Development and Characterization of Mycelium-Based Composite Using Agro-Industrial Waste and Ganoderma lucidum as Insulating Material
Abstract
1. Introduction
2. Materials and Methods
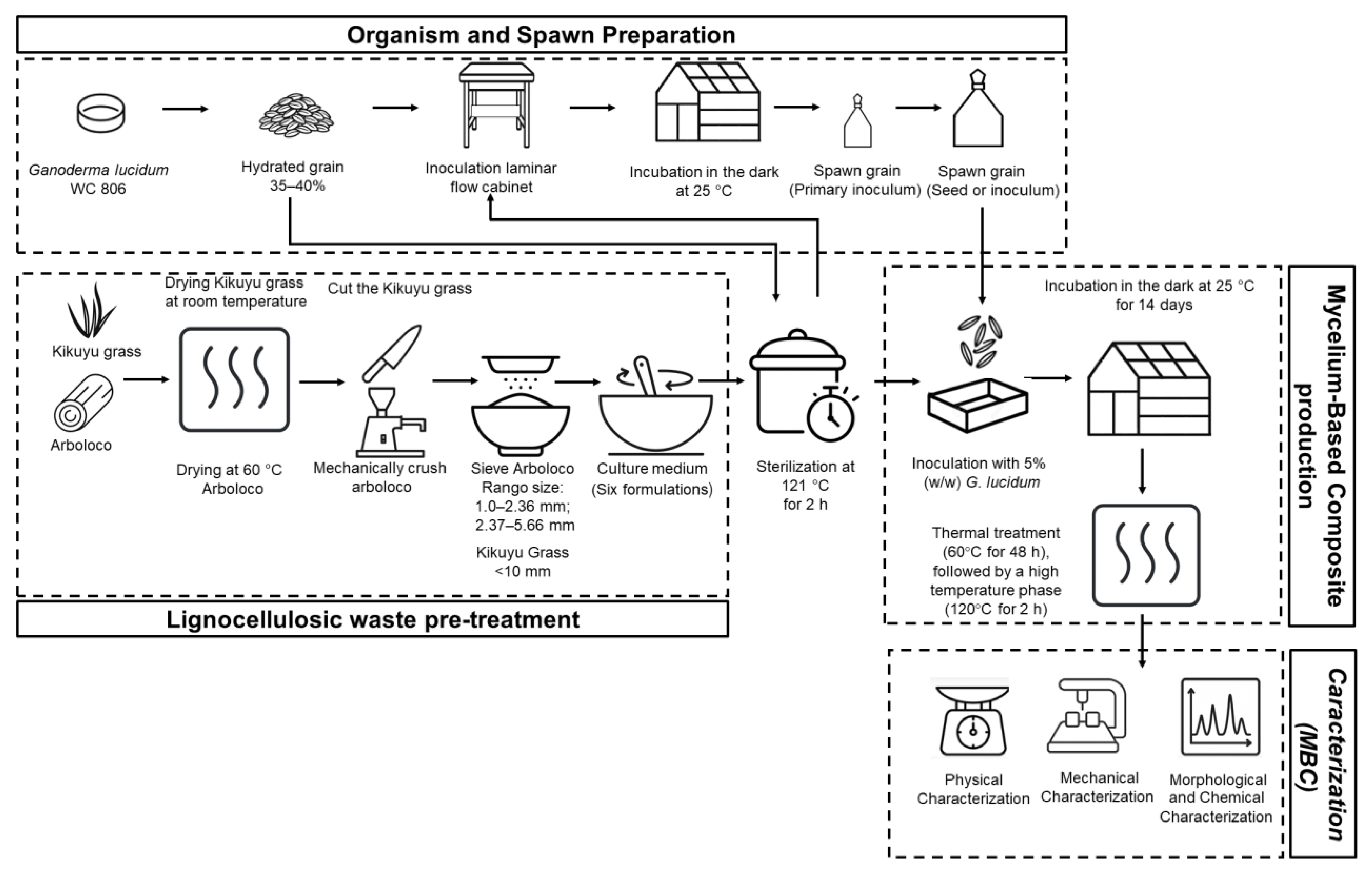
2.1. Organism and Spawn Preparation
2.2. Culture Medium to Produce Mycelium-Based Composite (MBC)
2.3. MBC Physical Characterization
2.3.1. Apparent Density
2.3.2. Water Absorption
2.3.3. Shrinkage
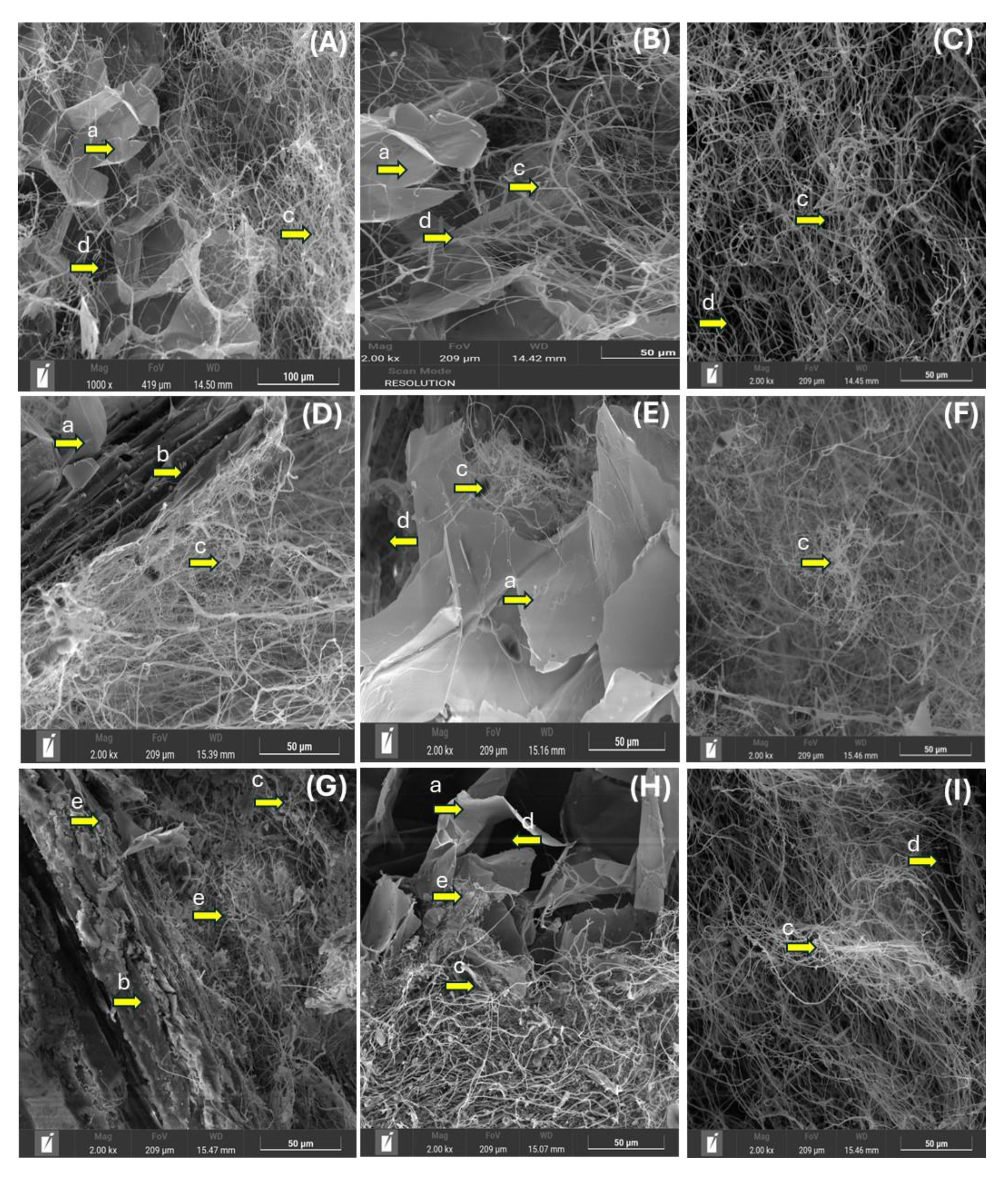
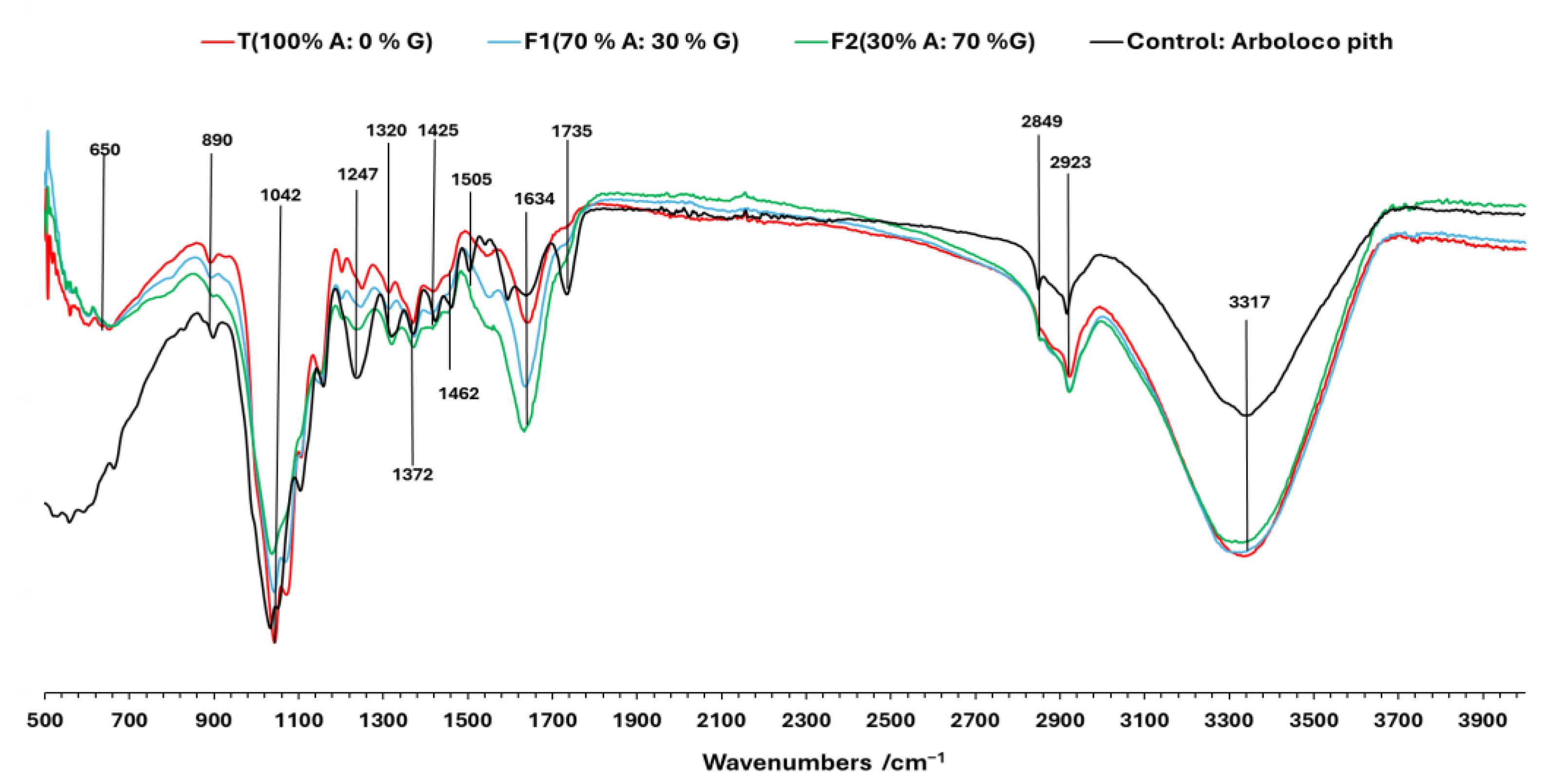
2.4. MBC Morphological and Chemical Characterization
2.5. MBC Mechanical Characterization
2.5.1. Compression Strength
2.5.2. Flexural Strength
- D: deflection at the center of the sample (mm);ε: strain (mm/mm);L: support span (mm);d: depth of the beam (mm).
- P: load at a given point on the load–deflection curve (N);b: width of the beam tested (mm);
2.6. MBC Thermal Characterization
3. Results and Discussion
3.1. Morphological Characteristics
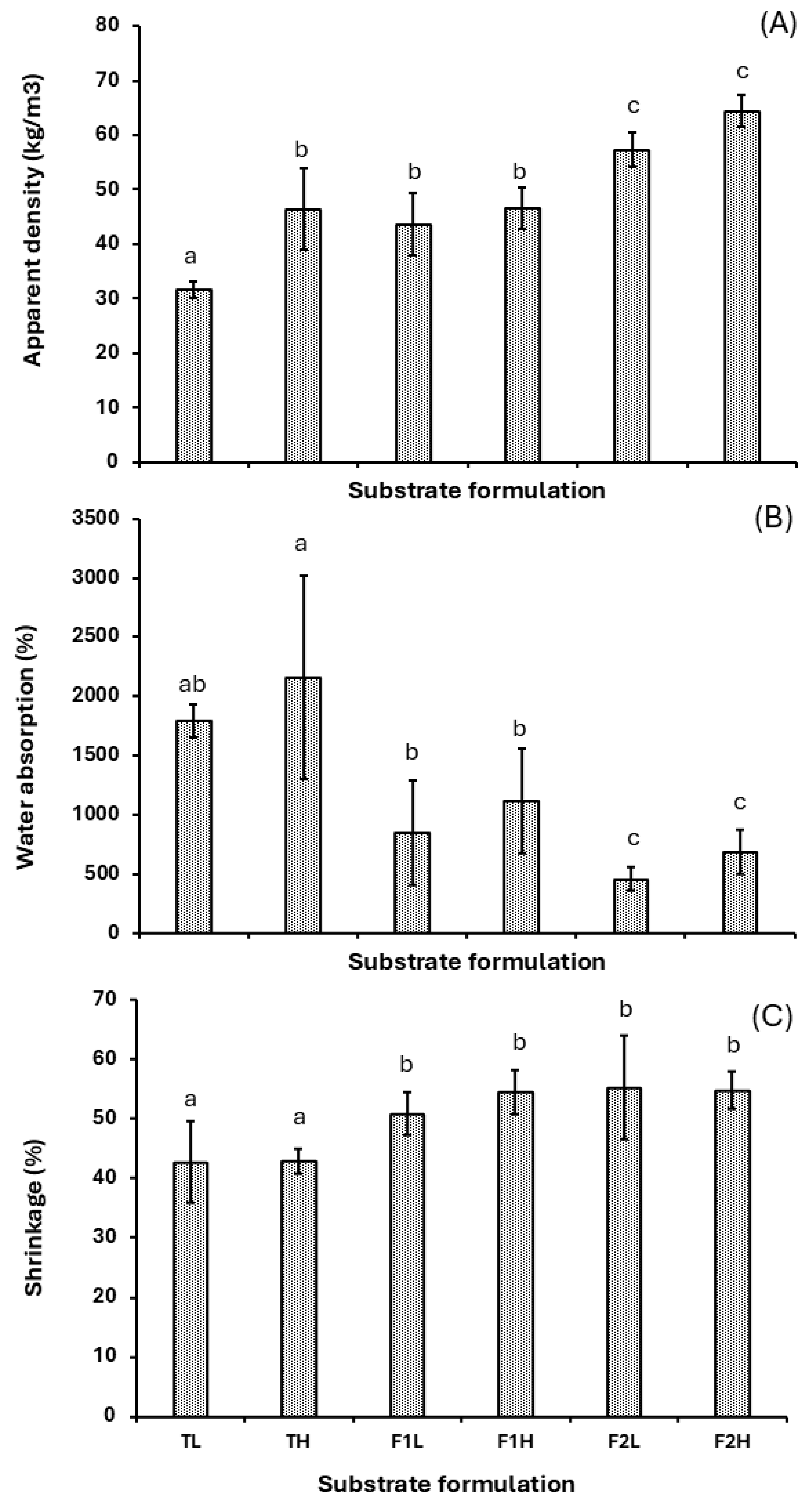
3.2. Physical Properties
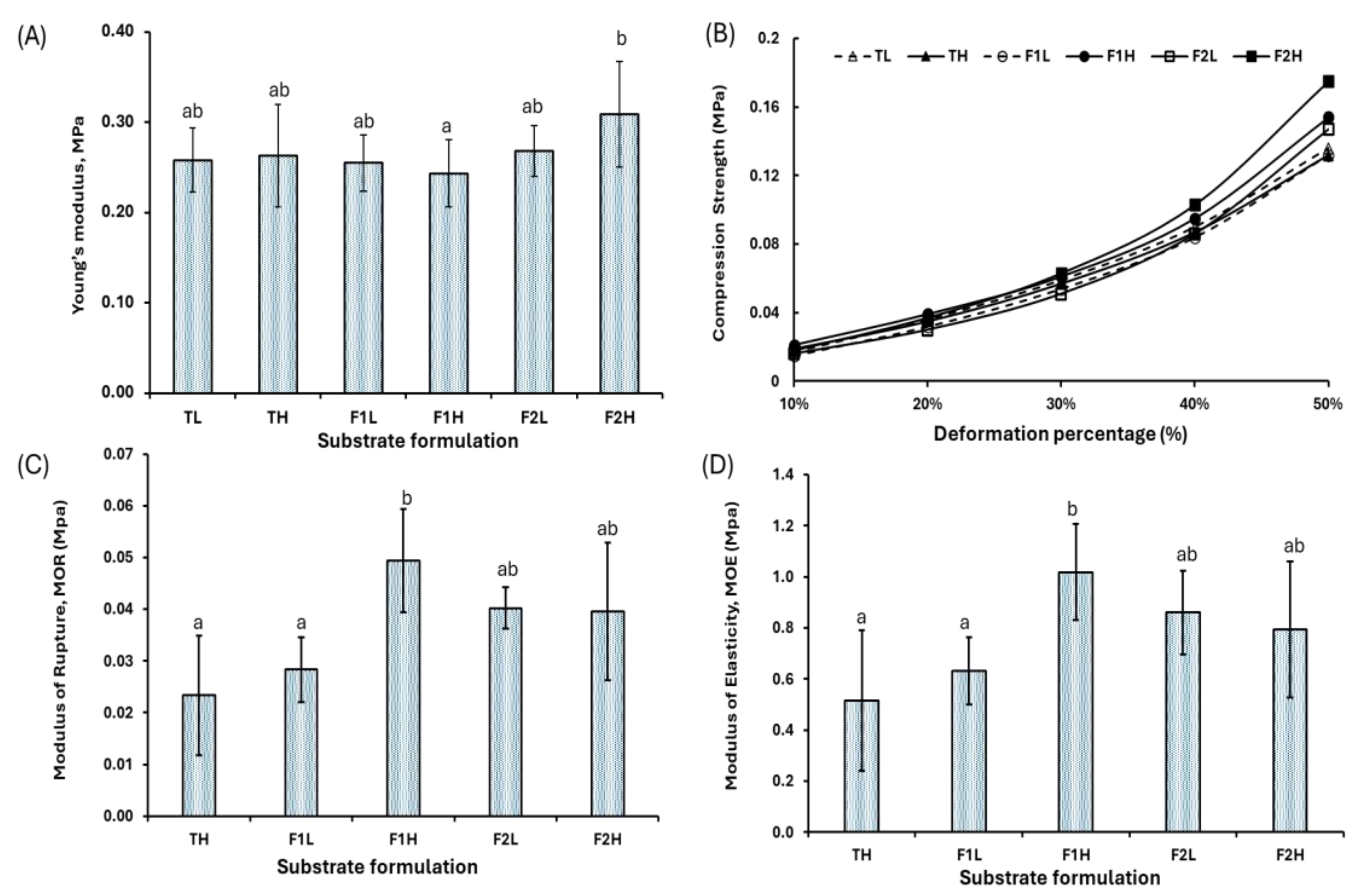
3.3. Mechanical Properties
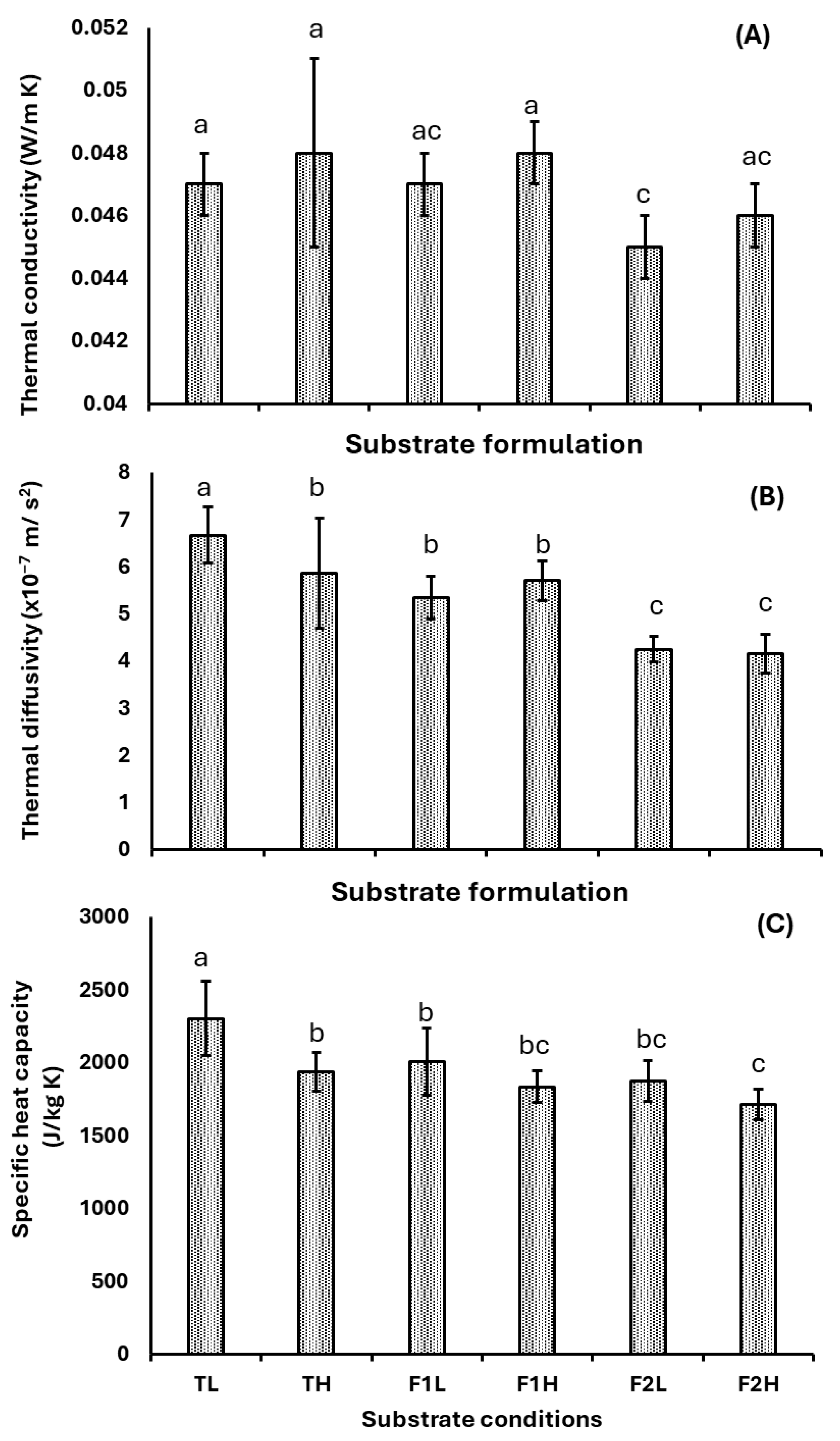
3.4. Thermal Properties
4. Conclusions
- The development of mycelium-based composite materials (MBCs) using Ganoderma lucidum and Colombian agro-industrial wastes (Arboloco and Kikuyu grass) illustrates significant potential as a lightweight thermal insulation material with a low environmental impact, consistent with the principles of sustainable construction.
- Formulation F2, consisting of 30% Arboloco and 70% Kikuyu grass, exhibited an outstanding thermal performance, with a thermal conductivity of 0.045 W m−1 K−1 and a specific heat capacity of 1714 ± 105 J kg−1 K−1. Moreover, the low apparent density (60.4 ± 4.5 kg·m−3) positions it as a promising lightweight insulating material that could serve as an alternative to synthetic insulation materials like expanded polystyrene. These results establish our MBC as a viable energy-efficient option.
- Despite the thermal and ecological advantages of these biomaterials, significant challenges, including high volumetric shrinkage (53.6%) and elevated water absorption (556 ± 184%), must be addressed, as they limit the materials’ direct applicability without additional treatment. These findings highlight the necessity of incorporating post-processing techniques, such as hot pressing, to enhance dimensional stability and developing strategies aimed at reducing water absorption capacity.
- Future research should focus on substrate optimization by exploring hybrid combinations of lignocellulosic biomass to enhance mechanical performance without compromising eco-design principles. Additionally, the standardization of growth and treatment protocols will be essential for achieving controlled and reproducible scaling of MBC production.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yadav, M.; Agarwal, M. Biobased building materials for sustainable future: An overview. Mater. Today Proc. 2021, 43, 2895–2902. [Google Scholar] [CrossRef]
- Cintura, E.; Faria, P.; Duarte, M.; Nunes, L. Eco-efficient boards with agro-industrial wastes—Assessment of different adhesives. Constr. Build. Mater. 2023, 404, 132665. [Google Scholar] [CrossRef]
- Xu, P.; Zhu, J.; Li, H.; Wei, Y.; Xiong, Z.; Xu, X. Are Bamboo Construction Materials Environmentally Friendly? A Life Cycle Environmental Impact Analysis. Environ. Impact Assess. Rev. 2022, 96, 106853. [Google Scholar] [CrossRef]
- Wang, R.; Haller, P. Applications of Wood Ash as a Construction Material in Civil Engineering: A Review. Biomass Convers. Biorefin 2022. [Google Scholar] [CrossRef]
- Sun, X.; He, M.; Li, Z. Novel Engineered Wood and Bamboo Composites for Structural Applications: State-of-Art of Manufacturing Technology and Mechanical Performance Evaluation. Constr. Build. Mater. 2020, 249, 118751. [Google Scholar] [CrossRef]
- Ginga, C.P.; Ongpeng, J.M.C.; Daly, M.K.M. Circular Economy on Construction and Demolition Waste: A Literature Review on Material Recovery and Production. Materials 2020, 13, 2970. [Google Scholar] [CrossRef]
- Haque, S.E.; Nahar, N.; Chowdhury, N.N.; Gazi-Khan, L.; Sayanno, T.K.; Muktadir, M.A.; Haque, M.S. Identification of Recycling Potential of Construction and Demolition Waste: Challenges and Opportunities in the Greater Dhaka Area. Environ. Monit. Assess. 2025, 197, 646. [Google Scholar] [CrossRef]
- Nyika, J.; Dinka, M. Recycling Plastic Waste Materials for Building and Construction Materials: A Minireview. Mater. Today Proc. 2022, 62, 3257–3262. [Google Scholar] [CrossRef]
- Wang, C.; Liu, J.; Lu, B.; Zhang, Y.; Ma, Z. Stiffness Degradation and Mechanical Behavior of Microfiber-Modified High-Toughness Recycled Aggregate Concrete under Constant Load Cycling. Eng. Fract. Mech. 2024, 312, 110608. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, B.; Zhang, Z.; Zhang, Y.; Wang, C. New Insights into the Effects of Silicate Modulus, Alkali Content and Modification on Multi-Properties of Recycled Brick Powder-Based Geopolymer. J. Build. Eng. 2024, 97, 110989. [Google Scholar] [CrossRef]
- Abu-Jdayil, B.; Mourad, A.H.; Hittini, W.; Hassan, M.; Hameedi, S. Traditional, state-of-the-art and renewable thermal building insulation materials: An overview. Constr. Build. Mater. 2019, 214, 709–735. [Google Scholar] [CrossRef]
- Vašatko, H.; Gosch, L.; Jauk, J.; Stavric, M. Basic Research of Material Properties of Mycelium-Based Composites. Biomimetics 2022, 7, 51. [Google Scholar] [CrossRef]
- Contreras, E.; Flores, R.; Gutiérrez, A.; Cerro, D.; Sepúlveda, L.A. Agro-Industrial Wastes Revalorization as Feedstock: Production of Lignin-Modifying Enzymes Extracts by Solid-State Fermentation Using White Rot Fungi. Prep. Biochem. Biotechnol. 2023, 53, 488–499. [Google Scholar] [CrossRef] [PubMed]
- Ekblad, A.; Wallander, H.; Godbold, D.L.; Cruz, C.; Johnson, D.; Baldrian, P.; Björk, R.G.; Epron, D.; Kieliszewska-Rokicka, B.; Kjøller, R.; et al. The production and turnover of extramatrical mycelium of ectomycorrhizal fungi in forest soils: Role in carbon cycling. Plant Soil. 2013, 366, 1–27. [Google Scholar] [CrossRef]
- Aiduang, W.; Suwannarach, N.; Kumla, J.; Thamjaree, W.; Lumyong, S. Valorization of agricultural waste to produce myco-composite materials from mushroom mycelia and their physical properties. Agric. Nat. Resour. 2022, 56, 1083–1090. [Google Scholar]
- Chulikavit, N.; Wang, C.; Huynh, T.; Yuen, A.C.Y.; Khatibi, A.; Kandare, E. Fireproofing Flammable Composites Using Mycelium: Investigating the Effect of Deacetylation on the Thermal Stability and Fire Reaction Properties of Mycelium. Polym. Degrad. Stab. 2023, 215, 110419. [Google Scholar] [CrossRef]
- Irbe, I.; Kirpluks, M.; Kampuss, M.; Andze, L.; Milbreta, U.; Filipova, I. Assessing the Conformity of Mycelium Biocomposites for Ecological Insulation Solutions. Materials 2024, 17, 6111. [Google Scholar] [CrossRef]
- Elsacker, E.; Vandelook, S.; Brancart, J.; Peeters, E.; De Laet, L. Mechanical, physical and chemical characterisation of mycelium-based composites with different types of lignocellulosic substrates. PLoS ONE 2019, 14, 7. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, J.; Fan, X.; Yu, X. Naturally grown mycelium-composite as sustainable building insulation materials. J. Clean. Prod. 2022, 342, 130784. [Google Scholar] [CrossRef]
- Camilleri, E.; Narayan, S.; Lingam, D.; Blundell, R. Mycelium-Based Composites: An Updated Comprehensive—Overview. Biotechnol. Adv. 2025, 79, 108517. [Google Scholar] [CrossRef]
- Jin, Q.; Zhang, Z.; Chen, J. A Study on the Thermal Performance of Pleurotus ostreatus/Straw Mycelium Composites and Its Application in Building Envelopes. J. Build. Eng. 2024, 92, 109646. [Google Scholar] [CrossRef]
- Nussbaumer, M.; Van Opdenbosch, D.; Engelhardt, M.; Briesen, H.; Benz, J.P.; Karl, T. Material characterization of pressed and unpressed wood–mycelium composites derived from two Trametes species. Environ. Technol. Innov. 2023, 30, 103063. [Google Scholar] [CrossRef]
- Sun, W.; Tajvidi, M.; Howell, C.; Hunt, C.G. Insight into mycelium-lignocellulosic bio-composites: Essential factors and properties. Compos. Part. A Appl. Sci. Manuf. 2022, 161, 107125. [Google Scholar] [CrossRef]
- Yang, L.; Park, D.; Qin, Z. Material Function of Mycelium-Based Bio-Composite: A Review. Front. Mater. 2021, 8, 737377. [Google Scholar] [CrossRef]
- Voutetaki, M.E.; Mpalaskas, A.C. Natural Fiber-Reinforced Mycelium Composite for Innovative and Sustainable Construction Materials. Fibers 2024, 12, 57. [Google Scholar] [CrossRef]
- Kohphaisansombat, C.; Jongpipitaporn, Y.; Laoratanakul, P.; Tantipaibulvut, S.; Euanorasetr, J.; Rungjindamai, N.; Chuaseeharonnachai, C.; Kwantong, P.; Somrithipol, S.; Boonyuen, N. Fabrication of Mycelium (Oyster mushroom)-Based Composites Derived from Spent Coffee Grounds with Pineapple Fibre Reinforcement. Mycology 2024, 15, 665–682. [Google Scholar] [CrossRef] [PubMed]
- Álvarez Mejía, L.M. Biología, uso y Manejo del Arboloco (Montanoa quadrangularis), 1st ed.; Editorial Universidad de Caldas: Manizales, Colombia, 2006; pp. 223–305. [Google Scholar]
- Montoya, S.; López, D.M.; Segura, B. Influencia de la luz azul sobre la productividad del cultivo sólido de Ganoderma lucidum. Rev. Colomb Biotecnol. 2018, 1, 51–58. [Google Scholar] [CrossRef]
- ASTM, D3575-20; Standard Test Methods for Flexible Cellular Materials Made From Olefin Polymers. ASTM International: West Conshohocken, PA, USA, 2020; 15, pp. 1–11.
- ASTM D570; Test Method for Water Absorption of Plastics. ASTM International: West Conshohocken, PA, USA, 2022.
- ASTM C165-23; Test Method for Measuring Compressive Properties of Thermal Insulations. ASTM International: West Conshohocken, PA, USA, 2023.
- ASTM C203-22; Test Methods for Breaking Load and Flexural Properties of Block-Type Thermal. ASTM International: West Conshohocken, PA, USA, 2022.
- ASTM D5334-22; Test Method for Determination of Thermal Conductivity of Soil and Rock by Thermal Needle Probe Procedure. ASTM International: West Conshohocken, PA, USA, 2022.
- Pesciaroli, L.; Petruccioli, M.; Federici, F.; D’Annibale, A. Pleurotus ostreatus Biofilm-Forming Ability and Ultrastructure Are Significantly Influenced by Growth Medium and Support Type. J. Appl. Microbiol. 2013, 114, 1750–1762. [Google Scholar] [CrossRef]
- Jimenes Obando, G.; Tolosa Correa, R.; Arcila Henao, J.S.; Montoya Barreto, S. Isolamento Térmico Natural: Médula de Montanoa quadrangularis Shultz BIP. In Proceedings of the 5 Congresso Luso-Brasileiro de Materiais de Construcao sustentáveis: Congresso Construcao 2024, Lisboa, Portugal, 6–8 November 2024. [Google Scholar]
- Pandey, K.K.; Pitman, A.J. FTIR studies of the changes in wood chemistry following decay by brown-rot and white-rot fungi. Int. Biodeterior. Biodegrad. 2003, 52, 151–160. [Google Scholar] [CrossRef]
- Qi, J.; Li, F.; Jia, L.; Zhang, X.; Deng, S.; Luo, B.; Zhou, Y.; Fan, M.; Xia, Y. Fungal Selectivity and Biodegradation Effects by White and Brown Rot Fungi for Wood Biomass Pretreatment. Polymers 2023, 15, 1957. [Google Scholar] [CrossRef]
- Supramani, S.; Rejab, N.A.; Ilham, Z.; Ahmad, R.; Show, P.L.; Ibrahim, M.F.; Wan-Mohtar, W.A.A.Q.I. Performance of Biomass and Exopolysaccharide Production from the Medicinal Mushroom Ganoderma lucidum in a New Fabricated Air-L-Shaped Bioreactor (ALSB). Processes 2023, 11, 670. [Google Scholar] [CrossRef]
- Hamza, A.; Khalad, A.; Kumar, D.S. Enhanced Production of Mycelium Biomass and Exopolysaccharides of Pleurotus ostreatus by Integrating Response Surface Methodology and Artificial Neural Network. Bioresour. Technol. 2024, 399, 130577. [Google Scholar] [CrossRef]
- Brazkova, M.; Angelova, G.; Goranov, B. Food Science and Applied Biotechnology Effect of the lignocellulose substrate type on mycelium growth and biocomposite formation by Ganoderma lucidum GA3P. Food Sci. Appl. Biotechnol. 2022, 5, 211–218. [Google Scholar]
- Cai, J.; Han, J.; Ge, F.; Lin, Y.; Pan, J.; Ren, A. Development of impact-resistant mycelium-based composites (MBCs) with agricultural waste straws. Constr. Build. Mater. 2023, 389, 131730. [Google Scholar] [CrossRef]
- Dénes, T.O.; Iştoan, R.; Tǎmaş-Gavrea, D.R.; Manea, D.L.; Hegyi, A.; Popa, F.; Vasile, O. Analysis of Sheep Wool-Based Composites for Building Insulation. Polymers 2022, 14, 2109. [Google Scholar] [CrossRef] [PubMed]
- Neila González, F.J. Arquitectura Bioclimática en un Entorno Sostenible, 1st ed.; Editorial Munilla-Lería: Madrid, Spain, 2004; pp. 89–99. [Google Scholar]
- Alaneme, K.K.; Anaele, J.U.; Oke, T.M.; Kareem, S.A.; Adediran, M.; Ajibuwa, O.A.; Anabaranze, Y.O. Mycelium based composites: A review of their bio-fabrication procedures, material properties and potential for green building and construction applications. Alex. Eng. J. 2023, 83, 234–250. [Google Scholar] [CrossRef]
- Jones, M.; Mautner, A.; Luenco, S.; Bismarck, A.; John, S. Engineered mycelium composite construction materials from fungal biorefineries: A critical review. Mat. Des. 2020, 187, 108397. [Google Scholar] [CrossRef]
- Aiduang, W.; Kumla, J.; Srinuanpan, S.; Thamjaree, W.; Lumyong, S.; Suwannarach, N. Mechanical, Physical, and Chemical Properties of Mycelium-Based Composites Produced from Various Lignocellulosic Residues and Fungal Species. J. Fungi 2022, 8, 1125. [Google Scholar] [CrossRef]
- Islam, M.R.; Tudryn, G.; Bucinell, R.; Schadler, L.; Picu, R.C. Morphology and mechanics of fungal mycelium. Sci. Rep. 2017, 7, 13070. [Google Scholar] [CrossRef]
- Walsh, J.; Kim, H.I.; Suhr, J. Low velocity impact resistance and energy absorption of environmentally friendly expanded cork core-carbon fiber sandwich composites. Compos. Part. A Appl. Sci. Manuf. 2017, 101, 290–296. [Google Scholar] [CrossRef]
- Vidholdová, Z.; Kormúthová, D.; Ždinsky, J.I.; Lagaña, R. Compressive Resistance of the Mycelium Composite. Ann. WULS For. Wood Technol. 2020, 107, 31–36. [Google Scholar] [CrossRef]
- Sisti, L.; Gioia, C.; Totaro, G.; Verstichel, S.; Cartabia, M.; Camere, S.; Celli, A. Valorization of wheat bran agro-industrial byproduct as an upgrading filler for mycelium-based composite materials. Ind. Crops Prod. 2021, 170, 113742. [Google Scholar] [CrossRef]
- Etinosa, P.O.; Salifu, A.A.; Salifu, T.A.; Obayemi, J.D.; Onche, E.O.; Aina, T.; Soboyejo, W.O. Self-organized mycelium biocomposites: Effects of geometry and laterite composition on compressive behavior. J. Mech. Behav. Biomed. Mater. 2023, 142, 105831. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Yi, J.; Yang, X.; Xie, J.; Chen, C. Development and characterization of mycelium bio-composites by utilization of different agricultural residual byproducts. J. Bioresour. Bioprod. 2022, 8, 78–89. [Google Scholar] [CrossRef]
- Sivaprasad, S.; Byju, S.K.; Prajith, C.; Shaju, J.; Rejeesh, C.R. Development of a novel mycelium bio-composite material to substitute for polystyrene in packaging applications. Mater. Today Proc. 2021, 47, 5038–5044. [Google Scholar] [CrossRef]
- Appels, F.V.W.; Camere, S.; Montalti, M.; Karana, E.; Jansen, K.M.B.; Dijksterhuis, J.; Krijgsheld, P.; Wöstenet, H.A. Fabrication factors influencing mechanical, moisture- and water-related properties of mycelium-based composites. Mater. Des. 2019, 161, 64–71. [Google Scholar] [CrossRef]
- Rigobello, A.; Ayres, P. Compressive behaviour of anisotropic mycelium-based composites. Sci. Rep. 2022, 12, 1–13. [Google Scholar] [CrossRef]
- Fu, H.; Ding, Y.; Li, M.; Li, H.; Huang, X.; Wang, Z. Research on thermal performance and hygrothermal behavior of timber-framed walls with different external insulation layer: Insulation Cork Board and anti-corrosion pine plate. J. Build. Eng. 2020, 28, 101069. [Google Scholar] [CrossRef]
- Hadini, M.H.; Susanto, D.; Chalid, M.; Alkadri, M.F. Investigating the mechanical performance of mycelium biocomposite using Oil Palm Empty Fruit Bunch (OPEFB) fiber and pine sap bioresin as sandwich insulation panels. Constr. Build. Mater. 2024, 448, 138173. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Z.; Zhang, R.; Peng, Y.; Wang, M.; Cao, J. Lightweight, thermal insulation, hydrophobic mycelium composites with hierarchical porous structure: Design, manufacture and applications. Compos. B Eng. 2023, 266, 111003. [Google Scholar] [CrossRef]
- Bonga, K.B.; Bertolacci, L.; Contardi, M.; Paul, U.C.; Zafar, M.S.; Mancini, G.; Marini, L.; Ceseracciu, L.; Fragouli, D.; Athanassiou, A. Mycelium Agrowaste-Bound Biocomposites as Thermal and Acoustic Insulation Materials in Building Construction. Macromol. Mater. Eng. 2024, 309, 202300449. [Google Scholar] [CrossRef]
- Mehrez, I.; Hachem, H.; Jemni, A. Thermal insulation potential of wood-cereal straws/plaster composite. Case Studies in Constr. Mat. 2022, 17, e01353. [Google Scholar] [CrossRef]
- Li, X.; Tabil, L.G.; Oguocha, I.N.; Panigrahi, S. Thermal diffusivity, thermal conductivity, and specific heat of flax fiber-HDPE biocomposites at processing temperatures. Compos. Sci. Technol. 2008, 68, 1753–1758. [Google Scholar] [CrossRef]
- Ghazi Wakili, K.; Rädle, W.; Rohner, T. Measuring the Specific Heat Capacity of Thermal Insulation Materials Used in Buildings by Means of a Guarded Hot Plate Apparatus. Int. J. Thermophys. 2023, 44, 15. [Google Scholar] [CrossRef]
- Yousefi, Y.; Tariku, F. Thermal conductivity and specific heat capacity of insulation materials at different mean temperatures. J. Phys. Conf. Ser. 2021, 2069, 012090. [Google Scholar] [CrossRef]
- Shah, D.U.; Bock, M.C.D.; Mulligan, H.; Ramage, M.H. Thermal conductivity of engineered bamboo composites. J. Mater. Sci. 2016, 51, 2991–3002. [Google Scholar] [CrossRef]
- Alazzawi, S.; Mahmood, W.A.; Shihab, S.K. Comparative study of natural fiber-Reinforced composites for sustainable thermal insulation in construction. Int. J. Thermofluids 2024, 24, 100839. [Google Scholar] [CrossRef]
- Xing, Y.; Brewer, M.; El-Gharabawy, H.; Griffith, G.; Jones, P. Growing and testing mycelium bricks as building insulation materials. IOP Conf. Ser. Earth Environ. Sci. 2018, 121, 022032. [Google Scholar] [CrossRef]
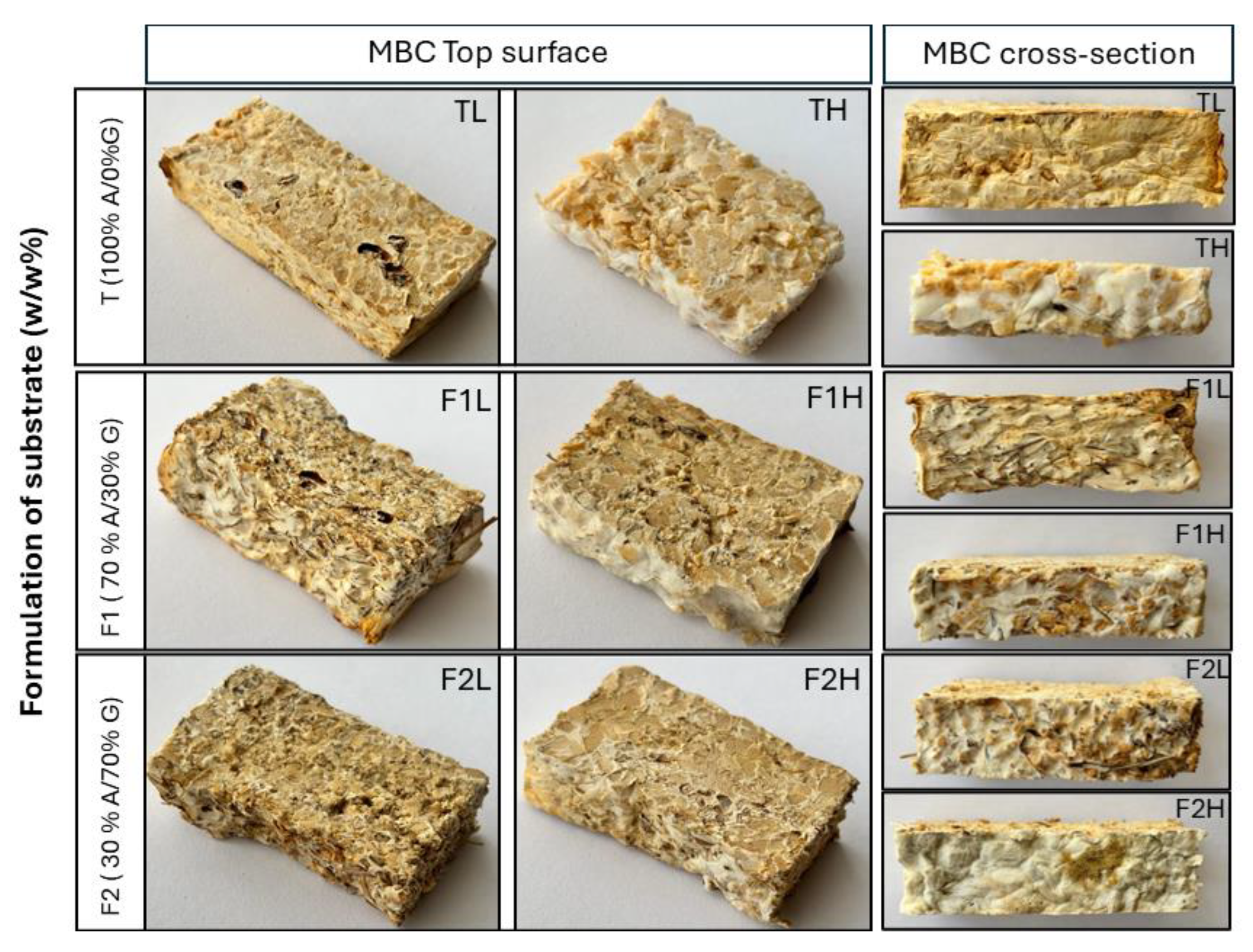
| Particle Size, mm | F1 (70%A/30% G) | F2 (30% A/70% G) | T (100% A/0% G) |
|---|---|---|---|
| L: 1.0–2.36 | 70 | 30 | 100 |
| H: 2.37–5.66 | 30 | 70 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Obando, G.; Arcila, J.S.; Tolosa-Correa, R.A.; Valencia-Cardona, Y.L.; Montoya, S. Development and Characterization of Mycelium-Based Composite Using Agro-Industrial Waste and Ganoderma lucidum as Insulating Material. J. Fungi 2025, 11, 460. https://doi.org/10.3390/jof11060460
Jiménez-Obando G, Arcila JS, Tolosa-Correa RA, Valencia-Cardona YL, Montoya S. Development and Characterization of Mycelium-Based Composite Using Agro-Industrial Waste and Ganoderma lucidum as Insulating Material. Journal of Fungi. 2025; 11(6):460. https://doi.org/10.3390/jof11060460
Chicago/Turabian StyleJiménez-Obando, Gustavo, Juan Sebastian Arcila, Ricardo Augusto Tolosa-Correa, Yenny Leandra Valencia-Cardona, and Sandra Montoya. 2025. "Development and Characterization of Mycelium-Based Composite Using Agro-Industrial Waste and Ganoderma lucidum as Insulating Material" Journal of Fungi 11, no. 6: 460. https://doi.org/10.3390/jof11060460
APA StyleJiménez-Obando, G., Arcila, J. S., Tolosa-Correa, R. A., Valencia-Cardona, Y. L., & Montoya, S. (2025). Development and Characterization of Mycelium-Based Composite Using Agro-Industrial Waste and Ganoderma lucidum as Insulating Material. Journal of Fungi, 11(6), 460. https://doi.org/10.3390/jof11060460









