; Beukes, I.; Fraser, C.; Dwyer, J.; Murray, H.; Amisse, J.; Mutabuana, E.L.; Tazan, G.; et al. Occurrence and spread of the banana fungus Fusarium oxyporum f. sp. cubense TR4 in Mozambique. S. Afr. J. Sci. 2020, 116. [Google Scholar] [CrossRef] [PubMed]
- Mmadi, M.; Azali, H.A.; Mostert, D.; Robène, I.; Viljoen, A. First report of Fusarium wilt of Cavendish bananas caused by Fusarium oxysporum f. sp. cubense Tropical Race 4 in the Grande Comoros Island. Plant Dis. 2023, 107, 4029. [Google Scholar] [CrossRef]
- García-Bastidas, F.A.; Quintero-Vargas, J.C.; Ayala-Vasquez, M.; Schermer, T.; Seidl, M.F.; Santos-Paiva, M.; Noguera, A.M.; Aguilera-Galvez, C.; Wittenberg, A.; Hofstede, R.; et al. First report of Fusarium Wilt Tropical race 4 in Cavendish Bananas Caused by Fusarium odoratissimum in Colombia. Plant Dis. 2020, 104, 994. [Google Scholar] [CrossRef]
- SENASA Confirma Brote de Fusarium Raza 4 Tropical en Piura. 12 April 2021. Available online: https://www.gob.pe/institucion/senasa/noticias/429832-senasa-confirma-brote-de-fusarium-raza-4-tropical-en-piura (accessed on 15 January 2025).
- Herrera, R.M.; Hernández, Y.; Magdama, F.; Mostert, D.; Bothma, S.; Paredes Salgado, E.M.; Terán, D.; González, E.; Angulo, R.; Angel, L.; et al. First report of Fusarium wilt of Cavendish bananas caused by Fusarium oxysporum f. sp. cubense Tropical Race 4 in Venezuela. Plant Dis. 2023, 107, 3297. [Google Scholar] [CrossRef]
- FAO. Banana Market Review 2023; FAO: Rome, Italy, 2024; p. 20. [Google Scholar]
- FAOSTAT FAO Statistical Database. 2024. Available online: http://www.fao.org/ (accessed on 24 October 2024).
- Chittarath, K.; Mostert, D.; Crew, K.S.; Viljoen, A.; Kong, G.; Molina, A.B.; Thomas, J.E. First report of Fusarium oxysporum f. sp. cubense Tropical Race 4 (VCG 01213/16) associated with Cavendish bananas in Laos. Plant Dis. 2018, 102, 449. [Google Scholar] [CrossRef]
- Hung, T.N.; Hung, N.Q.; Mostert, D.; Viljoen, A.; Chao, C.P.; Molina, A.B. First report of Fusarium wilt on Cavendish bananas, caused by Fusarium oxysporum f. sp. cubense Tropical Race 4 (VCG 01213/16) in Vietnam. Plant Dis. 2018, 102, 448. [Google Scholar] [CrossRef]
- International Plant Protection Convection (IPPC). Detection of Fusarium oxysporum f. sp. cubense Tropical Race 4 in Thailand; Plant Quarantine Research Group, Plant Protection Research and Development Office, Department of Agriculture: Bangkok, Thailand, 2019. [Google Scholar]
- Thi, L.L.; Mertens, A.; Vu, D.T.; Vu, T.D.; Minh, P.L.A.; Duc, H.N.; de Backer, S.; Swennen, R.; Vandelook, F.; Panis, B.; et al. Diversity of Fusarium associated banana wilt in northern Viet Nam. MycoKeys 2022, 87, 53–76. [Google Scholar] [CrossRef]
- Chittarath, K.; Nguyen, C.H.; Bailey, W.C.; Zheng, S.J.; Mostert, D.; Viljoen, A.; Tazuba, A.F.; Ocimati, W.; Kearsley, E.; Chi, T.Y.; et al. Geographical Distribution and Genetic Diversity of the Banana Fusarium Wilt Fungus in Laos and Vietnam. J. Fungi 2022, 8, 46. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pham, H.H.; Tran, D.T.; Nguyen, T.T.; Thi Thanh Nguyen, M.; Pham, D.T.; Duong, H.V.; Nguyen, C.X. Molecular identification of Fusarium oxysporum f. sp. cubense (Foc) causing Fusarium wilt disease of Tieu hong banana cultivar in the Red River Delta of Vietnam. Arch. Phytopathol. Plant Prot. 2024, 57, 470–490. [Google Scholar] [CrossRef]
- Subbaraya, U.; Iyyakutty, R.; Mayilvaganan, M.; Thangavelu, R.; Karpagam, C. Evaluations of ITC Accessions for Their Reaction to Foc R1 and TR4. Technical Report; ICAR—National Research Centre for Banana: Ariyavoor-Ukkadaiariyavoor, India, 2024; Available online: https://www.crop-diversity.org/mgis/content/evaluations-itc-accessions-their-reaction-foc-r1-and-tr4 (accessed on 30 January 2025).
- Martínez de la Parte, E.; Pérez-Vicente, L.; García-Bastidas, F.; Bermúdez-Caraballoso, I.; Schnabel, S.; Meijer, H.J.G.; Kema, G.H.J. The Vulnerability of Cuban Banana Production to Fusarium Wilt Caused by Tropical Race 4. Phytopathology 2024, 114, 111–118. [Google Scholar] [CrossRef]
- Thangavelu, R.; Amaresh, H.; Gopi, M.; Loganathan, M.; Nithya, B.; Ganga Devi, P.; Anuradha, C.; Thirugnanavel, A.; Patil, K.B.; Blomme, G.; et al. Geographical distribution, host range and genetic diversity of Fusarium oxysporum f. sp. cubense causing Fusarium wilt of banana in India. J. Fungi 2024, 10, 887. [Google Scholar] [CrossRef]
- Thangavelu, R.; Loganathan, M.; Backiyarani, S.; Saraswathi, M.S.; Uma, S.; Edwinraj, E.; Durai, P.; Nithya, B.; Roux, N.; Selvarajan, R. Screening of exotic banana accessions for their resistance to fusarium wilt race 1 and tropical race 4 in India. Sci. Rep. 2025, 15, 25060. [Google Scholar] [CrossRef] [PubMed]
- Viljoen, A.; Mahuku, G.S.; Massawe, C.; Tendo Ssali, R.; Kimunye, J.; Mostert, G.; Ndayihanzamaso, P.; Coyne, D. Banana Diseases and Pests: Field Guide for Diagnostics and Data Collection; IITA: Ibadan, Nigeria, 2017; p. 73. [Google Scholar]
- González-Mendoza, D.; Argumedo-Delira, R.; Morales-Trejo, A.; Pulido-Herrera, A.; Cervantes-Díaz, L.; Grimaldo-Juarez, O.; Alarcón, A. A rapid method for isolation of total DNA from pathogenic filamentous plant fungi. Genet. Mol. Res. 2010, 9, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Dita, M.A.; Waalwijk, C.; Buddenhagen, I.W.; Souza, M.T., Jr.; Kema, G.H.J. A molecular diagnostic for tropical race 4 of the banana fusarium wilt pathogen. Plant Pathol. 2010, 59, 348–357. [Google Scholar] [CrossRef]
- Fourie, G.; Steenkamp, E.T.; Gordon, T.R.; Viljoen, A. Evolutionary relationships among the Fusarium oxysporum f. sp. cubense vegetative compatibility groups. Appl. Environ. Microbiol. 2009, 75, 4770–4781. [Google Scholar] [CrossRef]
- Ndayihanzamaso, P.; Mostert, D.; Matthews, M.C.; Mahuku, G.; Jomanga, K.; Mpanda, H.J.; Mduma, H.; Brown, A.; Uwimana, B.; Swennen, R.; et al. Evaluation of Mchare and Matooke Bananas for Resistance to Fusarium oxysporum f. sp. cubense Race 1. Plants 2020, 9, 1082. [Google Scholar] [CrossRef]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual; Blackwell Publishing Professional: Ames, IA, USA, 2006; p. 388. [Google Scholar]
- Hwang, S.C.; Ko, W.-H. Cavendish banana cultivars resistant to Fusarium wilt acquired through somaclonal variation in Taiwan. Plant Dis. 2004, 88, 580–587. [Google Scholar] [CrossRef]
- Ndayihanzamaso, P.; Bothma, S.; Mostert, D.; Mahuku, G.; Viljoen, A. An Optimized Greenhouse Protocol for Screening Banana Plants for Fusarium Wilt Resistance. In Efficient Screening Techniques to Identify Mutants with TR4 Resistance in Banana; Jankowicz-Cieslak, J., Ingelbrecht, I.L., Eds.; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar] [CrossRef]
- Dita, M.; Teixeira, L.; Li, C.; Zheng, S.; O’Neill, W.; Daniels, J.; Pérez-Vicente, L.; Carreel, F.; Roussel, V.; Carlier, J.; et al. (Eds.) Practical Guidelines for Early Screening and Field Evaluation of Banana Against Fusarium Wilt, Pseudocercospora Leaf Spots and Drought; Bioversity International: Montpellier, France, 2021; 83p, ISBN 978-92-9255-192-6. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 30 January 2025).
- Guo, L.; Yang, L.; Liang, C.; Wang, G.; Dai, Q.; Huang, J. Differential Colonization Patterns of Bananas (Musa spp.) by Physiological Race 1 and Race 4 Isolates of Fusarium oxysporum f.sp. cubense. J. Phytopathol. 2015, 163, 807–817. [Google Scholar] [CrossRef]
- World Banana Forum (WBF) and FAO Subregional Office for Mesoamerica. TR4-Resistant Banana Varieties: From Selection to Market Demand. Report of the TR4 Global Network [Webinar]. 2022. Available online: https://openknowledge.fao.org/server/api/core/bitstreams/f00decd9-9d78-4907-b61d-384460a1935d/content (accessed on 19 January 2025).
- Mostert, D.; Molina, A.B.; Daniells, J.; Fourie, G.; Hermanto, C.; Chao, C.-P.; Fabregar, E.; Sinohin, V.G.; Masdek, N.; Thangavelu, R.; et al. The distribution and host range of Fusarium oxysporum f. sp. cubense vegetative compatibility groups in Asia. PLoS ONE 2017, 12, e0181630. [Google Scholar] [CrossRef]
- Somrith, A.; Singburaudom, N.; Piasai, O. Vegetative Compatibility Groups of Fusarium oxysporum F.SP. cubense in Thailand. Kasetsart J. Nat. Sci. 2011, 45, 451–460. [Google Scholar]
- Munhoz, T.; Vargas, J.; Teixeira, L.; Staver, C.; Dita, M. Fusarium Tropical Race 4 in Latin America and the Caribbean: Status and global research advances towards disease management. Front. Plant Sci. 2024, 15, 1397617. [Google Scholar] [CrossRef]
- Blomme, G.; Mahuku, G.; Kearsley, E.; Dita, M. Towards the integrated management of Fusarium wilt of banana. J. Fungi 2024, 10, 683. [Google Scholar] [CrossRef]
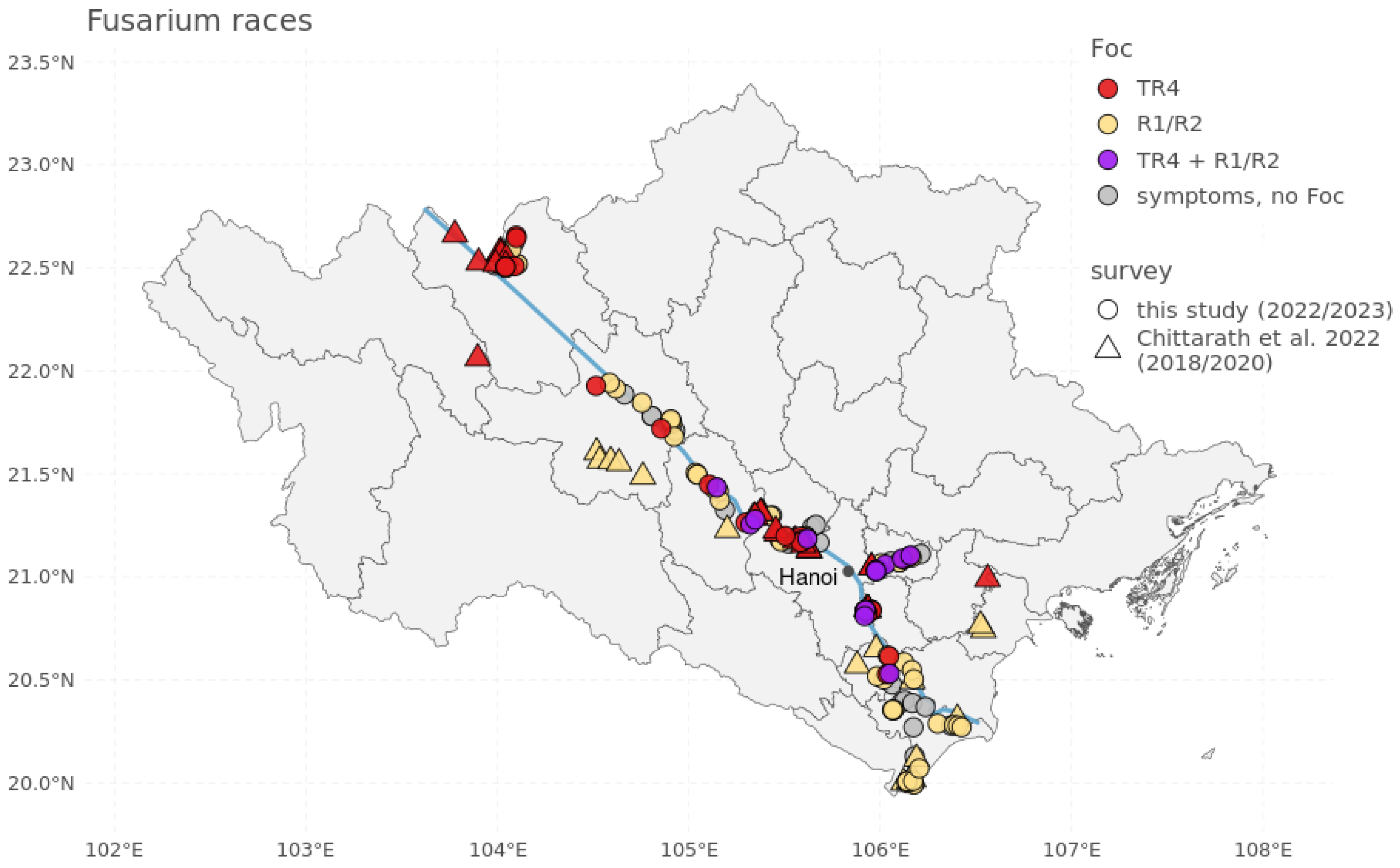

| Production System | Identified Infections | Cavendish | Pisang Awak | Pisang Mas | ||
|---|---|---|---|---|---|---|
| TR4 | R1/R2 | TR4 | R1/R2 | R1/R2 | ||
| Backyard garden | TR4 | - | 3 | - | - | |
| Backyard garden | R1/R2 | - | - | 19 | ||
| Backyard garden | TR4 + R1/R2 | 1 | 1 | |||
| Plantation | TR4 | 25 | - | 7 | - | - |
| Plantation | R1/R2 | - | - | 24 | 1 | |
| Plantation | TR4 + R1/R2 | 5 | - | 3 | 8 | |
| Village | R1/R2 | - | - | 4 | ||
| Along road | R1/R2 | - | - | 6 | ||
| Fusarium Strain | VCG | Cavendish | Pisang Awak | Pisang Mas |
|---|---|---|---|---|
| Foc TR4 | 01213/16 | 35 | 14 | |
| Foc R1/R2 | 0124 | 1 | ||
| 0124/5 | 19 | |||
| 0124/22 | 1 | 36 | 1 | |
| 0124/5/22 | 3 | |||
| 0124/8 | 1 | |||
| 0124/8/22 | 3 | |||
| 0124/5/8/22 | 1 | |||
| 0128/22 | 7 | |||
| 01218 | 1 | |||
| Unknown | 4 |
| Accession | Local Name | Leaf Score | Rhizome Score |
|---|---|---|---|
| Red banana | Chuối đỏ | 3.1 ± 0.7 b | 5.2 ± 0.8 b |
| Green Cavendish | Tiêu xanh | 2.5 ± 0.9 ab | 5.6 ± 0.7 ab |
| Pink Cavendish | Tiêu Hồng | 2.5 ± 0.9 ab | 5.6 ± 0.5 ab |
| Pisang Awak | Tây Thái | 2.1 ± 0.3 a | 5.2 ± 0.8 b |
| Chuoi sap | 1.9 ± 0.3 a | 5.8 ± 0.6 a | |
| Pisang Mas | Chuối ngự | 1.9 ± 0.3 a | 5.6 ± 0.4 b |
| Formosana | 1.8 ± 0.4 a | 3.2 ± 1.4 c | |
| p-value | <0.001 | <0.001 | |
| eta-squared[H] | 0.230 | 0.316 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, C.H.; Nguyen, T.T.; Mostert, D.; Viljoen, A.; Kearsley, E.; Blomme, G. The Evolving Threat of Fusarium Wilt TR4 to Small-Scale Mixed Cultivar Banana Production in the Red River Basin of Northern Vietnam. J. Fungi 2025, 11, 653. https://doi.org/10.3390/jof11090653
Nguyen CH, Nguyen TT, Mostert D, Viljoen A, Kearsley E, Blomme G. The Evolving Threat of Fusarium Wilt TR4 to Small-Scale Mixed Cultivar Banana Production in the Red River Basin of Northern Vietnam. Journal of Fungi. 2025; 11(9):653. https://doi.org/10.3390/jof11090653
Chicago/Turabian StyleNguyen, Chung Huy, Thi Tho Nguyen, Diane Mostert, Altus Viljoen, Elizabeth Kearsley, and Guy Blomme. 2025. "The Evolving Threat of Fusarium Wilt TR4 to Small-Scale Mixed Cultivar Banana Production in the Red River Basin of Northern Vietnam" Journal of Fungi 11, no. 9: 653. https://doi.org/10.3390/jof11090653
APA StyleNguyen, C. H., Nguyen, T. T., Mostert, D., Viljoen, A., Kearsley, E., & Blomme, G. (2025). The Evolving Threat of Fusarium Wilt TR4 to Small-Scale Mixed Cultivar Banana Production in the Red River Basin of Northern Vietnam. Journal of Fungi, 11(9), 653. https://doi.org/10.3390/jof11090653






