Species Distribution and Antifungal Susceptibility Patterns of Invasive Candidiasis in a Belgian Tertiary Center: A 7-Year Retrospective Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Episodes
2.2. Isolation and Species-Specific Identification of Yeast Isolates
2.3. Antifungal Susceptibility Testing
2.4. Statistical Analysis
3. Results
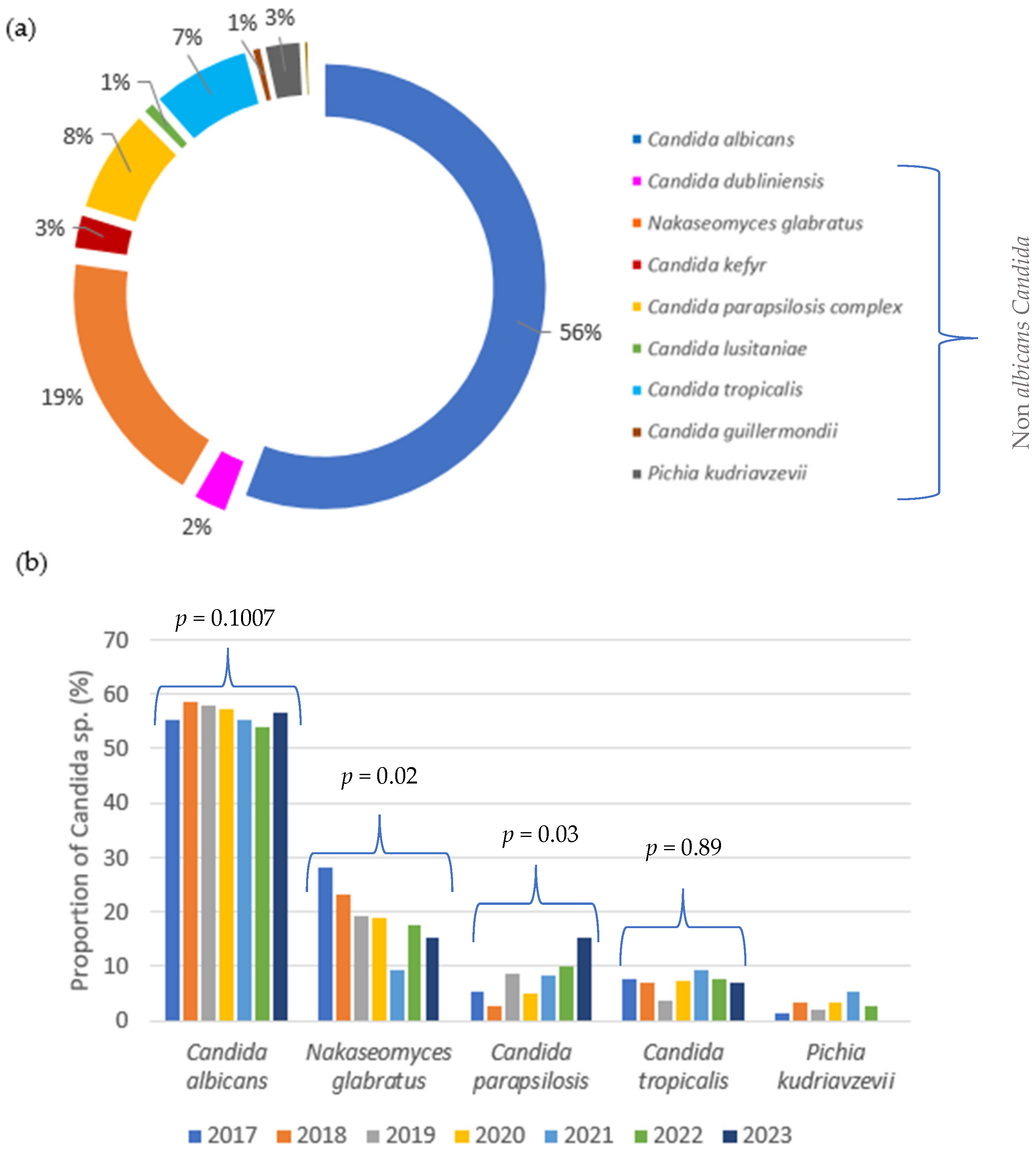
3.1. Temporal Trends and Distribution of Candida Species over Seven Years
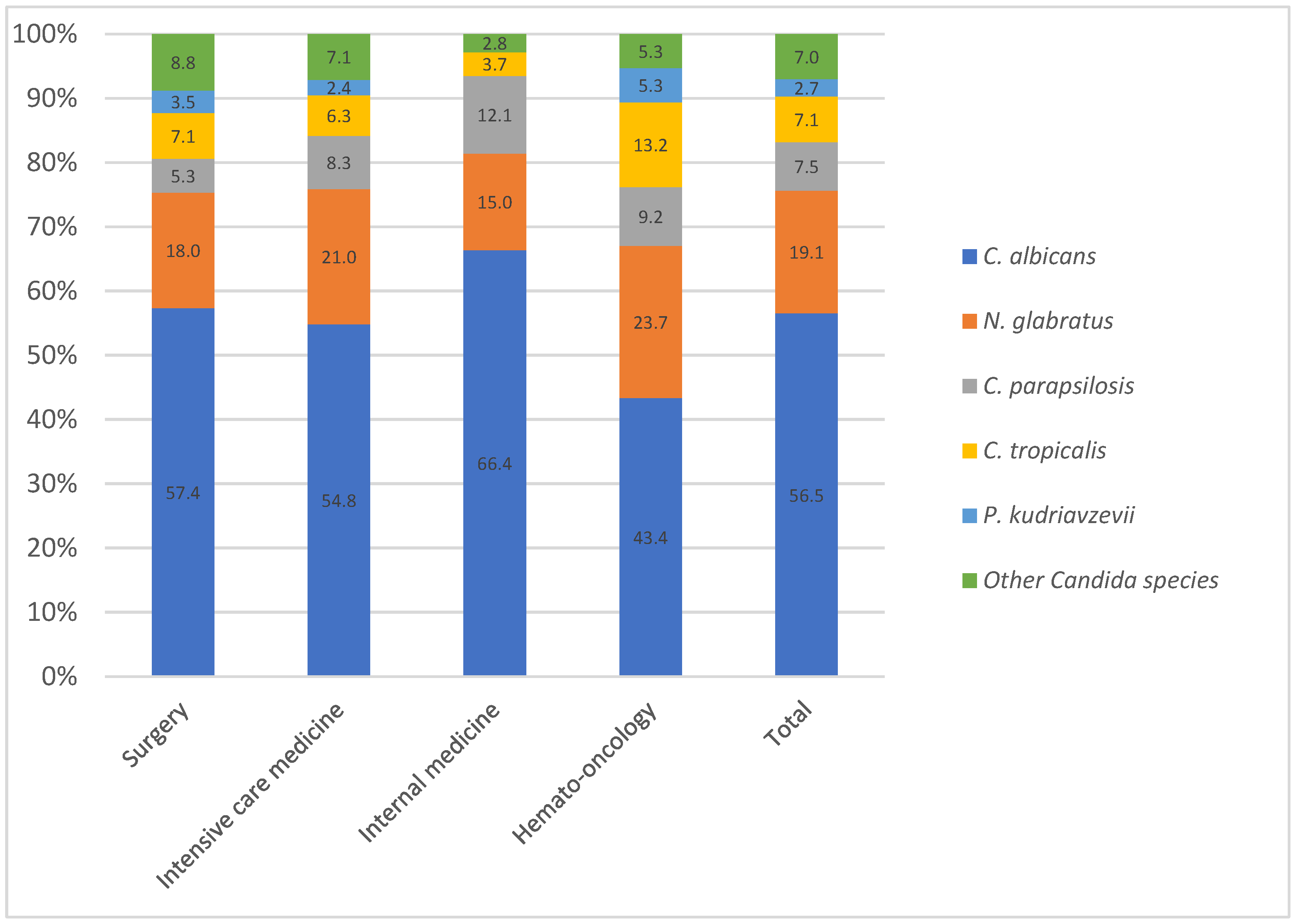
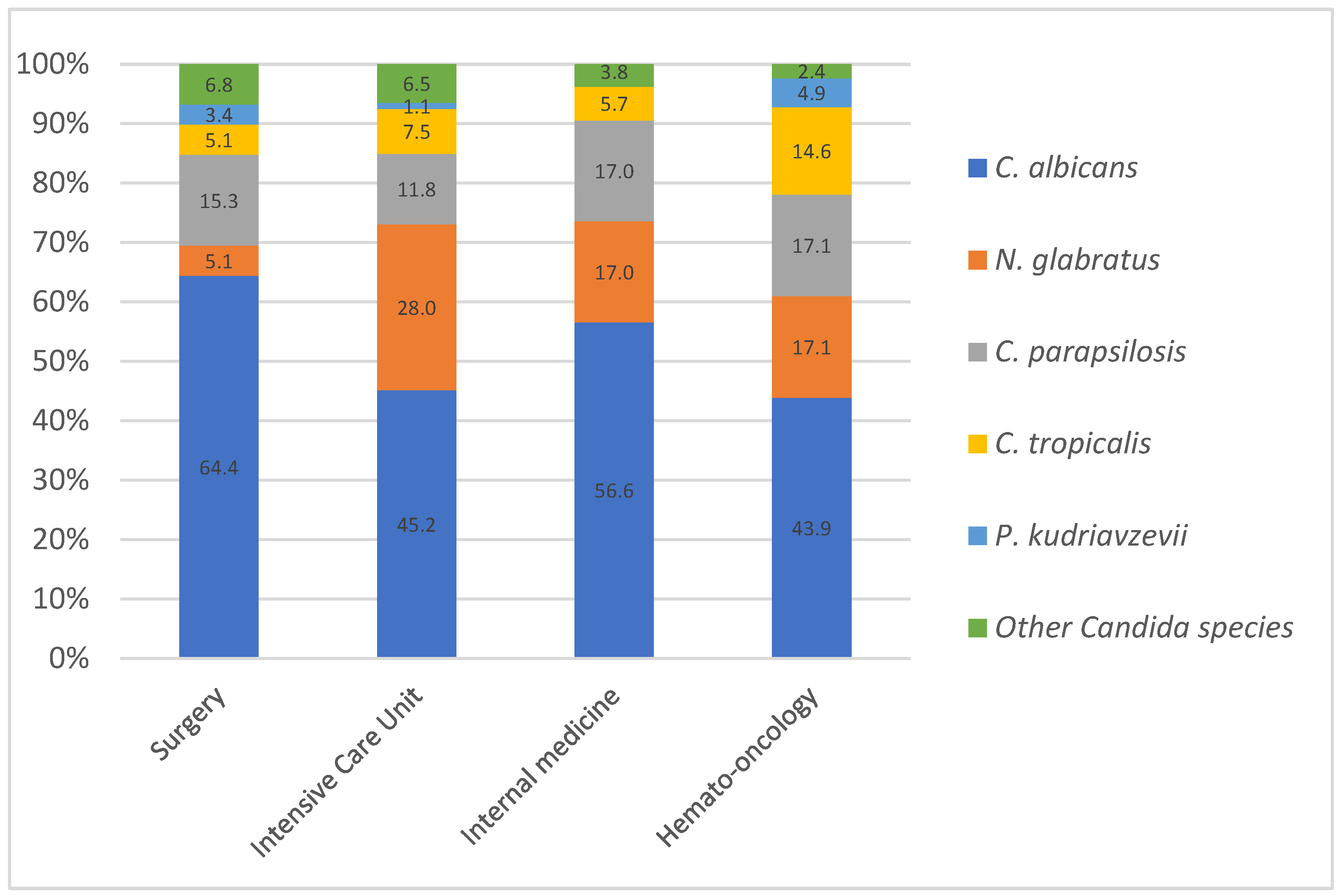
3.2. Species Distribution and Epidemiological Trends in Bloodstream Candida Infection
3.3. Antifungal Susceptibility Patterns of Candida Species: Azole Resistance and Echinocandin Activity
Fluconazole Susceptibility and Temporal Trends in C. parapsilosis
3.4. Isolated Detection of C. auris in a Single Imported Case
4. Discussion
4.1. Temporal Distribution of Candida Species
4.2. Distribution Across Medical Wards: Influence of Azole in High-Risk Patients
4.3. Trends in Antifungal Resistance: A Focus on Candida Species and Emerging Resistance Patterns
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Denning, D.W. Global Incidence and Mortality of Severe Fungal Disease. Lancet Infect. Dis. 2024, 24, e428–e438. [Google Scholar] [CrossRef] [PubMed]
- Wolfgruber, S.; Sedik, S.; Klingspor, L.; Tortorano, A.; Gow, N.A.R.; Lagrou, K.; Gangneux, J.-P.; Maertens, J.; Meis, J.F.; Lass-Flörl, C.; et al. Insights from Three Pan-European Multicentre Studies on Invasive Candida Infections and Outlook to ECMM Candida IV. Mycopathologia 2024, 189, 70. [Google Scholar] [CrossRef] [PubMed]
- WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action, 1st ed.; World Health Organization: Geneva, Switzerland, 2022.
- Bassetti, M.; Giacobbe, D.R.; Agvald-Ohman, C.; Akova, M.; Alastruey-Izquierdo, A.; Arikan-Akdagli, S.; Azoulay, E.; Blot, S.; Cornely, O.A.; Cuenca-Estrella, M.; et al. Invasive Fungal Diseases in Adult Patients in Intensive Care Unit (FUNDICU): 2024 Consensus Definitions from ESGCIP, EFISG, ESICM, ECMM, MSGERC, ISAC, and ISHAM. Intensive Care Med. 2024, 50, 502–515. [Google Scholar] [CrossRef] [PubMed]
- Soriano, A.; Honore, P.M.; Puerta-Alcalde, P.; Garcia-Vidal, C.; Pagotto, A.; Gonçalves-Bradley, D.C.; Verweij, P.E. Invasive Candidiasis: Current Clinical Challenges and Unmet Needs in Adult Populations. J. Antimicrob. Chemother. 2023, 78, 1569–1585. [Google Scholar] [CrossRef]
- Kullberg, B.J.; Arendrup, M.C. Invasive Candidiasis. N. Engl. J. Med. 2016, 374, 793–795. [Google Scholar] [CrossRef]
- Risum, M.; Astvad, K.; Johansen, H.K.; Schønheyder, H.C.; Rosenvinge, F.; Knudsen, J.D.; Hare, R.K.; Datcu, R.; Røder, B.L.; Antsupova, V.S.; et al. Update 2016–2018 of the Nationwide Danish Fungaemia Surveillance Study: Epidemiologic Changes in a 15-Year Perspective. J. Fungi 2021, 7, 491. [Google Scholar] [CrossRef]
- Escribano, P.; Guinea, J. Fluconazole-Resistant Candida Parapsilosis: A New Emerging Threat in the Fungi Arena. Front. Fungal Biol. 2022, 3, 1010782. [Google Scholar] [CrossRef]
- Daneshnia, F.; De Almeida Júnior, J.N.; Ilkit, M.; Lombardi, L.; Perry, A.M.; Gao, M.; Nobile, C.J.; Egger, M.; Perlin, D.S.; Zhai, B.; et al. Worldwide Emergence of Fluconazole-Resistant Candida Parapsilosis: Current Framework and Future Research Roadmap. Lancet Microbe 2023, 4, e470–e480. [Google Scholar] [CrossRef]
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive Candidiasis. Nat. Rev. Dis. Primer 2018, 4, 18026. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekema, D.J.; Turnidge, J.D.; Castanheira, M.; Jones, R.N. Twenty Years of the SENTRY Antifungal Surveillance Program: Results for Candida Species From 1997–2016. Open Forum Infect. Dis. 2019, 6, S79–S94. [Google Scholar] [CrossRef]
- Adam, K.-M.; Osthoff, M.; Lamoth, F.; Conen, A.; Erard, V.; Boggian, K.; Schreiber, P.W.; Zimmerli, S.; Bochud, P.-Y.; Neofytos, D.; et al. Trends of the Epidemiology of Candidemia in Switzerland: A 15-Year FUNGINOS Survey. Open Forum Infect. Dis. 2021, 8, ofab471. [Google Scholar] [CrossRef] [PubMed]
- Franconi, I.; Rizzato, C.; Tavanti, A.; Falcone, M.; Lupetti, A. Paradigm Shift: Candida Parapsilosis Sensu Stricto as the Most Prevalent Candida Species Isolated from Bloodstream Infections with Increasing Azole-Non-Susceptibility Rates: Trends from 2015–2022 Survey. J. Fungi 2023, 9, 1012. [Google Scholar] [CrossRef] [PubMed]
- Franconi, I. The S862C Amino Acid Change in CpMrr1 Confers Fluconazole Resistance in Candida Parapsilosis. JAC Antimicrob. Resist. 2025, 7, dlaf051. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, A.; Sharma, C.; Meis, J.F. Candida Auris: A Rapidly Emerging Cause of Hospital-Acquired Multidrug-Resistant Fungal Infections Globally. PLoS Pathog. 2017, 13, e1006290. [Google Scholar] [CrossRef]
- Lockhart, S.R.; Etienne, K.A.; Vallabhaneni, S.; Farooqi, J.; Chowdhary, A.; Govender, N.P.; Colombo, A.L.; Calvo, B.; Cuomo, C.A.; Desjardins, C.A.; et al. Simultaneous Emergence of Multidrug-Resistant Candida auris on 3 Continents Confirmed by Whole-Genome Sequencing and Epidemiological Analyses. Clin. Infect. Dis. 2017, 64, 134–140. [Google Scholar] [CrossRef]
- CLSI M27M44S; Performance Standards for Antifungal Susceptibility Testing of Yeasts. CLSI: Wayne, PA, USA, 2022.
- Cornely, O.A.; Sprute, R.; Bassetti, M.; Chen, S.C.-A.; Groll, A.H.; Kurzai, O.; Lass-Flörl, C.; Ostrosky-Zeichner, L.; Rautemaa-Richardson, R.; Revathi, G.; et al. Global Guideline for the Diagnosis and Management of Candidiasis: An Initiative of the ECMM in Cooperation with ISHAM and ASM. Lancet Infect. Dis. 2025, 25, e280–e293. [Google Scholar] [CrossRef]
- Parslow, B.Y.; Thornton, C.R. Continuing Shifts in Epidemiology and Antifungal Susceptibility Highlight the Need for Improved Disease Management of Invasive Candidiasis. Microorganisms 2022, 10, 1208. [Google Scholar] [CrossRef]
- De Francesco, M.A.; Piccinelli, G.; Gelmi, M.; Gargiulo, F.; Ravizzola, G.; Pinsi, G.; Peroni, L.; Bonfanti, C.; Caruso, A. Invasive Candidiasis in Brescia, Italy: Analysis of Species Distribution and Antifungal Susceptibilities During Seven Years. Mycopathologia 2017, 182, 897–905. [Google Scholar] [CrossRef]
- Delaloye, J.; Calandra, T. Invasive Candidiasis as a Cause of Sepsis in the Critically Ill Patient. Virulence 2014, 5, 161–169. [Google Scholar] [CrossRef]
- Lamoth, F.; Lockhart, S.R.; Berkow, E.L.; Calandra, T. Changes in the Epidemiological Landscape of Invasive Candidiasis. J. Antimicrob. Chemother. 2018, 73 (Suppl. S1), i4–i13. [Google Scholar] [CrossRef]
- Chapman, B.; Slavin, M.; Marriott, D.; Halliday, C.; Kidd, S.; Arthur, I.; Bak, N.; Heath, C.H.; Kennedy, K.; Morrissey, C.O.; et al. Changing Epidemiology of Candidaemia in Australia. J. Antimicrob. Chemother. 2016, 72, 1103–1108. [Google Scholar] [CrossRef] [PubMed]
- Arendrup, M.C.; Arikan-Akdagli, S.; Jørgensen, K.M.; Barac, A.; Steinmann, J.; Toscano, C.; Arsenijevic, V.A.; Sartor, A.; Lass-Flörl, C.; Hamprecht, A.; et al. European Candidaemia Is Characterised by Notable Differential Epidemiology and Susceptibility Pattern: Results from the ECMM Candida III Study. J. Infect. 2023, 87, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Tsay, S.V.; Mu, Y.; Williams, S.; Epson, E.; Nadle, J.; Bamberg, W.M.; Barter, D.M.; Johnston, H.L.; Farley, M.M.; Harb, S.; et al. Burden of Candidemia in the United States, 2017. Clin. Infect. Dis. 2020, 71, e449–e453. [Google Scholar] [CrossRef] [PubMed]
- Hesstvedt, L.; Gaustad, P.; Andersen, C.T.; Haarr, E.; Hannula, R.; Haukland, H.H.; Hermansen, N.-O.; Larssen, K.W.; Mylvaganam, H.; Ranheim, T.E.; et al. Twenty-Two Years of Candidaemia Surveillance: Results from a Norwegian National Study. Clin. Microbiol. Infect. 2015, 21, 938–945. [Google Scholar] [CrossRef]
- Goemaere, B.; Becker, P.; Van Wijngaerden, E.; Maertens, J.; Spriet, I.; Hendrickx, M.; Lagrou, K. Increasing Candidaemia Incidence from 2004 to 2015 with a Shift in Epidemiology in Patients Preexposed to Antifungals. Mycoses 2018, 61, 127–133. [Google Scholar] [CrossRef]
- Trouvé, C.; Blot, S.; Hayette, M.-P.; Jonckheere, S.; Patteet, S.; Rodriguez-Villalobos, H.; Symoens, F.; Van Wijngaerden, E.; Lagrou, K. Epidemiology and Reporting of Candidaemia in Belgium: A Multi-Centre Study. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 649–655. [Google Scholar] [CrossRef]
- Bassetti, M.; Merelli, M.; Righi, E.; Diaz-Martin, A.; Rosello, E.M.; Luzzati, R.; Parra, A.; Trecarichi, E.M.; Sanguinetti, M.; Posteraro, B.; et al. Epidemiology, Species Distribution, Antifungal Susceptibility, and Outcome of Candidemia across Five Sites in Italy and Spain. J. Clin. Microbiol. 2013, 51, 4167–4172. [Google Scholar] [CrossRef]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1–e50. [Google Scholar] [CrossRef]
- Tortorano, A.M.; Prigitano, A.; Morroni, G.; Brescini, L.; Barchiesi, F. Candidemia: Evolution of Drug Resistance and Novel Therapeutic Approaches. Infect. Drug Resist. 2021, 14, 5543–5553. [Google Scholar] [CrossRef]
- Bays, D.; Jenkins, E.; Lyman, M.; Chiller, T.; Strong, N.; Ostrosky-Zeichner, L.; Hoenigl, M.; Pappas, P.; Thompson, G. Epidemiology of Invasive Candidiasis. Clin. Epidemiol. 2024, 16, 549–566. [Google Scholar] [CrossRef]
- Vu, B.G.; Moye-Rowley, W.S. Azole-Resistant Alleles of ERG11 in Candida Glabrata Trigger Activation of the Pdr1 and Upc2A Transcription Factors. Antimicrob. Agents Chemother. 2022, 66, e02098-21. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, S.R. Current Epidemiology of Candida Infection. Clin. Microbiol. Newsl. 2014, 36, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Hii, I.-M.; Chang, H.-L.; Lin, L.-C.; Lee, Y.-L.; Liu, Y.-M.; Liu, C.-E.; Chen, C.-H.; Cheng, Y.-R.; Chang, C.-Y. Changing Epidemiology of Candidemia in a Medical Center in Middle Taiwan. J. Microbiol. Immunol. Infect. 2015, 48, 306–315. [Google Scholar] [CrossRef] [PubMed]
| MIC Range (µg/mL) | MIC50 (µg/mL) | MIC90 (µg/mL) | Number of Isolates (%) | ||||
|---|---|---|---|---|---|---|---|
| S/WT * | SDD | I | R/Non-WT ** | ||||
| C. albicans (n = 509) | |||||||
| Amphotericin B | ≤0.12–4 | 0.5 | 1 | 508 (99.8) | - | - | 1 (0.2) |
| Fluconazole | ≤0.12–16 | 0.25 | 1 | 503 (98.8) | 1 (0.2) | - | 5 (1) |
| Voriconazole | ≤0.008–0.5 | 0.015 | 0.03 | 507 (99.6) | 2 (0.4) | 0 | |
| Itraconazole | - | - | - | - | - | - | - |
| Caspofungin | ≤0.008–0.25 | 0.14 | 0.12 | 509 (100) | - | 0 | 0 |
| Anidulafungin | ≤0.015–0.25 | 0.06 | 0.12 | 509 (100) | - | 0 | 0 |
| N. glabratus (n = 168) | |||||||
| Amphotericin B | ≤0.12–2 | 0.5 | 1 | 168 (100) | - | - | - |
| Fluconazole | 0.25–≥256 | 16 | 32 | - | 151 (89.9) | - | 17 (10.1) |
| Voriconazole | ≤0.015–8 | 0.25 | 1 | - | 79 (45.3) | - | 92 (54.7) |
| Itraconazole | 0.5–16 | 0.5 | 1 | - | 160 (95.3) | - | 8 (4.7) |
| Caspofungin | ≤0.0008–8 | 0.12 | 0.12 | 161 (95.8) | - | 5 (3) | 2 (1.2) |
| Anidulafungin | ≤0.015–2 | 0.12 | 0.12 | 167 (99.4) | - | 0 | 1 (0.6) |
| C. parapsilosis sensu stricto (n = 69) | |||||||
| Amphotericin B | ≤0.12–2 | 0.25 | 0.5 | 69 (100) | - | - | - |
| Fluconazole | 0.25–8 | 0.5 | 1 | 67 (97.1) | - | - | 2 (2.9) |
| Voriconazole | ≤0.015–0.25 | 0.03 | 0.12 | 68 (98.5) | - | 1 (1.5) | - |
| Itraconazole | ≤0.008–0.25 | 0.015 | 0.03 | 69 (100) | - | - | - |
| Caspofungin | 0.12–4 | 0.5 | 1 | 67 (97.1) | - | 2 (2.9) | 0 |
| Anidulafungin | 0.12–4 | 1 | 2 | 65 (95.6) | - | 4 (4.4) | 0 |
| C. tropicalis (n = 65) | |||||||
| Amphotericin B | ≤0.12–2 | 0.5 | 1 | 65 (100) | - | - | |
| Fluconazole | 0.25–≥256 | 0.25 | 4 | 44 (67.7) | 10 (15.4) | - | 11 (16.9) |
| Voriconazole | 0.015–8 | 0.25 | 0.5 | 58 (89.2) | - | 0 | 7 (10.8)) |
| Itraconazole | 0.03–8 | 0.12 | 0.5 | 57 (87.7) | - | 1 (1.5) | 7 (10.8) |
| Caspofungin | 0.015–0.5 | 0.06 | 0.5 | 59 (90.7) | - | 6 (9.3) | - |
| Anidulafungin | 0.015–0.5 | 0.12 | 0.25 | 63 (96.9) | - | 2 (3.1) | - |
| P. kudriavzevii (n = 25) | |||||||
| Amphotericin B | ≤0.25–2 | 1 | 2 | 25 (100) | - | - | |
| Fluconazole | 8–128 | 64 | 128 | - | - | - | 25 (100) |
| Voriconazole | 0.12–1 | 0.5 | 0.5 | 24 (96) | - | 1 (4) | |
| Itraconazole | 0.12–1 | 0.5 | 0.5 | 24 (96) | - | - | 1 (4) |
| Caspofungin | 0.12–1 | 0.5 | 0.5 | 11 (44) | - | 13 (52) | 1 (4) |
| Anidulafungin | ≤0.015–4 | 0.06 | 0.12 | 24 (96) | - | 1 (4) | - |
| Antifungal | Susceptibility | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 |
|---|---|---|---|---|---|---|---|---|
| Fluconazole | S | 7/7 (100%) | 4/4 (100%) | 11/12 (91.7%) | 6/6 (100%) | 11/11 (100%) | 13/14 (92.9%) | 15/15 (100%) |
| SDD | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| R | 0 | 0 | 1/12 (8.3%) | 0 | 0 | 1/14 (7.1%) | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cugnata, S.; Sacheli, R.; Layios, N.; Hayette, M.-P. Species Distribution and Antifungal Susceptibility Patterns of Invasive Candidiasis in a Belgian Tertiary Center: A 7-Year Retrospective Analysis. J. Fungi 2025, 11, 465. https://doi.org/10.3390/jof11060465
Cugnata S, Sacheli R, Layios N, Hayette M-P. Species Distribution and Antifungal Susceptibility Patterns of Invasive Candidiasis in a Belgian Tertiary Center: A 7-Year Retrospective Analysis. Journal of Fungi. 2025; 11(6):465. https://doi.org/10.3390/jof11060465
Chicago/Turabian StyleCugnata, Sarah, Rosalie Sacheli, Nathalie Layios, and Marie-Pierre Hayette. 2025. "Species Distribution and Antifungal Susceptibility Patterns of Invasive Candidiasis in a Belgian Tertiary Center: A 7-Year Retrospective Analysis" Journal of Fungi 11, no. 6: 465. https://doi.org/10.3390/jof11060465
APA StyleCugnata, S., Sacheli, R., Layios, N., & Hayette, M.-P. (2025). Species Distribution and Antifungal Susceptibility Patterns of Invasive Candidiasis in a Belgian Tertiary Center: A 7-Year Retrospective Analysis. Journal of Fungi, 11(6), 465. https://doi.org/10.3390/jof11060465







