Biocontrol Potential of Entomopathogenic Fungi Against Plant-Parasitic Nematodes: A Caenorhabditis elegans-Based Screening and Mechanistic Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Maintenance
2.2. Preparation of Culture Filtrates and Nematodes
2.3. Pot Experiment
2.4. Reproduction and Lethality Assays
2.5. Locomotor Behaviour Assays
2.6. Oxidative Stress, Intestinal Damage and Autophagy Evaluation
2.7. Transcriptome and qRT-PCR Analysis
2.8. Data Analysis
3. Results
3.1. Evaluation of a C. elegans-Based Screening Platform
3.2. Effects of the CQMa421 Culture Filtrate on C. elegans Survival, Locomotion and Reproduction
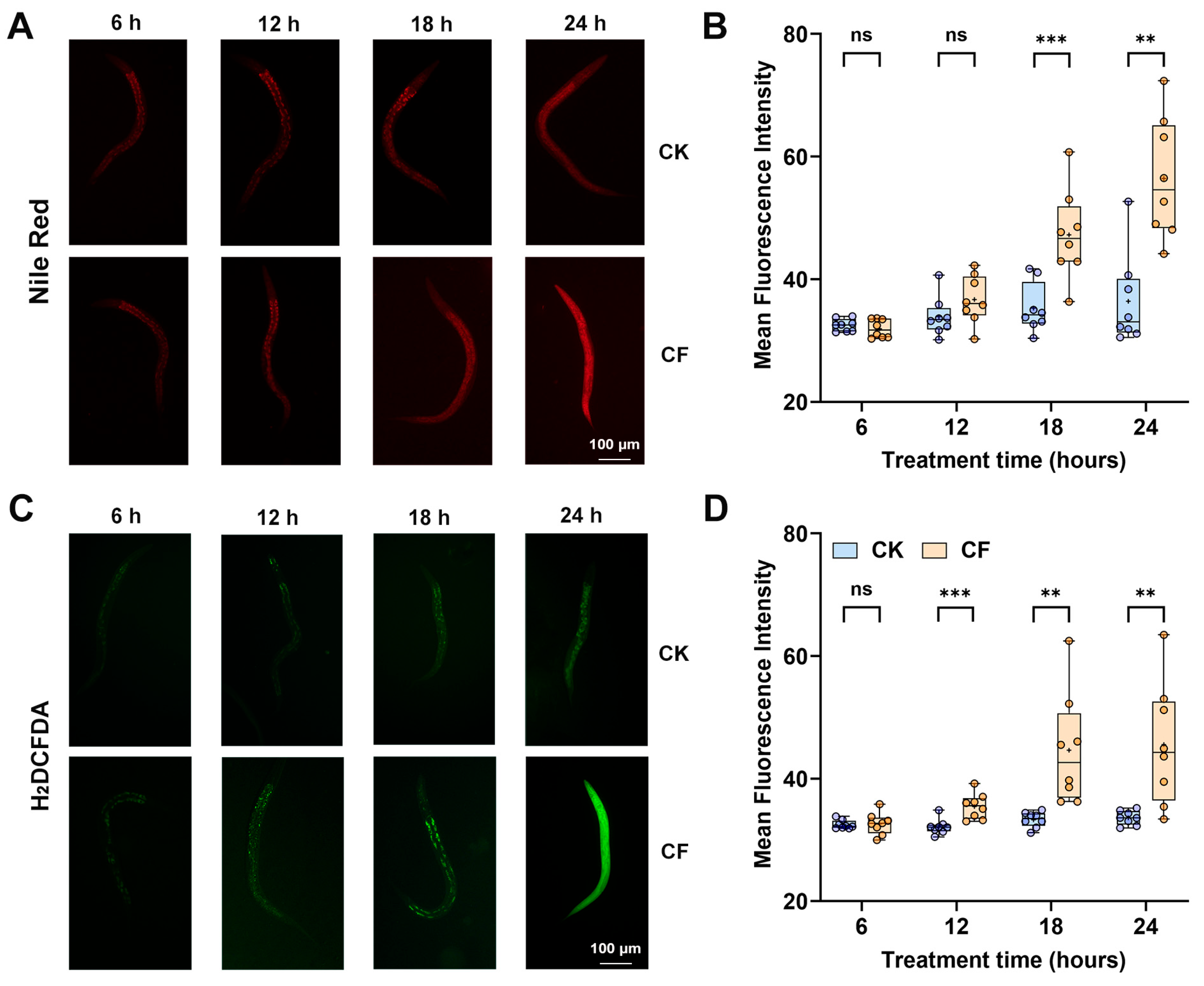
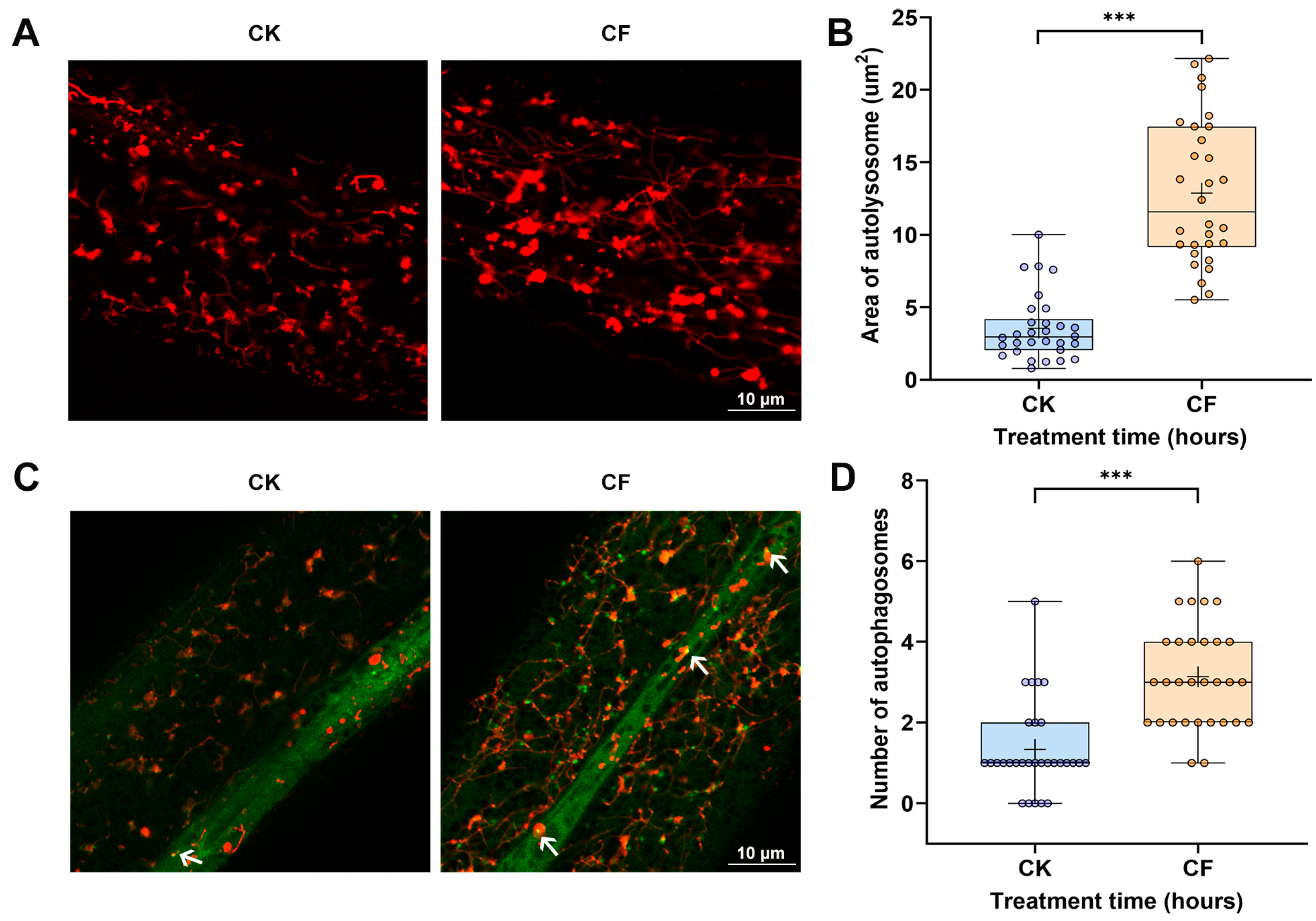
3.3. CQMa421 Culture Filtrate Induces Intestinal Damage, Oxidative Stress and Autophagy in C. elegans
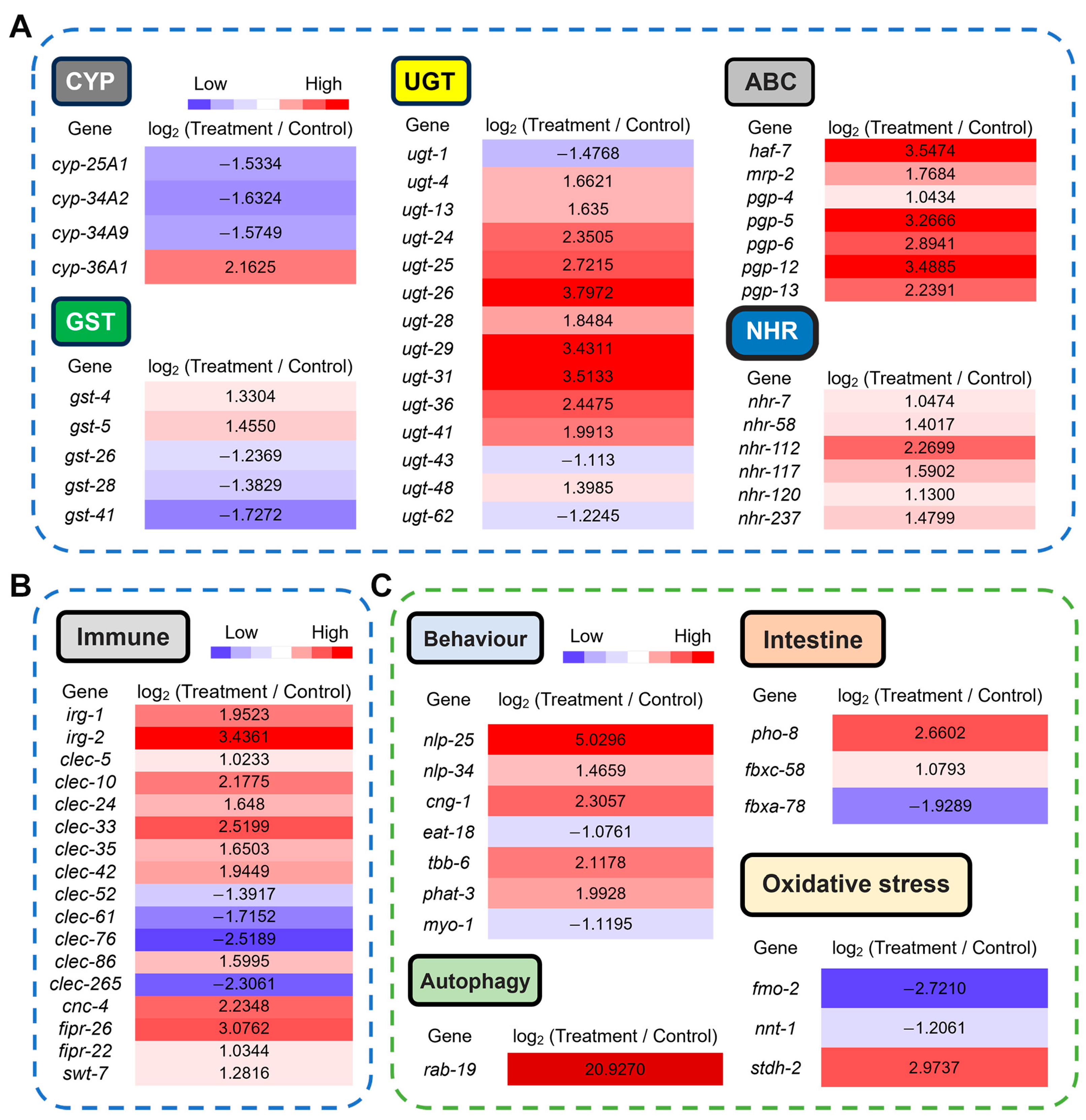
3.4. Transcriptomic Profiling of C. elegans Exposed to the CQMa421 Culture Filtrate
3.5. Transcriptional Basis of CQMa421-Induced Phenotypic and Metabolic Toxicity in C. elegans
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, S.; Singh, B.; Singh, A.P. Nematodes: A threat to sustainability of agriculture. Procedia Environ. Sci. 2015, 29, 215–216. [Google Scholar] [CrossRef]
- Decraemer, W.; Hunt, D.J. Structure and classification. In Plant Nematology, 2nd ed.; Perry, R.N., Moens, M., Eds.; CABI Publishing: Wallingford, UK, 2013; pp. 3–39. [Google Scholar] [CrossRef]
- Jones, J.T.; Haegeman, A.; Danchin, E.G.J.; Gaur, H.S.; Helder, J.; Jones, M.G.; Kikuchi, T.; Manzanilla-López, R.; Palomares-Rius, J.E.; Wesemael, W.M.; et al. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef]
- Warmerdam, S.; Sterken, M.G.; van Schaik, C.; Oortwijn, M.E.; Sukarta, O.C.; Lozano-Torres, J.L.; Dicke, M.; Helder, J.; Kammenga, J.E.; Goverse, A.; et al. Genome-wide association mapping of the architecture of susceptibility to the root-knot nematode Meloidogyne incognita in Arabidopsis thaliana. New Phytol. 2018, 218, 724–737. [Google Scholar] [CrossRef] [PubMed]
- Whitehorn, P.R.; O’Connor, S.; Wackers, F.L.; Goulson, D. Neonicotinoid pesticide reduces bumble bee colony growth and queen production. Science 2012, 336, 351–352. [Google Scholar] [CrossRef]
- Oh, M.; Han, J.W.; Lee, C.; Choi, G.J.; Kim, H. Nematicidal and plant growth-promoting activity of Enterobacter asburiae HK169: Genome analysis provides insight into its biological activities. J. Microbiol. Biotechnol. 2018, 28, 968–975. [Google Scholar] [CrossRef]
- Yao, X.; Guo, H.; Zhang, K.; Zhao, M.; Ruan, J.; Chen, J. Trichoderma and its role in biological control of plant fungal and nematode disease. Front. Microbiol. 2023, 14, 1160551. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, S.S.; Li, H.X.; Wang, R.; Zhang, K.Q.; Xu, J. Fungal-nematode interactions: Diversity, ecology and biocontrol prospects in agriculture. J. Fungi 2020, 6, 206. [Google Scholar] [CrossRef]
- Zimmermann, G. The entomopathogenic fungus Metarhizium anisopliae and its potential as a biocontrol agent. Pestic. Sci. 1993, 37, 375–379. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y.; Wang, B. Entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae play roles of maize (Zea mays) growth promoter. Sci. Rep. 2022, 12, 15706. [Google Scholar] [CrossRef]
- Roberts, D.W.; St Leger, R.J. Metarhizium spp., cosmopolitan insect-pathogenic fungi: Mycological aspects. Adv. Appl. Microbiol. 2004, 54, 1–70. [Google Scholar] [CrossRef]
- Liu, B.L.; Tzeng, Y.M. Development and applications of destruxins: A review. Biotechnol. Adv. 2012, 30, 1242–1254. [Google Scholar] [CrossRef]
- Mantzoukas, S.; Daskalaki, E.; Kitsiou, F.; Papantzikos, V.; Servis, D.; Bitivanos, S.; Patakioutas, G.; Eliopoulos, P.A. Dual action of Beauveria bassiana (Hypocreales; Cordycipitaceae) endophytic strains as biocontrol agents against sucking pests and plant growth biostimulants on melon and strawberry field plants. Microorganisms 2022, 10, 2306. [Google Scholar] [CrossRef] [PubMed]
- Karabörklü, S.; Dura, O. The potential of Beauveria bassiana and Metarhizium anisopliae in controlling the root-knot nematode Meloidogyne incognita in tomato and cucumber. J. Asia-Pacif. Entomol. 2022, 25, 101846. [Google Scholar] [CrossRef]
- Thongkaewyuan, A.; Chairin, T. Biocontrol of Meloidogyne incognita by Metarhizium guizhouense and its protease. Biol. Control 2018, 126, 142–146. [Google Scholar] [CrossRef]
- Khan, M.; Tanaka, K. Purpureocillium lilacinum for plant growth promotion and biocontrol against root-knot nematodes infecting eggplant. PLoS ONE 2023, 18, e0283550. [Google Scholar] [CrossRef]
- Long, N.P.; Kang, J.S.; Kim, H.M. Caenorhabditis elegans: A model organism in the toxicity assessment of environmental pollutants. Environ. Sci. Pollut. Res. Int. 2023, 30, 39273–39287. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Xiang, M.; Liu, S.; Che, Y.; Liu, X. A novel high-throughput nematicidal assay using embryo cells and larvae of Caenorhabditis elegans. Exp. Parasitol. 2014, 139, 33–41. [Google Scholar] [CrossRef]
- Burns, A.R.; Baker, R.J.; Kitner, M.; Knox, J.; Cooke, B.; Volpatti, J.R.; Vaidya, A.S.; Puumala, E.; Palmeira, B.M.; Redman, E.M.; et al. Selective control of parasitic nematodes using bioactivated nematicides. Nature 2023, 618, 102–109. [Google Scholar] [CrossRef]
- Costa, J.C.; Lilley, C.J.; Atkinson, H.J.; Urwin, P.E. Functional characterisation of a cyst nematode acetylcholinesterase gene using Caenorhabditis elegans as a heterologous system. Int. J. Parasitol. 2009, 39, 849–858. [Google Scholar] [CrossRef]
- Coke, M.C.; Bell, C.A.; Urwin, P.E. The Use of Caenorhabditis elegans as a Model for Plant-Parasitic Nematodes: What Have We Learned? Annu. Rev. Phytopathol. 2024, 62, 157–172. [Google Scholar] [CrossRef]
- Abad, P.; Gouzy, J.; Aury, J.M.; Castagnone-Sereno, P.; Danchin, E.G.; Deleury, E.; Perfus-Barbeoch, L.; Anthouard, V.; Artiguenave, F.; Blok, V.C.; et al. Genome sequence of the metazoan plant-parasitic nematode Meloidogyne incognita. Nat. Biotechnol. 2008, 26, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Gillet, F.X.; Bournaud, C.; Antonino de Souza Júnior, J.D.; Grossi-de-Sa, M.F. Plant-parasitic nematodes: Towards understanding molecular players in stress responses. Ann. Bot. 2017, 119, 775–789. [Google Scholar] [CrossRef] [PubMed]
- Hussey, R.S.; Janssen, G.J.W. Root-knot nematodes: Meloidogyne species. In Plant Resistance to Parasitic Nematodes, 2nd ed.; Starr, J.L., Cook, R., Bridge, J., Eds.; CABI Publishing: Wallingford, UK, 2002; pp. 43–70. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, L.; Khan, R.A.A.; Song, X.; Najeeb, S.; Zhao, J.; Yang, Y.; Ling, J.; Mao, Z.; Jiang, X.; et al. Methylorubrum rhodesianum M520 as a biocontrol agent against Meloidogyne incognita (Tylenchida: Heteroderidae) J2s infecting cucumber roots. J. Appl. Microbiol. 2023, 134, lxad001. [Google Scholar] [CrossRef]
- Widaad, A.; Zulkipli, I.N.; Petalcorin, M.I. Anthelmintic effect of Leucaena leucocephala extract and its active compound, mimosine, on vital behavioral activities in Caenorhabditis elegans. Molecules 2022, 27, 1875. [Google Scholar] [CrossRef]
- Zhang, W.J.; Zhang, N.; Zheng, S.; Zhang, W.; Liu, J.; He, L.; Ezemaduka, A.N.; Li, G.; Ning, J.; Xian, B.; et al. Effects of commercial beverages on the neurobehavioral motility of Caenorhabditis elegans. PeerJ 2022, 14, 10.e13563. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.G.; Teng, J.F.; Wong, V.K.; Zhou, X.G.; Qiu, W.Q.; Tang, Y.; Wu, J.M.; Xiong, R.; Pan, R.; Wang, Y.L.; et al. Novel steroidal saponin isolated from Trillium tschonoskii maxim. Exhibits anti-oxidative effect via autophagy induction in cellular and Caenorhabditis elegans models. Phytomedicine 2019, 65, 153088. [Google Scholar] [CrossRef]
- Chen, H.; Chen, X.; Ding, P.; Gu, Y.; Jiang, Y.; Li, X.; Hu, G.; Li, L.; Wang, C.; Yu, J.; et al. Photoaging enhances combined toxicity of microplastics and tetrabromobisphenol a by inducing intestinal damage and oxidative stress in Caenorhabditis elegans. Sci. Total Environ. 2024, 912, 169259. [Google Scholar] [CrossRef]
- Jenzer, C.; Simionato, E.; Legouis, R. Tools and methods to analyze autophagy in C. elegans. Methods 2015, 75, 162–171. [Google Scholar] [CrossRef]
- Tang, H.; Pang, S. Proline Catabolism Modulates Innate Immunity in Caenorhabditis elegans. Cell Rep. 2016, 17, 2837–2844. [Google Scholar] [CrossRef]
- Park, A.R.; Duraisamy, K.; Vo, D.D.; Kim, J.C. Elucidation of the nematicidal mode of action of grammicin on Caenorhabditis elegans. Pestic. Biochem. Physiol. 2022, 188, 105244. [Google Scholar] [CrossRef]
- Huang, X.; Pan, W.; Kim, W.; White, A.; Li, S.; Li, H.; Lee, K.; Fuchs, B.B.; Zeng, K.; Mylonakis, E. Caenorhabditis elegans mounts a p38 MAPK pathway-mediated defence to Cutibacterium acnes infection. Cell. Microbiol. 2020, 22, e13234. [Google Scholar] [CrossRef] [PubMed]
- Hart, A. Behavior. Wormbook. Available online: http://www.wormbook.org/chapters/www_behavior/behavior.html (accessed on 9 June 2024).
- Dierking, K.; Yang, W.; Schulenburg, H. Antimicrobial effectors in the nematode Caenorhabditis elegans: An outgroup to the Arthropoda. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150299. [Google Scholar] [CrossRef]
- Smith, S.W.; Latta, L.C.; Denver, D.R.; Estes, S. Endogenous ROS levels in C. elegans under exogenous stress support revision of oxidative stress theory of life-history tradeoffs. BMC Evol. Biol. 2014, 14, 161. [Google Scholar] [CrossRef]
- Meléndez, A.; Levine, B. Autophagy in C. elegans. Wormbook. Available online: http://www.wormbook.org/chapters/www_autophagy/autophagy.html (accessed on 12 September 2023).
- Liu, T.; Wang, L.; Duan, Y.X.; Wang, X. Nematicidal activity of culture filtrate of Beauveria bassiana against Meloidogyne hapla. World J. Microbiol. Biotechnol. 2008, 24, 113–118. [Google Scholar] [CrossRef]
- Youssef, M.M.; El-Nagdi, W.M.; Lotfy, D.E. Evaluation of the fungal activity of Beauveria bassiana, Metarhizium anisopliae and Paecilomyces lilacinus as biocontrol agents against root-knot nematode, Meloidogyne incognita on cowpea. Bull. Natl. Res. Cent. 2020, 44, 112. [Google Scholar] [CrossRef]
- Peng, G.; Zhang, S.; Xia, Y. Metarhizium anisopliae CQMa421 and Its Application Statuss. Chin. J. Biol. Control 2020, 36, 850. [Google Scholar] [CrossRef]
- Lindblom, T.H.; Dodd, A.K. Xenobiotic detoxification in the nematode Caenorhabditis elegans. J. Exp. Zool. Part A Comp. Exp. Biol. 2006, 305, 720–730. [Google Scholar] [CrossRef]
- Larigot, L.; Mansuy, D.; Borowski, I.; Coumoul, X.; Dairou, J. Cytochromes P450 of Caenorhabditis elegans: Implication in biological functions and metabolism of xenobiotics. Biomolecules 2022, 12, 342. [Google Scholar] [CrossRef]
- Leiers, B.; Kampkötter, A.; Grevelding, C.G.; Link, C.D.; Johnson, T.E.; Henkle-Dührsen, K. A stress-responsive glutathione S-transferase confers resistance to oxidative stress in Caenorhabditis elegans. Free Radic. Biol. Med. 2003, 34, 1405–1415. [Google Scholar] [CrossRef]
- Di, R.; Zhang, H.; Lawton, M.A. Transcriptome analysis of C. elegans reveals novel targets for DON cytotoxicity. Toxins 2018, 10, 262. [Google Scholar] [CrossRef]
- Yan, R.; Urdaneta-Marquez, L.; Keller, K.; James, C.E.; Davey, M.W.; Prichard, R.K. The role of several ABC transporter genes in ivermectin resistance in Caenorhabditis elegans. Vet. Parasitol. 2012, 190, 519–529. [Google Scholar] [CrossRef]
- Estes, K.A.; Dunbar, T.L.; Powell, J.R.; Ausubel, F.M.; Troemel, E.R. bZIP transcription factor zip-2 mediates an early response to Pseudomonas aeruginosa infection in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2010, 107, 2153–2158. [Google Scholar] [CrossRef] [PubMed]
- How, C.M.; Li, Y.S.; Huang, W.Y.; Wei, C.C. Early-life exposure to mycotoxin zearalenone exacerbates aberrant immune response, oxidative stress, and mortality of Caenorhabditis elegans under pathogen Bacillus thuringiensis infection. Ecotoxicol. Environ. Saf. 2024, 272, 116085. [Google Scholar] [CrossRef]
- Souza, A.C.R.; Burgwyn Fuchs, B.; de Souza Alves, V.; Jayamani, E.; Colombo, A.L.; Mylonakis, E. Pathogenesis of the Candida parapsilosis complex in the model host Caenorhabditis elegans. Genes 2018, 9, 401. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Deng, Y.; Wang, S.; Du, H.; Xiao, G.; Wang, D. Assessment of nanopolystyrene toxicity under fungal infection condition in Caenorhabditis elegans. Ecotoxicol. Environ. Saf. 2020, 197, 110625. [Google Scholar] [CrossRef]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Chávez, V.; Mohri-Shiomi, A.; Maadani, A.; Vega, L.A.; Garsin, D.A. Oxidative stress enzymes are required for DAF-16-mediated immunity due to generation of reactive oxygen species by Caenorhabditis elegans. Genetics 2007, 176, 1567–1577. [Google Scholar] [CrossRef]
- Liu, S.; Wu, Q.; Zhong, Y.; He, Z.; Wang, Z.; Li, R.; Wang, M. Fosthiazate exposure induces oxidative stress, nerve damage, and reproductive disorders in nontarget nematodes. Environ. Sci. Pollut. Res. Int. 2022, 30, 12522–12531. [Google Scholar] [CrossRef]
- McGhee, J.D. The C. elegans intestine. WormBook. Available online: http://www.wormbook.org/chapters/www_intestine/intestine.html (accessed on 11 June 2024).
- Reiner, D.J.; Lundquist, E.A. Small GTPases. WormBook. Available online: http://www.wormbook.org/chapters/www_smallGTPases.2/smallGTPases.2.html (accessed on 21 June 2024).
- Dou, T.; Chen, J.; Wang, R.; Pu, X.; Wu, H.; Zhao, Y. Complementary protective effects of autophagy and oxidative response against graphene oxide toxicity in Caenorhabditis elegans. Ecotoxicol. Environ. Saf. 2022, 248, 114289. [Google Scholar] [CrossRef]
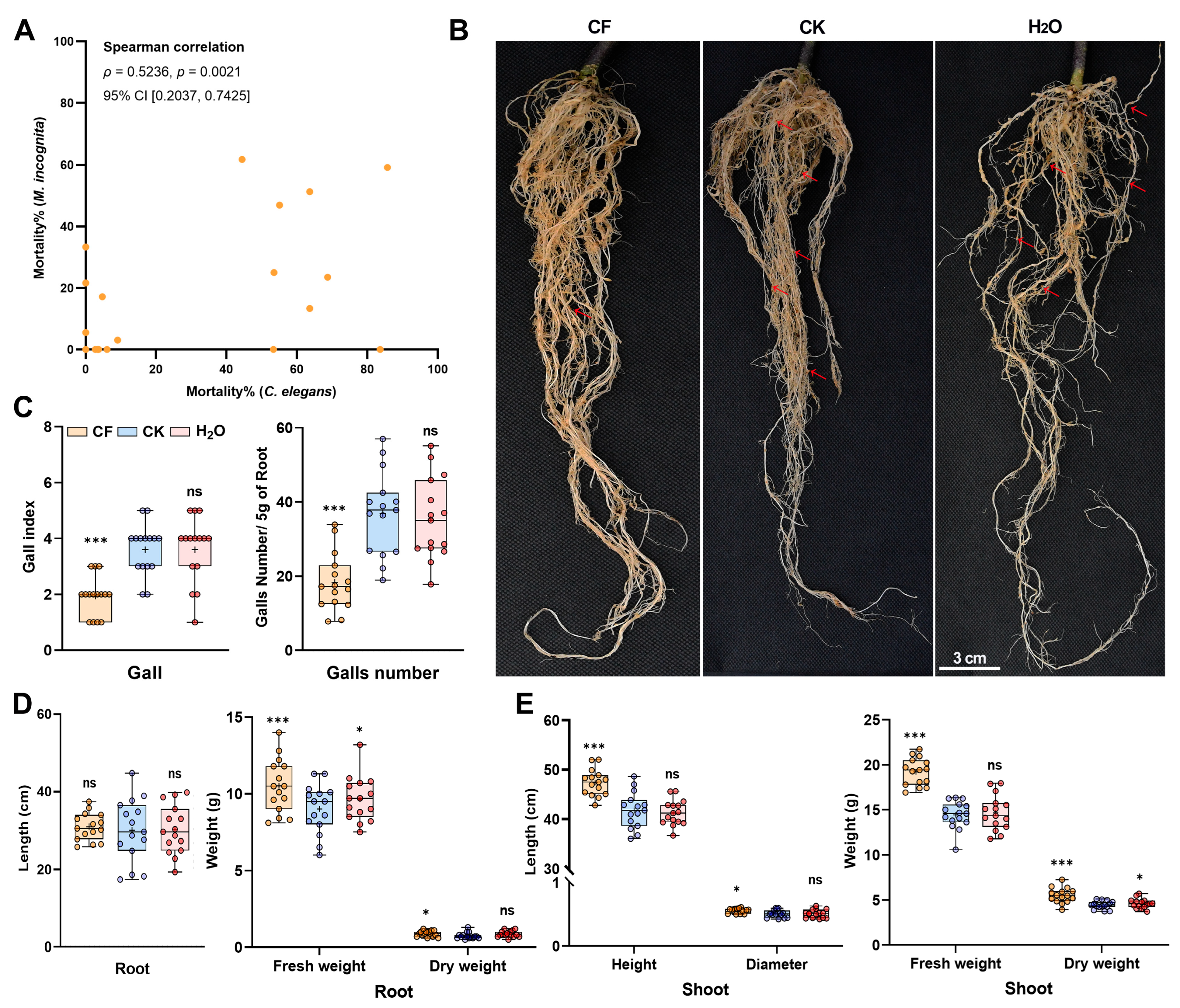


| No. | Strains | C. elegans | M. incognita | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean (%) | SD (%) | Median (%) | Letter Group | Mean (%) | SD (%) | Median (%) | Letter Group | ||
| 1 | SDAY | 0.00 | 0.00 | 0.00 | e | 0.00 | 0.00 | 0.00 | e |
| 2 | GB31.000127 | 3.70 | 5.43 | 6.06 | e | 0.00 | 0.00 | 0.00 | e |
| 3 | GB31.000128 | 48.06 | 12.87 | 53.33 | b | 2.59 | 4.13 | 0.00 | e |
| 4 | GB3100289 | 82.22 | 9.87 | 85.71 | a | 58.62 | 8.23 | 59.14 | a |
| 5 | GB3100290 | 61.02 | 9.45 | 63.64 | c | 14.30 | 4.01 | 13.33 | d |
| 6 | GB3100293 | 0.00 | 0.00 | 0.00 | e | 2.93 | 4.02 | 0.00 | e |
| 7 | GB3100311 | 1.11 | 2.72 | 0.00 | e | 23.23 | 6.82 | 21.62 | c |
| 8 | GB3100312 | 2.41 | 3.65 | 0.00 | e | 0.00 | 0.00 | 0.00 | e |
| 9 | GB3100313 | 63.54 | 8.91 | 63.64 | c | 53.30 | 7.94 | 51.30 | a |
| 10 | GB3100314 | 53.95 | 4.38 | 53.49 | c | 26.61 | 6.45 | 25.00 | c |
| 11 | GB3100315 | 58.60 | 7.82 | 55.10 | c | 44.86 | 9.73 | 46.91 | b |
| 12 | GB3100317 | 2.74 | 3.01 | 3.33 | e | 0.00 | 0.00 | 0.00 | e |
| 13 | GB3100318 | 68.75 | 15.29 | 68.75 | b | 23.24 | 7.12 | 23.50 | c |
| 14 | GB3100319 | 47.52 | 8.26 | 44.44 | d | 58.02 | 10.89 | 61.73 | a |
| 15 | GB3100320 | 1.85 | 2.96 | 0.00 | e | 0.83 | 2.04 | 0.00 | e |
| 16 | GB3100321 | 0.79 | 1.93 | 0.00 | e | 32.86 | 8.32 | 33.33 | c |
| 17 | GB3100322 | 78.33 | 11.34 | 83.67 | a | 0.00 | 0.00 | 0.00 | e |
| 18 | GB3200001 | 1.41 | 2.54 | 0.00 | e | 0.94 | 2.31 | 0.00 | e |
| 19 | GB3200002 | 0.67 | 1.63 | 0.00 | e | 1.47 | 3.60 | 0.00 | e |
| 20 | GB3200003 | 2.36 | 3.87 | 0.00 | e | 4.53 | 2.76 | 5.56 | e |
| 21 | GB3200004 | 0.00 | 0.00 | 0.00 | e | 0.76 | 1.85 | 0.00 | e |
| 22 | GB3200005 | 0.93 | 2.27 | 0.00 | e | 0.00 | 0.00 | 0.00 | e |
| 23 | GB3200006 | 3.28 | 3.28 | 2.70 | e | 0.00 | 0.00 | 0.00 | e |
| 24 | GB3200007 | 0.00 | 0.00 | 0.00 | e | 0.00 | 0.00 | 0.00 | e |
| 25 | GB3200008 | 3.87 | 3.02 | 3.70 | e | 0.00 | 0.00 | 0.00 | e |
| 26 | GB3200009 | 0.00 | 0.00 | 0.00 | e | 4.95 | 4.12 | 5.77 | e |
| 27 | GB3200010 | 2.09 | 2.73 | 0.00 | e | 0.00 | 0.00 | 0.00 | e |
| 28 | GB3200011 | 4.76 | 3.89 | 4.76 | e | 17.76 | 5.33 | 17.14 | c |
| 29 | GB3200023 | 0.00 | 0.00 | 0.00 | e | 0.00 | 0.00 | 0.00 | e |
| 30 | GB3200028 | 10.02 | 3.67 | 9.09 | d | 3.28 | 4.03 | 3.03 | e |
| 31 | GB3200029 | 0.00 | 0.00 | 0.00 | e | 0.00 | 0.00 | 0.00 | e |
| 32 | GB3200030 | 0.52 | 1.28 | 0.00 | e | 0.00 | 0.00 | 0.00 | e |
| 33 | GB3200031 | 0.00 | 0.00 | 0.00 | e | 0.93 | 1.63 | 0.00 | e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, C.; Zhang, R.; Wang, Y.; Yang, S.; Yu, W.; Xia, Y. Biocontrol Potential of Entomopathogenic Fungi Against Plant-Parasitic Nematodes: A Caenorhabditis elegans-Based Screening and Mechanistic Study. J. Fungi 2025, 11, 381. https://doi.org/10.3390/jof11050381
Cheng C, Zhang R, Wang Y, Yang S, Yu W, Xia Y. Biocontrol Potential of Entomopathogenic Fungi Against Plant-Parasitic Nematodes: A Caenorhabditis elegans-Based Screening and Mechanistic Study. Journal of Fungi. 2025; 11(5):381. https://doi.org/10.3390/jof11050381
Chicago/Turabian StyleCheng, Cheng, Renjun Zhang, Yanzhen Wang, Shuo Yang, Wenhao Yu, and Yuxian Xia. 2025. "Biocontrol Potential of Entomopathogenic Fungi Against Plant-Parasitic Nematodes: A Caenorhabditis elegans-Based Screening and Mechanistic Study" Journal of Fungi 11, no. 5: 381. https://doi.org/10.3390/jof11050381
APA StyleCheng, C., Zhang, R., Wang, Y., Yang, S., Yu, W., & Xia, Y. (2025). Biocontrol Potential of Entomopathogenic Fungi Against Plant-Parasitic Nematodes: A Caenorhabditis elegans-Based Screening and Mechanistic Study. Journal of Fungi, 11(5), 381. https://doi.org/10.3390/jof11050381






