CVF1 Promotes Invasive Candida albicans Infection via Inducing Ferroptosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Fungal Strains and Mice
2.3. Construction of Strains
2.4. Mouse Infection Assay
2.5. Infection of BMDMs with C. albicans
2.6. LDH Assays
2.7. Macrophage-Mediated Killing of C. albicans
2.8. Measurement of Cytokine Production
2.9. Measurement of GSH, GPX4, 4-HNE and MDA Levels
2.10. Statistical Analysis
3. Results
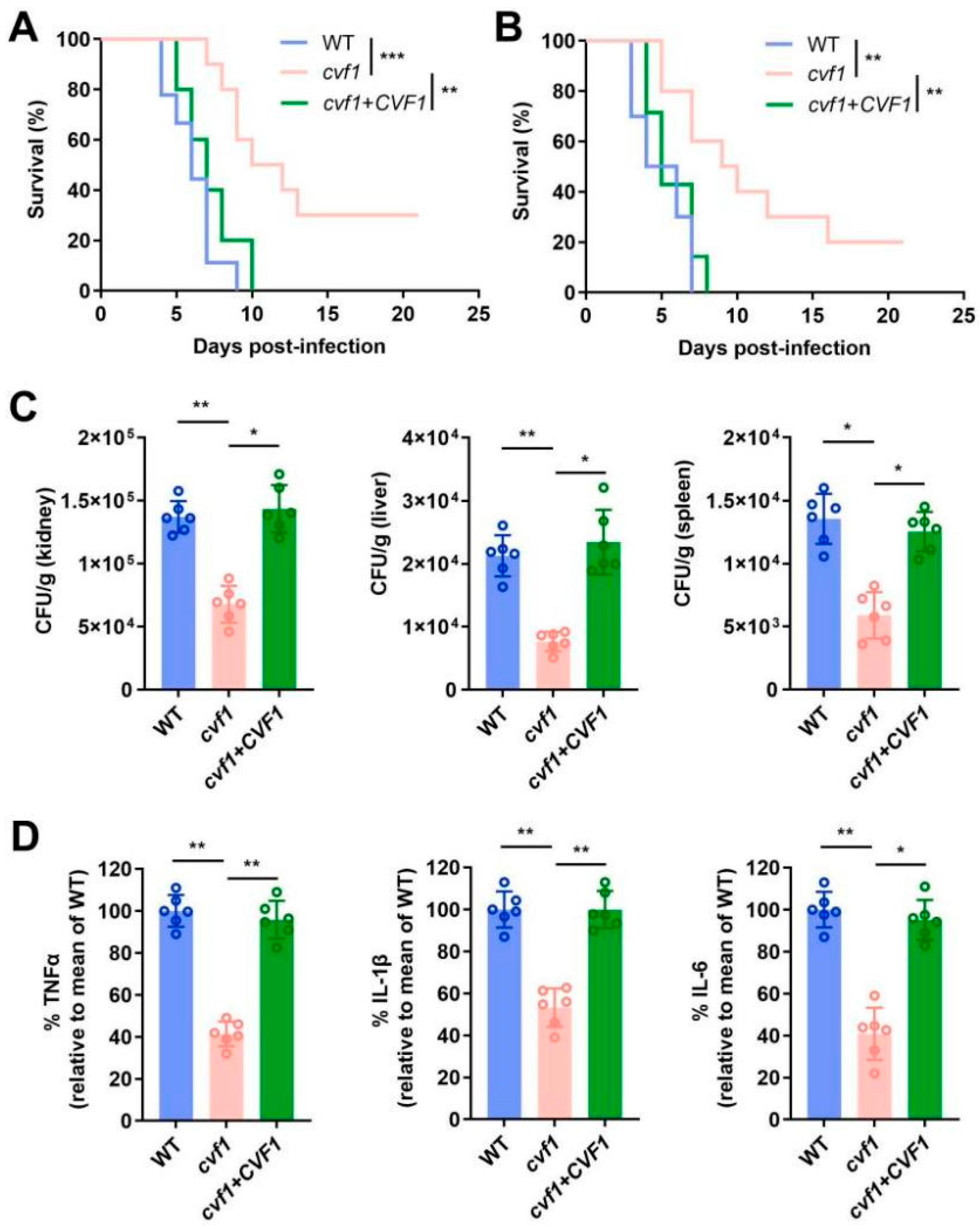
3.1. CVF1 Is Essential for C. albicans Pathogenicity During Systemic Infection
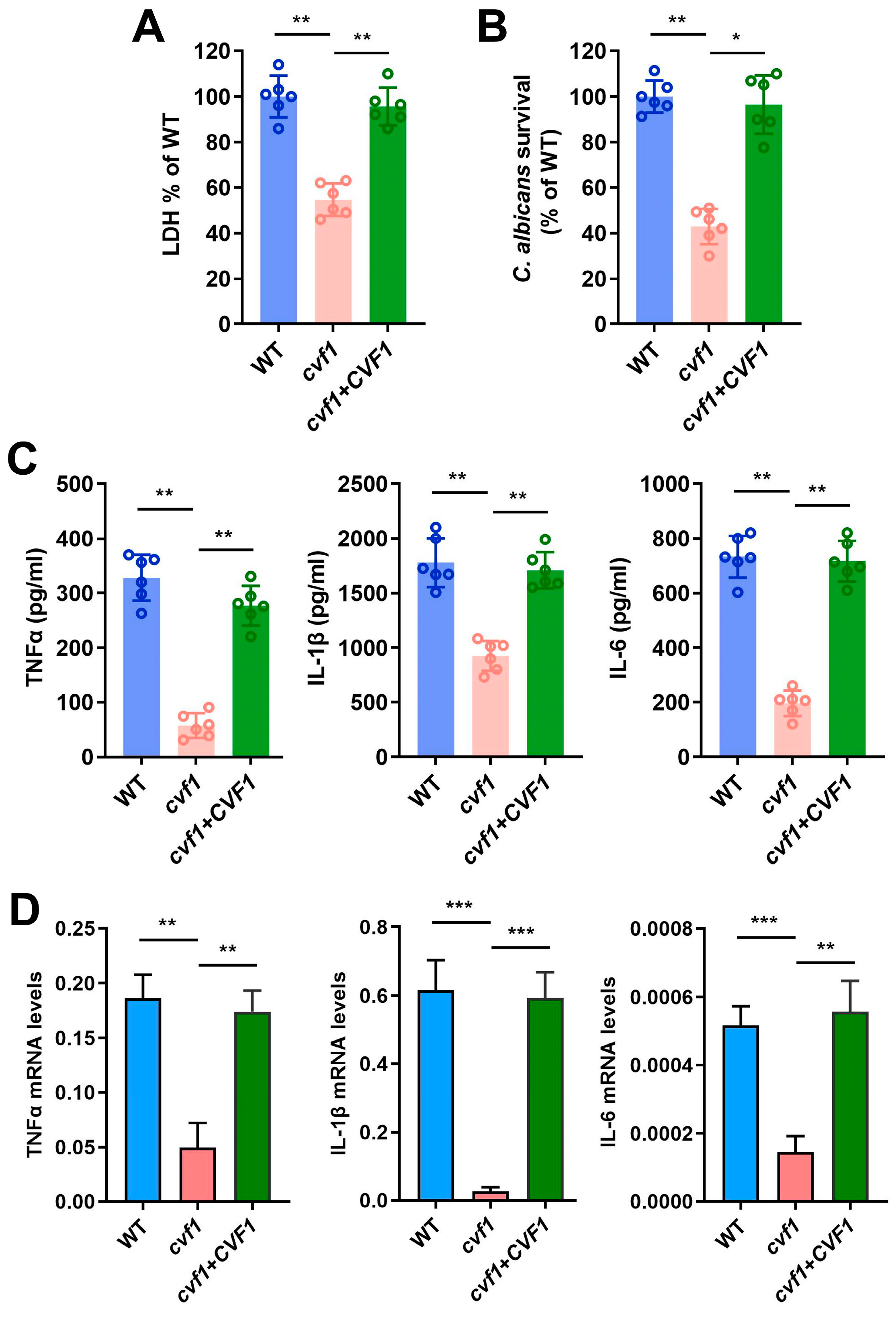
3.2. CVF1 Induces the Death of C. albicans-Infected Macrophages
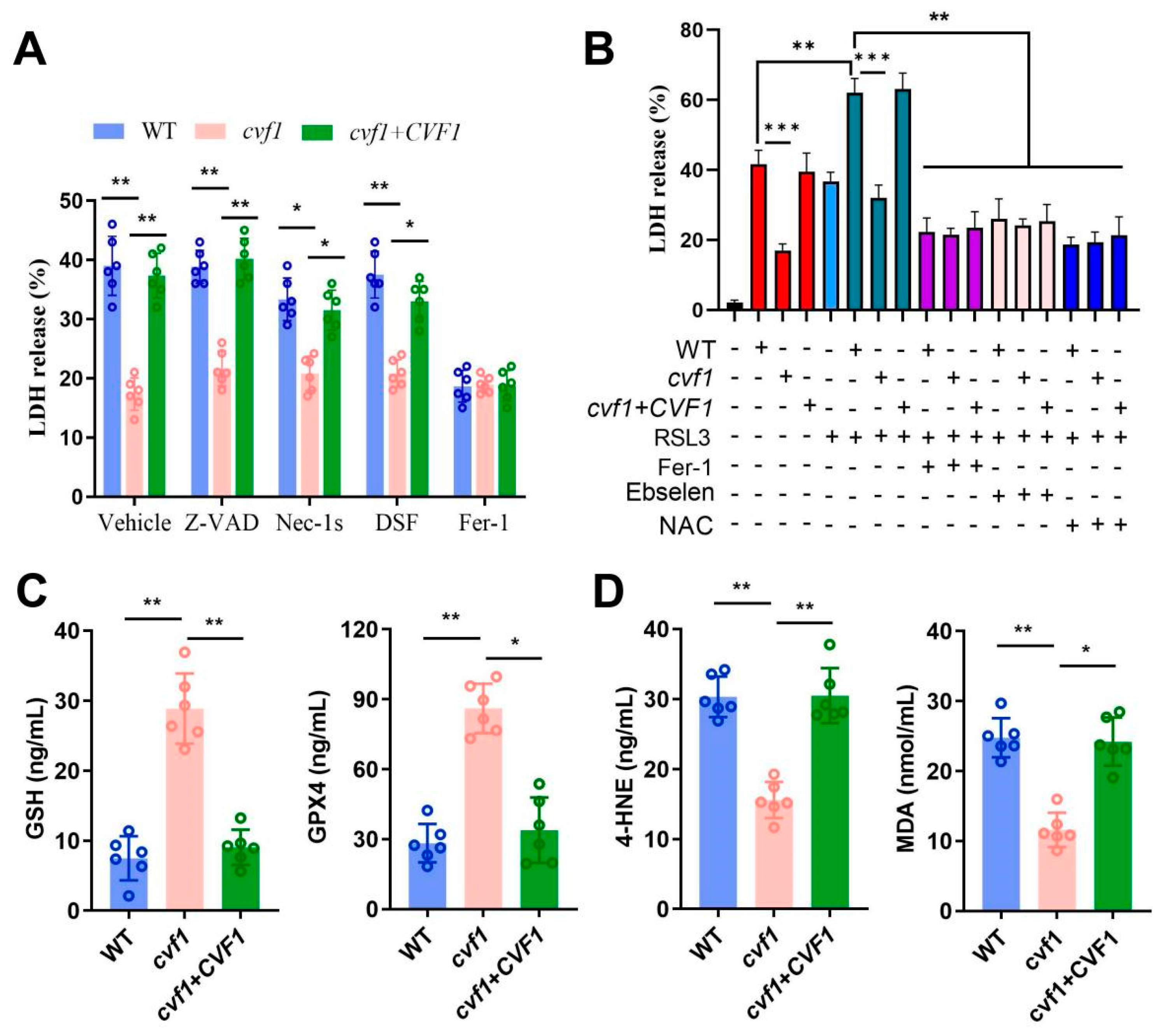
3.3. CVF1-Induced Macrophage Death Correlates with a Peroxidized Lipid Signature
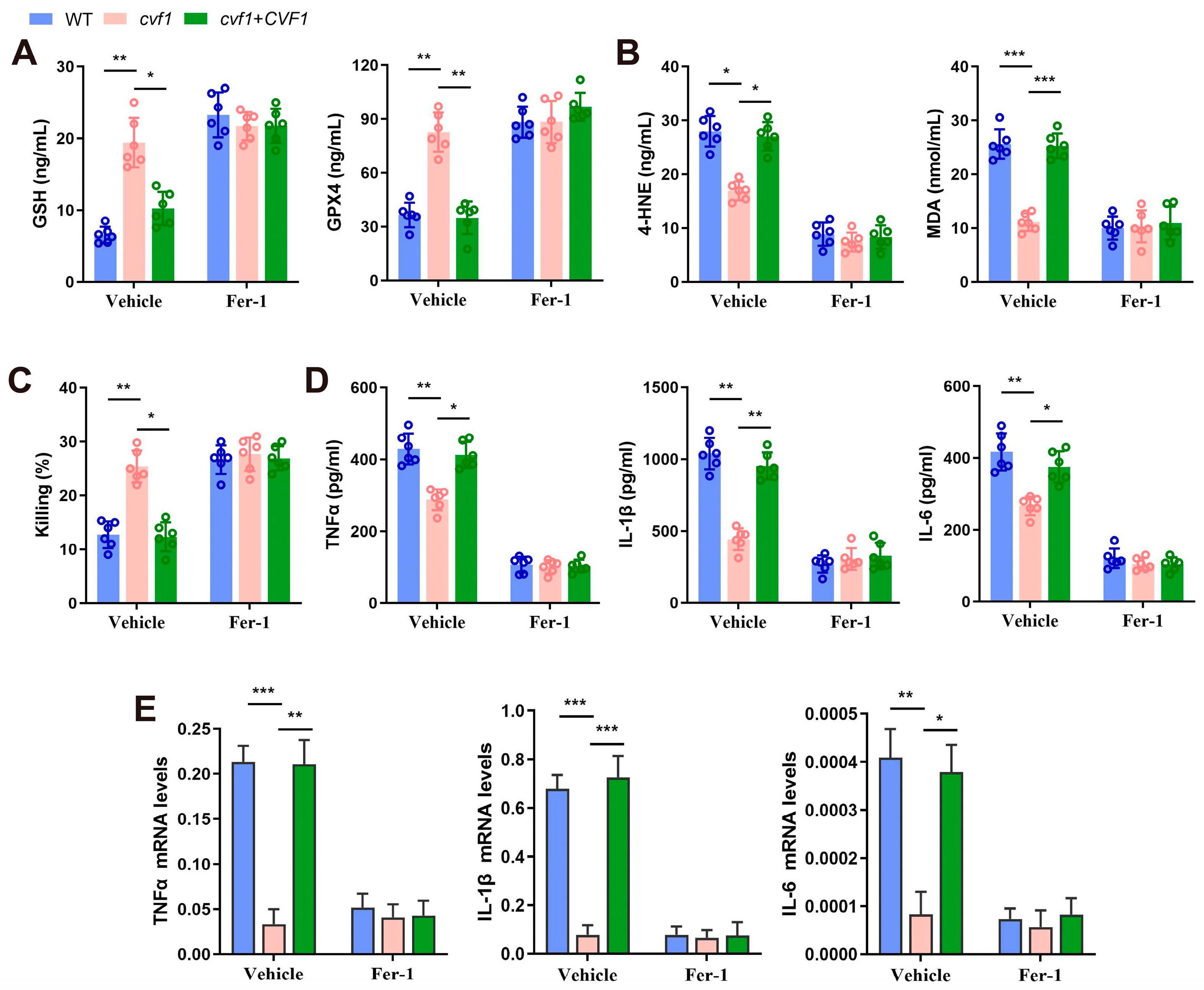
3.4. CVF1-Induced Ferroptosis Decreases Fungal Killing and Promotes Inflammation in Macrophages
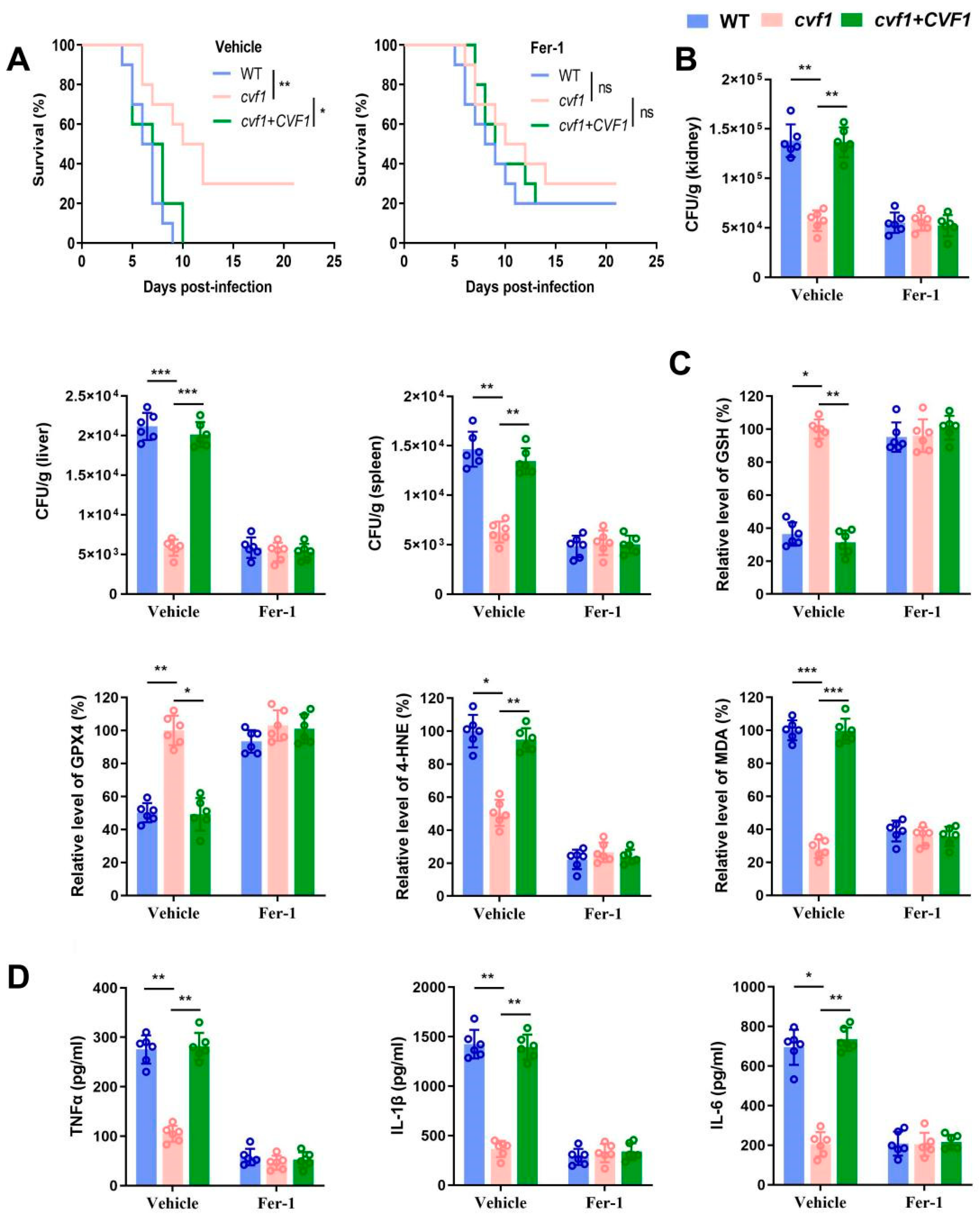
3.5. CVF1 Induces Ferroptosis to Enhance C. albicans Pathogenicity and Dissemination In Vivo
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Denning, D.W.; Bromley, M.J. How to bolster the antifungal pipeline. Science 2015, 347, 1414–1416. [Google Scholar] [CrossRef]
- Ding, X.; Kambara, H.; Guo, R.; Kanneganti, A.; Acosta-Zaldívar, M.; Li, J.; Liu, F.; Bei, T.; Qi, W.; Xie, X.; et al. Inflammasome-mediated GSDMD activation facilitates escape of Candida albicans from macrophages. Nat. Commun. 2021, 12, 6699. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Denning, D.W.; Gow, N.A.R.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden killers: Human fungal infections. Sci. Transl. Med. 2012, 4, 165rv113. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of invasive candidiasis: A persistent public health problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef] [PubMed]
- Lass-Flörl, C.; Kanj, S.S.; Govender, N.P.; Thompson, G.R., III; Ostrosky-Zeichner, L.; Govrins, M.A. Invasive candidiasis. Nat. Rev. Dis. Primerss. 2024, 10, 20. [Google Scholar] [CrossRef]
- Katsipoulaki, M.; Stappers, M.H.; Malavia-Jones, D.; Brunke, S.; Hube, B.; Gow, N.A. Candida albicans and Candida glabrata: Global priority pathogens. Microbiol. Mol. Biol. Rev. 2024, 88, e0002123. [Google Scholar] [CrossRef]
- Erwig, L.P.; Gow, N.A. Interactions of fungal pathogens with phagocytes. Nat. Rev. Microbiol. 2016, 14, 163–176. [Google Scholar] [CrossRef]
- Domingues, N.; Gonçalves, T.; Girao, H. Phagolysosomal remodeling to confine Candida albicans in the macrophage. Trends Microbiol. 2022, 30, 519–523. [Google Scholar] [CrossRef]
- Austermeier, S.; Kasper, L.; Westman, J.; Gresnigt, M.S. I want to break free—Macrophage strategies to recognize and kill Candida albicans, and fungal counter-strategies to escape. Curr. Opin. Microbiol. 2020, 58, 15–23. [Google Scholar] [CrossRef]
- Gaspar, M.L.; Pawlowska, T.E. Innate immunity in fungi: Is regulated cell death involved? PLoS Pathog. 2022, 18, e1010460. [Google Scholar] [CrossRef]
- Gao, J.; Wang, Q.; Tang, Y.-D.; Zhai, J.; Hu, W.; Zheng, C. When ferroptosis meets pathogenic infections. Trends Microbiol. 2023, 31, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, B.R. Ferroptosis turns 10: Emerging mechanisms, physiological functions, and therapeutic applications. Cell 2022, 185, 2401–2421. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Kang, R.; Kroemer, G.; Tang, D. Ferroptosis in infection, inflammation, and immunity. J. Exp. Med. 2021, 218, e20210518. [Google Scholar] [CrossRef]
- Dixon, S.J.; Pratt, D.A. Ferroptosis: A flexible constellation of related biochemical mechanisms. Mol. Cell 2023, 83, 1030–1042. [Google Scholar] [CrossRef]
- Bell, H.N.; Stockwell, B.R.; Zou, W. Ironing out the role of ferroptosis in immunity. Immunity 2024, 57, 941–956. [Google Scholar] [CrossRef]
- Dar, H.H.; Tyurina, Y.Y.; Mikulska-Ruminska, K.; Shrivastava, I.; Ting, H.-C.; Tyurin, V.A.; Krieger, J.; Croix, C.M.S.; Watkins, S.; Bayir, E.; et al. Pseudomonas aeruginosa utilizes host polyunsaturated phosphatidylethanolamines to trigger theft-ferroptosis in bronchial epithelium. J. Clin. Investig. 2018, 128, 4639–4653. [Google Scholar] [CrossRef]
- Amaral, E.P.; Costa, D.L.; Namasivayam, S.; Riteau, N.; Kamenyeva, O.; Mittereder, L.; Mayer-Barber, K.D.; Andrade, B.B.; Sher, A. A major role for ferroptosis in Mycobacterium tuberculosis-induced cell death and tissue necrosis. J. Exp. Med. 2019, 216, 556–570. [Google Scholar] [CrossRef] [PubMed]
- Qiang, L.; Zhang, Y.; Lei, Z.; Lu, Z.; Tan, S.; Ge, P.; Chai, Q.; Zhao, M.; Zhang, X.; Li, B.; et al. A mycobacterial effector promotes ferroptosis-dependent pathogenicity and dissemination. Nat. Commun. 2023, 14, 1430. [Google Scholar] [CrossRef]
- Amaral, E.P.; Namasivayam, S.; Queiroz, A.T.L.; Fukutani, E.; Hilligan, K.L.; Aberman, K.; Fisher, L.; Bomfim, C.C.B.; Kauffman, K.; Buchanan, J.; et al. BACH1 promotes tissue necrosis and Mycobacterium tuberculosis susceptibility. Nat. Microbiol. 2024, 9, 120–135. [Google Scholar] [CrossRef]
- Dangol, S.; Chen, Y. Iron- and Reactive Oxygen Species-Dependent Ferroptotic Cell Death in Rice-Magnaporthe oryzae Interactions. Plant Cell 2019, 31, 189–209. [Google Scholar] [CrossRef]
- Millet, N.; Solis, N.V.; Aguilar, D.; Lionakis, M.S.; Wheeler, R.T.; Jendzjowsky, N.; Swidergall, M. IL-23 signaling prevents ferroptosis-driven renal immunopathology during candidiasis. Nat. Commun. 2022, 13, 5545. [Google Scholar] [CrossRef] [PubMed]
- Noble, S.M.; Johnson, A.D. Strains and strategies for large-scale gene deletion studies of the diploid human fungal pathogen Candida albicans. Eukaryot. Cell 2005, 4, 298–309. [Google Scholar] [CrossRef]
- Noble, S.M.; French, S.; Kohn, L.A.; Chen, V.; Johnson, A.D. Systematic screens of a Candida albicans homozygous deletion library decouple morphogenetic switching and pathogenicity. Nat. Genet. 2010, 42, 590–598. [Google Scholar] [CrossRef]
- Kasper, L.; König, A.; Koenig, P.A.; Gresnigt, M.S.; Westman, J.; Drummond, R.A.; Lionakis, M.S.; Groß, O.; Ruland, J.; Naglik, J.R.; et al. The fungal peptide toxin Candidalysin activates the NLRP3 inflammasome and causes cytolysis in mononuclear phagocytes. Nat. Commun. 2018, 9, 4260. [Google Scholar] [CrossRef]
- Silao, F.G.S.; Ryman, K. Glutamate dehydrogenase (Gdh2)-dependent alkalization is dispensable for escape from macrophages and virulence of Candida albicans. PLoS Pathog. 2020, 16, e1008328. [Google Scholar] [CrossRef]
- Wang, W.; Deng, Z.; Wu, H.; Zhao, Q.; Li, T.; Zhu, W.; Wang, X.; Tang, L.; Wang, C.; Cui, S.-Z.; et al. A small secreted protein triggers a TLR2/4-dependent inflammatory response during invasive Candida albicans infection. Nat. Commun. 2019, 10, 1015. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, Y.; Chen, X.; Li, H.; Yin, Z.; Zhang, B.; Xu, Y.; Zhang, Y.; Zhang, R.; Huang, X.; et al. Innate immune responses against the fungal pathogen Candida auris. Nat. Commun. 2022, 13, 3553. [Google Scholar] [CrossRef]
- Amorim-Vaz, S.; Tran, V.D.T.; Pradervand, S.; Pagni, M.; Coste, A.T.; Sanglard, D. RNA Enrichment Method for Quantitative Transcriptional Analysis of Pathogens In Vivo Applied to the Fungus Candida albicans. mBio 2015, 6, e00942-15. [Google Scholar] [CrossRef]
- Moyes, D.L.; Wilson, D.; Richardson, J.P.; Mogavero, S.; Tang, S.X.; Wernecke, J.; Höfs, S.; Gratacap, R.L.; Robbins, J.; Runglall, M.; et al. Candidalysin is a fungal peptide toxin critical for mucosal infection. Nature 2016, 532, 64–68. [Google Scholar] [CrossRef]
- Phan, Q.T.; Myers, C.L.; Fu, Y.; Sheppard, D.C.; Yeaman, M.R.; Welch, W.H.; Ibrahim, A.S.; Edwards, J.E., Jr.; Filler, S.G. Als3 is a Candida albicans invasin that binds to cadherins and induces endocytosis by host cells. PLoS Biol. 2007, 5, e64. [Google Scholar] [CrossRef]
- Banerjee, M.; Thompson, D.S.; Lazzell, A.; Carlisle, P.L.; Pierce, C.; Monteagudo, C.; Lopez-Ribot, J.L.; Kadosh, D. UME6, a novel filament-specific regulator of Candida albicans hyphal extension and virulence. Mol. Biol. Cell 2008, 19, 1354–1365. [Google Scholar] [CrossRef]
- Peroumal, D.; Manohar, K.; Patel, S.K. Virulence and pathogenicity of a Candida albicans mutant with reduced filamentation. Cell Microbiol. 2019, 21, e13103. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Zhang, J.; Wang, T.; Cui, H.; Bai, Y.; Luo, J.; Zhang, J.; Zhang, M.; Di, L.; Yuan, Y.; et al. A human commensal-pathogenic fungus suppresses host immunity via targeting TBK1. Cell Host Microbe 2024, 32, 1536–1551. [Google Scholar] [CrossRef]
- Ou, Y.; Wang, S.J.; Li, D.; Chu, B.; Gu, W. Activation of SAT1 engages polyamine metabolism with p53-mediated ferroptotic responses. Proc. Natl. Acad. Sci. USA 2016, 113, E6806–E6812. [Google Scholar] [CrossRef] [PubMed]
- Ousingsawat, J.; Cabrita, I.; Wanitchakool, P.; Sirianant, L.; Krautwald, S.; Linkermann, A.; Schreiber, R.; Kunzelmann, K. Ca2+ signals, cell membrane disintegration, and activation of TMEM16F during necroptosis. Cell. Mol. Life Sci. 2017, 74, 173–181. [Google Scholar] [CrossRef]
- Hu, J.J.; Liu, X. FDA-approved disulfiram inhibits pyroptosis by blocking gasdermin D pore formation. Nat. Immunol. 2020, 21, 736–745. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Bagayoko, S.; Leon-Icaza, S.A.; Pinilla, M.; Hessel, A.; Santoni, K.; Péricat, D.; Bordignon, P.J.; Moreau, F.; Eren, E.; Boyancé, A.; et al. Host phospholipid peroxidation fuels ExoU-dependent cell necrosis and supports Pseudomonas aeruginosa-driven pathology. PLoS Pathog. 2021, 17, e1009927. [Google Scholar] [CrossRef]
- Camilli, G.; Blagojevic, M.; Naglik, J.R.; Richardson, J.P. Programmed Cell Death: Central Player in Fungal Infections. Trends Cell Biol. 2021, 31, 179–196. [Google Scholar] [CrossRef]
- Noble, S.M.; Gianetti, B.A.; Witchley, J.N. Candida albicans cell-type switching and functional plasticity in the mammalian host. Nat. Rev. Microbiol. 2017, 15, 96–108. [Google Scholar] [CrossRef]
- Cairns, T.C.; Studholme, D.J.; Talbot, N.J.; Haynes, K. New and Improved Techniques for the Study of Pathogenic Fungi. Trends Microbiol. 2016, 24, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Goranov, A.I.; Madhani, H.D. Functional profiling of human fungal pathogen genomes. Cold Spring Harb. Perspect. Med. 2014, 5, a019596. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Moni, S.S.; Ali, M.; Mohan, S.; Jan, H.; Rasool, S.; A Kamal, M.; Alshahrani, S.; Halawi, M.; A Alhazmi, H. Antifungal Activity of Plant Secondary Metabolites on Candida albicans: An Updated Review. Curr. Mol. Pharmacol. 2023, 16, 15–42. [Google Scholar] [CrossRef]
- Basso, P.; Dang, E.V.; Urisman, A.; Cowen, L.E.; Madhani, H.D.; Noble, S.M. Deep tissue infection by an invasive human fungal pathogen requires lipid-based suppression of the IL-17 response. Cell Host Microbe 2022, 30, 1589–1601.e1585. [Google Scholar] [CrossRef]
- Satala, D.; Juszczak, M.; Wronowska, E.; Surowiec, M.; Kulig, K.; Kozik, A.; Rapala-Kozik, M.; Karkowska-Kuleta, J. Similarities Differences among Species Closely Related to Candida albicans: C. tropicalis, C. dubliniensis, and C. auris. Cell. Microbiol. 2022, 2022, 2599136. [Google Scholar] [CrossRef]
- Vaillancourt, M.; Galdino, A.C.M.; Limsuwannarot, S.P.; Celedonio, D.; Dimitrova, E.; Broerman, M.; Bresee, C.; Doi, Y.; Lee, J.S.; Parks, W.C.; et al. A compensatory RNase E variation increases Iron Piracy and Virulence in multidrug-resistant Pseudomonas aeruginosa during Macrophage infection. PLoS Pathog. 2023, 19, e1010942. [Google Scholar] [CrossRef]
- Dang, E.V.; Lei, S.; Radkov, A.; Volk, R.F.; Zaro, B.W.; Madhani, H.D. Secreted fungal virulence effector triggers allergic inflammation via TLR4. Nature 2022, 608, 161–167. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, G.; Ma, Y.; Chen, C.; Hu, Y.; Yan, C.; Wang, D.; Wang, C.; Wang, Y.; Yu, X.; Sibirny, A.; et al. CVF1 Promotes Invasive Candida albicans Infection via Inducing Ferroptosis. J. Fungi 2025, 11, 342. https://doi.org/10.3390/jof11050342
Luo G, Ma Y, Chen C, Hu Y, Yan C, Wang D, Wang C, Wang Y, Yu X, Sibirny A, et al. CVF1 Promotes Invasive Candida albicans Infection via Inducing Ferroptosis. Journal of Fungi. 2025; 11(5):342. https://doi.org/10.3390/jof11050342
Chicago/Turabian StyleLuo, Gang, Yongman Ma, Chunyi Chen, Yudie Hu, Chunchun Yan, Di Wang, Cong Wang, Yanyan Wang, Xichen Yu, Andriy Sibirny, and et al. 2025. "CVF1 Promotes Invasive Candida albicans Infection via Inducing Ferroptosis" Journal of Fungi 11, no. 5: 342. https://doi.org/10.3390/jof11050342
APA StyleLuo, G., Ma, Y., Chen, C., Hu, Y., Yan, C., Wang, D., Wang, C., Wang, Y., Yu, X., Sibirny, A., Yuan, J., & Kang, Y. (2025). CVF1 Promotes Invasive Candida albicans Infection via Inducing Ferroptosis. Journal of Fungi, 11(5), 342. https://doi.org/10.3390/jof11050342





