Bacillus velezensis Isolate X5 Stimulates the Resistance of Resistant and Susceptible Banana Varieties to Foc Through Different Mechanisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Material and Inoculation
2.2. Assessment of Infection Symptoms
2.3. Collection and Determination of Root Exudates
2.4. Effects of Differentially Abundant Components in Root Exudates on the Growth of X5 and Foc4
2.5. Extraction, Detection, Quality Inspection, and Library Construction of Banana Root RNA
2.6. Identification of DEGs and Their Enrichment Analysis
2.7. Reverse Transcription–Quantitative PCR (RT-qPCR) Analysis
2.8. Statistical Analysis
3. Results
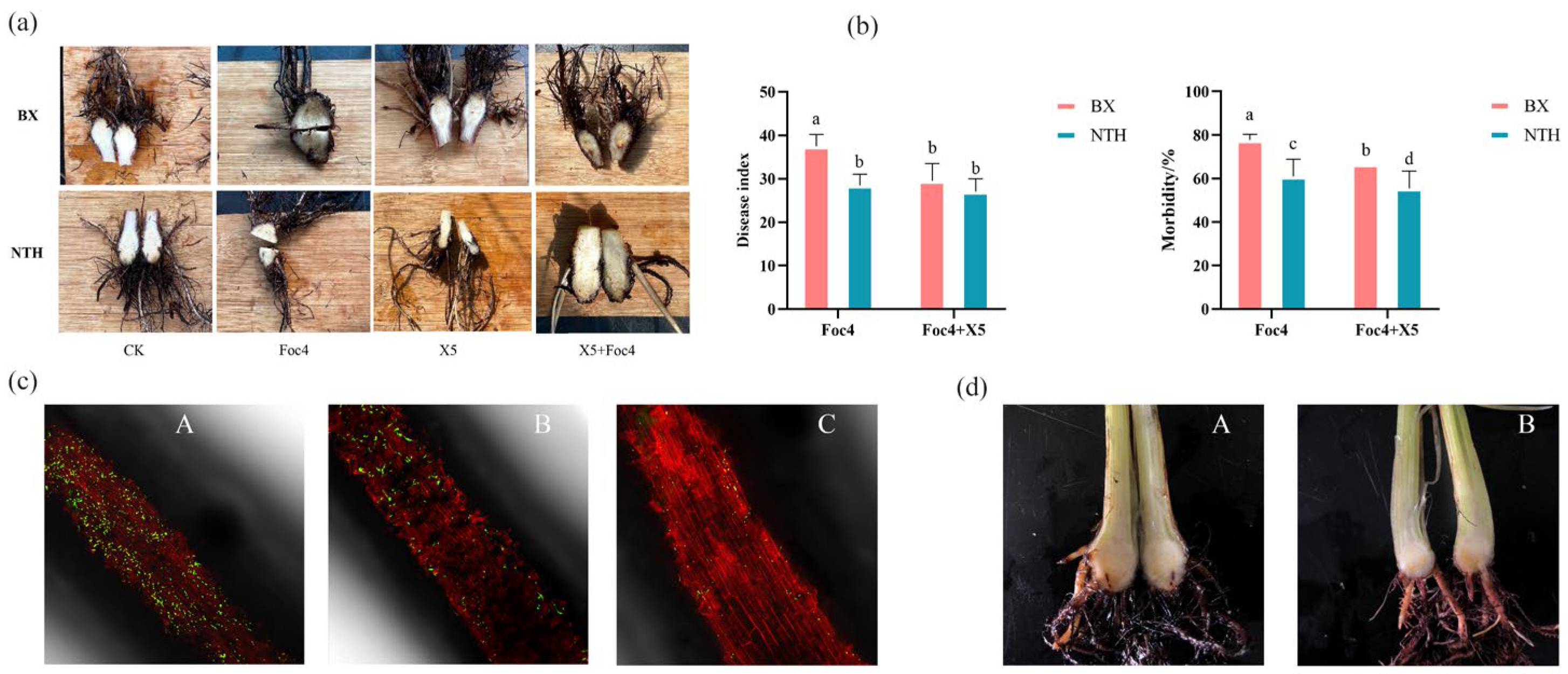
3.1. Resistance of BX and NTH to Pathogen Foc4
3.2. Identification and Analysis of Free Amino Acids in Root Exudates of Bananas with Different Resistances
3.3. Identification and Analysis of Soluble Sugars in Root Exudates of Different Resistant Bananas
3.4. Identification and Analysis of Organic Acids in Root Exudates of Bananas with Different Resistances
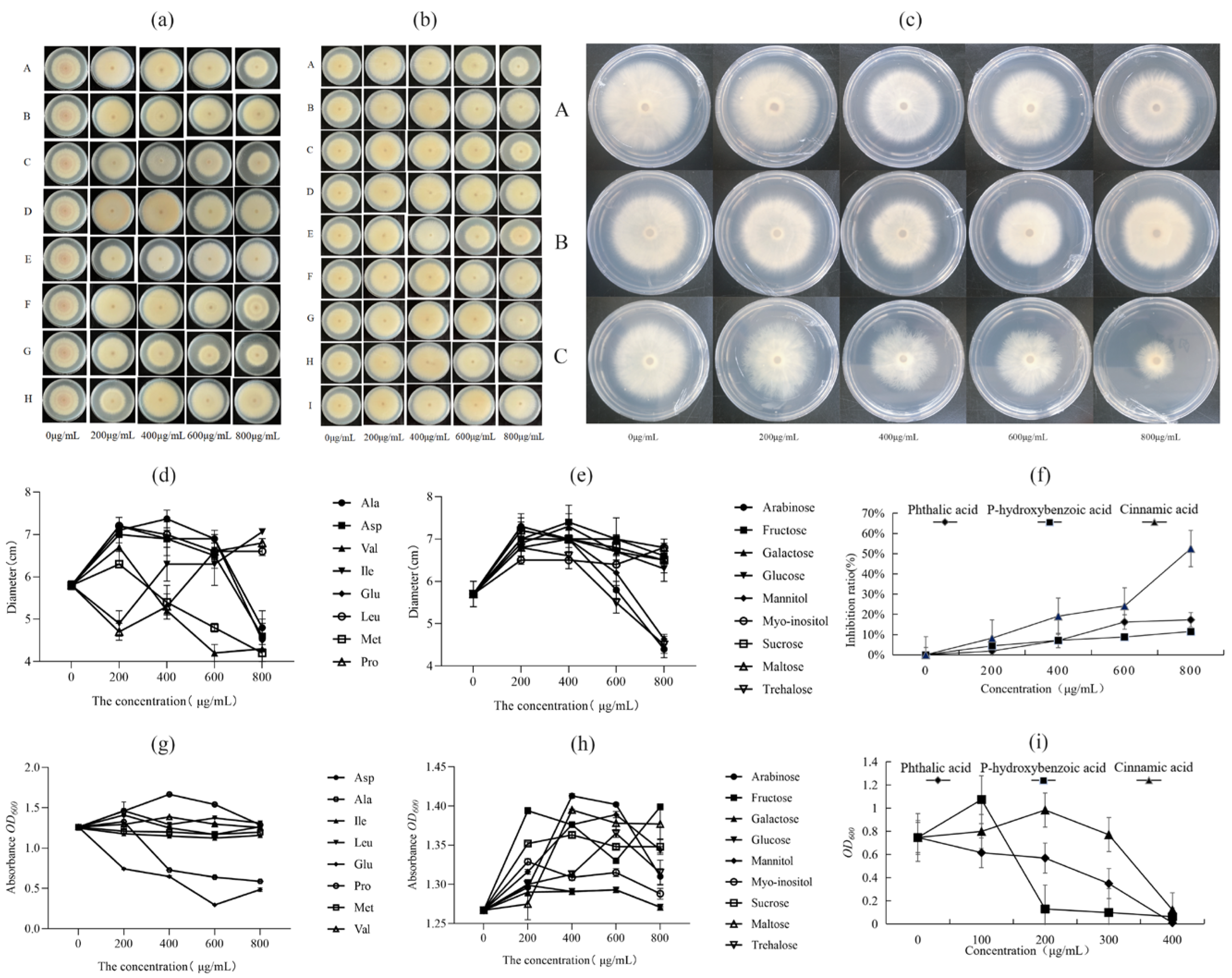
3.5. Effect of the Exogenous Addition of Differential Metabolites on the Growth of Foc4 and X5
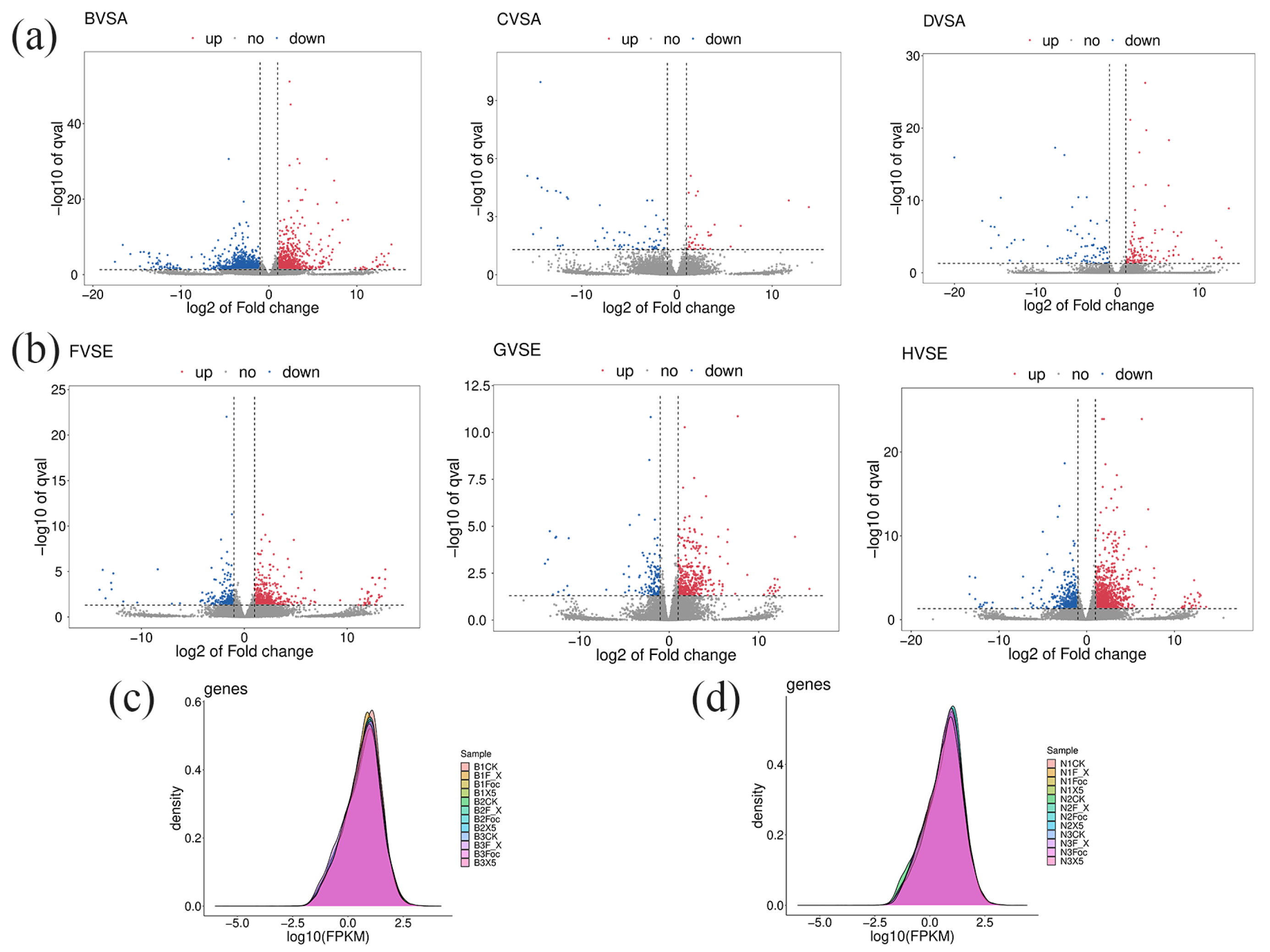
3.6. Sequencing and Analysis of the Root Transcriptome of BX and NTH Under Each Treatment
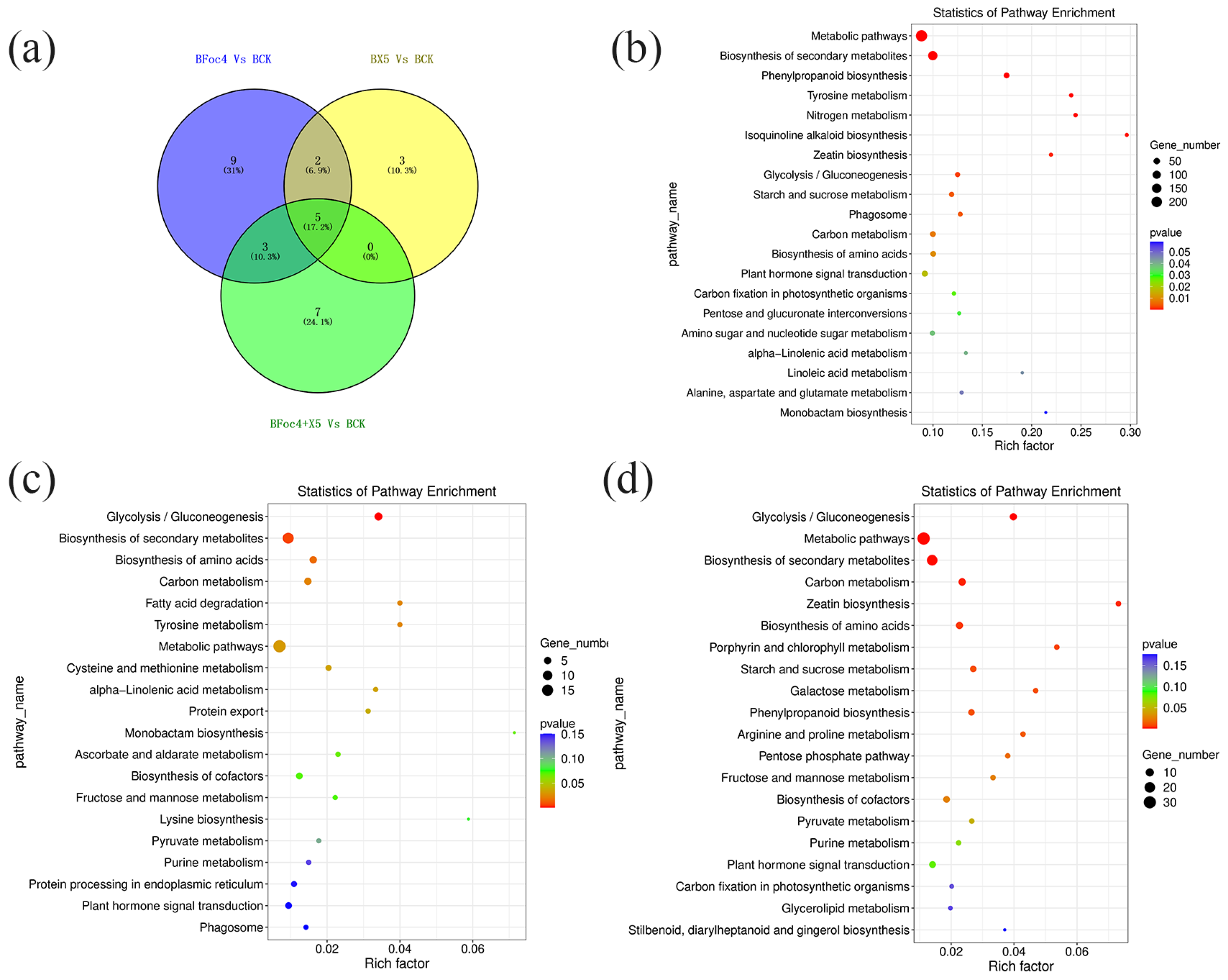
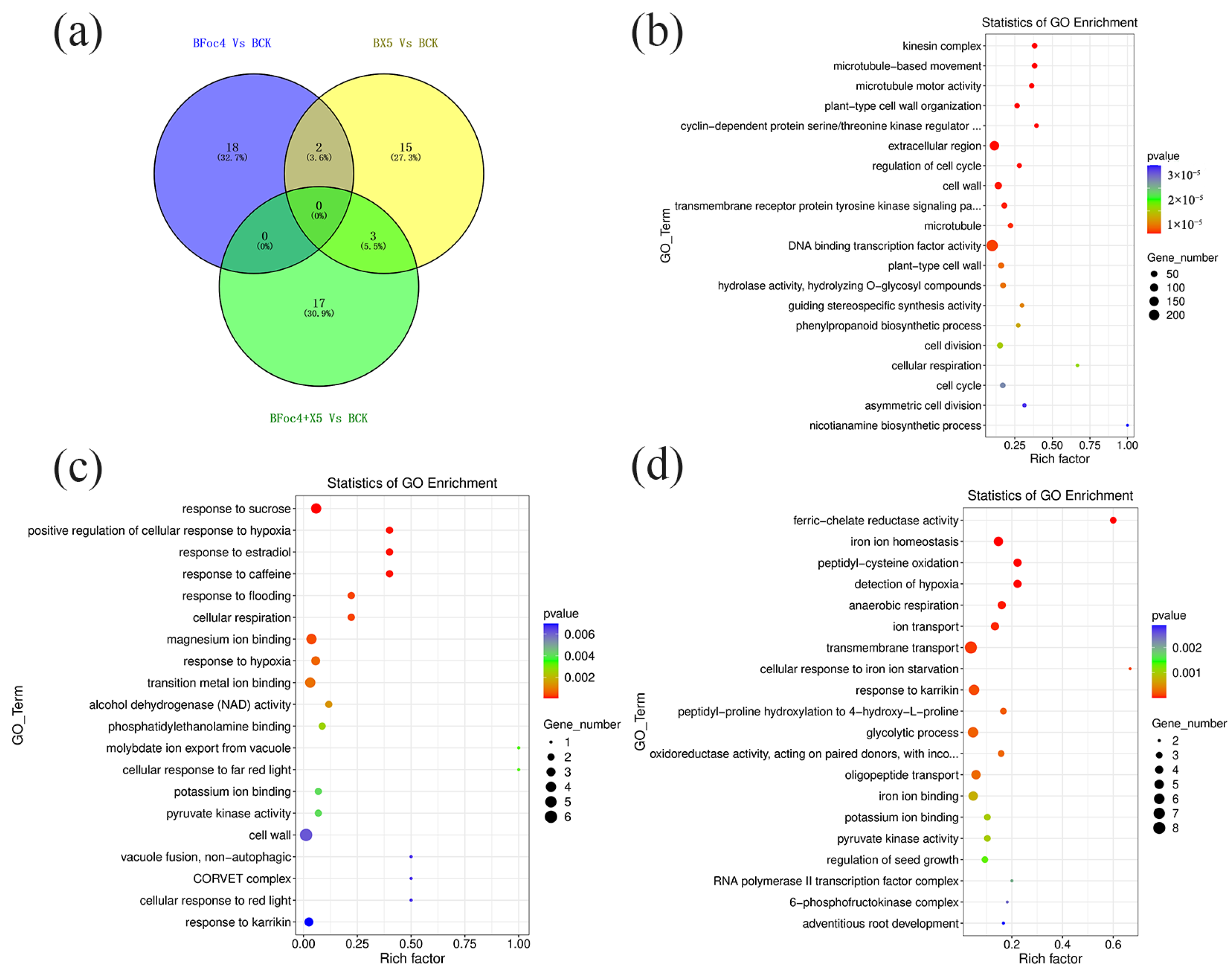
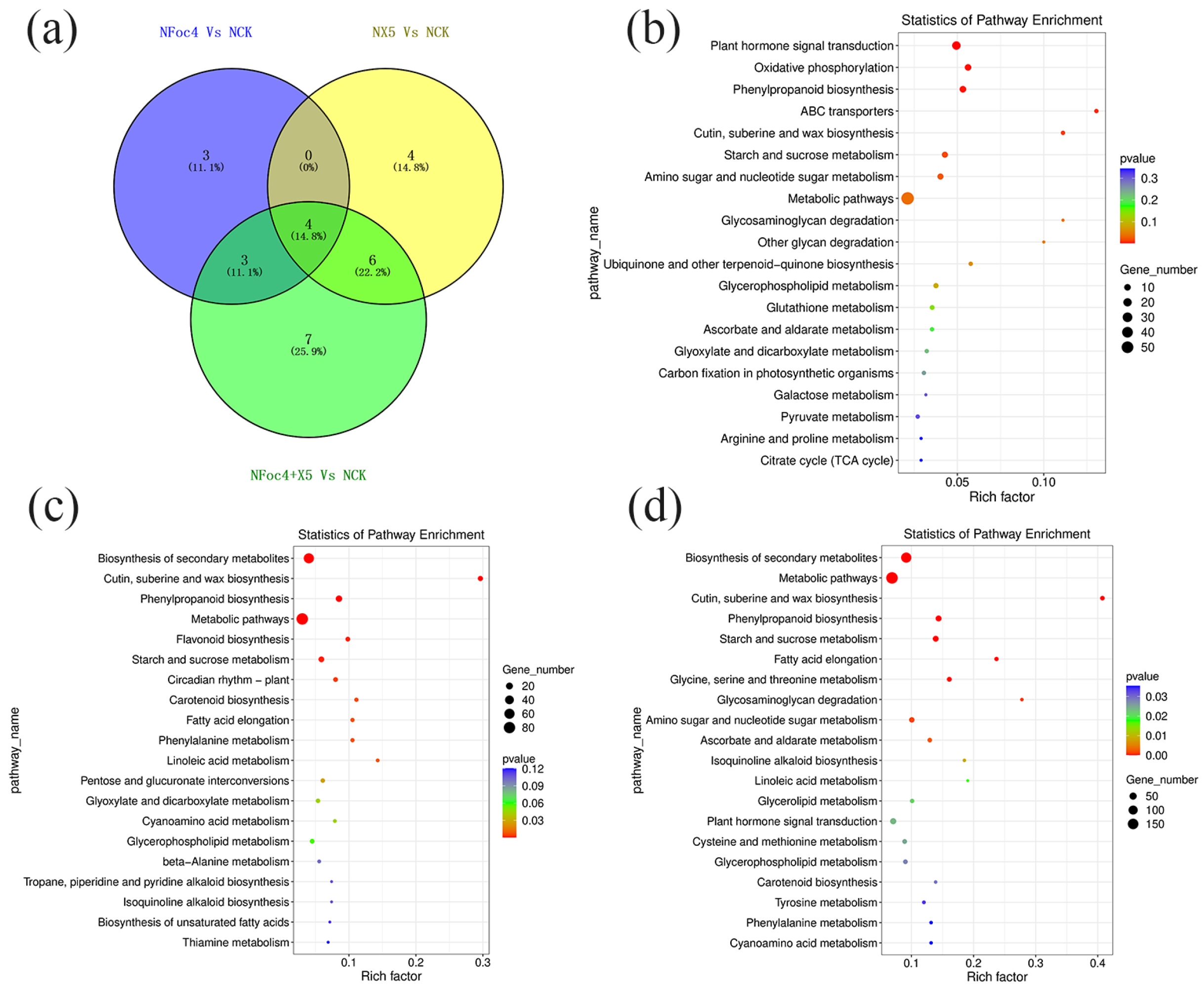
3.7. Transcriptomic Analysis and Functional Analysis of DEGs
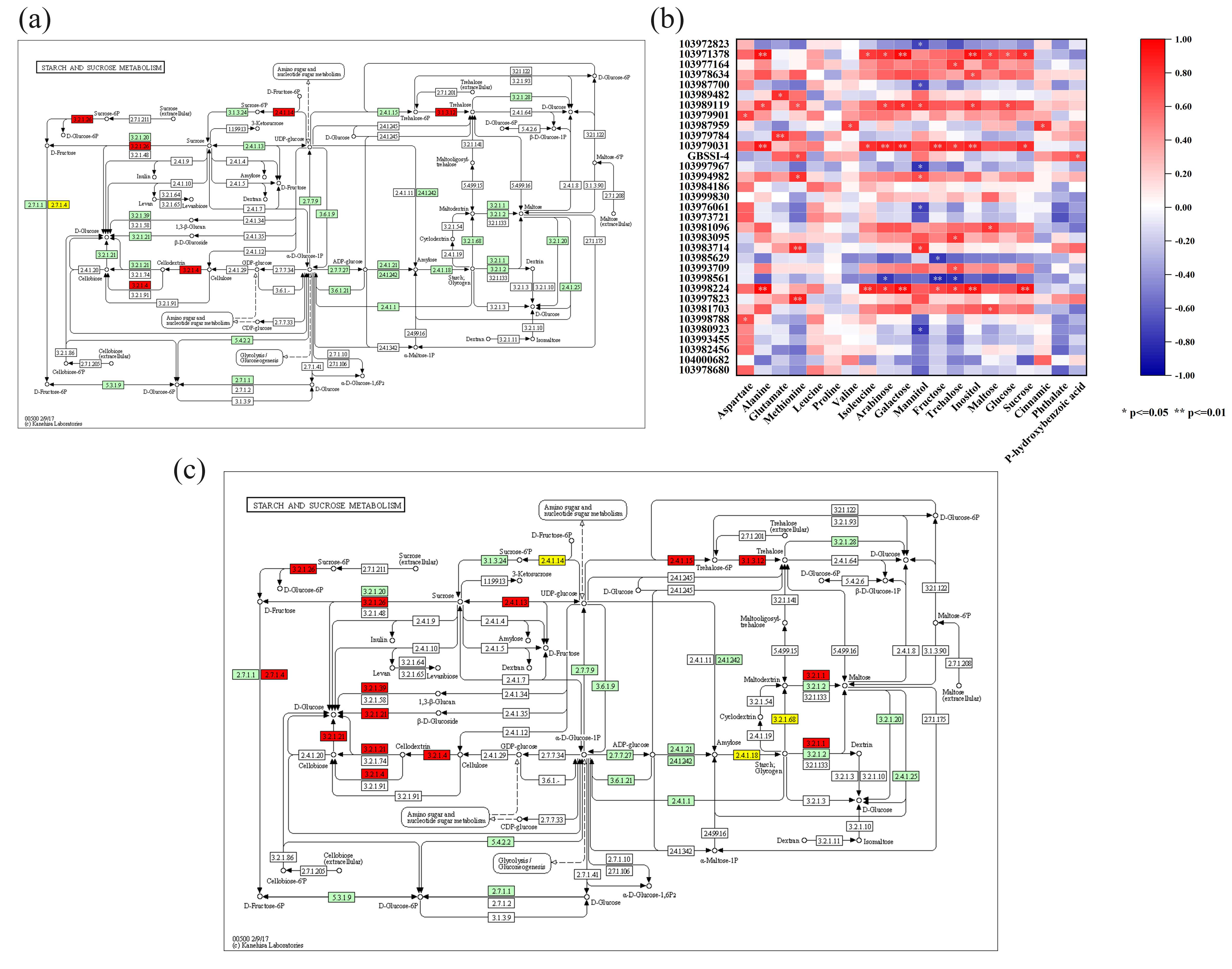
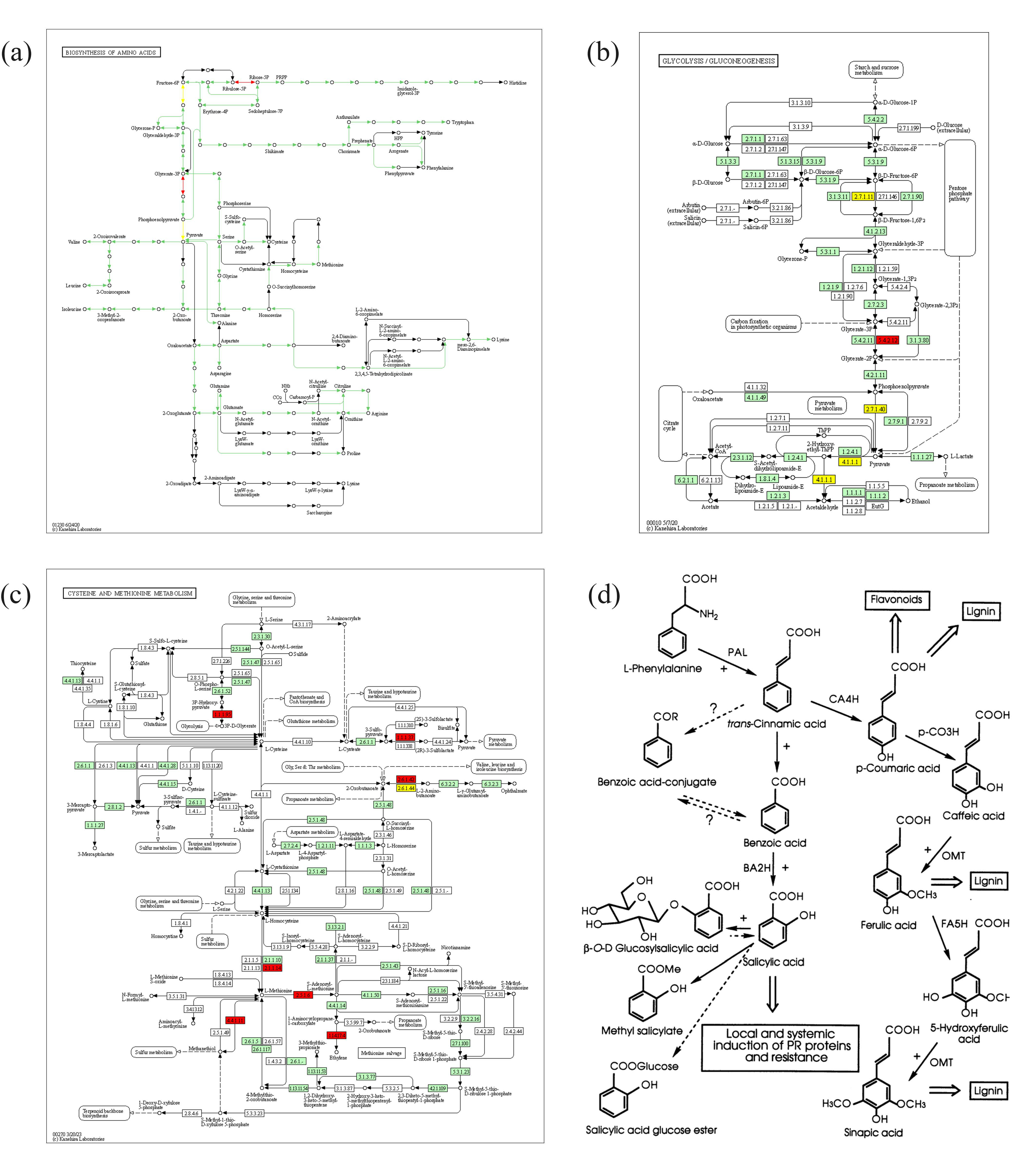
3.8. Correlation Analysis of the Transcriptomic and Metabolomic Data
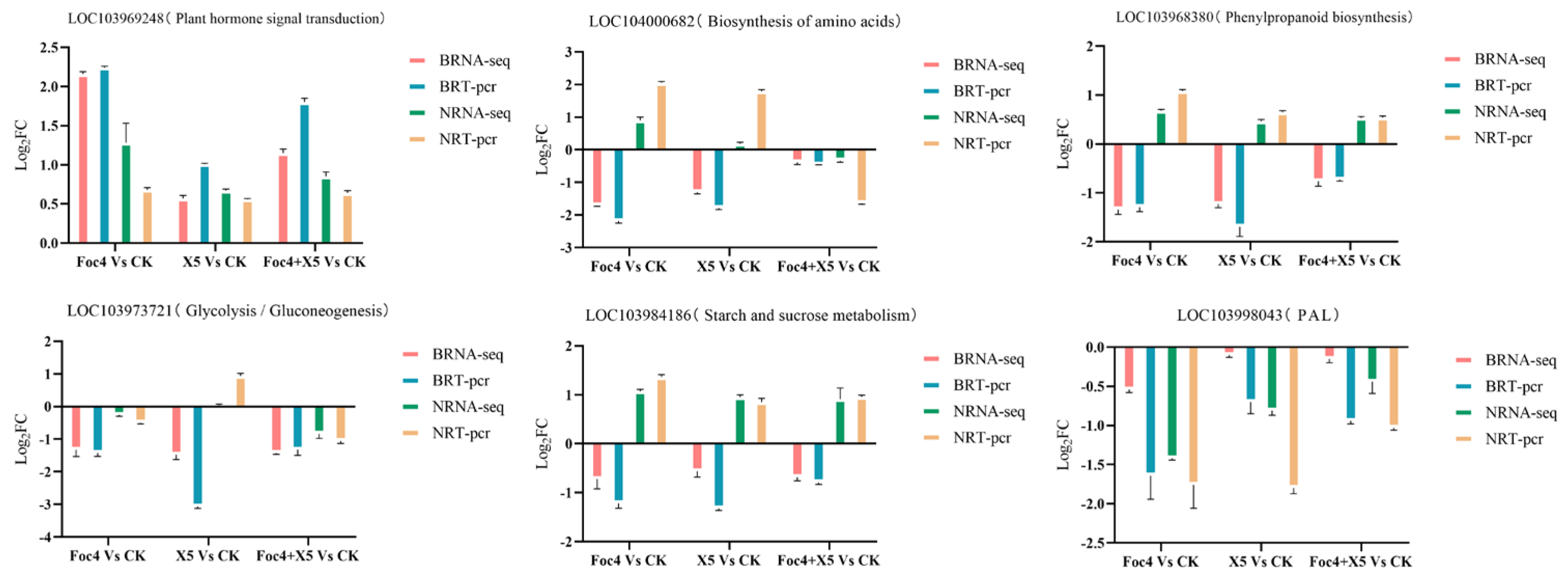
3.9. RT-qPCR Verification of the Transcriptome Data
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Q. Study on The Industrial Cluster of Tropical Bananas Based on Gem Model. Acta Univ. Cibiniensis. Ser. E Food Technol. 2017, 21, 69–74. [Google Scholar] [CrossRef]
- Yang, D.; Du, C.J.; Zhang, J.; Pan, L.F.; Wei, S.L.; Jiang, S.B.; Li, C.Y.; San, C.; Huy, N.D.; Ye, Y.F.; et al. Validation and application of a molecular detection system for Fusarium wilt of banana in China. Plant Dis. 2023, 107, 3687–3692. [Google Scholar] [CrossRef] [PubMed]
- Bidabadi, S.S.; Zheng, S.J. Banana fusarium wilt (Fusarium oxysporum f. sp. cubense) control and resistance, in the context of developing wilt-resistant bananas within sustainable production systems. Hortic. Plant J. 2018, 4, 208–218. [Google Scholar]
- Bancroft, J. Report of the board appointed to enquire into the cause of disease affecting livestock and plants. Votes Proc. 1876, 3, 1011–1038. [Google Scholar]
- Stover, R.H.; Ploetz, R.C. Wilt of banana: Some history and current status of the disease. In Fusarium Wilt of Banana; APS Press: St. Paul, MN, USA, 1990; pp. 1–7. [Google Scholar]
- Hwang, S.C.; Ko, W.H. Selection of improved cavendish banana mutants resistant to race 4 of Fusarium oxysporum f. sp. cubense. Acta Hortic. 1990, 1, 417–424. [Google Scholar] [CrossRef]
- Xu, L.; Huang, B.; Wu, Y.; Huang, Y.; Dong, T. The Cost-Benefit Analysis for Bananas Diversity Production in China Foc. Zones. Am. J. Plant Sci. 2011, 2, 561–568. [Google Scholar] [CrossRef]
- Mon, Y.Y.; Bidabadi, S.S.; Oo, K.S.; Zheng, S. The antagonistic mechanism of rhizosphere microbes and endophytes on the interaction between banana and Fusarium oxysporum f. sp. cubense. Physiol. Mol. Plant Pathol. 2021, 116, 101733. [Google Scholar] [CrossRef]
- Abo-Elyousr, K.A.M.; Hashem, M.; Ali, E.H. Integrated control of cotton root rot disease by mixing fungal biocontrol agents and resistance inducers. Crop Prot. 2009, 28, 295–301. [Google Scholar] [CrossRef]
- Ayyadurai, N.; Naik, P.R.; Rao, M.S.; Kumar, R.S.; Samrat, S.K.; Manohar, M.; Sakthivel, N. Isolation and characterization of a novel banana rhizosphere bacterium as fungal antagonist and microbial adjuvant in micropropagation of banana. J. Appl. Microbiol. 2006, 100, 926–937. [Google Scholar] [CrossRef]
- Li, X.J.; Li, K.; Zhou, D.B.; Zhang, M.Y.; Qi, D.; Jing, T.; Zang, X.P.; Qi, C.L.; Wang, W.; Xie, J.H. BiologicalControl of Banana Wilt Disease Caused by Fusarium oxysporum f. sp. Cubense using Streptomyces sp. H4. Biol. Control 2020, 155, 104524. [Google Scholar]
- Li, C.Y.; Yi, G.J.; Hu, C.H.; Yang, Q.S.; Bi, F.C.; Gao, H.; Sheng, O.; Deng, G.M.; Dong, T.; Dou, T.X. Combating Fusarium wilt of banana by developing cultivars with resistance to Fusarium oxysporum f. sp. cubense tropical race 4. Acta Hortic. 2020, 1272, 57–66. [Google Scholar] [CrossRef]
- Wang, R.; Gao, M.; Ji, S.; Wang, S.; Meng, Y.; Zhou, Z. Carbon allocation, osmotic adjustment, antioxidant capacity and growth in cotton under long-term soil drought during flowering and boll-forming period. Plant Physiol. Biochem. 2016, 107, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Zhao, J.; Wen, T.; Zhao, M.L.; Li, R.; Goossens, P.; Huang, Q.W.; Bai, Y.; Vivanco, J.M.; Kowalchuk, G.A.; et al. Root exudates drive the soil-borne legacy of aboveground pathogen infection. Microbiome 2018, 6, 156. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.F.; Robert, C.A.M.; Cadot, S.; Zhang, X.; Ye, M.; Li, B.B.; Manzo, D.; Chervet, N.; Steinger, T.; Heijden, M.G.A.V.D.; et al. Root exudate metabolites drive plant-soil feedbacks on growth and defense by shaping the rhizosphere microbiota. Nat. Commun. 2018, 9, 2738. [Google Scholar] [CrossRef]
- David, T.; Ghalid, M.E.; Rossi, F.; Pietro, A.D. Fungal pathogen uses sex pheromone receptor for chemotropic sensing of host plant signals. Nature 2015, 527, 521–524. [Google Scholar]
- Faure, D.; Vereecke, D.; Leveau, J.H.J. Molecular communication in the rhizosphere. Plant Soil 2009, 321, 279–303. [Google Scholar] [CrossRef]
- Chen, Y.; Cao, S.; Chai, Y.; Clardy, J.; Kolter, R.; Guo, J.H.; Losick, R. A bacillus subtilis sensor kinase involved in triggering biofilm formation on the roots of tomato plants. Mol. Microbiol. 2012, 85, 418–430. [Google Scholar] [CrossRef]
- Rudrappa, T.; Czymmek, K.J.; Pare, P.W.; Bais, H.P. Root-secreted malic acid recruits beneficial soil bacteria. Plant Physiol. 2008, 148, 1547–1556. [Google Scholar] [CrossRef]
- Liu, Y.P.; Zhang, N.; Qiu, M.H.; Feng, H.C.; Vivanco, J.M.; Shen, Q.; Zhang, R.F. Enhanced rhizosphere colonization of beneficial Bacillus amyloliquefaciens SQR9 by pathogen infection. FEMS Microbiol. Lett. 2014, 353, 49–56. [Google Scholar] [CrossRef]
- Mwita, L.; Chan, W.Y.; Pretorius, T.; Lyantagaye, S.L.; Lapa, S.V.; Avdeeva, L.V.; Reva, O.N. Gene expression regulation in the plant growth promoting Bacillusatrophaeus UCMB-5137 stimulated by maize root exudates. Gene 2016, 590, 18–28. [Google Scholar] [CrossRef]
- Ling, N.; Huang, Q.; Guo, S.; Shen, Q. Paenibacillus polymyxa SQR-21 systemically affects root exudates of watermelon to decrease the conidial germination of Fusarium oxysporum f. sp. niveum. Plant Soil 2011, 341, 485–493. [Google Scholar] [CrossRef]
- Hao, W.; Ren, L.; Ran, W.; Shen, Q. Allelopathic effects of root exudates from watermelon and rice plants on Fusarium oxysporum f. sp. niveum. Plant Soil 2010, 336, 485–497. [Google Scholar] [CrossRef]
- Tang, H.; Wang, Y. Metabonomics: A revolution in progress. Prog. Biochem. Biophys. 2006, 33, 401–417. [Google Scholar]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef]
- Niu, Y.; Hu, B.; Li, X.; Chen, H.; Takáˇc, T.; Šamaj, J.; Xu, C.X. Comparative Digital Gene Expression Analysis of Tissue-Cultured Plantlets of Highly Resistant and Susceptible Banana Cultivars in Response to Fusarium oxysporum. Int. J. Mol. Sci. 2018, 19, 350. [Google Scholar] [CrossRef]
- Kasote, D.M.; Jayaprakasha, G.K.; Ong, K.; Crosby, K.M.; Patil, B.S. Hormonal and metabolites responses in Fusarium wilt-susceptible and-resistant watermelon plants during plant-pathogen interactions. BMC Plant Biol. 2020, 20, 481. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Bai, L.; Sun, H.B.; Yang, C.; Cai, B.Y. Transcriptomic and Proteomic Analysis Revealed the Effect of Funneliformis mosseae in Soybean Roots Differential Expression Genes and Proteins. J. Proteome Res. 2020, 19, 9. [Google Scholar] [CrossRef]
- Yang, L.Y.; Guo, L.J.; Mao, C.; Liu, L.; Wang, F.Y.; Xie, Y.P.; Huang, J.S. Using a banana hydroponic culture system to determine the pathogenicity of Fusarium wilt pathogen. J. Plant Pathol. 2014, 44, 671–678. [Google Scholar]
- Mohamed, A.A.; Mak, C.; Liew, K.W.; Ho, Y.W. Early Evaluation of Banana Plants at Nursery Stage for Fusarium Wilt Tolerance; Food and Agriculture Organization of the United Nations: Los Banos, Philippines, 2001; pp. 174–185. [Google Scholar]
- Xu, W.; Zhang, Z.; Lei, Y.; Li, H. Determination of banana root exudates by gas chromatography-mass spectrometry. J. Trop. Crops 2010, 31, 1403–1408. [Google Scholar]
- Li, S.; Hu, M.; Gao, Z.; Yang, F. Identification of Dendrobium candidum strains and detection of amino acids in its fermentation broth. J. Trop. Biol. 2015, 6, 485–490. [Google Scholar]
- Qu, S. Determination of Eight Phenolic Acid Compounds in Plant Root Secretions by Solid Phase Extraction High Performance Liquid Chromatography. Master’s Thesis, Shandong Agricultural University, Tai’an, China, 2018. [Google Scholar]
- Wu, C.W.; Wu, X.J.; Wen, C.; Peng, B.; Peng, X.X.; Chen, X.H.; Li, H. Fructose promotes growth and antifungal activity of Penicillium citrinum. Protein Cell 2016, 7, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Park, C.; Bennett, C.; Thornton, M.; Kim, D. Rapid and accurate alignment of nucleotide conversion sequencing reads with HISAT-3N. Genome Res. 2021, 31, 1290–1295. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: Alanguage Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Ni, K.S.; Dai, M.H.; Lu, X.K.; Zhang, Y.X.; Fan, Y.P.; Xu, N.; Feng, X.X.; Huang, H.; Wang, J.; Rui, C.; et al. Pretreatment of NaCl enhances the drought resistance of cotton by regulating the biosynthesis of carotenoids and abscisic acid. Front. Environ. Sci. 2022, 10, 998141. [Google Scholar] [CrossRef]
- Dejene, W.; Yusuf, Z.; Desta, M.; Idris, M.; Seyida, S.; Dugasa, D. Physicochemical Properties, Antioxidant and Antimicrobial Activities of Unripe Green and Ripened Yellow Banana (Musa sp.) Peel and Pulp Oil Extracts. Curr. Biotechnol. 2022, 11, 140–147. [Google Scholar] [CrossRef]
- Esan, A.M.; Akeredolu, O.E.; Adeniji, A.O.; Olaiya, C.O. Comparative effects of gibberellic acid, salicylic acid and Bacillus subtilis on oxidative stress marker and antioxidant potential of Musa sapientum Linn. Arch. Phytopathol. Plant Prot. 2022, 55, 549–563. [Google Scholar] [CrossRef]
- Wang, Z.; Yao, X.M.; Jia, C.H.; Xu, B.Y.; Wang, J.Y.; Liu, J.H.; Jin, Z.Q. Identification and analysis of lignin biosynthesis genes related to fruit ripening and stress response in banana (Musa acuminata L.AAA group, cv. Cavendish). Front. Plant Sci. 2023, 14, 1072086. [Google Scholar]
- Tzeng, D.D.; DeVay, J.E. Biocidal Activity of Mixtures of Methionine and Riboflavin Against Plant Pathogenic Fungi and Bacteria and Possible Modes of Action. Mycologia 1989, 81, 404–412. [Google Scholar] [CrossRef]
- Lee, H.I.; León, J.; Raskin, I. Biosynthesis and metabolism of salicylic acid. Proc. Natl. Acad. Sci. USA 1995, 92, 4076–4079. [Google Scholar] [CrossRef]
- Jaiswal, P.; Pandey, A.; Agrawal, M.; Agrawal, S.B. Photosynthetic, Biochemical and Secondary Metabolite Changes in a Medicinal Plant Chlorophytum borivillianum (Safed musli) against Low and High Doses of UV-B Radiation. Photochem. Photobiol. 2022, 99, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Vora, S.M.; Ankati, S.; Patole, C.; Podile, A.R.; Archana, G. Alterations of Primary Metabolites in Root Exudates of Intercropped Cajanus cajan-Zea mays Modulate the Adaptation and Proteome of Ensifer (Sinorhizobium) fredii NGR234. Microb. Ecol. 2021, 83, 1008–1025. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Dai, Y.L.; Yang, X.J.; Du, Y.X.; Shi, N.N.; Ruan, H.C.; Chen, F.R. Biological effects of root exudates from resistant and susceptible banana varieties on Fusarium oxysporum f. sp. cubense and Bacillus subtilis. Ying Yong Sheng Tai Xue Bao=J. Appl. Ecol. 2020, 31, 2279–2286. [Google Scholar]
- Acin-Albiac, M.; García-Jiménez, B.; Garrido, C.M.; Casas, E.B.; Velasco-Alvarez, J.; Serra, N.S.; Acedo, A. Lettuce Soil Microbiome Modulated by an L-α-Amino Acid-Based Biostimulant. Agriculture 2023, 13, 344. [Google Scholar] [CrossRef]
- Li, X.G.; Liu, B.; Heia, S.; Liu, D.D.; Han, Z.M.; Zhou, K.X.; Cui, J.J.; Luo, J.Y.; Zheng, Y.P. The effect of root exudates from two transgenic insect-resistant cotton lines on the growth of Fusarium oxysporum. Transgenic Res. 2009, 18, 757–767. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants; Academic Press: London, UK, 1986; pp. 341–368. [Google Scholar]
- Wang, W.; Long, Y. A review of biocontrol agents in controlling late blight of potatoes and tomatoes caused by Phytophthora infestans and the underlying mechanisms. Pest Manag. Sci. 2023, 79, 4715–4725. [Google Scholar] [CrossRef]
- Usman, S.; Ge, X.W.; Xu, Y.Q.; Qin, Q.J.; Xie, J.; Wang, B.; Jin, B.; Fang, W.X. Loss of Phosphomannose Isomerase Impairs Growth, Perturbs Cell Wall Integrity, and Reduces Virulence of Fusarium oxysporum f. sp. cubense on Banana Plants. Plants. J. Fungi 2023, 9, 478. [Google Scholar] [CrossRef]
- Sivaram, K.A.; Logeshwaran, P.; Lockington, R.; Naidu, R.; Megharaj, M. The impact of low molecular weight organic acids from plants with C3 and C4 photosystems on the rhizoremediation of polycyclic aromatic hydrocarbons contaminated soil. Environ. Technol. Innov. 2020, 19, 100957. [Google Scholar] [CrossRef]
- Li, X.; Zhang, T.; Wang, X.; Hua, K.; Zhao, L.; Han, Z. The composition of root exudates from two different resistant peanut cultivars and their effects on the growth of soil-borne pathogen. Int. J. Biol. Sci. 2013, 9, 164–173. [Google Scholar] [CrossRef]
- Kaushal, M.; Mahuku, G.; Swennen, R. Comparative Transcriptome and Expression Profiling of Resistant and Susceptible Banana Cultivars during Infection by Fusarium oxysporum. Int. J. Mol. Sci. 2021, 22, 3002. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, A.; Morcillo, R.J.L.; Yu, G.; Xue, H.; Rufian, J.S.; Sang, Y.Y.; Macho, A.P. A bacterial effector protein uncovers a plant metabolic pathway involved in tolerance to bacterial wilt disease. Mol. Plant 2021, 14, 1281–1296. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.H.; Huang, B.Z.; Cheng, P.; Li, C.J.; Chen, Y.H.; Li, Y.J.; Zheng, L.; Xing, J.J.; Dong, Z.Y.; Yu, G.H. Endophytic Bacillus subtilis TR21 Improves Banana Plant Resistance to Fusarium oxysporum f. sp. cubense and Promotes Root Growth by Upregulating the Jasmonate and Brassinosteroid Biosynthesis Pathways. Phytopathology 2021, 112, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Rofiatun, R.; Febri, A.S.; Joko, T.; Nuringtyas, T.R.; Purwestri, Y.A. Phenylalanine ammonia lyase (PAL) contributes to the resistance of black rice against Xanthomonas Oryzae pv. oryzae. J. Plant Pathol. 2020, 2020, 102. [Google Scholar]
- Terrettaz, C.; Cabete, B.; Geiser, J.; Valentini, M.; Gonzalez, D. KaiC-like proteins contribute to stress resistance and biofilm formation in environmental Pseudomonas species. Environ. Microbiol. 2022, 25, 894–913. [Google Scholar] [CrossRef]
- Raouani, N.E.H.; Claverie, E.; Randoux, B.; Chaveriat, L.; Yaseen, Y.; Yada, B.; Martin, P.; Cabrera, J.C.; Jacques, P.; Reignault, P.; et al. Bio-Inspired Rhamnolipids, Cyclic Lipopeptides and a Chito-Oligosaccharide Confer Protection against Wheat Powdery Mildew and Inhibit Conidia Germination. Molecules 2022, 27, 6672. [Google Scholar] [CrossRef]
- Pramanik, M.H.R.; Imai, R. Functional identification of a trehalose 6-phosphate phosphatase gene that is involved in transient induction of trehalose biosynthesis during chilling stress in rice. Plant Mol. Biol. 2005, 58, 751–762. [Google Scholar] [CrossRef]
- Simões, M.S.; Carvalho, G.G.; Ferreira, S.S.; Cesarino, I. Toward the identification of class III peroxidases potentially involved in lignification in the model C4 grass Setaria viridis. Theor. Exp. Plant Physiol. 2023, 35, 111–131. [Google Scholar] [CrossRef]
- Muhammad, I.; Jing, X.Q.; Shalmani, A.; Ali Muhammad Yi, S.; Gan, P.F.; Li, W.Q.; Liu, W.T.; Chen, K.M. Comparative in Silico Analysis of Ferric Reduction Oxidase (FRO) Genes Expression Patterns in Response to Abiotic Stresses, Metal and Hormone Applications. Molecules 2018, 23, 1163. [Google Scholar] [CrossRef]
- Sun, X.Z.; Zhang, C.C.; Bei, S.K.; Wang, G.Z.; Geisen, S.; Bedoussac, L.; Christie, P.; Zhang, J.L. High bacterial diversity and siderophore-producing bacteria collectively suppress Fusarium oxysporum in maize/faba bean intercropping. Front. Microbiol. 2022, 13, 972587. [Google Scholar] [CrossRef]
- Wang, H.; Ma, F.; Cheng, L. Metabolism of organic acids, nitrogen and amino acids in chlorotic leaves of ‘Honeycrisp’ apple (Malus domestica Borkh) with excessive accumulation of carbohydrates. Planta 2010, 232, 511–522. [Google Scholar] [CrossRef]
- Zhu, H.H.; Ai, H.L.; Hu, Z.R.; Du, D.Y.; Sun, J.; Chen, K.; Chen, L. Comparative transcriptome com-bined with metabolome analyses revealed key factors involved in nitric oxide (NO)-regulated cadmium stress adaptation in tall fescue. BMC Genom. 2020, 21, 601. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xiang, Z.P.; Liu, M.; Wang, S.Y.; Zhang, L.; Cai, D.; Huang, Y.; Mao, D.D.; Fu, J.; Chen, L.B. ABA biosynthesis gene OsNCED3 contributes to preharvest sprouting resistance and grain development in rice. Plant Cell Environ. 2023, 46, 1384–1401. [Google Scholar] [CrossRef] [PubMed]
- Grover, A. Plant Chitinases: Genetic Diversity and Physiological Roles. Crit. Rev. Plant Sci. 2012, 31, 57–73. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Wang, J.; Tian, G.; Liang, C.; Zhou, Y.; Guo, L.; Yang, Y.; Yang, L. Bacillus velezensis Isolate X5 Stimulates the Resistance of Resistant and Susceptible Banana Varieties to Foc Through Different Mechanisms. J. Fungi 2025, 11, 379. https://doi.org/10.3390/jof11050379
Xu Y, Wang J, Tian G, Liang C, Zhou Y, Guo L, Yang Y, Yang L. Bacillus velezensis Isolate X5 Stimulates the Resistance of Resistant and Susceptible Banana Varieties to Foc Through Different Mechanisms. Journal of Fungi. 2025; 11(5):379. https://doi.org/10.3390/jof11050379
Chicago/Turabian StyleXu, Yunlong, Jun Wang, Guangxiang Tian, Changcong Liang, You Zhou, Lijia Guo, Yang Yang, and Laying Yang. 2025. "Bacillus velezensis Isolate X5 Stimulates the Resistance of Resistant and Susceptible Banana Varieties to Foc Through Different Mechanisms" Journal of Fungi 11, no. 5: 379. https://doi.org/10.3390/jof11050379
APA StyleXu, Y., Wang, J., Tian, G., Liang, C., Zhou, Y., Guo, L., Yang, Y., & Yang, L. (2025). Bacillus velezensis Isolate X5 Stimulates the Resistance of Resistant and Susceptible Banana Varieties to Foc Through Different Mechanisms. Journal of Fungi, 11(5), 379. https://doi.org/10.3390/jof11050379




