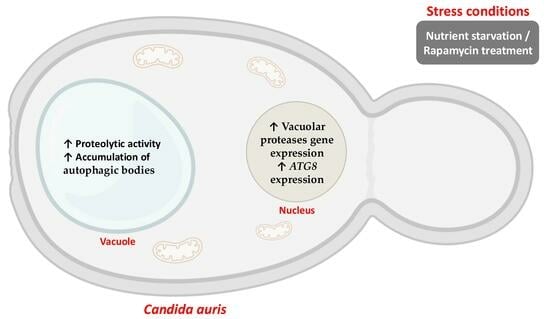
Vacuolar Proteases of Candida auris from Clades III and IV and Their Relationship with Autophagy
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains, Media, and Growth Conditions
2.2. Determination of Specific Protease Activity
2.3. Effect of Peptidase Inhibitors
2.4. RNA Extraction and cDNA Synthesis
2.5. RT-qPCR
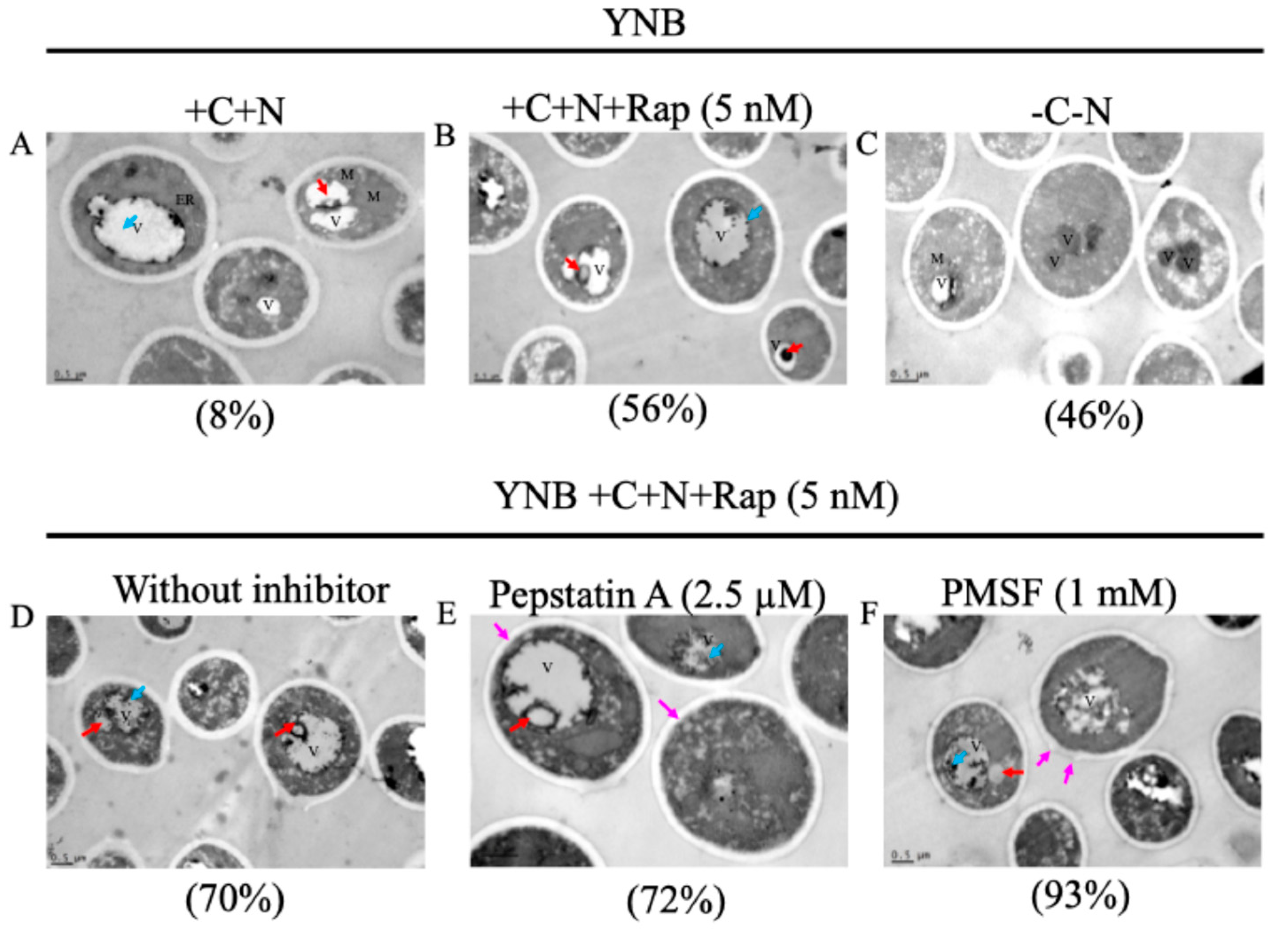
2.6. Microscopy of C. auris by TEM
2.7. Bioinformatic Analysis
2.8. Data Analysis
3. Results
3.1. C. auris Orthologs of S. cerevisiae Vacuolar Peptidases
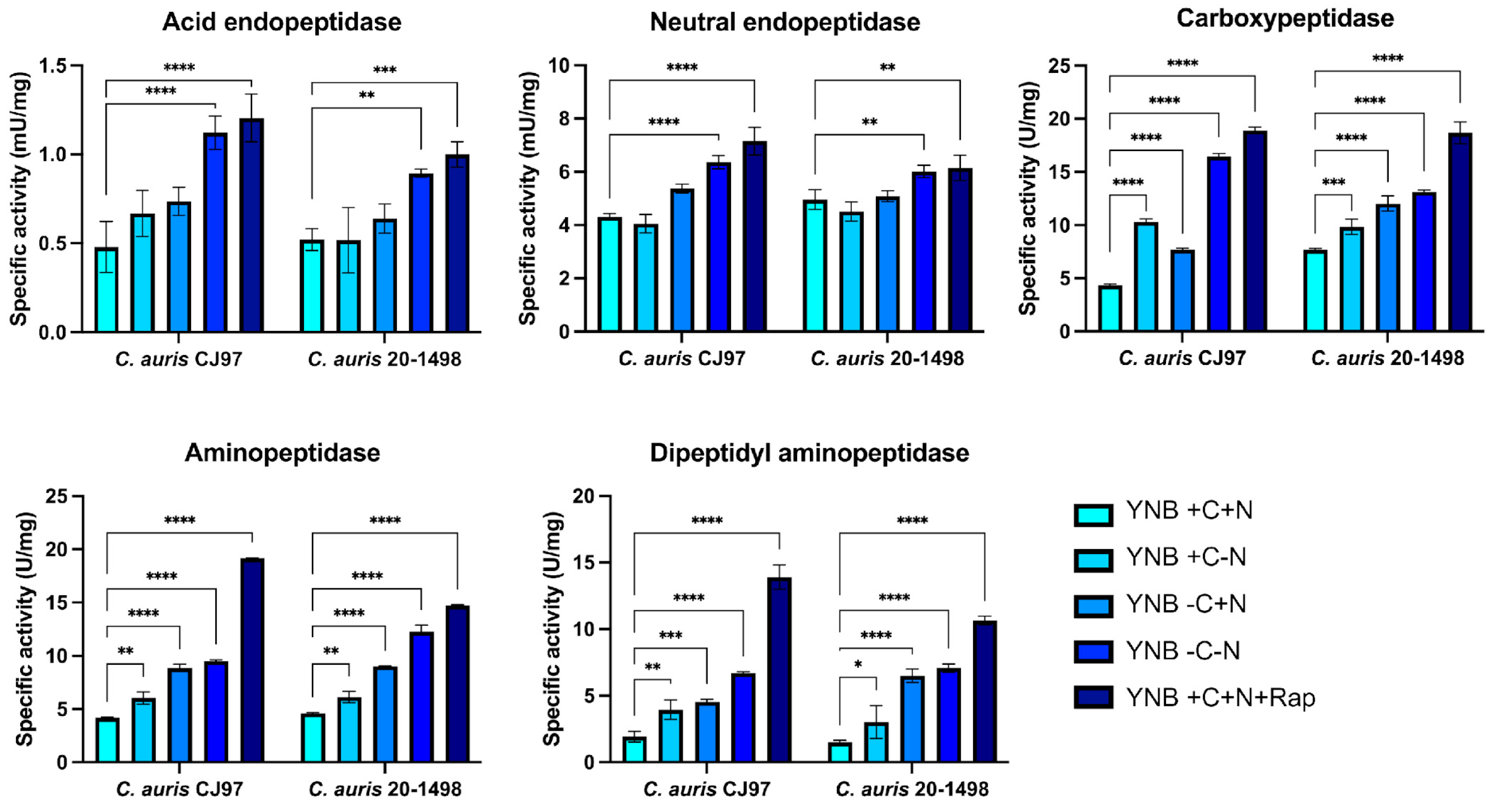
3.2. Proteolysis Under Nutritional Stress
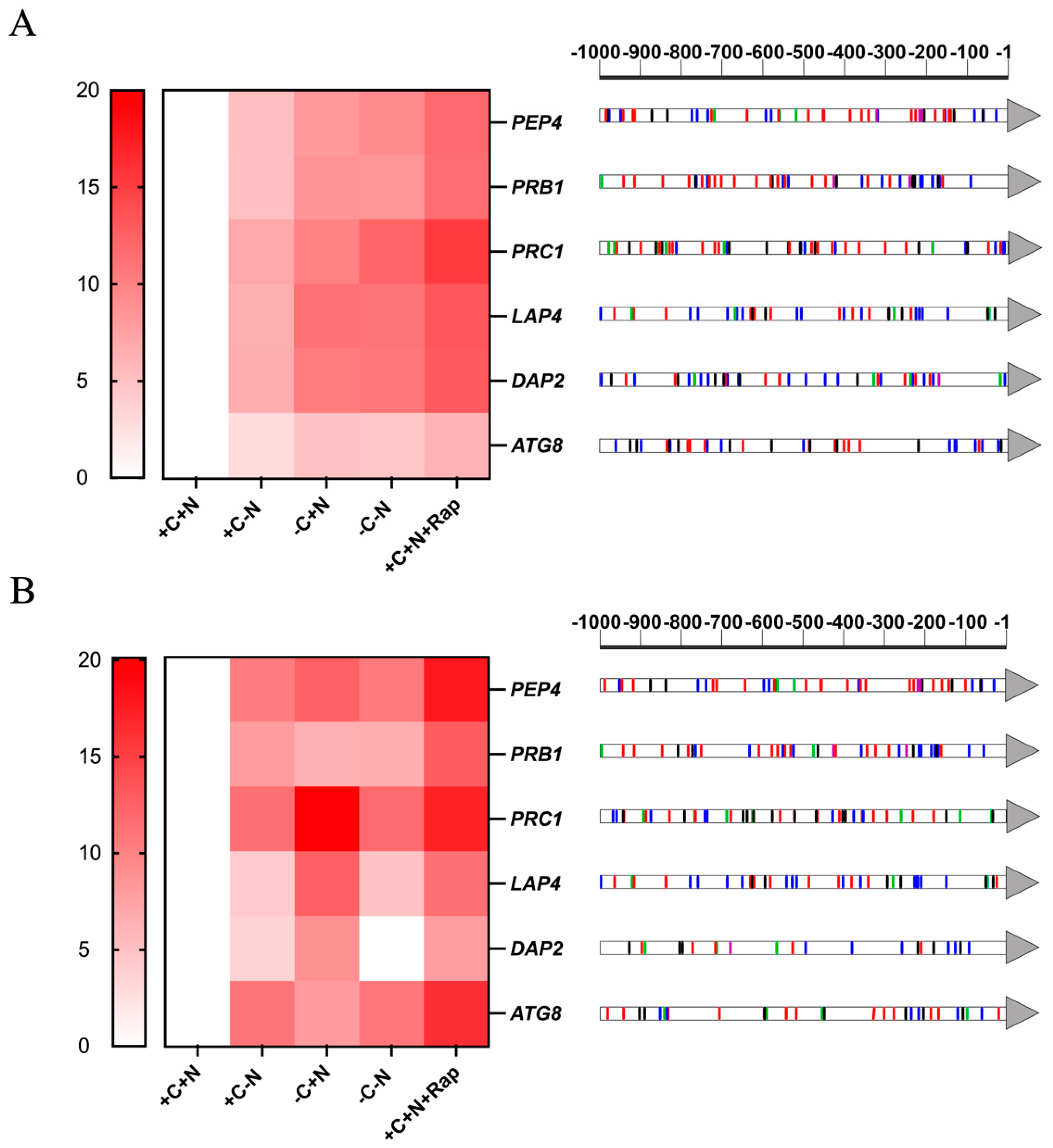
3.3. Genes Encoding Putative Vacuolar Peptidases of C. auris Are Overexpressed Under Nutritional Stress Conditions
3.4. Vacuolar Morphology of C. auris Under Nutritional Stress and Peptidase Inhibitor Treatment
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Santana, D.J.; Zhao, G.; O’Meara, T.R. The many faces of Candida auris: Phenotypic and strain variation in an emerging pathogen. PLoS Pathog. 2024, 20, e1012011. [Google Scholar] [CrossRef]
- Suphavilai, C.; Ko, K.K.K.; Lim, K.M.; Tan, M.G.; Boonsimma, P.; Chu, J.J.K.; Goh, S.S.; Rajandran, P.; Lee, L.C.; Tan, K.Y.; et al. Detection and characterization of a sixth Candida auris clade in Singapore: A genomic and phenotypic study. Lancet Microbe 2024, 5, 100878. [Google Scholar] [CrossRef] [PubMed]
- Spruijtenburg, B.; Badali, H.; Abastabar, M.; Mirhendi, H.; Khodavaisy, S.; Sharifisooraki, J.; Taghizadeh, M.; de Groot, T.; Meis, J.F. Confirmation of fifth Candida auris clade by whole genome sequencing. Emerg. Microbes Infect. 2022, 1, 2405–2411. [Google Scholar] [CrossRef] [PubMed]
- Brandt, P.; Mirhakkak, M.H.; Wagner, L.; Driesch, D.; Möslinger, A.; Fänder, P.; Schäuble, S.; Panagiotou, G.; Vylkova, S. High-Throughput profiling of Candida auris isolates reveals clade-specific metabolic differences. Microbiol. Spectr. 2023, 11, e00498-23. [Google Scholar] [CrossRef] [PubMed]
- Forgács, L.; Borman, A.M.; Prépost, E.; Tóth, Z.; Kardos, G.; Kovács, R.; Szekely, A.; Nagy, F.; Kovacs, I.; Majoros, L. Comparison of in vivo pathogenicity of four Candida auris clades in a neutropenic bloodstream infection murine model. Emerg. Microbes Infect. 2020, 9, 1160–1169. [Google Scholar] [CrossRef]
- Lockhart, S.R. Candida auris and multidrug resistance: Defining the new normal. Fungal Genet. Biol. 2019, 131, 103243. [Google Scholar] [CrossRef]
- Kwon, Y.J.; Shin, J.H.; Byun, S.A.; Choi, M.J.; Won, E.J.; Lee, D.; Lee, S.Y.; Chun, S.; Lee, J.H.; Choi, H.J.; et al. Candida auris clinical isolates from South Korea: Identification, antifungal susceptibility, and genotyping. J. Clin. Microbiol. 2019, 57, e01624-18. [Google Scholar] [CrossRef]
- Dakalbab, S.; Hamdy, R.; Holigová, P.; Abuzaid, E.J.; Abu-Qiyas, A.; Lashine, Y.; Mohammad, M.G.; Soliman, S.S.M. Uniqueness of Candida auris cell wall in morphogenesis, virulence, resistance, and immune evasion. Microbiol. Res. 2024, 286, 127797. [Google Scholar] [CrossRef]
- Chybowska, A.D.; Childers, D.S.; Farrer, R.A. Nine things genomics can tell us about Candida auris. Front. Genet. 2020, 11, 351. [Google Scholar] [CrossRef]
- Burchacka, E.; Pięta, P.; Łupicka-Słowik, A. Recent advances in fungal serine protease inhibitors. Biomed. Pharmacother. 2022, 146, 112523. [Google Scholar] [CrossRef]
- Barrett, A.J.; Rawlings, N.D.; Woessner, J.F. Handbook of Proteases, 3rd ed.; Academic Press: New York, NY, USA, 2012. [Google Scholar]
- Silva, N.C.; Nery, J.M.; Dias, A.L. Aspartic proteinases of Candida spp.: Role in pathogenicity and antifungal resistance. Mycoses 2014, 57, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Parra-Ortega, B.; Villa-Tanaca, L.; Hernandez-Rodriguez, C. Evolution of GPI-aspartyl proteinases (yapsines) of Candida spp. InTech 2011, 16, 289–314. [Google Scholar] [CrossRef]
- Cortez-Sánchez, J.L.; Cortés-Acosta, E.; Cueto-Hernández, V.M.; Reyes-Maldonado, E.; Hernández-Rodríguez, C.; Villa-Tanaca, L.; Ibarra, J.A. Activity and expression of Candida glabrata vacuolar proteases in autophagy-like conditions. FEMS Yeast Res. 2018, 18, foy006. [Google Scholar] [CrossRef]
- Kerstens, W.; Van Dijck, P.A. Cinderella story: How the vacuolar proteases Pep4 and Prb1 do more than cleaning up the cell’s mass degradation processes. Microb. Cell 2018, 5, 438–443. [Google Scholar] [CrossRef]
- Parzych, K.R.; Ariosa, A.; Mari, M.; Klionsky, D.J. A newly characterized vacuolar serine carboxypeptidase, Atg42/Ybr139w, is required for normal vacuole function and the terminal steps of autophagy in the yeast Saccharomyces cerevisiae. Mol. Biol. Cell 2018, 29, 1089–1099. [Google Scholar] [CrossRef] [PubMed]
- Hecht, K.A.; O’Donnell, A.F.; Brodsky, J.L. The proteolytic landscape of the yeast vacuole. Cell Logist. 2014, 4, e28023. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Abdel-Aziz, A.K.; Abdelfatah, S.; Abdellatif, M.; Abdoli, A.; Abel, S.; Abeliovich, H.; Abildgaard, M.H.; Abudu, Y.P.; Acevedo-Arozena, A.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition). Autophagy 2021, 17, 1–382. [Google Scholar] [CrossRef] [PubMed]
- Juárez-Montiel, M.; Clark-Flores, D.; Tesillo-Moreno, P.; de la Vega-Camarillo, E.; Andrade-Pavón, D.; Hernández-García, J.A.; Hernández-Rodríguez, C.; Villa-Tanaca, L. Vacuolar proteases and autophagy in phytopathogenic fungi: A review. Front. Fungal Biol. 2022, 3, 948477. [Google Scholar] [CrossRef]
- Saheki, T.; Holzer, H. Proteolytic activities in yeast. Biochim. Biophy. Acta 1975, 384, 203–214. [Google Scholar] [CrossRef]
- Hirsch, H.H.; Suárez, P.; Achstetter, T.; Wolf, D.H. Aminopeptidase yscII of yeast. Isolation of mutants and their biochemical and genetic analysis. Eur. J. Biochem. 1988, 173, 589–598. [Google Scholar] [CrossRef]
- Sepúlveda-González, M.E.; Parra-Ortega, B.; Betancourt-Cervantes, Y.; Hernández-Rodríguez, C.; Xicohtencatl-Cortes, J.; Villa-Tanaca, L. Vacuolar proteases from Candida glabrata: Acid aspartic protease PrA, neutral serine protease PrB and serine carboxypeptidase CpY. The nitrogen source influences their level of expression. Rev. Iberoamer Micol. 2016, 33, 26–33. [Google Scholar] [CrossRef]
- Sambrook, J.; Russell, D.W. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2001. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Srivastava, V.; Ahmad, A. Abrogation of pathogenic attributes in drug resistant Candida auris strains by farnesol. PLoS ONE 2020, 15, e0233102. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Casimiro-Ramos, A.; Bautista-Crescencio, C.; Vidal-Montiel, A.; González, G.M.; Hernández-García, J.A.; Hernández-Rodríguez, C.; Villa-Tanaca, L. Comparative genomics of the first resistant Candida auris strain isolated in Mexico: Phylogenomic and pan-genomic analysis and mutations associated with antifungal resistance. J. Fungi 2024, 10, 392. [Google Scholar] [CrossRef] [PubMed]
- Abascal, F.; Zardoya, R.; Posada, D. ProtTest: Selection of best-fit models of protein evolution. Bioinformatics 2005, 21, 2104–2105. [Google Scholar] [CrossRef]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 2020, 37, 1530. [Google Scholar] [CrossRef] [PubMed]
- Crooks, G.E.; Hon, G.; Chandonia, J.M.; Brenner, S.E. WebLogo: A sequence logo generator. Genome Res. 2004, 14, 1188–1190. [Google Scholar] [CrossRef]
- Campanella, J.J.; Bitincka, L.; Smalley, J. MatGAT: An application that generates similarity/identity matrices using protein or DNA sequences. BMC Bioinformatics 2003, 4, 29. [Google Scholar] [CrossRef]
- Teufel, F.; Almagro, J.J.; Johansen, A.R.; Gíslason, M.H.; Pihl, S.I.; Tsirigos, K.D.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 6.0 predicts all five types of signal peptides using protein language models. Nat. Biotechnol. 2022, 40, 1023–1025. [Google Scholar] [CrossRef]
- Krogh, A.; Larsson, B.; von Heijne, G.; Sonnhammer, E.L. Predicting transmembrane protein topology with a hidden Markov model: Application to complete genomes. J. Mol. Biol. 2001, 305, 567–580. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Abdulrehman, D.; Monteiro, P.T.; Teixeira, M.C.; Mira, N.P.; Lourenço, A.B.; dos Santos, S.C.; Cabrito, T.R.; Francisco, A.P.; Madeira, S.C.; Aires, R.S.; et al. YEASTRACT: Providing a programmatic access to curated transcriptional regulatory associations in Saccharomyces cerevisiae through a web services interface. Nucleic Acids Res. 2011, 39, D136–D140. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Gaitán, A.C.; Cantón, E.; Fernández-Rivero, M.E.; Ramírez, P.; Pemán, J. Outbreak of Candida auris in Spain: A comparison of antifungal activity by three methods with published data. Int. J. Antimicrob. Agents 2019, 53, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Gaytán, J.J.; Montoya, A.M.; Martínez-Resendez, M.F.; Guajardo-Lara, C.E.; de J. Treviño-Rangel, R.; Salazar-Cavazos, L.; Llaca-Díaz, J.M.; González, G.M. First case of Candida auris isolated from the bloodstream of a Mexican patient with serious gastrointestinal complications from severe endometriosis. Infection 2021, 49, 523–525. [Google Scholar] [CrossRef]
- Soberanes-Gutiérrez, C.V.; Juárez-Montiel, M.; Olguín-Rodríguez, O.; Hernández-Rodríguez, C.; Ruiz-Herrera, J.; Villa-Tanaca, L. The pep4 gene encoding proteinase A is involved in dimorphism and pathogenesis of Ustilago maydis. Mol. Plant Path 2015, 16, 837–846. [Google Scholar] [CrossRef]
- Li, S.C.; Kane, P.M. The yeast lysosome-like vacuole: Endpoint and crossroads. Biochim. Biophys. Acta 2009, 1793, 650–663. [Google Scholar] [CrossRef]
- Mijaljica, D.; Prescott, M.; Klionsky, D.J.; Devenish, R.J. Autophagy and vacuole homeostasis: A case for self-degradation? Autophagy 2007, 3, 417–421. [Google Scholar] [CrossRef]
- Richards, A.; Gow, N.A.; Veses, V. Identification of vacuole defects in fungi. J. Microbiol. Methods 2012, 91, 155–163. [Google Scholar] [CrossRef][Green Version]
- Lv, Q.; Yan, L.; Jiang, Y. The importance of vacuolar ion homeostasis and trafficking in hyphal development and virulence in Candida albicans. Front. Microbiol. 2021, 12, 779176. [Google Scholar] [CrossRef]
- Jones, E.W. Vacuolar proteases in yeast Saccharomyces cerevisiae. Methods Enzymol. 1990, 185, 372–386. [Google Scholar] [CrossRef]
- Teichert, U.; Mechler, B.; Müller, H.; Wolf, D.H. Lysosomal (vacuolar) proteinases of yeast are essential catalysts for protein degradation, differentiation, and cell survival. J. Biol. Chem. 1989, 264, 16037–16045. [Google Scholar] [CrossRef] [PubMed]
- Palmer, G.E.; Kelly, M.N.; Sturtevant, J.E. The Candida albicans vacuole is required for differentiation and efficient macrophage killing. Eukaryot. Cell 2005, 4, 1677–1686. [Google Scholar] [CrossRef]
- Roetzer, A.; Gratz, N.; Kovarik, P.; Schüller, C. Autophagy supports Candida glabrata survival during phagocytosis. Cell Microbiol. 2010, 12, 199–216. [Google Scholar] [CrossRef] [PubMed]
- Olmedo-Monfil, V.; Mendoza-Mendoza, A.; Gómez, I.; Cortés, C.; Herrera-Estrella, A. Multiple environmental signals determine the transcriptional activation of the mycoparasitism related gene prb1 in Trichoderma atroviride. Mol. Gen. Genom. 2002, 267, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Reichard, U.; Cole, G.T.; Rüchel, R.; Monod, M. Molecular cloning and targeted deletion of PEP2 which encodes a novel aspartic proteinase from Aspergillus fumigatus. Int. J. Med. Microbiol. 2000, 290, 85–96. [Google Scholar] [CrossRef]
- Satoh, K.; Makimura, K.; Hasumi, Y.; Nishiyama, Y.; Uchida, K.; Yamaguchi, H. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol. Immunol. 2009, 53, 41–44. [Google Scholar] [CrossRef]
- Du, H.; Bing, J.; Hu, T.; Ennis, C.L.; Nobile, C.J.; Huang, G. Candida auris: Epidemiology, biology, antifungal resistance, and virulence. PLoS Pathog. 2020, 16, e1008921. [Google Scholar] [CrossRef]
- Pearson, W.R. An introduction to sequence similarity ("homology") searching. Curr. Protoc. Bioinform. 2013, 3, 3.1.1–3.1.8. [Google Scholar] [CrossRef]
- Brown, S.M. A Biologist´s Guide to Biocomputing and the Internet, 1st ed.; Eaton Publishing: New York, NY, USA, 2000. [Google Scholar]
- Parr, C.L.; Keates, R.A.; Bryksa, B.C.; Ogawa, M.; Yada, R.Y. The structure and function of Saccharomyces cerevisiae proteinase A. Yeast 2007, 24, 467–480. [Google Scholar] [CrossRef]
- Cooper, J.B.; Khan, G.; Taylor, G.; Tickle, I.J.; Blundell, T.L. X-ray analyses of aspartic proteinases. II. Three-dimensional structure of the hexagonal crystal form of porcine pepsin at 2.3 A resolution. J. Mol. Biol. 1990, 214, 199–222. [Google Scholar] [CrossRef] [PubMed]
- Gensberg, K.; Jan, S.; Matthews, G.M. Subtilisin-related serine proteases in the mammalian constitutive secretory pathway. Semin. Cell Dev. Biol. 1998, 9, 11–17. [Google Scholar] [CrossRef]
- Rawlings, N.D.; Barrett, A.J. Families of serine peptidases. Methods Enzymol. 1994, 244, 19–61. [Google Scholar] [CrossRef]
- Spormann, D.O.; Heim, J.; Wolf, D.H. Carboxypeptidase yscS: Gene structure and function of the vacuolar enzyme. Eur. J. Biochem. 1991, 197, 399–405. [Google Scholar] [CrossRef]
- Taylor, A. Aminopeptidases: Structure and function. FASEB J. 1993, 7, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Strahl, T.; Hama, H.; DeWald, D.B.; Thorner, J. Yeast phosphatidylinositol 4-kinase, Pik1, has essential roles at the Golgi and in the nucleus. J. Cell Biol. 2005, 171, 967–979. [Google Scholar] [CrossRef] [PubMed]
- Spedale, G.; Mischerikow, N.; Heck, A.J.; Timmers, H.T.; Pijnappel, W.W. Identification of Pep4p as the protease responsible for formation of the SAGA-related SLIK protein complex. J. Biol. Chem. 2010, 285, 22793–22799. [Google Scholar] [CrossRef]
- Ossareh-Nazari, B.; Bonizec, M.; Cohen, M.; Dokudovskaya, S.; Delalande, F.; Schaeffer, C.; Van Dorsselaer, A.; Dargemont, C. Cdc48 and Ufd3, new partners of the ubiquitin protease Ubp3, are required for ribophagy. EMBO Rep. 2010, 11, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Bruun, A.W.; Svendsen, I.; Sørensen, S.O.; Kielland-Brandt, M.C.; Winther, J.R. A high-affinity inhibitor of yeast carboxypeptidase Y is encoded by TFS1 and shows homology to a family of lipid binding proteins. Biochemistry 1998, 37, 3351–3357. [Google Scholar] [CrossRef]
- Lynch-Day, M.A.; Klionsky, D.J. The Cvt pathway as a model for selective autophagy. FEBS Lett. 2010, 584, 1359–1366. [Google Scholar] [CrossRef]
- Hu, Y.M.; Boehm, D.M.; Chung, H.; Wilson, S.; Bird, A.J. Zinc-dependent activation of the Pho8 alkaline phosphatase in Schizosaccharomyces pombe. J. Biol. Chem. 2019, 294, 12392–12404. [Google Scholar] [CrossRef] [PubMed]
- Sarry, J.E.; Chen, S.; Collum, R.P. Analysis of the vacuolar luminal proteome of Saccharomyces cerevisiae. FEBS J. 2007, 274, 4287–4305. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, S.R.; Etienne, K.A.; Vallabhaneni, S.; Farooqi, J.; Chowdhary, A.; Govender, N.P.; Colombo, A.L.; Calvo, B.; Cuomo, C.A.; Desjardins, C.A.; et al. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin. Infect. Dis. 2017, 64, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, A.; Jain, K.; Chauhan, N. Candida auris genetics and emergence. Annu. Rev. Microbiol. 2023, 77, 583–602. [Google Scholar] [CrossRef]
- Shpilka, T.; Weidberg, H.; Pietrokovski, S.; Elazar, Z. Atg8: An autophagy-related ubiquitin-like protein family. Genome Biol. 2011, 12, 226. [Google Scholar] [CrossRef]
- Gasch, A.P.; Spellman, P.T.; Kao, C.M.; Carmel-Harel, O.; Eisen, M.B.; Storz, G.; Botstein, D.; Brown, P.O. Genomic expression programs in the response of yeast cells to environmental changes. Mol. Biol. Cell 2020, 11, 4241–4257. [Google Scholar] [CrossRef]
- Müller, M.; Schmidt, O.; Angelova, M.; Faserl, K.; Weys, S.; Kremser, L.; Pfaffenwimmer, T.; Dalik, T.; Kraft, C.; Trajanoski, Z.; et al. The coordinated action of the MVB pathway and autophagy ensures cell survival during starvation. eLife 2015, 4, e07736. [Google Scholar] [CrossRef]
- Takeshige, K.; Baba, M.; Tsuboi, S.; Noda, T.; Ohsumi, Y. Autophagy in yeast demonstrated with proteinase-deficient mutants and conditions for its induction. J. Cell Biol. 1992, 119, 301–311. [Google Scholar] [CrossRef]
- Bauerová, V.; Pichová, I.; Hrušková-Heidingsfeldová, O. Nitrogen source and growth stage of Candida albicans influence expression level of vacuolar aspartic protease Apr1p and carboxypeptidase Cpy1p. Can. J. Microbiol. 2012, 58, 678–681. [Google Scholar] [CrossRef]
- Horton, M.V.; Holt, A.M.; Nett, J.E. Mechanisms of pathogenicity for the emerging fungus Candida auris. PLoS Pathog. 2023, 19, e1011843. [Google Scholar] [CrossRef]
- Takeda, K.; Yanagida, M. In quiescence of fission yeast, autophagy and the proteasome collaborate for mitochondrial maintenance and longevity. Autophagy 2010, 4, 564–565. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Gresham, D. Cellular quiescence in budding yeast. Yeast 2021, 38, 12–29. [Google Scholar] [CrossRef] [PubMed]
- Marini, G.; Nüske, E.; Leng, W.; Alberti, S.; Pigino, G. Reorganization of budding yeast cytoplasm upon energy depletion. Mol. Biol. Cell 2020, 31, 1232–1245. [Google Scholar] [CrossRef] [PubMed]
- Klosinska, M.M.; Crutchfield, C.A.; Bradley, P.H.; Rabinowitz, J.D.; Broach, J.R. Yeast cells can access distinct quiescent states. Genes Dev. 2011, 25, 336–349. [Google Scholar] [CrossRef]
- Li, M.; Khambu, B.; Zhang, H.; Kang, J.H.; Chen, X.; Chen, D.; Vollmer, L.; Liu, P.Q.; Vogt, A.; Yin, X.M. Suppression of lysosome function induces autophagy via a feedback down-regulation of MTOR complex 1 (MTORC1) activity. J. Biol. Chem. 2013, 288, 35769–35780. [Google Scholar] [CrossRef]
- Krysan, D.J.; Ting, E.L.; Abeijon, C.; Kroos, L.; Fuller, R.S. Yapsins are a family of aspartyl proteases required for cell wall integrity in Saccharomyces cerevisiae. Eukaryot. Cell 2005, 4, 1364–1374. [Google Scholar] [CrossRef]
- Komano, H.; Rockwell, N.; Wang, G.T.; Krafft, G.A.; Fuller, R.S. Purification and characterization of the yeast glycosylphosphatidylinositol-anchored, monobasic-specific aspartyl protease yapsin 2 (Mkc7p). J. Biol. Chem. 1999, 274, 24431–24437. [Google Scholar] [CrossRef][Green Version]
- Bairwa, G.; Kaur, R. A novel role for a glycosylphosphatidylinositol-anchored aspartyl protease, CgYps1, in the regulation of pH homeostasis in Candida glabrata. Mol. Microbiol. 2011, 79, 900–913. [Google Scholar] [CrossRef]
- Pärnänen, P.; Meurman, J.H.; Nikula-Ijäs, P. A novel Candida glabrata cell wall associated serine protease. Biochem. Biophys. Res. Commun. 2015, 457, 676–680. [Google Scholar] [CrossRef]
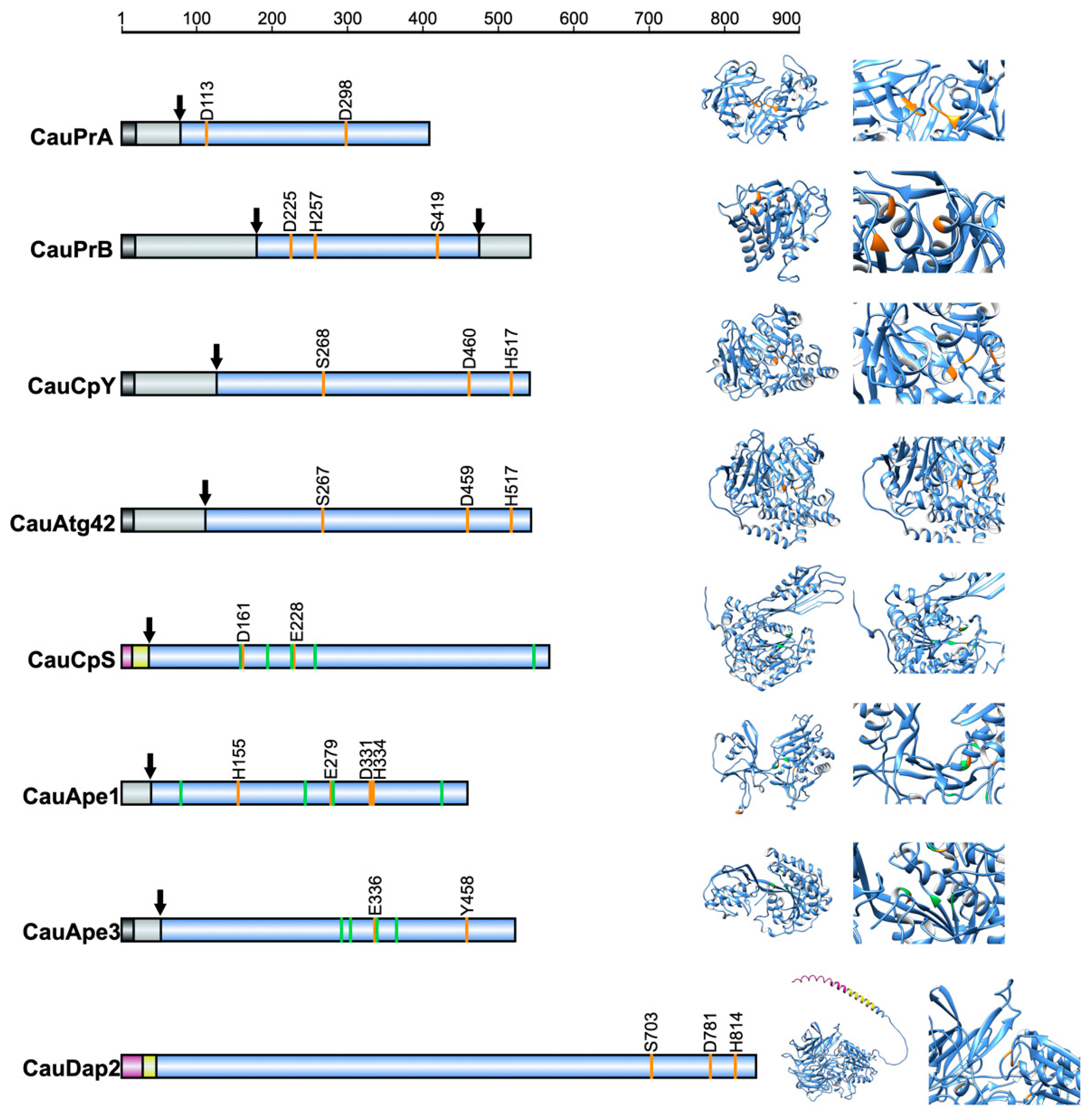


| Gen/Protein | C. auris CJ97 (Clade III) | C. auris 20-1498 (Clade IV) | ||
|---|---|---|---|---|
| Gene Length (bp) | Protein Length (aa)/Molecular Mass (kDa) | Gene Length (bp) | Protein Length (aa)/Molecular Mass (kDa) | |
| PEP4/PrA | 1230 | 409/44.33 | 1230 | 409/44.33 |
| PRB1/PrB | 1632 | 543/57.4 | 1632 | 543/57.4 |
| PRC1/CpY | 1629 | 542/61.42 | 1629 | 542/61.42 |
| ATG42/Atg42 | 1707 | 568/63.74 | 1707 | 568/63.76 |
| CPS/CpS | 1635 | 544/61.28 | 1635 | 544/61.29 |
| LAP4/Ape1 | 1383 | 460/50.61 | 1383 | 460/50.5 |
| APE3/Ape3 | 1572 | 523/57.37 | 1572 | 523/57.379 |
| DAP2/Dap2 | 2379 | 842/95.68 | 2379 | 792/89.98 |
| Residual Activity (%) | |||
|---|---|---|---|
| Enzymatic Activity (Catalytic Type) | Inhibitors (Concentration) | C. auris 20-1498 | C. auris CJ97 |
| Acidic proteinase (aspartyl peptidase) | Pepstatin A: 2.5/5/25 μM | 36.66/25.11/0.0 | 59.53/0.0/0.0 |
| Neutral proteinase (serine peptidase) | PMSF: 1/5 mM | 45.6/33.23 | 66.56/37.8 |
| Carboxypeptidase (serine peptidase) | PMSF: 1/5 mM | 46.44/36.82 | 32.71/21.7 |
| EDTA: 1/10 mM | 95.39/78.67 | 76.23/69.44 | |
| E-64: 1/10 μM | 96.4/41.99 | 100/35.63 | |
| Aminopeptidase (metallo-aminopeptidase) | Bestatin: 100/250 μM | 94.47/63.87 | 93.71/59.01 |
| EDTA: 1/10 mM | 100/72. 05 | 90.75/57.63 | |
| 1,10 phenanthroline: 2.5/7.5 mM | 80.49/23.25 | 81.66/19.16 | |
| Dipeptidyl aminopeptidase (metal-ion-dependent and serine aminopeptidase) | PMSF: 1/5 mM | 100/67.17 | 100/83.67 |
| EDTA: 1/10 mM | 59.7/55.73 | 40.06/32.5 | |
| E-64: 1/10 mM | 100/100 | 100/100 | |
| Bestatin: 100/250 μM | 100/32.83 | 100/28.11 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clark-Flores, D.; Vidal-Montiel, A.; Mondragón-Flores, R.; Valentín-Gómez, E.; Hernández-Rodríguez, C.; Juárez-Montiel, M.; Villa-Tanaca, L. Vacuolar Proteases of Candida auris from Clades III and IV and Their Relationship with Autophagy. J. Fungi 2025, 11, 388. https://doi.org/10.3390/jof11050388
Clark-Flores D, Vidal-Montiel A, Mondragón-Flores R, Valentín-Gómez E, Hernández-Rodríguez C, Juárez-Montiel M, Villa-Tanaca L. Vacuolar Proteases of Candida auris from Clades III and IV and Their Relationship with Autophagy. Journal of Fungi. 2025; 11(5):388. https://doi.org/10.3390/jof11050388
Chicago/Turabian StyleClark-Flores, Daniel, Alvaro Vidal-Montiel, Ricardo Mondragón-Flores, Eulogio Valentín-Gómez, César Hernández-Rodríguez, Margarita Juárez-Montiel, and Lourdes Villa-Tanaca. 2025. "Vacuolar Proteases of Candida auris from Clades III and IV and Their Relationship with Autophagy" Journal of Fungi 11, no. 5: 388. https://doi.org/10.3390/jof11050388
APA StyleClark-Flores, D., Vidal-Montiel, A., Mondragón-Flores, R., Valentín-Gómez, E., Hernández-Rodríguez, C., Juárez-Montiel, M., & Villa-Tanaca, L. (2025). Vacuolar Proteases of Candida auris from Clades III and IV and Their Relationship with Autophagy. Journal of Fungi, 11(5), 388. https://doi.org/10.3390/jof11050388