Hidden Treasures of Colombia’s Pacific Mangrove: New Fungal Species and Records of Macrofungi (Basidiomycota)
Abstract
1. Introduction
2. Materials and Methods
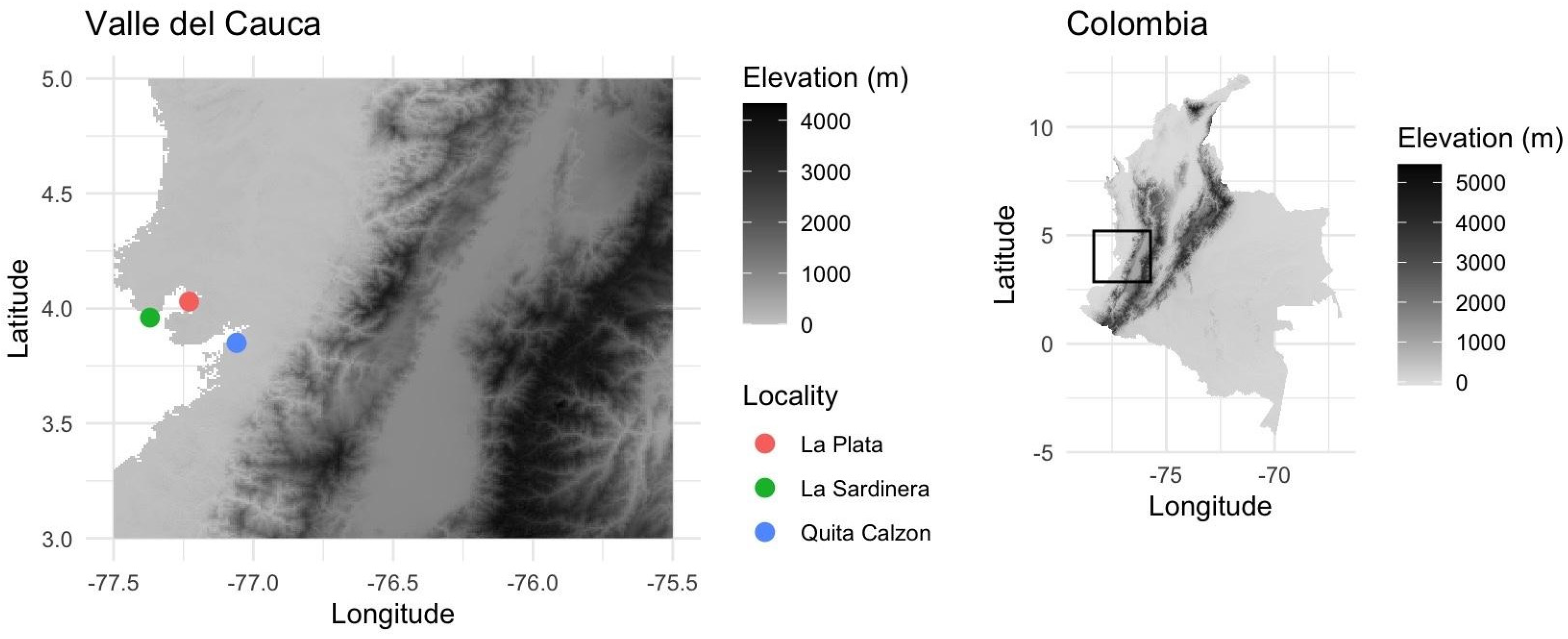
2.1. Sampling Area and Morphological Characterization
2.2. DNA Extraction, Barcode Loci Amplification, and Sequencing
2.3. Phylogenetic Inference
3. Results
3.1. Molecular Phylogeny
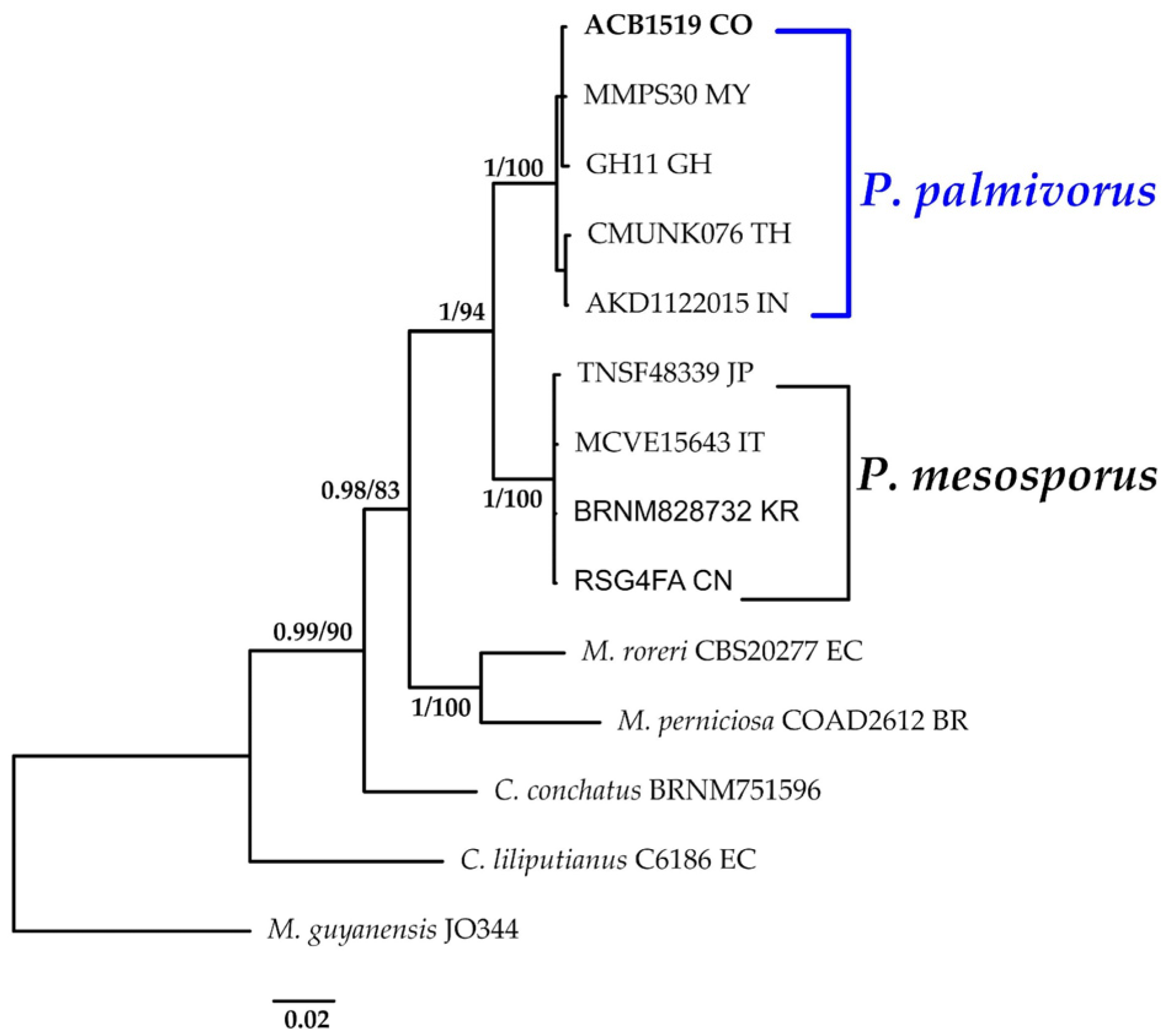
3.1.1. Paramarasmius Phylogeny Based on Combined ITS+nLSU Sequence Data
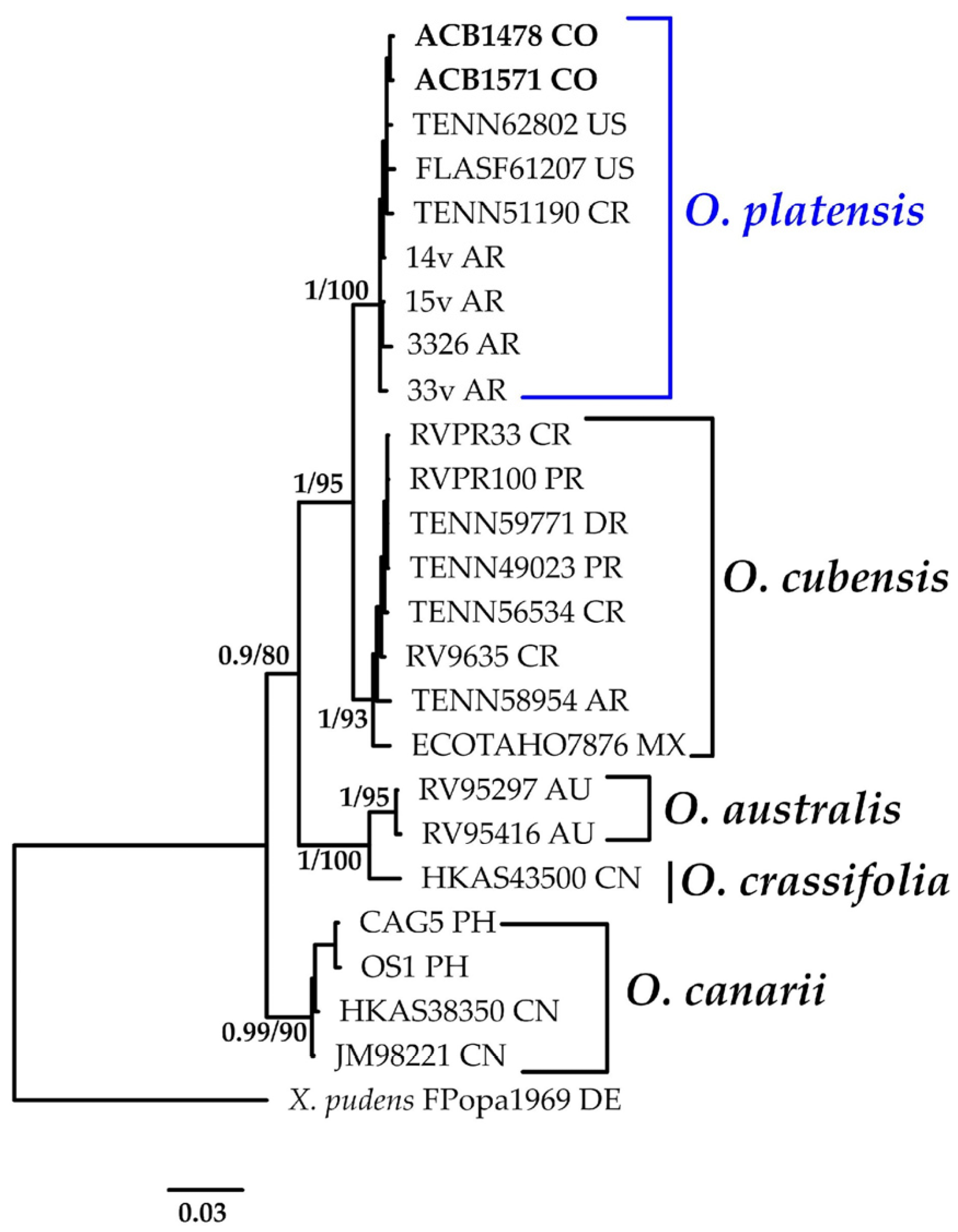
3.1.2. Oudemansiella Phylogeny Based on Combined ITS+nLSU Sequence Data
3.1.3. Punctularia Phylogeny Based on Combined ITS+nLSU Sequence Data
3.1.4. Resinicium Phylogeny Based on Combined ITS+nLSU Sequence Data
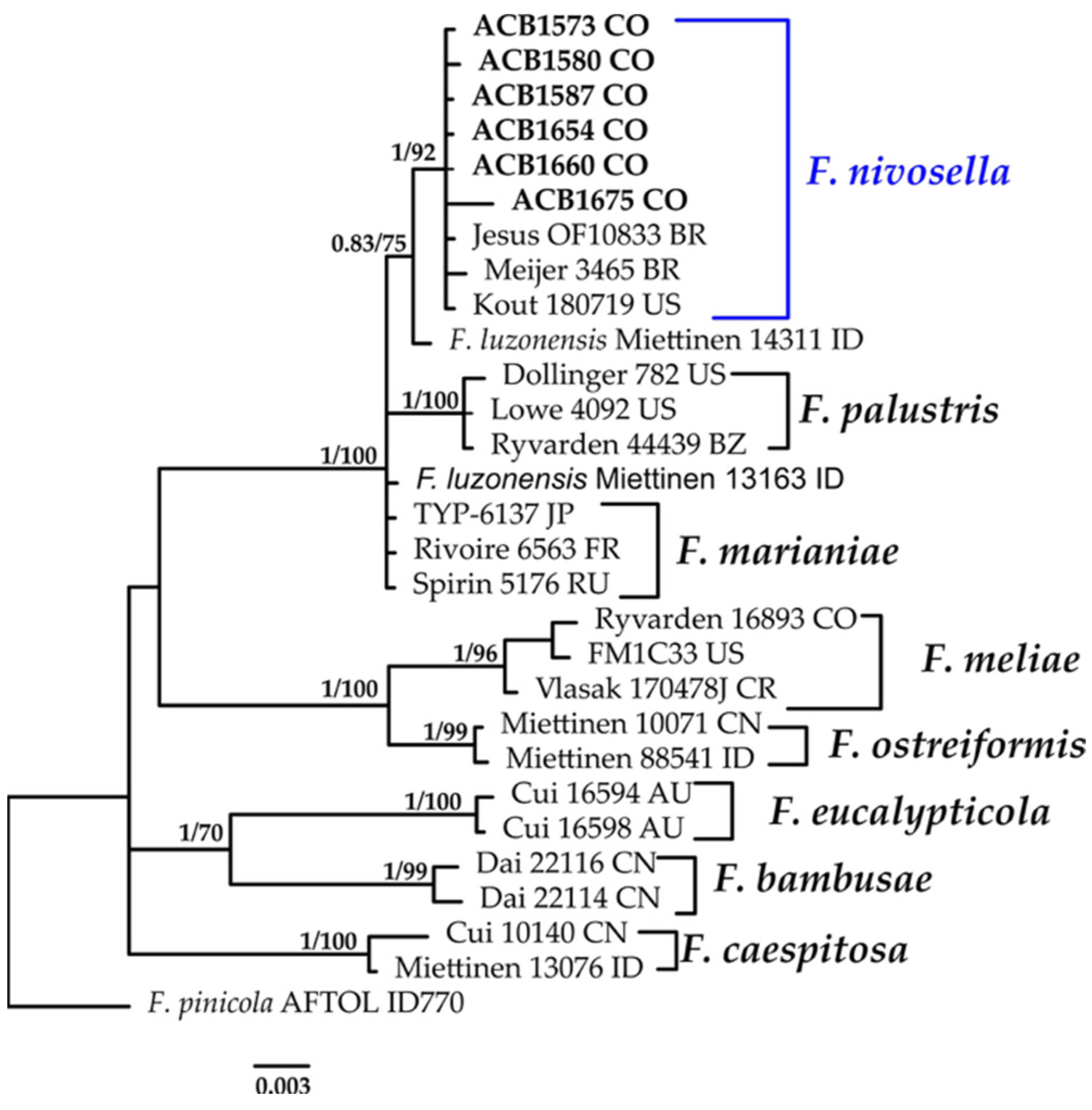
3.1.5. Fomitopsis Phylogeny Based on Combined ITS+nLSU Sequence Data
3.1.6. Neohypochnicium Phylogeny Based on Combined ITS+nLSU Sequence Data
3.1.7. Phlebiopsis Phylogeny Based on Combined ITS+nLSU Sequence Data
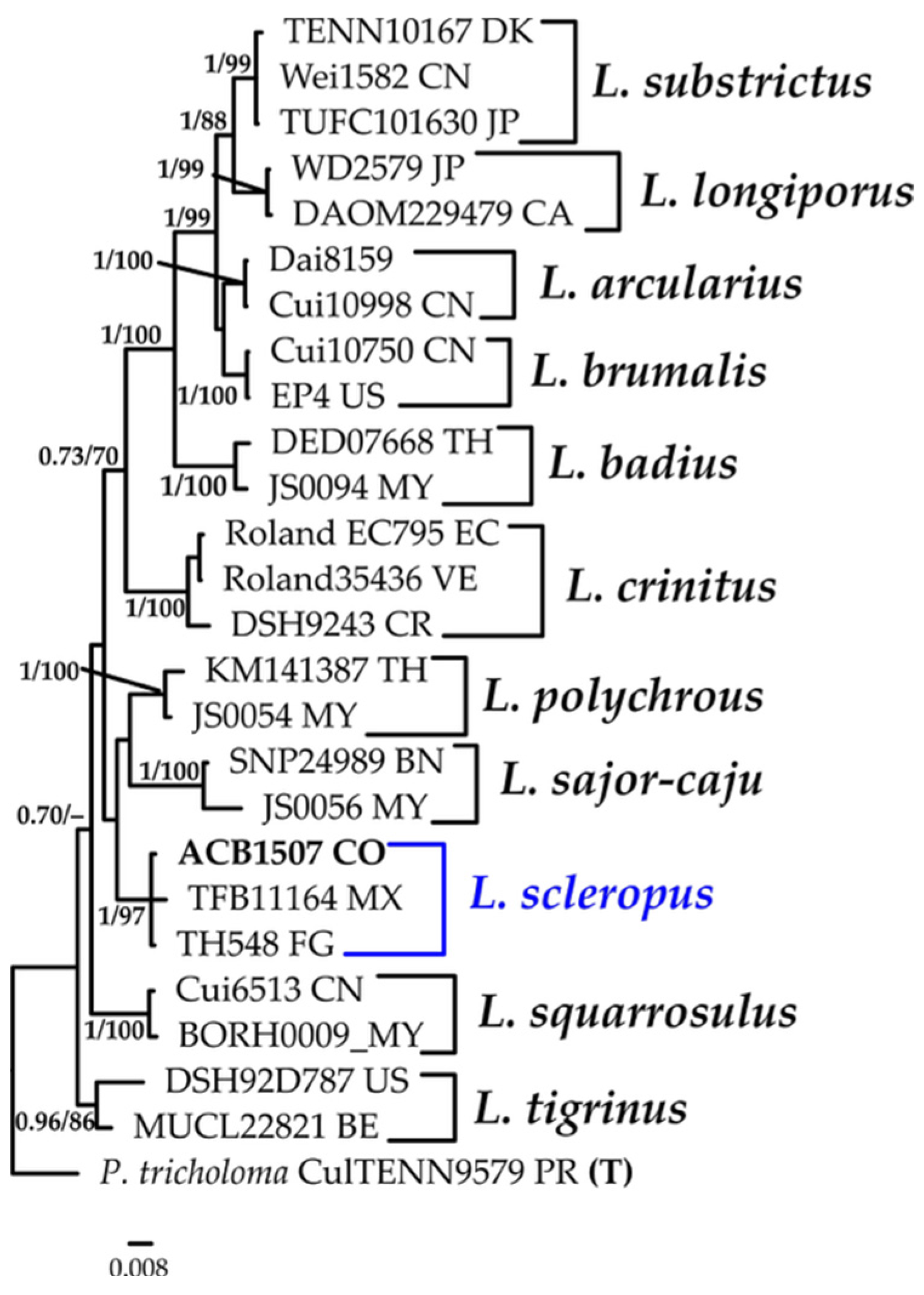
3.1.8. Lentinus Phylogeny Based on Combined ITS+nLSU Sequence Data
3.1.9. Microporus Phylogeny Based on Combined ITS+nLSU Sequence Data
3.1.10. Porogramme Phylogeny Based on Combined ITS+nLSU Sequence Data
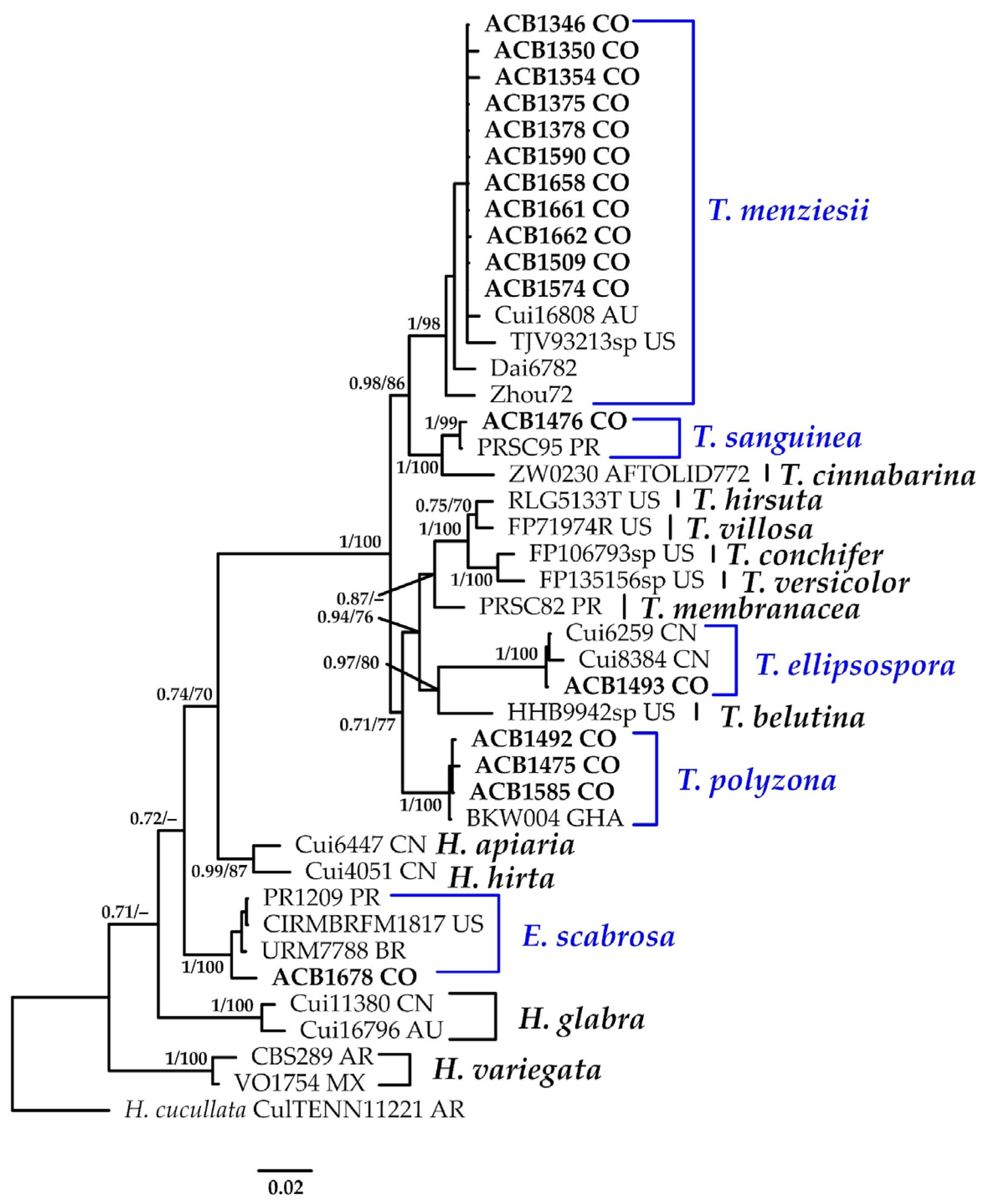
3.1.11. Trametes Phylogeny Based on Combined ITS+nLSU Sequence Data
3.2. Taxonomy
- ORDER AGARICALES
- Family Marasmiaceae
- Paramarasmius palmivorus (Sharples) Antonín, Hosaka & Kolařík, Pl. Biosystems: 2 (2022).
- ≡Marasmius palmivorus Sharples, Malay. agric. Journal 16 (nos. 9–10): (1928). Basionym.
- ≡Marasmiellus palmivorus (Sharples) Desjardin, Mycologia 97(3): 670 (2005). Synonyms.
- Family Physalacriaceae
- Oudemansiella platensis (Speg.) Speg. Anal. Soc. Cient. Argent. 12(1): 24 (1881).
- ≡Agaricus platensis Speg. Anal. Soc. Cient. Argent. 9(4): 161: (1880). Basionym.
- ≡Psalliota platensis (Speg.) Herter, Estudios Botánicos Región Uruguaya, III Florula Uruguayensis. Plantae Avasculares (Montevideo): 43 (1993). Synonym.
- Family Pleurotaceae
- Pleurotus djamor (Rumph. ex Fr.) Boedijn, Rumphius Memorial Volume: 292 (1959).
- ≡Agaricus djamor Rumph. ex Fr., Syst. mycol. (Lundae) 1: 185 (1821). Basionym.
- =Agaricus pacificus Berk., London J. Bot. 1: 451 (1842). Synonym.
- =Agaricus placentodes Berk. Hooker’s J. Bot. Kew Gard. Misc. 4: 104 (1852). Synonym.
- Family Schizophyllaceae
- Schizophyllum commune Fr., Observ. mycol. (Havniae) 1: 103 (1815).
- ORDER CORTICIALES
- Family Punctulariaceae
- Punctularia strigosozonata (Schweinn.) P.H.B. Talbot, Bothalia 7(1): 143 (1958).
- ≡Merulius strigosozonatus Schwein., Trans. Am. Phil. Soc., New Series 4(2): 160 (1832). Basionym.
- ≡Stereum strigosozonatum (Schwein.) G. Cunn., Trans. Roy. Soc. N.Z. 84: 213 (1956). Synonym.
- ORDER DACRYMYCETALES
- Family Dacrymycetaceae
- Dacrymyces spathularia (Schwein.) Alvarenga, Mycol. Progr. 21(12, no. 96): 6 (2022).
- ≡Merulius spathularia Schwein., Schr. naturf. Ges. Leipzig 1: 92 [66 of repr.] (1822). Basionym.
- ≡Dacryopinax spathularia (Schwin.) G.W. Martin, Lloydia 11: 116 (1948). Synonym.
- ORDER HYMENOCHAETALES
- Family Rickenellaceae
- Resinicium grandisporum G. Gruhn, S. Dumez & E. Schimann, Cryptog. Mycol. 38(4): 472 (2017).
- Family Schizoporaceae
- Xylodon flaviporus (Berk. & M.A. Curtis) Riebesehl & Langer, Mycol. Progr. 16(6): 646 (2017).
- ≡Poria flavipora Berk. & M.A. Curtis ex Cooke, Grevillea 15 (no. 73): 25 (1886). Basionym.
- ≡Hyphodontia flavipora (Berk. & M.A. Curtis ex Cooke) Sheng H. Wu, Mycotaxon 76: 54 (2000). Synonym.
- =Polystictus subiculoides Lloyd, Mycol. Writ. (Cincinnati) 7 (Letter 74): 1331 (1924). Synonym.
- ORDER POLYPORALES
- Family Fomitopsidaceae
- Fomitopsis nivosella (Murrill) Spirin & Vlasák, Stud. Mycol. 107: 217 (2024).
- ≡Tyromyces nivosellus Murrill, North American Flora (New York) 9(1): 32 (1907). Basionym.
- ≡Polyporus nivosellus (Murrill) Sacc. & Troter, Syll. fung. (Abellini) 21: 280 (1912). Synonym.
- =Tyromyces palmarum Murrill, North American Flora 9:32 (1907). Synonym.
- =Polyporus durescens Overh. ex J. Lowe, Mycotaxon 2(1): 65 (1975). Synonym.
- Family Neohypochniciaceae
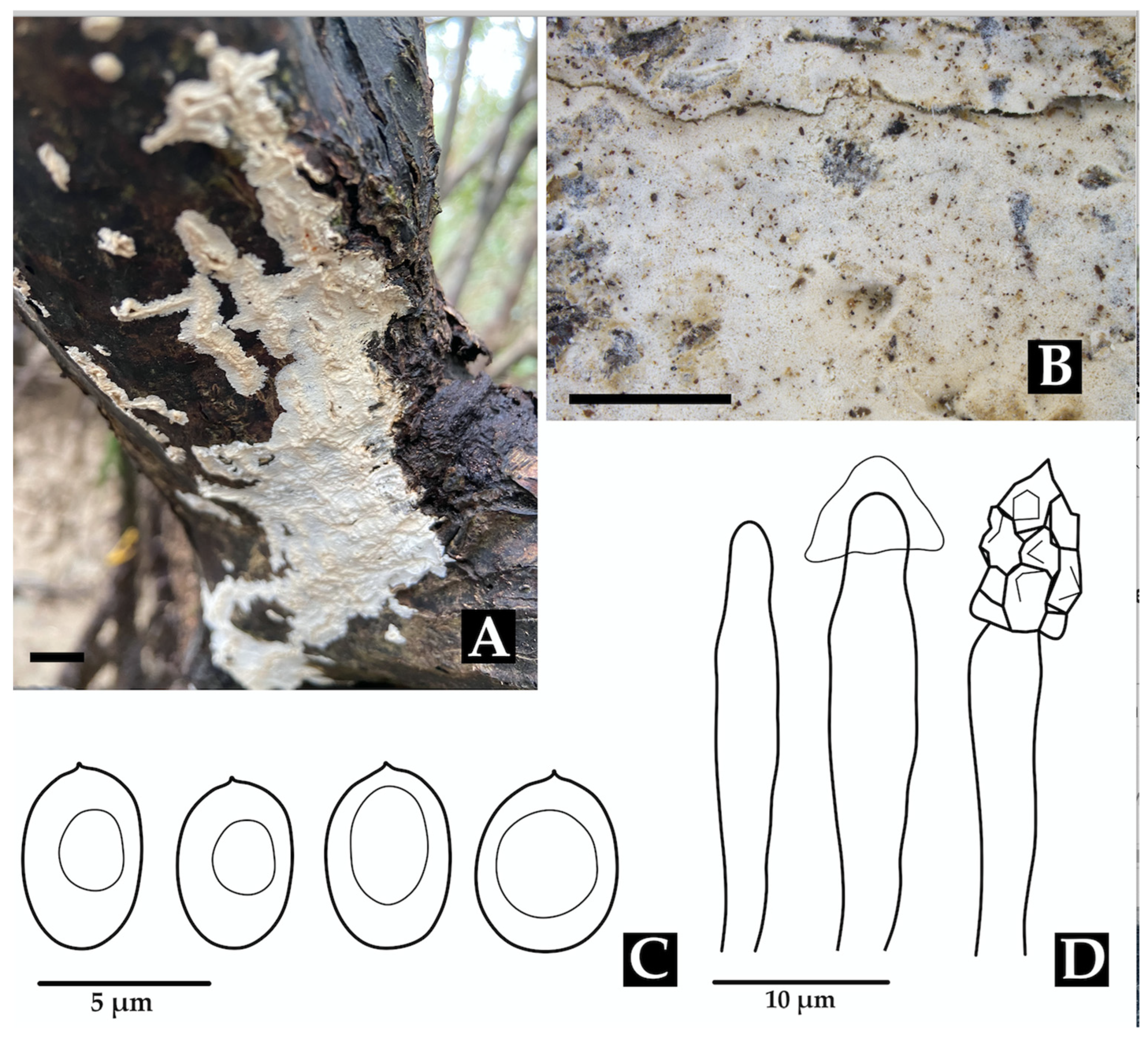
- Neohypochnicium manglarense Motato-Vásq. & Bolaños-Rojas sp. nov.
- Mycobank No: 858653.
- Family Panaceae
- Cymatoderma dendriticum (Pers.) D.A. Reid, Kew Bull. (13)(3): 523, 1959 [1958].
- ≡Thelephora dendritica Pers., in Gaudichaud-Beaupré in Freycinet, Voy, Uranie., Bot. 4: 176 (1827) [1826,1827,1828,1829,1830]. Basionym.
- ≡Cladoderris dendritica (Pers.) Berk., London J. Bot. 1(3): 152 (1842). Synonym.
- =Cladoderris australis Kalchbr. Symb. mycol. austr. 61: 442 (1878). Synonym.
- Family Phanerochaetaceae
- Phlebiopsis flavidoalba (Cooke) Hjortstam, Windahlia 17: 58 (1987).
- ≡Peniophora falvidoalba Cooke, Grevillea 8 (no. 45): 21 (1879). Basionym.
- ≡Phanerochaete flavidoalba (Cooke) S.S. Rattan, Biblthca Mycol. 60: 262 (1977).
- ≡Phlebia flavidoalba (Cooke) Maleçon & Bertault, Acta Phytotax. Barcino. 11: 27 (1972). Synonyms.
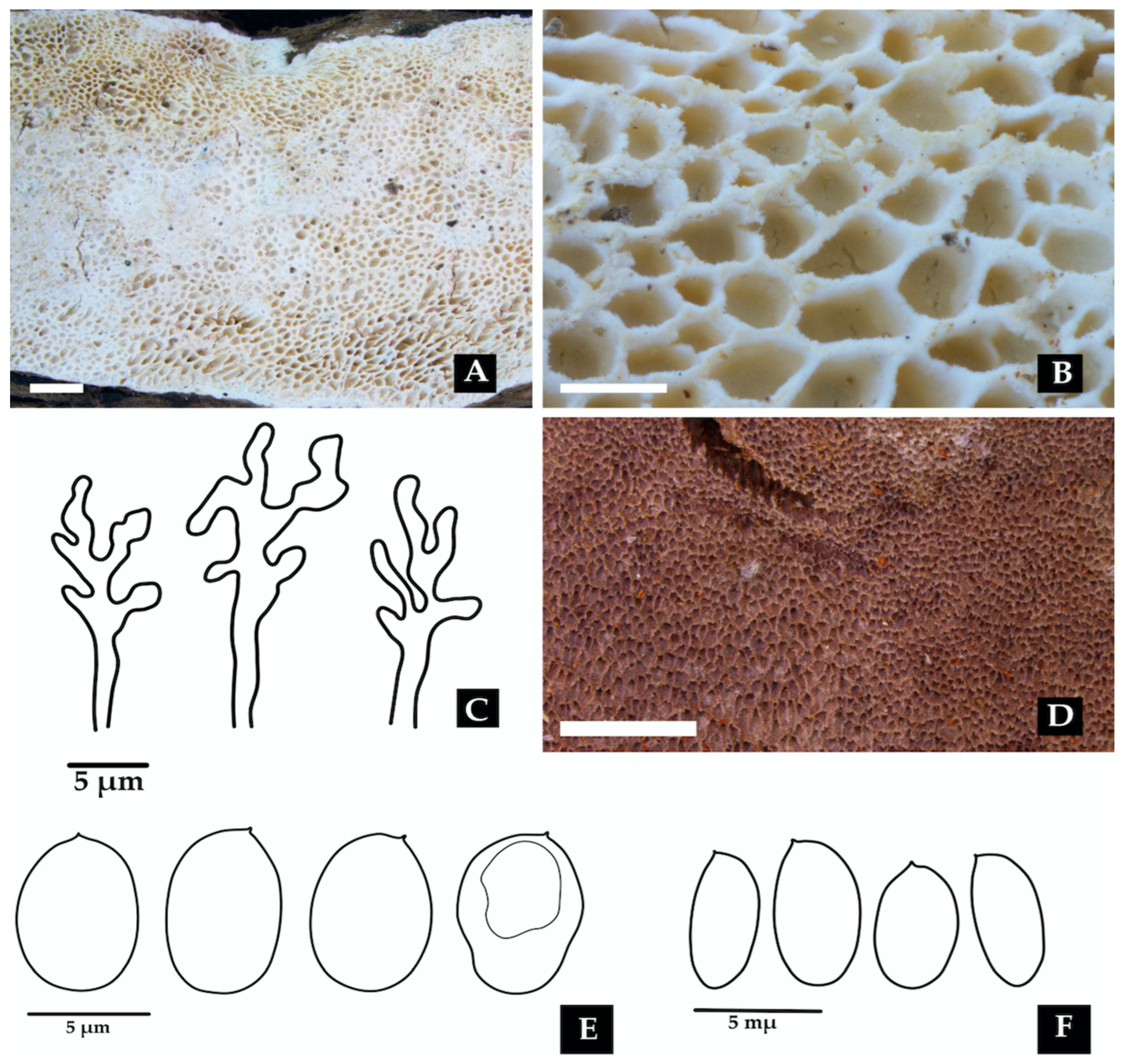
- Phlebiopsis colombiana Motato-Vásq. & Bolaños-Rojas sp. nov.
- MycoBank No: 858655.
- Family Polyporaceae
- Earliella scabrosa (Pers.) Gilb. & Ryvarden, Mycotaxon 22(2): 364 (1985).
- ≡Polyporus scabrosus Pers., in Gaudichaud-Beaupré in Freycinet, Voy. Uranie., Bot. (Paris) 4: 172 (1827) [1826,1827,1828,1829,1830]. Basionym.
- =Daedalea conchata Bres., Bull. Soc. mycol. Fr. 6(1): 166 (1854). Synonym.
- =Earliella cubensis Murrill, Bull. Torrey bot. Club 32(9): 479 (1905). Synonym.
- Porogramme bononiae Bolaños-Rojas & Motato-Vásq. sp. nov.
- Mycobank No: 858656.
- Porogramme brasiliensis (Ryvarden) Y.C. Dai, W.L. Mao & Yuan Yuan, IMA Fungus 14 (no. 5): 10 (2023).
- ≡Grammothele brasiliensis Ryvarden, Syn. Fung. (Oslo) 33: 38 (2015). Basionym.
- Trametes ellipsospora Ryvarden, Mycotaxon 28(2): 539 (1987).
- Trametes menziesii (Berk.) Ryvarden, Norw. Jl. Bot. 19(3–4): 236 (1972).
- ≡Polyporus menziesii Berk., Ann. Mag. nat. Hist., Ser. 1 10: 378 (1843). Basionym.
- ≡Cubamyces menziesii (Berk.) Lücking, Willdenowia 50(3): 396 (2020). Synonym.
- =Trametes grisea Pat., J. Bot., Paris 11: 341 (1897). Synonym.
- Trametes polyzona (Pers.) Justo, Taxon 60(6): 1580 (2011).
- ≡Polyporus polyzonus Pers. in Gaudichaud-Beaupré in Freycinet, Voy. Uranie., Bot. (Paris) 4: 171 (1827). Basionym.
- ≡Coriolopsis polyzona (Pers.) Ryvarden, Norw. Jl. Bot. 19: 230 (1972). Synonym.
- ≡Daedalea polyzona (Pers.) Pers., Mycol. eur. (Erlanga) 3: 8 (1828) Synonym.
- Trametes sanguinea
- ≡Boletus sanguineus L. Sp. pl., Edn 2 2(2): 1646 (1763). Basionym.
- ≡Fabisporus sanguineus (L.) Zmitr., Mycena 1(1): 93 (2001). Synonym.
- ≡Pycnoporus sanguineus (L.) Murrill, Bull. Torrey bot. Club 31(8): 421 (1904). Synonym.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alongi, D.M. Present State and Future of the World’s Mangrove Forests. Environ. Conserv. 2002, 29, 331–349. [Google Scholar] [CrossRef]
- Sridhar, K.R.; Alias, S.A.; Pang, K.-L. Mangrove Fungi. In Marine Fungi and Fungal-Like Organisms; DE GRUYTER: Vienna, Austria, 2012; pp. 253–272. [Google Scholar]
- FAO. The World’s Mangroves 2000–2020; FAO: Rome, Italy, 2023; ISBN 9789251380048. [Google Scholar]
- Leal, M.; Spalding, M.D. The State of the World’s Mangroves 2024; Global Mangrove Alliance: Washington, DC, USA, 2024. [Google Scholar]
- Lee, N.Y.L.; Huang, D.; Quek, Z.B.; Lee, J.N.; Wainwright, B.J. Mangrove-Associated Fungal Communities Are Differentiated by Geographic Location and Host Structure. Front. Microbiol. 2019, 10, 2456. [Google Scholar] [CrossRef]
- Macintosh, D.; Ashton, E. Review of Mangrove Biodiversity Conservation and Management A Review of Mangrove Biodiversity Conservation and Management; Centre for Tropical Ecosystems Research: Aarhus, Denmark, 2002. [Google Scholar]
- Stevens, F.L. New or Noteworthy Porto Rican Fungi. Bot. Gaz. 1920, 70, 399–402. [Google Scholar] [CrossRef]
- Cribb, A.B.; Cribb, J.W. Marine Fungi from Queensland-1; The University of Queensland Press: Queensland, Australia, 1955; Volume 3, pp. 77–81. [Google Scholar]
- Schmit, J.P.; Shearer, C.A. Geographic and Host Distribution of Lignicolous Mangrove Microfungi. Bot. Mar. 2004, 47, 496–500. [Google Scholar] [CrossRef]
- Hyde, K.D.; Lee, S.Y. Ecology of Mangrove Fungi and Their Role in Nutrient Cycling: What Gaps Occur in Our Knowledge? Hydrobiologia 1995, 295, 107–118. [Google Scholar] [CrossRef]
- Donnini, D.; Gargano, M.L.; Perini, C.; Savino, E.; Murat, C.; Di Piazza, S.; Altobelli, E.; Salerni, E.; Rubini, A.; Rana, G.L.; et al. Wild and Cultivated Mushrooms as a Model of Sustainable Development. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2013, 147, 226–236. [Google Scholar] [CrossRef]
- Maekawa, N.; Suhara, H.; Kondo, R.; Kinjo, K. Corticioid Fungi (Basidiomycota) in Mangrove Forests of the Islands of Iriomote and Okinawa, Japan. Mycoscience 2003, 44, 403–409. [Google Scholar] [CrossRef]
- Maekawa, N.; Suhara, H.; Kinjo, K.; Kondo, R.; Hoshi, Y. Haloaleurodiscus mangrovei Gen. Sp. Nov. (Basidiomycota) from Mangrove Forests in Japan. Mycol. Res. 2005, 109, 825–832. [Google Scholar] [CrossRef]
- Gilbert, G.S.; Sousa, W.P. Host Specialization among Wood-Decay Polypore Fungi in a Caribbean Mangrove Forest. Biotropica 2002, 34, 396–404. [Google Scholar] [CrossRef]
- Gilbert, G.S.; Gorospe, J.; Ryvarden, L. Host and Habitat Preferences of Polypore Fungi in Micronesian Tropical Flooded Forests. Mycol. Res. 2008, 112, 674–680. [Google Scholar] [CrossRef]
- Sotão, H.M.P.; Bononi, V.L.R.; Figueiredo, T.S. Basidiomycetes de Manguezais Da Ilha de Maracá, Amapá, Brasil. Bol. Mus. Para. Emílio Goeldi Série Botânica 1991, 7, 109–114. [Google Scholar]
- Sotão, H.M.P.; Lopes De Campos, E.; Costa, S.P.; Melo, O.; Azevedo, J.S. Basidiomycetes Macroscópicos de Manguezais de Bragança, Pará, Brasil. Hoehnea 2002, 29, 215–224. [Google Scholar]
- Sotão, H.M.P.; Campos, E.L.; Gugliotta, A.M.; Costa, S.P.S.E.; Fernandes, M.E.B. Fungos Macroscópicos: Basidiomycetes; Fernandes, M.E.B., Ed.; Fundação Rio Bacanga: São Luís, Brazil, 2003; ISBN 85-89547-01-9. [Google Scholar]
- Campos, E.; Sotão, H.M.P.; Cavalcanti, M.A.; Luz, A. Basidiomycetes de Manguezais Da APA de Algodoal-Maiandeua, Pará, Brasil. Bol. Mus. Para. Emílio Goeldi, sér. Ciências Naturais, Belém 2005, 1, 141–146. [Google Scholar]
- Raveendran, K.; Manimohan, P. Marine Fungi of Kerala A Preliminary Floristic and Ecological Study, 1st ed.; Malabar Natural History Society: Kerala, India, 2007; ISBN 9789351373155. [Google Scholar]
- Maria, G.L.; Sridhar, K.R. Diversity of Filamentous Fungi on Woody Litter of Five Mangrove Plant Species from the Southwest Coast of India. Fungal Divers. 2003, 14, 109–126. [Google Scholar]
- Tan, T.K.; Leong, W.F.; Jones, E.B.G. Succession of Fungi on Wood of Avicennia alba and A. Lanata in Singapore. Can. J. Bot. 1989, 67, 2687–2691. [Google Scholar] [CrossRef]
- Leong, W.F.; Tan, T.K.; Jones, E.B.G. Fungal Colonization of Submerged Bruguiera cylindrica and Rhizophora apiculata Wood. Botanica Marina 1991, 34, 69–76. [Google Scholar] [CrossRef]
- Baltazar, J.M.; Trierveiler-Pereira, L.; Loguercio-Leite, C. A Checklist of Xylophilous Basidiomycetes (Basidiomycota) in Mangroves. Mycotaxon 2009, 107, 221–224. [Google Scholar] [CrossRef]
- Blanco-Libreros, J.F.; López-Rodríguez, S.R.; Valencia-Palacios, A.M.; Perez-Vega, G.F.; Álvarez-León, R. Mangroves From Rainy to Desert Climates: Baseline Data to Assess Future Changes and Drivers in Colombia. Front. For. Glob. Change 2022, 5, 772271. [Google Scholar] [CrossRef]
- Correa, I.; Morton, R. Pacific Coast of Colombia. In Encyclopedia of the World’s Coastal Landforms; Springer: Dordrecht, The Netherlands, 2010; pp. 193–198. [Google Scholar]
- Álvarez-León, R. Los Manglares de Colombia y La Recuperación de Sus Áreas Degradadas: Revisión Bibliográfica y Nuevas Experiencias; Institute of Ecology: Xalapa, Mexico, 2003; Volume 9. [Google Scholar]
- Hijmans, R.J.; Barbosa, M.; Ghosh, A.; Mandel, A. Geographic Data; Lund University: Lund, Sweden, 2024; Volume 32. [Google Scholar]
- Wickman, H.; Chang, W.; Henry, L.; Pedersen, T.L.; Takahashi, K.; Wilke, C.; Woo, K.; Yutani, H.; Dunnington, D.; van der Brand, T. Create Elegant Data Visualisations Using the Grammar of Graphics. 2024. Available online: https://ggplot2.tidyverse.org/reference/ggplot2-package.html (accessed on 12 January 2025).
- Wilke, C.O. Streamlined Plot Theme and Plot Annotations for “Ggplot2”. 2024. Available online: https://wilkelab.org/cowplot/ (accessed on 15 January 2025).
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. PCR Protoc. 1990, 18, 315–322. [Google Scholar] [CrossRef]
- Haynes, K.; Westerneng, T.; Fell, J.; Moens, W. Rapid Detection and Identification of Pathogenic Fungi by Polymerase Chain Reaction Amplification of Large Subunit Ribosomal DNA. Med. Mycol. 1995, 33, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Vilgalys, R.; Hester, M. Rapid Genetic Identification and Mapping of Enzymatically Amplified Ribosomal DNA From Several Cryptococcus Species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, A.; Riesner, D. Purification of Nucleic Acids by Selective Precipitation with Polyethylene Glycol 6000. Anal. Biochem. 2006, 354, 311–313. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An Integrated and Extendable Desktop Software Platform for the Organization and Analysis of Sequence Data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT Online Service: Multiple Sequence Alignment, Interactive Sequence Choice and Visualization. Brief. Bioinform. 2018, 20, 1160–1166. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Larsson, A. AliView: A Fast and Lightweight Alignment Viewer and Editor for Large Datasets. Bioinformatics 2014, 30, 3276–3278. [Google Scholar] [CrossRef]
- Maddison, W.; Maddison, D. MESQUITE: A Modular System for Evolutionary Analysis. 2009. Available online: https://www.cs.mcgill.ca/~birch/birchhomedir/doc/mesquite/doc/MesquiteManual.pdf (accessed on 30 March 2025).
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. Mrbayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. JModelTest 2: More Models, New Heuristics and Parallel Computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; Von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast Model Selection for Accurate Phylogenetic Estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New Algorithms and Methods to Estimate Maximum-Likelihood Phylogenies: Assessing the Performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Kossmann, T.; Costa-Rezende, D.H.; Góes-Neto, A.; Drechsler-Santos, E.R. A New and Threatened Species of Trichaptum (Basidiomycota, Hymenochaetales) from Urban Mangroves of Santa Catarina Island, Southern Brazil. Phytotaxa 2021, 482, 197–207. [Google Scholar] [CrossRef]
- Desjardin, D.E.; Perry, B.A. The Gymnopoid Fungi (Basidiomycota, Agaricales) from the Republic of São Tomé and Príncipe, West Africa. Mycosphere 2017, 8, 1317–1391. [Google Scholar] [CrossRef]
- Sridhar, P.; Venkateshbabu, G.; Hemalakshmi, D.; Kirthika, V.M.; Palani, P. New Disease and First Report of Marasmioid Fungus, Marasmius palmivorus (Sharples), Causing White Root Rot in Arachis hypogaea L. Lett. Appl. Microbiol. 2022, 75, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Sharples, A. Palm Diseases in Malaya. Malay. Agric. J. 1928, 16, 313–360. [Google Scholar]
- Carvalho, E.; Farias dos Santos, T. Doenças Da Palma de Óleo No Contexto Da Expansão Do Cultivo No Estado Do Pará; Embrapa Eastern Amazon: Belém, Brazil, 2013. [Google Scholar]
- Antonín, V.; Hosaka, K.; Kolařík, M. Taxonomy and Phylogeny of Paramarasmius Gen. Nov. and Paramarasmius mesosporus, a Worldwide Distributed Fungus with a Strict Ecological Niche. Plant Biosyst. 2023, 157, 286–293. [Google Scholar] [CrossRef]
- Almaliky, S.B.; Kadir, J.; Wong, M.-Y. Pathogenicity of Marasmiellus palmivorus (Sharples) Desjardin Comb. Prov. on Oil Palm Elaeis guineensis. Wulfenia 2012, 19, 1–17. [Google Scholar]
- Maizatul-Suriza, M.; Suhanah, J.; Madihah, A.Z.; Idris, A.S.; Mohidin, H. Phylogenetic and Pathogenicity Evaluation of the Marasmioid Fungus Marasmius palmivorus Causing Fruit Bunch Rot Disease of Oil Palm. For. Pathol. 2021, 51, e12660. [Google Scholar] [CrossRef]
- Pham, M.T.; Huang, C.M.; Kirschner, R. First Report of the Oil Palm Disease Fungus Marasmius palmivorus from Taiwan Causing Stem Rot Disease on Native Formosa Palm Arenga engleri as New Host. Lett. Appl. Microbiol. 2020, 70, 143–150. [Google Scholar] [CrossRef]
- Dutta, A.K.; Acharya, K. A New Host for the Parasitic Macrofungus Marasmius palmivorus Sharples (Marasmiaceae). Curr. Sci. 2018, 114, 1400. [Google Scholar] [CrossRef]
- Bononi, V.L.R.; Trufem, S.; Grandi, R. Fungos Macroscópicos Do Parque Estadual Das Fontes Do Ipiranga, São Paulo, Brasil, Depositados No Herbário Do Instituto de Botânica. Rickia 1981, 9, 37–53. [Google Scholar]
- Petersen, R.H.; Desjardin, D.E.; Krüger, D. Three Type Specimens Designated in Oudemansiella Dirk Krüger. Fungal Divers. 2008, 32, 81–96. [Google Scholar]
- Yang, Z.L.; Zhang, L.F.; Mueller, G.; Kost, G.; Rexer, K. A New Systematic Arrangement of the Genus Oudemansiella s. Str. (Physalacriaceae, Agaricales). Mycosystema 2009, 28, 1–13. [Google Scholar]
- Corner, E.H.J. On the Agaric Genera Hohenbuehelia and Oudemansiella. Part. II: Oudemansiella Speg. Gard. Bul. 1994, 46, 49–75. [Google Scholar]
- Lechner, B.E.; Wright, J.E.; Albertó, E. The Genus Pleurotus in Argentina; The Mycological Society of America: San Diego, CA, USA, 2004; Volume 96. [Google Scholar]
- Menolli, N.; Breternitz, B.S.; Capelari, M. The Genus Pleurotus in Brazil: A Molecular and Taxonomic Overview. Mycoscience 2014, 55, 378–389. [Google Scholar] [CrossRef]
- Bolaños, A.C.; Soto-Medina, E. Macrohongos Comestibles y Medicinales Comunes En La Vegetación de La Universidad Del Valle, Colombia. Rev. Cienc. 2011, 15, 31–38. [Google Scholar] [CrossRef]
- Ruan-Soto, F.; Cifuentes, J.; Garibay-Orijel, R.; Caballero, J. Comparative Availability of Edible Mushrooms in the Highlands and Lowlands of Chiapas, Mexico, and Its Implications in Traditional Management Strategies. Acta Bot. Mex. 2021, 128, 1–22. [Google Scholar] [CrossRef]
- Nieves-Rivera, A.M. Coastal Mycology of Puerto Rico: A Survey and Biological Aspects of Marine, Estuarine, and Mangrove Fungi. Ph.D. Thesis, University of Puerto Rico, San Juan, PR, USA, 2005. [Google Scholar]
- Nogueira-Melo, G.S.; Santos, P.J.P.; Gibertoni, T.B. The Community Structure of Macroscopic Basidiomycetes (Fungi) in Brazilian Mangroves Influenced by Temporal and Spatial Variations. Rev. Biol. Trop 2014, 62, 1587–1595. [Google Scholar] [CrossRef]
- Nogueira-Melo, G.S.; Santos, P.J.P.; Gibertoni, T.B. Host-Exclusivity and Host-Recurrence by Wood Decay Fungi (Basidiomycota-Agaricomycetes) in Brazilian Mangroves. Acta Bot. Bras. 2017, 31, 566–570. [Google Scholar] [CrossRef]
- Sharif, W.R.; Da Silva, P.N. Macrofungal Diversity in Selected Coastal Mangrove Ecosystems in Guyana, South America. Eur. Mod. Stud. J. 2023, 7, 384–400. [Google Scholar] [CrossRef]
- Karun, N.; Sridhar, K.R. A Preliminary Study on Macrofungal Diversity in an Arboretum and Three Plantations of the Southwest Coast of India. Curr. Res. Environ. Appl. Mycol. 2014, 4, 173–187. [Google Scholar] [CrossRef]
- Nieves-Rivera, A.M.; Lodge, D.J. Contributions to the Study of Gasteromycetes of Puerto Rico. McIlvainea 1998, 13, 50–58. [Google Scholar]
- Raper, C.A. Schizophyllum commune, a Model for Genetic Studies of the Basidiomycotina. Adv. Plant Pathol. 1988, 6, 511–522. [Google Scholar]
- Carreño-Ruiz, S.D.; Lázaro, A.A.Á.; García, S.C.; Hernández, R.G.; Chen, J.I.E.; Navarro, G.K.G.; Fajardo, L.V.G.; Pérez, N.D.C.J.; Delacruz, M.T.; Blanco, J.C.; et al. New Record of Schizophyllum (Schizophyllaceae) from Mexico and the Confirmation of Its Edibility in the Humid Tropics. Phytotaxa 2019, 413, 137–148. [Google Scholar] [CrossRef]
- Wojewoda, W. Punctularia strigosozonata (Fungi, Corticiaceae) in Poland and North Korea. Fragm. Flor. Geobot. 2000, 45, 501–507. [Google Scholar]
- Ginns, J.; Lefebvre, M.N. Lignicolous Corticioid Fungi (Basidiomycota) of North America. Mycol. Mem. 1993, 19, 1–247. [Google Scholar]
- Ryvarden, L. (Ed.) Stereoid Fungi of America; Fungiflora: Oslo, Norway, 2009; ISBN 978-82-90724-42-4. [Google Scholar]
- Eriksson, J.; Hjortstam, K.; Ryvarden, L. The Corticiaceae of North Europe. Phlebia to Sarcodontia; Fungiflora: Oslo, Norway, 1981. [Google Scholar]
- Jiilich, W.; Stalpers, J.A. The Resupinate Nonporoid Aphyllophorales of the Temperate Northern Hemisphere; North-Holland: Amsterdam, The Netherlands, 1980. [Google Scholar]
- García-Martínez, Y.A.; Abarca, G.H.; Guzmán-Guillermo, J.; Valenzuela, R.; Raymundo, T. Fungi Associated with the Red Mangrove Rhizophora Mangle (Rhizophoraceae) in Cozumel Island Biosphere Reserve, Quintana Roo, Mexico. Acta Bot. Mex. 2021, 128, 1–27. [Google Scholar] [CrossRef]
- Das, K.; Aminuzzaman, F.M.; Das, K.; Akhtar, N. Diversity of Fleshy Macro Fungi in Mangrove Forest Regions of Bangladesh. J. Biol. Nat. 2016, 6, 218–241. [Google Scholar]
- Ghate, S.D.; Sridhar, K.R.; Karun, N.C. Macrofungi on the Coastal Sand Dunes of South-Western India. Mycosphere 2014, 5, 144–151. [Google Scholar] [CrossRef]
- Ghate, S.D.; Sridhar, K.R. Contribution to the Knowledge on Macrofungi in Mangroves of the Southwest India. Plant Biosyst. 2015, 150, 977–986. [Google Scholar] [CrossRef]
- Castro-Santiuste, S.; Sierra, S.; Guzmán-Dávalos, L.; Luna-Vega, I. A Review of the Taxonomy and Species Diversity in Dacrymycetes (Fungi, Basidiomycota) in Mexico. Nova Hedwig. 2017, 105, 365–384. [Google Scholar] [CrossRef]
- Alvarenga, R.L.M.; Xavier-Santos, S. New Records of Dacrymycetes (Fungi: Basidiomycota) from the Cerrado Biome (Brazilian Savanna) and Midwest Region, Brazil. Check List. 2017, 13, 335–342. [Google Scholar] [CrossRef]
- Gérald, G.; Dumez, S.; Moreau, P.-A. The Genus Resinicium in French Guiana and the West Indies: A Morphological and Molecular Survey, Revealing Resinicium grandisporum Sp. Nov. Cryptogam. Mycol. 2017, 38, 469–483. [Google Scholar]
- Paulus, B.; Hallenberg, N.; Buchanan, P.K.; Chambers, G.K. A Phylogenetic Study of the Genus Schizopora (Basidiomycota) Based on ITS DNA Sequences. Mycol. Res. 2000, 104, 1155–1163. [Google Scholar] [CrossRef]
- Ryvarden, L.; Melo, I. Poroid Fungi of Europe; Synopsis Fungorum Vol. 31; Fungiflora: Oslo, Norway, 2014. [Google Scholar]
- Wu, S. Studies on Schizopora flavipora s. l., with Special Emphasis on Specimens from Taiwan. Mycotaxon 2000, 76, 51–66. [Google Scholar]
- Hjortstam, K.; Ryvarden, L. Corticioid Species (Basidiomycotina, Aphyllophorales) from Colombia Collected by Leif Ryvarden. Mycotaxon 1997, 64, 229–241. [Google Scholar]
- Setliff, E.C.; Ryvarden, L. Los Hongos de Colombia VII: Some Aphyllophoraceous Wood-Inhabiting Fungi. Mycotaxon 1983, 18, 509–525. [Google Scholar]
- Chikowski, R.S.; de Lira, C.R.S.; Larsson, K.H.; Gibertoni, T.B. A Checklist of Corticioid Fungi (Agaricomycetes, Basidiomycota) from Brazil. Mycotaxon 2020, 135, 467. [Google Scholar] [CrossRef]
- Abrahão, M.C.; Gugliotta, A.D.M.; Bononi, V.L.R. Xylophilous Agaricomycetes (Basidiomycota) of the Brazilian Cerrado. CheckList 2012, 8, 1102–1116. [Google Scholar] [CrossRef]
- Abrahão, M.C.; Pires, R.M.; Gugliotta, A.d.M.; Gomes, E.P.C.; Bononi, V.L.R. Wood-Decay Fungi (Agaricomycetes, Basidiomycota) in Three Physiognomies in the Savannah Region in Brazil. Hoehnea 2019, 46, 1. [Google Scholar] [CrossRef]
- Gilbertoni, T.; Gomes-Silva, A.C.; Chikowski, R.S.; Lira, C.R.S.; Soares, A.M.S.; Melo, G.S.N.; Araújo Neta, L.; Gugliotta, A.M.; Medeiros, P.S.; Silva, V.F.; et al. Hymenochaetales in Lista de Espécies Da Flora Do Brasil. Jardim Botânico do Rio de Janeiro. 2015. Available online: https://floradobrasil2015.jbrj.gov.br/FB92820 (accessed on 12 January 2025).
- Riebesehl, J.; Yurchenko, E.; Nakasone, K.K.; Langer, E. Phylogenetic and Morphological Studies in Xylodon (Hymenochaetales, Basidiomycota) with the Addition of Four New Species. MycoKeys 2019, 47, 97–137. [Google Scholar] [CrossRef] [PubMed]
- Baltazar, J.M.; Trierveiler-Pereira, L.; Da Silveira, R.M.B.; Loguercio-Leite, C. Santa Catarina Island Mangroves 5: Corticioid Fungi and an Updated Checklist of Xylophilous Fungi and Pseudofungi1. J. Torrey Bot. Soc. 2017, 144, 230–238. [Google Scholar] [CrossRef]
- Spirin, V.; Runnel, K.; Vlasák, J.; Viner, I.; Barrett, M.D.; Ryvarden, L.; Bernicchia, A.; Rivoire, B.; Ainsworth, A.M.; Grebenc, T.; et al. The Genus Fomitopsis (Polyporales, Basidiomycota) Reconsidered. Stud. Mycol. 2024, 107, 149–249. [Google Scholar] [CrossRef] [PubMed]
- Telleria, M.T.; Dueñas, M.; Melo, I.; Hallenberg, N.; Martín, M.P. A Re-Evaluation of Hypochnicium (Polyporales) Based on Morphological and Molecular Characters. Mycologia 2010, 102, 1426–1436. [Google Scholar] [CrossRef]
- Hattori, T.; Yamashita, S.; Lee, S.S. Diversity and Conservation of Wood-Inhabiting Polypores and Other Aphyllophoraceous Fungi in Malaysia. Biodivers. Conserv. 2012, 21, 2375–2396. [Google Scholar] [CrossRef]
- Trierveiler-Pereira, L.; Marcon, J.; Clarice, B. Santa Catarina Island Mangroves 4-Xylophilous Basidiomycetes. Mycotaxon 2009, 109, 107–110. [Google Scholar] [CrossRef]
- Popoff, O.F.; Wright, J.E. Fungi of Paraguay. I. Preliminary Check-List of Wood-Inhabiting Polypores (Aphyllophorales, Basidiomycota). Mycotaxon 1998, 68, 323–340. [Google Scholar]
- Burdsall, H.H. A Contribution to the Taxonomy of the Genus Phanerochaete (Corticiaceae, Aphyllophorales). Mycol. Mem. 1985, 10, 165. [Google Scholar]
- Bononi, V.L. Basidiomicetos Do Parque Estadual Da Ilha Do Cardoso: IV. Adicoes as Familias Hymenochaetaceae, Stereaceae e Thelephoraceae. Rickia 1984, 11, 43–52. [Google Scholar]
- Gilbertson, R.L.; Adaskaveg, J.E. Studies on Wood-Rotting Basidiomycetes of Hawaii. Mycotaxon 1993, 49, 369–397. [Google Scholar]
- Gazzano, S. Notas Sobre Basidiomycetes Xilófilos Del Uruguay. V. Nuevos Registros de Corticiaceae s.l. (Aphyllophorales) de La Región Litoral Platense. Com. Bot. Mus. Hist. Nat. Montev. 1992, 99, 1–7. [Google Scholar]
- Zhao, Y.N.; He, S.H.; Nakasone, K.K.; Wasantha Kumara, K.L.; Chen, C.C.; Liu, S.L.; Ma, H.X.; Huang, M.R. Global Phylogeny and Taxonomy of the Wood-Decaying Fungal Genus Phlebiopsis (Polyporales, Basidiomycota). Front. Microbiol. 2021, 12, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Prasher, I.B.; Lalita, L. A Checklist of Wood Rotting Fungi (Non-Gilled Agaricomycotina) of Uttarakhand. J. New Biol. Rep. 2013, 2, 108–123. [Google Scholar]
- Hjortstam, K.; Ryvarden, L. Checklist of Corticioid Fungi (Basidiomycotina) from the Tropics, Subtropics, and the Southern Hemisphere. Synop. Fungorum. 2007, 22, 27–146. [Google Scholar]
- Gazzano, S. Notas Sobre Basidiomycetes Xilófilos Del Uruguay. VIII.Registro de Aphyllophorales y Sus Sustratos Arbóreos. Com. Bot. Mus. Hist. Nat. Montev. 1998, 109, 1–12. [Google Scholar]
- Gilbertson, R.L.; Ryvarden, L. North American Polypores. Vol. I. Abortiporus-Lindtneria; Fungiflora: Oslo, Norway, 1986. [Google Scholar]
- Ryvarden, L. Neotropical Polypores. Part 2, Polyporaceae Abortiporus—Nigroporus; Ryvarden, L., Ed.; Fungiflora: Oslo, Norway, 2015; ISBN 9788290724509. [Google Scholar]
- Menolli, J.N.; Alves-Silva, G. Lentinus Scleropus. In The IUCN Red List of Threatened Species; International Union for Conservation of Nature and Natural Resources: Gland, Switzerland, 2024; E.T265094980A265150983. [Google Scholar]
- Ryvarden, L. Studies in Neotropical Polypores 40 A Note on the Genus Grammothele; Fungiflora: Oslo, Norway, 2015. [Google Scholar]
- Olou, B.A.; Langer, E.; Ryvarden, L.; Krah, F.S.; Hounwanou, G.B.; Piepenbring, M.; Yorou, N.S. New Records and Barcode Sequence Data of Wood-Inhabiting Polypores in Benin with Notes on Their Phylogenetic Placements and Distribution. Fungal Syst. Evol. 2023, 11, 11–42. [Google Scholar] [CrossRef]
- Hyde, K.D.; Tennakoon, D.S.; Jeewon, R.; Bhat, D.J.; Maharachchikumbura, S.S.N.; Rossi, W.; Leonardi, M.; Lee, H.B.; Mun, H.Y.; Houbraken, J.; et al. Fungal Diversity Notes 1036–1150: Taxonomic and Phylogenetic Contributions on Genera and Species of Fungal Taxa. Fungal Divers. 2019, 96, 1–242. [Google Scholar] [CrossRef]
- Ryvarden, L. Neotropical Polypores. Part 3. Polyporaceae Obba-Wrightoporia. Synopsis Fungorum 36; Fungiflora: Oslo, Norway, 2016. [Google Scholar]
- Bononi, V.L.R.; De Oliveira, A.K.M.; Gugliotta, A.D.M.; De Quevedo, J.R. Agaricomycetes (Basidiomycota, Fungi) Diversity in a Protected Area in the Maracaju Mountains, in the Brazilian Central Region. Hoehnea 2017, 44, 361–377. [Google Scholar] [CrossRef]
- Li, H.J.; He, S.H. Notes on Trametes (Basidiomycota) in China. Mycotaxon 2011, 116, 265–281. [Google Scholar] [CrossRef]
- Gore, V.; Mali, V.P. On the Diversity and Taxonomic Evaluation of Wood-Decaying Fungi from Ajanta Forest Caves, Maharashtra, India. Biosci. Biotechnol. Res. Commun. 2023, 16, 241–248. [Google Scholar] [CrossRef]
- Bustillos, R.G.; Dulay, R.M.R.; Kalaw, S.P.; Reyes, R.G. Diversity of Macrofungi along Elevation Gradients in Mt. Arayat Protected Landscape, Arayat, Pampanga, The Philippines. Stud. Fungi 2024, 9, e017. [Google Scholar] [CrossRef]
- Ryvarden, L.; Johansen, I. A Preliminary Polypore Fora of East Africa. Fungiflora 1980, 73, 1020. [Google Scholar]
- Gilbertson, R.L.; Ryvarden, R. North American Polypores 2; Fungiflora: Oslo, Norway, 1987. [Google Scholar]
- Justo, A.; Hibbett, D.S. Phylogenetic Classification of Trametes (Basidiomycota, Polyporales) Based on a Five-Marker Dataset. Taxon 2011, 60, 1567–1583. [Google Scholar] [CrossRef]
- Lücking, R.; Truong, B.V.; Huong, D.T.T.; Le, N.H.; Nguyen, Q.D.; Nguyen, V.D.; Von Raab-Straube, E.; Bollendorff, S.; Govers, K.; Di Vincenzo, V. Caveats of Fungal Barcoding: A Case Study in Trametes s. Lat. (Basidiomycota: Polyporales) in Vietnam Reveals Multiple Issues with Mislabelled Reference Sequences and Calls for Third-Party Annotations. Willdenowia 2020, 50, 383–403. [Google Scholar] [CrossRef]
- Adarsh, C.K.; Vidyasagaran, K.; Ganesh, P.N. The Diversity and Distribution of Polypores (Basidiomycota: Aphyllophorales) in Wet Evergreen and Shola Forests of Silent Valley National Park, Southern Western Ghats, India, with Three New Records. J. Threat. Taxa 2019, 11, 13886–13909. [Google Scholar] [CrossRef]
- Miguel López-Guzmán, L.; Chacón, S.; Bautista-Gálvez, A. Adiciones al Conocimiento Sobre La Diversidad de Los Hongos (Macromicetes) de Chiapas, México Additions to the Knowledge on the Diversity of Fungi (Macromycetes) from Chiapas, Mexico. Sci. Fungorum 2017, 45, 27–35. [Google Scholar] [CrossRef]
- Gugliotta, A.M.; Gibertoni, T.B.; Dreschsler-Santos, E.R.; Silveira, R.M.B.; Chikowski, R.S.; Pires, R.M.; Montoya, C.A.S.; Souza, J.F.; Palacio, M.; Rezende, D.H.C. Polyporales in Lista de Espécies Da Flora Do Brasil; Jardim Botânico Do Rio de Janeiro: Rio de Janeiro, Brazil, 2015. [Google Scholar]
- Welti, S.; Moreau, P.A.; Favel, A.; Courtecuisse, R.; Haon, M.; Navarro, D.; Taussac, S.; Lesage-Meessen, L. Molecular Phylogeny of Trametes and Related Genera, and Description of a New Genus Leiotrametes. Fungal Divers. 2012, 55, 47–64. [Google Scholar] [CrossRef]
- Ondo, I.; Dhanjal-Adams, K.L.; Pironon, S.; Silvestro, D.; Colli-Silva, M.; Deklerck, V.; Grace, O.M.; Monro, A.K.; Nicolson, N.; Walker, B.; et al. Plant Diversity Darkspots for Global Collection Priorities. New Phytol. 2024, 244, 719–733. [Google Scholar] [CrossRef]
- Thatoi, H.; Behera, B.C.; Mishra, R.R. Ecological Role and Biotechnological Potential of Mangrove Fungi: A Review. Mycology 2013, 4, 54–71. [Google Scholar] [CrossRef]
- Elias, S.G.; Salvador-Montoya, C.A.; Costa-Rezende, D.H.; Guterres, D.C.; Fernandes, M.; Olkoski, D.; Klabunde, G.H.F.; Drechsler-Santos, E.R. Studies on the Biogeography of Phellinotus piptadeniae (Hymenochaetales, Basidiomycota): Expanding the Knowledge on Its Distribution and Clarifying Hosts Relationships. Fungal Ecol. 2020, 45, 100912. [Google Scholar] [CrossRef]
- Devadatha, B.; Jones, E.B.G.; Pang, K.L.; Abdel-Wahab, M.A.; Hyde, K.D.; Sakayaroj, J.; Bahkali, A.H.; Calabon, M.S.; Sarma, V.V.; Sutreong, S.; et al. Occurrence and Geographical Distribution of Mangrove Fungi; Springer: Dordrecht, The Netherlands, 2021; Volume 106, ISBN 0123456789. [Google Scholar]
- Sridhar, K.R. Highlights on the Macrofungi of South West Coast of Karnataka, India. Int. J. of. Life Sci. 2018, 9, 37–42. [Google Scholar]
- Alam, S.A.; Shamsi, S.; Ahmed, A. New Records of Macrofungi of the Mangrove Ecosystem of Sundarbans of Bangladesh. Bangladesh J. Plant Taxon. 2024, 31, 293–299. [Google Scholar] [CrossRef]
- Maekawa, N.; Sugawara, R.; Kogi, H.; Norikura, S.; Sotome, K.; Endo, N.; Nakagiri, A.; Ushijima, S. Hypochnicium Sensu Lato (Polyporales, Basidiomycota) from Japan, with Descriptions of a New Genus and Three New Species. Mycoscience 2023, 64, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Trejos, M.; Farnum, F. Estimacindeladiversidaddemacro-Hongoscomoindicadoresdelacalidad. CENTROS 2014, 3, 143–155. [Google Scholar]
- Andreakis, N.; Høj, L.; Kearns, P.; Hall, M.R.; Ericson, G.; Cobb, R.E.; Gordon, B.R.; Evans-Illidge, E.; Kellogg, C.A. Diversity of Marine-Derived Fungal Cultures Exposed by DNA Barcodes: The Algorithm Matters. PLoS ONE 2015, 10, e0136130. [Google Scholar] [CrossRef]
- Bonugli-Santos, R.C.; dos Santos Vasconcelos, M.R.; Passarini, M.R.Z.; Vieira, G.A.L.; Lopes, V.C.P.; Mainardi, P.H.; dos Santos, J.A.; de Azevedo Duarte, L.; Otero, I.V.R.; da Silva Yoshida, A.M.; et al. Marine-Derived Fungi: Diversity of Enzymes and Biotechnological Applications. Front. Microbiol. 2015, 6, 269. [Google Scholar] [CrossRef]
- Spalding, M.D.; Leal, M.; Longley-Wood, K.; Ahmadia, G.N.; Aigrette, L.; Andradi-Brown, D.A.; Arquiza, Y.; Balidy, H.; Bandeira, S.; Beck, M.W.; et al. The State of Mangroves World’s Mangroves 2021; Global Mangrove Alliance: Washington, DC, USA, 2021. [Google Scholar]
- Pellizzer, E.P.; Giovanella, P.; Faria, A.U.D.; Sette, L.D. Marine-Derived Fungus Paramarasmius palmivorus CBMAI 1062 Applied to Sulphur Indigo Blue Decolorization, Degradation and Detoxification. An. Acad. Bras. Ciências 2024, 96, e20230315. [Google Scholar] [CrossRef]
- Bahamón-Méndez, T. Science Contributes to the Preservation of Mangroves, the Coastal “Lungs” That Are under Threat. Period. UNAL Medio Ambiente, 9 June 2023. [Google Scholar]
- Zhou, M.; Wang, C.G.; Wu, Y.D.; Liu, S.; Yuan, Y. Two New Brown Rot Polypores from Tropical China. MycoKeys 2021, 82, 173–197. [Google Scholar] [CrossRef]
- Li, H.J.; Han, M.L.; Cui, B.K. Two New Fomitopsis Species from Southern China Based on Morphological and Molecular Characters. Mycol. Prog. 2013, 12, 709–718. [Google Scholar] [CrossRef]
- Ortiz-Santana, B.; Lindner, D.L.; Miettinen, O.; Justo, A.; Hibbett, D.S. A Phylogenetic Overview of the Antrodia Clade (Basidiomycota, Polyporales). Mycologia 2013, 105, 1391–1411. [Google Scholar] [CrossRef]
- Liu, S.; Song, C.G.; Cui, B.K. Morphological Characters and Molecular Data Reveal Three New Species of Fomitopsis (Basidiomycota). Mycol. Prog. 2019, 18, 1317–1327. [Google Scholar] [CrossRef]
- Han, M.L.; Chen, Y.Y.; Shen, L.L.; Song, J.; Vlasák, J.; Dai, Y.C.; Cui, B.K. Taxonomy and Phylogeny of the Brown-Rot Fungi: Fomitopsis and Its Related Genera. Fungal Divers. 2016, 80, 343–373. [Google Scholar] [CrossRef]
- Zhou, J.L.; Zhu, L.; Chen, H.; Cui, B.K. Taxonomy and Phylogeny of Polyporus Group Melanopus (Polyporales, Basidiomycota) from China. PLoS ONE 2016, 11. [Google Scholar] [CrossRef]
- Sathiya Seelan, J.S.; Justo, A.; Nagy, L.G.; Grand, E.A.; Redhead, S.A.; Hibbett, D. Phylogenetic Relationships and Morphological Evolution in Lentinus, Polyporellus and Neofavolus, Emphasizing Southeastern Asian Taxa. Mycologia 2015, 107, 460–474. [Google Scholar] [CrossRef]
- Grand, E.A.; Hughes, K.W.; Petersen, R.H. Relationships within Lentinus Subg. Lentinus (Polyporales, Agaricomycetes), with emphasis on Sects. Lentinus and Tigrini. Mycol. Prog. 2011, 10, 399–413. [Google Scholar] [CrossRef]
- Sotome, K.; Hattori, T.; Ota, Y.; Kakishima, M. Second Report of Polyporus longiporus and Its Phylogenetic Position. Mycoscience 2009, 50, 415–420. [Google Scholar] [CrossRef]
- Krueger, D. Studies in the Genus Polyporus (Basidiomycotina). Doctoral Dissertation, University of Tennessee, Knoxville, TN, USA, 2002. [Google Scholar]
- Matozaki, T.; Hattori, T.; Kuwahara, T.; Boonlue, S.; Maekawa, N.; Nakagiri, A.; Endo, N.; Sotome, K. First Report of Polyporus ciliatus in Japan, and Taxonomic Re-Evaluation of Synonymous Species Described from Japan. Mushroom Sci. Biotechnol. 2019, 27, 140–147. [Google Scholar]
- Li, H.J.; Cui, B.K.; Dai, Y.C. Taxonomy and Multi-Gene Phylogeny of Datronia (Polyporales, Basidiomycota). Persoonia Mol. Phylogeny Evol. Fungi 2014, 32, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Jang, S.; Min, M.; Hong, J.H.; Lee, H.; Lee, H.; Lim, Y.W.; Kim, J.J. Comparison of the Diversity of Basidiomycetes from Dead Wood of the Manchurian Fir (Abies holophylla) as Evaluated by Fruiting Body Collection, Mycelial Isolation, and 454 Sequencing. Microb. Ecol. 2015, 70, 634–645. [Google Scholar] [CrossRef]
- Matozaki, T.; Hattori, T.; Maekawa, N.; Nakagiri, A.; Ishikawa, N.K.; Sotome, K. Hirticrusta Gen. Nov. Segregated from Neofomitella in Polyporaceae (Polyporales). Mycoscience 2020, 61, 240–248. [Google Scholar] [CrossRef]
- Henrik Nilsson, R.; Hallenberg, N. Phylogeny of the Hypochnicium punctulatum Complex as Inferred from ITS Sequence Data. Mycologia 2003, 95, 54–60. [Google Scholar] [CrossRef]
- Paulus, B.; Nilsson, H.; Hallenberg, N. Phylogenetic Studies in Hypochnicium (Basidiomycota), with Special Emphasis on Species from New Zealand. N. Z. J. Bot. 2007, 45, 139–150. [Google Scholar] [CrossRef]
- Vu, D.; Groenewald, M.; de Vries, M.; Gehrmann, T.; Stielow, B.; Eberhardt, U.; Al-Hatmi, A.; Groenewald, J.Z.; Cardinali, G.; Houbraken, J.; et al. Large-Scale Generation and Analysis of Filamentous Fungal DNA Barcodes Boosts Coverage for Kingdom Fungi and Reveals Thresholds for Fungal Species and Higher Taxon Delimitation. Stud. Mycol. 2019, 92, 135–154. [Google Scholar] [CrossRef] [PubMed]
- Justo, A.; Miettinen, O.; Floudas, D.; Ortiz-Santana, B.; Sjökvist, E.; Lindner, D.; Nakasone, K.; Niemelä, T.; Larsson, K.H.; Ryvarden, L.; et al. A Revised Family-Level Classification of the Polyporales (Basidiomycota). Fungal Biol. 2017, 121, 798–824. [Google Scholar] [CrossRef]
- Larsson, K.H. Molecular Phylogeny of Hyphoderma and the Reinstatement of Peniophorella. Mycol. Res. 2007, 111, 186–195. [Google Scholar] [CrossRef]
- Menkis, A.; Allmer, J.; Vasiliauskas, R.; Lygis, V.; Stenlid, J.; Finlay, R. Ecology and Molecular Characterization of Dark Septate Fungi from Roots, Living Stems, Coarse and Fine Woody Debris. Mycol. Res. 2004, 108, 965–973. [Google Scholar] [CrossRef]
- Jang, Y.; Lee, S.W.; Lim, Y.W.; Lee, J.S.; Hallenberg, N.; Kim, J.J. Hypochnicium Pini, a New Corticioid Basidiomycete in East Asia. Mycotaxon 2013, 124, 209–217. [Google Scholar] [CrossRef]
- Sjökvist, E.; Larsson, E.; Eberhardt, U.; Ryvarden, L.; Larsson, K.H. Stipitate Stereoid Basidiocarps Have Evolved Multiple Times. Mycologia 2012, 104, 1046–1055. [Google Scholar] [CrossRef]
- Mueller, G.M.; Wu, Q.X.; Huang, Y.Q.; Guo, S.Y.; Aldana-Gomez, R.; Vilgalys, R. Assessing Biogeographic Relationships between North American and Chinese Macrofungi. J. Biogeogr. 2001, 28, 271–281. [Google Scholar] [CrossRef]
- Qin, J.; Horak, E.; Popa, F.; Rexer, K.H.; Kost, G.; Li, F.; Yang, Z.L. Species Diversity, Distribution Patterns, and Substrate Specificity of Strobilurus. Mycologia 2018, 110, 584–604. [Google Scholar] [CrossRef] [PubMed]
- Antonín, V.; Ryoo, R.; Ka, K.H.; Sou, H.D. Three New Species of Crinipellis and One New Variety of Moniliophthora (Basidiomycota, Marasmiaceae) Described from the Republic of Korea. Phytotaxa 2014, 170, 86–102. [Google Scholar] [CrossRef]
- Bodensteiner, P.; Binder, M.; Moncalvo, J.M.; Agerer, R.; Hibbett, D.S. Phylogenetic Relationships of Cyphelloid Homobasidiomycetes. Mol. Phylogenet Evol. 2004, 33, 501–515. [Google Scholar] [CrossRef]
- Zhao, D.L.; Wang, D.; Tian, X.Y.; Cao, F.; Li, Y.Q.; Zhang, C.S. Anti-Phytopathogenic and Cytotoxic Activities of Crude Extracts and Secondary Metabolites of Marine-Derived Fungi. Mar. Drugs 2018, 16, 36. [Google Scholar] [CrossRef]
- de Oliveira, J.J.S.; Moncalvo, J.M.; Margaritescu, S.; Capelari, M. Phylogenetic and Morphological Analyses of Species of Marasmius Sect. Marasmius from the Atlantic Rainforest, Brazil. Plant Syst. Evol. 2020, 306. [Google Scholar] [CrossRef]
- Chen, C.C.; Chen, C.Y.; Wu, S.H. Species Diversity, Taxonomy and Multi-Gene Phylogeny of Phlebioid Clade (Phanerochaetaceae, Irpicaceae, Meruliaceae) of Polyporales. Fungal Divers. 2021, 111, 337–442. [Google Scholar] [CrossRef]
- Xavier de Lima, V.; Lira, C.d.S.; Chikowski, R.d.S.; Santos, C.; Lima, N.; Gibertoni, T.B. Additions to Neotropical Stereoid Fungi (Polyporales, Basidiomycota): One New Species of Lopharia and One New Combination in Phlebiopsis. Mycol. Prog. 2020, 19, 31–40. [Google Scholar] [CrossRef]
- Miettinen, O.; Spirin, V.; Vlasák, J.; Rivoire, B.; Stenroos, S.; Hibbett, D.S. Polypores and Genus Concepts in Phanerochaetaceae (Polyporales, Basidiomycota). MycoKeys 2016, 17, 1–46. [Google Scholar] [CrossRef]
- Floudas, D.; Hibbett, D.S. Revisiting the Taxonomy of Phanerochaete (Polyporales, Basidiomycota) Using a Four Gene Dataset and Extensive ITS Sampling. Fungal Biol. 2015, 119, 679–719. [Google Scholar] [CrossRef]
- Dong, J.H.; Li, Q.; Yuan, Q.; Luo, Y.X.; Zhang, X.C.; Dai, Y.F.; Zhou, Q.; Liu, X.F.; Deng, Y.L.; Zhou, H.M.; et al. Species Diversity, Taxonomy, Molecular Systematics and Divergence Time of Wood-Inhabiting Fungi in Yunnan-Guizhou Plateau, Asia. Mycosphere 2024, 15, 1110–1293. [Google Scholar] [CrossRef]
- Wu, S.H.; Nilsson, H.R.; Chen, C.T.; Yu, S.Y.; Hallenberg, N. The White-Rotting Genus Phanerochaete Is Polyphyletic and Distributed throughout the Phleboid Clade of the Polyporales (Basidiomycota). Fungal Divers. 2010, 42, 107–118. [Google Scholar] [CrossRef]
- Mao, W.L.; Wu, Y.D.; Liu, H.G.; Yuan, Y.; Dai, Y.C. A Contribution to Porogramme (Polyporaceae, Agaricomycetes) and Related Genera. IMA Fungus 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.S.; Wan, X.Z. Morphological and ITS RDNA-Based Phylogenetic Identification of Two New Species in Tinctoporellus. Mycol. Prog. 2012, 11, 947–952. [Google Scholar] [CrossRef]
- Wei, C.L.; Chen, C.C.; He, S.H.; Wu, S.H. Dendrocorticiopsis orientalis Gen. et Sp. Nov. of the Punctulariaceae (Corticiales, Basidiomycota) Revealed by Molecular Data. MycoKeys 2022, 90, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Acero, D.; Khan, F.S.T.; Medina-Ortiz, A.J.; Rivero-Cruz, I.; Raja, H.A.; Flores-Bocanegra, L.; Fajardo-Hernández, C.A.; Wan, B.; Franzblau, S.G.; Hematian, S.; et al. New Terpenoids from the Corticioid Fungus Punctularia atropurpurascens and Their Antimycobacterial Evaluation. Planta Med. 2022, 88, 729–734. [Google Scholar] [CrossRef]
- Guan, Q.-X.; Zhao, W.; Zhao, C.-L. A New Species of Punctularia (Punctulariaceae, Basidiomycota) from Southwest China. Phytotaxa 2021, 489, 285–292. [Google Scholar] [CrossRef]
- Ghobad-Nejhad, M.; Nilsson, R.H.; Hallenberg, N. Phylogeny and Taxonomy of the Genus Vuilleminia (Basidiomycota) Based on Molecular and Morphological Evidence, with New Insights into Corticiales. Taxon 2010, 59, 1519–1534. [Google Scholar] [CrossRef]
- Muhammad, A.; Deng, Y.; Dai, Y.; Su, J.; Zhao, C. Phylogenetic and Taxonomic Evidence Reveal Punctulariopsis yunnanensis Sp. Nov. (Punctulariaceae, Basidiomycota) from Southwest China. Phytotaxa 2024, 663, 59–68. [Google Scholar] [CrossRef]
- Yu, J.; Wang, X.W.; Liu, S.L.; Shen, S.; Zhou, L.W. Taxonomy and Phylogeny of Resinicium Sensu Lato from Asia-Pacific Revealing a New Genus and Five New Species (Hymenochaetales, Basidiomycota). IMA Fungus 2021, 12. [Google Scholar] [CrossRef]
- Wang, X.W.; Liu, S.L.; Zhou, L.W. An Updated Taxonomic Framework of Hymenochaetales (Agaricomycetes, Basidiomycota). Mycosphere 2023, 14, 452–496. [Google Scholar] [CrossRef]
- Brandon Matheny, P.; Wang, Z.; Binder, M.; Curtis, J.M.; Lim, Y.W.; Henrik Nilsson, R.; Hughes, K.W.; Hofstetter, V.; Ammirati, J.F.; Schoch, C.L.; et al. Contributions of Rpb2 and Tef1 to the Phylogeny of Mushrooms and Allies (Basidiomycota, Fungi). Mol. Phylogenet Evol. 2007, 43, 430–451. [Google Scholar] [CrossRef] [PubMed]
- Nakasone, K.K. Morphological and Molecular Studies on Resinicium s. Str. Can. J. Bot. 2007, 85, 420–436. [Google Scholar] [CrossRef]
- Miettinen, O.; Larsson, K.H. Sidera, a New Genus in Hymenochaetales with Poroid and Hydnoid Species. Mycol. Prog. 2011, 10, 131–141. [Google Scholar] [CrossRef]
- Du, R.; Wu, F.; Gate, G.M.; Dai, Y.C.; Tian, X.M. Taxonomy and Phylogeny of Sidera (Hymenochaetales, Basidiomycota): Four New Species and Keys to Species of the Genus. MycoKeys 2020, 68, 115–135. [Google Scholar] [CrossRef] [PubMed]
- Hage, H.; Miyauchi, S.; Virágh, M.; Drula, E.; Min, B.; Chaduli, D.; Navarro, D.; Favel, A.; Norest, M.; Lesage-Meessen, L.; et al. Gene Family Expansions and Transcriptome Signatures Uncover Fungal Adaptations to Wood Decay. Env. Microbiol. 2021, 23, 5716–5732. [Google Scholar] [CrossRef]
- de Pádua, A.P.S.L.; Freire, K.T.L.d.S.; de Oliveira, T.G.L.; da Silva, L.F.; Araújo-Magalhães, G.R.; Agamez-Montalvo, G.S.; da Silva, I.R.; Bezerra, J.D.P.; de Souza-Motta, C.M. Fungal Endophyte Diversity in the Leaves of the Medicinal Plant Myracrodruon urundeuva in a Brazilian Dry Tropical Forest and Their Capacity to Produce L-Asparaginase. Acta Bot. Bras. 2019, 33, 39–49. [Google Scholar] [CrossRef]
- Ji, X.; Wu, D.M.; Song, C.G.; Liu, S.; Si, J.; Cui, B.K. Two New Neofomitella Species (Polyporaceae, Basidiomycota) Based on Morphological and Molecular Evidence. Mycol. Prog. 2019, 18, 593–602. [Google Scholar] [CrossRef]
- Zhao, C.L.; Cui, B.K.; Steffen, K.T. Yuchengia, a New Polypore Genus Segregated from Perenniporia (Polyporales) Based on Morphological and Molecular Evidence. Nord. J. Bot. 2013, 31, 331–338. [Google Scholar] [CrossRef]
- Yu, J.; Lai, J.; Neal, B.M.; White, B.J.; Banik, M.T.; Dai, S.Y. Genomic Diversity and Phenotypic Variation in Fungal Decomposers Involved in Bioremediation of Persistent Organic Pollutants. J. Fungi 2023, 9, 418. [Google Scholar] [CrossRef]









| Specimen | Voucher | Locality | GenBank Accession No. | |
|---|---|---|---|---|
| ITS | nLSU | |||
| F. nivosella (Murrill) Spirin & Vlasák | ACB1573 | CO | PV330456 | – |
| ACB1580 | CO | PV330457 | PV299508 | |
| ACB1587 | CO | PV330458 | PV299509 | |
| ACB1654 | CO | PV330459 | PV299510 | |
| ACB1660 | CO | PV330460 | PV299511 | |
| ACB1675 | CO | PV330461 | PV299512 | |
| Lentinus scleropus (Pers.) Fr. | ACB1507 | CO | PV330462 | PV299513 |
| M. affinis (Blume & T. Nees) Kuntze | ACB1512 | CO | PV330463 | PV299514 |
| N. manglarense sp. nov. | ACB1490 * | CO | PV330464 | – |
| O. platensis (Speg.) Speg. | ACB1478 | CO | PV330465 | PV299515 |
| ACB1571 | CO | PV330467 | PV299517 | |
| Paramarasmius palmivorus (Sharples) Antonín & Kolarík | ACB1519 | CO | PV330467 | PV299517 |
| P. amethystea (Hjortstam & Ryvarden) R.S. Chikowski & C.R.S. de Lira | MV534 | BR | PV562813 | PV562973 |
| AL93 | BR | PV562814 | PV562974 | |
| MV474 | BR | PV562815 | PV562975 | |
| P. colombiana sp. nov. | ACB1508 * | CO | PV330468 | PV299518 |
| MV396 | BR | PV562816 | PV562976 | |
| MV650 | CO | PV562817 | – | |
| ACB1655 | CO | PV562818 | – | |
| P. crassa (Lév.) Floudas y Hibbett | MV 397 | BR | PV562819 | PV562977 |
| MV 442 | BR | PV562820 | – | |
| P. flavidoalba (Cooke) Hjortstam | ACB1495 | CO | PV330469 | PV299519 |
| P. bononiae sp. nov. | ACB1486 | CO | PV330470 | PV299520 |
| ACB1494 * | CO | PV330471 | PV299521 | |
| P. brasiliensis (Ryvarden) Y.C. Dai, W.L. Mao & Yuan Yuan | ACB1510 | CO | PV330472 | PV299522 |
| P. micropora (A.M.S. Soares & W.K.S. Waxier) Y.C. Dai, W.L. Mao & Yuan Yuan | MV955 | BR | PV562821 | PV562978 |
| P. strigosozonata (Schwein.) P.H. Talbot | ACB 1592 | CO | PV330473 | PV299523 |
| Resinicium grandisporum G. Gruhn, Dumez & Schimann | ACB1491 | CO | PV330475 | PV299525 |
| Earliella scabrosa (Pers.) Gilb. & Ryvarden | ACB1678 | CO | PV330455 | PV299507 |
| T. ellipsospora Ryvarden | ACB1493 | CO | PV330477 | PV299528 |
| T. menziesii (Berk.) Ryvarden | ACB1350 | CO | PV330478 | PV299529 |
| ACB1354 | CO | PV330479 | PV299530 | |
| ACB1375 | CO | PV330480 | PV299531 | |
| ACB1378 | CO | PV330481 | PV299532 | |
| ACB1574 | CO | PV330482 | PV299533 | |
| ACB1590 | CO | PV330483 | PV299534 | |
| ACB1658 | CO | PV330484 | PV299535 | |
| ACB1661 | CO | PV330485 | PV299536 | |
| ACB1662 | CO | PV330486 | PV299537 | |
| T. polyzona (Pers.) Justo | ACB1475 | CO | PV330487 | PV299538 |
| ACB1492 | CO | PV330488 | PV299539 | |
| ACB1585 | CO | PV330489 | PV299540 | |
| T. sanguinea (Klotzsch) Pat. | ACB1476 | CO | PV330474 | PV299524 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motato-Vásquez, V.; Vinasco-Diaz, L.K.; Londoño-Caicedo, J.M.; Bolaños-Rojas, A.C. Hidden Treasures of Colombia’s Pacific Mangrove: New Fungal Species and Records of Macrofungi (Basidiomycota). J. Fungi 2025, 11, 459. https://doi.org/10.3390/jof11060459
Motato-Vásquez V, Vinasco-Diaz LK, Londoño-Caicedo JM, Bolaños-Rojas AC. Hidden Treasures of Colombia’s Pacific Mangrove: New Fungal Species and Records of Macrofungi (Basidiomycota). Journal of Fungi. 2025; 11(6):459. https://doi.org/10.3390/jof11060459
Chicago/Turabian StyleMotato-Vásquez, Viviana, Lina Katherine Vinasco-Diaz, Jorge M. Londoño-Caicedo, and Ana C. Bolaños-Rojas. 2025. "Hidden Treasures of Colombia’s Pacific Mangrove: New Fungal Species and Records of Macrofungi (Basidiomycota)" Journal of Fungi 11, no. 6: 459. https://doi.org/10.3390/jof11060459
APA StyleMotato-Vásquez, V., Vinasco-Diaz, L. K., Londoño-Caicedo, J. M., & Bolaños-Rojas, A. C. (2025). Hidden Treasures of Colombia’s Pacific Mangrove: New Fungal Species and Records of Macrofungi (Basidiomycota). Journal of Fungi, 11(6), 459. https://doi.org/10.3390/jof11060459






