Foliar Epichloë gansuensis Endophyte and Root-Originated Bacillus subtilis LZU7 Increases Biomass Accumulation and Synergistically Improve Nitrogen Fixation in Achnatherum inebrians
Abstract
1. Introduction
2. Methods and Materials
2.1. Plant Materials
2.2. Detection and Isolation of E. gansuensis Endophyte
2.3. Isolation and Identification of Endophytic Bacteria
2.4. Functional Characterization of Endophytic Bacteria
2.4.1. IAA Determination
2.4.2. The Measurement of Siderophores
2.4.3. Determination of Nitrogen Fixation Potential
2.4.4. Real-Time Amplification Assay of nifH
2.5. Plate Confrontation Experiments Between Epichloë Endophyte and Endophytic Bacterial Strains
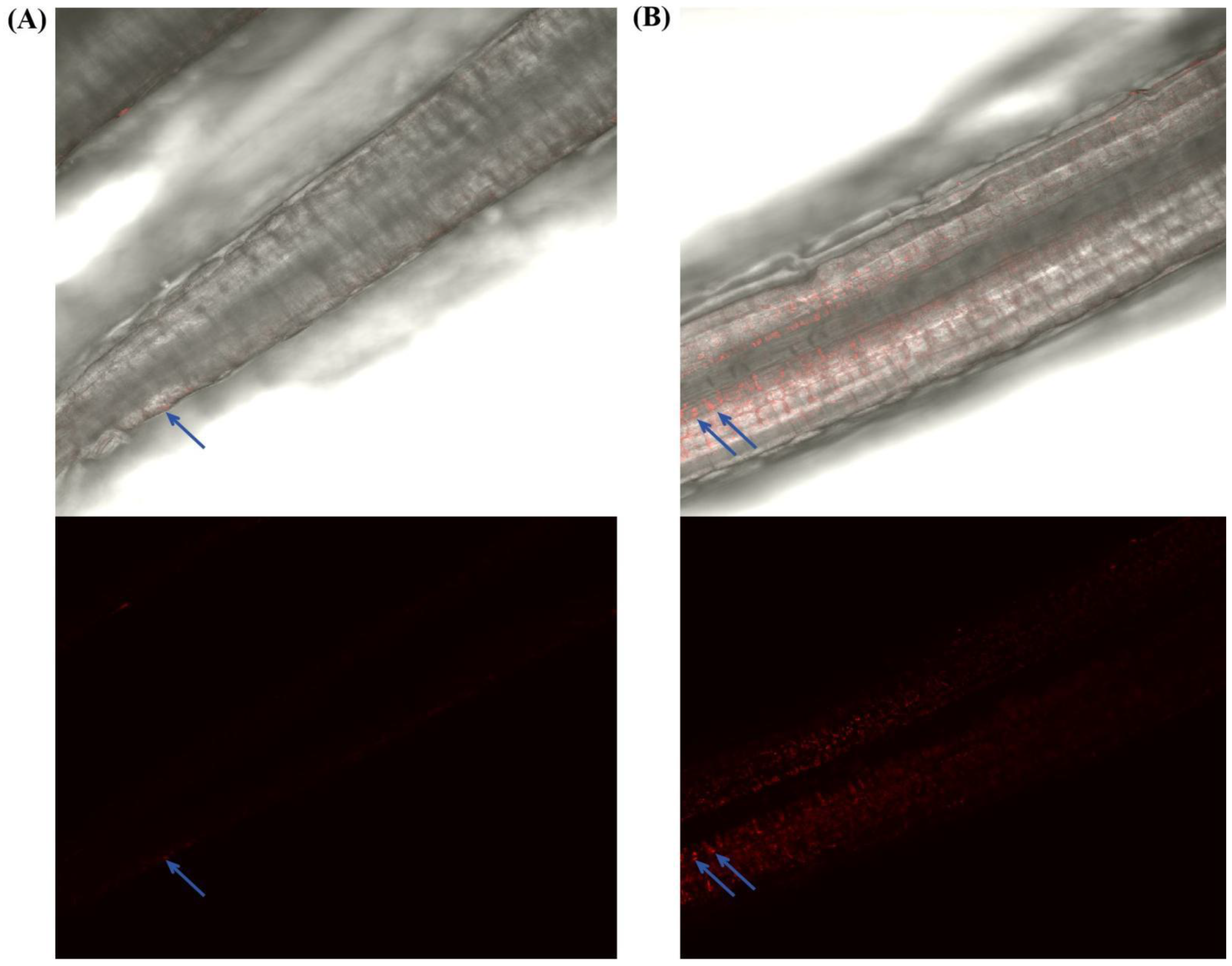
2.6. Colonization of A. inebrians Roots by GFP-Tagged Endophytic Bacteria
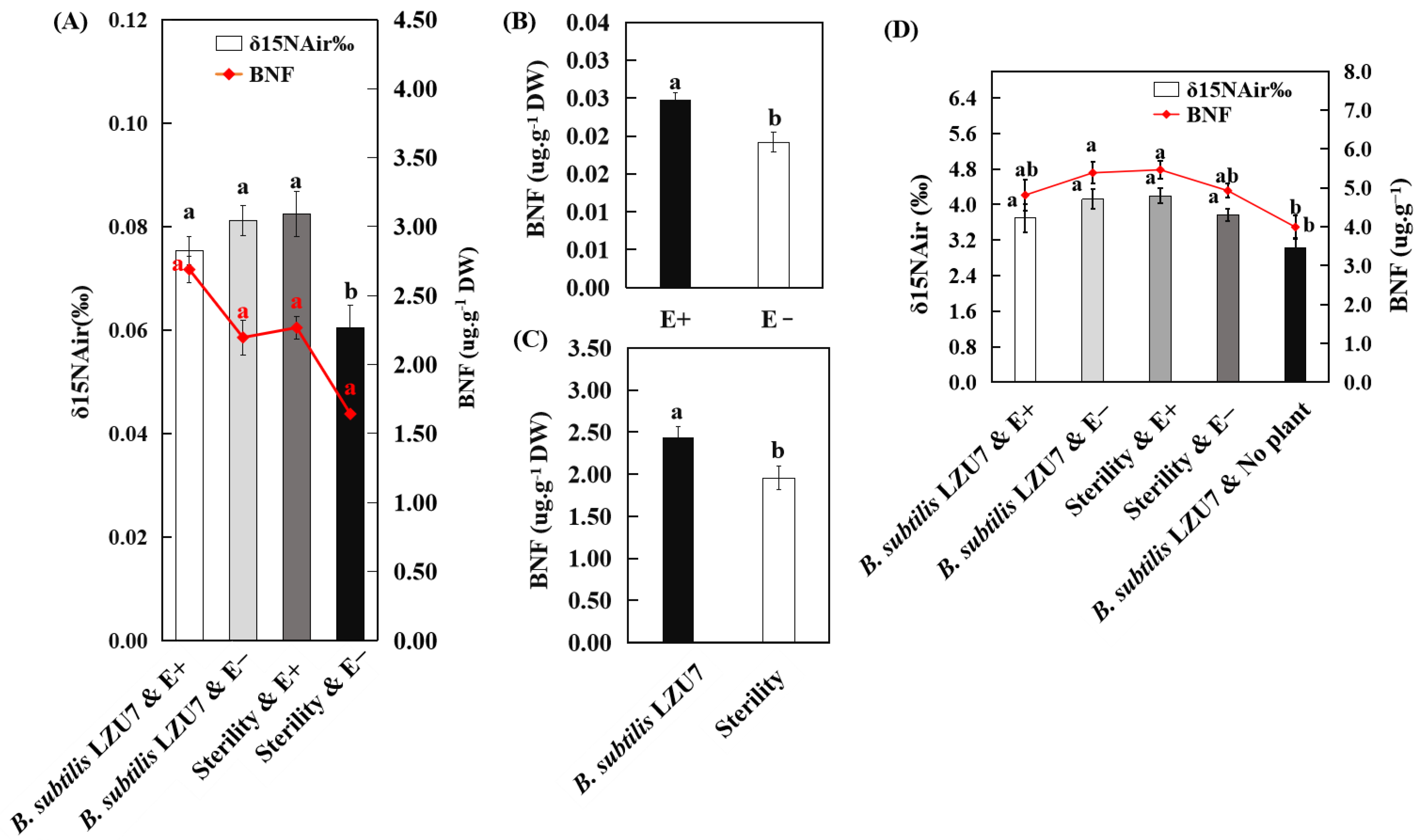
2.7. 15N Isotope Dilution Method
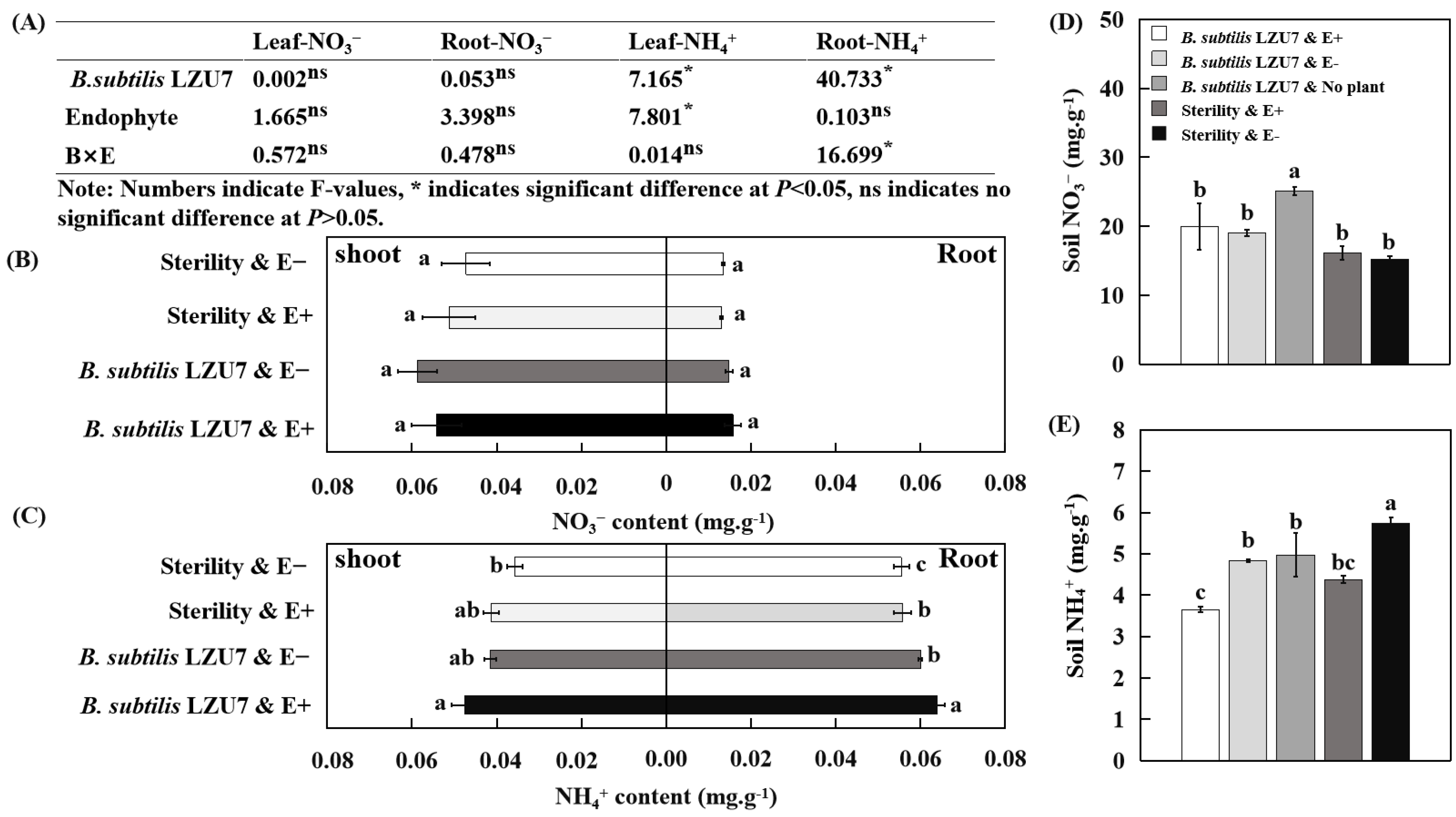
2.8. Plant and Soil Nutrient Determination
2.9. Statistical Analysis
3. Results
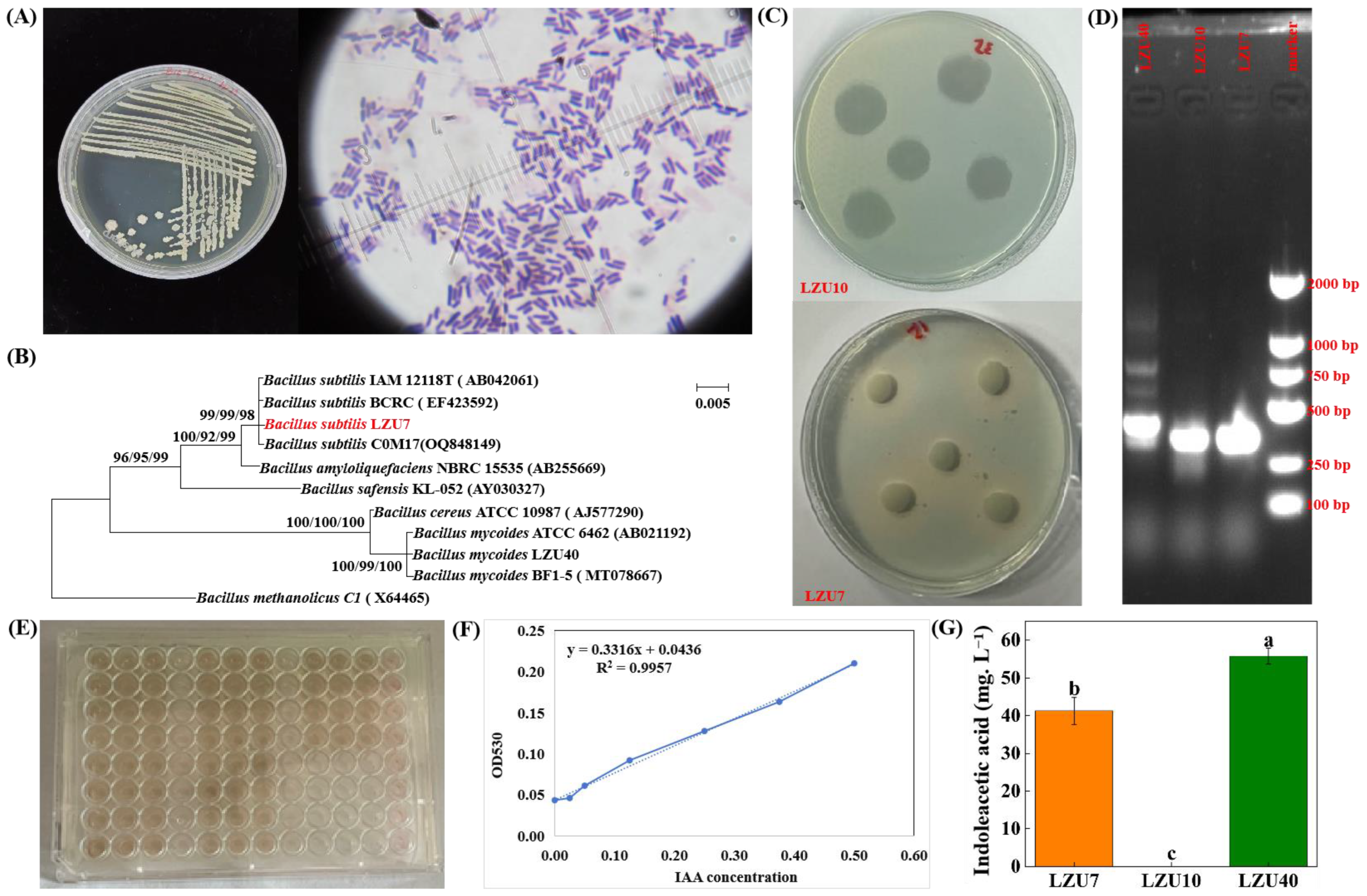
3.1. Community Structure and Function of Endophytic Bacteria in Roots of A. inebrians
3.2. E. gansuensis Endophyte Promotes the Growth and Colonization of Bacillus subtilis LZU7
3.3. E. gansuensis Endophyte and B. subtilis LZU7 Promote A. inebrians Growth and Increase Yield
3.4. Interactions Between E. gansuensis and B. subtilis LZU7 Enhance Nitrogen Fixation in A. inebrians
3.5. E. gansuensis Endophyte Increased the Nitrogen and Biomass Accumulation of A. inebrians by Interacting with B. subtilis LZU7
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chepick, G.P.; Cho, R. Interactive effects of fungal endophyte infection and host genotype on growth and storage in Lolium perenne. New Phytol. 2003, 158, 183–191. [Google Scholar] [CrossRef]
- Rey, T.; Chatterjee, A.; Buttay, M.; Toulotte, J.; Schornack, S. Medicago truncatula symbiosis mutants affected in the interaction with a biotrophic root pathogen. New Phytol. 2015, 206, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, S.; Harrison, M.J. Receptor-associated kinases control the lipid provisioning program in plant-fungal symbiosis. Science 2024, 383, 443–448. [Google Scholar] [CrossRef]
- Parniske, M. Arbuscular mycorrhiza: The mother of plant root endosymbioses. Nat. Rev. Microbiol. 2008, 6, 763–765. [Google Scholar] [CrossRef] [PubMed]
- Schardl, C.L.; Leuchtmann, A.; Spiering, M.J. Symbioses of grasses with seedborne fungal endophytes. Annu. Rev. Plant Biol. 2004, 55, 315–340. [Google Scholar] [CrossRef]
- Clay, K. Fungal endophyte symbiosis and plant diversity in successional fields. Science 1999, 285, 1742–1744. [Google Scholar] [CrossRef]
- Leuchtmann, A. Systematics, distribution, and host specificity of grass endophytes. Nat. Toxins 1992, 1, 150–162. [Google Scholar] [CrossRef]
- Popay, A.J.; Thom, E.R. Endophyte effects on major insect pests in Waikato dairy pasture. N. Z. Grassl. Assoc. 2009, 71, 121–126. [Google Scholar] [CrossRef]
- Vázquez-de-Aldana, B.R.; García-Ciudad, A.; García-Criado, B.; Vicente-Tavera, S.; Zabalgogeazcoa, I. Fungal endophyte (Epichloë festucae) alters the nutrient content of Festuca rubra regardless of water availability. PLoS ONE 2013, 18, e84539. [Google Scholar] [CrossRef]
- Guo, J.Q.; Mcculley, R.L.; Phillips, T.D.; Mcnear, D.H. Fungal endophyte and tall fescue cultivar interact to differentially affect bulk and rhizosphere soil processes governing c and n cycling. Soil Biol. Biochem. 2016, 101, 165–174. [Google Scholar] [CrossRef]
- Cavazos, B.R.; Bohner, T.F.; Donald, M.L.; Sneck, M.E.; Shadow, A.; Omacini, M.; Rudgers, J.A.; Miller, T.E.X. Testing the roles of vertical transmission and drought stress in the prevalence of heritable fungal endophytes in annual grass populations. New Phytol. 2018, 219, 1075–1084. [Google Scholar] [CrossRef]
- Oberhofer, M.; Güsewell, S.; Leuchtmann, A. Effects of natural hybrid and non-hybrid Epichloë endophytes on the response of Hordelymus europaeus to drought stress. New Phytol. 2024, 201, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Pereira, E.C.; Aldana, B.R.V.D.; Arellano, J.B.; Zabalgogeazcoa, I. The Role of fungal microbiome components on the adaptation to salinity of Festuca rubra subsp. pruinosa. Front. Plant Sci. 2021, 12, 695717–695732. [Google Scholar] [CrossRef] [PubMed]
- Dupont, P.Y.; Eaton, C.J.; Wargent, J.J.; Fechtner, S.; Solomon, P.; Schmid, J.; Day, R.C.; Scott, B.; Cox, M. Fungal endophyte infection of ryegrass reprograms host metabolism and alters development. New Phytol. 2015, 208, 1227–1240. [Google Scholar] [CrossRef]
- Purahong, W.; Hyde, K.D. Effects of fungal endophytes on grass and non-grass litter decomposition rates. Fungal Divers. 2011, 47, 1–7. [Google Scholar] [CrossRef]
- Young, C.A.; Hume, D.E.; McCulley, R.L. Forages and pastures symposium: Fungal endophytes of tall fescue and perennial ryegrass: Pasture friend or foe? J. Anim. Sci. 2013, 91, 2379–2394. [Google Scholar] [CrossRef]
- Li, F.; Deng, J.; Nzabanita, C.; Li, Y.Z.; Duan, T.Y. Growth and physiological responses of perennial ryegrass to an AMF and an Epichloë endophyte under different soil water contents. Symbiosis 2019, 79, 151–161. [Google Scholar] [CrossRef]
- Guo, S.; Geisen, S.; Mo, Y.N.; Yan, X.Y.; Huang, R.L.; Liu, H.J.; Gao, Z.L.; Tao, C.Y.; Deng, X.H.; Xiong, W.; et al. Predatory protists impact plant performance by promoting plant growth-promoting rhizobacterial consortia. ISME J. 2024, 18, wrae180. [Google Scholar] [CrossRef]
- Kennedy, I.R.; Choudhury, A.T.M.A.; Kecskés, M.L. Non-symbiotic bacterial diazotrophs in crop-farming systems: Can their potential for plant growth promotion be better exploited? Soil Biol. Biochem. 2004, 36, 1229–1244. [Google Scholar] [CrossRef]
- Lugtenberg, B.; Kamilova, F. Plant-growth-promoting rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef]
- Wang, W.B.; Portal-Gonzalez, N.; Wang, X.; Li, J.L.; Li, H.; Portieles, R.; Borras-Hidalgo, O.; He, W.X.; Santos-Berrmudez, R. Metabolome-driven microbiome assembly determining the health of ginger crop (Zingiber officinalel. roscoe) against rhizome rot. Microbiome 2024, 12, 167–187. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, R.; Hashem, A.; Abd_Allah, E.F. Bacillus: A biological tool for crop improvement through bio-molecular changes in adverse environments. Front. Physiol. 2017, 8, 667. [Google Scholar] [CrossRef] [PubMed]
- Figueredo, M.S.; Tonelli, M.L.; Taurian, T.; Angelini, J.; Ibañez, F.; Valetti, L.; Muñoz, V.; Anzuay, S.; Ludueña, L.; Fabra, A. Interrelationships between Bacillus sp. CHEP5 and Bradyrhizobium sp. SEMIA6144 in the induced systemic resistance against Sclerotium rolfsii and symbiosis on peanut plants. J. Biosci. 2014, 39, 877–885. [Google Scholar] [CrossRef]
- Kaschuk, G.; Auler, A.C.; Vieira, C.E.; Dakora, F.D.; Jaiswal, S.K.; Da-Cruz, S.P. Coinoculation impact on plant growth promotion: A review and meta-analysis on coinoculation of rhizobia and plant growth-promoting bacilli in grain legumes. Braz. J. Microbiol. 2022, 53, 2027–2037. [Google Scholar] [CrossRef]
- Kalantari, S.; Marefat, A.; Naseri, B.; Hemmati, R. Improvement of bean yield and Fusarium root rot biocontrol using mixtures of Bacillus, Pseudomonas and Rhizobium. Trop. Plant Pathol. 2018, 43, 499–505. [Google Scholar] [CrossRef]
- Wang, T.Q.; Chen, Q.Q.; Liang, Q.; Zhao, Q.; Lu, X.; Tian, J.H.; Guan, Z.D.; Liu, C.; Li, J.F.; Zho, M.; et al. Bacillus suppresses nitrogen efficiency of soybean–rhizobium symbiosis through regulation of nitrogen-related transcriptional and microbial patterns. Plant Cell Environ. 2024, 47, 4305–4322. [Google Scholar] [CrossRef]
- Soundararajan, S.; Jedd, G.; Li, X.L.; Ramos-Pamploña, M.; Chua, N.H.; Naqvia, N.I. Woronin body function in magnaporthe grisea is essential for efficient pathogenesis and for survival during nitrogen starvation stress. Plant Cell 2004, 16, 1564–1574. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Cheng, Z.Q.; Jin, L.Y.; Zhang, T.; Liang, L.M.; Cheng, L.J.; Zhang, Q.Y.; Xu, X.H.; Lan, C.H.; et al. Microbiota and functional analyses of nitrogen-fixing bacteria in root-knot nematode parasitism of plants. Microbiome 2023, 11, 48. [Google Scholar] [CrossRef]
- Jin, Y.Y.; Chen, Z.J.; White, J.F.; Malik, K.; Li, C.J. Interactions between Epichloë endophyte and the plant microbiome impact nitrogen responses in host Achnatherum inebrians plants. Microbiol. Spectr. 2024, 12, e0257423. [Google Scholar] [CrossRef]
- Liu, L.; Gundersen, P.; Zhang, T.; Mo, J.M. Effects of phosphorus addition on soil microbial biomass and community composition in three forest types in tropical China. Soil Biol. Biochem. 2012, 44, 31–38. [Google Scholar] [CrossRef]
- Yang, H.J.; Li, Y.; Wu, M.Y.; Zhang, Z.; Li, L.H.; Wan, S.Q. Plant community responses to nitrogen addition and increased precipitation: The importance of water availability and species traits. Glob. Change Biol. 2011, 17, 2936–2944. [Google Scholar] [CrossRef]
- Faeth, S.H.; Fagan, W.F. Fungal endophytes: Common host plant symbionts but uncommon mutualists. Integr. Comp. Biol. 2002, 42, 360–368. [Google Scholar] [CrossRef]
- Schardl, C.L.; Young, C.A.; Pan, J.; Florea, S.; Takach, J.E.; Panaccione, D.G.; Farman, M.L.; Webb, J.S.; Jaromczyk, J.; Charlton, N.D.; et al. Currencies of mutualisms: Sources of alkaloid genes in vertically transmitted Epichloë. Toxins 2013, 5, 1064–1088. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.Y.; Chen, Z.J.; He, Y.L.; White, J.F.; Malik, K.; Chen, T.X.; Li, C.J. Effects of Achnatherum inebrians ecotypes and endophyte status on plant growth, plant nutrient, soil fertility and soil microbial community. Soil Sci. Soc. Am. J. 2022, 86, 1028–1042. [Google Scholar] [CrossRef]
- Zhao, H.T.; Nie, X.M.; Zhang, W.; Zhang, X.X.; Ju, Y.W.; Li, Y.Z.; Christesen, M.J. Effects of interaction of Epichloë gansuensis and Bacillus strains on the seed germination and seedling growth in Achnatherum inebrians plants. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Jin, Y.Y. Microbial Mechanisms of Nitrogen Effective Use in Achnatherum inebrians-Fungal Endphyte Symbiont. Ph.D. Dissertation, Lanzhou University, Lanzhou, China, 2024. [Google Scholar]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.P.; Fan, K.K.; Li, G.L.; Gao, S.J.; Chang, D.N.; Liang, T.; Li, S.; Liang, H.; Zhang, J.D.; Che, Z.X.; et al. Synergistic effects of diazotrophs and arbuscular mycorrhizal fungi on soil biological nitrogen fixation after three decades of fertilization. iMeta 2023, 2, e81. [Google Scholar] [CrossRef] [PubMed]
- Cheplick, G.P.; Clay, K.; Marks, S. Interactions between fungal endophyte infection and nutrient limitation in the grasses Lolium perenne and Festuca arundinacea. New Phytol. 1989, 111, 89–98. [Google Scholar] [CrossRef]
- Chen, T.X.; Simpson, W.R.; Nan, Z.B.; Li, C.J. NaCl stress modifies the concentrations of endophytic fungal hyphal and peramine in Hordeum brevisubulatum seedlings. Crop Pasture Sci. 2021, 73, 214–221. [Google Scholar] [CrossRef]
- Patchett, A.; Newman, J.A. Comparison of plant metabolites in root exudates of Lolium perenne infected with different strains of the fungal endophyte Epichloë festucae var. lolii. J. Fungi 2021, 7, 148–177. [Google Scholar] [CrossRef]
- Liang, J.J.; Gao, G.Y.; Zhong, R.; Liu, B.W.; Christensen, M.J.; Ju, Y.W.; Zhang, W.; Wu, Z.; Li, C.; Li, C.J.; et al. Effect of Epichloë gansuensis endophyte on seed-borne microbes and seed metabolites in Achnatherum inebrians. Microbiol. Spectr. 2023, 11, e01350-22. [Google Scholar] [CrossRef] [PubMed]
- Rojas, X.; Guo, J.Q.; Leff, J.W.; McNear, D.; Fierer, N.; McCulley, R.L. Infection with a shoot-specific fungal endophyte (Epichloë) alters tall fescue soil microbial communities. Microb. Ecol. 2016, 72, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Brem, D.; Leuchtmann, A. Epichloë grass endophytes increase herbivore resistance in the woodland grass Brachypodium sylvaticum. Oecologia 2001, 126, 522–530. [Google Scholar] [CrossRef]
- Bultman, T.L.; Bell, G.; Martin, W.D. A fungal endophyte mediates reversal of wound induced resistance and constrains tolerance in a grass. Ecology 2004, 85, 679–685. [Google Scholar] [CrossRef]
- Powell, R.G.; Petroski, R.J. Alkaloid toxins in endophyte-infected grasses. Nat. Toxins 2010, 1, 163–170. [Google Scholar] [CrossRef]
- Njoroge Kimotho, R.; Zheng, X.; Li, F.R.; Chen, Y.J.; Li, X.F. A potent endophytic fungus Purpureocillium lilacinum YZ1 protects against Fusarium infection in field-grown wheat. New Phytol. 2024, 243, 1899–1916. [Google Scholar] [CrossRef]
- Hou, W.P.; Xia, C.; Christensen, M.J.; Wang, J.F.; Li, X.Z.; Chen, T.; Nan, Z.B. Effect of Epichloë gansuensis endophyte on rhizosphere bacterial communities and nutrient concentrations and ratios in the perennial grass species Achnatherum inebrians during three growth seasons. Crop Pasture Sci. 2020, 71, 1050–1066. [Google Scholar] [CrossRef]
- Wang, J.F.; Nan, Z.B.; Christensen, M.J.; Zhang, X.X.; Tian, P.; Zhang, Z.X.; Niu, X.L.; Gao, P.; Chen, T.; Ma, L.X. Effect of Epichloë gansuensis endophyte on the nitrogen metabolism, nitrogen use efficiency, and stoichiometry of Achnatherum inebrians under nitrogen limitation. J. Agric. Food Chem. 2018, 66, 4022–4031. [Google Scholar] [CrossRef]
- Ren, A.Z.; Wei, M.Y.; Yin, L.J.; Wu, L.J.; Zhou, Y.; Li, X.; Cao, Y.B. Benefits of a fungal endophyte in Leymus chinensis depend more on water than on nutrient availability. Environ. Exp. Bot. 2014, 108, 71–78. [Google Scholar] [CrossRef]
- Li, X.; Zhou, Y.; Mace, W.; Qin, J.H.; Liu, H.; Chen, W.; Ren, A.Z.; Gao, Y.B. Endophyte species influence the biomass production of the native grass Achnatherum sibiricum (L.) keng under high nitrogen availability. Ecol. Evol. 2016, 6, 8595–8606. [Google Scholar] [CrossRef]
- Murphy, B.R.; Doohan, F.M.; Hodkinson, T.R. Yield increase induced by the fungal root endophyte Piriformospora indica in barley grown at low temperature is nutrient limited. Symbiosis 2014, 62, 29–39. [Google Scholar] [CrossRef]
- Hamilton, C.E.; Dowling, T.E.; Faeth, S.H. Hybridization in endophyte symbionts alters host response to moisture and nutrient treatments. Microb. Ecol. 2010, 59, 768–775. [Google Scholar] [CrossRef] [PubMed]




| Bacteria Coating | Treatment | 10−5 | 10−6 |
|---|---|---|---|
| B. subtilis LZU7 | Fungal endophyte fluid | 59.25 ± 1.31 a | 19.25 ± 3.98 a |
| PDA culture medium | 44.50 ± 3.88 b | 12.00 ± 3.13 b | |
| Sterile water | 44.50 ± 3.88 b | 7.00 ± 0.91 bc | |
| B. mycoides LZU40 | Fungal endophyte fluid | 36.00 ± 5.30 b | 8.00 ± 0.91 bc |
| PDA culture medium | 40.25 ± 4.03 b | 7.75 ± 0.63 bc | |
| Sterile water | 19.75 ± 0.85 c | 4.00 ± 250.85 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Y.; Chen, Z.; Malik, K.; Li, C. Foliar Epichloë gansuensis Endophyte and Root-Originated Bacillus subtilis LZU7 Increases Biomass Accumulation and Synergistically Improve Nitrogen Fixation in Achnatherum inebrians. J. Fungi 2025, 11, 466. https://doi.org/10.3390/jof11070466
Jin Y, Chen Z, Malik K, Li C. Foliar Epichloë gansuensis Endophyte and Root-Originated Bacillus subtilis LZU7 Increases Biomass Accumulation and Synergistically Improve Nitrogen Fixation in Achnatherum inebrians. Journal of Fungi. 2025; 11(7):466. https://doi.org/10.3390/jof11070466
Chicago/Turabian StyleJin, Yuanyuan, Zhenjiang Chen, Kamran Malik, and Chunjie Li. 2025. "Foliar Epichloë gansuensis Endophyte and Root-Originated Bacillus subtilis LZU7 Increases Biomass Accumulation and Synergistically Improve Nitrogen Fixation in Achnatherum inebrians" Journal of Fungi 11, no. 7: 466. https://doi.org/10.3390/jof11070466
APA StyleJin, Y., Chen, Z., Malik, K., & Li, C. (2025). Foliar Epichloë gansuensis Endophyte and Root-Originated Bacillus subtilis LZU7 Increases Biomass Accumulation and Synergistically Improve Nitrogen Fixation in Achnatherum inebrians. Journal of Fungi, 11(7), 466. https://doi.org/10.3390/jof11070466







