Silencing of MNT1 and PMT2 Shows the Importance of O-Linked Glycosylation During the Sporothrix schenckii–Host Interaction
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms, Strains, and Culture Conditions
2.2. PMT2 and MNT1 Silencing
2.3. Analysis of Gene Expression and Insertional Events Within the Fungal Genome by qRT-PCR
2.4. Growth Curves and Microscopic Analysis of MNT1 and PMT2 Mutants
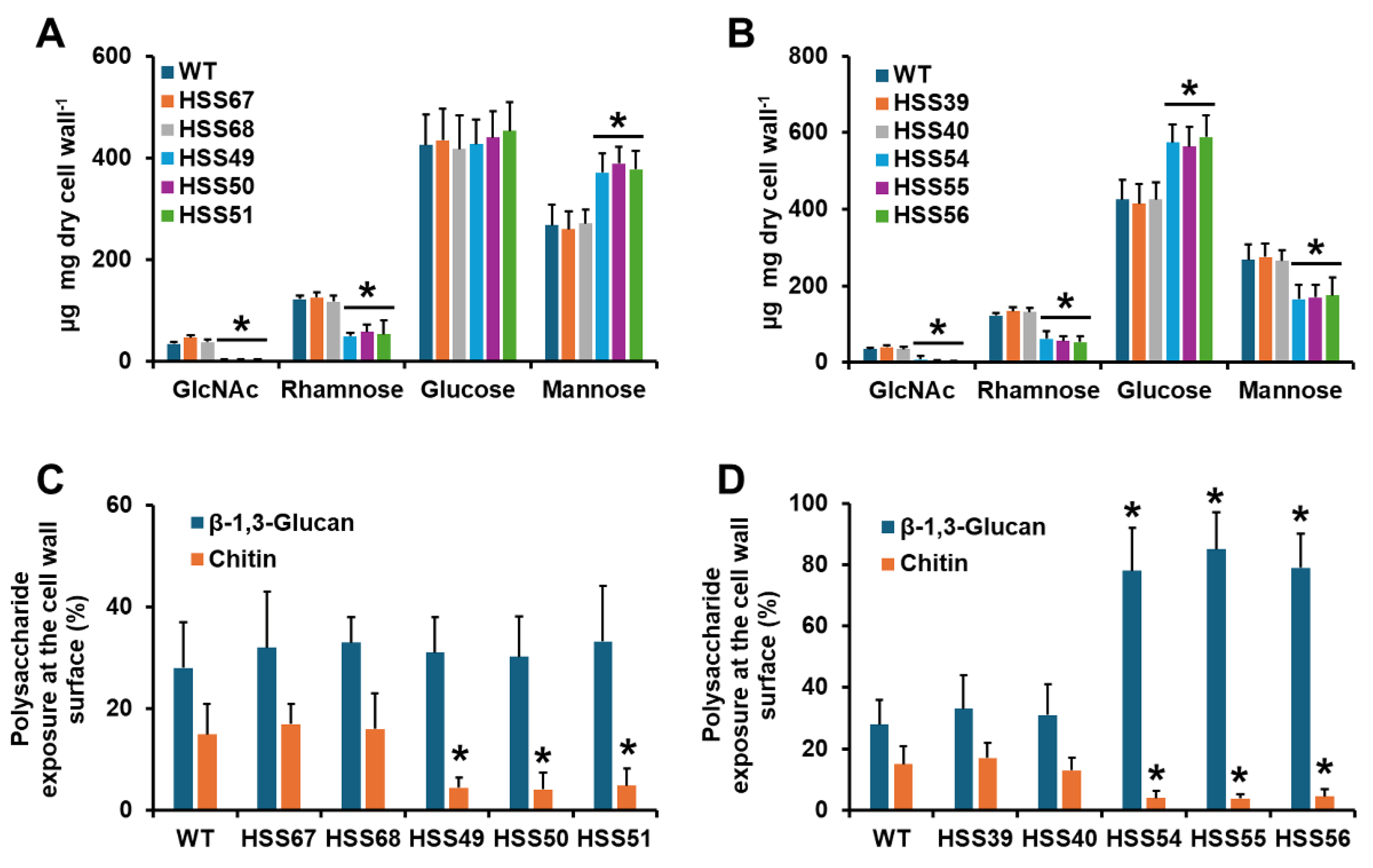
2.5. Cell Wall Analysis
2.6. Alcian Blue Binding Assay
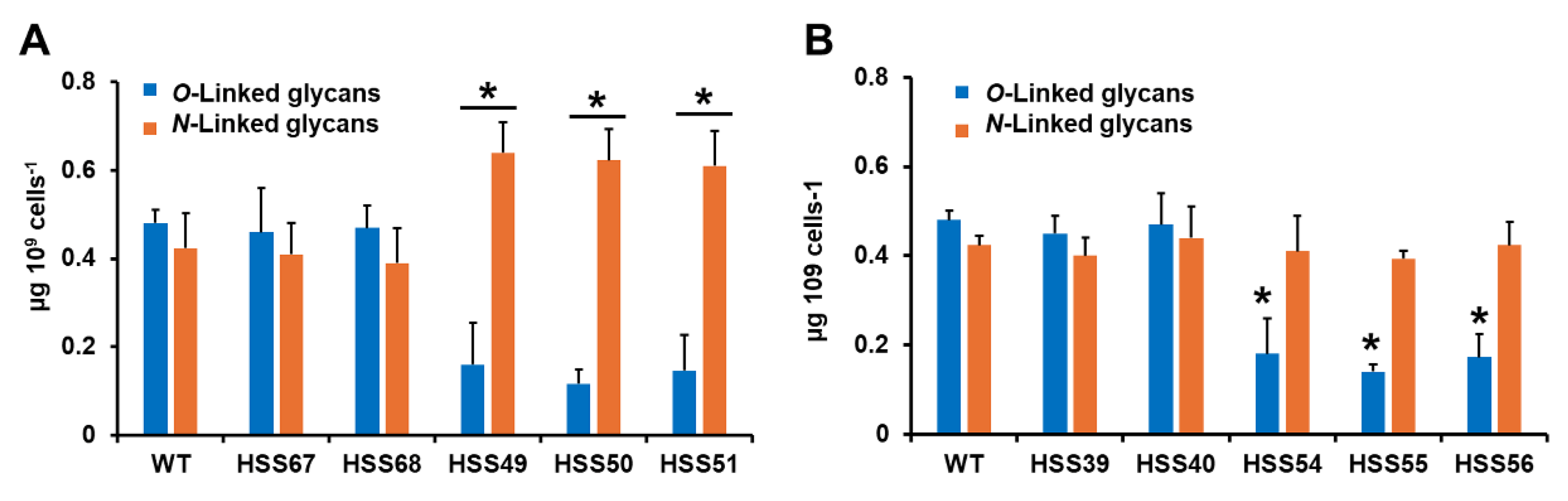
2.7. Analysis of N-Linked and O-Linked Glycans
2.8. Adhesion Assays
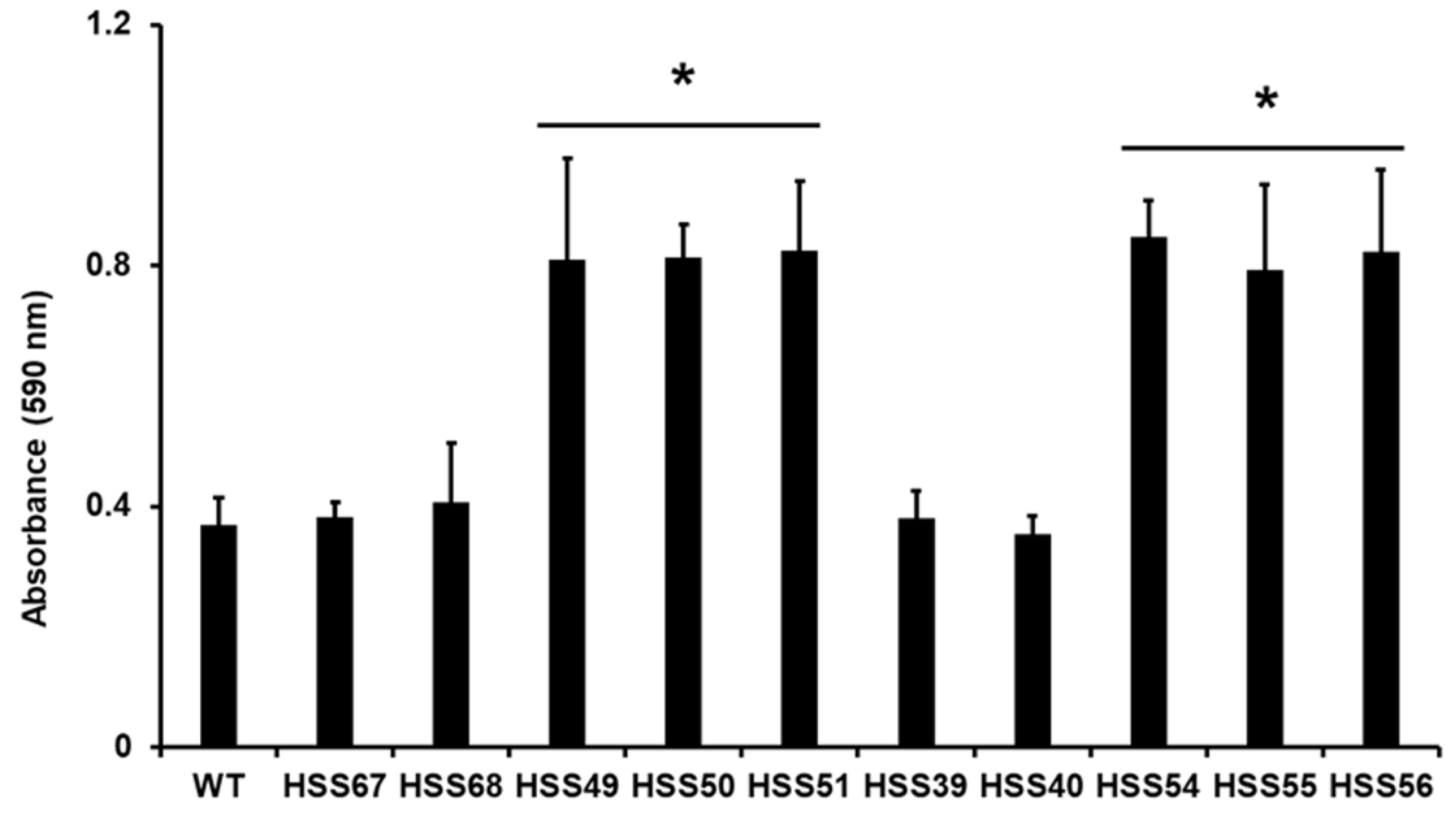
2.9. Biofilm Formation
2.10. Protease and Lipase Activity
2.11. Ethical Considerations
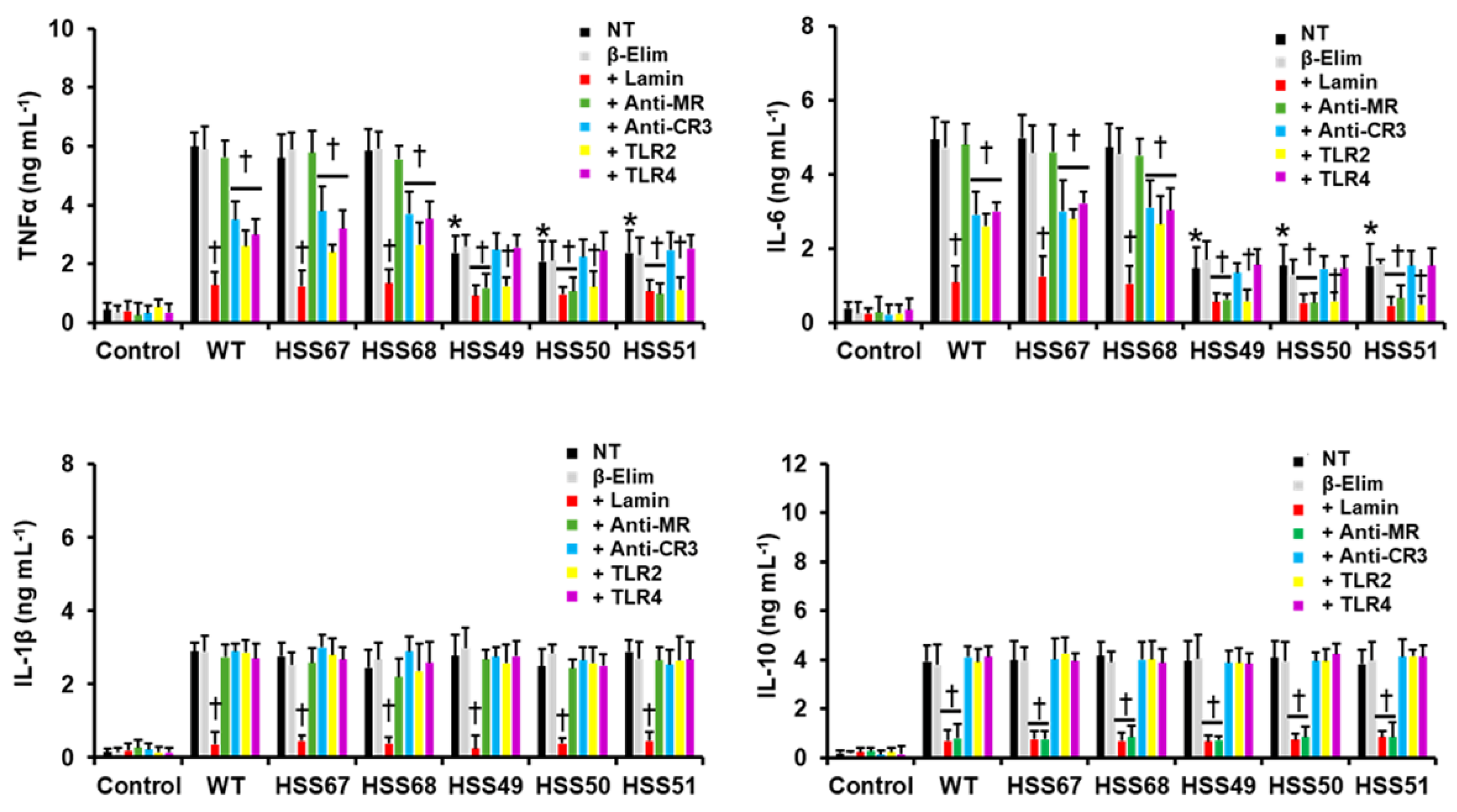
2.12. Human Peripheral Blood Mononuclear Cells Isolation and Cytokine Stimulation
2.13. Phagocytosis by Human Monocyte-Derived Macrophages
2.14. Analysis of Neutrophils’ Extracellular Traps
2.15. Analysis of Virulence
2.16. Statistical Analyses
3. Results
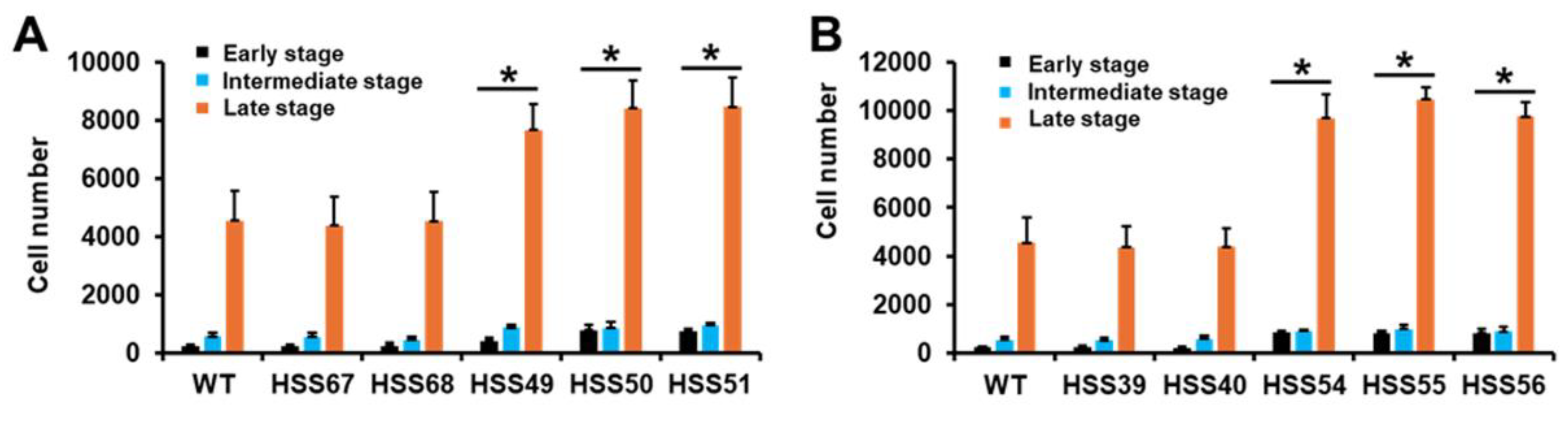
3.1. Silencing of Sporothrix schenckii MNT1 and PMT2 and Morphology Abnormalities
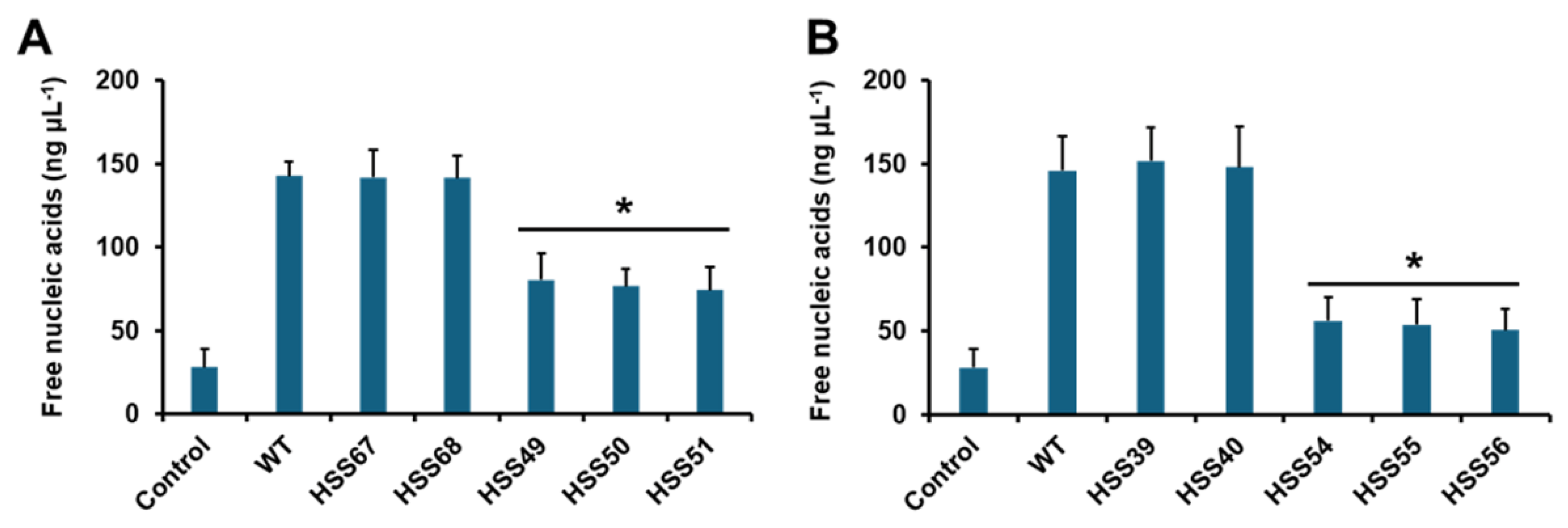
3.2. Silencing of Sporothrix schenckii MNT1 or PMT2 Affected the Cell Wall Composition and Protein Glycosylation
3.3. Silencing of Sporothrix schenckii MNT1 or PMT2 Affected Cell Adhesion, Biofilm Formation, and Secreted Protease and Lipase
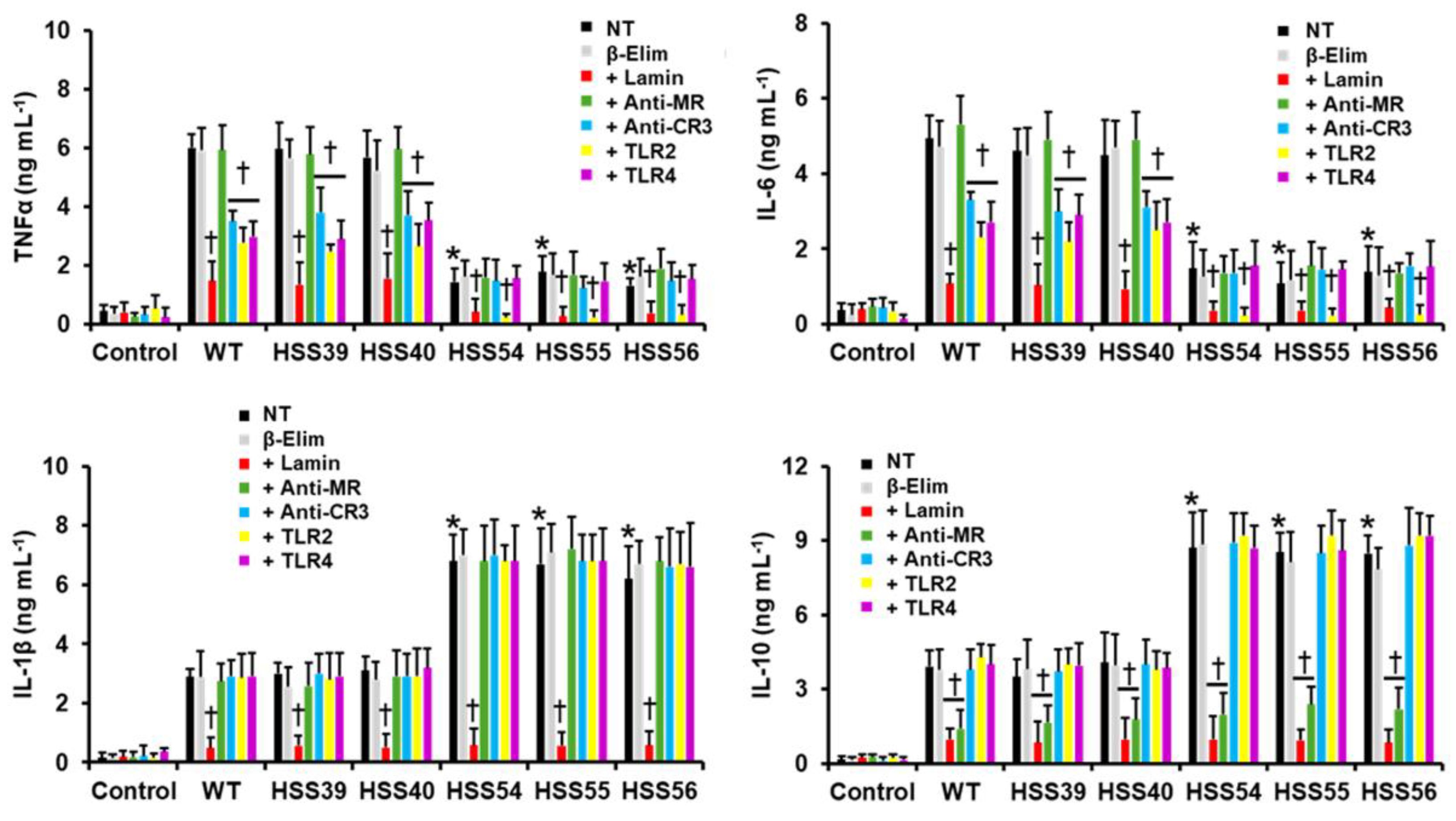
3.4. Human Innate Immune Cell–Sporothrix schenckii Interaction Is Affected by MNT1 or PMT2 Silencing
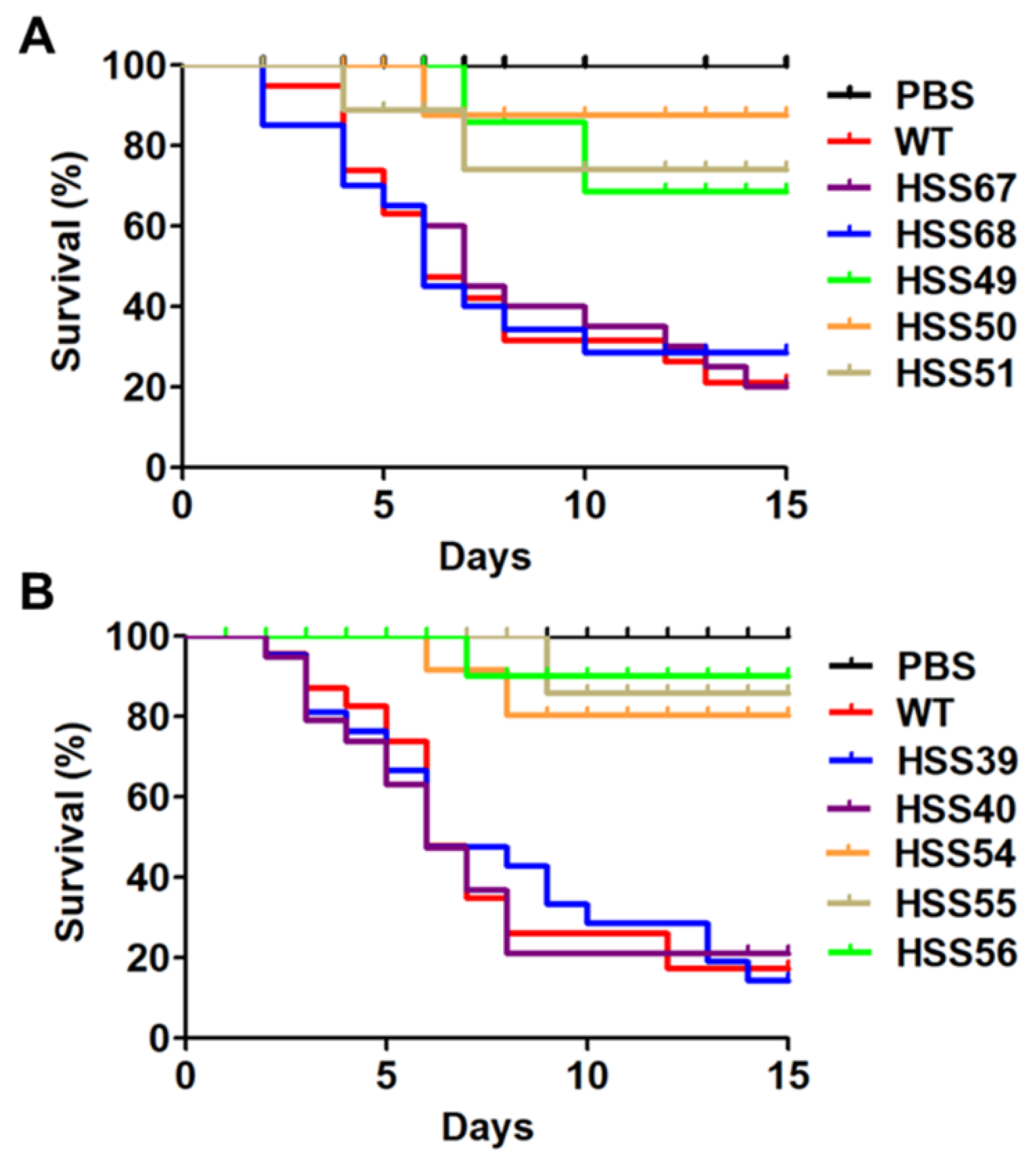
3.5. Virulence Is Attenuated in the Sporothrix schenckii MNT1- and PMT2-Silenced Mutans
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodrigues, A.M.; de Hoog, S.; de Camargo, Z.P. Emergence of pathogenicity in the Sporothrix schenckii complex. Med. Mycol. 2013, 51, 405–412. [Google Scholar] [CrossRef]
- Queiroz-Telles, F.; Fahal, A.H.; Falci, D.R.; Caceres, D.H.; Chiller, T.; Pasqualotto, A.C. Neglected endemic mycoses. Lancet Infect. Dis. 2017, 17, e367–e377. [Google Scholar] [CrossRef] [PubMed]
- de Lima Barros, M.B.; Schubach, A.O.; de-Vasconcellos Carvalhaes De-Oliveira, R.; Martins, E.B.; Teixeira, J.L.; Wanke, B. Treatment of cutaneous sporotrichosis with Itraconazole—Study of 645 patients. Clin. Infect. Dis. 2011, 52, e200–e206. [Google Scholar] [CrossRef]
- Rangel-Gamboa, L.; Martínez-Hernandez, F.; Maravilla, P.; Arenas-Guzmán, R.; Flisser, A. Update of phylogenetic and genetic diversity of Sporothrix schenckii sensu lato. Med. Mycol. 2016, 54, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Bezerra, L.M.; Walker, L.A.; Niño-Vega, G.; Mora-Montes, H.M.; Neves, G.W.P.; Villalobos-Duno, H.; Barreto, L.; Garcia, K.; Franco, B.; Martínez-Álvarez, J.A.; et al. Cell walls of the dimorphic fungal pathogens Sporothrix schenckii and Sporothrix brasiliensis exhibit bilaminate structures and sloughing of extensive and intact layers. PLoS Negl. Trop. Dis. 2018, 12, e0006169. [Google Scholar] [CrossRef]
- Gómez-Gaviria, M.; Vargas-Macías, A.P.; García-Carnero, L.C.; Martínez-Duncker, I.; Mora-Montes, H.M. Role of Protein Glycosylation in Interactions of Medically Relevant Fungi with the Host. J. Fungi 2021, 7, 875. [Google Scholar] [CrossRef]
- Diaz-Jimenez, D.F.; Mora-Montes, H.M.; Hernandez-Cervantes, A.; Luna-Arias, J.P.; Gow, N.A.; Flores-Carreon, A. Biochemical characterization of recombinant Candida albicans mannosyltransferases Mnt1, Mnt2 and Mnt5 reveals new functions in O- and N-mannan biosynthesis. Biochem. Biophys. Res. Commun. 2012, 419, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Goto, M. Protein O-glycosylation in fungi: Diverse structures and multiple functions. Biosci. Biotechnol. Biochem. 2007, 71, 1415–1427. [Google Scholar] [CrossRef]
- Lommel, M.; Strahl, S. Protein O-mannosylation: Conserved from bacteria to humans. Glycobiology 2009, 19, 816–828. [Google Scholar] [CrossRef]
- Martínez-Duncker, I.; Díaz-Jímenez, D.F.; Mora-Montes, H.M. Comparative analysis of protein glycosylation pathways in humans and the fungal pathogen Candida albicans. Int. J. Microbiol. 2014, 2014, 267497. [Google Scholar] [CrossRef]
- Munro, C.A.; Bates, S.; Buurman, E.T.; Hughes, H.B.; Maccallum, D.M.; Bertram, G.; Atrih, A.; Ferguson, M.A.; Bain, J.M.; Brand, A.; et al. Mnt1p and Mnt2p of Candida albicans are partially redundant alpha-1,2-mannosyltransferases that participate in O-linked mannosylation and are required for adhesion and virulence. J. Biol. Chem. 2005, 280, 1051–1060. [Google Scholar] [CrossRef]
- Gentzsch, M.; Tanner, W. Protein-O-glycosylation in yeast: Protein-specific mannosyltransferases. Glycobiology 1997, 7, 481–486. [Google Scholar] [CrossRef]
- Timpel, C.; Strahl-Bolsinger, S.; Ziegelbauer, K.; Ernst, J.F. Multiple functions of Pmt1p-mediated protein O-mannosylation in the fungal pathogen Candida albicans. J. Biol. Chem. 1998, 273, 20837–20846. [Google Scholar] [CrossRef]
- Leitao, E.A.; Bittencourt, V.C.; Haido, R.M.; Valente, A.P.; Peter-Katalinic, J.; Letzel, M.; de Souza, L.M.; Barreto-Bergter, E. Beta-galactofuranose-containing O-linked oligosaccharides present in the cell wall peptidogalactomannan of Aspergillus fumigatus contain immunodominant epitopes. Glycobiology 2003, 13, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Prill, S.K.; Klinkert, B.; Timpel, C.; Gale, C.A.; Schröppel, K.; Ernst, J.F. PMT family of Candida albicans: Five protein mannosyltransferase isoforms affect growth, morphogenesis and antifungal resistance. Mol. Microbiol. 2005, 55, 546–560. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Hu, H.; Zhang, L.; Li, R.; Ouyang, H.; Ming, J.; Jin, C. O-Mannosyltransferase 1 in Aspergillus fumigatus (AfPmt1p) is crucial for cell wall integrity and conidium morphology, especially at an elevated temperature. Eukaryot. Cell 2007, 6, 2260–2268. [Google Scholar] [CrossRef]
- Hernández-Chávez, M.J.; Martínez-Duncker, I.; Clavijo-Giraldo, D.M.; López-Ramirez, L.A.; Mora-Montes, H.M. Candida tropicalis PMT2 Is a dispensable gene for viability but required for proper interaction with the host. J. Fungi 2024, 10, 502. [Google Scholar] [CrossRef] [PubMed]
- Jin, C. Protein glycosylation in Aspergillus fumigatus is essential for cell wall synthesis and serves as a promising model of multicellular eukaryotic development. Int. J. Microbiol. 2012, 2012, 654251. [Google Scholar] [CrossRef]
- Lee, D.J.; Bahn, Y.S.; Kim, H.J.; Chung, S.Y.; Kang, H.A. Unraveling the novel structure and biosynthetic pathway of O-linked glycans in the Golgi apparatus of the human pathogenic yeast Cryptococcus neoformans. J. Biol. Chem. 2015, 290, 1861–1873. [Google Scholar] [CrossRef]
- Wagener, J.; Echtenacher, B.; Rohde, M.; Kotz, A.; Krappmann, S.; Heesemann, J.; Ebel, F. The putative alpha-1,2-mannosyltransferase AfMnt1 of the opportunistic fungal pathogen Aspergillus fumigatus is required for cell wall stability and full virulence. Eukaryot. Cell 2008, 7, 1661–1673. [Google Scholar] [CrossRef]
- Kadooka, C.; Hira, D.; Tanaka, Y.; Chihara, Y.; Goto, M.; Oka, T. Mnt1, an α-(1 → 2)-mannosyltransferase responsible for the elongation of N-glycans and O-glycans in Aspergillus fumigatus. Glycobiology 2022, 32, 1137–1152. [Google Scholar] [CrossRef] [PubMed]
- Mora-Montes, H.M.; Bates, S.; Netea, M.G.; Castillo, L.; Brand, A.; Buurman, E.T.; Diaz-Jimenez, D.F.; Jan Kullberg, B.; Brown, A.J.; Odds, F.C.; et al. A multifunctional mannosyltransferase family in Candida albicans determines cell wall mannan structure and host-fungus interactions. J. Biol. Chem. 2010, 285, 12087–12095. [Google Scholar] [CrossRef]
- López-Ramírez, L.A.; Martínez-Duncker, I.; Márquez-Márquez, A.; Vargas-Macías, A.P.; Mora-Montes, H.M. Silencing of ROT2, the encoding gene of the endoplasmic reticulum glucosidase II, affects the cell wall and the Sporothrix schenckii-host interaction. J. Fungi 2022, 8, 1220. [Google Scholar] [CrossRef] [PubMed]
- Lozoya-Pérez, N.E.; Casas-Flores, S.; de Almeida, J.R.F.; Martínez-Álvarez, J.A.; López-Ramírez, L.A.; Jannuzzi, G.P.; Trujillo-Esquivel, E.; Estrada-Mata, E.; Almeida, S.R.; Franco, B.; et al. Silencing of OCH1 unveils the role of Sporothrix schenckii N-linked glycans during the host-fungus interaction. Infect. Drug Resist. 2019, 12, 67–85. [Google Scholar] [CrossRef]
- Lopes-Bezerra, L.M. Sporothrix schenckii cell wall peptidorhamnomannans. Front. Microbiol. 2011, 2, 243. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Wakabayashi, K.; Nisizawa, K. Xylanase activity of an endo-cellulase of carboxymethyl-cellulase type from Irpex lacteus (Polyporus tulipiferae). J. Biochem. 1976, 79, 989–995. [Google Scholar] [CrossRef]
- Bauer, W.D.; Talmadge, K.W.; Keegstra, K.; Albersheim, P. The structure of plant cell walls: II. the hemicellulose of the walls of suspension-cultured Sycamore cells. Plant Physiol. 1973, 51, 174–187. [Google Scholar] [CrossRef]
- Nakayashiki, H.; Hanada, S.; Nguyen, B.Q.; Kadotani, N.; Tosa, Y.; Mayama, S. RNA silencing as a tool for exploring gene function in ascomycete fungi. Fungal Genet. Biol. 2005, 42, 275–283. [Google Scholar] [CrossRef]
- Tamez-Castrellón, A.K.; Romo-Lucio, R.; Martínez-Duncker, I.; Mora-Montes, H.M. Generation of a synthetic binary plasmid that confers resistance to nourseothricin for genetic engineering of Sporothrix schenckii. Plasmid 2018, 100, 1–5. [Google Scholar] [CrossRef]
- Lozoya-Pérez, N.E.; Casas-Flores, S.; Martínez-Álvarez, J.A.; López-Ramírez, L.A.; Lopes-Bezerra, L.M.; Franco, B.; Mora-Montes, H.M. Generation of Sporothrix schenckii mutants expressing the green fluorescent protein suitable for the study of host-fungus interactions. Fungal Biol. 2018, 122, 1023–1030. [Google Scholar] [CrossRef]
- Tamez-Castrellón, A.K.; van der Beek, S.L.; López-Ramírez, L.A.; Martínez-Duncker, I.; Lozoya-Pérez, N.E.; van Sorge, N.M.; Mora-Montes, H.M. Disruption of protein rhamnosylation affects the Sporothrix schenckii-host interaction. Cell Surf. 2021, 7, 100058. [Google Scholar] [CrossRef] [PubMed]
- Trujillo-Esquivel, E.; Franco, B.; Flores-Martínez, A.; Ponce-Noyola, P.; Mora-Montes, H.M. Purification of single-stranded cDNA based on RNA degradation treatment and adsorption chromatography. Nucleosides Nucleotides Nucleic Acids 2016, 35, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Trujillo-Esquivel, E.; Martínez-Álvarez, J.A.; Clavijo-Giraldo, D.M.; Hernández, N.V.; Flores-Martínez, A.; Ponce-Noyola, P.; Mora-Montes, H.M. The Sporothrix schenckii gene encoding for the ribosomal protein L6 has constitutive and stable expression and works as an endogenous control in gene expression analysis. Front. Microbiol. 2017, 8, 1676. [Google Scholar] [CrossRef] [PubMed]
- Mora-Montes, H.M.; Bates, S.; Netea, M.G.; Diaz-Jimenez, D.F.; Lopez-Romero, E.; Zinker, S.; Ponce-Noyola, P.; Kullberg, B.J.; Brown, A.J.; Odds, F.C.; et al. Endoplasmic reticulum alpha-glycosidases of Candida albicans are required for N glycosylation, cell wall integrity, and normal host-fungus interaction. Eukaryot. Cell 2007, 6, 2184–2193. [Google Scholar] [CrossRef]
- Martínez-Álvarez, J.A.; Pérez-García, L.A.; Mellado-Mojica, E.; López, M.G.; Martínez-Duncker, I.; Lópes-Bezerra, L.M.; Mora-Montes, H.M. Sporothrix schenckii sensu stricto and Sporothrix brasiliensis are differentially recognized by human peripheral blood mononuclear cells. Front. Microbiol. 2017, 8, 843. [Google Scholar] [CrossRef]
- Marakalala, M.J.; Vautier, S.; Potrykus, J.; Walker, L.A.; Shepardson, K.M.; Hopke, A.; Mora-Montes, H.M.; Kerrigan, A.; Netea, M.G.; Murray, G.I.; et al. Differential adaptation of Candida albicans in vivo modulates immune recognition by dectin-1. PLoS Pathog. 2013, 9, e1003315. [Google Scholar] [CrossRef]
- Mora-Montes, H.M.; Netea, M.G.; Ferwerda, G.; Lenardon, M.D.; Brown, G.D.; Mistry, A.R.; Kullberg, B.J.; O’Callaghan, C.A.; Sheth, C.C.; Odds, F.C.; et al. Recognition and blocking of innate immunity cells by Candida albicans chitin. Infect. Immun. 2011, 79, 1961–1970. [Google Scholar] [CrossRef]
- Hobson, R.P.; Munro, C.A.; Bates, S.; MacCallum, D.M.; Cutler, J.E.; Heinsbroek, S.E.; Brown, G.D.; Odds, F.C.; Gow, N.A. Loss of cell wall mannosylphosphate in Candida albicans does not influence macrophage recognition. J. Biol. Chem. 2004, 279, 39628–39635. [Google Scholar] [CrossRef]
- Navarro-Arias, M.J.; Defosse, T.A.; Dementhon, K.; Csonka, K.; Mellado-Mojica, E.; Dias Valério, A.; González-Hernández, R.J.; Courdavault, V.; Clastre, M.; Hernández, N.V.; et al. Disruption of protein mannosylation affects Candida guilliermondii cell wall, immune sensing, and virulence. Front. Microbiol. 2016, 7, 1951. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Mora-Montes, H.M.; McKenzie, C.; Bain, J.M.; Lewis, L.E.; Erwig, L.P.; Gow, N.A. Interactions between macrophages and cell wall oligosaccharides of Candida albicans. Methods Mol. Biol. 2012, 845, 247–260. [Google Scholar]
- Lima, O.C.; Figueiredo, C.C.; Pereira, B.A.; Coelho, M.G.; Morandi, V.; Lopes-Bezerra, L.M. Adhesion of the human pathogen Sporothrix schenckii to several extracellular matrix proteins. Braz. J. Med. Biol. Res. 1999, 32, 651–657. [Google Scholar] [CrossRef]
- García-Carnero, L.C.; Salinas-Marín, R.; Lozoya-Pérez, N.E.; Wrobel, K.; Wrobel, K.; Martínez-Duncker, I.; Niño-Vega, G.A.; Mora-Montes, H.M. The Heat shock protein 60 and Pap1 participate in the Sporothrix schenckii-host interaction. J. Fungi 2021, 7, 960. [Google Scholar] [CrossRef] [PubMed]
- Clavijo-Giraldo, D.M.; Pérez-García, L.A.; Hernández-Chávez, M.J.; Martínez-Duncker, I.; Mora-Montes, H.M. Contribution of N-linked mannosylation pathway to Candida parapsilosis and Candida tropicalis biofilm formation. Infect. Drug Resist. 2023, 16, 6843–6857. [Google Scholar] [CrossRef]
- Smolenski, G.; Sullivan, P.A.; Cutfield, S.M.; Cutfield, J.F. Analysis of secreted aspartic proteinases from Candida albicans: Purification and characterization of individual Sap1, Sap2 and Sap3 isoenzymes. Microbiology 1997, 143, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Mora-Montes, H.M.; López-Romero, E.; Zinker, S.; Ponce-Noyola, P.; Flores-Carreón, A. Hydrolysis of Man9GlcNAc2 and Man8GlcNAc2 oligosaccharides by a purified alpha-mannosidase from Candida albicans. Glycobiology 2004, 14, 593–598. [Google Scholar] [CrossRef]
- Endres, S.; Ghorbani, R.; Lonnemann, G.; van der Meer, J.W.; Dinarello, C.A. Measurement of immunoreactive interleukin-1 beta from human mononuclear cells: Optimization of recovery, intrasubject consistency, and comparison with interleukin-1 alpha and tumor necrosis factor. Clin. Immunol. Immunopathol. 1988, 49, 424–438. [Google Scholar] [CrossRef]
- Gómez-Gaviria, M.; Martínez-Duncker, I.; García-Carnero, L.C.; Mora-Montes, H.M. Differential recognition of Sporothrix schenckii, Sporothrix brasiliensis, and Sporothrix globosa by human monocyte-derived macrophages and dendritic cells. Infect. Drug Resist. 2023, 16, 4817–4834. [Google Scholar] [CrossRef]
- Schwartz, S.N.; Medoff, G.; Kobayashi, G.S.; Kwan, C.N.; Schlessinger, D. Antifungal properties of polymyxin B and its potentiation of tetracycline as an antifungal agent. Antimicrob. Agents Chemother. 1972, 2, 36–40. [Google Scholar] [CrossRef]
- Neves, G.W.P.; Wong, S.S.W.; Aimanianda, V.; Simenel, C.; Guijarro, J.I.; Walls, C.; Willment, J.A.; Gow, N.A.R.; Munro, C.A.; Brown, G.D.; et al. Complement-Mediated Differential Immune Response of Human Macrophages to Sporothrix Species Through Interaction With Their Cell Wall Peptidorhamnomannans. Front. Immunol. 2021, 12, 749074. [Google Scholar] [CrossRef] [PubMed]
- Perez-Garcia, L.A.; Csonka, K.; Flores-Carreon, A.; Estrada-Mata, E.; Mellado-Mojica, E.; Nemeth, T.; Lopez-Ramirez, L.A.; Toth, R.; Lopez, M.G.; Vizler, C.; et al. Role of protein glycosylation in Candida parapsilosis cell wall integrity and host interaction. Front. Microbiol. 2016, 7, 306. [Google Scholar] [CrossRef]
- Hernández-Chávez, M.J.; Franco, B.; Clavijo-Giraldo, D.M.; Hernández, N.V.; Estrada-Mata, E.; Mora-Montes, H.M. Role of protein phosphomannosylation in the Candida tropicalis–macrophage interaction. FEMS Yeast Res. 2018, 18, foy053. [Google Scholar] [CrossRef]
- Galván-Hernández, A.K.; Gómez-Gaviria, M.; Martínez-Duncker, I.; Martínez-Álvarez, J.A.; Mora-Montes, H.M. Differential recognition of clinically relevant Sporothrix species by human granulocytes. J. Fungi 2023, 9, 986. [Google Scholar] [CrossRef]
- Clavijo-Giraldo, D.M.; Matinez-Alvarez, J.A.; Lopes-Bezerra, L.M.; Ponce-Noyola, P.; Franco, B.; Almeida, R.S.; Mora-Montes, H.M. Analysis of Sporothrix schenckii sensu stricto and Sporothrix brasiliensis virulence in Galleria mellonella. J. Microbiol. Methods 2016, 122, 73–77. [Google Scholar] [CrossRef]
- García-Carnero, L.C.; Clavijo-Giraldo, D.M.; Gómez-Gaviria, M.; Lozoya-Pérez, N.E.; Tamez-Castrellón, A.K.; López-Ramírez, L.A.; Mora-Montes, H.M. Early virulence predictors during the Candida species-Galleria mellonella Interaction. J. Fungi 2020, 6, 152. [Google Scholar] [CrossRef] [PubMed]
- Lozoya-Pérez, N.E.; Clavijo-Giraldo, D.M.; Martínez-Duncker, I.; García-Carnero, L.C.; López-Ramírez, L.A.; Niño-Vega, G.A.; Mora-Montes, H.M. Influences of the culturing media in the virulence and cell wall of Sporothrix schenckii, Sporothrix brasiliensis, and Sporothrix globosa. J. Fungi 2020, 6, 323. [Google Scholar] [CrossRef]
- Hernández-Cervantes, A.; Mora-Montes, H.M.; Álvarez-Vargas, A.; Jiménez, D.F.D.; Robledo-Ortiz, C.I.; Flores-Carreón, A. Isolation of Sporothrix schenckii MNT1 and the biochemical and functional characterization of the encoded α1,2-mannosyltransferase activity. Microbiology 2012, 158, 2419–2427. [Google Scholar] [CrossRef] [PubMed]
- López-Ramírez, L.A.; Hernández, N.V.; Lozoya-Pérez, N.E.; Lopes-Bezerra, L.M.; Mora-Montes, H.M. Functional characterization of the Sporothrix schenckii Ktr4 and Ktr5, mannosyltransferases involved in the N-linked glycosylation pathway. Res. Microbiol. 2018, 169, 188–197. [Google Scholar] [CrossRef]
- Ferreira, B.H.; Ramírez-Prado, J.H.; Neves, G.W.P.; Torrado, E.; Sampaio, P.; Felipe, M.S.S.; Vasconcelos, A.T.; Goldman, G.H.; Carvalho, A.; Cunha, C.; et al. Ploidy determination in the pathogenic fungus Sporothrix spp. Front. Microbiol. 2019, 10, 284. [Google Scholar] [CrossRef]
- Lopes-Bezerra, L.M.; Mora-Montes, H.M.; Zhang, Y.; Nino-Vega, G.; Rodrigues, A.M.; de Camargo, Z.P.; de Hoog, S. Sporotrichosis between 1898 and 2017: The evolution of knowledge on a changeable disease and on emerging etiological agents. Med. Mycol. 2018, 56, S126–S143. [Google Scholar] [CrossRef]
- Tamez-Castrellón, A.K.; Romeo, O.; García-Carnero, L.C.; Lozoya-Pérez, N.E.; Mora-Montes, H.M. Virulence factors in Sporothrix schenckii, one of the causative agents of sporotrichosis. Curr. Protein Pept. Sci. 2020, 21, 295–312. [Google Scholar] [CrossRef]
- Lima, O.C.; Figueiredo, C.C.; Previato, J.O.; Mendonça-Previato, L.; Morandi, V.; Lopes Bezerra, L.M. Involvement of fungal cell wall components in adhesion of Sporothrix schenckii to human fibronectin. Infect. Immun. 2001, 69, 6874–6880. [Google Scholar] [CrossRef] [PubMed]
- López-Ramírez, L.A.; Martínez-Álvarez, J.A.; Martínez-Duncker, I.; Lozoya-Pérez, N.E.; Mora-Montes, H.M. Silencing of Sporothrix schenckii GP70 reveals its contribution to fungal adhesion, virulence, and the host-fungus interaction. J. Fungi 2024, 10, 302. [Google Scholar] [CrossRef]
- Pierce, C.G.; Thomas, D.P.; López-Ribot, J.L. Effect of tunicamycin on Candida albicans biofilm formation and maintenance. J. Antimicrob. Chemother. 2009, 63, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Peeters, E.; Nelis, H.J.; Coenye, T. Comparison of multiple methods for quantification of microbial biofilms grown in microtiter plates. J. Microbiol. Methods 2008, 72, 157–165. [Google Scholar] [CrossRef] [PubMed]
- García-Carnero, L.C.; Martínez-Duncker, I.; Gómez-Gaviria, M.; Mora-Montes, H.M. Differential recognition of clinically relevant Sporothrix species by human mononuclear cells. J. Fungi 2023, 9, 448. [Google Scholar] [CrossRef]
- White, P.C.; Chicca, I.J.; Ling, M.R.; Wright, H.J.; Cooper, P.R.; Milward, M.R.; Chapple, I.L. Characterization, quantification, and visualization of neutrophil extracellular traps. Methods Mol. Biol. 2017, 1537, 481–497. [Google Scholar] [CrossRef]
- Reis, N.F.; de Jesus, M.C.S.; de Souza, L.C.d.S.V.; Alcântara, L.M.; Rodrigues, J.A.d.C.; Brito, S.C.P.; Penna, P.d.A.; Vieira, C.S.; Silva, J.R.S.; Penna, B.d.A.; et al. Sporothrix brasiliensis infection modulates antimicrobial peptides and stress management gene expression in the invertebrate biomodel Galleria mellonella. J. Fungi 2023, 9, 1053. [Google Scholar] [CrossRef]
- Aroonvuthiphong, V.; Bangphoomi, N. Therapeutic alternatives for sporotrichosis induced by wild-type and non-wild-type Sporothrix schenckii through in vitro and in vivo assessment of enilconazole, isavuconazole, posaconazole, and terbinafine. Sci. Rep. 2025, 15, 3230. [Google Scholar] [CrossRef]
- Li, S.; Tang, Z.; Liu, Z.; Lv, S.; Yao, C.; Wang, S.; Li, F. Antifungal activity of indolicidin-derived peptide In-58 against Sporothrix globosa in vitro and in vivo. Front. Med. 2024, 11, 1458951. [Google Scholar] [CrossRef] [PubMed]
- Borba-Santos, L.P.; Barreto, T.L.; Vila, T.; Chi, K.D.; Dos Santos Monti, F.; de Farias, M.R.; Alviano, D.S.; Alviano, C.S.; Futuro, D.O.; Ferreira, V.; et al. In vitro and in vivo antifungal activity of buparvaquone against Sporothrix brasiliensis. Antimicrob. Agents Chemother. 2021, 65, e0069921. [Google Scholar] [CrossRef]
- Díaz-Jiménez, D.F.; Pérez-García, L.A.; Martínez-Álvarez, J.A.; Mora-Montes, H.M. Role of the fungal cell wall in pathogenesis and antifungal resistance. Curr. Fungal Infect. Rep. 2012, 6, 275–282. [Google Scholar] [CrossRef]
- Latge, J.P.; Beauvais, A. Functional duality of the cell wall. Curr. Opin. Microbiol. 2014, 20, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Toustou, C.; Walet-Balieu, M.-L.; Kiefer-Meyer, M.-C.; Houdou, M.; Lerouge, P.; Foulquier, F.; Bardor, M. Towards understanding the extensive diversity of protein -glycan structures in eukaryotes. Biol. Rev. 2022, 97, 732–748. [Google Scholar] [CrossRef]
- Mouyna, I.; Kniemeyer, O.; Jank, T.; Loussert, C.; Mellado, E.; Aimanianda, V.; Beauvais, A.; Wartenberg, D.; Sarfati, J.; Bayry, J.; et al. Members of protein O-mannosyltransferase family in Aspergillus fumigatus differentially affect growth, morphogenesis and viability. Mol. Microbiol. 2010, 76, 1205–1221. [Google Scholar] [CrossRef]
- Willer, T.; Brandl, M.; Sipiczki, M.; Strahl, S. Protein O-mannosylation is crucial for cell wall integrity, septation and viability in fission yeast. Mol. Microbiol. 2005, 57, 156–170. [Google Scholar] [CrossRef]
- Willger, S.D.; Ernst, J.F.; Alspaugh, J.A.; Lengeler, K.B. Characterization of the PMT gene family in Cryptococcus neoformans. PLoS ONE 2009, 4, e6321. [Google Scholar] [CrossRef]
- Buurman, E.T.; Westwater, C.; Hube, B.; Brown, A.J.; Odds, F.C.; Gow, N.A. Molecular analysis of CaMnt1p, a mannosyl transferase important for adhesion and virulence of Candida albicans. Proc. Natl. Acad. Sci. USA 1998, 95, 7670–7675. [Google Scholar] [CrossRef]
- Ernst, J.F.; Prill, S.K. O-glycosylation. Med. Mycol. 2001, 39 (Suppl. S1), 67–74. [Google Scholar] [CrossRef]
- Olson, G.M.; Fox, D.S.; Wang, P.; Alspaugh, J.A.; Buchanan, K.L. Role of protein O-mannosyltransferase Pmt4 in the morphogenesis and virulence of Cryptococcus neoformans. Eukaryot. Cell 2007, 6, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Levin, D.E. Regulation of cell wall biogenesis in Saccharomyces cerevisiae: The cell wall integrity signaling pathway. Genetics 2011, 189, 1145–1175. [Google Scholar] [CrossRef] [PubMed]
- Dichtl, K.; Samantaray, S.; Wagener, J. Cell wall integrity signalling in human pathogenic fungi. Cell Microbiol. 2016, 18, 1228–1238. [Google Scholar] [CrossRef] [PubMed]
- de Nobel, H.; Ruiz, C.; Martin, H.; Morris, W.; Brul, S.; Molina, M.; Klis, F.M. Cell wall perturbation in yeast results in dual phosphorylation of the Slt2/Mpk1 MAP kinase and in an Slt2-mediated increase in FKS2-lacZ expression, glucanase resistance and thermotolerance. Microbiology 2000, 146 Pt 9, 2121–2132. [Google Scholar] [CrossRef]
- Cantero, P.D.; Ernst, J.F. Damage to the glycoshield activates PMT-directed O-mannosylation via the Msb2-Cek1 pathway in Candida albicans. Mol. Microbiol. 2011, 80, 715–725. [Google Scholar] [CrossRef]
- Neubert, P.; Strahl, S. Protein O-mannosylation in the early secretory pathway. Curr. Opin. Cell Biol. 2016, 41, 100–108. [Google Scholar] [CrossRef]
- Sánchez-Herrera, R.; Flores-Villavicencio, L.L.; Pichardo-Molina, J.L.; Castruita-Domínguez, J.P.; Aparicio-Fernández, X.; Sabanero López, M.; Villagómez-Castro, J.C. Analysis of biofilm formation by Sporothrix schenckii. Med. Mycol. 2021, 59, 31–40. [Google Scholar] [CrossRef]
- Brown, G.D.; Herre, J.; Williams, D.L.; Willment, J.A.; Marshall, A.S.; Gordon, S. Dectin-1 mediates the biological effects of beta-glucans. J. Exp. Med. 2003, 197, 1119–1124. [Google Scholar] [CrossRef]
- Reid, D.M.; Gow, N.A.; Brown, G.D. Pattern recognition: Recent insights from Dectin-1. Curr. Opin. Immunol. 2009, 21, 30–37. [Google Scholar] [CrossRef]
- Netea, M.G.; Gow, N.A.; Munro, C.A.; Bates, S.; Collins, C.; Ferwerda, G.; Hobson, R.P.; Bertram, G.; Hughes, H.B.; Jansen, T.; et al. Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and Toll-like receptors. J. Clin. Investig. 2006, 116, 1642–1650. [Google Scholar] [CrossRef]


| Microorganisms | Strain | Genotype |
|---|---|---|
| Escherichia coli | DH5α | F- Φ80lacZΔM15 Δ(lacZYAargF) U169 recA1 endA1 hsdR17 (rk-, mk+) phoA supE44 λ-thi-1 gyrA96 relA1 |
| Agrobacterium tumefaciens | AGL1 | AGL0 (C58 pTiBo542) recA::bla, T-region deleted Mop (+) Cb(R) |
| S. schenckii | 1099-18 ATCC MYA 4821 | Wild-Type |
| S. schenckii | HSS49 HSS50 HSS51 | Strain 1099-18 ATCC MYA 4821 transformed with pCambia-Nou-MNT1 |
| S. schenckii | HSS54 HSS55 HSS56 | Strain 1099-18 ATCC MYA 4821 transformed with pBGgHg-PMT2 |
| S. schenckii | HSS39 HSS40 | Strain 1099-18 ATCC MYA 4821 transformed with pBGgHg |
| S. schenckii | HSS67 HSS68 | Strain 1099-18 ATCC MYA 4821 transformed with pCambia-Nou |
| Strain | Cell Wall Protein Content (µg mg Cell Wall−1) * | Alcian Blue Bound (µg OD600nm = 1.0−1) * |
|---|---|---|
| WT | 186.5 ± 32.4 | 113.5 ± 12.5 |
| HSS67 | 195.3 ± 28.4 | 118.1 ± 11.2 |
| HSS68 | 188.6 ± 35.6 | 116.8 ± 19.2 |
| HSS49 | 245.6 ± 48.2 † | 6.5 ± 5.3 † |
| HSS50 | 239.8 ± 36.4 † | 12.4 ± 7.9 † |
| HSS51 | 249.2 ± 41.1 † | 9.8 ± 5.6 † |
| HSS39 | 188.5 ± 26.8 | 115.9 ± 21.5 |
| HSS40 | 195.2 ± 35.5 | 110.5 ± 18.5 |
| HSS54 | 268.5 ± 42.3 † | 40.7 ± 21.4 † |
| HSS55 | 258.2 ± 39.5 † | 32.4 ± 12.5 † |
| HSS56 | 271.3 ± 33.8 † | 35.7 ± 17.7 † |
| Strain | Secreted Protease Activity (U) * | Intracellular Protease Activity (U) | Secreted Lipase Activity (U) | Intracellular Lipase Activity (U) |
|---|---|---|---|---|
| WT | 1280.1 ± 258.8 | 3956.1 ± 358.6 | 412.5 ± 48.5 | 386.4 ± 68.5 |
| HSS67 | 1145.6 ± 285.7 | 3845.3 ± 324.5. | 435.6 ± 56.8 | 378.5 ± 56.8 |
| HSS68 | 1205.5 ± 305.0 | 4102.5 ± 389.6 | 422.5 ± 42.1 | 401.0 ± 48.1 |
| HSS49 | 656.2 ± 225.3 † | 4258.2 ± 412.5 | 98.5 ± 36.5 † | 423.4 ± 63.5 |
| HSS50 | 708.4 ± 306.5 † | 4125.3 ± 435.2 | 77.8 ± 45.5 † | 389.7 ± 45.7 |
| HSS51 | 777.5 ± 215.8 † | 4356.1 ± 386.4 | 102.5 ± 48.7 † | 405.2 ± 66.5 |
| HSS39 | 1178.3 ± 296.7 | 4025.4 ± 401.5 | 435.6 ± 26.5 | 398.4 ± 78.9 |
| HSS40 | 1258.4 ± 298.5 | 3953.5 ± 385.7 | 425.8 ± 29.8 | 412.4 ± 85.7 |
| HSS54 | 325.1 ± 301.5 † ‡ | 5199.2 ± 356.2 † ‡ | 45.8 ± 45.6 † ‡ | 658.4 ± 96.4 † ‡ |
| HSS55 | 268.1 ± 369.2 † ‡ | 5258.3 ± 478.5 † ‡ | 56.7 ± 52.4 † ‡ | 703.1 ± 55.5 † ‡ |
| HSS56 | 298.5 ± 333.1 † ‡ | 5124.5 ± 402.8 † ‡ | 49.5 ± 47.8 † ‡ | 688.7 ± 88.4 † ‡ |
| Strain | Colony-Forming Units (×105) a | Cytotoxicity (%) b | Hemocytes (×106) mL−1 | Melanin c | Phenoloxidase d |
|---|---|---|---|---|---|
| PBS e | 0.0 ± 0.0 | 11.8 ± 3.4 | 3.4 ± 0.6 | 1.4 ± 0.8 | 0.5 ± 0.3 |
| WT f | 3.4 ± 0.7 | 96.1 ± 8.9 | 8.0 ± 0.4 | 5.6 ± 0.8 | 3.9 ± 0.8 |
| HSS67 | 3.4 ± 0.6 | 91.2 ± 6.6 | 7.7 ± 0.9 | 5.8 ± 0.4 | 3.9 ± 0.5 |
| HSS68 | 3.1 ± 0.4 | 98.0 ± 9.7 | 7.9 ±0.7 | 5.9 ± 0.9 | 3.4 ± 0.9 |
| HSS49 | 3.2 ± 0.8 | 22.4 ± 5.5 * | 3.9 ± 0.6 * | 2.2 ± 0.6 * | 1.3 ± 0.4 * |
| HSS50 | 2.9 ± 1.0 | 30.5 ± 9.9 * | 4.0 ± 0.6 * | 2.4 ± 0.2 * | 1.0 ± 0.3 * |
| HSS51 | 3.1± 0.8 | 22.1 ± 7.7 * | 3.5 ± 0.8 * | 1.9 ± 0.6 * | 1.1 ± 0.9 * |
| HSS39 | 3.3 ± 0.8 | 94.9 ± 7.9 | 7.9 ± 0.4 | 5.3 ± 0.8 | 4.1 ± 0.9 |
| HSS40 | 3.5 ± 0.5 | 97.5 ± 7.7 | 8.2 ± 0.9 | 5.4 ± 0.7 | 3.7 ± 0.8 |
| HSS54 | 3.0 ± 0.9 | 18.4 ± 7.7 * | 3.4 ± 0.5 * | 1.5 ± 0.8 * | 0.9 ± 0.3 * |
| HSS55 | 3.3 ± 0.7 | 12.5 ± 6.8 * | 3.8 ± 0.7 * | 1.6 ± 0.2 * | 0.7 ± 0.2 * |
| HSS56 | 3.2 ± 0.9 | 17.5 ± 5.4 * | 3.9 ± 0.9 * | 1.9 ± 0.7 * | 1.1 ± 0.8 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Gaviria, M.; Martínez-Álvarez, J.A.; Martínez-Duncker, I.; Baptista, A.R.d.S.; Mora-Montes, H.M. Silencing of MNT1 and PMT2 Shows the Importance of O-Linked Glycosylation During the Sporothrix schenckii–Host Interaction. J. Fungi 2025, 11, 352. https://doi.org/10.3390/jof11050352
Gómez-Gaviria M, Martínez-Álvarez JA, Martínez-Duncker I, Baptista ARdS, Mora-Montes HM. Silencing of MNT1 and PMT2 Shows the Importance of O-Linked Glycosylation During the Sporothrix schenckii–Host Interaction. Journal of Fungi. 2025; 11(5):352. https://doi.org/10.3390/jof11050352
Chicago/Turabian StyleGómez-Gaviria, Manuela, José A. Martínez-Álvarez, Iván Martínez-Duncker, Andrea Regina de Souza Baptista, and Héctor M. Mora-Montes. 2025. "Silencing of MNT1 and PMT2 Shows the Importance of O-Linked Glycosylation During the Sporothrix schenckii–Host Interaction" Journal of Fungi 11, no. 5: 352. https://doi.org/10.3390/jof11050352
APA StyleGómez-Gaviria, M., Martínez-Álvarez, J. A., Martínez-Duncker, I., Baptista, A. R. d. S., & Mora-Montes, H. M. (2025). Silencing of MNT1 and PMT2 Shows the Importance of O-Linked Glycosylation During the Sporothrix schenckii–Host Interaction. Journal of Fungi, 11(5), 352. https://doi.org/10.3390/jof11050352







