1. Introduction
The cultivation of grapevines, covering approximately 7 million hectares worldwide, is one of the most economically significant agricultural activities globally [
1]. Historically rooted in Europe, with Italy, France, and Spain as leading producers, grapevine cultivation has also expanded significantly to regions such as the United States, Australia, Chile, and South Africa. The grapevine (
Vitis vinifera L.) is highly susceptible to various pathogens, with downy mildew, caused by the oomycete
Plasmopara viticola Berl. & De Toni, being among the most devastating in warm, humid climates characterized by frequent rainfall during flowering and fruit set [
2]. While resistant varieties have been developed through crossbreeding with American vine species, their adoption remains limited [
3], necessitating reliance on careful and costly chemical pesticide applications to protect crops.
From 1890 until the end of the Second World War, the fight against downy mildew relied primarily on copper-based products, such as Bordeaux mixture, discovered serendipitously by Millardet in 1882 [
4]. After the war, the introduction of multi-site synthetic fungicides with contact action, such as dithiocarbamates (e.g., maneb, mancozeb, metiram, propineb, thiram, ziram, and zineb), simplified disease management due to their lower phytotoxicity compared to copper. However, these products now face strict regulations because of their ecotoxicological impacts. In the 1980s, the diffusion on the market of fungicides with cytotropic and systemic capability like cymoxanil and metalaxyl, offering preventive and curative effects, revolutionized disease control. These products, still central to conventional and integrated agriculture, are prone to the development of resistance by pathogens due to their single-site mode of action [
5]. Moreover, their environmental consequences, including soil and water contamination and potential entry into the food chain, have caused growing concern among consumers about the presence of fungicide residues in food products [
6]. In organic agriculture, copper remains the cornerstone of downy mildew management. Although copper is not subject to the evolution of resistance by major pathogens, this heavy metal accumulates in the soil causing phytotoxicity and disrupting soil microbiomes and fauna. As a result, its use is quantitatively restricted and targeted for replacement within the European Union.
Significant research efforts have focused on alternative solutions to replace or complement copper. Biocontrol agents such as
Trichoderma harzianum T39 [
7],
Epicoccum nigrum [
8],
Fusarium proliferatum [
9],
Alternaria alternata [
10],
Lysobacter capsica [
11], and the hyperparasite
Trichothecium plasmoparae [
12] have been extensively studied. Resistance inducers like the non-protein amino acid BABA, chitosan, laminarin, and plant extracts have also received considerable attention [
2,
13]. However, these alternatives have seen limited commercial adoption due to their inconsistent efficacy, particularly during periods of high pathogen pressure. The unique autecology of
P. viticola, which is exclusively associated with liquid films during the infection phase and may complete the penetration in just 2.5 h at 24 °C, likely provide one explanation for the ineffectiveness of fungal biocontrol agents. In fact, the majority of biocontrol fungi are still in the early stages of germination during
P. viticola infection, indicating that a key component of successful biocontrol is the rate at which the fungus colonizes when wet leaves are present. The importance of developing new defense systems is emphasized by the recent epidemic of 2023 in Central and Southern Italy that experienced over 30 rainy days and more than 300 mm of rainfall during May and June, resulting in downy mildew-related losses of 50% to 100% on organic farms. These challenges highlight the urgent need for effective and reliable copper alternatives that can withstand adverse weather conditions, including frequent and heavy rainfall.
In this context, compost tea presents a promising strategy for suppressing plant pathogens. Compost tea is produced through the short-term aerobic or anaerobic brewing of various organic feedstocks in water and is well-known for its plant growth-promoting properties [
14]. Both researchers and farmers have shown significant interest in its potential to suppress numerous plant diseases. In their seminal review, Scheuerell and Mahaffee [
15] highlighted the ability of compost tea to control a range of foliar and soil-borne diseases caused by biotrophic and necrotrophic pathogens. Subsequent studies have reported its suppressive effects on pathogens such as
Pythium ultimum [
15] and
Rhizoctonia solani [
16], as well as foliar diseases caused by
Botrytis cinerea and
Alternaria alternata [
17],
Phytophthora infestans [
18], and various powdery mildew species [
19,
20]. However, the suppressive effect of compost tea is variable, with studies reporting positive effects and others indicating limited efficacy and in others even an increase in the incidence of diseases. The lack of standardization in the raw feedstocks for the production of compost tea as well as the process conditions are elements that certainly contribute to the variability of the results reported in the literature. Surprisingly, despite this growing body of research, there are no published studies, aside from anecdotal reports in gray literature, on the suppressive effects of compost tea against grapevine downy mildew. This gap underscores the need for targeted research to explore its potential as a sustainable alternative for managing this economically significant disease.
The primary objective of this study is to assess the suppressive capacity of compost tea against grapevine downy mildew in an open vineyard setting when combined with synthetic fungicides currently employed to manage this disease. Additionally, the study investigates the mechanisms underlying the suppressive effects of compost tea through a combination of pre- and post-infection assays, extensive bacteriome characterization across vineyard compartments (soil, rhizosphere, and phyllosphere), and a metabolomic analysis to evaluate the grapevine’s physiological response to compost tea application. To ensure the stability and replicability of the results, a fully chemically and microbiologically characterized starting feedstock was used and the brewing conditions were defined both in terms of temperature and volume of oxygen supplied. The study focuses on the following specific hypotheses:
- i.
Compost tea reduces the severity and incidence of grapevine downy mildew in open vineyard conditions when integrated with synthetic fungicides;
- ii.
The suppressive effects of compost tea are mediated by the ability of its bacterial community to establish and persist within the rhizosphere and phyllosphere, enhancing the plant’s natural defense barriers;
- iii.
Compost tea application induces specific metabolomic changes in grapevines, including the upregulation of key metabolic pathways associated with enhanced resistance to P. viticola.
2. Materials and Methods
2.1. Compost Tea Preparation, Chemical and Microbiological Analysis
The commercial compost tea Stimol-C
®, produced by GWA (GimaWater & Air S.r.l., Anagni, Italy), was utilized in this study. The solid organic feedstock comprised hay from polyphyte meadows (20%), compost from cow and horse manure and straw (30%) sourced from organic farms, and dried sunflower residues (50%). The organic fraction of the compost tea used in the study was recently analyzed by
13C CPAMS NMR [
21]. Before the aerobic brewing process, standard techniques were employed to analyze the solid fraction’s properties, including water content, pH, organic carbon content, humic and fulvic acid concentrations, total nitrogen, C/N ratio, and electrical conductivity.
Compost tea was prepared in a 1000 L polyethylene, non-biodegradable container by mixing tap water with the organic feedstock at a ratio of 1:100 (w/v). The solid fraction, enclosed in a porous bag with a 0.2 mm mesh, was submerged in the water and brewed for 24 h. During the process the liquid was aerated with an air pump (Secoh Air Pump, model JDK-40, Wholesale Septic Supply, Dayton, TX, USA) that introduced 60 L of air per minute and generated very fine air bubbles. After the 24 h brewing period, compost tea was immediately applied to the plants for agronomic studies, without undergoing storage. Compost tea was freshly brewed every ten days and promptly sprayed on crops following preparation. The compost tea was applied at 600 L per hectare by means of a 3-point mounted sprayers model CIMA, sprayer New Plus 55 (CIMA SpA, Pavia, Italy).
The compost tea’s chemical properties, including pH, electrical conductivity (EC), dissolved organic carbon (DOC), dissolved organic nitrogen (DON), biological oxygen demand (BOD), dissolved oxygen, nitrate, and ammonium, were measured. Dissolved oxygen, EC, and pH were determined using a multi-parametric probe (M40+ instrument, Crison, Alella, Spain). The IRSA-CNR 5110 protocol was employed to analyze DOC, DON, and BOD, while the respirometric approach utilized the Oxitop OC100 system (OXITO-C) (WTW
® Xylem
® OxiTop
® OC100 Xylem Inc.301 Water Street SE, Suite 200, Washington, DC, USA). Nitrate and ammonium concentrations were assessed using a DR 3900 Spectrophotometer (Hach, Loveland, CO, USA) with manufacturer kits LCK 340 (assay range 5–35 mg L
−1) for nitrate and LCK 303 (assay range 2–47 mg L
−1) for ammonium. The chemical characteristics of compost tea at the time of applications are reported in
Table 1.
Concerning compost tea safety, according to the official procedures established by the Italian Ministry of Agricultural Food and Forestry Policies (MIPAF 2014), the presence of human pathogens, specifically Salmonella spp. and Escherichia coli, has been evaluated on organic feedstock. In this regard, Salmonella spp. and E. coli were not detected in the solid feedstock by our systematic microbiological investigations.
2.2. Field Evaluation of Compost Tea for Managing Downy Mildew in Vineyards
The field experiment was conducted in the spring and summer of 2023 in an 18-year-old Montepulciano vineyard located in Cerignola, Puglia, Southern Italy, with a planting density of 2500 plants per hectare. All the viticultural practices, with the exception of disease control, including fertilization, irrigation, and pruning, were comparable to the standard agronomical management adopted in the surrounding area. The soil is poor in terms of organic carbon (1.4%), with pH of 7.8, EC of 220 μS cm−1, total nitrogen (N) content of 1.1 g kg−1, phosphorus pentoxide (P2O5) content of 2.8 mg kg−1, total limestone of 153.7 g kg−1, Magnesium of 0.2 g kg−1, exchangeable Sodium of 0.02 g kg−1, Potassium of 1.34 g kg−1, Iron of 43.2 mg kg−1, Copper of 22.0 mg kg−1, Zinc of 5.4 mg kg−1, and Manganese of 6.0 mg kg−1.
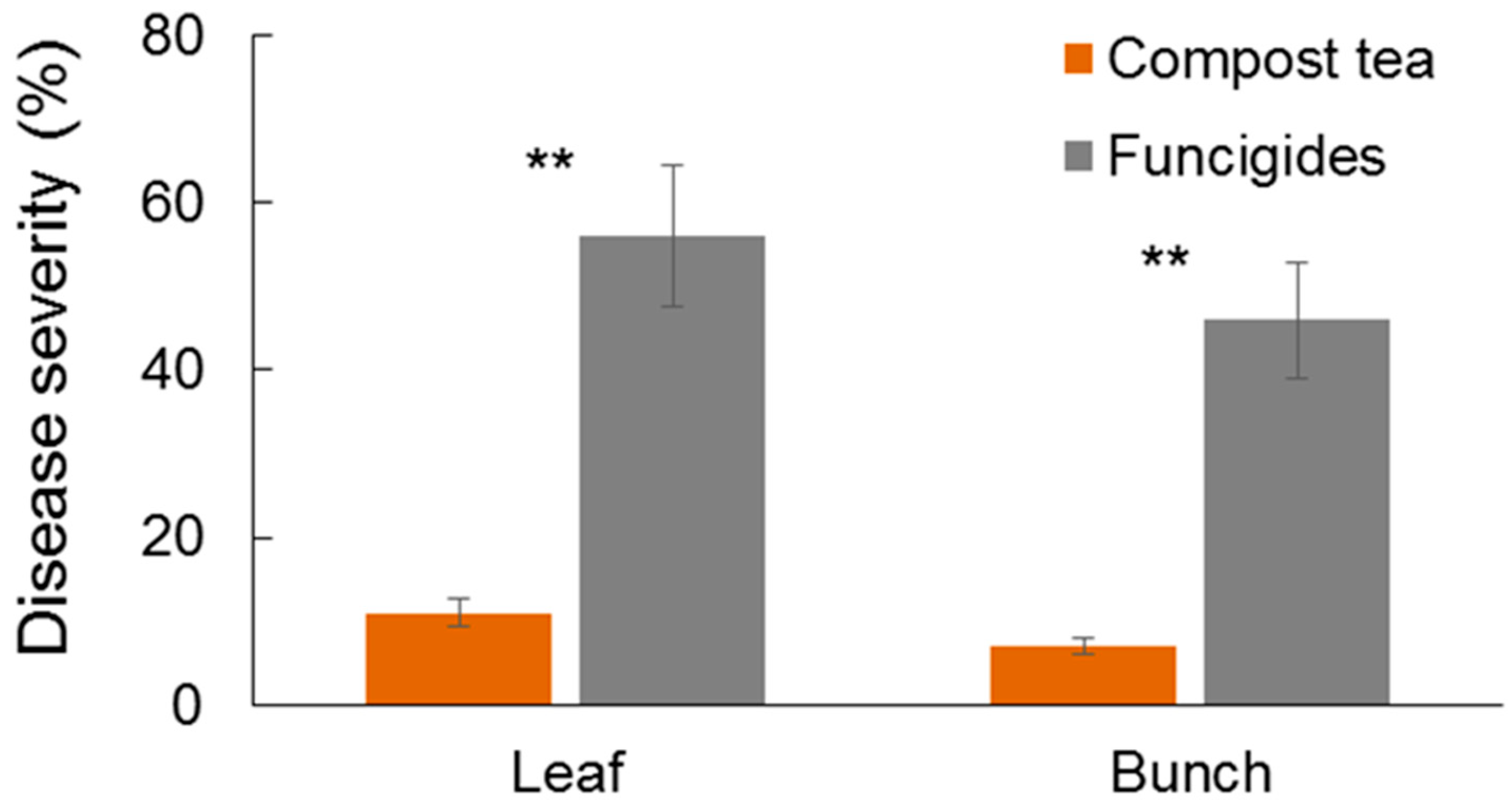
The objective was to test the effectiveness of compost tea in controlling downy mildew under field conditions. Two phytosanitary management strategies were compared. The first strategy involved conventional farm management, which relied on synthetic fungicides such as cymoxanil, metalaxyl, strobilurins, and zoxamide, applied every 10 to 14 days depending on climatic conditions. Due to frequent rains in 2023 [
22], a total of 15 applications were made from mid-April to late July. The second strategy utilized compost tea, applied at a rate of 600 L per hectare every 10 to 14 days, supplemented by two cymoxanil applications during the post-flowering and fruit-setting phases. In total, 13 compost tea applications and two cymoxanil applications were performed. Notably, no copper-based products were used in either plant protection strategy. The fungicides were applied at the dosage reported on the label and applied by means of 3-point mounted sprayers (model CIMA, sprayer New Plus 55).
The experiment was set up with three replicates per strategy, with each replicate consisting of 100 plants arranged across four vineyard rows for a total of 600 plants. At the end of July 2023 and 2024, the severity of downy mildew on both leaves and grape bunches was visually assessed. In each block, 20 plants were randomly selected, and data were collected on the number and size of downy mildew spots on leaves, as well as the presence and extent of infection on grape bunches. Statistical analysis of the differences between the two defense strategies was performed using a t-test.
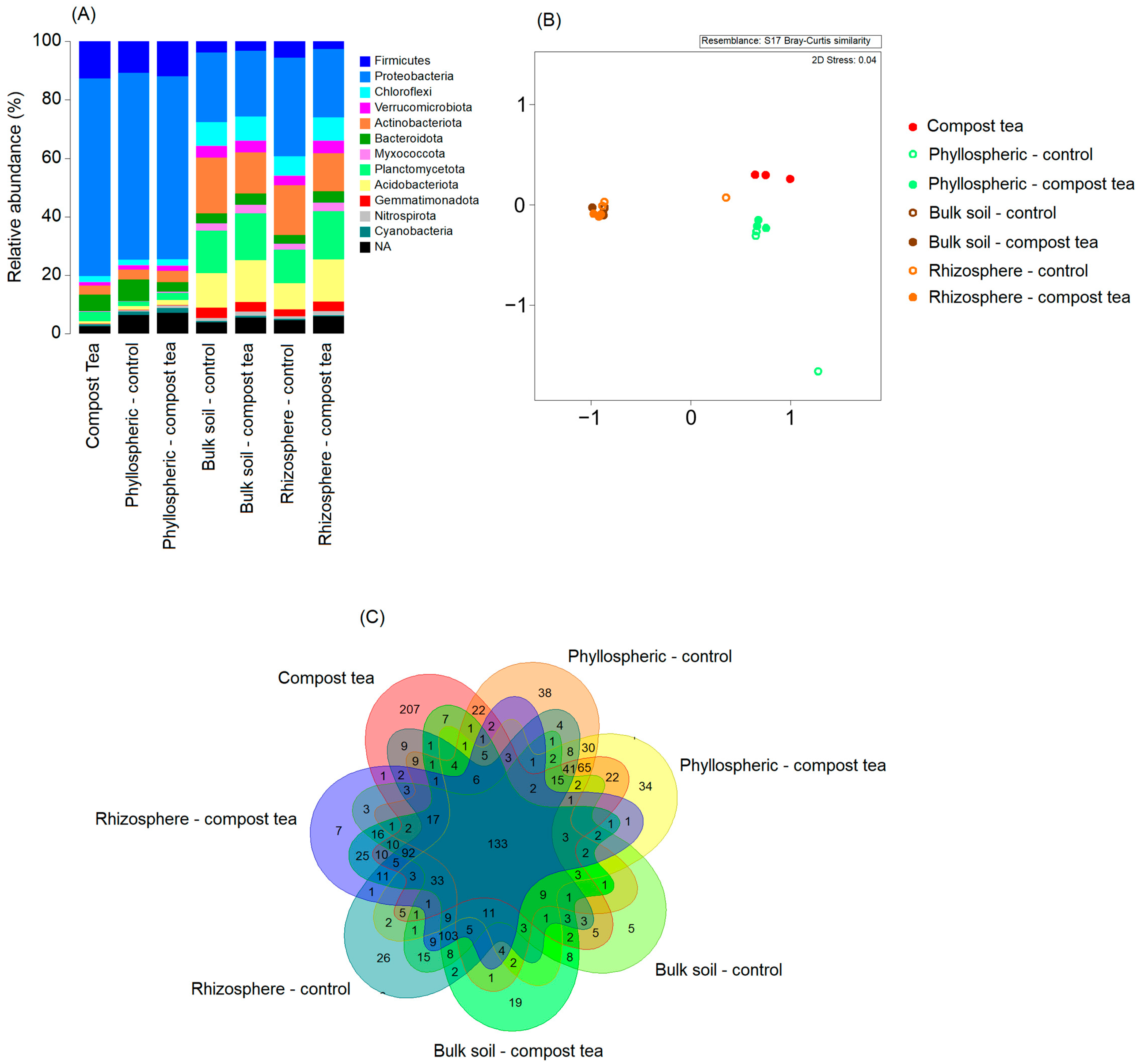
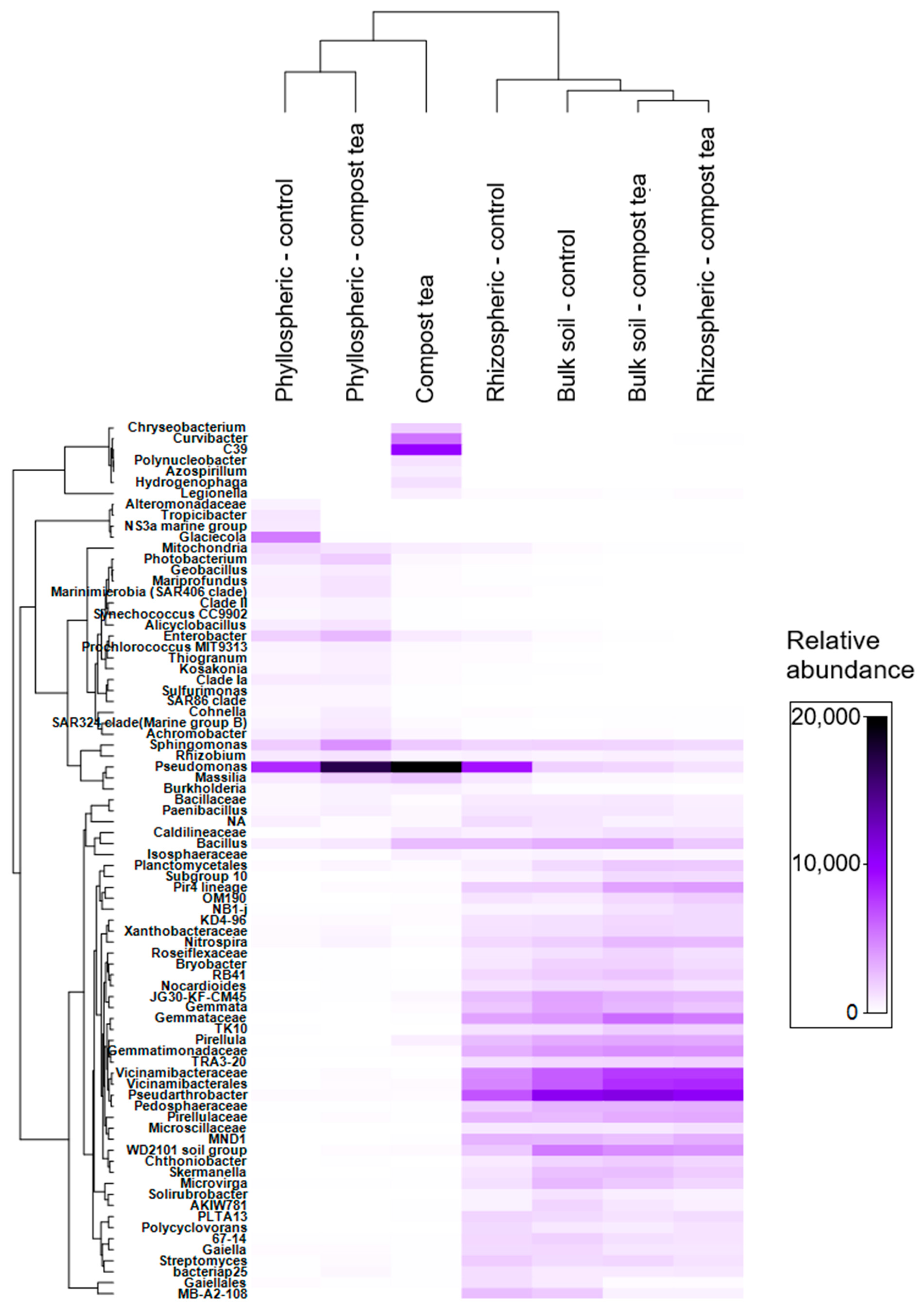
2.3. Grapevine and Compost Tea Bacteriome
The objective of this activity was twofold: first, to characterize the bacterial community of the compost tea itself, and second, to compare the bacteriome of the vineyard ecosystem’s main compartments, soil, rhizosphere, and phyllosphere, under treatments with either compost tea or synthetic fungicides. In late July 2023, samples were collected for analysis. Freshly brewed compost tea was sampled by taking 1 L at the end of the brewing process conducted on the farm. For the vineyard bacteriome, 50 fully expanded, non-senescent leaves were collected from each of the 20 plants previously selected for downy mildew assessment. Bulk soil samples were taken from five locations within each block, combined to form one composite sample per block. Soil was collected from the top 30 cm of the profile after removing surface litter and weeds. Rhizosphere samples were obtained by carefully excavating soil to expose vine roots and collecting the soil adhering to them. All samples, phyllosphere, rhizosphere, and bulk soil, were placed in polyethylene bags, kept refrigerated at 4 °C during transport to the laboratory on the same day, and subsequently stored at −20 °C until DNA extraction.
DNA was extracted from the collected samples, including compost tea, bulk soil, rhizosphere soil, and phyllosphere, using the DNeasy PowerSoil Kit (Qiagen, Germantown, MD, USA) according to the manufacturer’s protocol. For compost tea, 500 mL of the sample was filtered through a 0.2 μm membrane to concentrate bacterial cells. The membrane was cut into small pieces and processed directly using the PowerSoil kit. For bulk soil samples, 0.25 g of soil was used for each extraction, ensuring representative sampling. Rhizosphere samples were carefully prepared by processing only the soil tightly adhering to the vine roots. For the phyllosphere, bacterial cells were detached by washing leaves in sterile phosphate-buffered saline by vortexing for 5 min. The wash solution was filtered through a 0.2 μm membrane, which was then processed with the PowerSoil kit. The quantity of extracted DNA was measured using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA), and quality was verified via agarose gel electrophoresis. PCR amplification targeted the V3–V4 region of the 16S rRNA gene (approximately 460 bp) to profile bacterial communities. Bacterial 16S rRNA amplification was performed using primers S-D-Bact-0341-b-S-17 and S-D-Bact-0785-a-A-21. Amplification conditions followed the protocols described in the respective studies. PCR products were purified using Agencourt AMPure XP beads (Beckman Coulter, Milan, Italy) and quantified with an AF2200 Plate Reader (Eppendorf, Milan, Italy). Libraries were prepared according to Illumina’s standard protocols, and sequencing was performed on the MiSeq platform (Illumina, Milan, Italy), generating paired end reads of 2 × 250 bp.
2.4. Effects of Pre- and Post-Infection Applications of Compost Tea on P. viticola
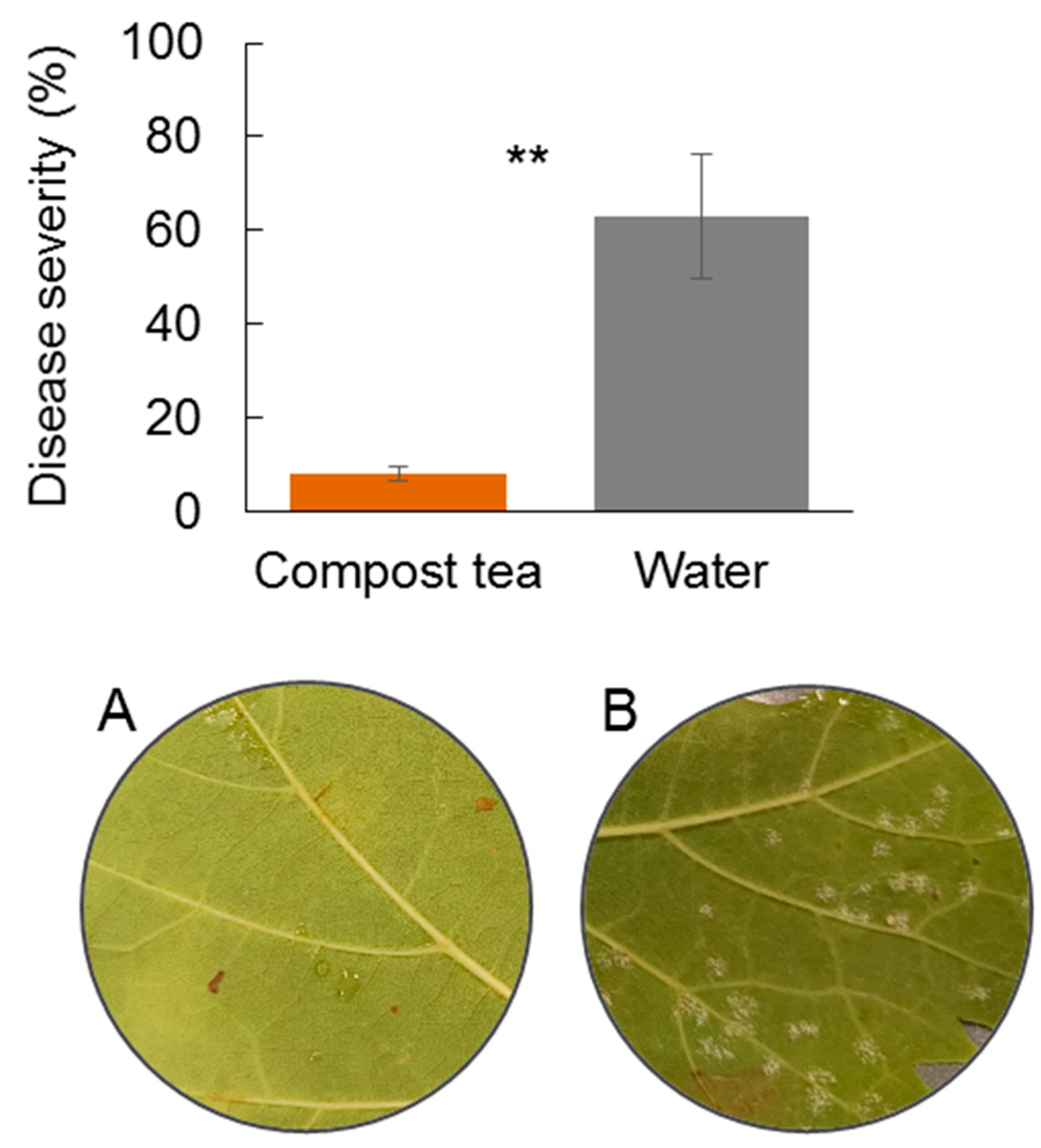
In order to further understand the suppressive effect of compost tea on downy mildew observed in open fields, two experiments were conducted in spring 2024 under controlled conditions to investigate the potential effect on the infection and sporulation process of P. viticola. The experiments were performed using whole leaves in one case and leaf disk in the second case in the laboratory. Briefly, three-year-old plants of the V. vinifera cv. Montepulciano were grown under shade-greenhouse conditions in large, 30 L pots. A P. viticola population was collected in a vineyard not treated located in the Department of Agriculture in May 2024 and maintained by periodical inoculations on potted grapevine grown in shade-house. The P. viticola inoculum was prepared collecting disease leaf with evident symptoms that were incubated overnight in darkness at 100% RH and 22 ± 2 °C to promote the sporulation of the pathogen. Sporangia were then collected by gently washing the abaxial leaf surfaces bearing freshly sporulating lesions with distilled water. The inoculum concentration was adjusted to 1 × 105 sporangia mL−1 by light microscope using a hemocytometer. The sporangia suspension, in the experiments, was applied to the abaxial leaf until the surface was completely wet. For the inoculum, grape leaves collected from V. vinifera cv. Montepulciano were surface sterilized by immerging them for 1 min in 1% v/v solution of sodium hypochlorite. After that, the leaves were abundantly rinsed three times with distilled water and dried on filter paper. Entire excised leaf or leaf disk with a 15 mm diameter were cut using a scissor and placed, abaxial side up, in Petri dishes (9 cm), over moistened filter paper layer. After the inoculum, Petri dishes were sealed with parafilm and incubated in a growth chamber at 24 ± 2 °C in the dark. Disease severity was quantified at 5 days after inoculation (dpi) as percentage of leaf area that was covered by P. viticola lesion and sporulation. Twenty replicates were assessed for each treatment and each experiment was carried out twice.
In the first experiment, aimed at investigating the infection process, entire and excised grapevine leaves prepared as described above were sprayed with compost tea or distilled water as control and left to dry for 2 h. Following this treatment, the leaves were inoculated with sporangia as described above and incubated in dark conditions at 22 ± 2 °C, with disease severity quantified after 5 days of dpi. In the second experiment, aimed at investigating the effect on sporulation, infected grapevine leaves with evident sporulation on the abaxial leaf surface were sampled. Leaf disks were cut with scissors at the points of abundant sporulation and emerged in the previous 24 h. These leaf disks were sprayed with compost tea or distilled water and subsequently incubated as described. After two days the state of the sporulated areas was evaluated using an optical microscope.
2.5. Metabolomics of Grapevine Leaves Treated with Compost Tea
For metabolomic analyses, grape leaves were collected from Vitis vinifera cv. Montepulciano grown in greenhouses at the Department of Agriculture, located in the Royal Park of Portici (40°48′40.3″ N, 14°20′33.8″ E; 75 m a.s.l.). The leaves were categorized into four groups: (1) healthy grapevine leaves not treated with compost tea (water), (2) healthy grapevine leaves treated with compost tea (compost tea), (3) grapevine leaves affected by downy mildew but not treated with compost tea (water inoculated), and (4) grapevine leaves affected by downy mildew and treated with compost tea (compost tea inoculated). The plant material was freeze-dried (Zirbus VaCo 2, ZIRBUS Technology GmbH, Bad Grund, Germany) and ground using a mortar and pestle. For each sample, 50 mg of the powdered material was extracted using three sequential solvents: methanol, dichloromethane, and n-hexane (500 µL each). After the addition of each solvent, the suspension was vortexed for 30 s and centrifuged at 13,000 rpm for 10 min. The supernatants were pooled and evaporated to dryness using a vacuum concentrator (Savant™ SpeedVac™ SPD130, Thermo Fisher Scientific Inc., USA). The dried extracts were derivatized by adding 1 mL of N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA, Merck KGaA, Darmstadt, Germany). The reaction mixture was incubated in an ultrasonic bath (Sonorex, Bandelin Electronic GmbH & Co. KG, Berlin, Germany) at room temperature for 30 min.
2.6. GC-MS Analysis
The trimethylsilyl derivatives were analyzed using an Agilent 8890 GC system (Agilent Technologies, Santa Clara, CA, USA) coupled with an Agilent 5977B Inert MS detector. The instrument was equipped with an HP-5MS capillary column (5%-phenyl)-methylpolysiloxane stationary phase) for compound separation. The GC oven was programmed with the following temperature gradient: an initial temperature of 90 °C, increasing at a rate of 10 °C/min to a final temperature of 300 °C, which was held for 10 min. The solvent delay was set to 5 min. The injector operated in splitless mode at 250 °C, and helium was used as the carrier gas at a flow rate of 1 mL/min. The injection volume was 1 µL. Mass spectrometry measurements were performed in full scan mode (m/z 35–550) with electron impact (EI) ionization at 70 eV. The ion source and quadrupole mass filter temperatures were maintained at 230 °C and 150 °C, respectively. Metabolite identification was performed by comparing the acquired mass spectra with those stored in the NIST20 library. Identification was considered successful when the match factor associated with the comparison was above 800.
2.7. Data Processing and Statistical Analysis
Metabolomic statistical analysis was performed using Mass Profile Professional software, version 13.1.1 (Agilent Technologies). Raw data were grouped by experimental condition and subjected to univariate analysis between paired conditions using Student’s t-test (p-value < 0.05), with a fold change threshold of ≥2.0. Metabolite identification was achieved by comparing the obtained mass spectra with those in the NIST20 library (National Institute of Standards and Technology). Aligned abundance values were exported to R for further analysis. Principal Coordinates Analysis (PCoA) was conducted using the vegan package (function capscale) to explore relationships between experimental conditions based on Spearman rank correlations. The results were visualized as a 2D scatter plot using the ggplot2 package. Diversity indices were calculated using the vegan package (function diversity), and box plots representing the distribution of the indices were generated with ggplot2. Hierarchical clustering analysis was performed using the pheatmap package to generate a heatmap of metabolite relative abundance. Clustering was performed using the hclust function with Euclidean distance and complete linkage. Venn diagrams to compare the overlap of identified metabolites across different experimental conditions were created using the VennDiagram package, specifically with the venn.diagram function.
For bacterial community composition analysis, relative abundance data were visualized as stacked bar plots using ggplot2. PCoA was performed on Bray–Curtis dissimilarity matrices using the vegan package (functions vegdist for distance calculation and ordinate for ordination). The resulting plot was created using ggplot2. Differences between groups were assessed using PERMANOVA (function adonis from the vegan package) with 999 permutations. Alpha diversity metrics, including species richness (observed ASVs), Shannon index, and Pielou’s evenness, were calculated using the vegan package (functions specaccum for species richness and diversity for Shannon index and Pielou’s evenness). Boxplots were generated using ggplot2, and statistical significance between experimental groups was determined using one-way ANOVA followed by post hoc Tukey’s HSD test for multiple comparisons. The Venn diagram for bacterial taxa overlap was created using the limma package (function voom for variance modeling and differential abundance analysis), with the overlap visualized using the Venn function from limma to highlight shared and unique ASVs between the experimental groups.
5. Conclusions
This study conclusively demonstrates that a repeated compost tea application, integrated with just two targeted cymoxanil treatments, represents a highly effective and sustainable strategy for managing grapevine downy mildew, even under severe disease pressure from climatically challenging conditions. Crucially, this innovative defense strategy achieved an over 80% reduction in synthetic fungicide use compared to conventional approaches, marking a significant advancement towards more environmentally conscious viticulture. Beyond its impressive field efficacy, our comprehensive investigation provides pivotal insights into the multifaceted mechanisms underlying compost tea’s suppressive power. We revealed its direct actions in limiting P. viticola infection and sporulation, thereby disrupting key phases of the pathogen’s life cycle. Furthermore, this research highlights compost tea’s profound ability to induce systemic resistance in grapevines, evidenced by the upregulation of defense-related metabolites like shikimic acid and the maintenance of essential compounds such as tartaric acid under stress. Concurrently, the repeated application of compost tea notably modifies the plant’s microbiome in both the rhizosphere and phyllosphere, enriching beneficial bacterial groups (e.g., Pseudomonas, Sphingomonas, Bacillus) that likely contribute to enhanced plant health and biocontrol potential. We also established that, with standardized feedstock and brewing, compost tea can exhibit a consistent and stable microbial composition, addressing a long-standing challenge in its practical application. While these promising results are specifically validated for the Montepulciano variety in the Puglia region, laying a strong foundation for sustainable viticulture, future research should broaden its scope. Investigating the efficacy of this integrated strategy across diverse viticultural contexts and grape varieties is essential for wider adoption. Additionally, further studies should evaluate the compatibility of compost tea with fungicides possessing different modes of action and explore its potential as a standalone or copper alternative in organic systems. Unraveling the precise molecular underpinnings of compost tea-induced resistance through the quantification of specific plant defense enzymes will further deepen our understanding and optimize its use.