Assessment of Entomopathogenic Fungi Activity from the Fiocruz Amazônia Collection in Anopheles aquasalis Mosquitoes †
Abstract
1. Introduction
2. Materials and Methods
2.1. Anopheles Aquasalis Colony
2.2. Fungi Species
2.3. Fungal Culture
2.4. Preparation and Viability of Fungal Suspensions
2.5. Evaluation of Entomopathogenic Activity of Each Selected Fungi: Survival
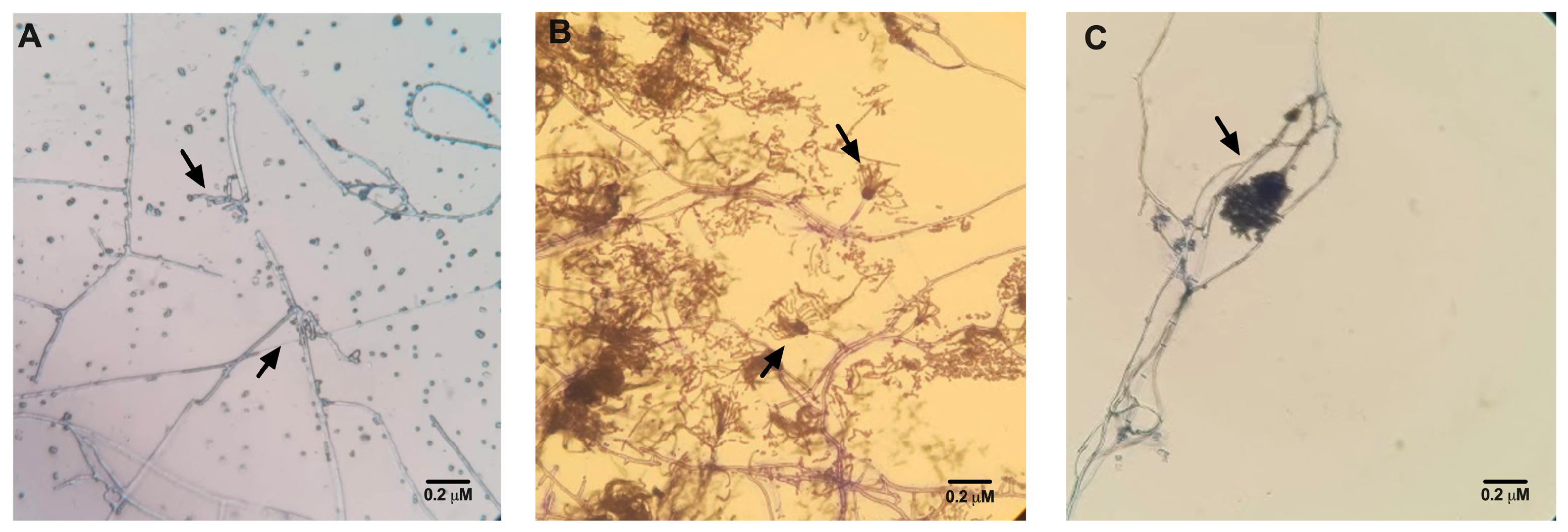
2.6. Confirmation of Fungal Species
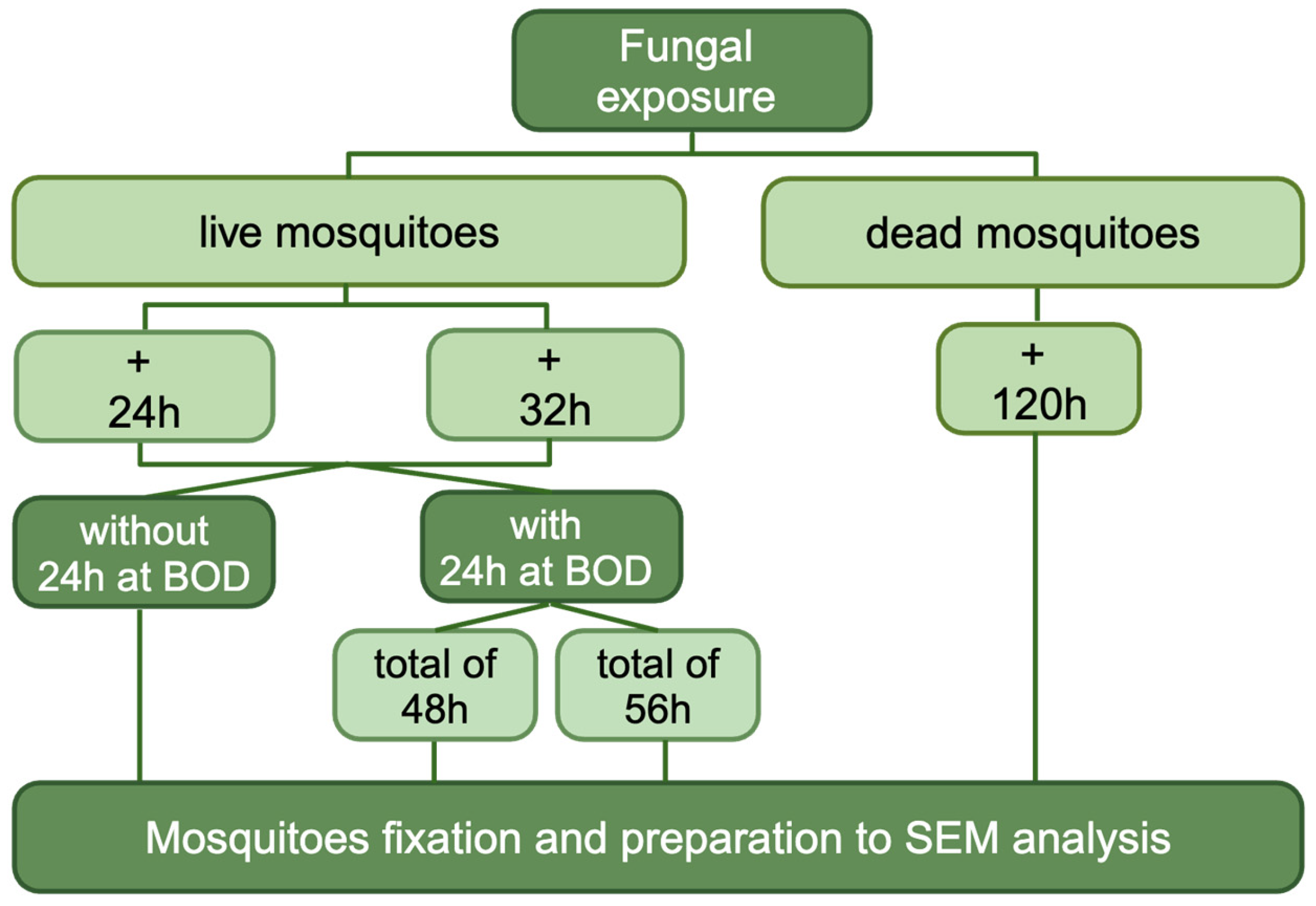
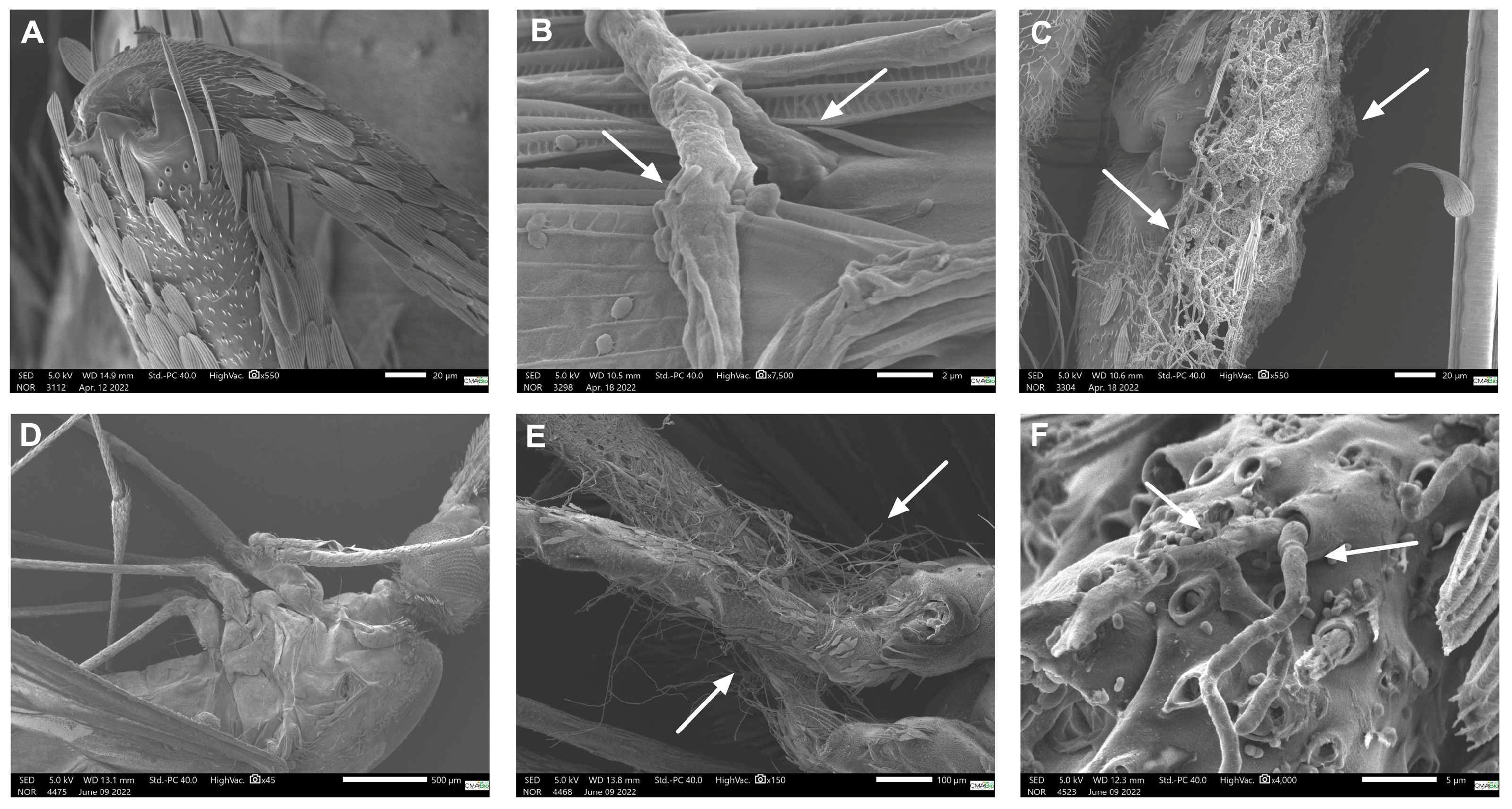
2.7. Scanning Electron Microscopy
2.8. Data Analysis
3. Results
3.1. Effect of Entomopathogenic Fungi on Anopheles aquasalis Mosquito Survival
3.2. Confirmation of Fungal Species
3.3. Scanning Electron Microscopy (SEM)
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. World Malaria Report 2024—Addressing Inequity in the Global Malaria Response; World Health Organization: Geneva, Switzerland, 2024; p. 320. [Google Scholar]
- Björkman, A.; Shakely, D.; Ali, A.S.; Morris, U.; Mkali, H.; Abbas, A.K.; Al-Mafazy, A.-W.; Haji, K.A.; Mcha, J.; Omar, R.; et al. From High to Low Malaria Transmission in Zanzibar—Challenges and Opportunities to Achieve Elimination. BMC Med. 2019, 17, 14. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Beier, J.C. Current Vector Control Challenges in the Fight against Malaria. Acta Trop. 2017, 174, 91–96. [Google Scholar] [CrossRef]
- Howard, A.F.; Koenraadt, C.J.; Farenhorst, M.; Knols, B.G.; Takken, W. Pyrethroid Resistance in Anopheles gambiae Leads to Increased Susceptibility to the Entomopathogenic Fungi Metarhizium anisopliae and Beauveria bassiana. Malar. J. 2010, 9, 168. [Google Scholar] [CrossRef] [PubMed]
- Araújo, J.P.M.; Hughes, D.P. Diversity of Entomopathogenic Fungi. In Advances in Genetics; Elsevier: Amsterdam, The Netherlands, 2016; Volume 94, pp. 1–39. ISBN 978-0-12-804694-4. [Google Scholar]
- Mora, M.A.E.; Castilho, A.M.C.; Fraga, M.E. Classification and Infection Mechanism of Entomopathogenic Fungi. Arq. Inst. Biol. 2018, 84, e0552015. [Google Scholar] [CrossRef]
- De Oliveira Barbosa Bitencourt, R.; Reis Dos Santos Mallet, J.; Mesquita, E.; Silva Gôlo, P.; Fiorotti, J.; Rita Elias Pinheiro Bittencourt, V.; Guedes Pontes, E.; Da Costa Angelo, I. Larvicidal Activity, Route of Interaction and Ultrastructural Changes in Aedes aegypti Exposed to Entomopathogenic Fungi. Acta Trop. 2021, 213, 105732. [Google Scholar] [CrossRef]
- Mondal, S.; Baksi, S.; Koris, A.; Vatai, G. Journey of Enzymes in Entomopathogenic Fungi. Pac. Sci. Rev. A Nat. Sci. Eng. 2016, 18, 85–99. [Google Scholar] [CrossRef]
- Fouda, A.; Awad, M.A.; Eid, A.M.; Saied, E.; Barghoth, M.G.; Hamza, M.F.; Awad, M.F.; Abdelbary, S.; Hassan, S.E.-D. An Eco-Friendly Approach to the Control of Pathogenic Microbes and Anopheles stephensi Malarial Vector Using Magnesium Oxide Nanoparticles (Mg-NPs) Fabricated by Penicillium chrysogenum. Int. J. Mol. Sci. 2021, 22, 5096. [Google Scholar] [CrossRef]
- Vivekanandhan, P.; Alarfaj, A.A.; Alfarraj, S.; Ansari, M.J.; Kamaraj, C. Biocontrol Toxicity of Trichoderma harzianum (Hypocreales: Hypocreaceae) Derived Chemical Molecules against Malarial Mosquito Anopheles stephensi with Molecular Docking Studies. Biotechnol. Lett. 2025, 47, 12. [Google Scholar] [CrossRef]
- Moosa-Kazemi, S.H.; Asgarian, T.S.; Sedaghat, M.M.; Javar, S. Pathogenic Fungi Infection Attributes of Malarial Vectors Anopheles maculipennis and Anopheles superpictus in Central Iran. Malar. J. 2021, 20, 393. [Google Scholar] [CrossRef]
- Mnyone, L.L.; Kirby, M.J.; Mpingwa, M.W.; Lwetoijera, D.W.; Knols, B.G.J.; Takken, W.; Koenraadt, C.J.M.; Russell, T.L. Infection of Anopheles gambiae Mosquitoes with Entomopathogenic Fungi: Effect of Host Age and Blood-Feeding Status. Parasitol. Res. 2011, 108, 317–322. [Google Scholar] [CrossRef]
- Ferreira, A.C.M.; Pereira, N.S.; Moya, K.N.; Fabbri, C.; Santana, R.A.; Rio-Velásquez, C.M.; Justiniano, S.C.; Aquino, P.F.; Lopes, S.C.P. Atividade entomopatogênica de fungos da coleção da Fiocruz Amazônia em mosquitos Anopheles aquasalis. In Proceedings of the Anais do II Simpósio FIOCRUZ/NIAID e XVII Reunião Nacional de Pesquisa em Malária—2024, Even3, Belém, PA, Brazil, 8 November 2024. [Google Scholar]
- Fabbri, C.; Trindade, A.O.; Andrade, F.S.; Souza, M.F.D.; Ríos-Velásquez, C.M.; Lacerda, M.V.G.D.; Monteiro, W.M.; Costa, F.T.M.; Amino, R.; Lopes, S.C.P. Transmission-Blocking Compound Candidates against Plasmodium vivax Using P. Berghei as an Initial Screening. Mem. Inst. Oswaldo Cruz 2021, 116, e200513. [Google Scholar] [CrossRef] [PubMed]
- Silva, K.N.d. Atividade Entomopatogênica de Espécies Paecilomyces Contra Ovos de Aedes (Stegomyia) Aegypti linnaeus, 1762 (Diptera: Culicidae). 2022. Available online: https://bdtd.ibict.br/vufind/Record/CRUZ_fa6eac44910655627cc83aa47711ae4d (accessed on 3 April 2025).
- De Oliveira, F.S.; Carvalho, B.F.D.; Martins, F.B.; Bezerra, N.V. Bioprospecção e Avaliação Do Potencial Antimicrobiano de Actinobactérias Do Solo Rizóide de Açaí (Euterpe oleracea) No Município de Igarapé-Açú No Estado Do Pará/Bioprospecting and Evaluation of the Antimicrobial Potential of Açaí Rhizoid Soil Actinobacteria (Euterpe oleracea) in the Municipality of Igarapé-Açú in the State of Pará. Braz. J. Dev. 2021, 7, 64309–64318. [Google Scholar] [CrossRef]
- Camargo, M.G.; Marciano, A.F.; Sá, F.A.; Perinotto, W.M.S.; Quinelato, S.; Gôlo, P.S.; Angelo, I.C.; Prata, M.C.A.; Bittencourt, V.R.E.P. Commercial Formulation of Metarhizium anisopliae for the Control of Rhipicephalus microplus in a Pen Study. Vet. Parasitol. 2014, 205, 271–276. [Google Scholar] [CrossRef]
- Mnyone, L.L.; Kirby, M.J.; Lwetoijera, D.W.; Mpingwa, M.W.; Knols, B.G.; Takken, W.; Russell, T.L. Infection of the Malaria Mosquito, Anopheles gambiae, with Two Species of Entomopathogenic Fungi: Effects of Concentration, Co-Formulation, Exposure Time and Persistence. Malar. J. 2009, 8, 309. [Google Scholar] [CrossRef]
- Jaber, S.; Mercier, A.; Knio, K.; Brun, S.; Kambris, Z. Isolation of Fungi from Dead Arthropods and Identification of a New Mosquito Natural Pathogen. Parasites Vectors 2016, 9, 491. [Google Scholar] [CrossRef] [PubMed]
- Vieira, L. Infection of Aedes aegypti (Diptera: Culicidae) Larvae and Adults by the Entomopathogenic Fungus Metarhizium anisopliae (Metschn.) Sorokin. BMRJ 2013, 3, 309–317. [Google Scholar] [CrossRef]
- Souza, W.D. Técnicas de Microscopia Eletrônica Aplicadas às Ciências Biológicas, 3rd ed.; Sociedade Brasileira de Microscopia: Rio de Janeiro, Brazil, 2007. [Google Scholar]
- Blanford, S.; Chan, B.H.K.; Jenkins, N.; Sim, D.; Turner, R.J.; Read, A.F.; Thomas, M.B. Fungal Pathogen Reduces Potential for Malaria Transmission. Science 2005, 308, 1638–1641. [Google Scholar] [CrossRef] [PubMed]
- Farenhorst, M.; Hunt, R.H.; Knols, B.G.J.; Takken, W.; Scholte, E.-J.; Farina, D.; Coetzee, M. African Water Storage Pots for the Delivery of the Entomopathogenic Fungus Metarhizium anisopliae to the Malaria Vectors Anopheles gambiae s.s. and Anopheles funestus. Am. J. Trop. Med. Hyg. 2008, 78, 910–916. [Google Scholar] [CrossRef]
- Begum, S.; Iqbal, M.; Iqbal, Z.; Shah, H.U.; Numan, M. Assessment of Mycelia Extract from Trichoderma harzianum for Its Antifungal, Insecticidal and Phytotoxic Importance. J. Plant Biochem. Physiol. 2018, 6, 1000209. [Google Scholar] [CrossRef]
- Shakeri, J.; Foster, H.A. Proteolytic Activity and Antibiotic Production by Trichoderma harzianum in Relation to Pathogenicity to Insects. Enzym. Microb. Technol. 2007, 40, 961–968. [Google Scholar] [CrossRef]
- Podder, D.; Ghosh, S.K. A New Application of Trichoderma asperellum as an Anopheline Larvicide for Eco Friendly Management in Medical Science. Sci. Rep. 2019, 9, 1108. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Podder, D.; Mukherjee, A. An Insight of Anopheline Larvicidal Mechanism of Trichoderma asperellum (TaspSKGN2). Sci. Rep. 2021, 11, 16029. [Google Scholar] [CrossRef]
- Maketon, M.; Amnuaykanjanasin, A.; Kaysorngup, A. A Rapid Knockdown Effect of Penicillium citrinum for Control of the Mosquito Culex quinquefasciatus in Thailand. World J. Microbiol. Biotechnol. 2014, 30, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, J.B.; Polonio, J.C.; Orlandelli, R.C.; Pamphile, J.A.; Golias, H.C. Atividade Larvicida de Fungos Endofíticos: Uma Revisão. Braz. J. Dev. 2020, 6, 35761–35774. [Google Scholar] [CrossRef]
- Lara Da Costa, G.; Cunha De Oliveira, P. Penicillium Species in Mosquitoes from Two Brazilian Regions. J. Basic Microbiol. 1998, 38, 343–347. [Google Scholar] [CrossRef]
- Mishra, S.; Nussenzweig, R.S.; Nussenzweig, V. Antibodies to Plasmodium Circumsporozoite Protein (CSP) Inhibit Sporozoite’s Cell Traversal Activity. J. Immunol. Methods 2012, 377, 47–52. [Google Scholar] [CrossRef]
- Barboza, M.R.; Silva, D.N.; Lustosa, S.B.C.; Hirose, E. Patogenicidade Do Fungo Entomopatogênico Beauveria bassiana Sobre o Percevejo Collaria scenica (Hemiptera: Miridae). Ambiência 2011, 7, 473–480. [Google Scholar] [CrossRef]
- Sun, X.; Yan, W.; Qin, W.; Zhang, J.; Niu, X.; Ma, G.; Li, F. Screening of Tropical Isolates of Metarhizium anisopliae for Virulence to the Red Palm Weevil Rhynchophorus ferrugineus Olivier (Coleoptera: Curculionidae). SpringerPlus 2016, 5, 1100. [Google Scholar] [CrossRef]
- Mantzoukas, S.; Kitsiou, F.; Natsiopoulos, D.; Eliopoulos, P.A. Entomopathogenic Fungi: Interactions and Applications. Encyclopedia 2022, 2, 646–656. [Google Scholar] [CrossRef]

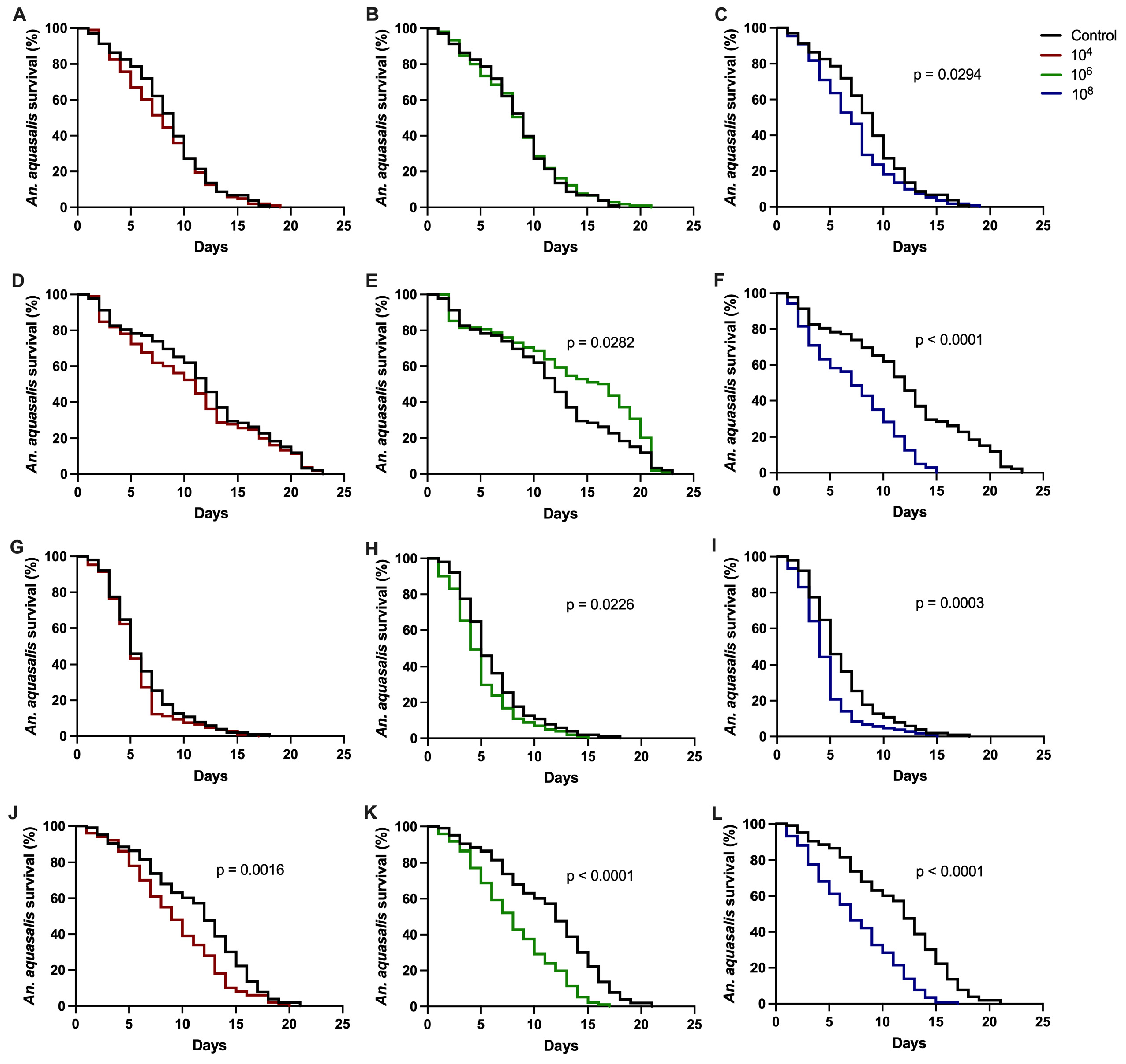
| Bioassay 1 (p-Value) | Bioassay 2 (p-Value) | |
|---|---|---|
| P. citrinum | ||
| C 1 × 104 | NS 2 | NS |
| C × 106 | NS | 0.0282 |
| C × 108 | 0.0294 | <0.0001 |
| T. harzianum | ||
| C × 104 | NS | 0.0016 |
| C × 106 | 0.0226 | <0.0001 |
| C × 108 | 0.0003 | <0.0001 |
| Species | Live Mosquitoes | Dead Mosquitoes | |||
|---|---|---|---|---|---|
| Without Incubation | With Incubation 1 | Without Incubation | |||
| 24 h | 32 h | 24 h | 32 h | 120 h (5 days) | |
| P. citrinum | no fungi | tibia | femur | femur and tibia | abdomen |
| T. harzianum | no fungi | no fungi | femur | femur | no fungi |
| Control group | no fungi presence in all tested conditions | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, N.S.; Fabbri, C.; Moya, K.N.; Ferreira, A.C.M.; Andrade, F.S.; Santana, R.A.; Ríos-Velásquez, C.M.; Aquino, P.F.d.; Lopes, S.C.P. Assessment of Entomopathogenic Fungi Activity from the Fiocruz Amazônia Collection in Anopheles aquasalis Mosquitoes. J. Fungi 2025, 11, 464. https://doi.org/10.3390/jof11060464
Pereira NS, Fabbri C, Moya KN, Ferreira ACM, Andrade FS, Santana RA, Ríos-Velásquez CM, Aquino PFd, Lopes SCP. Assessment of Entomopathogenic Fungi Activity from the Fiocruz Amazônia Collection in Anopheles aquasalis Mosquitoes. Journal of Fungi. 2025; 11(6):464. https://doi.org/10.3390/jof11060464
Chicago/Turabian StylePereira, Natalia Stefany, Camila Fabbri, Kemily Nunes Moya, Ana Carolina Monteiro Ferreira, Francy’s Sayara Andrade, Rosa Amélia Santana, Claudia Maria Ríos-Velásquez, Priscila Ferreira de Aquino, and Stefanie Costa Pinto Lopes. 2025. "Assessment of Entomopathogenic Fungi Activity from the Fiocruz Amazônia Collection in Anopheles aquasalis Mosquitoes" Journal of Fungi 11, no. 6: 464. https://doi.org/10.3390/jof11060464
APA StylePereira, N. S., Fabbri, C., Moya, K. N., Ferreira, A. C. M., Andrade, F. S., Santana, R. A., Ríos-Velásquez, C. M., Aquino, P. F. d., & Lopes, S. C. P. (2025). Assessment of Entomopathogenic Fungi Activity from the Fiocruz Amazônia Collection in Anopheles aquasalis Mosquitoes. Journal of Fungi, 11(6), 464. https://doi.org/10.3390/jof11060464







