Changing Climate, Changing Candida: Environmental and Social Pressures on Invasive Candidiasis and Antifungal Resistance in Latin America
Abstract
1. Introduction
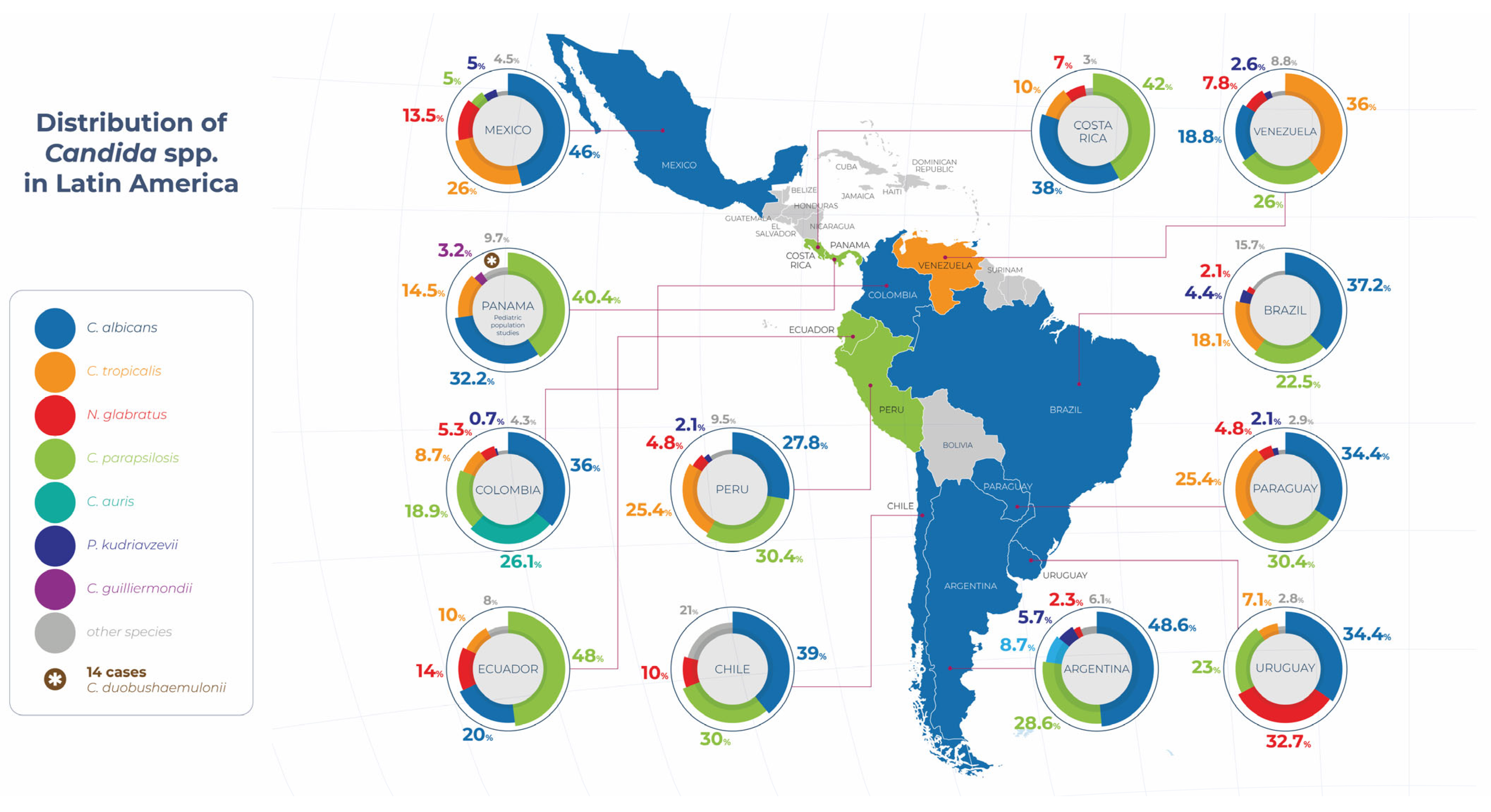
2. Epidemiology of IC in Latin America
The Global Comparative Epidemiology
3. Risk Factors for IC in Latin America
4. Diagnosis of IC in Resource-Limited Settings
The Situation in Latin America: Challenges, Barriers, and Obstacles
5. Regional Antifungal Resistance Patterns
5.1. Candida Parapsilosis
5.2. Candida Tropicalis
5.3. Nakaseomyces Glabratus (Formerly C. glabrata)
5.4. Pichia Kudriavzevii (Formerly C. krusei)
5.5. Other Emerging Yeast-like Strains
- Candida haemulonii complex: This yeast is emerging as an invasive pathogen, with reports in several hospitals in the region, including Mexico, Panama, and Brazil. In Brazil, it has been identified in 0.3% of cases, mainly from chronic wounds and blood cultures, and is associated with critically ill patients. It is characterized by a multidrug resistance profile, particularly to AmB and FCZ, and is often misidentified by conventional methods. Therefore, molecular diagnostics or MALDI-TOF are required for accurate identification, as well as active mycological surveillance and timely adjustment of empirical antifungal treatment [101,102].
- Candida duobushaemulonii: This yeast represents an emerging threat in the context of nosocomial infections, with a marked tendency to behave as an invasive, underdiagnosed, and multidrug-resistant pathogen. It should be considered an emerging yeast of relevance in the Latin American hospital setting. The national surveillance of C. auris carried out in Panama between November 2016 and May 2017 evidenced this, and a significant number of cases of invasive infections caused by this strain were unexpectedly identified. Of the 36 suspected isolates sent to the national reference laboratory, 17 (47%) were confirmed as C. duobushaemulonii, affecting 14 patients hospitalized in six health centers in the country [21].
- Meyerozyma guilliermondii complex (formerly Candida guilliermondii): This is considered an emerging group of opportunistic yeasts, especially in immunocompromised and hospitalized patients. Globally, it accounts for approximately 1% to 5% of candidemia cases, but its importance is increasing due to three main factors: its genetic and taxonomic diversity, an unfavorable antifungal profile, and the difficulties associated with its diagnostic identification. In Latin America, it accounts for up to 7% of candidemia cases in Peru, with the spread of M. caribbica and clade 2 of M. guilliermondii sensu stricto, both associated with azole resistance, being particularly noteworthy. In addition, multiple isolates with resistance to FCZ, AmB, and echinocandins have been documented in Brazil. Given this situation, it is crucial to incorporate molecular identification methods, establish robust antifungal surveillance systems, and adjust empirical antifungal treatment according to the local susceptibility profile. Azole resistance in this complex can vary between 40% and 70%, reinforcing the need for an individualized therapeutic approach [103].
- Candida rugosa: This emerging yeast has gained relevance in the context of invasive infections, particularly in cases of candidemia, due to its worrying resistance profile to azoles. It has established itself as an opportunistic pathogen of growing importance, especially in Latin America, where it has a prevalence approximately seven times higher than in other geographical regions. In Brazil, C. rugosa accounts for up to 2.7% of isolates in ICUs, with FCZ resistance rates reaching 64.9%, and AmB-resistant isolates have also been reported. Its low sensitivity to classic azoles—FCZ (35.7%) and VCZl (55.8%)—makes these antifungals high-risk therapeutic options [104].
5.6. Candidozyma auris (Candida auris)
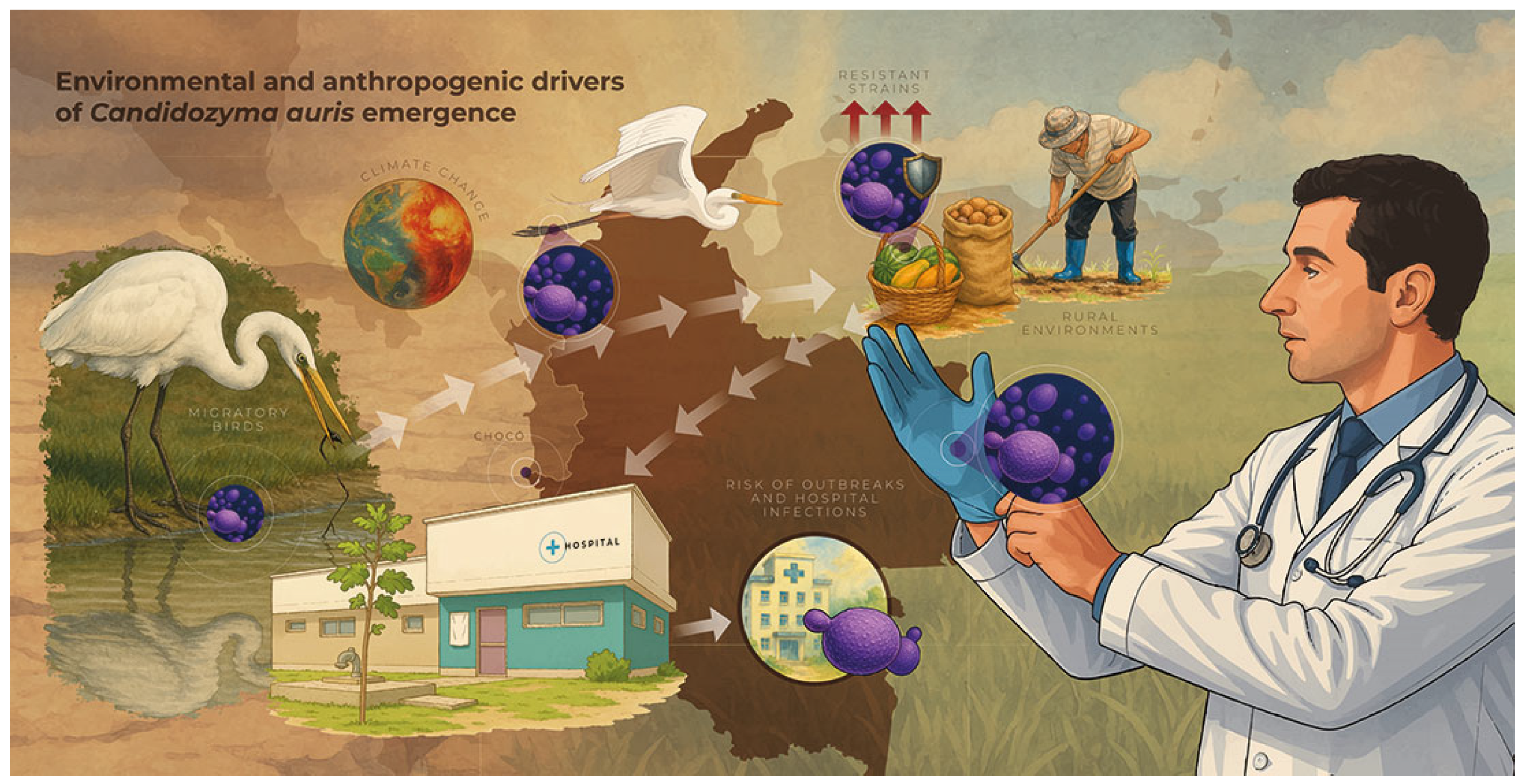
6. Impact of Climate Change on Fungal Infections (Invasive Candidiasis)
7. Access to Health Services and Inequalities in the Region
8. Impact of Housing and Environment on IC in Latin America
Socio-Environmental Hypotheses and Research Gaps
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campion, E.W.; Kullberg, B.J.; Arendrup, M.C. Invasive Candidiasis. N. Engl. J. Med. 2015, 373, 1445–1456. [Google Scholar] [CrossRef]
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive candidiasis. Nat. Rev. Dis. Primers 2018, 4, 18026. [Google Scholar] [CrossRef]
- Lass-Flörl, C.; Kanj, S.S.; Govender, N.P.; Thompson, G.R.; Ostrosky-Zeichner, L.; Govrins, M.A. Invasive candidiasis. Nat. Rev. Dis. Primers 2024, 10, 20. [Google Scholar] [CrossRef] [PubMed]
- Soriano, A.; Honore, P.M.; Puerta-Alcalde, P.; Garcia-Vidal, C.; Pagotto, A.; Gonçalves-Bradley, D.C.; Verweij, P.E. Invasive candidiasis: Current clinical challenges and unmet needs in adult populations. J. Antimicrob. Chemother. 2023, 78, 1569–1585. [Google Scholar] [CrossRef]
- Noppè, E.; Robert, J.; Eloff, P.; Keane, S.; Martin-Loeches, I. A Narrative Review of Invasive Candidiasis in the Intensive Care Unit. Ther. Adv. Pulm. Crit. Care Med. 2024, 19, 29768675241304684. [Google Scholar] [CrossRef]
- Koehler, P.; Stecher, M.; Cornely, O.A.; Koehler, D.; Vehreschild, M.J.G.T.; Bohlius, J.; Wisplinghoff, H.; Vehreschild, J. Morbidity and mortality of candidaemia in Europe: An epidemiologic meta-analysis. Clin. Microbiol. Infect. 2019, 25, 1200–1212. [Google Scholar] [CrossRef]
- Seagle, E.E.; Jackson, B.R.; Lockhart, S.R.; Jenkins, E.N.; Revis, A.; Farley, M.M.; Harrison, L.H.; Schaffner, W.; Markus, T.M.; A Pierce, R.; et al. Recurrent Candidemia: Trends and Risk Factors Among Persons Residing in 4 US States, 2011–2018. Open Forum Infect. Dis. 2022, 9, ofac545. [Google Scholar] [CrossRef]
- Toda, M.; Williams, S.R.; Berkow, E.L.; Farley, M.M.; Harrison, L.H.; Bonner, L.; Marceaux, K.M.; Hollick, R.; Zhang, A.Y.; Schaffner, W.; et al. Population-Based Active Surveillance for Culture-Confirmed Candidemia—Four Sites, United States, 2012–2016. MMWR Surveill. Summ. 2019, 68, 1–17. [Google Scholar] [CrossRef]
- Ricotta, E.E.; Lai, Y.L.; Babiker, A.; Strich, J.R.; Kadri, S.S.; Lionakis, M.S.; Prevots, D.R.; Adjemian, J. Invasive Candidiasis Species Distribution and Trends, United States, 2009–2017. J. Infect. Dis. 2020, 223, 1295. [Google Scholar] [CrossRef] [PubMed]
- Quindós, G. Epidemiology of candidaemia and invasive candidiasis. A changing face. Rev. Iberoam. Micol. 2014, 31, 42–48. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Y.C.; Hsueh, P.R. Epidemiology of candidemia and antifungal susceptibility in invasive Candida species in the Asia-Pacific region. Future Microbiol. 2016, 11, 1461–1477. [Google Scholar] [CrossRef]
- Giacobbe, D.R.; Maraolo, A.E.; Simeon, V.; Magnè, F.; Pace, M.C.; Gentile, I.; Chiodini, P.; Viscoli, C.; Sanguinetti, M.; Mikulska, M.; et al. Changes in the relative prevalence of candidaemia due to non-albicans Candida species in adult in-patients: A systematic review, meta-analysis and meta-regression. Mycoses 2020, 63, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019, a systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Frías-De-león, M.G.; Hernández-Castro, R.; Conde-Cuevas, E.; García-Coronel, I.H.; Vázquez-Aceituno, V.A.; Soriano-Ursúa, M.A.; Farfán-García, E.D.; Ocharán-Hernández, E.; Rodríguez-Cerdeira, C.; Arenas, R.; et al. Candida glabrata Antifungal Resistance and Virulence Factors, a Perfect Pathogenic Combination. Pharmaceutics 2021, 13, 1529. [Google Scholar] [CrossRef]
- Nucci, M.; Queiroz-Telles, F.; Alvarado-Matute, T.; Tiraboschi, I.N.; Cortes, J.; Zurita, J.; Guzman-Blanco, M.; Santolaya, M.E.; Thompson, L.; Sifuentes-Osornio, J.; et al. Epidemiology of Candidemia in Latin America: A Laboratory-Based Survey. PLoS ONE 2013, 8, e59373. [Google Scholar] [CrossRef] [PubMed]
- Nucci, M.; Queiroz-Telles, F.; Tobón, A.M.; Restrepo, A.; Colombo, A.L. Epidemiology of opportunistic fungal infections in latin America. Clin. Infect. Dis. 2010, 51, 561–570. [Google Scholar] [CrossRef] [PubMed]
- da Silva, R.B.; Neves, R.P.; Hinrichsen, S.L.; de Lima-Neto, R.G. Candidemia in a public hospital in Northeastern Brazil: Epidemiological features and risk factors in critically ill patients. Rev. Iberoam. Micol. 2019, 36, 181–185. [Google Scholar] [CrossRef]
- da Matta, D.A.; Souza, A.C.R.; Colombo, A.L. Revisiting Species Distribution and Antifungal Susceptibility of Candida Bloodstream Isolates from Latin American Medical Centers. J. Fungi 2017, 3, 24. [Google Scholar] [CrossRef]
- Wille, M.P.; Guimarães, T.; Campos Furtado, G.H.; Colombo, A.L. Historical trends in the epidemiology of candidaemia: Analysis of an 11-year period in a tertiary care hospital in Brazil. Mem. Inst. Oswaldo Cruz 2013, 108, 288. [Google Scholar] [CrossRef]
- Hamburger, F.G.; Gales, A.C.; Colombo, A.L. Systematic Review of Candidemia in Brazil: Unlocking Historical Trends and Challenges in Conducting Surveys in Middle-Income Countries. Mycopathologia 2024, 189. [Google Scholar] [CrossRef]
- Ramos, R.; Caceres, D.H.; Perez, M.; Garcia, N.; Castillo, W.; Santiago, E.; Borace, J.; Lockhart, S.R.; Berkow, E.L.; Hayer, L.; et al. Emerging Multidrug-Resistant Candida duobushaemulonii Infections in Panama Hospitals: Importance of Laboratory Surveillance and Accurate Identification. J. Clin. Microbiol. 2018, 56, e00371-18. [Google Scholar] [CrossRef] [PubMed]
- la Garza, P.R.-D.; Cruz, C.d.l.C.-D.l.; Bejarano, J.I.C.; Romo, A.E.L.; Delgado, J.V.; Ramos, B.A.; Neira, M.N.M.; Rodríguez, D.S.; Rodríguez, H.M.S.; Selvera, O.A.R. A multicentric outbreak of Candida auris in Mexico: 2020 to 2023. Am. J. Infect. Control. 2024, 52, 1384–1389. [Google Scholar] [CrossRef]
- Reyes-Montes Mdel, R.; Duarte-Escalante, E.; Martínez-Herrera, E.; Acosta-Altamirano, G.; Frías-De León, M.G. Current status of the etiology of candidiasis in Mexico. Rev. Iberoam. Micol. 2017, 34, 203–210. [Google Scholar] [CrossRef]
- Gousy, N.; Adithya Sateesh, B.; Denning, D.W.; Latchman, K.; Mansoor, E.; Joseph, J.; Honnavar, P. Fungal Infections in the Caribbean: A Review of the Literature to Date. J. Fungi 2023, 9, 1177. [Google Scholar] [CrossRef]
- Mesa, L.M.; Arcaya, N.M.; Pineda S, M.R.; Luengo, H.B.; Calvo, B.M. Candidemia en el Hospital Universitario de Maracaibo, Estado Zulia, Venezuela 2000–2002. Rev. Soc. Venez. Microbiol. 2005, 25, 109–113. [Google Scholar]
- Rodriguez, L.; Bustamante, B.; Huaroto, L.; Agurto, C.; Illescas, R.; Ramirez, R.; Diaz, A.; Hidalgo, J.; Coste, A.T. A multi-centric Study of Candida bloodstream infection in Lima-Callao, Peru: Species distribution, antifungal resistance and clinical outcomes. PLoS ONE 2017, 12, e0175172. [Google Scholar] [CrossRef]
- Aguilar, G.; Araujo, P.; Lird, G.; Insaurralde, S.; Kawabata, A.; Ayala, E.; Irala, J.; Argüello, R. Identificación y perfil de sensibilidad de Candida spp. aisladas de hemocultivos en hospitales de Paraguay. Rev. Panam. Salud Pública 2020, 44, e34. [Google Scholar] [CrossRef]
- Carbia, M.; Medina, V.; Bustillo, C.; Martínez, C.; González, M.P.; Ballesté, R. Study of Candidemia and its Antifungal Susceptibility Profile at the University Hospital of Montevideo, Uruguay. Mycopathologia 2023, 188, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Márquez, F.; Iturrieta, I.; Calvo, M.; Urrutia, M.; Godoy-Martínez, P. Epidemiología y susceptibilidad antifúngica de especies causantes de candidemia en la ciudad de Valdivia, Chile. Rev. Chil. Infectología 2017, 34, 441–446. [Google Scholar] [CrossRef]
- Tiraboschi, I.N.; Pozzi, N.C.; Farías, L.; García, S.; Fernández, N.B. Epidemiología, especies, resistencia antifúngica y evolución de las candidemias en un hospital universitario de Buenos Aires, Argentina, durante 16 años. Rev. Chil. Infectol. 2017, 34, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Roa, C.; Valderrama-Rios, M.C.; Sierra-Umaña, S.F.; Rodríguez, J.Y.; Muñetón-López, G.A.; Solórzano-Ramos, C.A.; Escandón, P.; Alvarez-Moreno, C.A.; Cortés, J.A. Mortality Caused by Candida auris Bloodstream Infections in Comparison with Other Candida Species, a Multicentre Retrospective Cohort. J. Fungi 2023, 9, 715. [Google Scholar] [CrossRef]
- de Oliveira, C.S.; Colombo, A.L.; Francisco, E.C.; de Lima, B.; Gandra, R.F.; de Carvalho, M.C.P.; Carrilho, C.M.D.d.M.; Petinelli, R.; Pelison, M.; Helbel, C.; et al. Clinical and epidemiological aspects of Candidemia in eight medical centers in the state of Parana, Brazil: Parana Candidemia Network. Braz. J. Infect. Dis. 2021, 25, 101041. [Google Scholar] [CrossRef]
- Cortés, J.A.; Ruiz, J.F.; Melgarejo-Moreno, L.N.; Lemos, E.V. Candidemia en Colombia. Biomédica 2020, 40, 195. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cesar, A.S. Características Clínicas, Microbiológicas Y Desenlaces de la Candidiasis Invasora en Adultos en UN Hospital de Alta Complejidad. Master’s Thesis, Universidad del Rosario, Bogotá, Colombia, 2020. [Google Scholar] [CrossRef]
- Riera, F.O.; Caeiro, J.P.; Denning, D.W. Burden of serious fungal infections in Argentina. J. Fungi 2018, 4, 51. [Google Scholar] [CrossRef]
- Macedo-Viñas, M.; Denning, D.W. Estimating the Burden of Serious Fungal Infections in Uruguay. J. Fungi 2018, 4, 37. [Google Scholar] [CrossRef]
- Corzo-León, D.E.; Perales-Martínez, D.; Martin-Onraet, A.; Rivera-Martínez, N.; Camacho-Ortiz, A.; Villanueva-Lozano, H. Monetary costs and hospital burden associated with the management of invasive fungal infections in Mexico: A multicenter study. Braz. J. Infect. Dis. 2018, 22, 360. [Google Scholar] [CrossRef] [PubMed]
- Corzo-León, D.E.; Armstrong-James, D.; Denning, D.W. Burden of serious fungal infections in Mexico. Mycoses 2015, 58, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Bays, D.J.; Jenkins, E.N.; Lyman, M.; Chiller, T.; Strong, N.; Ostrosky-Zeichner, L.; Hoenigl, M.; Pappas, P.G.; Thompson, G. Epidemiology of Invasive Candidiasis. Clin. Epidemiol. 2024, 16, 549–566. [Google Scholar] [CrossRef]
- Jenkins, E.N.; Gold, J.A.W.; Benedict, K.; Lockhart, S.R.; Berkow, E.L.; Dixon, T.; Shack, S.L.; Witt, L.S.; Harrison, L.H.; Seopaul, S.; et al. Population-Based Active Surveillance for Culture-Confirmed Candidemia—10 Sites, United States, 2017–2021. MMWR Surveill. Summ. 2025, 74, 1–15. [Google Scholar] [CrossRef]
- Kohlenberg, A.; Monnet, D.L.; Plachouras, D.; Willinger, B.; Lagrou, K.; Philipova, I.; Budimir, A.; Hadjihannas, L.; Marcou, M.; Bareková, L.; et al. Increasing number of cases and outbreaks caused by Candida auris in the EU/EEA, 2020 to 2021. Eurosurveillance 2022, 27, 2200846. [Google Scholar] [CrossRef]
- Huh, K.; Ha, Y.E.; Denning, D.W.; Peck, K.R. Serious fungal infections in Korea. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 957–963. [Google Scholar] [CrossRef]
- Bilal, H.; Shafiq, M.; Hou, B.; Islam, R.; Khan, M.N.; Khan, R.U.; Zeng, Y. Distribution and antifungal susceptibility pattern of Candida species from mainland China: A systematic analysis. Virulence 2022, 13, 1573–1589. [Google Scholar] [CrossRef]
- Bongomin, F.; Ekeng, B.E.; Kibone, W.; Nsenga, L.; Olum, R.; Itam-Eyo, A.; Kuate, M.P.N.; Pebolo, F.P.; Davies, A.A.; Manga, M.; et al. Invasive Fungal Diseases in Africa: A Critical Literature Review. J. Fungi 2022, 8, 1236. [Google Scholar] [CrossRef]
- Gebremicael, M.N.; Nuttall, J.J.C.; Tootla, H.D.; Khumalo, A.; Tooke, L.; Salie, S.; Muloiwa, R.; Rhoda, N.; Basera, W.; Eley, B.S. Candida bloodstream infection among children hospitalised in three public-sector hospitals in the Metro West region of Cape Town, South Africa. BMC Infect Dis 2023, 23, 67. [Google Scholar] [CrossRef]
- de Almeida, B.L.; Agnelli, C.; Guimarães, T.; Sukiennik, T.; Lima, P.R.P.; Salles, M.J.C.; Breda, G.L.; Queiroz-Telles, F.; Mendes, A.V.A.; Camargo, L.F.A.; et al. Candidemia in ICU Patients: What Are the Real Game-Changers for Survival? J. Fungi 2025, 11, 152. [Google Scholar] [CrossRef]
- Motoa, G.; Muñoz, J.S.; Oñate, J.; Pallares, C.J.; Hernández, C.; Villegas, M.V. Epidemiology of Candida isolates from Intensive Care Units in Colombia from 2010 to 2013. Rev. Iberoam Micol. 2016, 34, 17–22. [Google Scholar] [CrossRef]
- Ortíz Ruiz, G.; Osorio, J.; Valderrama, S.; Álvarez, D.; Elías Díaz, R.; Calderón, J.; Ballesteros, D.; Franco, A. Factores de riesgo asociados a candidemia en pacientes críticos no neutropénicos en Colombia. Med. Intensiva 2016, 40, 139–144. [Google Scholar] [CrossRef]
- Riera, F.O.; Caeiro, J.P.; Angiolini, S.C.; Vigezzi, C.; Rodriguez, E.; Icely, P.A.; Sotomayor, C.E. Invasive Candidiasis: Update and Current Challenges in the Management of This Mycosis in South America. Antibiotics 2022, 11, 877. [Google Scholar] [CrossRef]
- Vazquez, J.A.; Whitaker, L.; Zubovskaia, A. Invasive Candidiasis in the Intensive Care Unit: Where Are We Now? J. Fungi 2025, 11, 258. [Google Scholar] [CrossRef]
- Thomas-Rüddel, D.O.; Schlattmann, P.; Pletz, M.; Kurzai, O.; Bloos, F. Risk Factors for Invasive Candida Infection in Critically Ill Patients: A Systematic Review and Meta-analysis. Chest 2022, 161, 345–355. [Google Scholar] [CrossRef]
- Oñate, J.M.; Rivas, P.; Pallares, C.; Saavedra, C.H.; Martínez, E.; Coronell, W.; López, E.; Berrio, I.; Álvarez-Moreno, C.A.; Roncancio, G.E.; et al. Colombian consensus on the diagnosis, treatment, and prevention of Candida Spp. disease in children and adults. Infectio 2019, 23, 271–304. [Google Scholar] [CrossRef]
- Cornely, O.A.; Sprute, R.; Bassetti, M.; Chen, S.C.-A.; Groll, A.H.; Kurzai, O.; Lass-Flörl, C.; Ostrosky-Zeichner, L.; Rautemaa-Richardson, R.; Revathi, G.; et al. Global guideline for the diagnosis and management of candidiasis: An initiative of the ECMM in cooperation with ISHAM and ASM. Lancet Infect. Dis. 2025, 25, e280–e293. [Google Scholar] [CrossRef]
- Riera, F.; Cortes Luna, J.; Rabagliatti, R.; Scapellato, P.; Caeiro, J.P.; Chaves Magri, M.M.; Sotomayor, C.E.; Falci, D.R. Antifungal stewardship: The Latin American experience. Antimicrob. Steward. Healthc. Epidemiol. 2023, 3, e217. [Google Scholar] [CrossRef]
- Barantsevich, N.; Barantsevich, E. Diagnosis and Treatment of Invasive Candidiasis. Antibiotics 2022, 11, 718. [Google Scholar] [CrossRef]
- Bassetti, M.; Giacobbe, D.R.; Vena, A.; Wolff, M. Diagnosis and Treatment of Candidemia in the Intensive Care Unit. Semin. Respir. Crit. Care Med. 2019, 40, 524–539. [Google Scholar] [CrossRef]
- Keighley, C.; Garnham, K.; Harch, S.A.J.; Robertson, M.; Chaw, K.; Teng, J.C.; Chen, S.C.-A. Candida auris: Diagnostic Challenges and Emerging Opportunities for the Clinical Microbiology Laboratory. Curr. Fungal Infect. Rep. 2021, 15, 116. [Google Scholar] [CrossRef]
- Hsu, C.; Yassin, M. Diagnostic Approaches for Candida auris: A Comprehensive Review of Screening, Identification, and Susceptibility Testing. Microorganisms 2025, 13, 1461. [Google Scholar] [CrossRef]
- Falci, D.R.; Pasqualotto, A.C. Clinical mycology in Latin America and the Caribbean: A snapshot of diagnostic and therapeutic capabilities. Mycoses 2019, 62, 368–373. [Google Scholar] [CrossRef]
- Mikulska, M.; Calandra, T.; Sanguinetti, M.; Poulain, D.; Viscoli, C. The use of mannan antigen and anti-mannan antibodies in the diagnosis of invasive candidiasis: Recommendations from the Third European Conference on Infections in Leukemia. Crit. Care 2010, 14, R222. [Google Scholar] [CrossRef]
- Riera, F.; Caeiro, J.P.; Cornely, O.A.; Salmanton-García, J.; Argentinian IFI diagnostic and treatment capacity group. The Argentinian landscape of mycological diagnostic capacity and treatment accessibility. Med. Mycol. 2023, 61, 58. [Google Scholar] [CrossRef]
- Holm WVan Ghesquière, J.; Boon, N.; Verspecht, T.; Bernaerts, K.; Zayed, N.; Chatzigiannidou, I.; Teughels, W.; Kivisaar, M. A Viability Quantitative PCR Dilemma: Are Longer Amplicons Better? Appl. Environ. Microbiol. 2021, 87, 1–11. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Q.; Dong, Y.Q.; Yin, J.; Qiu, Y.Q. Diagnostic accuracy of metagenomic next-generation sequencing in diagnosing infectious diseases: A meta-analysis. Sci. Rep. 2022, 12, 21032. [Google Scholar] [CrossRef]
- Monday, L.M.; Acosta, T.P.; Alangaden, G. T2Candida for the Diagnosis and Management of Invasive Candida Infections. J. Fungi 2021, 7, 178. [Google Scholar] [CrossRef]
- Teke, L.; Barış, A.; Bayraktar, B. Comparative evaluation of the Bruker Biotyper and Vitek MS matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) systems for non-albicans Candida and uncommon yeast isolates. J. Microbiol. Methods 2021, 185, 106232. [Google Scholar] [CrossRef]
- Kassim, A.; Pflüger, V.; Premji, Z.; Daubenberger, C.; Revathi, G. Comparison of biomarker based Matrix Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry (MALDI-TOF MS) and conventional methods in the identification of clinically relevant bacteria and yeast. BMC Microbiol. 2017, 17, 128. [Google Scholar] [CrossRef]
- Van Veen, S.Q.; Claas, E.C.J.; Kuijper, E.J. High-throughput identification of bacteria and yeast by matrix-assisted laser desorption ionization-time of flight mass spectrometry in conventional medical microbiology laboratories. J. Clin. Microbiol. 2010, 48, 900–907. [Google Scholar] [CrossRef]
- Nguyen, M.H.; Wissel, M.C.; Shields, R.K.; Salomoni, M.A.; Hao, B.; Press, E.G.; Shields, R.M.; Cheng, S.; Mitsani, D.; Vadnerkar, A.; et al. Performance of candida real-time polymerase chain reaction, β-D-glucan assay, and blood cultures in the diagnosis of invasive candidiasis. Clin. Infect. Dis. 2012, 54, 1240–1248. [Google Scholar] [CrossRef]
- Cortés, J.A.; Valderrama-Rios, M.C.; Peçanha-Pietrobom, P.M.; Júnior, M.S.; Diaz-Brochero, C.; Robles-Torres, R.R.; Espinosa-Almanza, C.J.; Nocua-Báez, L.C.; Nucci, M.; Álvarez-Moreno, C.A.; et al. Evidence-based clinical standard for the diagnosis and treatment of candidemia in critically ill patients in the intensive care unit. Braz. J. Infect. Dis. 2024, 29, 104495. [Google Scholar] [CrossRef]
- Avni, T.; Leibovici, L.; Paul, M. PCR diagnosis of invasive candidiasis: Systematic review and meta-analysis. J. Clin. Microbiol. 2011, 49, 665–670. [Google Scholar] [CrossRef]
- Nieto, M.; Robles, J.C.; Causse, M.; Gutiérrez, L.; Cruz Perez, M.; Ferrer, R.; Xercavins, M.; Herrero, E.; Sirvent, E.; Fernández, C.; et al. Polymerase Chain Reaction Versus Blood Culture to Detect Candida Species in High-Risk Patients with Suspected Invasive Candidiasis: The MICAFEM Study. Infect. Dis. Ther. 2019, 8, 429–444. [Google Scholar] [CrossRef]
- Tang, D.L.; Chen, X.; Zhu, C.G.; Li, Z.W.; Xia, Y.; Guo, X.G. Pooled analysis of T2 Candida for rapid diagnosis of candidiasis. BMC Infect. Dis. 2019, 19, 1–8. [Google Scholar] [CrossRef]
- Mallick, D.C.; Kaushik, N.; Goyal, L.; Mallick, L.; Singh, P. A Comprehensive Review of Candidemia and Invasive Candidiasis in Adults: Focus on the Emerging Multidrug-Resistant Fungus Candida auris. Diseases 2025, 13, 93. [Google Scholar] [CrossRef]
- Sexton, D.J.; Bentz, M.L.; Welsh, R.M.; Litvintseva, A.P. Evaluation of a new T2 Magnetic Resonance assay for rapid detection of emergent fungal pathogen Candida auris on clinical skin swab samples. Mycoses 2018, 61, 786–790. [Google Scholar] [CrossRef] [PubMed]
- Cortés, J.A.; Reyes, P.; Gómez, C.H.; Cuervo, S.I.; Rivas, P.; Casas, C.A.; Sánchez, R. Clinical and epidemiological characteristics and risk factors for mortality in patients with candidemia in hospitals from Bogotá, Colombia. Braz. J. Infect. Dis. 2014, 18, 631–637. [Google Scholar] [CrossRef]
- Alvarez-Moreno, C.A.; Cortes, J.A.; Denning, D.W. Burden of fungal infections in Colombia. J. Fungi 2018, 4, 41. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Bustos, V.; Cabanero-Navalon, M.D.; Ruiz-Saurí, A.; Ruiz-Gaitán, A.C.; Salavert, M.; Tormo, M.; Pemán, J. What do we know about candida auris? State of the art, knowledge gaps, and future directions. Microorganisms 2021, 9, 2177. [Google Scholar] [CrossRef]
- Escandón, P.; Cáceres, D.H.; Lizarazo, D.; Lockhart, S.R.; Lyman, M.; Duarte, C. Laboratory-based surveillance of Candida auris in Colombia, 2016–2020. Mycoses 2022, 65, 222–225. [Google Scholar] [CrossRef]
- Escandón, P.; Lockhart, S.R.; Chow, N.A.; Chiller, T.M. Candida auris: A global pathogen that has taken root in Colombia. Biomédica 2023, 43, 278. [Google Scholar] [CrossRef]
- Escandón, P.; Chow, N.A.; Caceres, D.H.; Gade, L.; Berkow, E.L.; Armstrong, P.; Rivera, S.; Misas, E.; Duarte, C.; Moulton-Meissner, H.; et al. Molecular Epidemiology of Candida auris in Colombia Reveals a Highly Related, Countrywide Colonization with Regional Patterns in Amphotericin B Resistance. Clin. Infect. Dis. 2019, 68, 15–21. [Google Scholar] [CrossRef]
- Lanna, M.; Lovatto, J.; de Almeida, J.N.; Medeiros, E.A.; Colombo, A.L.; García-Effron, G. Epidemiological and Microbiological aspects of Candidozyma auris (Candida auris) in Latin America: A literature review. J. Med. Mycol. 2025, 35, 101546. [Google Scholar] [CrossRef]
- Martinez-Parada, I.; Agudelo-Quintero, E.; Prado-Molina, D.G.; Serna-Trejos, J.S. Current situation of Candida auris in Colombia, 2021. An. De La Fac. De Med. 2021, 82, 242–243. [Google Scholar] [CrossRef]
- Zuluaga Rodríguez, A.; de Bedout Gómez, C.; Agudelo Restrepo, C.A.; Hurtado Parra, H.; Arango Arteaga, M.; Restrepo Moreno, Á.; González Marín, Á. Sensibilidad a fluconazol y voriconazol de especies de Candida aisladas de pacientes provenientes de unidades de cuidados intensivos en Medellín, Colombia (2001–2007). Rev. Iberoam Micol. 2010, 27, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Colombo, A.L.; Cortes, J.A.; Zurita, J.; Guzman-Blanco, M.; Alvarado Matute, T.; de Queiroz Telles, F.; Santolaya, M.E.; Tiraboschi, I.N.; Echevarría, J.; Sifuentes, J.; et al. Recommendations for the diagnosis of candidemia in Latin America. Rev. Iberoam. Micol. 2013, 30, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Govrins, M.; Lass-Flörl, C. Candida parapsilosis complex in the clinical setting. Nat. Rev. Microbiol. 2023, 22, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Govender, N.P.; Patel, J.; Magobo, R.E.; Naicker, S.; Wadula, J.; Whitelaw, A.; Coovadia, Y.; Kularatne, R.; Govind, C.; Lockhart, S.R.; et al. Emergence of azole-resistant Candida parapsilosis causing bloodstream infection: Results from laboratory-based sentinel surveillance in South Africa. J. Antimicrob. Chemother. 2016, 71, 1994–2004. [Google Scholar] [CrossRef]
- Corzo-Leon, D.E.; Peacock, M.; Rodriguez-Zulueta, P.; Salazar-Tamayo, G.J.; MacCallum, D.M. General hospital outbreak of invasive candidiasis due to azole-resistant Candida parapsilosis associated with an Erg11 Y132F mutation. Med. Mycol. 2021, 59, 664–671. [Google Scholar] [CrossRef]
- Ceballos-Garzon, A.; Peñuela, A.; Valderrama-Beltrán, S.; Vargas-Casanova, Y.; Ariza, B.; Parra-Giraldo, C.M. Emergence and circulation of azole-resistant C. albicans, C. auris and C. parapsilosis bloodstream isolates carrying Y132F, K143R or T220L Erg11p substitutions in Colombia. Front. Cell Infect. Microbiol. 2023, 13, 1136217. [Google Scholar] [CrossRef]
- Franconi, I.; Rizzato, C.; Poma, N.; Tavanti, A.; Lupetti, A. Candida parapsilosis sensu stricto Antifungal Resistance Mechanisms and Associated Epidemiology. J. Fungi 2023, 9, 798. [Google Scholar] [CrossRef]
- Ning, Y.; Xiao, M.; Perlin, D.S.; Zhao, Y.; Lu, M.; Li, Y.; Luo, Z.; Dai, R.; Li, S.; Xu, J.; et al. Decreased echinocandin susceptibility in Candida parapsilosis causing candidemia and emergence of a pan-echinocandin resistant case in China. Emerg. Microbes Infect. 2023, 12, 2153086. [Google Scholar] [CrossRef]
- Daneshnia, F.; de Almeida Júnior, J.N.; Ilkit, M.; Lombardi, L.; Perry, A.M.; Gao, M.; Nobile, C.J.; Egger, M.; Perlin, D.S.; Zhai, B.; et al. Worldwide emergence of fluconazole-resistant Candida parapsilosis: Current framework and future research roadmap. Lancet Microbe 2023, 4, e470–e480. [Google Scholar] [CrossRef]
- Favarello, L.M.; Nucci, M.; Queiroz-Telles, F.; Guimarães, T.; Salles, M.J.; Sukiennik, T.C.T.; da Matta, D.A.; Melo, A.S.; Colombo, A.L. Trends towards lower azole susceptibility among 200 Candida tropicalis bloodstream isolates from Brazilian medical centres. J. Glob. Antimicrob. Resist. 2021, 25, 199–201. [Google Scholar] [CrossRef]
- Spruijtenburg, B.; Baqueiro, C.C.S.Z.; Colombo, A.L.; Meijer, E.F.J.; de Almeida, J.N.; Berrio, I.; Fernández, N.B.; Chaves, G.M.; Meis, J.F.; de Groot, T.; et al. Short Tandem Repeat Genotyping and Antifungal Susceptibility Testing of Latin American Candida tropicalis Isolates. J. Fungi 2023, 9, 207. [Google Scholar] [CrossRef]
- Khan, Z.; Ahmad, S.; Mokaddas, E.; Meis, J.F.; Joseph, L.; Abdullah, A.; Vayalil, S. Development of echinocandin resistance in Candida tropicalis following short-term exposure to caspofungin for empiric therapy. Antimicrob Agents Chemother 2018, 62, e01926-17. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.Y.; Chuang, Y.C.; Wu, U.I.; Sun, H.Y.; Wang, J.T.; Sheng, W.H.; Chen, Y.C.; Chang, S.C. Mechanisms of azole resistance and trailing in candida tropicalis bloodstream isolates. J. Fungi 2021, 7, 612. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Herrera, E.; Frías-De-león, M.G.; Hernández-Castro, R.; García-Salazar, E.; Arenas, R.; Ocharan-Hernández, E.; Rodríguez-Cerdeira, C. Antifungal Resistance in Clinical Isolates of Candida glabrata in Ibero-America. J. Fungi 2021, 8, 14. [Google Scholar] [CrossRef]
- Colombo, A.L.; Garnica, M.; Aranha Camargo, L.F.; Da Cunha, C.A.; Bandeira, A.C.; Borghi, D.; Campos, T.; Senna, A.L.; Didier, M.E.V.; Dias, V.C.; et al. Candida glabrata: An emerging pathogen in Brazilian tertiary care hospitals. Med. Mycol. 2013, 51, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Alexander, B.D.; Johnson, M.D.; Pfeiffer, C.D.; Jiménez-Ortigosa, C.; Catania, J.; Booker, R.; Castanheira, M.; Messer, S.A.; Perlin, D.S.; Pfaller, M.A. Increasing echinocandin resistance in candida glabrata: Clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin. Infect. Dis. 2013, 56, 1724–1732. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Kim, H.Y.; Stocker, S.; Kidd, S.; Alastruey-Izquierdo, A.; Dao, A.; Harrison, T.; Wahyuningsih, R.; Rickerts, V.; Perfect, J.; et al. Pichia kudriavzevii (Candida krusei): A systematic review to inform the World Health Organisation priority list of fungal pathogens. Med. Mycol. 2024, 62, myad132. [Google Scholar] [CrossRef]
- Jamiu, A.T.; Albertyn, J.; Sebolai, O.M.; Pohl, C.H. Update on Candida krusei, a potential multidrug-resistant pathogen. Med. Mycol. 2021, 59, 14–30. [Google Scholar] [CrossRef]
- Françoiseid, U.; Desnos-Ollivier, M.; Le Govic, Y.; Sitbon, K.; Valentino, R.; Peugny, S.; Chouaki, T.; Mazars, E.; Paugam, A.; Nicolas, M.; et al. Candida haemulonii complex, an emerging threat from tropical regions? PLoS Negl. Trop. Dis. 2023, 17, e0011453. [Google Scholar] [CrossRef]
- de Almeida, J.N.; Assy, J.G.P.L.; Levin, A.S.; del Negro, G.M.B.; Giudice, M.C.; Tringoni, M.P.; Thomaz, D.Y.; Motta, A.L.; Abdala, E.; Pierroti, L.C.; et al. Candida haemulonii Complex Species, Brazil, January 2010–March 2015. Emerg. Infect. Dis. 2016, 22, 561–563. [Google Scholar] [CrossRef]
- Ghasemi, R.; Lotfali, E.; Rezaei, K.; Madinehzad, S.A.; Tafti, M.F.; Aliabadi, N.; Kouhsari, E.; Fattahi, M. Meyerozyma guilliermondii species complex: Review of current epidemiology, antifungal resistance, and mechanisms. Braz. J. Microbiol. 2022, 53, 1761. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekema, D.J.; Colombo, A.L.; Kibbler, C.; Ng, K.P.; Gibbs, D.L.; Newell, V.A.; the Global Antifungal Surveillance Group. Candida rugosa, an Emerging Fungal Pathogen with Resistance to Azoles: Geographic and Temporal Trends from the ARTEMIS DISK Antifungal Surveillance Program. J. Clin. Microbiol. 2006, 44, 3578. [Google Scholar] [CrossRef]
- Misas, E.; Escandón, P.L.; Gade, L.; Caceres, D.H.; Hurst, S.; Le, N.; Min, B.; Lyman, M.; Duarte, C.; Chow, N.A.; et al. Genomic epidemiology and antifungal-resistant characterization of Candida auris, Colombia, 2016–2021. MSphere 2024, 9, e00577-23. [Google Scholar] [CrossRef]
- Chow, N.A.; Muñoz, J.F.; Gade, L.; Berkow, E.L.; Li, X.; Welsh, R.M.; Forsberg, K.; Lockhart, S.R.; Adam, R.; Alanio, A.; et al. Tracing the evolutionary history and global expansion of candida auris using population genomic analyses. MBio 2020, 11. [Google Scholar] [CrossRef]
- Sabino, R.; Veríssimo, C.; Pereira, Á.A.; Antunes, F. Candida auris, an agent of hospital-associated outbreaks: Which challenging issues do we need to have in mind? Microorganisms 2020, 8, 181. [Google Scholar] [CrossRef]
- Casadevall, A.; Kontoyiannis, D.P.; Robert, V. On the emergence of candida auris: Climate change, azoles, swamps, and birds. MBio 2019, 10. [Google Scholar] [CrossRef]
- Escandón, P. Novel Environmental Niches for Candida auris: Isolation from a Coastal Habitat in Colombia. J. Fungi 2022, 8, 748. [Google Scholar] [CrossRef]
- Du, H.; Bing, J.; Hu, T.; Ennis, C.L.; Nobile, C.J.; Huang, G. Candida auris: Epidemiology, biology, antifungal resistance, and virulence. PLoS Pathog. 2020, 16, e1008921. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Sood, P. On the emergence, spread and resistance of Candida auris: Host, pathogen and environmental tipping points. J. Med. Microbiol. 2021, 70, 001318. [Google Scholar] [CrossRef]
- Frías-De-León, M.G.; García-Salazar, E.; Reyes-Montes Mdel, R.; Duarte-Escalante, E.; Acosta-Altamirano, G. Opportunistic Yeast Infections and Climate Change: The Emergence of Candida auris. In The Impact of Climate Change on Fungal Diseases; Springer International Publishing: Cham, Switzerland, 2022; pp. 161–179. [Google Scholar] [CrossRef]
- Escandón, P.; Duarte, C.; Rivera, S. Alerta por Emergencia Global de Infecciones Invasivas Causadas por la Levadura Multirresistente, Candida auris; Instituto Nacional de Salud: Bogota, Colombia, 2016. [Google Scholar]
- Clara-Noguera, M.; Orozco, S.; Lizarazo, D.; Escandón, P.; Clara-Noguera, M.; Orozco, S.; Lizarazo, D.; Escandón, P. Prevalencia de Candida auris en departamentos de Colombia durante la vigilancia por el laboratorio (2018–2021). Infectio 2024, 28, 241–245. [Google Scholar] [CrossRef]
- Naz, S.; Fatima, Z.; Iqbal, P.; Khan, A.; Zakir, I.; Ullah, H.; Abbas, G.; Ahmed, M.; Mubeen, M.; Hussain, S.; et al. An introduction to climate change phenomenon. In Building Climate Resilience in Agriculture: Theory, Practice and Future Perspective; Springer International Publishing: Cham, Switzerland, 2021; pp. 3–16. [Google Scholar] [CrossRef]
- Jaramillo, A.; Mendoza-Ponce, A. Climate Change Overview. In The Impact of Climate Change on Fungal Diseases; Springer International Publishing: Cham, Switzerland, 2022; pp. 1–18. [Google Scholar] [CrossRef]
- Sedik, S.; Egger, M.; Hoenigl, M. Climate Change and Medical Mycology. Infect. Dis. Clin. N. Am. 2024, 39. [Google Scholar] [CrossRef]
- Van Rhijn, N.; Bromley, M. The consequences of our changing environment on life threatening and debilitating fungal diseases in humans. J. Fungi 2021, 7, 367. [Google Scholar] [CrossRef]
- Seidel, D.; Wurster, S.; Jenks, J.D.; Sati, H.; Gangneux, J.P.; Egger, M.; Alastruey-Izquierdo, A.; Ford, N.P.; Chowdhary, A.; Sprute, R.; et al. Impact of climate change and natural disasters on fungal infections. Lancet Microbe 2024, 5, e594–e605. [Google Scholar] [CrossRef]
- Sharma, C.; Kadosh, D. Perspective on the origin, resistance, and spread of the emerging human fungal pathogen Candida auris. PLoS Pathog. 2023, 19, e1011190. [Google Scholar] [CrossRef] [PubMed]
- Misseri, G.; Ippolito, M.; Cortegiani, A. Global warming “heating up” the ICU through Candida auris infections: The climate changes theory. Crit Care 2019, 23, 416. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Bustos, V.; Cabañero-Navalon, M.D.; Ruiz-Gaitán, A.; Salavert, M.; Tormo-Mas, M.Á.; Pemán, J. Climate change, animals, and Candida auris: Insights into the ecological niche of a new species from a One Health approach. Clin. Microbiol. Infect. 2023, 29, 858–862. [Google Scholar] [CrossRef]
- Salazar-Hamm, P.; Torres-Cruz, T.J. The Impact of Climate Change on Human Fungal Pathogen Distribution and Disease Incidence. Curr. Clin. Microbiol. Rep. 2024, 11, 140–152. [Google Scholar] [CrossRef]
- George, M.E.; Gaitor, T.T.; Cluck, D.B.; Henao-Martínez, A.F.; Sells, N.R.; Chastain, D.B. The impact of climate change on the epidemiology of fungal infections: Implications for diagnosis, treatment, and public health strategies. Ther. Adv. Infect. Dis. 2025, 12, 20499361251313840. [Google Scholar] [CrossRef]
- Kneale, M.; Bartholomew, J.S.; Davies, E.; Denning, D.W. Global access to antifungal therapy and its variable cost. J. Antimicrob. Chemother. 2016, 71, 3599–3606. [Google Scholar] [CrossRef]
- Valladales-Restrepo, L.F.; Ospina-Cano, J.A.; Aristizábal-Carmona, B.S.; López-Caicedo, D.F.; Toro-Londoño, M.; Gaviria-Mendoza, A.; Machado-Duque, M.E.; Machado-Alba, J.E. Study of Prescription-Indication of Outpatient Systemic Anti-Fungals in a Colombian Population. A Cross-Sectional Study. Antibiotics 2022, 11, 1805. [Google Scholar] [CrossRef]
- Van Zyl, C.; Badenhorst, M.; Hanekom, S.; Heine, M. Unravelling ‘low-resource settings’: A systematic scoping review with qualitative content analysis. BMJ Glob. Health 2021, 6, 5190. [Google Scholar] [CrossRef]
- Laks, M.; Guerra, C.M.; Miraglia, J.L.; Medeiros, E.A. Distance learning in antimicrobial stewardship: Innovation in medical education. BMC Med. Educ. 2019, 19, 191. [Google Scholar] [CrossRef]
- Barteit, S.; Guzek, D.; Jahn, A.; Bärnighausen, T.; Jorge, M.M.; Neuhann, F. Evaluation of e-learning for medical education in low- and middle-income countries: A systematic review. Comput. Educ. 2020, 145, 103726. [Google Scholar] [CrossRef]
- Otero, P.; Hersh, W.; Luna, D.; González Bernaldo De Quirós, F. A medical informatics distance-learning course for Latin America: Translation, implementation and evaluation. Methods Inf. Med. 2010, 49, 310–315. [Google Scholar] [CrossRef]
- GAFFI y OPS Unen Fuerzas Para Combatir las Infecciones Causadas por Hongos en América Latina y el Caribe-OPS/OMS | Organización Panamericana de la Salud n.d. Available online: https://www.paho.org/es/noticias/14-5-2024-gaffi-ops-unen-fuerzas-para-combatir-infecciones-causadas-por-hongos-america (accessed on 8 May 2025).
- De Fátima Dos Santos, A.; Alves, H.J.; Nogueira, J.T.; Torres, R.M.; Do Carmo Barros Melo, M. Telehealth distance education course in latin America: Analysis of an experience involving 15 countries. Telemed. E-Health 2014, 20, 736–741. [Google Scholar] [CrossRef]
- Jenks, J.D.; Aneke, C.I.; Al-Obaidi, M.M.; Egger, M.; Garcia, L.; Gaines, T.; Hoenigl, M.; Thompson, G.R.; Xue, C. Race and ethnicity: Risk factors for fungal infections? PLoS Pathog. 2023, 19, e1011025. [Google Scholar] [CrossRef] [PubMed]
- Jenks, J.D.; Prattes, J.; Wurster, S.; Sprute, R.; Seidel, D.; Oliverio, M.; Egger, M.; Del Rio, C.; Sati, H.; Cornely, O.A.; et al. Social determinants of health as drivers of fungal disease. EClinicalMedicine 2023, 66, 102325. [Google Scholar] [CrossRef] [PubMed]
- del Carmen Morales-Ramírez, K.; Avila-Sosa, R.; Cid-Pérez, T.S.; Avelino-Flores, F.; Duarte-Escalante, E.; Munguía-Pérez, R. Environmental and Social Determinants Related to Candidiasi. In Candida albicans—Epidemiology and Treatment; Behzadi, P., Ed.; IntechOpen: Tehran, Iran, 2024; Volume 1, pp. 1–122. [Google Scholar] [CrossRef]
- Graham, H.; White, P.C.L. Social determinants and lifestyles: Integrating environmental and public health perspectives. Public. Health 2016, 141, 270–278. [Google Scholar] [CrossRef]
- Brito-Santos, F.; Barbosa, G.G.; Trilles, L.; Nishikawa, M.M.; Wanke, B.; Meyer, W.; Carvalho-Costa, F.A.; Lazéra, M.d.S.; Nielsen, K. Environmental Isolation of Cryptococcus gattii VGII from Indoor Dust from Typical Wooden Houses in the Deep Amazonas of the Rio Negro Basin. PLoS ONE 2015, 10, e0115866. [Google Scholar] [CrossRef]
| Diagnostic Method | Main Advantages | Main Limitations | C. auris | Availability in Latin America | References |
|---|---|---|---|---|---|
| Blood culture (biochemical tests (VITEK, API, and microscanning)) | - Reference standard: isolation, identification, and susceptibility testing | - Prolonged duration (3–4 days) - Moderate sensitivity (21–71%) | Grows reliably at 37–40 °C but identification by conventional phenotypic methods often misidentifies C. auris; requires species-level confirmation | Widely available in most hospitals | [57,58] |
| 1,3-β-D-glucan (serum) | - High sensitivity for invasive candidiasis (92%) - Useful for ruling out systemic infection | - Low specificity (positive in other mycoses) - High cost | Sensitivity ~71% for C. auris candidemia (lower than other Candida spp.); frequent false negatives; not recommended as a standalone diagnostic marker for C. auris | Absent in most centers | [57,58,59] |
| Mannan/Antimannan (serum) | - Allows detection of circulating Candida antigens | - Reduced sensitivity (55–60%) - Interlaboratory variability | Very low sensitivity (<60%) and high inter-laboratory variability for non-albicans species; C. auris often yields negative or indeterminate results; rarely used for C. auris detection | Very scarce: infrequent clinical use | [57,58,60] |
| Quantitative PCR (in blood) | - Results can be obtained in a few hours—sensitivity 92%; specificity 95% | - Requires reference laboratory and highly trained personnel | Direct detection of C. auris DNA in blood within 3–6 h; sensitivity ~92–93%, and specificity ~95–96%; requires specialized equipment and trained personnel; available in ~20% of reference laboratories in Latin America | Only 20% of centers in Argentina | [57,58,61] |
| Conventional endpoint PCR + Sanger | Confirmation of amplicon size via gel electrophoresis, and enhanced sensitivity and specificity through Sanger sequencing of gel-purified products—especially beneficial for detecting longer DNA fragments that short-amplicon qPCR assays may overlook | - Longer turnaround time than qPCR - Requires sequencing capacity | Gold-standard molecular confirmation of C. auris via ITS or D1/D2 sequencing; 100% specificity; turnaround of 1–2 days; limited to labs with sequencing capacity; ideal for resolving ambiguous identifications | Variable; typically available only in reference or specialized labs | [57,58,62] |
| Metagenomic next-generation sequencing (mNGS) | - Broad, hypothesis-free pathogen detection - High sensitivity and specificity for fungi in multiple studies | - High cost, need for specialized infrastructure, bioinformatics expertise, potential contamination, longer turnaround | Culture-independent detection of C. auris (and co-pathogens) with high sensitivity and specificity directly from clinical samples; turnaround of 1–2 days | Limited; available in few references’ centers | [57,58,63] |
| T2Candida (magnetic resonance imaging) | - Direct and rapid detection (3–5 h) - Sensitivity 91%; specificity 99 | - Very high cost - Only five specific strains | Current commercial panel does not include C. auris and thus fails to detect this species; an investigational T2 C. auris panel exists but is not yet clinically available | Almost unavailable; very limited data | [57,58,64] |
| Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry | - MALDI-TOF is highly accurate (97–99%) for common species | - Expensive equipment - Requires previous cultivation | When using an updated library, it accurately distinguishes C. auris from related yeasts (>90% correct ID) within minutes of colony growth; requires prior culture | Available in 20–50% of high-volume laboratories [59] | [57,58,65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motta, J.C.; Rivas-Pinedo, P.; Onate, J.M. Changing Climate, Changing Candida: Environmental and Social Pressures on Invasive Candidiasis and Antifungal Resistance in Latin America. J. Fungi 2025, 11, 609. https://doi.org/10.3390/jof11090609
Motta JC, Rivas-Pinedo P, Onate JM. Changing Climate, Changing Candida: Environmental and Social Pressures on Invasive Candidiasis and Antifungal Resistance in Latin America. Journal of Fungi. 2025; 11(9):609. https://doi.org/10.3390/jof11090609
Chicago/Turabian StyleMotta, Juan Camilo, Pilar Rivas-Pinedo, and José Millan Onate. 2025. "Changing Climate, Changing Candida: Environmental and Social Pressures on Invasive Candidiasis and Antifungal Resistance in Latin America" Journal of Fungi 11, no. 9: 609. https://doi.org/10.3390/jof11090609
APA StyleMotta, J. C., Rivas-Pinedo, P., & Onate, J. M. (2025). Changing Climate, Changing Candida: Environmental and Social Pressures on Invasive Candidiasis and Antifungal Resistance in Latin America. Journal of Fungi, 11(9), 609. https://doi.org/10.3390/jof11090609







